Abnormal Doppler and perinatal outcomes according to the placental lesions of maternal and fetal vascular malperfusion in preterm fetal growth restriction
-
Young Eun Chung
, Jung-Sun Kim
and Cheong-Rae Roh
Abstract
Objectives
We aimed to identify the associations between specific pathologic placental findings and abnormal Doppler results as well as perinatal outcomes in preterm fetal growth restriction (FGR).
Methods
This retrospective study included 465 consecutive singleton pregnancies with FGR delivered between 22+0 and 36+6 weeks of gestation. Abnormal Doppler was defined as absent or reversed end-diastolic flow in the umbilical artery (UA) and a pulsatility index less than the 10th percentile in the middle cerebral artery (MCA). Placental pathology was evaluated focusing on villous or vascular findings of maternal (MVM) and fetal vascular malperfusion (FVM) based on Amsterdam criteria.
Results
The average gestational age at delivery and neonatal birth weight were 32 weeks (±4 weeks) and 1,299 g (±575 g), respectively. Placental MVM lesions were observed in the majority of cases (90.3 %) and were significantly related to abnormal UA and MCA Doppler. Meanwhile, FVM lesions was observed in about half of the cases (44.8 %) and were significantly related to abnormal MCA Doppler, but not abnormal UA Doppler. Both villous and vascular lesions of MVM and FVM were associated with lower neonatal birth weight. Among specific key findings of MVM and FVM, distal villous hypoplasia was highly associated with abnormal UA Doppler (crude OR 5.01, 95 % CI 1.27–19.68), while intramural fibrin deposition in large fetal vessels was significantly associated with abnormal MCA Doppler (crude OR 2.49, 95 % CI 1.01–6.13). Of note, intramural fibrin deposition of large fetal vessels was associated with neonatal mortality (crude OR 3.25, 95 % CI 1.52–6.92).
Conclusions
We identified key findings among placental MVM and FVM associated with abnormal UA and MCA Doppler as well as neonatal mortality in preterm FGR.
Introduction
Fetal growth restriction (FGR) is an important cause of perinatal mortality and morbidity and is associated with detrimental long-term health issues including hypertension and diabetes [1], 2]. In fact, placental dysfunction is a main pathophysiology underling FGR, and assessment of histopathologic changes in the placenta is crucial. The pathologic lesions in FGR are usually assessed by maternal vascular malperfusion (MVM), fetal vascular malperfusion (FVM), and chronic villitis of unknown etiology (VUE) according to the Amsterdam classification system [3], 4].
Antenatal Doppler assessment is used as a diagnostic and monitoring tool for pregnancies with FGR. Umbilical artery (UA) Doppler, reflecting vascular impedance in fetal-placental circulation, is especially important for assessing fetal decompensation status in FGR [5], 6]. Abnormal UA Doppler values are related to moderate damage to the placenta causing fetal hypoxemia and further neonatal mortality or cerebral palsy [7], 8]. The middle cerebral artery (MCA) reflects cerebral vasodilation as a result of fetal hypoxemia in FGR. Abnormal MCA Doppler results are an indication of compensatory blood flow redistribution due to fetal hypoxemia [6], 9].
Several studies suggest that abnormal Doppler, especially UA, is associated with placental pathologic findings in FGR [10], 11]. According to an earlier study including SGA (both preterm and full term), MVM but not FVM lesions were associated with abnormal UA Doppler [12]. Another study shows that late onset FGR; vascular lesions of MVM, including acute atherosis, fibrinoid necrosis, and mural hypertrophy; and villous lesions of FVM, including villous stromal-vascular karyorrhexis and avascular villi, were more prevalent in an abnormal cerebroplacental ratio (CPR) group than a healthy group [13]. Recently, another study including early onset FGR showed that severe MVM of the placenta is independently associated with abnormal UA Doppler PI and abnormal CRP. However, in that study, placental findings of FVM were not associated with abnormal Doppler findings, which may be due to the limited number of severe FVM cases included [14]. Collectively, the clinical significance and prevalence of abnormal placental pathology in FGR vary according to the characteristics of the study population and are affected by sample size.
With this background, we studied 465 consecutive pregnancies with FGR neonates delivered preterm in our institution over a period of 11 years. We investigated relationships among placental pathologic findings focusing on MVM and FVM, abnormal UA/MCA Doppler, and perinatal outcomes in FGR neonates delivered preterm. We also focused on specific key findings of MVM and FVM including distal villous hypoplasia, acute atherosis of basal plate arteries and/or decidual arterioles, fetal thrombotic vasculopathy, and intramural fibrin deposition of large fetal vessels.
Subjects and methods
This retrospective study included consecutive singleton pregnancies with FGR delivered between 22+0 and 36+6 weeks from January 2011 to December 2021 at our institution. FGR was defined as birth weight less than the 10th percentile on the birth weight chart according to gestational age in Korea [15]. We excluded stillbirths (n=38) and cases with accompanying major and chromosomal anomalies (n=30) or unavailable placental pathology (n=7). Finally, 465 pregnancies were included in this study.
We collected prenatal ultrasound findings including UA and MCA Doppler assessment measured within one week before delivery. Abnormal UA Doppler was defined as absent or reversed end-diastolic flow, and abnormal MCA Doppler was defined as measured pulsatility index less than the 10th percentile. UA Doppler was performed in 400 pregnancies and MCA Doppler was assessed in 379 pregnancies. Both UA and MCA Doppler assessments were conducted in 374 pregnancies.
