Abstract
Objectives
The purpose of the study is to explore the potential of novel genetic markers in predicting and diagnosing hypertensive conditions during pregnancy, focusing on their role in key pathological processes. The relevance of this study is determined by the necessity to improve methods for the early detection and effective management of pregnancy complications, particularly those associated with elevated blood pressure, which pose a threat to the health of both mother and foetus.
Methods
The research methodology involves a systematic analysis of scientific literature from the past five years, specifically from 2019 to 2024, emphasising the integration of data from various populations and research approaches within the field of molecular medicine.
Results
The main findings demonstrate that certain molecular markers reflecting gene activity exhibit high accuracy in predicting the risks of complications associated with hypertension of pregnancy. It is established that these markers can detect disturbances in the regulation of vascular tone, angiogenesis processes, and inflammatory responses long before the onset of clinical symptoms. This opens up opportunities for timely medical intervention and a personalised approach to pregnancy management.
Conclusions
The results of the study confirm the potential of utilising genetic markers in clinical practice to improve pregnancy outcomes and reduce risks for both mother and child. The practical value lies in the possibility of developing new screening programmes and therapeutic strategies based on the individual characteristics of patients, contributing to more effective management of hypertensive disorders during pregnancy.
Introduction
Hypertensive disorders during pregnancy, including pre-eclampsia and gestational hypertension, are among the leading causes of maternal and perinatal morbidity and mortality [1], [2], [3]. These conditions not only affect maternal health but can also have long-term consequences for the new-born, including an increased risk of cardiovascular diseases in the future. Therefore, the development of methods for early diagnosis, monitoring, and prognosis of hypertensive disorders during pregnancy is a critically important task in modern medicine. It is known that mRNA plays an essential role in intercellular communication, and its levels in the blood can change under the influence of pathological processes. Due to their specific profile, mRNA biomarkers are capable of reflecting processes such as impaired angiogenesis, inflammation, and endothelial dysfunction, which are fundamental to the pathogenesis of hypertensive disorders during pregnancy [4]. Furthermore, mRNA biomarkers present in plasma or exosomes have the advantage of being non-invasive indicators [5], 6]. Their examination can contribute to the development of precise molecular diagnostic methods, enabling the monitoring of pregnant women at risk even before the clinical manifestation of the disease.
Recent studies have significantly advanced our understanding of hypertensive disorders during pregnancy and their broader health implications. Ford et al. [1] highlighted an increase in the prevalence of these conditions from 13.3 % in 2017 to 15.9 % in 2019, noting a higher mortality rate among women of lower socio-economic status. However, the reliance on retrospective data may have led to certain nuances being overlooked. Melchiorre et al. [2] found that women with a history of pre-eclampsia face an elevated risk of future cardiovascular diseases due to structural and functional changes in the heart. Despite these insights, there remains a gap in standardised recommendations for long-term monitoring and management of these patients, posing challenges for effective postpartum care. In the realm of biomarker development, Wu et al. [5] identified new biomarkers for the early detection of pre-eclampsia, such as placental growth factor, which shows promise in predicting severe complications. However, the practical application of these biomarkers is often hindered by high testing costs, limiting their accessibility and widespread use. Kipnis et al. [6] confirmed the safety and effectiveness of antihypertensive medications like labetalol and nifedipine when used within recommended doses during pregnancy. Yet, there is a notable lack of data on the long-term effects of these therapies on newborns, underscoring the need for further research in this area.
Garovic et al. [7] revealed a doubled risk of developing conditions such as heart failure and strokes, particularly among women who had pre-eclampsia. These studies, while informative, often fall short in fully exploring the genetic factors contributing to these complications, indicating a need for more comprehensive genetic analyses. Efforts by Tagetti and Fava [8] to improve the diagnosis and classification of hypertensive disorders have emphasised the importance of accurate classification to reduce maternal mortality. Innovative methods for early risk detection have been proposed, although adapting these methods to low-resource settings remain a challenge, limiting their global applicability.
Thus, a large number of studies have been dedicated to examining hypertensive disorders; however, few works focus on mRNA biomarkers in pregnant women with such conditions, which determined the necessity of this study. By integrating data from different populations and methodologies, the study aims to create a comprehensive overview of the transcriptional landscape characterising these conditions, ultimately providing a valuable resource for both researchers and clinicians striving to improve maternal and foetal health outcomes.
This study significantly advances the understanding and management of hypertensive disorders during pregnancy by systematically analysing mRNA biomarkers, which are crucial for early diagnosis and prognosis of conditions like pre-eclampsia and gestational hypertension. By identifying key biomarkers such as miR-19a, miR-210, and miR-126, the research offers promising tools for early screening, potentially transforming clinical practice through personalised medicine approaches [9]. The insights into the pathophysiology of these disorders, including impaired angiogenesis and endothelial dysfunction, provide a foundation for developing targeted therapies. The integration of these biomarkers into clinical practice could lead to standardised diagnostic platforms, improving maternal health outcomes and reducing long-term cardiovascular risks. This study underscores the potential of mRNA biomarkers to enhance the precision and effectiveness of maternal healthcare, marking a pivotal step forward in obstetrics and personalised medicine.
Materials and methods
This study is a systematic review of the literature conducted between January 2019 and December 2024. The literature search was performed in five major scientific databases: PubMed, Scopus, Web of Science, Embase, and the Cochrane Library. Search queries included combinations of the following keywords: “mRNA biomarkers”, “hypertensive disorders”, “pregnancy”, “pre-eclampsia”, “hypertensive disorders in pregnancy”, “circulating mRNA”, “gestational hypertension”, “mRNA expression”, “placental mRNA”, and their equivalents in Kazakh. Boolean operators AND, OR, and NOT were applied to refine and expand the search. A protocol describing the rationale, objectives, and search strategies for this systematic review was developed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and registered with the International Prospective Register of Systematic Reviews under registration number CRD42024512410 [9]. An initial search identified 150 publications. After removing duplicates, 120 studies remained. Preliminary screening of titles and abstracts, based on the inclusion and exclusion criteria, reduced the number to 92 potentially relevant papers. Full-text analysis was conducted for these papers, resulting in 77 sources being included in the final analysis as they met all criteria (Figure 1).
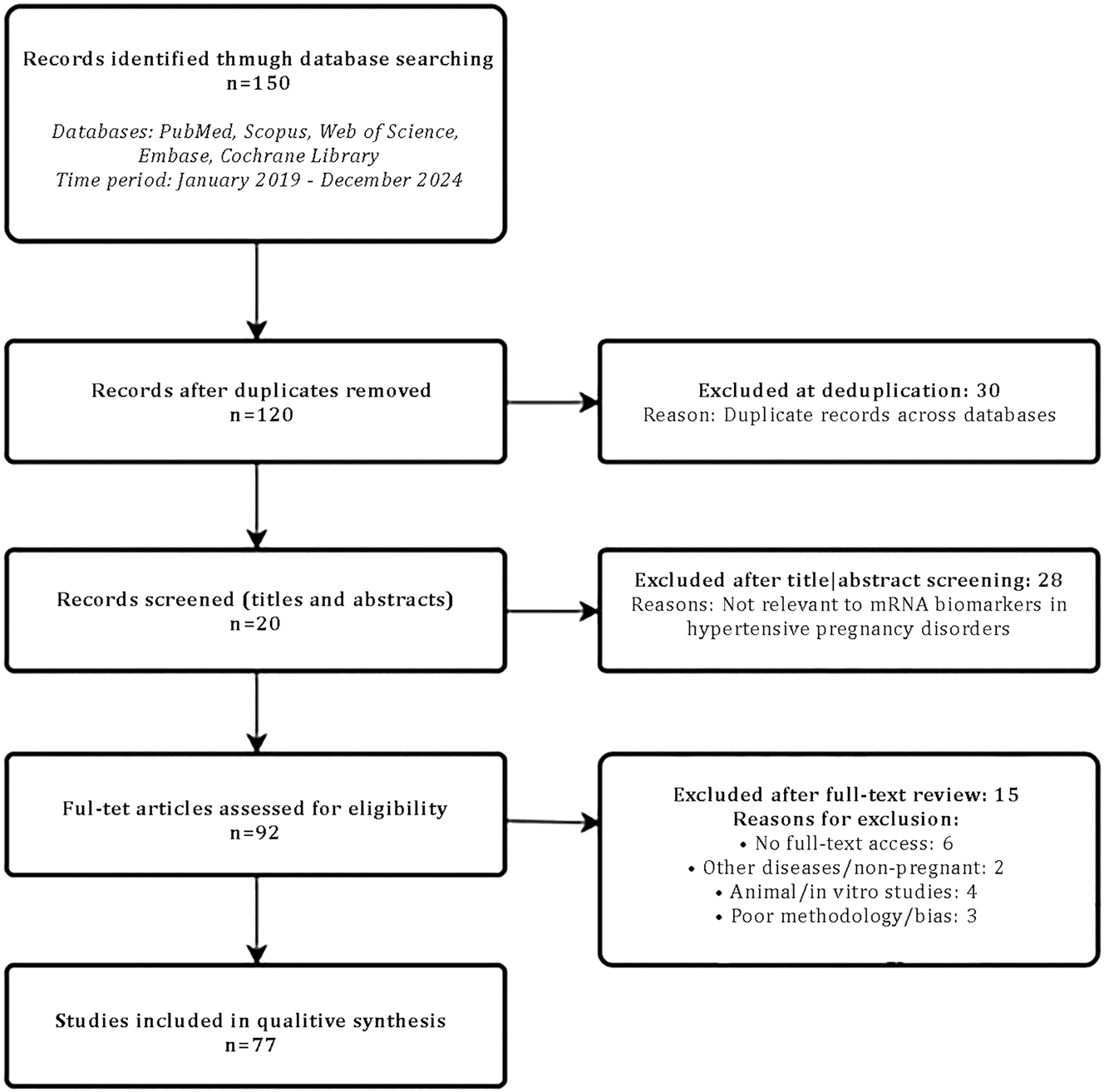
Flow diagram: Literature selection process.
