First-trimester maternal serum PAPP-A levels and hyperemesis gravidarum: unraveling the link – a meta-analysis
-
Antonia Varthaliti
, Vasilios Pergialiotis
, Vasilios Lygizos
, Marianna Theodora
, Dimitrios-Efthymios Vlachos
Abstract
Objectives
Hyperemesis gravidarum is a severe form of nausea and vomiting that affects approximately 0.3–2 % of pregnancies, leading to significant perinatal complications. This systematic review and meta-analysis aims to investigate the potential link between hyperemesis gravidarum and maternal serum pregnancy-associated plasma protein-A (PAPP-A) levels in the first trimester.
Methods
A thorough literature search of PubMed/MEDLINE, ScienceDirect, the Cochrane Library, and Google Scholar was conducted to identify relevant studies comparing PAPP-A levels in pregnant women diagnosed with hyperemesis gravidarum compared to healthy controls. Six studies met the inclusion criteria, with a total of 1,049 participants. Meta-analysis was performed to estimate the pooled mean difference in PAPP-A levels between hyperemesis gravidarum and control groups. A p-curve analysis and funnel plot assessment were conducted to evaluate publication bias and statistical power.
Results
The meta-analysis demonstrated a pooled mean difference of 0.16 (95 % CI: 0.07–0.25), indicating that PAPP-A levels were significantly higher in pregnancies affected by HG. The heterogeneity statistic (I2=46 %) suggested moderate variability among studies. P-curve analysis showed a right-skewed distribution of significant p-values (p=0.033), suggesting evidential value and ruling out selective reporting bias. However, the prediction interval (−0.08–0.4) indicated that some future studies might yield non-significant or even negative findings. Funnel plot analysis revealed minimal publication bias, though a slight asymmetry suggested potential underrepresentation of small, non-significant studies.
Conclusions
This study provides evidence that PAPP-A levels are elevated in pregnancies complicated by hyperemesis gravidarum, implicating potential placental dysfunction and hormonal influences in its pathogenesis. While the findings are statistically significant and robust against publication bias, moderate heterogeneity highlights the need for larger prospective studies with standardized methodologies to confirm this association and explore possible underlying mechanisms. Understanding the role of PAPP-A in hyperemesis gravidarum may contribute to improved screening and management strategies for affected pregnancies and as a result, improved perinatal care.
Introduction
Hyperemesis gravidarum (HG) is a severe form of nausea and vomiting that affects approximately 0.3–2 % of pregnancies, leading to significant maternal dehydration, electrolyte and metabolic imbalances, weight loss and even morbidity [1]. Unlike common pregnancy-related nausea and vomiting, hyperemesis gravidarum requires medical intervention, often including hospitalization, intravenous fluids, and pharmacological therapy. Despite its clinical significance, the exact eetiology remains unclear, though several hypotheses suggest an association with hormonal fluctuations, genetic predisposition, and placental factors [2], [3], [4], [5].
One of the key biomarkers of placental function is pregnancy-associated plasma protein-A (PAPP-A), a glycoprotein primarily produced by the syncytiotrophoblast. PAPP-A plays a crucial role in modulating insulin-like growth factor (IGF) activity, which is essential for placental development and fetal growth. Low levels of PAPP-A in the first trimester have been associated with adverse pregnancy outcomes, including fetal growth restriction, preeclampsia, and preterm birth. However, emerging evidence suggests that PAPP-A levels may also be altered in pregnant women with hyperemesis gravidarum [6], 7].
Several studies have explored the relationship between first-trimester maternal serum PAPP-A levels and HG, yet the findings remain inconsistent. Some investigations report elevated PAPP-A levels in women with HG, suggesting a potential link between placental dysfunction and the pathophysiology of the condition. Conversely, other studies have found no significant differences or even lower PAPP-A levels in HG pregnancies, making it challenging to draw definitive conclusions [8], 9].
Given the conflicting results in the literature, a comprehensive meta-analysis is warranted to synthesize available data and provide a clearer understanding of the association between HG and first-trimester PAPP-A levels. This systematic review and meta-analysis aims to evaluate whether there is a link between hyperemesis gravidarum and maternal serum PAPP-A concentrations and to assess the potential implications for pregnancy outcomes. By consolidating findings from multiple studies, this review seeks to contribute to the existing body of knowledge and inform clinical practice regarding the screening and management of hyperemesis gravidarum.
Materials and methods
The present systematic review and meta-analysis is designed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10]. It is based on previously published data from international literature; therefore, neither patient consent nor Institutional Review Board approval was necessary. This review was registered in the PROSPERO international database for systematic reviews (CRD420250651991).
