Abstract
Introduction
Fetal therapy has evolved into an established clinical specialty in fetal medicine, offering life-saving interventions for conditions with high intrauterine morbidity and mortality. Among various approaches, laser therapy has played a significant role in advancing fetal procedures.
Content
The most established application of laser is fetoscopic laser coagulation for twin-twin transfusion syndrome, which significantly reduces perinatal mortality and neurological complications. Other indications include twin anemia-polycythemia sequence and, as an intrafetal application, twin reversed arterial perfusion sequence. As an experimental approach, intrafetal laser therapy can be used to close the feeding blood vessel of unwanted tissue, thus preventing further tissue growth. Despite the advantages of fetal laser therapy, it presents challenges such as preterm premature rupture of membranes, preterm birth, technical difficulties, and the need for specialized expertise.
Summary
Laser therapy has become a cornerstone of fetal medicine, demonstrating significant clinical benefits. While laser therapy for TTTS is supported by high-level evidence, other applications rely on observational or experimental data.
Outlook
Future advancements, including improved imaging technologies, artificial intelligence, and robotic-assisted techniques, are expected to further enhance the precision, safety, and accessibility of fetal laser therapy. Large-scale studies through international collaborations will be essential to standardize protocols and improve outcomes.
Introduction
Fetal therapy has evolved significantly in recent decades, from an experimental field to an established clinical specialty with a significant impact on perinatal outcomes. A key factor is the selection of patients, as fetal surgical intervention is primarily used in conditions with a high risk of intrauterine fetal death or sequelae, since, unlike postnatal interventions, it is associated with additional risks such as iatrogenic preterm birth or maternal complications. The prenatal laser technique is primarily used to correct vascular anomalies, remove obstructions, and perform precise surgical interventions on the fetus or placenta. Its use in fetal medicine has significantly advanced the field of fetoscopy, allowing for minimally invasive procedures that can treat life-threatening conditions in utero. The first successful fetoscopic laser coagulations were performed in the early 1990s for twin-twin transfusion syndrome (TTTS) [1]. Today, fetoscopic laser has become the gold standard treatment for this condition, significantly reducing perinatal mortality and improving neurological outcomes [2], [3], [4], [5]. Since its introduction, the application of fetoscopic laser surgery in fetal medicine has expanded to include other complications of monochorionic twinning, including twin anemia-polycythemia sequence (TAPS) [6], 7]. In addition to its fetoscopic approach, laser therapy is now being used intrafetally, primarily to treat twin reversed arterial perfusion sequence (TRAP) [8], 9]. The other laser procedures discussed in this review are performed far less frequently and are reserved for rare or experimental indications, such as bronchopulmonary sequestration (BPS) [10], [11], [12], lower urinary tract obstructions (LUTO) [13] and sacrococcygeal teratomas [14]. Despite these advances, fetal laser therapy continues to face significant challenges. These include potential maternal and fetal complications such as preterm premature rupture of membranes (PPROM), spontaneous preterm birth, and procedure-related fetal loss [15]. Technical difficulties may arise in certain anatomical situations, particularly with anterior placentas or poor visualization conditions. Furthermore, the specialized training and expertise required for these procedures limit their widespread availability [16].
This narrative review provides a comprehensive overview of current laser applications in fetal medicine, explaining the fetal conditions that benefit from laser treatment and discussing its technical aspects, complications, and clinical outcomes.
Twin-to-twin transfusion syndrome
Pathophysiology and diagnosis of twin-to-twin transfusion syndrome
TTTS represents a complication that occurs in monochorionic multiples, affecting approximately 10–15 % of these cases [17]. The underlying cause of TTTS lies in the blood flow through placental anastomoses in monochorionic twins, which include arteriovenous (AV), arterioarterial (AA), and venovenous (VV) anastomoses. TTTS typically develops when multiple unidirectional AV anastomoses predominate in the absence of sufficient compensatory connections, leading to an imbalance of blood flow between the fetuses [18]. TTTS typically manifests between 16 and 26 weeks of gestation [19]. The donor twin experiences a blood volume deficit leading to oligohydramnios and decreased urinary output (oliguria). Conversely, the recipient twin develops blood volume overload, leading to polyhydramnios and increased urinary production (polyuria) [20]. To facilitate early detection of these sonographic signs, international guidelines recommend serial ultrasound examinations every two weeks from the 16th gestational week onward [21], [22], [23].
For clinical assessment and management purposes [24] several staging systems have been proposed to categorize TTTS severity. The Quintero classification remains the most widely implemented in clinical practice [25]. This system establishes five progressive stages based on ultrasonographic finding (Table 1 with Figure 1).
Staging of twin-to-twin transfusion syndrome (TTTS) according to Quintero [25].
| Stage | Ultrasound findings |
|---|---|
| I | Oligohydramnios in the donor twin’s sac and polyhydramnios in the recipient’s sac |
| II | + The donor twin’s bladder is undetectable |
| III | + Critically abnormal Doppler measurements in either twina |
| IV | + Hydrops fetalis in either twin |
| V | + Intrauterine death of one or both fetuses |
-
aCritically abnormal Doppler measurements is defined as umbilical artery Doppler with absent or reverse flow in end-diastole and/or ductus venosus Doppler with reverse a-wave (Figure 1) or and/or umbilical vein with pulsatile flow.
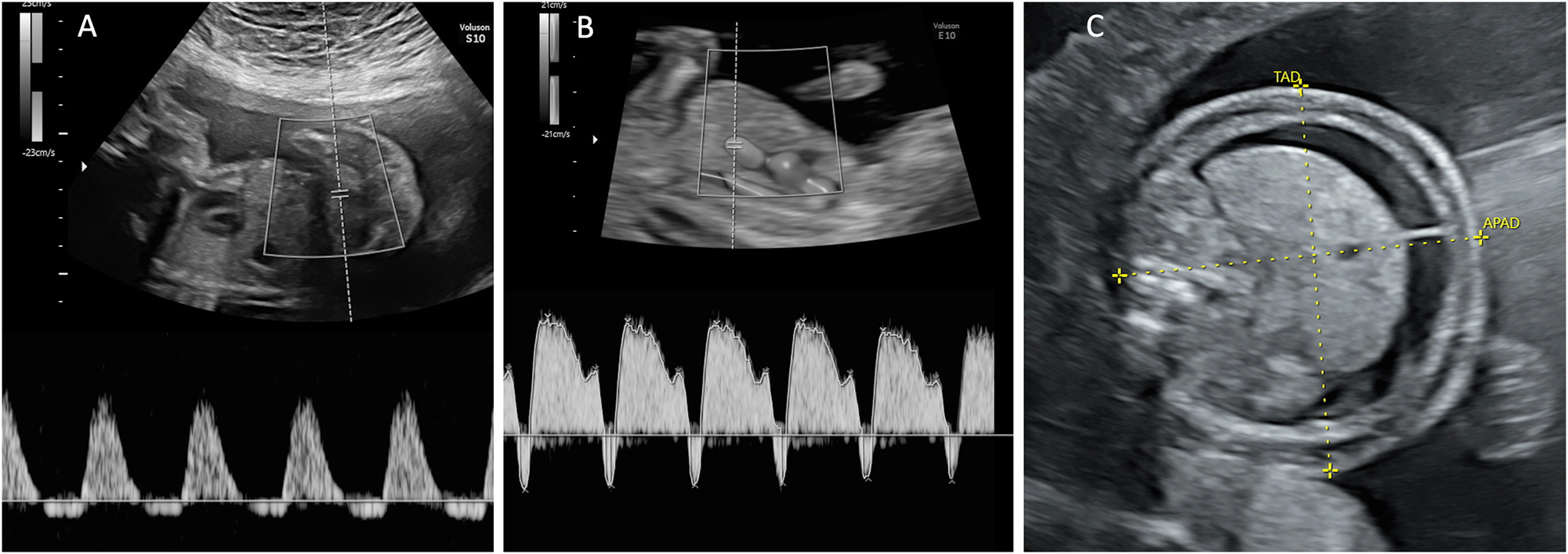
Ultrasound findings relevant for TTTS staging. (A) Umbilical artery Doppler with reverse flow in end-diastole and (B) ductus venosus Doppler with reverse a-wave. (C) Hydropic fetus with ascites and skin oedema.
Fetoscopic laser procedure for twin-to-twin transfusion syndrome
Fetoscopic laser therapy represents the only causal treatment and is considered the gold standard for TTTS management between 16 and 26 weeks of gestation, with some centers extending this window [26], [27], [28]. This is based on the multicenter, randomized, controlled landmark study published by the Eurofoetus group, which demonstrated the superiority of fetoscopic laser over serial amnioreduction. The study showed higher survival of at least one twin to age 28 days (76 % vs. 56 %; p=0.009), higher gestational age at delivery (33.3 weeks vs. 29.0 weeks; p=0.004), and higher survival rates without major neurological complications at 6 months (52 % vs. 31 %; p=0.003) [2].
Fetoscopic laser therapy for TTTS is a minimally invasive procedure. Selecting the optimal site for insertion of the fetoscope under ultrasound guidance into the recipient’s sac for laser treatment of TTTS is essential. The entry site technique varies depending on the position of the placenta, umbilical cord insertions, and fetal position. The entry site should be positioned opposite the likely vascular anastomoses on the placental surface. In the case of a posterior placenta, the entry site is close to the maternal navel. In cases where the placenta is anterior, the entry point is carefully selected along the side of the uterus to avoid coming into contact with the placenta. The authors use special scopes with 30° optics to visualize the entire vascular equator. The distal tip of the operative fetoscope is equipped with an Albarran steering lever, a mechanism inside the fetoscope that enables the laser fibre to be bent upwards (Figure 2). This allows it to target the anterior placenta and coagulate anastomoses in that area as well. With this technique, even anastomoses located close to the entry point of the fetoscope can be effectively coagulated. There is evidence that when using the 30° scopes with a special Albarran mechanism, the neonatal double survival rate is independent of the placental location [3]. The procedure is usually performed under local anesthesia with or without maternal additional sedation [29]. After identifying the anastomoses on the placental surface, these vascular connections are coagulated using a diode laser or Nd:YAG with wavelengths of 980 nm or 810 nm, thereby interrupting the pathological inter-twin blood flow (Figure 3). Following successful laser coagulation, amnioreduction is performed to normalize the amniotic fluid volume in the recipient’s sac. Studies have demonstrated that operator experience significantly influences outcomes, with higher success rates for experienced fetal surgeons [16]. Over the years, various technical approaches to fetoscopic laser coagulation for TTTS have been developed. An overview is provided below.