The risk of bias for included observational, case-control, or nested case-control studies was assessed using the modified Newcastle-Ottawa Scale [10]. Since randomised controlled trials were not included in this systematic review, common systematic biases (e.g., selection bias, allocation concealment bias, etc.) were not assessed for the selected papers. The interpretation of the obtained data was based on comparing results from various studies, considering the heterogeneity of the samples and methods. A narrative approach was employed for data synthesis, enabling the integration of information from diverse sources and identifying common trends regarding the role of mRNA biomarkers in the pathogenesis and diagnosis of hypertensive disorders during pregnancy. The results of the analysis contributed to achieving the purpose of the study by summarising current scientific data on mRNA biomarkers, which could be utilised for the early diagnosis and prognosis of hypertensive disorders in pregnancy.
Results and discussion
Types, mechanisms, and pathophysiology of hypertensive disorders during pregnancy
Hypertensive disorders during pregnancy, including pre-eclampsia and gestational hypertension, are major causes of maternal and foetal morbidity and mortality. Understanding the molecular basis of these disorders is crucial for early diagnosis and the development of targeted therapies. Profiling blood gene transcript signatures provides an innovative method for identifying the dynamic changes in gene expression associated with these pregnancy complications.
Most maternal and foetal health issues during pregnancy involve hypertension [11], 12]. Many locations worldwide have seen an increase in these situations. A French study found that these illnesses complicate 7.4 % of pregnancies, with pre-eclampsia being the most common [13]. A comprehensive study in China indicated an increase in gestational hypertension and pre-eclampsia, which are linked to obesity, age over 35, and inadequate physical activity [14]. In Africa, hypertensive problems were common in pregnancy, with 8 % of cases resulting in major consequences for the mother and baby, such as premature deliveries or low birth weight [15]. This epidemiological trend emphasises the importance of early detection and management to improve women’s and children’s health. Table 1 lists the main hypertensive disorders.
Main types of hypertensive disorders.
| Disorder name | Characteristics |
|---|---|
| Gestational hypertension | Elevated blood pressure first appearing after the 20th week of pregnancy without proteinuria (presence of protein in the urine) or other signs of pre-eclampsia |
| Pre-eclampsia | Hypertension after the 20th week of pregnancy with proteinuria (>300 mg of protein in urine over 24 h) or signs of systemic organ dysfunction |
| Eclampsia | Pre-eclampsia accompanied by seizures or coma not associated with other neurological disorders |
| Chronic hypertension with superimposed pre-eclampsia | Development of pre-eclampsia in women with pre-existing chronic hypertension, characterised by worsening blood pressure control and the onset of proteinuria and other symptoms |
| HELLP syndrome | A severe form of pre-eclampsia that includes haemolysis (destruction of red blood cells), elevated liver enzyme levels, and low platelet count |
Pregnancy-related hypertensive disorders, particularly pre-eclampsia, are characterised by complex pathophysiology involving disturbances at the placental, immune system, and systemic vascular tone levels [18], 19]. A key factor in the development of these conditions is impaired placentation. Normally, during pregnancy, spiral arteries remodel to ensure adequate blood supply to the foetus. In the case of pre-eclampsia, this process is incomplete, leading to impaired placental blood flow and placental hypoxia. Placental hypoxia stimulates the release of anti-angiogenic factors such as soluble fms-like tyrosine kinase 1 (sFLT1), which block the action of angiogenic proteins VEGF and PlGF. This impairs endothelial function, promotes vasoconstriction, and increases blood pressure, which are key features of pre-eclampsia [20].
The immune system also plays an important role in the development of these conditions. Impaired adaptation of the maternal immune system to foetal antigens may contribute to the activation of systemic inflammation. This initiates a cascade of oxidative stress that further damages the endothelium and worsens placental blood flow [21]. Systemic endothelial stress leads to the activation of the coagulation cascade, the formation of microthrombi in small vessels, and organ dysfunction, particularly affecting the kidneys, liver, and brain. This results in symptoms such as proteinuria, elevated liver enzyme levels, and, in severe cases, may lead to HELLP syndrome or eclampsia [22].
Considering the risk factors for hypertensive disorders in pregnancy is an important aspect of pregnancy planning and management. These factors not only affect the health of the mother and child but may also have long-term consequences for a woman’s cardiovascular system. Knowledge of the risks allows for timely preventive measures, reducing the likelihood of complications. Table 2 below outlines the key risk factors and their characteristics.
Main risk factors for developing hypertension disorders.
| Risk factor | Characteristics |
|---|---|
| Obesity | A higher-than-normal body mass index is associated with an increased risk of hypertension disorders and more severe manifestations of pre-eclampsia |
| Nulliparity | Pregnancy in women who have not had a previous birth is more likely to have complications, in particular due to the lower adaptive reserve of the body before pregnancy |
| Age over 35 years | Older women of reproductive age have an increased risk of hypertension disorders due to a decrease in vascular elasticity and an increase in concomitant diseases |
| Genetic predisposition | Having a family history of hypertension or other cardiovascular diseases significantly increases the risk of hypertensive disorders during pregnancy |
| Multiple pregnancy | Pregnancy with twins or triplets increases the volume of circulating blood and the load on the cardiovascular system, which increases the risk of pre-eclampsia and other hypertensive disorders |
| Previous history of abortions | Having one or more abortions in the anamnesis is associated with an increased risk of hypertension disorders during subsequent pregnancies |
| Chronic stress | Increased stress levels during pregnancy can affect the cardiovascular system due to increased stress hormones that contribute to hypertension |
| Diabetes mellitus | Diabetes increases the risk of hypertensive complications due to vascular wall damage and reduced vascular elasticity |
| Previous pre-eclampsia | Women who have experienced pre-eclampsia in a previous pregnancy are at a higher risk of developing hypertensive disorders in subsequent pregnancies. |
Thus, hypertensive disorders in pregnancy, including pre-eclampsia, gestational hypertension, and associated conditions, are major contributors to maternal and foetal morbidity, causing serious complications such as preterm birth, low birth weight, organ dysfunction in the mother, and even mortality. These disorders have a complex pathophysiology, including impaired placentation, where insufficient remodelling of the spiral arteries reduces placental blood supply, causing hypoxia. This, in turn, triggers the release of anti-angiogenic factors, vascular tone disturbances, and inflammatory reactions, leading to systemic endothelial dysfunction, vasoconstriction, and high blood pressure.
In addition to placental factors, the immune system plays a critical role in the development of these conditions. When the adaptation to foetal antigens is disrupted, chronic systemic inflammation may be triggered [25]. This leads to the activation of the coagulation cascade, microthrombi formation, and worsening microcirculation, further exacerbating organ failure. Among the severe complications of these conditions is HELLP syndrome, characterised by erythrocyte destruction, liver damage, thrombocytopenia, and eclampsia, a life-threatening seizure disorder for both the mother and child.
Epidemiological studies indicate a rising prevalence of hypertensive disorders in pregnancy worldwide, with significant risk factors including obesity, age over 35, genetic predisposition, and low physical activity levels. In many cases, these disorders are more common in a woman’s first pregnancy or in the presence of multiple pregnancies, which places additional strain on the maternal cardiovascular system. As a result, prevention is of paramount importance, involving the control of risk factors, regular medical monitoring, and early diagnosis of changes in blood pressure or other signs of complications.
Given the complex nature of these conditions, a crucial task for modern medicine is to gain a deeper understanding of the molecular mechanisms underlying their development. In particular, research into blood gene expression profiles may contribute to the development of innovative approaches for early risk detection and the refinement of targeted therapies. This is especially important as hypertensive disorders in pregnancy not only impact the course of pregnancy but also have long-term consequences for women’s health, increasing the risk of cardiovascular diseases in the future. Thus, a combination of screening strategies, monitoring, and prevention can help mitigate the negative impact of these conditions on both women and their children.