Eligibility criteria, information sources, search strategy
For this review, two reviewers, A.V. and V.L., systematically searched databases, including PubMed/MEDLINE, ScienceDirect, the Cochrane Library, and Google Scholar in February 2025 to identify relevant studies investigating the association between first-trimester maternal serum pregnancy-associated plasma protein-A (PAPP-A) levels and hyperemesis gravidarum (HG). The authors predetermined the eligibility criteria. This systematic review encompassed all observational studies involving human patients, including prospective and retrospective cohort studies, case-control studies, as well as cross-sectional studies, that examined the association of hyperemesis gravidarum and PAPP-A levels, measured at the first-trimester screening. The included studies examined both spontaneous and in vitro fertilization (IVF) pregnancies. Restrictions were applied to enhance the relevance and validity of the findings. Studies that did not report direct comparisons between HG and non-HG groups were excluded. Studies such as case reports, small case series, letters to the editor, animal studies, and review articles were excluded. Additionally, conference abstracts and proceedings were not considered, as they often lack critical details necessary for evaluating study limitations and the quality of evidence. Additionally, studies with insufficient data on PAPP-A levels or those reporting only adjusted associations without raw biomarker values were omitted. Articles were screened for eligibility using title and abstract review, followed by full-text assessment. Furthermore, only studies published in English were considered. This review is based on previously published, aggregated data and does not constitute a randomized controlled trial (RCT). PICO criteria for this study involve: P (Population): Pregnant women undergoing first-trimester screening; I (Intervention/Exposure): Women diagnosed with hyperemesis gravidarum; C (Comparison): Pregnant women without hyperemesis gravidarum (control group); O (Outcome): Comparison of PAPP-A levels between the two groups.
Our search included the following strategy: (Pregnancy-associated Plasma Protein-A) OR (PAPP-A) AND (hyperemesis gravidarum) and is presented in Figure 1. The final studies included, ranged from 2005 to 2025. The two independent reviewers worked together to identify eligible studies and reached a consensus on the final selection. They individually evaluated the search results, and any discrepancies were addressed through discussions with a third author (V.P.) before making the final decisions.
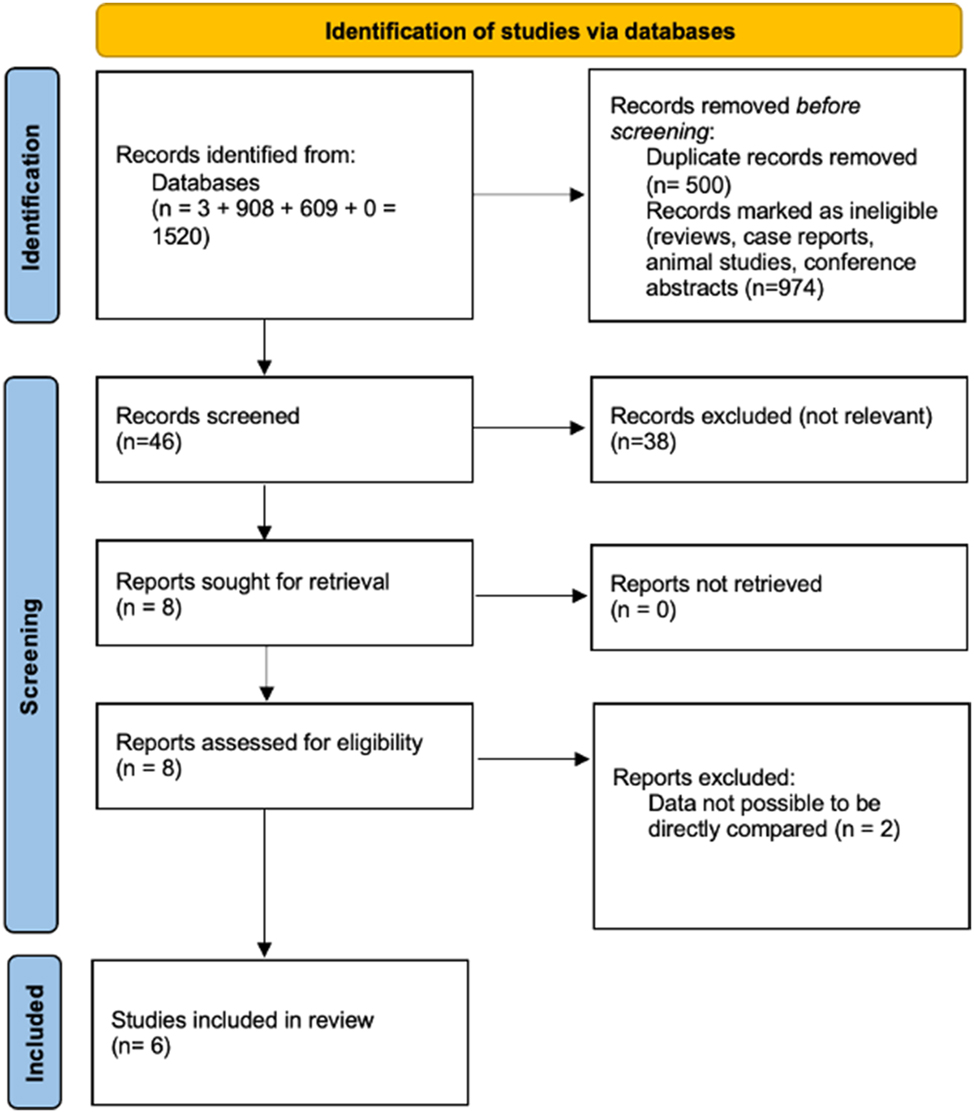
PRISMA guidelines.
Study selection
The study selection process was carried out in three sequential stages. First, duplicate articles were eliminated. Then, reviews, animal studies, case reports and conference abstracts were excluded. Subsequently, two authors (A.V. and V.L.) independently screened the abstracts of the remaining articles to evaluate their relevance. Finally, full-text reviews were conducted for studies deemed potentially eligible, and only those meeting the inclusion criteria were retained. Any disagreements in the final selection were resolved through consensus among all authors.