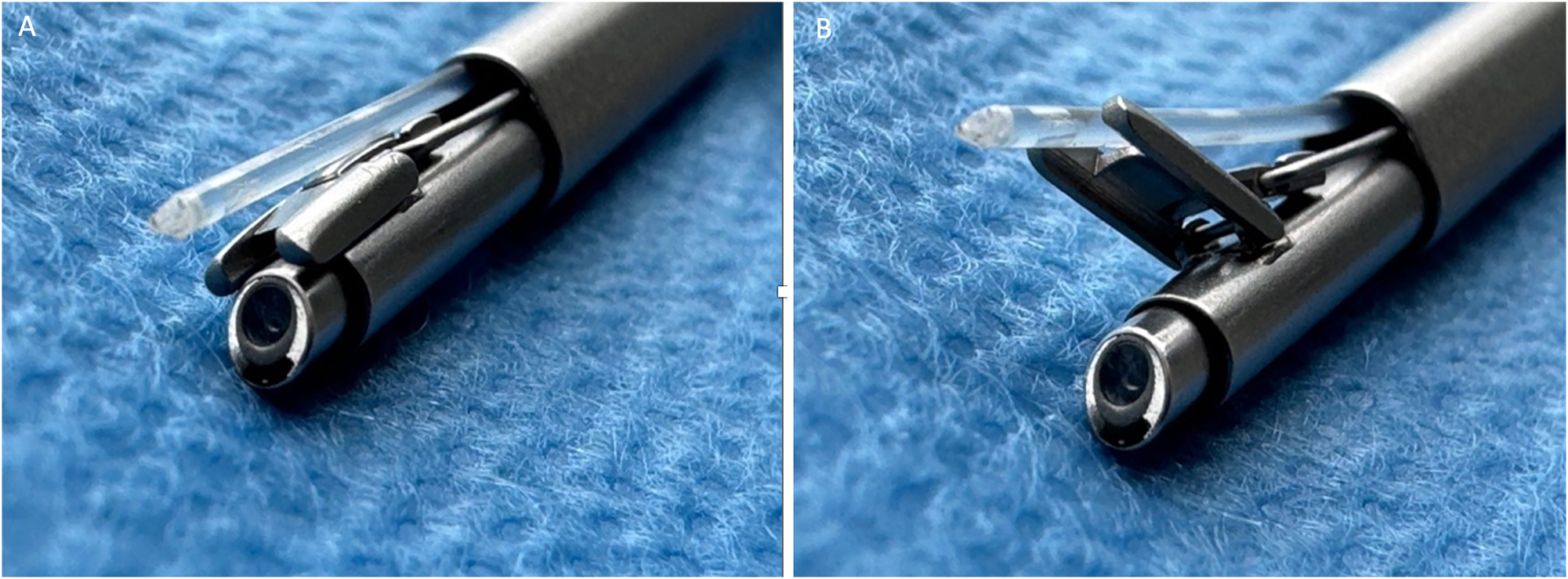
Distal tip of a 30° fetoscope with Albarran steering lever. (A) Laser fiber in resting position. (B) Laser fiber in deflected position.
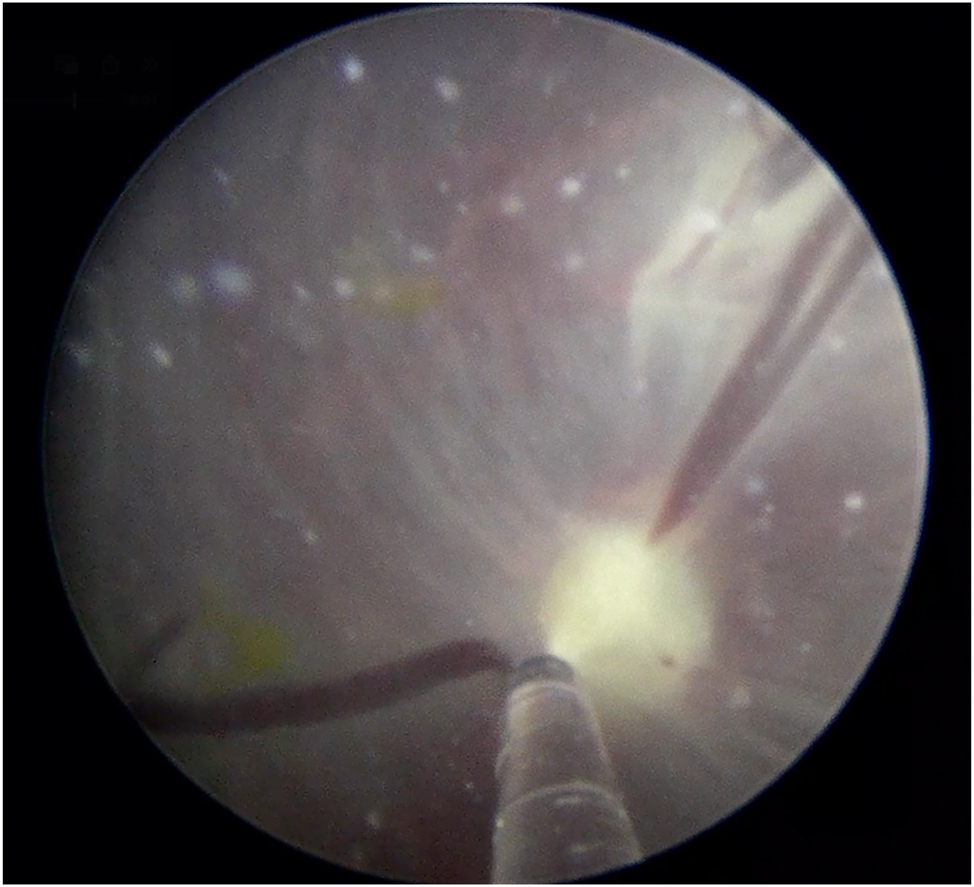
Fetoscopic laser coagulation of an anastomosis on the placental surface.
Selective laser coagulation
This approach specifically targets only the anastomoses connecting the two fetuses in no particular order while preserving vascular connections that remain within the territory of each individual fetus. This selective method improves fetal outcomes compared to a non-selective technique [30], in which all blood vessels on the placental surface crossing the intertwin membrane are coagulated without specifically targeting the actual vascular equator [31].
Solomon technique
The Solomon technique is a modified laser surgery method where a continuous laser line is made to connect all coagulated blood vessel connections (anastomoses) along the vascular equator (Figure 4). Randomized controlled trials have shown that this method significantly lowers the risk of leaving residual anastomoses [32], 33]. A landmark study by Slaghekke et al. found that the Solomon technique also reduces complications like post laser TAPS (Twin Anemia-Polycythemia Sequence) and recurrent TTTS. However, it does not significantly reduce the overall risk of death in affected pregnancies [32]. One concern is that the Solomon technique uses more laser energy, which has been linked to a higher chance of placental abruption (14 % compared to 3 % [34]). Also, Akkermans et al. found that damage to the placenta is linked to earlier births [35]. To reduce these risks, a modified approach has emerged, called the partial Solomon technique. In this version, the laser line skips large areas of the placenta that don’t have visible blood vessels [36]. The authors of this study prefer this approach.
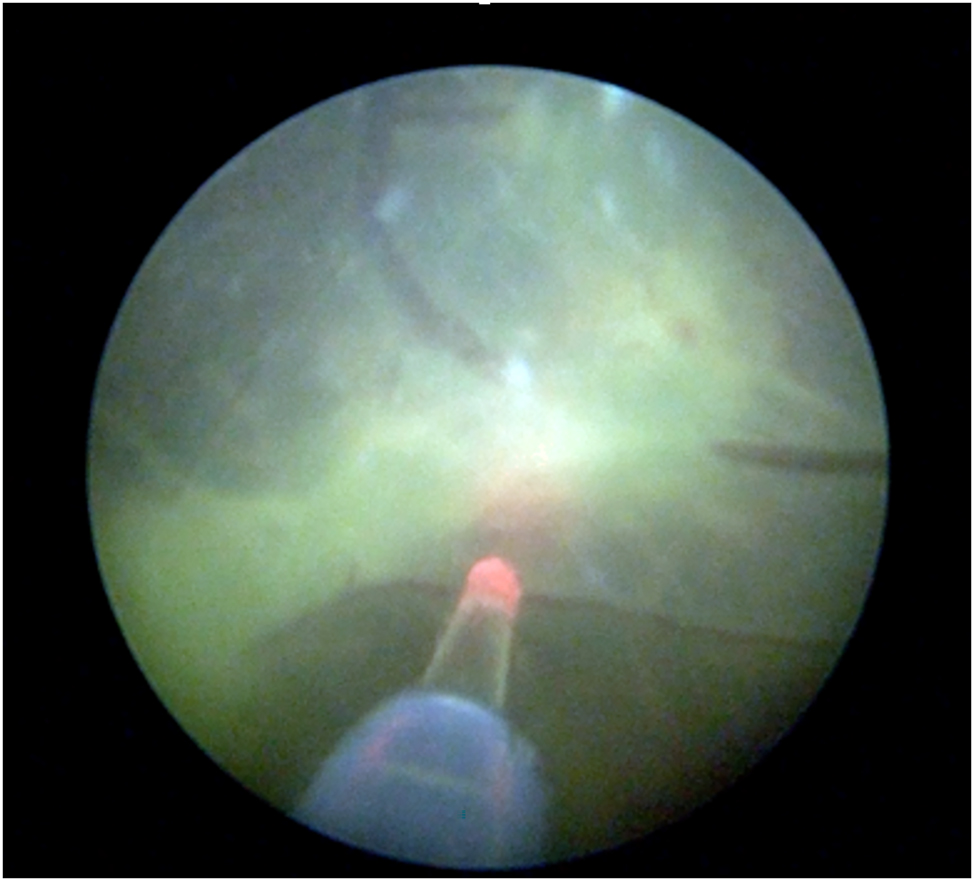
Fetoscopic image of the Solomon technique: continuous laser ablation along the vascular equator.
Sequential selective laser technique
This approach involves coagulating anastomoses in a specific predetermined sequence [37]. Arteriovenous (AV) anastomoses from donor to recipient are closed first, followed by AV anastomoses from recipient to donor and arterio-arterial (AA) and veno-venous (VV) anastomoses are lasered last. This technique is intended to reduce the risk of hypotension in the donor twin, thereby lowering the likelihood of intrauterine demise. Recently Chmait and coworkers published a randomized controlled trial suggesting that donor twin survival may improve with the sequential laser technique when AA anastomoses are present and/or the donor exhibits critical abnormal Doppler findings preoperatively (TTTS Stage III donor) [38].
The length of time it takes to complete laser surgery (to fully separate the shared blood vessels between the two babies) doesn’t seem to affect the survival of the recipient baby. However, the donor baby’s chances of survival do change depending on how long the surgery takes. If the surgery takes more than 10 min, the donor baby survives 78 % of the time. If it takes between 5 and 10 min, survival goes up to 85 %. And if the surgery is done in under 5 min, the survival rate is 92 % [39]. There is no doubt that all vascular anastomoses should be closed during the fetoscopic laser surgery, as this is the rational of the curative therapy.
Outcomes and complications of fetoscopic laser therapy for twin-to-twin transfusion syndrome
Without treatment, TTTS carries an extremely high mortality rate exceeding 80–90 %, primarily resulting from intrauterine death or pregnancy loss due to cervical insufficiency or premature membrane rupture secondary to polyhydramnios [19]. The implementation of laser therapy has dramatically improved survival rates compared to serial amnioreduction [2], with outcomes continuing to improve over recent decades [3], 40]. The largest single-center cohort study reported stage-dependent survival rates, with Quintero stages I and II achieving dual fetal survival in 75 % of cases and at least one survivor in 94.5 %. For more severe disease (stages III and IV), these rates decreased significantly to 62 % for dual survival and 88 % for at least one survivor [41]. These figures represent significant improvement over historical outcomes and demonstrate the efficacy of current laser treatment protocols.