Diagnostic value of RNA biomarkers in hypertensive disorders of pregnancy (HDP)
mRNA biomarkers are messenger RNA molecules that reflect gene expression in the body and can serve as indicators of biological processes or pathological conditions [26]. They are an integral part of the central dogma of molecular biology: the process where DNA is transcribed into mRNA, which then transports genetic information to ribosomes for protein synthesis. This process forms the basis of cellular functioning and regulates various physiological functions.
mRNA functions as a “mediator”, reading information from DNA and transferring it to ribosomes, where protein synthesis occurs. However, its functionality is not limited to this role. In many cases, mRNA interacts with other molecules, such as microRNAs, which can suppress or stimulate its activity, altering gene expression. This mechanism is key to regulating numerous biological processes, including cell proliferation, apoptosis, and stress response [27]. mRNA biomarkers can be tissue- or organ-specific. For example, certain mRNAs are expressed only in specific tissues (e.g., alpha-foetal haemoglobin in blood or SPINK5 in vaginal secretions), enabling their use in determining the source of biomaterial [28].
mRNA can exist freely or be packaged into vesicles, such as exosomes, which ensure its stability in biological fluids, including blood, urine, saliva, or plasma. These vesicles protect mRNA from the action of ribonucleases – enzymes that degrade RNA – allowing it to retain its functionality in the body for a longer period [29]. Furthermore, circular mRNAs, which form closed loops via “back-splicing”, are even more stable due to their structure. These molecules are increasingly considered promising biomarkers because they are stable, tissue-specific, and detectable in various biological fluids [30]. The ability of mRNA to reflect the condition of cells or organs makes them valuable tools for exploring the body’s functioning. For example, altered levels of mRNA expression can signal the presence of diseases, such as cancer, or the response to therapy. Due to their unique expression in response to disease, mRNA becomes an important indicator for early diagnosis or treatment monitoring [31].
mRNA biomarkers play an important role in the diagnosis and prognosis of diseases due to their capacity to reflect gene activity in various physiological and pathological states [32], 33]. They enable the detection of early disease stages, the prediction of disease progression, and the assessment of treatment effectiveness, providing high levels of specificity and sensitivity. The role of mRNA biomarkers in disease diagnosis and prognosis has become pivotal due to their ability to provide detailed information on molecular processes occurring within the body. They are an indispensable component of modern medicine, ensuring non-invasiveness, high specificity, and sensitivity in disease detection and progression assessment.
One of the most progressive areas is the utilisation of mRNA biomarkers in the diagnosis of cancer [34], 35]. For instance, in the study by Zhou et al. [36], a signature of three mRNAs (NPFFR2, XPNPEP2, and CELA3B) was identified, enabling the prognosis of serous cystadenocarcinoma of the ovaries. This signature provides an opportunity to distinguish between high-risk and low-risk patient groups, facilitating more precise treatment planning and improving outcomes. Circular RNAs, which form closed-loop structures, have become another promising type of biomarker due to their high stability in biological fluids and tissue specificity. In the study by S. Wang et al. [30], it was emphasised that circular RNAs demonstrate high sensitivity in detecting cancers such as breast, prostate, and lung cancer. Their expression profiles allow not only for disease detection but also for evaluating its progression and response to therapy.
Long non-coding RNAs also exhibit significant prognostic potential. In the study by Kunadirek et al. [37], it was determined that long non-coding RNAs MIR4435-2HG and lnc-POLD3-2 could serve as sensitive markers for diagnosing hepatocellular carcinoma, even among patients with normal levels of serum alpha-fetoprotein. High levels of these molecules correlate with poor prognosis, underscoring their value in predicting disease progression. Kumar et al. [38] demonstrated that circulating microRNAs, such as miR-320a and miR-375, have the ability to correlate with the stages of non-small cell lung cancer. This enables not only tumour classification by stage but also the evaluation of patients’ sensitivity to therapy and the prediction of potential recurrence. Panels of biomarkers, incorporating several types of RNA, are also used in diagnostics. In the study by Tan et al. [39], a novel biomarker, RN7SL1, for hepatocellular carcinoma was proposed, which demonstrates a high level of accuracy in detecting early-stage cancer. It not only provides high sensitivity but also correlates with patient survival, making it a valuable tool in clinical practice [40].
Some approaches use biosensors for electrochemical analysis. An independent hybridisation process yields lengthy double-stranded DNA with significant electrochemical signals, making hybridisation chain reaction biosensors sensitive to mRNA. This allows mRNA detection in complicated biological matrices with a 3 fM detection limit [41]. Electrochemical biosensors using fluorescent nanoparticles analyse mRNA without labelling. This technology detects numerous microRNAs in tissue or serum samples at once, saving time and resources [42]. Modern technologies like CRISPR/Cas12a use cascaded amplification events to detect microRNAs sensitively. This method is essential in clinical practice because to its low detection threshold and precise discrimination of closely related mRNA molecules. The latest innovations use light-harvesting nanoparticles to generate ultra-bright fluorescent signals in response to target mRNA. This method quantitatively assesses cell extract molecules and is compatible with portable fast diagnostic equipment [43].
Research has shown that mRNA biomarkers can diagnose pregnancy problems. The detection of foetal mRNA in maternal blood is significant. Transcripts of genes involved in foetal and placental development have been found in pregnant women’s blood but are absent or diminished postpartum, indicating foetal origin. PLAC3, PLAC4, and CRH transcripts were raised in pre-eclampsia, indicating a close link between placenta and maternal blood mRNA levels. The mRNAs can be used to diagnose pre-eclampsia without intrusive procedures [44]. Detecting maternal blood microRNAs has also improved diagnosis. MiR-142-5p and miR-1275 in the serum of pregnant women with foetuses with congenital cardiac abnormalities can be used to diagnose such illnesses non-invasively. These microRNAs differed significantly across cardiac defect and control groups, proving their biomarker specificity [45].
Maternal blood placental mRNA stability allows for longer monitoring. Research has revealed that these transcripts swiftly vanish from the bloodstream after delivery, allowing precise pregnancy sign identification. They can be used to evaluate the baby and placenta throughout pregnancy [46]. Another study found that long non-coding RNAs in early pregnancy indicate hypertension, including pre-eclampsia. Pregnant women with high blood levels of particular long non-coding RNAs had more problems and adverse outcomes for mother and baby, such as foetal growth limitation or placental abnormalities [47]. Thus, mRNA and microRNA are attractive non-invasive prenatal diagnostic tools since they can accurately identify pre-eclampsia, congenital heart abnormalities, and early pregnancy problems.
Modern mRNA biomarker detection and analysis technologies are more accurate, fast, and sensitive. These approaches provide genetic activity measurement, early illness diagnosis, and therapy response monitoring. Isothermal amplification, CRISPR/Cas12a-based systems, electrochemical biosensors, and light-harvesting nanoparticles are the core of current diagnostics due to their diversity, precision, and low detection limits. Their use in clinical practice, especially in predicting pregnancy problems, is intriguing. Pre-eclampsia is a primary cause of maternal and perinatal morbidity. mRNA biomarker detection technologies can detect early molecular changes in the placenta and vascular system of pregnant women, which may indicate the danger of these issues before clinical symptoms occur. This allows timely medical intervention, lowering mother-child risks. Thus, mRNA biomarkers are crucial for safe pregnancies and reducing problems.
Analysis of RNA biomarkers for diagnosing HDP
The analysis of mRNA biomarkers is gaining increasing importance in modern medicine, particularly in the context of diagnosing HDP. The use of mRNA as biomarkers opens new horizons in predicting and promptly diagnosing these conditions, as it provides information on genetic activity at the molecular level. Due to their high sensitivity and specificity, mRNA biomarkers allow for the detection of early changes accompanying pathological processes, even before the manifestation of clinical symptoms. The unique ability of these biomolecules to reflect dynamic changes in the pregnant woman’s body contributes to more accurate risk assessment and an individualised approach to treatment. The analysis of mRNA is not only a promising tool for medical research but also has the potential to transform clinical practice paradigms, enhancing the effectiveness of perinatal medicine and reducing risks associated with pregnancy complications.
Recent advances in RNA sequencing have revolutionised transcriptome research, enabling comprehensive analysis of RNA species, including mRNA, which reflects active biological processes in tissues such as the placenta [48]. The placenta plays a pivotal role during pregnancy, acting as the interface between mother and foetus, with dysfunctional placentation being a hallmark of hypertensive disorders during pregnancy [49]. Thus, analysing placental gene expression using RNA derived from blood can provide critical insights into pathophysiological mechanisms.
In pregnancies complicated by hypertensive disorders, changes in the transcriptome may result from disruptions in placental development and function [50]. Identifying specific mRNA signatures in maternal blood can facilitate understanding of how alterations in gene expression contribute to the onset and progression of conditions such as pre-eclampsia. Such information could pave the way for developing prognostic biomarkers that could be applied in clinical settings to manage and mitigate risks associated with affected pregnancies [51].
Over the past five years, comprehensive analyses of mRNA biomarkers relevant to the diagnosis, prognosis, and understanding of the pathogenesis of HDP, including pre-eclampsia and gestational hypertension, have been conducted. Particular attention has been given to microRNAs, as their expression in peripheral blood and tissues is associated with the key pathological processes underlying these conditions. For instance, miR-19a, miR-210, and miR-126 have been identified as reliable markers capable of detecting both the risk and severity of pre-eclampsia. A combination of miR-20a-5p, miR-143-3p, miR-145-5p, and miR-574-3p has demonstrated greater sensitivity in predicting pre-eclampsia at early stages of pregnancy compared to conventional screening methods [52]. Other studies confirm the value of circulating C19MC microRNAs as tools for early detection of gestational hypertension [53].