Data extraction
The main objective of this study was to evaluate the relationship between first-trimester PAPP-A levels and hyperemesis gravidarum. Studies that examined hyperemesis gravidarum in relation to other factors or lacked comparable data were excluded. Ultimately, a total of six studies were included in the meta-analysis. The outcome measures were defined at the outset during the study’s initial design.
Quality assessment
The methodological quality of all included studies was assessed using the Newcastle–Ottawa Scale (NOS), a widely recognized tool for evaluating the quality of non-randomized studies in systematic reviews and meta-analyses. This tool evaluates studies based on eight criteria, categorized into three main domains: selection of study groups, comparability of groups (considering maternal weight and gestational age), and assessment of either exposure (in case-control studies) or outcome (in cohort studies). A star-based rating system was applied to provide a quick visual representation of study quality, with the highest-quality studies receiving a maximum of nine stars. Two authors (A.V. and V.L.) independently conducted the quality assessment, and any disagreements were resolved through discussion with a third author (D.V.). The overall risk of bias was classified as good, fair, or poor (Figure 2).
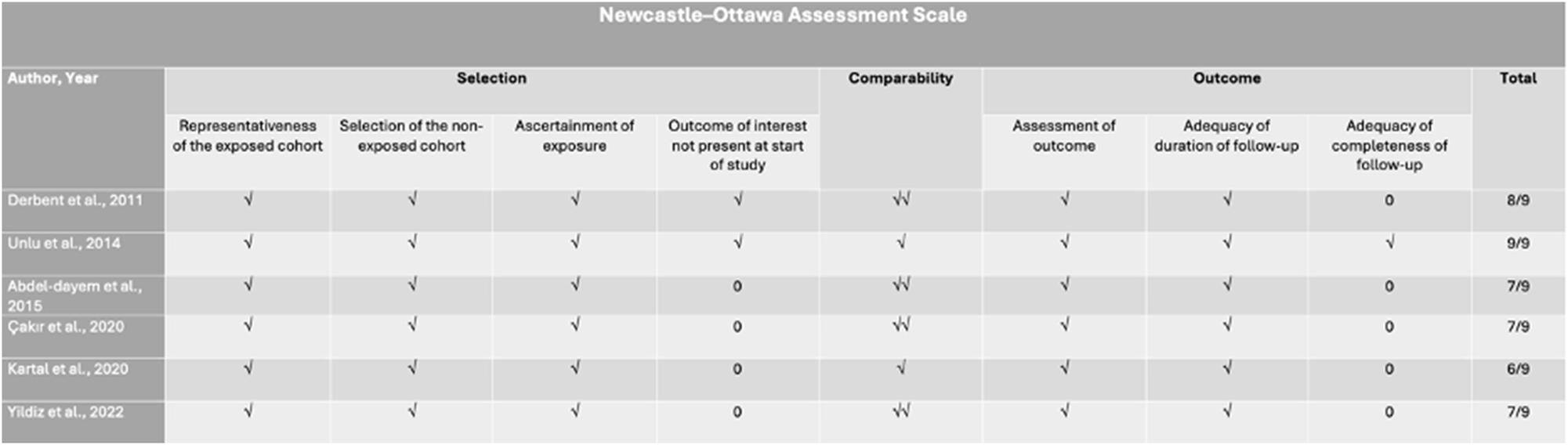
Newcastle–Ottawa assessment scale of the included studies. √: the assessed quality item was identified and confirmed within the study (applicable to selection and outcome categories); √: the study groups were adjusted for a single key factor; √√: the study groups were adjusted for two key factors.
Data synthesis, prediction intervals and trial sequential analysis
A statistical meta-analysis was conducted using RStudio with the meta function [RStudio Team (2015). RStudio: Integrated Development for R. RStudio, Inc., Boston, MA. URL http://www.rstudio.com/]. Given the substantial methodological heterogeneity among the included studies (Table 1), statistical heterogeneity was not factored into the selection of the appropriate analytical model (fixed-effects or random-effects), as assuming comparable effect sizes across studies was not feasible. Confidence intervals were set at 95 %. Pooled odds ratios (OR) and their corresponding 95 % confidence intervals (CI) were estimated using the Hartung–Knapp–Sidik–Jonkman method instead of the traditional Dersimonian–Laird random-effects model (REM). This approach was chosen based on recent evidence suggesting its superiority, particularly when dealing with studies of varying sample sizes and considerable between-study heterogeneity.
Methodological characteristics.