The most common complication following fetoscopic laser therapy for TTTS is spontaneous preterm labor and birth, with a median gestational age at delivery of around 32 weeks [40]. PPROM represents the primary cause, occurring in approximately 40 % of cases [15], 42]. This often happens because the small hole created in the amniotic sac during the laser procedure does not heal properly [20], 43]. However, premature labor can also occur without PPROM after laser therapy. In cases where a shortened cervical canal is already present at TTTS diagnosis (due to the mechanical stress of polyhydramnios in twin pregnancies), the risk of preterm delivery increases substantially. Even though this is a known risk, treatments like cervical cerclage or a supportive pessary have not clearly been shown to help in these cases [44], although high-quality randomized controlled trials addressing this question are still lacking. A comprehensive review found that the risk of severe neurodevelopmental impairment following prenatal laser therapy for TTTS, assessed at the ages of 2 and 4–6 years, ranges from approximately 6–18 % and 4–13 %, respectively [45]. This risk is higher if the baby is born very prematurely or exhibits abnormal Doppler findings before birth. Beyond neurological considerations, cardiovascular sequelae represent a significant potential complication. Right ventricular outflow tract anomalies have been observed in up to 11 % of recipient twins in TTTS cases [45].
Twin anemia-polycythemia sequence
Pathophysiology and diagnosis of twin anemia-polycythemia sequence
Twin Anemia-Polycythemia Sequence (TAPS) is a condition unique to monochorionic twin pregnancies, characterized by a significant difference in hemoglobin levels between the twins, without the typical amniotic fluid imbalance seen in TTTS [46]. TAPS arises from an uneven blood flow through very small vascular connections (anastomoses) in the placenta, located between the umbilical cord insertions of the two fetuses. Instead of causing rapid volume shifts, this results in a slow, chronic transfusion of blood from one twin to the other – leading to an anemic donor twin and a polycythemic recipient twin (with excess red blood cells). TAPS occurs spontaneously in about 5 % of monochorionic twin pregnancies, usually in the third trimester. It can also develop after laser surgery for TTTS in 2–13 % of cases [17], 47], 48], often due to tiny vascular connections that were either missed or could not be sealed completely during the procedure.
Antenatal diagnosis of TAPS relies on Doppler ultrasonography of the middle cerebral artery peak systolic velocity (MCA-PSV). Currently, there is no universally accepted international consensus regarding the precise cut-off values for MCA-PSV. Through a Delphi procedure, international experts agreed on diagnostic thresholds of ≥1.5 multiples of the median (MoM) for the donor twin and ≤0.8 MoM for the recipient twin [49]. Other studies have suggested ≤1.0 MoM as the threshold for the recipient twin [46]. The expert panel in the Delphi procedure also recognized a delta of ≥1.0 MoM in MCA-PSV difference between twins as a diagnostic criterion [49]. Setting the MCA-PSV difference to 0.5 MoM further improves diagnostic accuracy, but at the cost of a higher false-positive rate [50], 51].
Beyond MCA-PSV measurements, several additional ultrasound findings may suggest TAPS, including differences in placental thickness and echogenicity. Typically, the donor’s placental share appears hyperechoic and thick, while the recipient’s portion presents hypoechoic and thin (Figure 5) [52]. Another characteristic finding is the ‘starry-sky liver’ appearance in the recipient twin, characterized by a hypoechoic liver parenchyma with hyperechoic portal venous walls (Figure 6) [53]. Similar to TTTS, TAPS can be classified into five stages of severity (Table 2) [50]. For early detection of TAPS, ultrasound examinations are recommended fortnightly beginning at 16 weeks’ gestation. Upon diagnosis of TAPS, weekly ultrasound monitoring is indicated [49].
In TAPS, the donor’s placental share appears hyperechoic and thick, while the recipient’s placental share is hypoechoic and thin.
Ultrasound appearance of the liver in twin-to-twin transfusion syndrome. (A) Abdomen of the polycythemic recipient twin showing hypoechoic liver parenchyma with hyperechoic portal venous walls, known as the ‘starry-sky’ liver appearance, and (B) the abdomen with the normal liver of the anemic donor twin.
Staging of twin anemia-polycythemia sequence (TAPS) [50].
| Stage | Ultrasound findings |
|---|---|
| I | Difference of MCA‐PSV > 0.5 MoM between donor and recipient twin |
| II | Difference of MCA‐PSV > 0.7 MoM between donor and recipient twin |
| III | + Critically abnormal Doppler measurements in the donor twina |
| IV | + Hydrops of the donor twin |
| V | + Intrauterine death of one or both fetuses |
-
MCA‐PSV, middle cerebral artery peak systolic velocity; MoM, multiple of the median. aCritically abnormal Doppler measurements is defined as umbilical artery Doppler with absent or reverse flow in end-diastole and/or ductus venosus Doppler with reverse a-wave or and/or umbilical vein with pulsatile flow.
Fetoscopic laser procedure for twin anemia-polycythemia sequence
The therapeutic options for TAPS include expectant management, fetoscopic laser therapy, intrauterine blood transfusion, delivery, and rarely selective feticide. The selection of treatment modality depends on gestational age, disease severity, patient preferences, and physician expertise with significant variations in approach among international fetal therapy centers [6]. There is little evidence about optimal management of TAPS, therefore treatment options should be individualized and discussed with parents.
Laser therapy represents the only causative treatment option and can be performed up to 28 weeks of gestation [7] at stage II – IV [54]. The laser coagulation procedure for TAPS follows a similar protocol to that used in TTTS cases; however, it presents greater technical challenges. Access to the vascular equator is more challenging due to the absence of polyhydramnios, which reduces the maneuvering space for the fetoscope and causes the freely floating intertwin membrane to potentially obstruct visibility. In some cases, amnioinfusion helps to create an adequate operative field. An additional challenge in TAPS laser surgery is that amniotic fluid tends to be cloudier in the later gestational weeks when TAPS typically manifests. To aid orientation during the procedure, the difference in placental echogenicity between donor and recipient territories can serve as a useful landmark [52], as this represents the area of the vascular equator depicted by ultrasound. Since TAPS requires the closure of extremely small anastomoses, the Solomon technique, which involves coagulating the entire vascular equator rather than selectively targeting visible anastomoses, is considered essential for successful treatment. This is because not all anastomoses are visible to the naked eye and could otherwise be missed [55].
Outcomes and complications of fetoscopic laser therapy for twin anemia-polycythemia sequence
If TAPS is not treated, it can lead to serious complications for both babies. The anemic donor twin – the one losing blood – may develop heart failure, fluid buildup in the body (hydrops), low oxygen levels, brain development issues, hearing loss, or may even die before birth. The polycythemic recipient twin – the one receiving too much blood – can develop thick, sticky blood (hyperviscosity), increasing the risk of blood clots, bleeding, and damage to organs such as the skin, intestines, or brain. In 2020, the TAPS Registry published international data on outcomes following treatment for TAPS. The choice of treatment varied depending on the individual case and the experience of the Fetal Therapy Centre making the decision [6]. In this cohort, 110 fetuses with TAPS underwent laser surgery as therapy, with a perinatal mortality rate of 18 %, a severe neonatal morbidity rate of 31 % and a severe cerebral injury rate of 3 % Table 3 [6]. However, other reports describe a higher rate of overall neurodevelopmental impairment of 9 % [56], and systematically collected data on long-term neurological outcomes following laser therapy for TAPS remain insufficient and are still needed. Otherwise, the laser procedure for TAPS generally carries comparable risks in terms of PPROM and preterm birth as the laser treatment for TTTS.
Outcome data from the twin anemia-polycythemia sequence (TAPS) registry depending on the therapy used [6].
| Expectant management | Fetoscopic laser | Intrauterine blood transfusion (+/− partial exchange transfusion) | Delivery | Selective feticide | |
|---|---|---|---|---|---|
| Gestational age at delivery in weeks | 33.0 | 31.8 | 31.1 | 31.9 | 32.1 |
| Overall perinatal mortality | 17 % | 18 % | 18 % | 10 % | 7 % |
| Fetal demise | 11 % | 13 % | 13 % | 0 % | 7 % |
| Neonatal mortality | 7 % | 5 % | 6 % | 10 % | 0 % |
| Severe cerebral injury | 5 % | 3 % | 11 % | 10 % | 0 % |
| Severe neonatal morbidity | 31 % | 31 % | 46 % | 49 % | 25 % |
Twin reversed arterial perfusion sequence
Pathophysiology and diagnosis of twin reversed arterial perfusion sequence
The Twin Reversed Arterial Perfusion Sequence (TRAP) is another complication occurring in monochorionic twins, with an incidence of 1 in 35.000 pregnancies [57]. This is the most extreme form of interfetal transfusion. Through an AA anastomosis of the monochorionic placenta, one twin (pumping twin) pumps blood retrograde via the umbilical cord into the arterial system of the other twin (parasitic co-twin). The latter develops hydrops fetalis at an early stage, the heart regresses and pronounced malformations of the upper half of the body (acardius acranius) are the result. The sonographically unremarkable pumping twin supplies the parasitic co-twin with blood, and this severe circulatory strain often leads to hyperdynamic heart failure, hydrops fetalis, and intrauterine death. With conservative management, approximately half of pumping twins survive [57]. The TRAP sequence can be diagnosed by experienced prenatal diagnosticians as early as the first trimester of pregnancy (Figure 7). After diagnosis at 12 weeks of pregnancy, 33 % of pumping twins in utero have died spontaneously and unpredictably by 16 weeks of pregnancy [58]. Spontaneous cessation of retrograde perfusion of the acardius occurs in approximately 20 % of cases [58]. In untreated cohorts, mortality rates exceeding 50 % have been reported for the pump twin, primarily due to progressive high-output cardiac failure and resulting preterm delivery [57], 59].
Parasitic twin (acardius acranius) with absent heart and head, and retrograde umbilical artery perfusion.
Laser procedure for twin reversed arterial perfusion sequence
Various invasive therapies have been described in the first and second trimester to disconnect the acardius acranius from the pumping twin (Table 4).
Fetal surgical management options in twin reversed arterial perfusion sequence (TRAP).
| Fetoscopic procedure | Ultrasound-guided procedure |
|---|---|
| Laser coagulation of placental anastomoses | Intrafetal (interstitial) laser |
| Laser coagulation of the TRAP twin’s umbilical cord | Radiofrequency or microwave ablation |
| Cord clamping through bipolar umbilical cord coagulation | |
Traditionally, intervention is only performed after the 16th week of pregnancy. The overall survival rate is 80 % [8]. However, evidence suggests that earlier interventions might reduce the rate of very early preterm births [60]. The results of the large international TRAPIST study, which compares the outcomes of early and late interventions, are still pending (ClinicalTrials.gov NCT02621645). For the parasitic twin, there is no possibility of survival even with early prenatal diagnosis. The most commonly used causative management options are intrafetal interventions such as intrafetal (=interstitial) laser therapy, radiofrequency ablation, or microwave ablation [8], 9], 59], 61], 62]. Currently, there is no international consensus regarding which of these procedures is the best. However, the intrafetal approach is considered technically less demanding compared to fetoscopic approaches, such as cord coagulation or ultrasound-guided cord clamping with bipolar forceps [63], 64], and is associated with lower rates of preterm delivery and membrane rupture [65], 66]. A comparative study between interstitial laser and fetoscopic cord occlusion reported neurological disabilities in 12 % of cases following fetoscopic intervention, while no such complications were observed in the interstitial laser group [67]. Due to the higher complication rates, fetoscopic procedures are now less commonly used in TRAP.