The functions of mRNA biomarkers encompass the regulation of key pathophysiological processes. Specifically, miR-210 is critical for regulating hypoxia and oxidative stress in the placenta, which can lead to its dysfunction. This plays a central role in the development of pre-eclampsia, particularly in the context of disrupted oxygen and nutrient transport to the foetus [54]. Conversely, miR-126, which is typically responsible for maintaining endothelial function, exhibits reduced expression in women with severe HDP, indicating progressive vascular damage [55]. Other biomarkers, such as miR-20a-5p and miR-146a-5p, actively regulate inflammatory processes associated with pre-eclampsia. Disruptions in these processes manifest as elevated levels of pro-inflammatory cytokines, including IL-6 and IL-8, which, according to studies, impair placental functionality [56].
HDP are also characterised by significant disruptions in signalling pathways. The principal pathways include those related to angiogenesis (specifically the PlGF/sFlt-1 pathway), inflammatory responses, and endothelial metabolism. For instance, disruptions in the PlGF-to-sFlt-1 ratio directly affect vascular permeability and the formation of the placental barrier [57].
Ping et al. [58] investigated the integrated expression of mRNA and microRNA in the context of pre-eclampsia. Their findings indicate distinct signatures involving microRNAs such as miR-29b, which interact with mRNAs associated with angiogenesis and immune regulation. These data contribute to understanding the complex mechanisms of gene regulation in pre-eclampsia and complement prior discoveries regarding the role of mRNA biomarkers in placental dysfunction. Rasmussen et al. [59] presented a comprehensive analysis of RNA profiles in the blood of pregnant women, enabling the prediction of future disease risks. The study highlighted circulating mRNA and its role in predicting pre-eclampsia, particularly during early pregnancy stages. Their findings emphasised the importance of RNA profiles as predictors of maternal and foetal health, making them relevant for integration into contemporary research.
Tarca et al. [60] focused on mRNA profiles in maternal whole blood, identifying biomarkers capable of predicting early pre-eclampsia. They identified specific mRNA signatures associated with disruptions in angiogenesis and inflammatory processes, providing new tools for early intervention. These findings underscore the critical role of circulating mRNA in screening women at risk of hypertensive disorders.
Recent studies on genetic factors conducted by Mack et al. [61], associated with HDP, identified the significant role of polymorphisms in the retinoic acid receptor beta gene in the pathogenesis of these conditions. The retinoic acid receptor beta gene is responsible for regulating processes related to inflammatory immune responses, which play a crucial role in the development of pre-eclampsia. The examination of associations revealed that alterations in the retinoic acid receptor beta gene affect the biological mechanisms determining vascular system resilience to risk factors, including inflammatory cytokines, immune activation, and endothelial dysfunction. Polymorphisms in the retinoic acid receptor beta gene region were found to significantly increase the risk of developing pre-eclampsia and gestational hypertension. Specifically, the identified gene variants influenced the activation of inflammatory pathways and heightened the body’s response to angiotensin II, a key mediator of vascular tone. These findings add to the current understanding of how hereditary factors contribute to pregnancy complications, increasing the risks of placental dysfunction and hypoxia.
In addition to genetic factors, Hauspurg et al. [62] added that biochemical markers, particularly natriuretic peptides, may play a significant role in predicting HDP risks during early pregnancy stages. N-terminal pro-brain natriuretic peptide is a crucial indicator of subclinical cardiac dysfunction. Its concentration during early pregnancy reflects the efficiency of the maternal cardiovascular system’s adaptation to pregnancy-related changes. Elevated N-terminal pro-brain natriuretic peptide levels are associated with improved adaptive mechanisms, whereas reduced concentrations of this peptide may indicate a high risk of pre-eclampsia. It is noteworthy that N-terminal pro-brain natriuretic peptide also demonstrates prognostic significance for long-term cardiovascular health. In studies involving women who experienced HDP, this biomarker enabled the assessment of risks for developing hypertension, ischaemic heart disease, and other cardiovascular complications for several years post-partum.
These biomarkers exhibit significant potential for the early detection of diseases prior to the onset of clinical symptoms, enabling timely implementation of preventive or therapeutic measures. For instance, a study by Hromadnikova et al. [52] demonstrated that microRNAs concentrations in the blood of pregnant women correlate with the risk of developing pre-eclampsia. Specifically, microRNAs such as miR-20a-5p, miR-145-5p, and miR-181a-5p allow for the prediction of pre-eclampsia development with a sensitivity of 70–80 % and specificity of approximately 75 %.
Particular attention is drawn to the potential use of mRNA biomarkers for predicting complications such as foetal growth restriction or severe forms of pre-eclampsia. For example, a study by Zhou et al. [63] demonstrated that levels of miR-25-3p are significantly elevated in patients with hypertensive pregnancy disorders, with high levels associated with an increased risk of complications such as preterm birth or poor neonatal outcomes.
Significant attention has been devoted to the diagnostic characteristics of mRNA biomarkers. Sensitivity and specificity analyses of various microRNAs in diagnosing hypertensive pregnancy disorders have confirmed their high diagnostic efficacy. For instance, in studies by Jin et al. [55], microRNAs miR-19a, miR-126, and miR-210 exhibited a sensitivity of 81.2 % and a specificity of 73.5 %, with their levels correlating with disease severity. More complex biomarker panels combining multiple microRNAs, such as miR-31-5p and miR-214-3p, can achieve diagnostic accuracy of up to 90 % [64]. In addition, long non-coding RNAs have been successfully tested as markers for predicting the severity of pre-eclampsia. A reduction in lncRNA FAM99A levels has been associated with increased diastolic blood pressure, elevated urinary protein levels, and liver dysfunction, enabling the use of this biomarker to assess disease severity [65].
Thus, mRNA biomarkers demonstrate significant potential for both the early diagnosis of hypertensive pregnancy disorders and the prediction of their severity and complications. These findings underscore the potential of incorporating such approaches into routine medical practice following further large-scale clinical studies.
Clinical applications and prospects of RNA biomarkers in hypertensive pregnancy disorders
The integration of mRNA biomarkers into clinical practice for hypertensive disorders during pregnancy holds significant potential for improving the diagnosis, prognosis, and monitoring of these conditions. In particular, mRNA biomarkers may be utilised for the early identification of pregnant women at heightened risk of developing pre-eclampsia, enabling timely implementation of preventive and therapeutic measures. Furthermore, changes in mRNA expression may serve as dynamic indicators of disease progression or therapeutic efficacy. The analysis of mRNA biomarkers also facilitates the development of individualised predictions concerning potential complications, providing a more personalised approach to pregnancy management. For the incorporation of these methods into practice, further research is required to standardise detection methods, validate the clinical value of biomarkers, and develop guidelines for their use.
Scientific studies, particularly those by Zhang et al. [66], demonstrate that specific profiles of mRNA and non-coding RNA can reflect pathophysiological processes associated with endothelial dysfunction, inflammatory responses, and immune imbalances underlying conditions such as pre-eclampsia and gestational hypertension. The use of molecular panels, including differentially expressed mRNA, significantly enhances the sensitivity and specificity of early diagnosis of HDP, enabling risk prediction and personalised treatment at the early stages of gestation [47]. Furthermore, the application of multi-marker models incorporating mRNA expands diagnostic capabilities for assessing risks to both the mother and the foetus [67]. Key challenges in this field include standardising quantitative methods for mRNA analysis, developing cost-effective and rapid platforms for evaluation, and integrating these technologies into existing clinical protocols. Collectively, this evidence indicates that mRNA biomarkers hold considerable potential for improving the management of pregnancies complicated by hypertensive disorders.
Integrating mRNA biomarker testing into existing clinical protocols and workflows requires a systematic approach to ensure seamless adoption and effective utilisation within healthcare settings. Initially, healthcare providers should identify key points within the prenatal care timeline where biomarker testing can provide the most value, such as during initial screenings and follow-up visits for high-risk pregnancies [14], [15], [16], [17]. Collaboration with laboratory specialists is essential to establish standardised procedures for sample collection, handling, and analysis, ensuring that these processes align with current clinical operations. Training for healthcare staff on the interpretation of biomarker results and their implications for patient management is crucial to facilitate informed decision-making. Additionally, integrating biomarker data into electronic health record systems can enhance accessibility and allow for better tracking of patient outcomes over time. By embedding these tests into routine prenatal assessments and leveraging existing infrastructure, healthcare providers can enhance diagnostic accuracy and personalise patient care without significantly disrupting established workflows [57], [58], [59], [60], [61], [62].