| Author, year | Study type | Number of patients | Inclusion criteria | Exclusion criteria | Maternal age | Timing of sample collection | Outcome |
|---|---|---|---|---|---|---|---|
| Derbent et al. [11] | Prospective | 225 115 H G, 110 controls |
Singleton pregnancies, hospitalization for HG before 20 weeks | Hyperthyroidism, stomach disease, gastroenteritis, multiple pregnancies | HG 27.7 ± 4.1 vs. control 29.5 ± 4.9 p=0.01a | First trimester prenatal screening | Elevated PAPP-A and β-hCG in HG group |
| Unlu et al. [12] | Prospective | 301 169 H G, 132 controls |
Singleton pregnancy, HG with dehydration and ketonuria | Hyperthyroidism, stomach disease, gastroenteritis | HG 27.6 ± 5.14 vs. control 25.53 ± 5.11 p=0.002a | 12–14 weeks of gestation | Increased PAPP-A levels in HG group, no difference in β-hCG |
| Abdel-dayem et al. [13] | Prospective | 60 30 H G, 30 controls |
HG cases, admitted to hospital | N/A | HG vs. control p=0.483 | 11–13 weeks of gestation | No significant difference in PAPP-A and β-hCG between HG and control |
| Çakır et al. [14] | Retrospective | 108 54 H G, 54 controls |
HG diagnosed cases | Vomiting caused by other diseases | HG 27.62 ± 5.037 vs. control 28.33 ± 5.051 p=0.283 | 10–14 weeks of gestation | Higher PAPP-A and hCG levels in HG group |
| Kartal et al. [15] | Prospective | 80 40 H G, 40 controls |
Singleton pregnancy, HG diagnosis | Systemic diseases, chromosomal disorders, gastrointestinal diseases | HG 25.7 ± 5.3 vs. controls 27.4 ± 4.6 p=0.130 | 11–14 weeks of gestation | No significant difference in PAPP-A and β-hCG levels |
| Yildiz [16] | Retrospective | 265 93 H G, 172 controls |
Pregnant women undergoing first trimester screening | Multiple pregnancy, fetal anomaly, systemic disease, eccentric umbilical cord, age>45 y.o. or<18 y.o., CRL<45 or>85 mm | 29.54 ± 4.94 (min–max: 18–42) | 11–14 weeks of gestation | Higher β-hCG in HG group, no difference in PAPP-A |
-
aStatistically significant.
Prediction intervals (PI) were also determined using the meta function in RStudio to assess the anticipated effect size in future studies within this field. This estimation incorporates the variability between studies, representing the existing heterogeneity on the same scale as the outcome being analysed.
To assess the required information size, trial sequential analysis (TSA) was conducted. This method allows for the evaluation of type I error in the pooled results of meta-analyses focusing on the primary outcomes predefined in this study. A minimum of three studies was considered necessary to perform the analysis. Since repeated significance testing in meta-analyses increases the likelihood of type I errors, TSA adjusts the significance threshold using the O’Brien–Fleming α-spending function. Through sequential interim analyses, TSA evaluates the influence of each individual study on the overall meta-analysis findings. The risk of type I error was set at 5 %, while the type II error threshold was established at 20 %. TSA was conducted using TSA version 0.9.5.10 Beta software (http://www.ctu.dk/tsa/).
Results
Study selection and methodological characteristics
The extensive literature search resulted in 1,520 records: three from PubMed, 908 from ScienceDirect, 0 from the Cochrane Library, and 609 from Google Scholar. After eliminating 500 duplicate entries, 650 animal studies, 300 reviews, 24 conference abstracts, editorials and case reports, we screened 46 original studies based on their titles and abstracts. Out of those, 38 were excluded as they were not relevant to our study and two could not be retrieved. Lastly, six studies, published between 2005 and 2025, were included in our meta-analysis that involved 1,039 pregnant women, 501 with hyperemesis gravidarum and 538 controls, that underwent the first-trimester screening.
In Table 1, methodological characteristics of the included studies are depicted. The timing of sample collection for the assessment of PAPP-A occurred during the first-trimester screening, between 10 and 14 weeks of gestation, in all the included studies. The mean maternal age varied across studies but was comparable between HG and control groups. Three studies [11], 14], 16] found significantly higher PAPP-A levels in the hyperemesis gravidarum group, whereas others reported no significant difference.
Synthesis of results
This meta-analysis supports the hypothesis that PAPP-A levels are elevated in pregnancies affected by hyperemesis gravidarum. The forest plot in Figure 3 summarizes the findings from the six included studies, subgrouped as prospective and retrospective studies, displaying individual mean differences (MD) with 95 % confidence intervals (CI), along with the overall pooled estimate. The pooled mean difference random effects models was 0.12 (95 % CI: 0.00–0.24) for the prospective studies and 0.22 (95 % CI: 0.11–0.34) for the retrospective studies, indicating that PAPP-A levels were significantly higher in women with hyperemesis gravidarum compared to the control group. Notably, individual study estimates varied, with Abdel-Dayem et al. [13] reporting a non-significant mean difference (0.02, 95 % CI: −0.12–0.16), while Çakır et al. [14] reported the largest effect (0.36, 95 % CI: 0.07–0.65). The heterogeneity statistic (I2=46 % – I2=52 % for the prospective studies and I2=7 % for the retrospective studies) suggests moderate variability among studies, but it is not high enough to indicate strong inconsistency. The prediction interval (−0.08–0.4) suggests that while most future studies are expected to show a positive association, some may yield non-significant or even negative results.