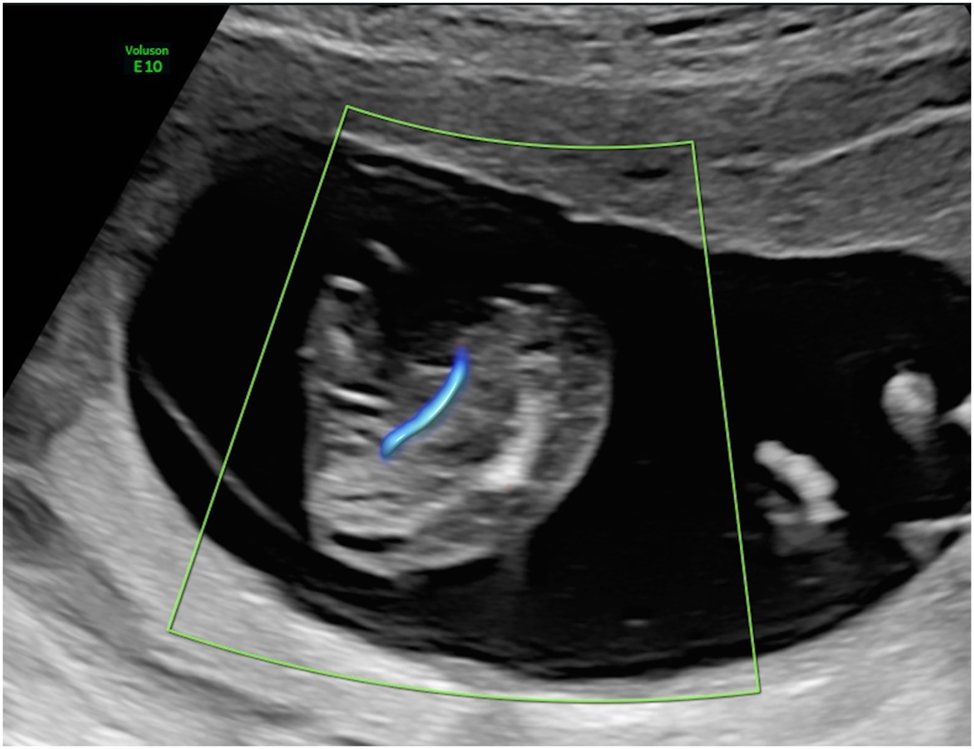
The procedure of intrafetal laser (Figure 8) involves inserting an 18-gauge needle through the maternal abdominal wall into the amniotic cavity under ultrasound guidance, after local anaesthetic has been administered. The needle is positioned directly into the parasitic co-twin at the level of umbilical cord insertion in the pelvic region. A laser fiber is then passed through the needle to coagulate the abnormal blood supply. The thin laser fiber allows precise control of the ablation zone, which is particularly advantageous at earlier gestational ages. The laser energy ablates the blood supply to the acardiac twin, with successful occlusion subsequently confirmed by color Doppler ultrasound [8], 9], 59].
Intrafetal laser therapy performed in the first trimester (A) procedural setup. (B) Ultrasound-guided insertion of an 18-gauge needle into the parasitic twin at the level of the pelvic region, targeting its vascularization. (C) Laser fiber insertion through the needle for coagulation of the vessel, resulting in complete occlusion.
Outcomes and complications of intrafetal laser therapy for twin reversed arterial perfusion sequence
Fetal interventions significantly reduce mortality rates, with recent research supporting therapeutic intervention as early as the first trimester [8], 59], 62] before the development of cardiac compromise in the pump twin can occur. This is further evidenced by a German study from 2020, which documented a 92 % survival rate of pump twins following first-trimester intervention [9]. However, other studies report significantly lower survival rates, such as the series by Roethlisberger et al. with a survival rate of 58 % [60]. Moreover, there seems to be an improvement over time due to the learning curve [68]. A meta-analysis from 2022 found an overall survival rate of 79 % following TRAP laser therapy, regardless of gestational age at intervention [69].
If pump twin demise occurs, it typically happens within a median of 72 h post-procedure [60]. If the pump twin survives the initial period after the procedure, many authors describe excellent outcomes, with healthy children and no relevant complications [9], 60]. There are no reports of abnormal neurological outcomes after interstitial laser therapy [67], whereas such cases have been described in conservatively managed pregnancies without intervention. The risk of preterm birth following intrafetal laser therapy appears to be low. Chaveeva reported that earlier interventions were associated with a lower risk of preterm birth [8]. Tavares and Roethlisberger observed no preterm births in their TRAP laser cohorts [9], 60], whereas Pagani et al. reported a rate of 10 % [59]. In monochorionic monoamniotic twins with TRAP, there is a risk that after laser treatment, the umbilical cord of the acardiac twin may become rigid and constrict the umbilical cord of the pump twin, potentially leading to pump twin demise. Fetoscopic transection of the typically short acardiac umbilical cord can be considered, though it carries significant risks [70]. Another complication is the interruption of the laser procedure. Pagani et al. reported that in three patients the procedure was stopped to avoid excessive use of laser energy and thus reduce the risk of aplasia cutis congenita, a complication previously described in laser ablation for embryo reduction. The repeat procedures were successful [59].
Bronchopulmonary sequestration
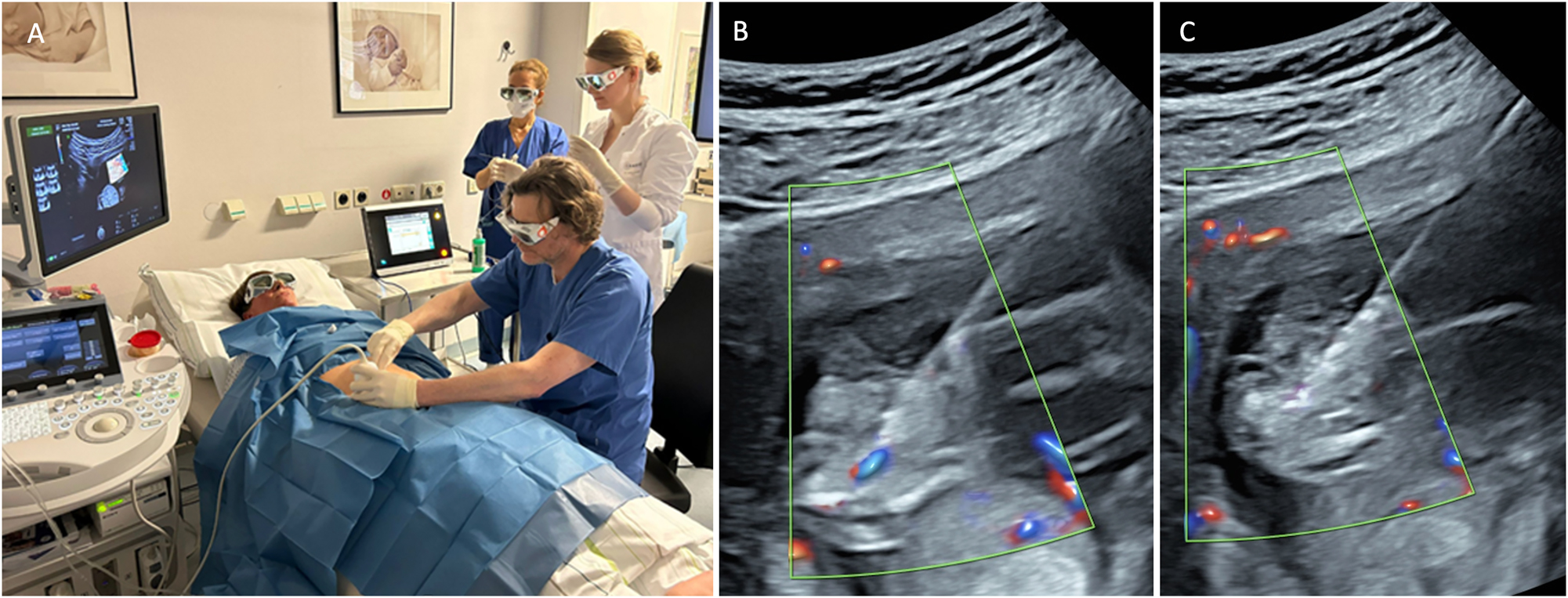
When an abnormal solid lung region, disconnected from the bronchial system, is supplied by a systemic arterial vessel, typically arising from the thoracoabdominal aorta, it is referred to as bronchopulmonary sequestration (BPS) [71] (Figure 9A). The natural history is variable. A large single-center study reported that approximately 70 % of fetuses with BPS did not develop hydrops, allowing for conservative management with favorable outcomes [72]. However, particularly with large lesions, there is a risk of hydrops. A possible cause for hydrops development under discussion is mediastinal shift, leading to compression of the systemic veins, increased central venous pressure, reduced cardiac output, and the subsequent development of hydrops [73]. In severe cases, this can result in intrauterine death if intervention is not undertaken.
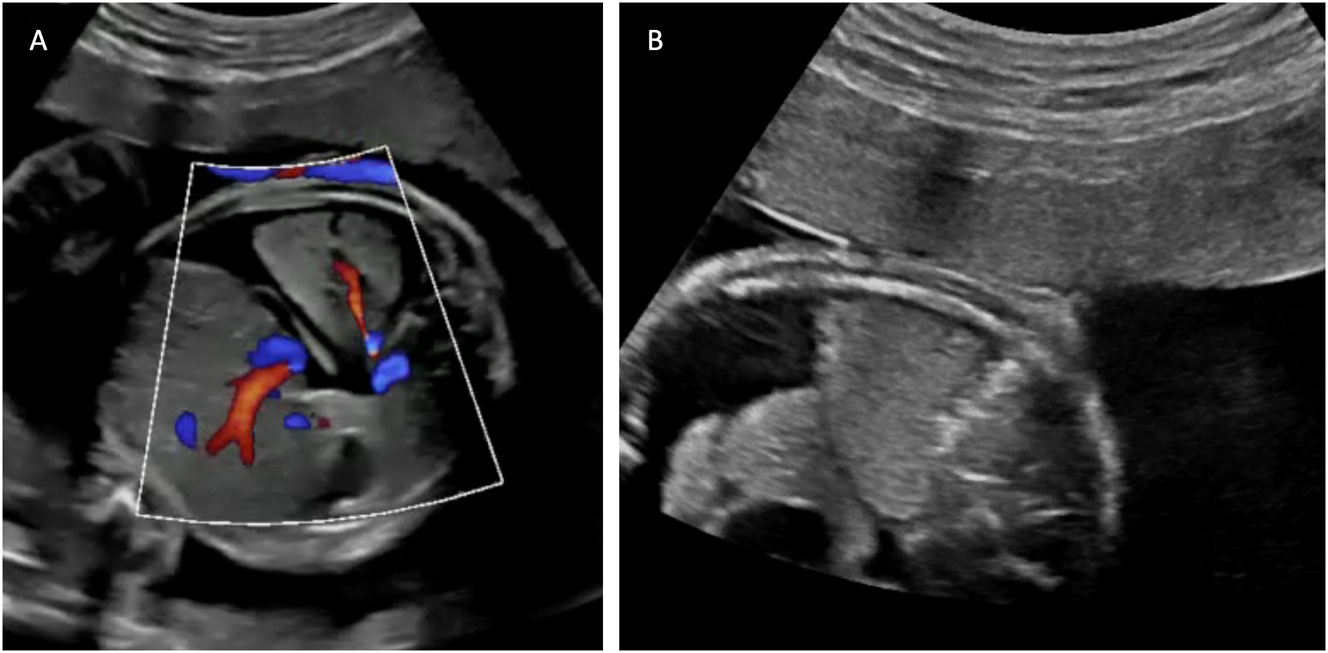
Ultrasound images depicting bronchopulmonary sequestration and its management. (A) Transverse thoracic view showing bronchopulmonary sequestration with aortic perfusion and fetal hydrops. (B) Intrafetal laser coagulation of the feeding vessel using a laser fiber inserted through an 18-gauge needle under ultrasound guidance. Courtesy of Prof. Dr. Ingo Gottschalk.