The integration of mRNA biomarker testing into healthcare systems presents both opportunities and challenges that necessitate careful consideration of infrastructure and training requirements. Implementing these advanced diagnostic tools requires healthcare facilities to invest in specialised equipment and laboratory infrastructure capable of handling and analysing genetic material with high precision. This includes the acquisition of high-throughput sequencing technologies and the establishment of secure data management systems to store and process sensitive genetic information efficiently [52], [53], [54], [55, 68].
Training for healthcare providers is a critical component of this integration process. Clinicians, laboratory technicians, and support staff must receive comprehensive training to understand the principles of mRNA biomarker testing, interpret test results accurately, and apply this information effectively in clinical decision-making. Continuous education programs and workshops can help ensure that healthcare professionals remain up-to-date with the latest advancements and best practices in genetic testing [69], 70]. Moreover, the incorporation of mRNA biomarker testing into existing clinical workflows may require adjustments to standard operating procedures and care pathways. Collaborative efforts among multidisciplinary teams, including geneticists, obstetricians, and bioinformaticians, are essential to develop guidelines and protocols that facilitate seamless integration and utilisation of these tests [67], 68], [71], [72], [73].
From a broader healthcare system perspective, the adoption of mRNA biomarker testing could lead to improved patient outcomes through earlier and more accurate diagnoses, potentially reducing the overall cost of care by preventing complications and enabling targeted interventions. However, healthcare systems must also prepare for the initial financial investment and ongoing costs associated with maintaining the necessary infrastructure and training programs.
The use of mRNA biomarkers for the diagnosis and monitoring of hypertensive disorders during pregnancy offers significant advantages and certain limitations compared to conventional methods, making this research area both highly promising and complex. Among the advantages, mRNA biomarkers enable early detection of high-risk pregnancy complications, allowing clinicians to adopt individualised approaches to antenatal care. For instance, sFLT-1 has been identified as a significant mRNA biomarker for predicting the progression of HDP. Its application can improve patient monitoring and reduce the incidence of severe complications [68]. Other mRNA biomarkers associated with the renin-angiotensin-aldosterone system have the potential for assessing pregnancy risks, particularly in patients at high risk of developing pre-eclampsia, thus optimising treatment strategies [72].
In addition, the use of biomarkers such as apelin and visfatin provides insights into the impact of hypertensive disorders on the overall health of pregnant women and predicts long-term health risks, including the development of cardiovascular diseases in the future. This approach goes beyond standard diagnostics, offering a deeper understanding of the pathophysiology of hypertensive disorders. Moreover, some studies suggest that biomarkers such as CA125 and alpha-fetoprotein may be effective for predicting the risk of pre-eclampsia at early stages of pregnancy, especially in the context of multivariate analysis [73].
Despite significant progress, the use of mRNA biomarkers in clinical practice faces several limitations. One of the main challenges is the high variability in biomarker levels, which can depend on individual patient characteristics, including their general health status and the presence of comorbidities. Furthermore, there is the issue of external and internal factors affecting biomarker levels, which, in turn, impact the accuracy of their interpretation. For example, changes in the functioning of the renin-angiotensin-aldosterone system can result from both the progression of pregnancy and the influence of comorbidities, creating additional difficulties for the routine application of these biomarkers in practice. In addition, small sample sizes used in many studies, such as those investigating sFLT-1, limit the generalisability of the results and necessitate further clinical trials involving a larger number of participants. The weak prognostic characteristics of individual biomarkers, such as CA125 and alpha-fetoprotein, also highlight the need to integrate these indicators with standard diagnostic methods, which may complicate their implementation in clinical practice [73].
Prokineticin 1 is a key protein ensuring the normal course of pregnancy, being involved in decidualisation, embryo implantation, and placental development. Its role is critical for maintaining healthy conditions at the maternal-foetal interface, and its dysregulation is associated with pathologies such as pre-eclampsia, recurrent miscarriages, and intrauterine growth restriction. These pathological states underscore the necessity for further investigation of Prokineticin 1 to develop potential therapeutic approaches and biomarkers for the diagnosis and treatment of such complications [74]. Research into the potential of quinoa proteins has revealed that bioactive peptides formed after simulated digestion exhibit antihypertensive properties. These peptides, in particular, are able to inhibit the activity of angiotensin converting enzyme, which is important in the regulation of blood pressure. Such properties open up prospects for using quinoa as a basis for creating new therapeutic agents that could be applied in the treatment of hypertensive disorders during pregnancy, particularly pre-eclampsia [75]. Ischaemia-modified albumin has proven to be an effective marker for assessing tissue hypoxia and oxidative stress. This is especially important for evaluating the risk of developing microvascular complications in patients with chronic conditions, such as type 2 diabetes, where hypoxia and oxidative stress play a pivotal role. Early studies indicate that this marker may also be relevant to conditions associated with HDP, such as pre-eclampsia, as these pathologies share similar mechanisms of tissue damage.
The use of mRNA biomarkers in the diagnosis and monitoring of hypertensive disorders during pregnancy opens new horizons in medical practice, providing a more precise and personalised approach to maternity care. Biomarkers such as sFLT-1, apelin, and visfatin demonstrate significant potential in predicting complications, early identification of risks, and improving outcomes for both mothers and new-borns. Furthermore, the integration of these innovative methods with conventional approaches can contribute to better pregnancy outcomes and a reduction in complications.
While limitations related to biomarker variability and the need for further research persist, these challenges do not diminish the opportunities they present. Efforts towards standardising analytical methodologies and increasing the representativeness of study samples will help address these shortcomings and make mRNA biomarkers an indispensable tool in modern medicine. Ultimately, their implementation not only enhances diagnostics but also lays the foundation for the long-term health of both mother and child, emphasising their unique value in the field of obstetrics.
Based on mRNA biomarkers, it is possible to propose the development of several promising therapeutic approaches for managing hypertensive disorders during pregnancy. Firstly, these biomarkers could be employed to create individualised treatment regimens that account for the genetic and epigenetic characteristics of the patient. This may include the development of drugs targeting the regulation of specific mRNA expressions associated with the pathogenesis of hypertension. Secondly, therapeutic strategies may focus on restoring the balance of biologically active substances, such as apelin or visfatin, through the administration of their analogues or the stimulation of their natural secretion. The use of these markers also appears promising for real-time monitoring of treatment effectiveness, allowing therapy to be adapted according to the disease’s progression. Thirdly, therapeutic interventions could involve correcting related metabolic or hormonal imbalances, such as hypersecretion of hCG or dysfunction of the renin-angiotensin-aldosterone system. This could be achieved through pharmacological modulation or gene therapy aimed at regulating the expression of relevant genes.
The utilisation of mRNA biomarkers in clinical practice, while promising for enhancing diagnostic and therapeutic strategies, also raises significant ethical concerns that warrant careful consideration. Privacy issues are paramount, as genetic information is highly sensitive, and its misuse could lead to potential breaches of confidentiality, impacting individuals’ personal and professional lives. RNA expression profiles can vary depending on the individual’s physiological state, meaning that the potential for identifying specific individuals based on these profiles is limited [8], [9], [10, 76]. While certain genetic variants may be identifiable, RNA profiles generally have restricted identifiable value. There is also the risk of genetic discrimination, where individuals might face unfair treatment by employers or insurance companies based on their genetic predispositions revealed through biomarker analysis. To mitigate these risks, it is crucial to establish robust legal frameworks and ethical guidelines that ensure the protection of genetic data, promote transparency in how this information is used, and prevent discriminatory practices. Additionally, informed consent processes must be clearly communicated to patients, ensuring they are fully aware of the implications of genetic testing and their rights regarding their genetic information. Addressing these ethical challenges is essential for responsibly integrating mRNA biomarkers into healthcare systems and maintaining public trust in genetic research and medicine [28], [29], [30], [31], [32], [33], [34], [35], [36].
The analysis of mRNA biomarkers in the context of hypertensive disorders during pregnancy, particularly pre-eclampsia and gestational hypertension, reveals significant insights into their diagnostic and prognostic potential. By identifying specific mRNA biomarkers such as miR-19a, miR-210, and miR-126, the study underscores their role in reflecting key pathophysiological processes, including impaired angiogenesis, endothelial dysfunction, and placental hypoxia, which are central to these conditions. The integration of these biomarkers into clinical practice offers a promising avenue for early detection and personalised management of hypertensive disorders, potentially improving maternal and fatal health outcomes. Furthermore, the examination of genetic and molecular mechanisms associated with these biomarkers enhances the understanding of disease progression and severity, paving the way for targeted therapeutic interventions. Despite the challenges related to variability in biomarker levels and the need for standardised detection methods, the findings highlight the transformative potential of mRNA biomarkers in advancing prenatal care and mitigating long-term health risks for both mother and child.
In this review, certain studies are highlighted for their higher quality and impact on the field of mRNA biomarkers in hypertensive disorders during pregnancy. Notably, Ford et al. [1] and Garovic et al. [7] stand out due to their large sample sizes, robust methodologies, and significant findings that have been replicated or validated in subsequent research, offering robust evidence and valuable insights into the prevalence, impact, and long-term consequences of these conditions. Rasmussen et al. [59] is also emphasised for its innovative approach and contributions to understanding RNA profiles in predicting pre-eclampsia. These high-quality studies provide a stronger evidence base and are recommended for readers seeking to delve deeper into the subject. Conversely, while studies such as Melchiorre et al. [2] and Wu et al. [5] provide useful data, they are considered of moderate quality due to limitations like smaller sample sizes or constraints in real-world application, guiding readers on where to focus for further exploration.