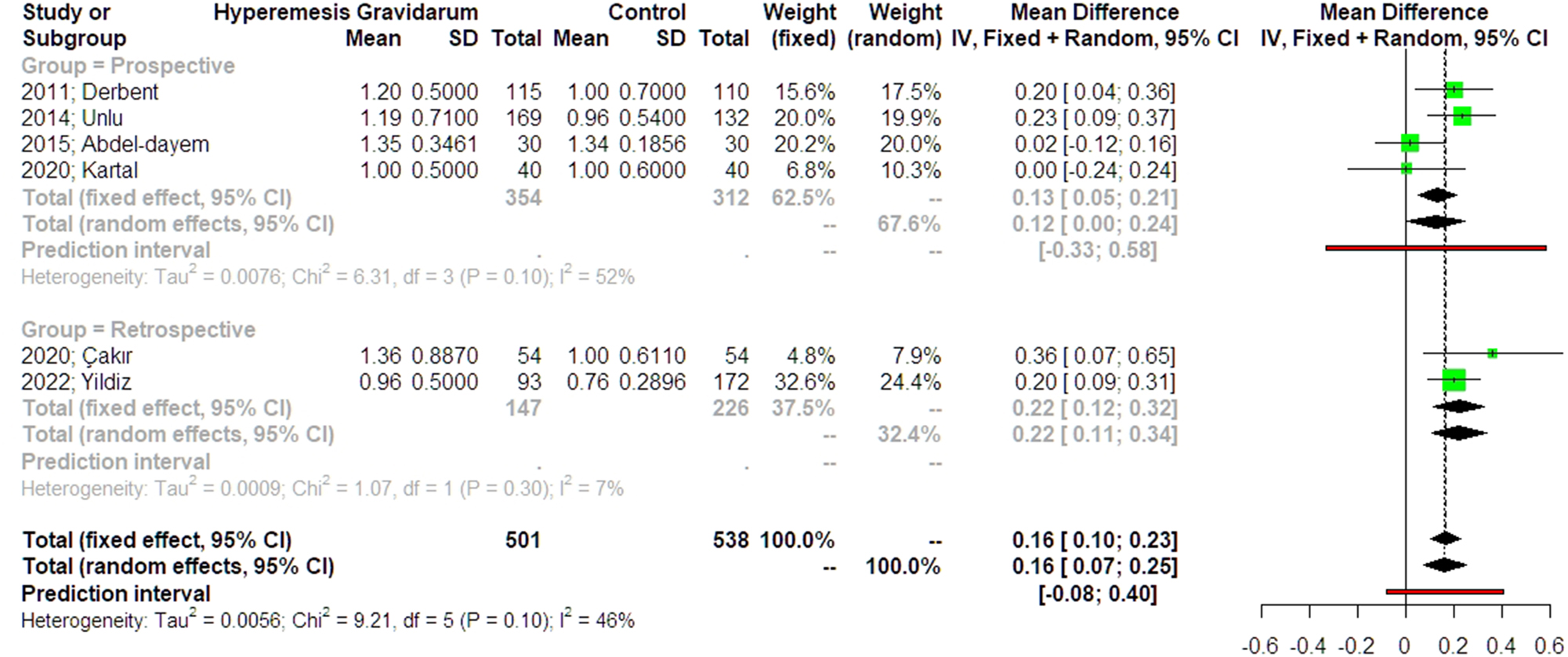
Forest plots of odds ratio for PAPP-A levels in the first-trimester screening in women with hyperemesis gravidarum compared to controls with 95 % confidence intervals (CI) and weighted pooled summary statistics using bivariate random-effects model. The studies are subgrouped into prospective and retrospective. Vertical line = “no difference” point between the two groups. Green squares=risk ratios; diamonds=pooled risk ratios and 95 confidence intervals for all studies; horizontal black lines=95 % CI; horizontal red line=pooled 95 % prediction intervals).
In order to evaluate the evidential value and potential bias, p-curve analysis was conducted, as shown in Figure 4. The observed p-curve (blue line) demonstrated a right-skewed distribution, with all statistically significant p-values (p<0.05) falling below 0.025. This right-skewness test was statistically significant (p=0.033, p=0.0462), suggesting that the results are likely due to true effects rather than selective reporting or p-hacking. The estimated statistical power of the included studies was 69 % (95 % CI: 20–94 %), indicating moderate power, though with some uncertainty. The lack of an excess of p-values between 0.03 and 0.05 reduces concerns about selective reporting bias. However, two additional non-significant results (p>0.05) were excluded from the p-curve, indicating that some studies did not find a significant association. Overall, the p-curve analysis supports the presence of a genuine association between PAPP-A levels and hyperemesis while highlighting the need for further studies with larger sample sizes to improve statistical power.