Emerging evidence suggests that laser ablation of the feeding vessel is the most effective therapy BPS complicated by hydrops. This procedure involves using interstitial laser to occlude the systemic arterial vessel supplying the sequestered lung tissue [10], [11], [12, 72], 74]. Historically, alternative approaches included thoraco-amniotic shunt placement or serial thoracocentesis for associated pleural effusions [72], 75], 76]. However, the limited studies available to date indicate that interstitial laser therapy is more effective and carries lower risks compared to shunt insertion [10], 72].
The laser procedure is performed as an intrafetal intervention, similar to the technique used in TRAP sequence. Following local anesthesia, an 18-gauge needle is guided under ultrasound visualization to the feeding vessel of the sequestration, where laser energy is applied to coagulate the vessel (Figure 9B) [10], 72], 77]. Critical to procedural success is avoiding puncture of other fetal organs, including healthy lung tissue, and applying only short laser pulses to prevent collateral damage. Gottschalk and colleagues reported that approximately 42 % of cases required a second intervention [11], highlighting the technical challenges of achieving complete vascular occlusion in a single procedure.
The success of laser intervention can be evaluated prenatally through ultrasound assessment, primarily by confirming the absence of blood flow in the feeding vessel and observing regression of hydrops. In one of the larger patient cohorts comprising 15 patients with BPS complicated by hydrops or hydrothorax, these conditions resolved at median intervals of 7.5 days for hydrops and 21 days for hydrothorax following interstitial laser therapy [78]. Additionally, lung volume typically normalizes approximately 10 weeks post-intervention [78].
Outcomes and complications of intrafetal laser therapy for bronchopulmonary sequestration
Clinical experience with intrafetal laser for hydropic BPS suggests that, when performed by experienced fetal surgeons, it carries relatively low risks. The majority of fetuses (>95 %) reported in published cohorts survived to live birth, with preterm birth rates ranging from 0 to 14 % [10], 72], 77], 78]. Notably, there have been no reported cases of intrauterine death, placental abruption, PPROM, significant bleeding, or requirement for tocolysis in the largest published series [77]. However, these favorable outcomes may partially reflect publication bias, with a tendency toward reporting predominantly positive results. Additionally, patient selection in fetal therapy centers likely favored cases with optimal technical conditions for the procedure, including suitable access routes and clear visualization of the feeding vessel. The long-term benefit of prenatal laser therapy for BPS is further demonstrated by the reduced need for postnatal surgical intervention. Only 0–24 % of prenatally laser-treated fetuses required postnatal surgery for sequestrectomy [10], 11], 72], 77], 78], which is significantly lower than reported rates following alternative interventions such as shunt placement [72]. Long-term outcome data on fetuses after successful prenatal interstitial laser therapy for BPS are not available.
Lower urinary tract obstruction
Laser therapy can also be used in combination with cystoscopy, which has been proposed for singletons with lower urinary tract obstruction (LUTO) due to posterior urethral valves as an alternative to vesicoamniotic shunting to prevent pulmonary hypoplasia and severe renal impairment [13]. Current indications for fetal cystoscopy are a massively enlarged urinary bladder and keyhole sign, bilateral hydronephrosis, severe oligohydramnios or anhydramnios (Figure 10). The procedure is performed under continuous ultrasound guidance, maternal epidural or local anesthesia and fetal anesthesia. A curved sheath and a 1.0–1.3 mm fetoscope are inserted percutaneously into the fetal bladder. If posterior urethral valves are present they are ablated using guidewires, hydroablation, or an ND:YAG/diode laser. The optimal technical approach has yet to be determined. However, fetal cystoscopic laser dilatation of the valves would be safely indicated and performed only if adequate visualization of the obstruction is observed. Otherwise, urethral trauma or urethrorectal fistulas may occur as complications of laser therapy. Vinit et al. examined the long-term survival, nephrological, and urological outcomes of children treated prenatally for LUTO using fetal cystoscopy (n=23) or vesicoamniotic shunting (n=25) [79]. There was no difference between cystoscopy and shunting in terms of survival (92 vs. 83 %, p=1), complication rate (74 vs. 92 %, p=0.88), or chronic kidney disease (58 vs. 50 %, p=1). The authors concluded, despite overly optimistic reports on cystoscopy, its reproducibility is limited due to inadequate visualization of the posterior urethra and suboptimal instrumentation. Flexible fetal video cystoscopy may help overcome these technical challenges [80].
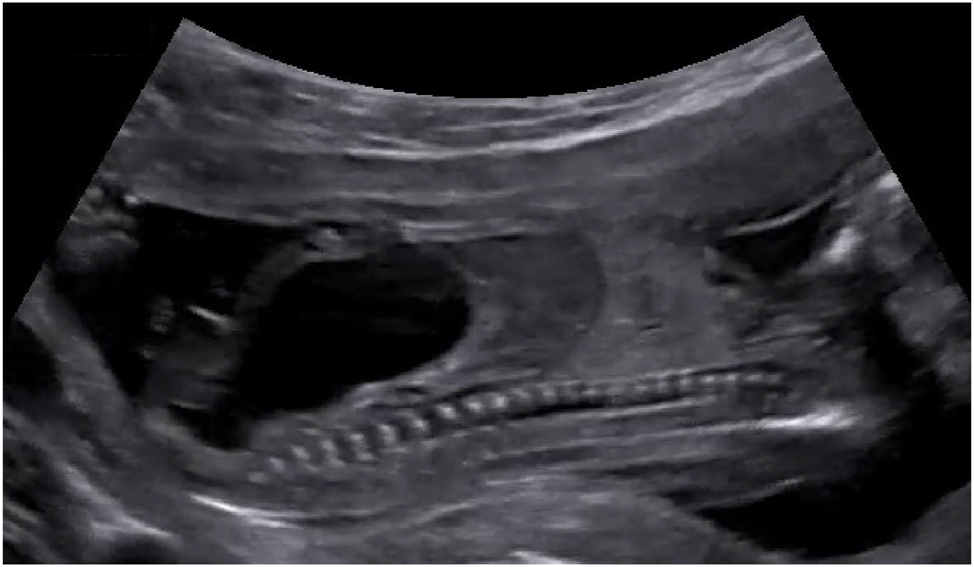
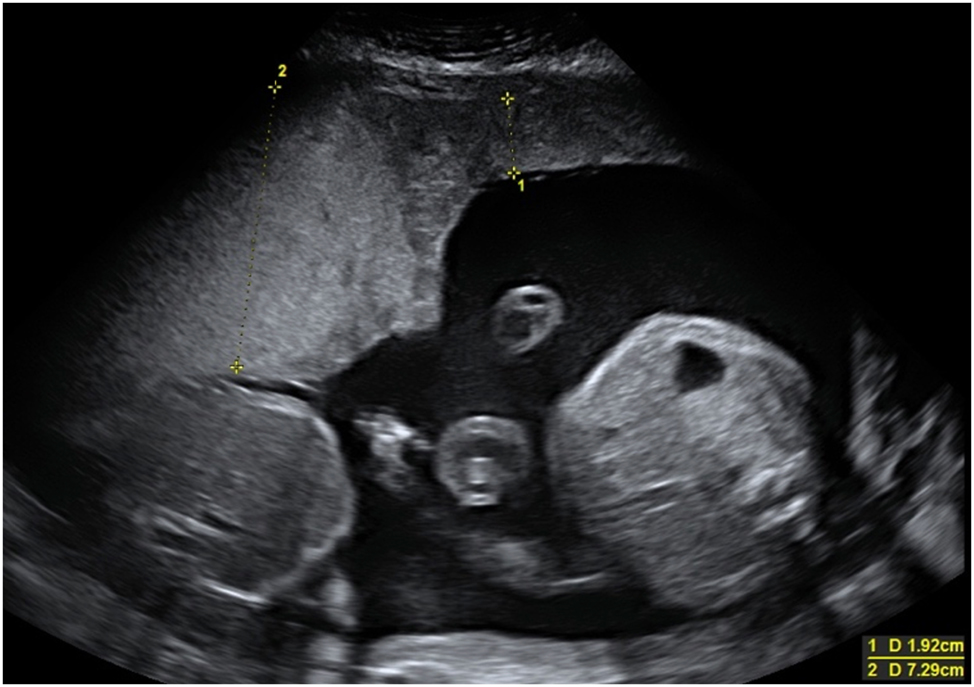
Sagittal view of a fetus with an enlarged bladder due to lower urinary tract obstruction (LUTO) caused by posterior urethral valves.
Other fetal malformations
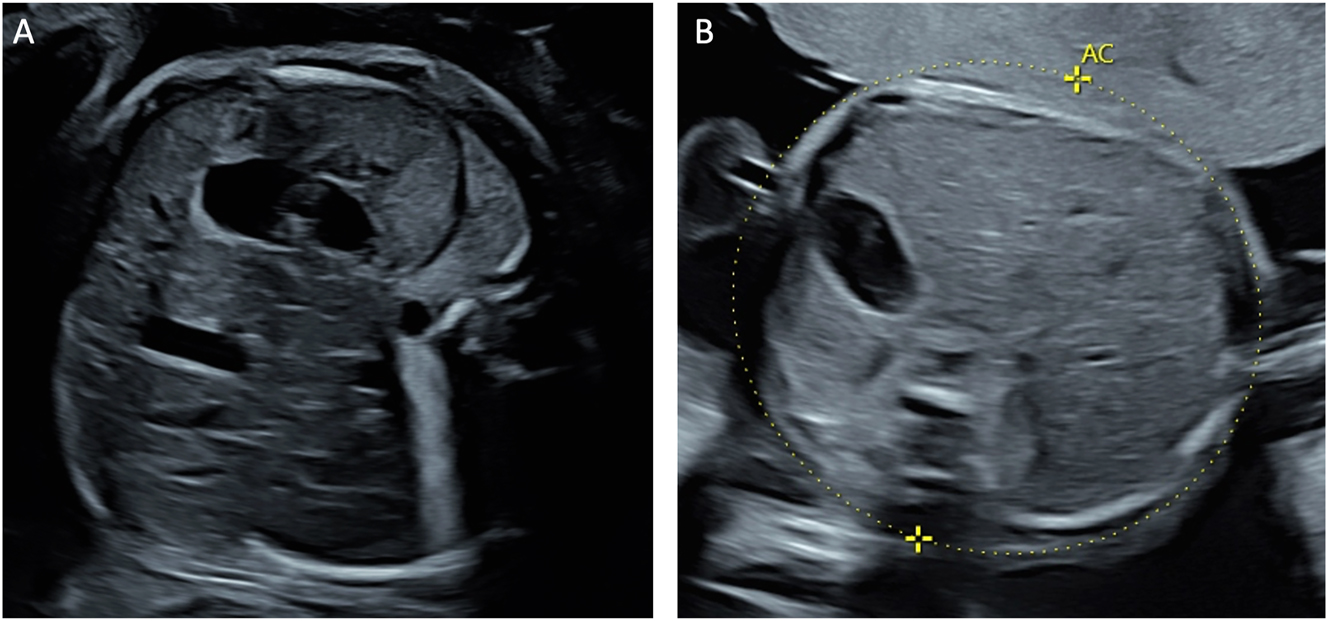
Laser therapy can also be offered as an individualized treatment approach for various other fetal and placental conditions with significant vascularization. For example, interstitial laser ablation may be offered as a treatment option in cases of decompensated sacrococcygeal teratomas (Figure 11) with large extracorporeal components and identifiable feeding vessels [14], 81], 82]. Large placental chorioangiomas associated with hyperdynamic circulation can also be treated by coagulation of their feeding vessels using either interstitial or fetoscopic laser techniques [83], [84], [85]. However, these laser applications often require multiple treatment sessions due to the high degree of vascularization and frequent development of collateral vessels. Overall, this is not a standard but an experimental therapy with a generally poor prognosis.