Conclusions
The analysis of mRNA biomarkers in hypertensive disorders during pregnancy, particularly pre-eclampsia and gestational hypertension, has provided significant insights into their potential for early diagnosis and personalised management. This study underscores the critical role of specific mRNA biomarkers such as miR-19a, miR-210, and miR-126 in reflecting key pathophysiological processes, including impaired angiogenesis, endothelial dysfunction, and placental hypoxia. These biomarkers offer promising tools for early screening and intervention, potentially transforming clinical practice and improving maternal and fatal health outcomes. The integration of mRNA biomarkers into clinical practice could lead to the development of standardised diagnostic platforms, enhancing the precision and effectiveness of maternal healthcare. By enabling early detection and monitoring of hypertensive disorders, these biomarkers can facilitate timely medical interventions, thereby reducing the risk of complications and long-term health issues for both mother and child.
The results are substantial because they suggest the potential for implementing mRNA biomarkers in clinical practice for early diagnosis, monitoring treatment efficacy, and assessing the severity of HDP. Further research should focus on standardising mRNA analysis methodologies, developing accessible diagnostic platforms, and investigating genetic and metabolic factors influencing biomarker expression. Special attention should be given to developing integrated screening programmes to personalise the approach to the prevention and treatment of HDP. A limitation of this study was the lack of standardisation in mRNA analysis methods, which may have affected the consistency of results across different studies. Furthermore, further validation of the obtained data in larger clinical cohorts is required.
Funding source: This research was funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. ÐÐ 19678324)
Award Identifier / Grant number: ÐÐ 19678324
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: This research was funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. АР19678324).
-
Data availability: Not applicable.
References
1. Ford, N, Cox, S, Ko, J, Ouyang, L, Romero, L, Colarusso, T, et al.. Hypertensive disorders in pregnancy and mortality at delivery hospitalization – United States, 2017–2019. MMWR (Morb Mortal Wkly Rep) 2022;71:585–91. https://doi.org/10.15585/mmwr.mm7117a1.Search in Google Scholar PubMed PubMed Central
2. Melchiorre, K, Thilaganathan, B, Giorgione, V, Ridder, A, Memmo, A, Khalil, A. Hypertensive disorders of pregnancy and future cardiovascular health. Front Cardiovasc Med 2020;7:59. https://doi.org/10.3389/fcvm.2020.00059.Search in Google Scholar PubMed PubMed Central
3. Benschop, L, Duvekot, J, van Lennep, J. Future risk of cardiovascular disease risk factors and events in women after a hypertensive disorder of pregnancy. Heart 2019;105:1273–8. https://doi.org/10.1136/heartjnl-2018-313453.Search in Google Scholar PubMed PubMed Central
4. Mora, A, Escudero, P, Marín, JG, Lozada, CC, Antique, PF. Hypertensive disorders of pregnancy: fetal hepatic artery pulsatility index. Gac Med Caracas 2024;132:392–407. https://doi.org/10.47307/GMC.2024.132.2.11.Search in Google Scholar
5. Wu, P, Green, M, Myers, J. Hypertensive disorders of pregnancy. BMJ 2023;381:e071653. https://doi.org/10.1136/bmj-2022-071653.Search in Google Scholar PubMed
6. Kipnis, C, Daly, P, Goodwin, E, Smith, D. Hypertensive conditions: hypertensive disorders in pregnancy. FP Essentials 2022;522:25–33.Search in Google Scholar
7. Garovic, V, White, W, Vaughan, L, Saiki, M, Parashuram, S, Garcia-Valencia, O, et al.. Incidence and long-term outcomes of hypertensive disorders of pregnancy. J Am Coll Cardiol 2020;75:2323–34. https://doi.org/10.1016/j.jacc.2020.03.028.Search in Google Scholar PubMed PubMed Central
8. Tagetti, A, Fava, C. Diagnosis of hypertensive disorders in pregnancy: an update. J Lab Precis. Med 2020;5:8. https://doi.org/10.21037/jlpm.2019.11.04.Search in Google Scholar
9. Smailov, M, Salimbayeva, D, Kurmanova, A. A systematic review of profiling of blood gene transcript signatures in pregnancies complicated by hypertensive disorders during pregnancy. 2024. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=512410.Search in Google Scholar
10. Wells, GA, Shea, B, O’Connell, D, Peterson, J, Welch, V, Losos, M, et al.. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000. https://Www.Ohri.Ca/Programs/Clinical_Epidemiology/Oxford.Asp.Search in Google Scholar
11. Novak-Mazepa, CO, Sachuk, NV, Marushchak, MI. Analysis of factors associated with arterial hypertension and the quality of patients’ life. Bulletin of Med Biological Res 2023;5:60–7. https://doi.org/10.11603/bmbr.2706-6290.2023.1.13350.Search in Google Scholar
12. Baibolova, M, Bolatbekov, BA, Trusheva, KS, Kuramysuly, KS, Bolatbekova, ZS, Yesenbekov, B. Effects of the cardiac rehabilitation program on the quality of life in patients after open-heart surgery. Heart Vessel Transplant 2024;8. https://doi.org/10.24969/hvt.2024.479.Search in Google Scholar
13. Olié, V, Moutengou, É, Grave, C, Deneux-Tharaux, C, Regnault, NK, Kretz, S, et al.. Prevalence of hypertensive disorders during pregnancy in France (2010–2018): the nationwide CONCEPTION study. J Clin Hypertens 2021;23:1344–53. https://doi.org/10.1111/jch.14254.Search in Google Scholar PubMed PubMed Central
14. Li, F, Qin, J, Zhang, S, Chen, L. Prevalence of hypertensive disorders in pregnancy in China: a systematic review and meta-analysis. Pregnancy Hypertension 2021;24:13–21. https://doi.org/10.1016/j.preghy.2021.02.001.Search in Google Scholar PubMed
15. Gemechu, K, Assefa, N, Mengistie, B. Prevalence of hypertensive disorders of pregnancy and pregnancy outcomes in Sub-Saharan Africa: a systematic review and meta-analysis. Womens Health 2020;16:1–25. https://doi.org/10.1177/1745506520973105.Search in Google Scholar PubMed PubMed Central
16. Wilkerson, R, Ogunbodede, A. Hypertensive disorders of pregnancy. Emerg Med Clin 2019;37:301–16. https://doi.org/10.1016/j.emc.2019.01.008.Search in Google Scholar PubMed
17. Agrawal, A, Wenger, N. Hypertension during pregnancy. Curr Hypertens Rep 2020;22:64. https://doi.org/10.1007/s11906-020-01070-0.Search in Google Scholar PubMed
18. Pasichnyk, M, Furdychko, A, Gorban, I, Fedun, I, Ilchyshyn, M. Condition of periodontal tissues in pregnant women of different age groups. Ukr J Med, Biol Sports 2021;6:244–8. https://doi.org/10.63341/ujmbs/1.2021.244.Search in Google Scholar
19. Pérez, AD, Perez, AR, Sanjuan, JZ, Rodelo, MM, Almanza, OH, Lozano, BD. Factors associated to the hypertensive disorders of pregnancy: a case-control study in women in the Colombian Caribbean. Gac Med Caracas 2024;132:30–46. https://doi.org/10.47307/GMC.2024.132.1.4.Search in Google Scholar
20. Rana, S, Lemoine, E, Granger, J, Karumanchi, S. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res 2019;124:1094–112. https://doi.org/10.1161/CIRCRESAHA.118.313276.Search in Google Scholar PubMed
21. Sanapo, L, Bublitz, M, Bourjeily, G. Sleep disordered breathing, a novel, modifiable risk factor for hypertensive disorders of pregnancy. Curr Hypertens Rep 2020;22:28. https://doi.org/10.1007/s11906-020-1035-7.Search in Google Scholar PubMed PubMed Central
22. Garovic, V, Dechend, R, Easterling, T, Karumanchi, S, Baird, S, Magee, L, et al.. Hypertension in pregnancy: diagnosis, blood pressure goals, and pharmacotherapy: a scientific statement from the American heart association. Hypertension 2021;79:e21–41. https://doi.org/10.1161/HYP.0000000000000208.Search in Google Scholar PubMed PubMed Central