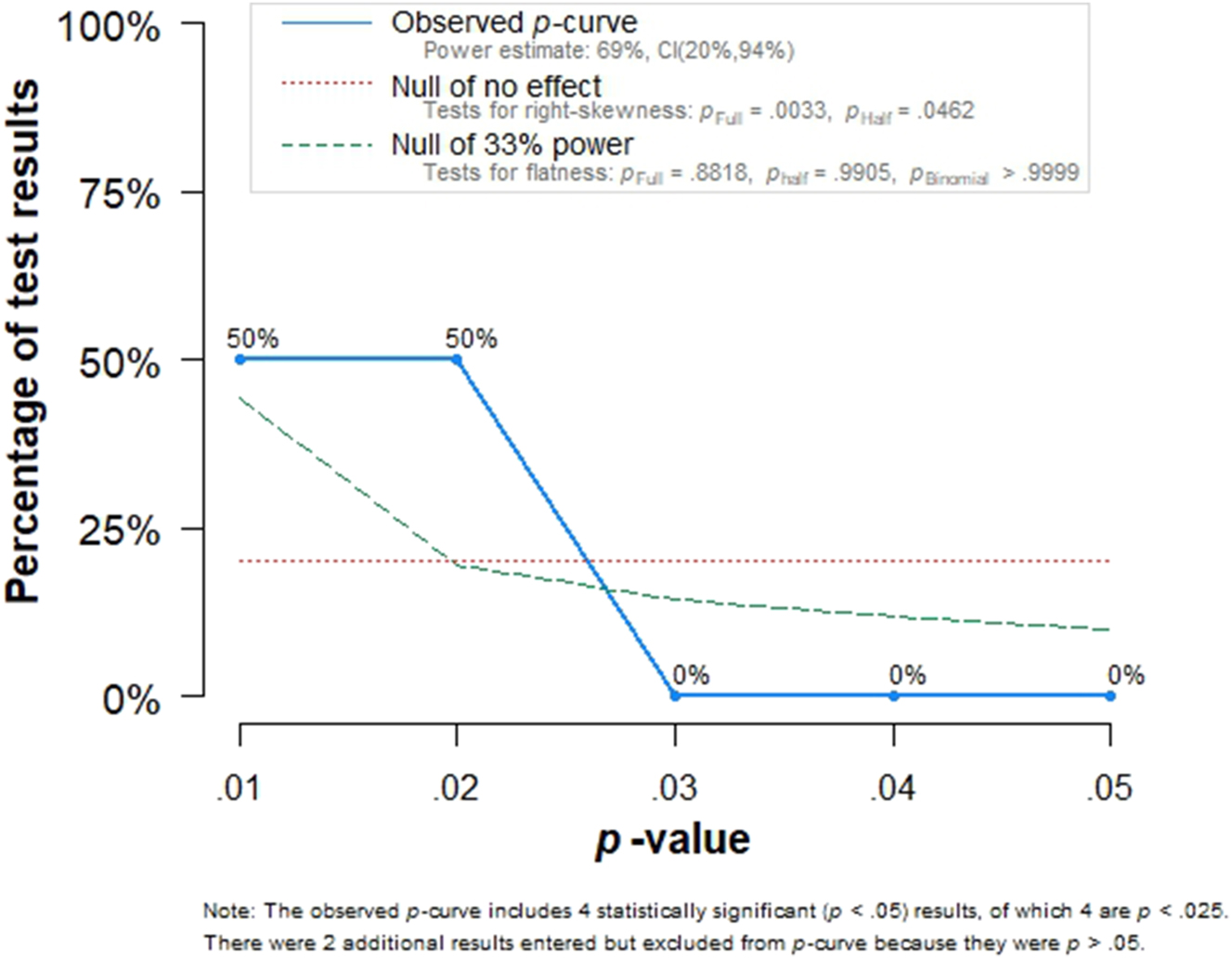
p-Curve analysis demonstrating the distribution of significant p-Values from the included studies. The right-skewed curve suggests evidential value for a real effect, while power is estimated at 69 % (95 % CI: 20–94 %).
A contour-enhanced funnel plot, depicted in Figure 5, was used to assess publication bias in the meta-analysis of PAPP-A levels and hyperemesis gravidarum. The plot showed a reasonably symmetric distribution of studies around the pooled mean difference (∼0.16), suggesting minimal publication bias. Most studies fell within regions indicating statistical significance (p<0.05), further supporting the robustness of the findings. However, a slight asymmetry, with fewer studies reporting negative or null results, suggests the potential underrepresentation of small, non-significant studies. Despite this, the overall pattern does not indicate a strong small-study effect, reinforcing the reliability of the meta-analysis conclusions.
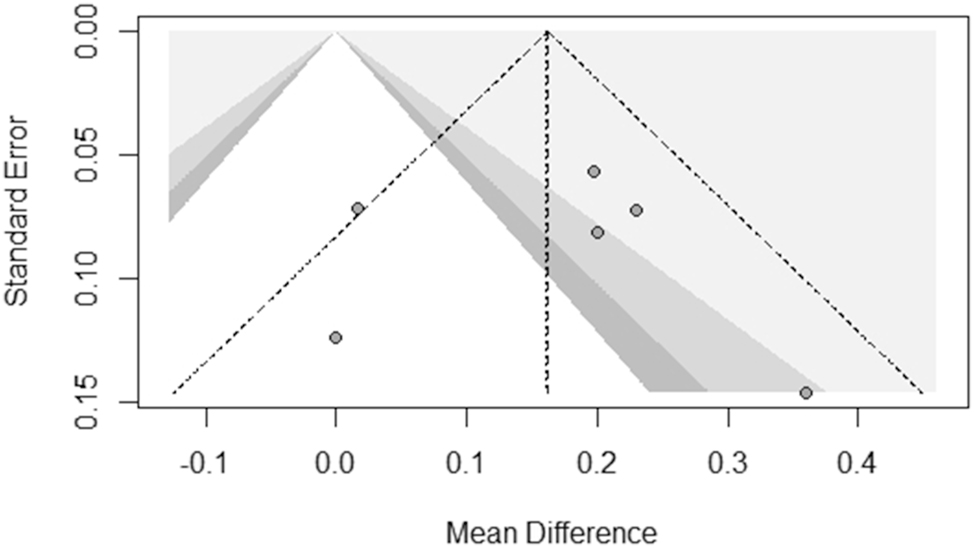
Contour-enhanced funnel plot for publication bias assessment in the meta-analysis of PAPP-A levels and hyperemesis gravidarum. The X-axis represents the mean difference, and the Y-axis represents the standard error. The dashed triangle indicates expected study distribution in the absence of bias. Shaded regions represent significance thresholds (darker=more significant). The plot suggests minimal publication bias, though a slight asymmetry indicates potential underreporting of small, non-significant studies.