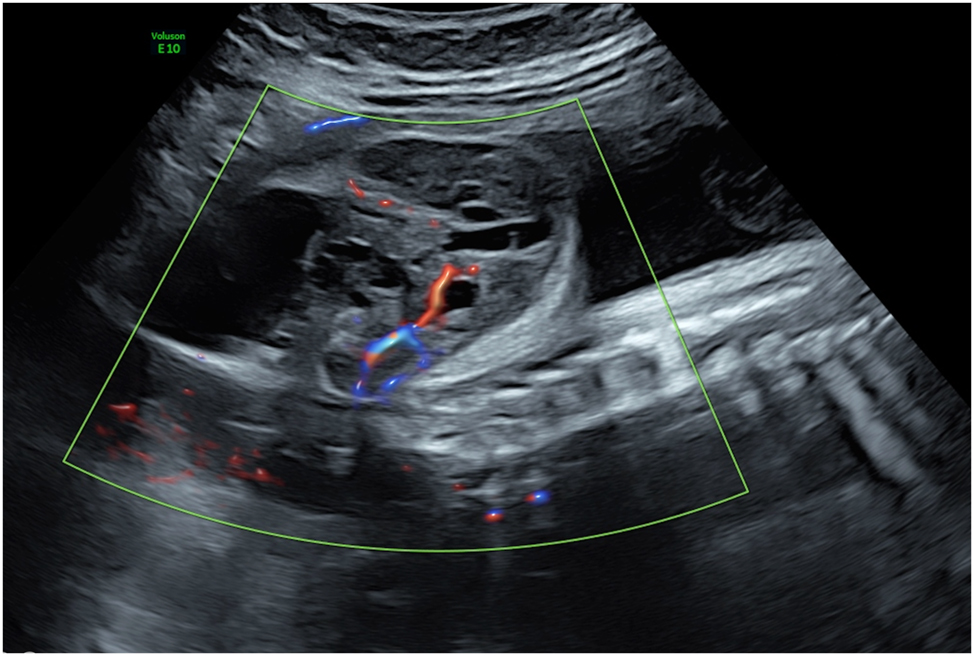
Sagittal view of a fetus with a sacrococcygeal teratoma demonstrating a feeding vessel suitable for laser ablation.
Summary and outlook
The use of laser therapy in fetal medicine has significantly improved the prognosis in selected cases. The strength of evidence supporting these interventions varies: Fetoscopic laser coagulation for TTTS is the most common fetal surgery procedure, supported by high-quality evidence from randomized controlled trials. In contrast, management of TAPS and TRAP is based on moderate-quality evidence from cohort studies, while laser therapy for BPS has shown significant clinical benefit despite limited evidence. Although laser therapy has shown promising results, challenges remain, including procedural risks, operator expertise, and access to specialized centers. The future of laser therapy in fetal medicine lies in advances such as enhanced imaging modalities, integration of artificial intelligence, and robotic-assisted procedures to improve precision and safety. Further randomized trials, international collaborations, and registry development are essential to increase sample sizes, improve statistical power, and establish standardized protocols with long-term follow-up. In conclusion, laser therapy remains a cornerstone of modern fetal interventions, offering hope for improved perinatal outcomes in complex prenatal conditions.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. De Lia, JE, Cruikshank, DP, Keye, WRJr. Fetoscopic neodymium:YAG laser occlusion of placental vessels in severe twin-twin transfusion syndrome. Obstet Gynecol 1990;75:1046–53.Search in Google Scholar
2. Senat, MV, Deprest, J, Boulvain, M, Paupe, A, Winer, N, Ville, Y. Endoscopic laser surgery versus serial amnioreduction for severe twin-to-twin transfusion syndrome. N Engl J Med 2004;351:136–44. https://doi.org/10.1056/nejmoa032597.Search in Google Scholar
3. Diehl, W, Diemert, A, Grasso, D, Sehner, S, Wegscheider, K, Hecher, K. Fetoscopic laser coagulation in 1020 pregnancies with twin-twin transfusion syndrome demonstrates improvement in double-twin survival rate. Ultrasound Obstet Gynecol 2017;50:728–35. https://doi.org/10.1002/uog.17520.Search in Google Scholar PubMed
4. Rossi, AC, Vanderbilt, D, Chmait, RH. Neurodevelopmental outcomes after laser therapy for twin-twin transfusion syndrome: a systematic review and meta-analysis. Obstet Gynecol 2011;118:1145–50. https://doi.org/10.1097/aog.0b013e318231827f.Search in Google Scholar
5. Schou, KV, Lando, AV, Ekelund, CK, Jensen, LN, Jorgensen, C, Norgaard, LN, et al.. Long-term neurodevelopmental outcome of monochorionic twins after laser therapy or umbilical cord occlusion for twin-twin transfusion syndrome. Fetal Diagn Ther 2019;46:20–7. https://doi.org/10.1159/000491787.Search in Google Scholar PubMed
6. Tollenaar, LSA, Slaghekke, F, Lewi, L, Ville, Y, Lanna, M, Weingertner, A, et al.. Treatment and outcome of 370 cases with spontaneous or post-laser twin anemia-polycythemia sequence managed in 17 fetal therapy centers. Ultrasound Obstet Gynecol 2020;56:378–87. https://doi.org/10.1002/uog.22042.Search in Google Scholar PubMed PubMed Central
7. Slaghekke, F, Favre, R, Peeters, SH, Middeldorp, JM, Weingertner, AS, van Zwet, EW, et al.. Laser surgery as a management option for twin anemia-polycythemia sequence. Ultrasound Obstet Gynecol 2014;44:304–10. https://doi.org/10.1002/uog.13382.Search in Google Scholar PubMed
8. Chaveeva, P, Poon, LC, Sotiriadis, A, Kosinski, P, Nicolaides, KH. Optimal method and timing of intrauterine intervention in twin reversed arterial perfusion sequence: case study and meta-analysis. Fetal Diagn Ther 2014;35:267–79. https://doi.org/10.1159/000358593.Search in Google Scholar PubMed
9. Tavares de Sousa, M, Glosemeyer, P, Diemert, A, Bamberg, C, Hecher, K. First-trimester intervention in twin reversed arterial perfusion sequence. Ultrasound Obstet Gynecol 2020;55:47–9. https://doi.org/10.1002/uog.20860.Search in Google Scholar PubMed
10. Litwinska, M, Litwinska, E, Szaflik, K, Debska, M, Szajner, T, Janiak, K, et al.. Management options for fetal bronchopulmonary sequestration. J Clin Med 2022;11. https://doi.org/10.3390/jcm11061724.Search in Google Scholar PubMed PubMed Central
11. Gottschalk, I, Strizek, B, Mallmann, MR, Muller, A, Geipel, A, Gembruch, U, et al.. Outcome of bronchopulmonary sequestration with massive pleural effusion after intrafetal vascular laser ablation. Fetal Diagn Ther 2018;44:149–55. https://doi.org/10.1159/000479793.Search in Google Scholar PubMed
12. Kosinski, P, Tavares de Sousa, M, Wielgos, M, Hecher, K. Intrauterine ultrasound-guided laser coagulation of the feeding artery in fetal bronchopulmonary sequestration. Ultraschall Med 2017;38:583–6. https://doi.org/10.1055/s-0043-100505.Search in Google Scholar PubMed
13. Pierucci, UM, Paraboschi, I, Lanfranchi, G, Peycelon, M, Pelizzo, G, Ruano, R. Indications and outcomes of fetal cystoscopy for lower urinary tract obstruction: a comprehensive review. Prenat Diagn 2025;45:89–101. https://doi.org/10.1002/pd.6721.Search in Google Scholar PubMed PubMed Central
14. Van Mieghem, T, Al-Ibrahim, A, Deprest, J, Lewi, L, Langer, JC, Baud, D, et al.. Minimally invasive therapy for fetal sacrococcygeal teratoma: case series and systematic review of the literature. Ultrasound Obstet Gynecol 2014;43:611–9. https://doi.org/10.1002/uog.13315.Search in Google Scholar PubMed
15. Snowise, S, Mann, LK, Moise, KJJr., Johnson, A, Bebbington, MW, Papanna, R. Preterm prelabor rupture of membranes after fetoscopic laser surgery for twin-twin transfusion syndrome. Ultrasound Obstet Gynecol 2017;49:607–11. https://doi.org/10.1002/uog.15958.Search in Google Scholar PubMed
16. Peeters, SH, Akkermans, J, Bustraan, J, Middeldorp, JM, Lopriore, E, Devlieger, R, et al.. Operator competence in fetoscopic laser surgery for twin-twin transfusion syndrome: validation of a procedure-specific evaluation tool. Ultrasound Obstet Gynecol 2016;47:350–5. https://doi.org/10.1002/uog.15734.Search in Google Scholar PubMed
17. Lewi, L, Jani, J, Blickstein, I, Huber, A, Gucciardo, L, Van Mieghem, T, et al.. The outcome of monochorionic diamniotic twin gestations in the era of invasive fetal therapy: a prospective cohort study. Am J Obstet Gynecol 2008;199:514 e1–8. https://doi.org/10.1016/j.ajog.2008.03.050.Search in Google Scholar PubMed
18. Bermudez, C, Becerra, CH, Bornick, PW, Allen, MH, Arroyo, J, Quintero, RA. Placental types and twin-twin transfusion syndrome. Am J Obstet Gynecol 2002;187:489–94. https://doi.org/10.1067/mob.2002.124280.Search in Google Scholar PubMed
19. Berghella, V, Kaufmann, M. Natural history of twin-twin transfusion syndrome. J Reprod Med 2001;46:480–4.Search in Google Scholar
20. Bamberg, C, Hecher, K. Twin-to-twin transfusion syndrome: controversies in the diagnosis and management. Best Pract Res Clin Obstet Gynaecol 2022;84:143–54. https://doi.org/10.1016/j.bpobgyn.2022.03.013.Search in Google Scholar PubMed
21. Khalil, A, Rodgers, M, Baschat, A, Bhide, A, Gratacos, E, Hecher, K, et al.. ISUOG Practice Guidelines: role of ultrasound in twin pregnancy. Ultrasound Obstet Gynecol 2016;47:247–63. https://doi.org/10.1002/uog.15821.Search in Google Scholar PubMed
22. Oepkes, D, Sueters, M. Antenatal fetal surveillance in multiple pregnancies. Best Pract Res Clin Obstet Gynaecol 2017;38:59–70. https://doi.org/10.1016/j.bpobgyn.2016.09.004.Search in Google Scholar PubMed
23. Society for Maternal-Fetal, M, Simpson, LL. Twin-twin transfusion syndrome. Am J Obstet Gynecol 2013;208:3–18.10.1016/j.ajog.2012.10.880Search in Google Scholar PubMed
24. Bamberg, C. Handlungsalgorithmus: Zwillingstransfusionssyndrom – Diagnose und Therapie Algorithm for action: twin-to-twin transfusion syndrome—diagnosis and treatment. Die Gynäkologie 2025;58:262–4. https://doi.org/10.1007/s00129-025-05342-1.Search in Google Scholar
25. Quintero, RA, Morales, WJ, Allen, MH, Bornick, PW, Johnson, PK, Kruger, M. Staging of twin-twin transfusion syndrome. J Perinatol 1999;19:550–5. https://doi.org/10.1038/sj.jp.7200292.Search in Google Scholar PubMed
26. Baud, D, Windrim, R, Keunen, J, Kelly, EN, Shah, P, van Mieghem, T, et al.. Fetoscopic laser therapy for twin-twin transfusion syndrome before 17 and after 26 weeks’ gestation. Am J Obstet Gynecol 2013;208:197 e1–7. https://doi.org/10.1016/j.ajog.2012.11.027.Search in Google Scholar PubMed
27. Mustafa, HJ, Aghajani, F, Patrick, E, Baerz, MM, Arias-Sanchez, P, Khalil, A. Perinatal outcomes following fetoscopic laser surgery for early twin-to-twin transfusion syndrome: systematic review and meta-analysis. Acta Obstet Gynecol Scand 2024;103:824–31. https://doi.org/10.1111/aogs.14806.Search in Google Scholar PubMed PubMed Central