23. Haug, E, Horn, J, Markovitz, A, Fraser, A, Klykken, B, Dalen, H, et al.. Association of conventional cardiovascular risk factors with cardiovascular disease after hypertensive disorders of pregnancy. JAMA Cardiol 2019;4:628–35. https://doi.org/10.1001/jamacardio.2019.1746.Search in Google Scholar PubMed PubMed Central
24. Groenhof, T, Zoet, G, Franx, A, Gansevoort, R, Bots, M, Groen, H, et al.. Trajectory of cardiovascular risk factors after hypertensive disorders of pregnancy: an argument for follow-up. Hypertension 2019;73:171–8. https://doi.org/10.1161/HYPERTENSIONAHA.118.11726.Search in Google Scholar PubMed
25. Yager, J, Kay, J. The kvetch: assessment, pathogenesis, and treatment of patients who are clinically impaired by chronic complaining. J Nerv Ment Dis 2024;212:4–11. https://doi.org/10.1097/NMD.0000000000001717.Search in Google Scholar PubMed
26. Gunasekaran, P, Luther, J, Byrd, J. For what factors should we normalize urinary extracellular mRNA biomarkers? Biomol Detect Quantification 2019;17:100090. https://doi.org/10.1016/j.bdq.2019.100090.Search in Google Scholar PubMed PubMed Central
27. Cheung, K, Choi, S, Lee, L, Lee, N, Tsang, H, Cheng, Y, et al.. The potential of circulating cell free RNA as a biomarker in cancer. Expert Rev Mol Diagn 2019;19:579–90. https://doi.org/10.1080/14737159.2019.1633307.Search in Google Scholar PubMed
28. Liu, B, Song, F, Yang, Q, Zhou, Y, Shao, C, Shen, Y, et al.. Characterization of tissue-specific biomarkers with the expression of circRNAs in forensically relevant body fluids. Int J Leg Med 2019;133:1321–31. https://doi.org/10.1007/s00414-019-02027-y.Search in Google Scholar PubMed
29. Moon, J, Lim, J, Lee, S, Son, H, Rho, H, Kim, H, et al.. Urinary exosomal mRNA detection using novel isothermal gene amplification method based on three-way junction. Biosens Bioelectron 2020;167:112474. https://doi.org/10.1016/j.bios.2020.112474.Search in Google Scholar PubMed
30. Wang, S, Zhang, K, Tan, S, Xin, J, Yuan, Q, Xu, H, et al.. Circular RNAs in body fluids as cancer biomarkers: the new Frontier of liquid biopsies. Mol Cancer 2021;20:13. https://doi.org/10.1186/s12943-020-01298-z.Search in Google Scholar PubMed PubMed Central
31. Ahn, S, Kang, S, Min, D. Direct monitoring of cancer-associated mRNAs in living cells to evaluate the therapeutic RNAi efficiency using fluorescent nanosensor. ACS Sens 2019;4:1174–9. https://doi.org/10.1021/acssensors.8b01498.Search in Google Scholar PubMed
32. Shulgau, Z, Sergazy, S, Krivoruchko, T, Kenzhebayeva, N, Sagindykova, B, Gulyayev, A. Osteoprotective properties of RNA-containing drug osteochondrin s on the model of insufficiency of sex Hormones in rats. Int J Morphol 2017;35:1233–8. https://doi.org/10.4067/S0717-95022017000401233.Search in Google Scholar
33. Shulgau, Z, Sergazy, S, Krivoruchko, T, Sagindykova, B, Gulyayev, A. Study of hepatoprotective properties of RNA-containing drug rn-13 on the model of acute tetrahllormethane induced hepatitis in rats. Georgian Med News 2017;265:104–9. https://pubmed.ncbi.nlm.nih.gov/28574392/.Search in Google Scholar
34. Goxharaj, A, Suyunov, N, Nikolaev, E, Bazhanova, A, Li, N. Current developments and innovations in early detection and subsequent treatment of cancer. J Cancer Res Updates 2024;13:85–99. https://doi.org/10.30683/1929-2279.2024.13.12.Search in Google Scholar
35. Navruzov, SN, Polatova, DS, Gafoor-Akhunov, MA, Gabdikarimov, KH. The value of marker proteins p53, bcl-2, Ki-67 in predicting the effectiveness of treatment for osteogenic sarcoma of tubular bones. Vopr Onkol 2012;58:691–3.Search in Google Scholar
36. Zhou, J, Yi, Y, Wang, C, Su, C, Luo, Y. Identification of a 3-mRNA signature as a novel potential prognostic biomarker in patients with ovarian serous cystadenocarcinoma in G2 and G3. Oncol Lett 2019;18:3545–52. https://doi.org/10.3892/ol.2019.10701.Search in Google Scholar PubMed PubMed Central
37. Kunadirek, P, Pinjaroen, N, Nookaew, I, Tangkijvanich, P, Chuaypen, N. Transcriptomic analyses reveal long non-coding RNA in peripheral blood mononuclear cells as a novel biomarker for diagnosis and prognosis of hepatocellular carcinoma. Int J Mol Sci 2022;23:7882. https://doi.org/10.3390/ijms23147882.Search in Google Scholar PubMed PubMed Central
38. Kumar, S, Sharawat, S, Ali, A, Gaur, V, Malik, P, Kumar, S, et al.. Identification of differentially expressed circulating serum microRNA for the diagnosis and prognosis of Indian non-small cell lung cancer patients. Curr Probl Cancer 2020;44:100540. https://doi.org/10.1016/j.currproblcancer.2020.100540.Search in Google Scholar PubMed
39. Tan, C, Cao, J, Chen, L, Xi, X, Wang, S, Zhu, Y, et al.. Noncoding RNAs serve as diagnosis and prognosis biomarkers for hepatocellular carcinoma. Clin Chem 2019;65:905–15. https://doi.org/10.1373/clinchem.2018.301150.Search in Google Scholar PubMed
40. Li, H, Warden, A, Su, W, He, J, Zhi, X, Wang, K, et al.. Highly sensitive and portable mRNA detection platform for early cancer detection. J Nanobiotechnol 2021;19:287. https://doi.org/10.1186/s12951-021-01039-4.Search in Google Scholar PubMed PubMed Central
41. Cheng, Y, Liu, S, Jiang, J. Enzyme-free electrochemical biosensor based on amplification of proximity-dependent surface hybridization chain reaction for ultrasensitive mRNA detection. Talanta 2021;222:121536. https://doi.org/10.1016/j.talanta.2020.121536.Search in Google Scholar PubMed
42. Egloff, S, Melnychuk, N, Reisch, A, Martin, S, Klymchenko, A. Enzyme-free amplified detection of cellular microRNA by light-harvesting fluorescent nanoparticle probes. Biosens Bioelectron 2021;179:113084. https://doi.org/10.1016/j.bios.2021.113084.Search in Google Scholar PubMed
43. Li, X, Zhang, D, Gan, X, Liu, P, Zheng, Q, Yang, T, et al.. A cascade signal amplification based on dynamic DNA nanodevices and CRISPR/Cas12a trans-cleavage for highly sensitive MicroRNA sensing. ACS Synth Biol 2021;10:1481–9. https://doi.org/10.1021/acssynbio.1c00064.Search in Google Scholar PubMed
44. Paiva, P, Whitehead, C, Sağlam, B, Palmer, K, Tong, S. Measurement of mRNA transcripts of very high placental expression in maternal blood as biomarkers of preeclampsia. J Clin Endocrinol Metab 2011;96:E1807–15. https://doi.org/10.1210/jc.2011-1233.Search in Google Scholar PubMed
45. Gu, H, Chen, L, Xue, J, Huang, T, Wei, X, Liu, D, et al.. Expression profile of maternal circulating microRNAs as non-invasive biomarkers for prenatal diagnosis of congenital heart defects. Biomed Pharmacother 2019;109:823–30. https://doi.org/10.1016/j.biopha.2018.10.110.Search in Google Scholar PubMed
46. Ng, E, Tsui, N, Lau, T, Leung, T, Chiu, R, Panesar, N, et al.. mRNA of placental origin is readily detectable in maternal plasma. Proc Natl Acad Sci USA 2003;100:4748–53. https://doi.org/10.1073/pnas.0637450100.Search in Google Scholar PubMed PubMed Central
47. Dai, C, Zhao, C, Xu, M, Sui, X, Sun, L, Liu, Y, et al.. Serum lncRNAs in early pregnancy as potential biomarkers for the prediction of pregnancy-induced hypertension, including preeclampsia. Mol Ther Nucleic Acids 2021;24:416–25. https://doi.org/10.1016/j.omtn.2021.03.010.Search in Google Scholar PubMed PubMed Central
48. Xu, J, Shi, X, Lin, S, Singh, S, Haddox, S, Phung, C, et al.. Chimeric RNA landscape in the placenta: a transcriptomic analysis revealing novel diagnostic biomarkers for preeclampsia. Genes & Dis 2025;12:101242. https://doi.org/10.1016/j.gendis.2024.101242.Search in Google Scholar PubMed PubMed Central
49. Boychuk, AV, Berehulyak, SO, Berehulyak, OO, Yakumchuk, YB. Statistical analysis of the SARS-CoV-2 effect on the gestation and childbirth course. Bulletin of Med Biol Res 2022;4:131–5. https://doi.org/10.11603/bmbr.2706-6290.2022.1.12981.Search in Google Scholar