Discussion
This meta-analysis included several studies which assessed the association between hyperemesis gravidarum and first-trimester maternal serum factors, which include pregnancy-associated PAPP-A, in relation to potential implications for placental function, fetal growth, and pregnancy outcomes. Our findings support the hypothesis that HG serves as a potential marker of compromised placental development, contributing to adverse fetal outcomes, as we found a statistically significant relationship between hyperemesis gravidarum and elevated levels of PAPP-A, which indicates a shared pathophysiological background.
Previous studies have shown that hyperemesis gravidarum correlates with placental abnormalities [3], 5]. While high levels of PAPP-A itself do not constitute an etiology for HG, the presence of HG may represent a significant underlying placental pathology, indicating abnormalities in placentation, which, in first trimester of pregnancy, correlates with an increased PAPP-A and later with placental dysfunction. Considering this, we believe that hyperemesis gravidarum may be added, in the future, in predictive algorithms of conditions, such as preeclampsia, related to abnormal placentation. Çakır et al. [14] and Bolin et al. [17] conducted a population-based cohort study in Sweden, demonstrating that women with HG had increased risks of preterm preeclampsia, placental abruption, and SGA births, particularly when HG extended into the second trimester. Their results indicated a threefold increased risk of placental abruption and a 39 % heightened likelihood of SGA birth in HG pregnancies. Similarly, Moberg et al. [18] found that women with HG exhibited elevated odds of adverse placental outcomes, including FGR, preterm delivery, and preeclampsia, reinforcing the pathogenic link between HG and placental insufficiency [19].
One of the strengths of this study was that all studies utilized standardized definitions for HG and biochemical markers, which allow for comparability [11], 16]. Furthermore, the use of various study populations in different geographic regions supports the generalizability of our conclusions [14]. Despite these strengths, several limitations should be acknowledged. The most important limitation is the sample size. Further research is required in order to ensure the correlation between HG and PAPP-A. Additionally, differences in sample sizes and study designs create opportunity for bias. Some of the included studies were retrospective, which raises the possibility of selection bias [13]. Furthermore, confounders like maternal comorbidities or background characteristics, were not uniformly controlled for across all studies [15], 16]. While many studies show a link between HG and placental dysfunction, there are some inconsistencies. Koudijs et al. [20] found no difference in preeclampsia or neonatal outcomes between women with and without HG, except for lower birth weights in HG pregnancies.
In conclusion, this meta-analysis indicates a significant association between HG and high first-trimester values of maternal serum PAPP-A. HG is associated with an increased risk of placental dysfunction disorders, particularly in cases of severe or prolonged HG [3], 5]. However, rather than being an etiological factor for HG, high PAPP-A levels may reflect underlying placental pathology. The presence of HG in pregnancies with elevated PAPP-A should be recognized as a potential indicator of future placental dysfunction. Given the possible implications that HG has for placental and fetal well-being, early diagnosis and targeted management strategies, such as close monitoring of the fetus, may reduce any risks involved with HG pregnancies. Although these results highlight the hormonal foundation of HG, additional studies are needed to clarify the exact pathophysiology and clinical implications of the biomarkers towards HG treatment [14], 15]. A better understanding of the pathophysiological underpinnings of HG, as well as further studies that examine the correlation between HG and placental abnormalities, would enable the design of screening tools with predisposing factors, which would allow for focused therapeutic intervention and the optimization of maternal and fetal outcomes.
Conclusions
This systematic review and meta-analysis provide evidence that PAPP-A levels are elevated in pregnancies complicated by hyperemesis gravidarum, supporting the hypothesis that placental dysfunction and endocrine alterations contribute to its pathophysiology. While the results are statistically significant and robust against publication bias, the moderate heterogeneity and wide prediction interval indicate potential variability in future studies. The findings emphasize the need for larger, well-powered prospective studies with standardized methodologies to confirm the association and explore underlying mechanisms. Understanding the role of PAPP-A in hyperemesis gravidarum could have clinical implications for early prediction and management, ultimately improving maternal and fetal outcomes.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: Conceptualization, A.V. and V.P.; methodology, A.V. and V.P.; software, D.E.V.; validation, A.V., V.L and V.P.; formal analysis, A.V.; investigation, A.V., P.A. and M.T.; resources, M.T.; data curation, D.E.V.; writing: original draft preparation, A.V., V.P. and V.L.; writing: review and editing, A.V. and M.-A.D.; visualization, P.A.; supervision, G.D., P.A. and V.P.; project administration, V.P. and G.D. All authors have read and agreed to the published version of the manuscript.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Sanu, O, Lamont, RF. Hyperemesis gravidarum: pathogenesis and the use of antiemetic agents. Expet Opin Pharmacother 2011;12:737–48. https://doi.org/10.1517/14656566.2010.537655.Search in Google Scholar PubMed