28. Krispin, E, Javinani, A, Odibo, A, Carreras, E, Emery, SP, Sepulveda Gonzalez, G, et al.. Consensus protocol for management of early and late twin-twin transfusion syndrome: Delphi study. Ultrasound Obstet Gynecol 2024;63:371–7. https://doi.org/10.1002/uog.27446.Search in Google Scholar PubMed
29. Van Der Veeken, L, Couck, I, Van Der Merwe, J, De Catte, L, Devlieger, R, Deprest, J, et al.. Laser for twin-to-twin transfusion syndrome: a guide for endoscopic surgeons. Facts Views Vis Obgyn 2019;11:197–205.Search in Google Scholar
30. Quintero, RA, Comas, C, Bornick, PW, Allen, MH, Kruger, M. Selective versus non-selective laser photocoagulation of placental vessels in twin-to-twin transfusion syndrome. Ultrasound Obstet Gynecol 2000;16:230–6. https://doi.org/10.1046/j.1469-0705.2000.00265.x.Search in Google Scholar PubMed
31. Ville, Y, Hyett, J, Hecher, K, Nicolaides, K. Preliminary experience with endoscopic laser surgery for severe twin-twin transfusion syndrome. N Engl J Med 1995;332:224–7. https://doi.org/10.1056/nejm199501263320404.Search in Google Scholar
32. Slaghekke, F, Lopriore, E, Lewi, L, Middeldorp, JM, van Zwet, EW, Weingertner, AS, et al.. Fetoscopic laser coagulation of the vascular equator versus selective coagulation for twin-to-twin transfusion syndrome: an open-label randomised controlled trial. Lancet 2014;383:2144–51. https://doi.org/10.1016/s0140-6736(13)62419-8.Search in Google Scholar
33. Slaghekke, F, Lewi, L, Middeldorp, JM, Weingertner, AS, Klumper, FJ, Dekoninck, P, et al.. Residual anastomoses in twin-twin transfusion syndrome after laser: the Solomon randomized trial. Am J Obstet Gynecol 2014;211:285 e1–7. https://doi.org/10.1016/j.ajog.2014.05.012.Search in Google Scholar PubMed
34. Lanna, MM, Faiola, S, Consonni, D, Rustico, MA. Increased risk of placental abruption after solomon laser treatment of twin-twin transfusion syndrome. Placenta 2017;53:54–6. https://doi.org/10.1016/j.placenta.2017.03.018.Search in Google Scholar PubMed
35. Akkermans, J, de Vries, SM, Zhao, D, Peeters, SHP, Klumper, FJ, Middeldorp, JM, et al.. What is the impact of placental tissue damage after laser surgery for twin-twin transfusion syndrome? A secondary analysis of the Solomon trial. Placenta 2017;52:71–6. https://doi.org/10.1016/j.placenta.2017.02.023.Search in Google Scholar PubMed
36. Bamberg, C, Hecher, K. Update on twin-to-twin transfusion syndrome. Best Pract Res Clin Obstet Gynaecol 2019;58:55–65. https://doi.org/10.1016/j.bpobgyn.2018.12.011.Search in Google Scholar PubMed
37. Quintero, RA, Ishii, K, Chmait, RH, Bornick, PW, Allen, MH, Kontopoulos, EV. Sequential selective laser photocoagulation of communicating vessels in twin-twin transfusion syndrome. J Matern Fetal Neonatal Med 2007;20:763–8. https://doi.org/10.1080/14767050701591827.Search in Google Scholar PubMed
38. Chmait, RH, Korst, LM, Llanes, AS, Rallo, KR, Chon, AH, Monson, MA, et al.. Randomized controlled trial of twin-twin transfusion syndrome laser surgery: the sequential trial. Am J Obstet Gynecol 2024;231:365 e1–15. https://doi.org/10.1016/j.ajog.2024.06.009.Search in Google Scholar PubMed
39. Habli, M, Khaled, O, Lim, F, Polzin, W, Vanhook, J, David, L, et al.. 421: the impact of duration of laser time and operative time on pregnancy outcome and survival in twin-twin transfusion syndrome treated by fetoscopic laser. Am J Obstet Gynecol 2011;206:195.10.1016/j.ajog.2011.10.439Search in Google Scholar
40. Akkermans, J, Peeters, SH, Klumper, FJ, Lopriore, E, Middeldorp, JM, Oepkes, D. Twenty-five years of fetoscopic laser coagulation in twin-twin transfusion syndrome: a systematic review. Fetal Diagn Ther 2015;38:241–53. https://doi.org/10.1159/000437053.Search in Google Scholar PubMed
41. Bamberg, C, Diehl, W, Diemert, A, Sehner, S, Hecher, K. Differentiation between TTTS Stages I vs II and III vs IV does not affect probability of double survival after laser therapy. Ultrasound Obstet Gynecol 2021;58:201–6. https://doi.org/10.1002/uog.23131.Search in Google Scholar PubMed
42. Stirnemann, J, Djaafri, F, Kim, A, Mediouni, I, Bussieres, L, Spaggiari, E, et al.. Preterm premature rupture of membranes is a collateral effect of improvement in perinatal outcomes following fetoscopic coagulation of chorionic vessels for twin-twin transfusion syndrome: a retrospective observational study of 1092 cases. BJOG 2018;125:1154–62. https://doi.org/10.1111/1471-0528.15147.Search in Google Scholar PubMed
43. Devlieger, R, Millar, LK, Bryant-Greenwood, G, Lewi, L, Deprest, JA. Fetal membrane healing after spontaneous and iatrogenic membrane rupture: a review of current evidence. Am J Obstet Gynecol 2006;195:1512–20. https://doi.org/10.1016/j.ajog.2006.01.074.Search in Google Scholar PubMed PubMed Central
44. Buskmiller, C, Bergh, EP, Brock, C, Miller, J, Baschat, A, Galan, H, et al.. Interventions to prevent preterm delivery in women with short cervix before fetoscopic laser surgery for twin-twin transfusion syndrome. Ultrasound Obstet Gynecol 2022;59:169–76. https://doi.org/10.1002/uog.23708.Search in Google Scholar PubMed
45. Hecher, K, Gardiner, HM, Diemert, A, Bartmann, P. Long-term outcomes for monochorionic twins after laser therapy in twin-to-twin transfusion syndrome. Lancet Child Adolesc Health 2018;2:525–35. https://doi.org/10.1016/s2352-4642(18)30127-5.Search in Google Scholar PubMed
46. Slaghekke, F, Kist, WJ, Oepkes, D, Pasman, SA, Middeldorp, JM, Klumper, FJ, et al.. Twin anemia-polycythemia sequence: diagnostic criteria, classification, perinatal management and outcome. Fetal Diagn Ther 2010;27:181–90. https://doi.org/10.1159/000304512.Search in Google Scholar PubMed
47. Robyr, R, Lewi, L, Salomon, LJ, Yamamoto, M, Bernard, JP, Deprest, J, et al.. Prevalence and management of late fetal complications following successful selective laser coagulation of chorionic plate anastomoses in twin-to-twin transfusion syndrome. Am J Obstet Gynecol 2006;194:796–803. https://doi.org/10.1016/j.ajog.2005.08.069.Search in Google Scholar PubMed
48. Habli, M, Bombrys, A, Lewis, D, Lim, FY, Polzin, W, Maxwell, R, et al.. Incidence of complications in twin-twin transfusion syndrome after selective fetoscopic laser photocoagulation: a single-center experience. Am J Obstet Gynecol 2009;201:417 e1–7. https://doi.org/10.1016/j.ajog.2009.07.046.Search in Google Scholar PubMed
49. Khalil, A, Gordijn, S, Ganzevoort, W, Thilaganathan, B, Johnson, A, Baschat, AA, et al.. Consensus diagnostic criteria and monitoring of twin anemia-polycythemia sequence: Delphi procedure. Ultrasound Obstet Gynecol 2020;56:388–94. https://doi.org/10.1002/uog.21882.Search in Google Scholar PubMed
50. Tollenaar, LSA, Lopriore, E, Middeldorp, JM, Haak, MC, Klumper, FJ, Oepkes, D, et al.. Improved prediction of twin anemia-polycythemia sequence by delta middle cerebral artery peak systolic velocity: new antenatal classification system. Ultrasound Obstet Gynecol 2019;53:788–93. https://doi.org/10.1002/uog.20096.Search in Google Scholar PubMed PubMed Central
51. Tavares de Sousa, M, Fonseca, A, Hecher, K. Role of fetal intertwin difference in middle cerebral artery peak systolic velocity in predicting neonatal twin anemia-polycythemia sequence. Ultrasound Obstet Gynecol 2019;53:794–7. https://doi.org/10.1002/uog.20116.Search in Google Scholar PubMed
52. Bamberg, C, Diemert, A, Glosemeyer, P, Hecher, K. Quantified discordant placental echogenicity in twin anemia-polycythemia sequence (TAPS) and middle cerebral artery peak systolic velocity. Ultrasound Obstet Gynecol 2018;52:373–7. https://doi.org/10.1002/uog.17535.Search in Google Scholar PubMed
53. Segev, Y, Goldberg, Y, Riskin-Mashiah, S, Berdicef, M, Lavie, O, Auslender, R. Starry sky pattern of fetal liver sonogram as first sign of twin-twin transfusion syndrome. Ultrasound Obstet Gynecol 2012;39:723–5. https://doi.org/10.1002/uog.10063.Search in Google Scholar PubMed
54. Tollenaar, LS, Slaghekke, F, Middeldorp, JM, Klumper, FJ, Haak, MC, Oepkes, D, et al.. Twin anemia polycythemia sequence: current views on pathogenesis, diagnostic criteria, perinatal management, and outcome. Twin Res Hum Genet 2016;19:222–33. https://doi.org/10.1017/thg.2016.18.Search in Google Scholar PubMed
55. Lewi, L, Jani, J, Cannie, M, Robyr, R, Ville, Y, Hecher, K, et al.. Intertwin anastomoses in monochorionic placentas after fetoscopic laser coagulation for twin-to-twin transfusion syndrome: is there more than meets the eye? Am J Obstet Gynecol 2006;194:790–5. https://doi.org/10.1016/j.ajog.2005.08.062.Search in Google Scholar PubMed