50. Guo, F, Zhang, B, Yang, H, Fu, Y, Wang, Y, Huang, J, et al.. Systemic transcriptome comparison between early – and late-onset pre-eclampsia shows distinct pathology and novel biomarkers. Cell Prolif 2021;54:e12968. https://doi.org/10.1111/cpr.12968.Search in Google Scholar PubMed PubMed Central
51. Zhou, S, Li, J, Yang, W, Xue, P, Yin, Y, Wang, Y, et al.. Noninvasive preeclampsia prediction using plasma cell-free RNA signatures. Am J Obstet Gynecol 2023;229:553.e1–553.e16. https://doi.org/10.1016/j.ajog.2023.05.015.Search in Google Scholar PubMed
52. Hromadnikova, I, Kotlabova, K, Krofta, L. Cardiovascular disease-associated microrna dysregulation during the first trimester of gestation in women with chronic hypertension and normotensive women subsequently developing gestational hypertension or preeclampsia with or without fetal growth restriction. Biomedicines 2022;10:256. https://doi.org/10.3390/biomedicines10020256.Search in Google Scholar PubMed PubMed Central
53. Kondracka, A, Jaszczuk, I, Koczkodaj, D, Kondracki, B, Frąszczak, K, Oniszczuk, A, et al.. Analysis of circulating C19MC MicroRNA as an early marker of hypertension and preeclampsia in pregnant patients: a systematic review. J Clin Med 2022;11:7051. https://doi.org/10.3390/jcm11237051.Search in Google Scholar PubMed PubMed Central
54. Ntsethe, A, Mackraj, I. An investigation of exosome concentration and exosomal microRNA (miR-155 and miR-222) expression in pregnant women with gestational hypertension and preeclampsia. Int J Wom Health 2022;14:1681–9. https://doi.org/10.2147/IJWH.S382836.Search in Google Scholar PubMed PubMed Central
55. Jin, Y, Jia, T, Wu, X, Wang, Y, Sun, W, Chen, Y, et al.. The predictive value of microRNA in early hypertensive disorder complicating pregnancy (HDCP). Am J Tour Res 2021;13:7288–93.Search in Google Scholar
56. Reyes-Aguilar, S, Poblete-Naredo, I, Rodríguez-Yáñez, Y, Corona-Núñez, R, Ortiz-Robles, C, Calderón-Aranda, E, et al.. CYP1A1, GSTT1, IL-6 and IL-8 transcription and IL-6 secretion on umbilical endothelial cells from hypertensive pregnant women: preliminary results. Pregnancy Hypertens 2019;18:63–6. https://doi.org/10.1016/j.preghy.2019.09.002.Search in Google Scholar PubMed
57. Giorgione, V, Fabrizio, C, Giallongo, E, Khalil, A, O’Driscoll, J, Whitley, G, et al.. Angiogenic markers and maternal echocardiographic indices in women with hypertensive disorders of pregnancy. Ultrasound Obstet Gynecol 2023;63:206–13. https://doi.org/10.1002/uog.27474.Search in Google Scholar PubMed
58. Ping, Z, Feng, Y, Lu, Y, Ai, L, Jiang, H. Integrated analysis of microRNA and mRNA expression profiles in Preeclampsia. BMC Med Genom 2023;16:309. https://doi.org/10.1186/s12920-023-01740-3.Search in Google Scholar PubMed PubMed Central
59. Rasmussen, M, Reddy, M, Nolan, R, Camunas-Soler, J, Khodursky, A, Scheller, NM, et al.. RNA profiles reveal signatures of future health and disease in pregnancy. Nature 2022;601:422–7. https://doi.org/10.1038/s41586-021-04249-w.Search in Google Scholar PubMed PubMed Central
60. Tarca, AL, Romero, R, Erez, O, Gudicha, DW, Than, NG, Benshalom-Tirosh, N, et al.. Maternal whole blood mRNA signatures identify women at risk of early preeclampsia: a longitudinal study. J Matern Fetal Neonatal Med 2020;34:3463–74. https://doi.org/10.1080/14767058.2019.1685964.Search in Google Scholar PubMed PubMed Central
61. Mack, J, Burkholder, A, Akhtari, F, House, J, Sovio, U, Smith, G, et al.. A multi-ancestry genome-wide association study identifies novel candidate loci in the RARB gene associated with hypertensive disorders of pregnancy. 2023. https://doi.org/10.1101/2023.10.30.23297806.Search in Google Scholar
62. Hauspurg, A, Marsh, D, McNeil, R, Merz, C, Greenland, P, Straub, A, et al.. Association of N-terminal pro-brain natriuretic peptide concentration in early pregnancy with development of hypertensive disorders of pregnancy and future hypertension. JAMA Cardiol 2022;7:268–76. https://doi.org/10.1001/jamacardio.2021.5617.Search in Google Scholar PubMed PubMed Central
63. Zhou, D, Qu, B, Zhang, X. Diagnostic value of serum miR-25-3p in hypertensive disorders in pregnancy. Women Health 2021;62:818–26. https://doi.org/10.1080/03630242.2022.2108193.Search in Google Scholar PubMed
64. Kim, S, Park, M, Kim, J, Kim, T, Hwang, J, Ha, K, et al.. Circulating miRNAs associated with dysregulated vascular and trophoblast function as target-based diagnostic biomarkers for preeclampsia. Cells 2020;9:2003. https://doi.org/10.3390/cells9092003.Search in Google Scholar PubMed PubMed Central
65. Ge, Q, Zhao, J, Qu, F. Expression of serum long noncoding RNA FAM99A in patients with hypertensive disorder complicating and its clinical significance. Blood Pres Monit 2022;27:233–8. https://doi.org/10.1097/MBP.0000000000000591.Search in Google Scholar PubMed
66. Zhang, L, Wang, Y, Yang, R, Shan, L, Wang, L, Zhang, Y, et al.. mRNA expression profiles and molecular biomarkers involved in inflammatory response for essential hypertension in Kazakh population from Xinjian. Am J Hypertens 2023;36:354. https://doi.org/10.1093/ajh/hpad015.Search in Google Scholar
67. Mirzakhani, H, Handy, D, Lu, Z, Oppenheimer, B, Litonjua, A, Loscalzo, J, et al.. Integration of circulating microRNAs and transcriptome signatures identifies early‐pregnancy biomarkers of preeclampsia. Clin Transl Med 2023;13:E1446. https://doi.org/10.1002/ctm2.1446.Search in Google Scholar PubMed PubMed Central
68. Clifford, C, Hesson, A, Sangtani, A, Langen, ES, Ganesh, SK. Can biomarkers help identify women who will have progressively worsening hypertensive disorders of pregnancy? Am J Obstet Gynecol 2022;226:S296–7. https://doi.org/10.1016/j.ajog.2021.11.500.Search in Google Scholar
69. Nowak, R, Grzybowski, A. Outcome of an outreach microsurgical project in rural Nepal. Saudi J Ophthalmol 2013;27. https://doi.org/10.1016/j.sjopt.2012.09.002.Search in Google Scholar PubMed PubMed Central
70. Shevchenko, O, Holovkova, T, Onul, N, Kramaryova, Y, Shtepa, O, Shchudro, S. Preventive medicine as a component of objective structured clinical examination. Ukr J Med, Biol Sports 2023;8:258–64. https://doi.org/10.63341/ujmbs/4.2024.258.Search in Google Scholar
71. Briana, DD, Malamitsi-Puchner, A. Hypertension in pregnancy is associated with adverse outcomes for both mothers and fetuses. Angiology 2019;71:94–5. https://doi.org/10.1177/0003319719875633.Search in Google Scholar PubMed
72. Shoemaker, R, Poglitsch, M, Huang, H, Vignes, K, Patel, N, Bauer, JA, et al.. Abstract P350: profiling of the renin angiotensin aldosterone system in high risk human pregnancy. Hypertension 2022;79:AP350. https://doi.org/10.1161/hyp.79.suppl_1.P350.Search in Google Scholar
73. Al-Memar, M, Fourie, H, Vaulet, T, Bobdiwala, S, Saso, S, De Moor, B, et al.. EP03.07: early pregnancy biomarkers and prediction of hypertensive disorders of pregnancy. Ultrasound Obstet Gynecol 2019;54:247. https://doi.org/10.1002/uog.21153.Search in Google Scholar
74. Cheng, X, Li, M, Peng, T. Novel insights into prokineticin 1 role in pregnancy-related diseases. Int J Med Sci 2024;21:27–36. https://doi.org/10.7150/ijms.76817.Search in Google Scholar PubMed PubMed Central
75. Chirinos, R, Rodriguez-Diaz, J, Anticona, S, Aguilar-Galvez, A, Pedreschi, R, Campos, D. Antihypertensive and antidiabetic peptides derived from in silico simulated gastrointestinal digestion of quinoa (Chenopodium quinoa) globulins and molecular docking study. Quim Nova 2024;47:e-20230126. https://doi.org/10.21577/0100-4042.20230126.Search in Google Scholar
76. Riise, H, Sulo, G, Tell, G, Igland, J, Egeland, G, Nygård, O, et al.. Hypertensive pregnancy disorders increase the risk of maternal cardiovascular disease after adjustment for cardiovascular risk factors. Int J Cardiol 2019;282:81–7. https://doi.org/10.1016/j.ijcard.2019.01.097.Search in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