2. Erick, M, Cox, JT, Mogensen, KM. ACOG practice Bulletin 189: nausea and vomiting of pregnancy. Obstet Gynecol 2018;131:935. https://doi.org/10.1097/aog.0000000000002604.Search in Google Scholar PubMed
3. Fejzo, MS, Trovik, J, Grooten, IJ, Sridharan, K, Roseboom, TJ, Vikanes, Å, et al.. Nausea and vomiting of pregnancy and hyperemesis gravidarum. Nat Rev Dis Primers 2019;5:62. https://doi.org/10.1038/s41572-019-0110-3.Search in Google Scholar PubMed
4. Martinez De Tejada, B, Vonzun, L, Von Mandach, DU, Burch, A, Yaron, M, Hodel, M, et al.. Nausea and vomiting of pregnancy, hyperemesis gravidarum. Eur J Obstet Gynecol Reprod Biol 2025;304:115–20. https://doi.org/10.1016/j.ejogrb.2024.11.006.Search in Google Scholar PubMed
5. Philip, B. Hyperemesis gravidarum: literature review. Wis Med J 2003;102:46–51.Search in Google Scholar
6. Conover, CA, Oxvig, C. The pregnancy-associated plasma protein-A (PAPP-A) story. Endocr Rev 2023;44:1012–28. https://doi.org/10.1210/endrev/bnad017.Search in Google Scholar PubMed
7. Fialova, L, Malbohan, IM. Pregnancy-associated plasma protein A (PAPP-A): theoretical and clinical aspects. Bratisl Lek Listy 2002;103:194–205.Search in Google Scholar
8. Kaňková, Š, Hlaváčová, J, Roberts, K, Benešová, J, Havlíček, J, Calda, P, et al.. Associations between nausea and vomiting in pregnancy, disgust sensitivity, and first-trimester maternal serum free β-hCG and PAPP-A. Horm Behav 2023;152:105360. https://doi.org/10.1016/j.yhbeh.2023.105360.Search in Google Scholar PubMed
9. Spencer, CA, Allen, VM, Flowerdew, G, Dooley, K, Dodds, L. Low levels of maternal serum PAPP‐A in early pregnancy and the risk of adverse outcomes. Prenat Diagn 2008;28:1029–36. https://doi.org/10.1002/pd.2116.Search in Google Scholar PubMed
10. Page, MJ, Moher, D, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al.. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. Br Med J 2021;372:n160. https://doi.org/10.1136/bmj.n160.Search in Google Scholar PubMed PubMed Central
11. Derbent, AU, Yanik, FF, Simavli, S, Atasoy, L, Ürün, E, Kuşçu, ÜE, et al.. First trimester maternal serum PAPP‐A and free β‐HCG levels in hyperemesis gravidarum. Prenat Diagn 2011;31:450–3. https://doi.org/10.1002/pd.2715.Search in Google Scholar PubMed
12. Unlu, BS, Energin, H, Yildiz, Y, Unlu, E, Eyi, EGY. Maternal serum pregnancy-associated plasma protein-A levels in hyperemesis gravidarum: a prospective case control study. Clin Exp Obstet Gynecol 2014;41:534–6. https://doi.org/10.12891/ceog17382014.Search in Google Scholar
13. Abdel-Dayem, TM, El-Agwany, AS, Soliman, TI, Kholief, A, El-Sawy, MM. Double marker test and uterine doppler in cases with hyperemesis gravidarum. EBWHJ 2015;5:134–9. https://doi.org/10.1097/01.ebx.0000466751.03382.4a.Search in Google Scholar
14. Çakır, AT, Boran, AB. Evaluation of maternal serum PAPP-A and hCG levels at 10–14 weeks of gestation in hyperemesis gravidarum. Haseki 2020;58:465–9. https://doi.org/10.4274/haseki.galenos.2020.6499.Search in Google Scholar
15. Kartal, D, Arıcı Yurtkul, A, Şenkaya, AR. The role of uterine artery Doppler index and nuchal translucency measurement in predicting perinatal problems and the gestational age in patients diagnosed with hyperemesis gravidarum. Aegean J Obstet Gynecol 2020;2:19–22. https://doi.org/10.46328/aejog.v2i3.63.Search in Google Scholar
16. Yıldız, G. Hyperemesis gravidarum and its relationships with placental thickness, PAPP-A and free beta-HCG: a case control study. South Clin Ist Euras 2022;33:406–12. https://doi.org/10.14744/scie.2021.93546.Search in Google Scholar
17. Bolin, M, Åkerud, H, Cnattingius, S, Stephansson, O, Wikström, A. Hyperemesis gravidarum and risks of placental dysfunction disorders: a population‐based cohort study. BJOG 2013;120:541–7. https://doi.org/10.1111/1471-0528.12132.Search in Google Scholar PubMed PubMed Central
18. Moberg, T, Van Der Veeken, L, Persad, E, Hansson, SR, Bruschettini, M. Placenta-associated adverse pregnancy outcomes in women experiencing mild or severe hyperemesis gravidarum – a systematic review and meta-analysis. BMC Pregnancy Childbirth 2023;23:375. https://doi.org/10.1186/s12884-023-05691-6.Search in Google Scholar PubMed PubMed Central
19. Odibo, AO, Zhong, Y, Goetzinger, KR, Odibo, L, Bick, JL, Bower, CR, et al.. First-trimester placental protein 13, PAPP-A, uterine artery doppler and maternal characteristics in the prediction of pre-eclampsia. Placenta 2011;32:598–602. https://doi.org/10.1016/j.placenta.2011.05.006.Search in Google Scholar PubMed PubMed Central
20. Koudijs, HM, Savitri, AI, Browne, JL, Amelia, D, Baharuddin, M, Grobbee, DE, et al.. Hyperemesis gravidarum and placental dysfunction disorders. BMC Pregnancy Childbirth 2016;16:374. https://doi.org/10.1186/s12884-016-1174-7.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