56. Slaghekke, F, van Klink, JM, Koopman, HM, Middeldorp, JM, Oepkes, D, Lopriore, E. Neurodevelopmental outcome in twin anemia-polycythemia sequence after laser surgery for twin-twin transfusion syndrome. Ultrasound Obstet Gynecol 2014;44:316–21. https://doi.org/10.1002/uog.13387.Search in Google Scholar PubMed
57. Moore, TR, Gale, S, Benirschke, K. Perinatal outcome of forty-nine pregnancies complicated by acardiac twinning. Am J Obstet Gynecol 1990;163:907–12. https://doi.org/10.1016/0002-9378(90)91094-s.Search in Google Scholar PubMed
58. Lewi, L, Valencia, C, Gonzalez, E, Deprest, J, Nicolaides, KH. The outcome of twin reversed arterial perfusion sequence diagnosed in the first trimester. Am J Obstet Gynecol 2010;203:213 e1–4. https://doi.org/10.1016/j.ajog.2010.04.018.Search in Google Scholar PubMed
59. Pagani, G, D’Antonio, F, Khalil, A, Papageorghiou, A, Bhide, A, Thilaganathan, B. Intrafetal laser treatment for twin reversed arterial perfusion sequence: cohort study and meta-analysis. Ultrasound Obstet Gynecol 2013;42:6–14. https://doi.org/10.1002/uog.12495.Search in Google Scholar PubMed
60. Roethlisberger, M, Strizek, B, Gottschalk, I, Mallmann, MR, Geipel, A, Gembruch, U, et al.. First-trimester intervention in twin reversed arterial perfusion sequence: does size matter? Ultrasound Obstet Gynecol 2017;50:40–4. https://doi.org/10.1002/uog.16013.Search in Google Scholar PubMed
61. Meng, X, Yuan, P, Gong, L, Wang, X, Wu, T, Wei, Y, et al.. Forty-five consecutive cases of complicated monochorionic multiple pregnancy treated with microwave ablation: a single-center experience. Prenat Diagn 2019;39:293–8. https://doi.org/10.1002/pd.5423.Search in Google Scholar PubMed
62. Vitucci, A, Fichera, A, Fratelli, N, Sartori, E, Prefumo, F. Twin reversed arterial perfusion sequence: current treatment options. Int J Womens Health 2020;12:435–43. https://doi.org/10.2147/ijwh.s214254.Search in Google Scholar
63. Robyr, R, Yamamoto, M, Ville, Y. Selective feticide in complicated monochorionic twin pregnancies using ultrasound-guided bipolar cord coagulation. BJOG 2005;112:1344–8. https://doi.org/10.1111/j.1471-0528.2005.00746.x.Search in Google Scholar PubMed
64. Hecher, K, Lewi, L, Gratacos, E, Huber, A, Ville, Y, Deprest, J. Twin reversed arterial perfusion: fetoscopic laser coagulation of placental anastomoses or the umbilical cord. Ultrasound Obstet Gynecol 2006;28:688–91. https://doi.org/10.1002/uog.3816.Search in Google Scholar PubMed
65. Tan, TY, Sepulveda, W. Acardiac twin: a systematic review of minimally invasive treatment modalities. Ultrasound Obstet Gynecol 2003;22:409–19. https://doi.org/10.1002/uog.224.Search in Google Scholar PubMed
66. Stellon, MA, Joshi, DS, Beninati, MJ, Leverson, G, Yang, Q, Antony, KM, et al.. Management of twin reversed arterial perfusion sequence: a systematic review and meta-analysis. Fetal Diagn Ther 2024:1–16. https://doi.org/10.1159/000542841.Search in Google Scholar PubMed
67. Walasik, I, Litwinska, M, Janiak, K, Szaflik, K, Kaczmarek, P, Ludwin, A, et al.. Outcome of monochorionic diamniotic twins with twin reversed arterial perfusion sequence: interstitial laser versus endoscopic cord occlusion. J Clin Med 2023;12. https://doi.org/10.3390/jcm12206593.Search in Google Scholar PubMed PubMed Central
68. Weber, EC, Recker, F, Gottschalk, I, Strizek, B, Geipel, A, Gembruch, U, et al.. Outcome of TRAP sequence treated in the first trimester - a ten-year single-center experience. Ultraschall Med 2022;43:614–8. https://doi.org/10.1055/a-1526-1775.Search in Google Scholar PubMed
69. Vitucci, A, Fratelli, N, Fichera, A, Sartori, E, Prefumo, F. Timing of intra-fetal laser therapy for twin reversed arterial perfusion (TRAP) sequence: retrospective series and systematic review and meta-analysis. Int J Gynaecol Obstet 2022;159:833–40. https://doi.org/10.1002/ijgo.14221.Search in Google Scholar PubMed PubMed Central
70. Weber, EC, Recker, F, Gottschalk, I, Strizek, B, Geipel, A, Gembruch, U, et al.. Outcome of monochorionic monoamniotic twin reversed arterial perfusion sequence diagnosed in the first trimester. Fetal Diagn Ther 2021;48:778–84. https://doi.org/10.1159/000519860.Search in Google Scholar PubMed
71. Ruano, R, Benachi, A, Aubry, MC, Revillon, Y, Emond, S, Dumez, Y, et al.. Prenatal diagnosis of pulmonary sequestration using three-dimensional power Doppler ultrasound. Ultrasound Obstet Gynecol 2005;25:128–33. https://doi.org/10.1002/uog.1797.Search in Google Scholar PubMed
72. Mallmann, MR, Geipel, A, Bludau, M, Matil, K, Gottschalk, I, Hoopmann, M, et al.. Bronchopulmonary sequestration with massive pleural effusion: pleuroamniotic shunting vs intrafetal vascular laser ablation. Ultrasound Obstet Gynecol 2014;44:441–6. https://doi.org/10.1002/uog.13304.Search in Google Scholar PubMed
73. Achiron, R, Hegesh, J, Yagel, S. Fetal lung lesions: a spectrum of disease. New classification based on pathogenesis, two-dimensional and color Doppler ultrasound. Ultrasound Obstet Gynecol 2004;24:107–14. https://doi.org/10.1002/uog.1110.Search in Google Scholar PubMed
74. Ruano, R, da Silva, MM, Salustiano, EM, Kilby, MD, Tannuri, U, Zugaib, M. Percutaneous laser ablation under ultrasound guidance for fetal hyperechogenic microcystic lung lesions with hydrops: a single center cohort and a literature review. Prenat Diagn 2012;32:1127–32. https://doi.org/10.1002/pd.3969.Search in Google Scholar PubMed
75. Salomon, LJ, Audibert, F, Dommergues, M, Vial, M, Frydman, R. Fetal thoracoamniotic shunting as the only treatment for pulmonary sequestration with hydrops: favorable long-term outcome without postnatal surgery. Ultrasound Obstet Gynecol 2003;21:299–301. https://doi.org/10.1002/uog.76.Search in Google Scholar PubMed
76. Hayashi, S, Sago, H, Kitano, Y, Kuroda, T, Honna, T, Nakamura, T, et al.. Fetal pleuroamniotic shunting for bronchopulmonary sequestration with hydrops. Ultrasound Obstet Gynecol 2006;28:963–7. https://doi.org/10.1002/uog.3861.Search in Google Scholar PubMed
77. Cruz-Martinez, R, Mendez, A, Duenas-Riano, J, Ordorica-Flores, R, Nieto-Zermeno, J, Malagon-Salazar, P, et al.. Fetal laser surgery prevents fetal death and avoids the need for neonatal sequestrectomy in cases with bronchopulmonary sequestration. Ultrasound Obstet Gynecol 2015;46:627–8. https://doi.org/10.1002/uog.14921.Search in Google Scholar PubMed
78. Cruz-Martinez, R, Nieto-Castro, B, Martinez-Rodriguez, M, Gamez-Varela, A, Ahumada-Angulo, E, Luna-Garcia, J, et al.. Thoracic changes after full laser ablation of the feeding artery in fetuses with bronchopulmonary sequestration. Fetal Diagn Ther 2018;44:166–72. https://doi.org/10.1159/000481170.Search in Google Scholar PubMed
79. Vinit, N, Gueneuc, A, Bessieres, B, Dreux, S, Heidet, L, Salomon, R, et al.. Fetal cystoscopy and vesicoamniotic shunting in lower urinary tract obstruction: long-term outcome and current technical limitations. Fetal Diagn Ther 2020;47:74–83. https://doi.org/10.1159/000500569.Search in Google Scholar PubMed
80. Cruz-Martinez, R, Gil, PS, Villalobos-Gomez, R, Martinez-Rodriguez, M, Gamez-Varela, A, Lopez-Briones, H, et al.. Flexible video fetoscopy: feasibility and outcomes of a novel modality for laser therapy in twin-to-twin transfusion syndrome presenting with inaccessible anterior placenta. Fetal Diagn Ther 2023;50:106–14. https://doi.org/10.1159/000528815.Search in Google Scholar PubMed
81. Ruano, R, Duarte, S, Zugaib, M. Percutaneous laser ablation of sacrococcygeal teratoma in a hydropic fetus with severe heart failure--too late for a surgical procedure? Fetal Diagn Ther 2009;25:26–30. https://doi.org/10.1159/000188663.Search in Google Scholar PubMed
82. Ding, J, Chen, Q, Stone, P. Percutaneous laser photocoagulation of tumour vessels for the treatment of a rapidly growing sacrococcygeal teratoma in an extremely premature fetus. J Matern Fetal Neonatal Med 2010;23:1516–8. https://doi.org/10.3109/14767051003678085.Search in Google Scholar PubMed
83. Bouchghoul, H, Benachi, A, Senat, MV. Prenatal percutaneous fetoscopic laser photocoagulation of chorioangioma: report of two cases and review of the literature. Fetal Diagn Ther 2021;48:633–9. https://doi.org/10.1159/000517392.Search in Google Scholar PubMed
84. Mendez-Figueroa, H, Papanna, R, Popek, EJ, Byrd, RH, Goldaber, K, Moise, KJJr., et al.. Endoscopic laser coagulation following amnioreduction for the management of a large placental chorioangioma. Prenat Diagn 2009;29:1277–8. https://doi.org/10.1002/pd.2400.Search in Google Scholar PubMed
85. Bhide, A, Prefumo, F, Sairam, S, Carvalho, J, Thilaganathan, B. Ultrasound-guided interstitial laser therapy for the treatment of placental chorioangioma. Obstet Gynecol 2003;102:1189–91. https://doi.org/10.1097/00006250-200311001-00024.Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