Maternal characteristics were maternal age, parity, pre-pregnancy BMI (kg/m2), assisted reproductive technology (ART), diagnosis of placenta abruptio and preeclampsia, and Cesarean section. Perinatal outcomes comprised gestational age at delivery (GAD), birth weight, cord pH, Apgar score (1-min and 5-min), and neonatal mortality. All data including maternal characteristics and perinatal outcomes were collected by medical record review. Placenta abruptio was diagnosed clinically (sudden onset of vaginal bleeding or abdominal pain) and confirmed after delivery. Preeclampsia was diagnosed when a pregnant woman had blood pressure higher than 140/90 mmHg after 20 weeks with one of the following features: proteinuria, thrombocytopenia, renal insufficiency, abnormal liver function, cerebral symptoms, severe symptoms, or pulmonary edema [16].
In our institution, placental pathology is assessed according to the Amsterdam classification as one of four major categories [17], 18]: (a) findings consistent with amniotic fluid infection (acute chorioamnionitis) including maternal response and fetal response; (b) findings consistent with MVM including villous changes (villous infarct, increased syncytial knot, villous agglutination, increased intervillous fibrin, decreased placental weight/increased fetoplacental weight ratio, distal villous hypoplasia) and vascular lesions (persistent muscularization of basal plate arteries, mural hypertrophy of decidual arterioles, acute atherosis of basal plate arteries and/or decidual arterioles); (c) findings consistent with FVM including villous changes (villous stromal-vascular karyorrhexis, hyalinized avascular villi, fetal thrombotic vasculopathy (average >15 avascular villi/section)) and vascular lesions (thrombi in large fetal vessels, intramural fibrin deposition of large fetal vessels, fibromuscular sclerosis of intermediate-sized fetal vessels); or (d) chronic inflammatory lesions (chronic villitis with obliterative fetal vasculopathy, chronic chorioamnionitis, chronic deciduitis). For analytic purposes, a case was classified as villous or vascular change of MVM or FVM-positive if at least one lesion among the corresponding categories was identified. All placental pathology specimens, including those obtained prior to the study period, were retrospectively re-evaluated by a single experienced pathologist in accordance with the Amsterdam Placental Workshop Group Consensus Statement (2016) [18]. This protocol ensured consistent lesion classification throughout the study period.
Statistical analysis
Continuous variables were compared by Student’s t-test and summarized as mean and standard deviation. Categorical variables were tested by the Chi-square test or Fisher’s exact test and summarized as frequency and percentage. We calculated odds ratio (OR) using a binary logistic regression model. Crude OR was calculated for the univariable model, and adjusted OR was calculated for the multivariable model after controlling for GAD. OR and 95 % confidence interval (CI) were expressed visually using forest plots. p-values less than 0.05 were considered statistically significant. All statistical analyses were conducted using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA), and R software, version 4.4.0 (R Project for Statistical Computing).
Results
Baseline characteristics of the study population
Among the study population, the average GAD and neonatal birth weight were 32 weeks (±4 weeks) and 1,299 g (±575 g), respectively. The smallest baby was 230 g at 24+5 weeks, and the most premature baby was born at 23+0 weeks with a birth weight of 380 g. In our study population, preeclampsia was comorbid in 48.4 % of cases (225/465). With regard to placental findings, lesions of the MVM or FVM were observed in 90.3 % (419/495) and 44.8 % (208/495) of patients, respectively. Among cases with available Doppler results, abnormal UA Doppler was found in 32.8 % (131/400) and abnormal MCA Doppler was found in 70.4 % (267/379).
Villous and vascular lesions of MVM or FVM in the placenta
We compared maternal characteristics, Doppler findings, and perinatal outcomes according to villous and vascular lesions of MVM or FVM in the placenta (Table 1). Both villous and vascular (both p<0.001) lesions of MVM were associated with higher rates of preeclampsia. Interestingly, villous lesions of FVM were associated with a significantly lower rate of preeclampsia (32.6 vs. 52.7 %, p<0.001), whereas vascular lesions of FVM showed a trend toward lower prevalence (37.7 vs. 50.3 %, p=0.054), although this trend did not reach significance. Villous lesions of MVM tended to be associated with advanced maternal age (p=0.057), whereas vascular lesions of MVM were associated with higher pre-pregnancy BMI (p=0.002).
Maternal characteristics, Doppler findings, and perinatal outcomes according to placental findings of maternal vascular malperfusion (MVM) and fetal vascular malperfusion (FVM).
| Villous change of MVM | Vascular change of MVM | Villous change of FVM | Vascular change of FVM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes (n=412) | No (n=53) | p-Value | Yes (n=158) | No (n=307) | p-Value | Yes (n=101) | No (n=364) | p-Value | Yes (n=69) | No (n=396) | p-Value | |
| Maternal characteristics | ||||||||||||
| Maternal age, year | 33.5 ± 3.9 | 32.4 ± 4.3 | 0.057 | 33.1 ± 3.9 | 33.5 ± 4 | 0.374 | 33.2 ± 4.3 | 33.4 ± 3.9 | 0.655 | 33.2 ± 4.3 | 33.4 ± 3.9 | 0.698 |
| Pre-pregnancy BMI, kg/m2 | 21.9 ± 3.5 | 21.1 ± 3.8 | 0.135 | 22.6 ± 3.7 | 21.4 ± 3.4 | 0.002 | 21.8 ± 3.2 | 21.9 ± 3.7 | 0.880 | 22 ± 3.3 | 21.8 ± 3.6 | 0.750 |
| Multiparity | 142 (34.5 %) | 12 (22.6 %) | 0.085 | 54 (34.2 %) | 100 (32.6 %) | 0.728 | 27 (26.7 %) | 127 (34.9 %) | 0.123 | 16 (23.2 %) | 138 (34.8 %) | 0.058 |
| ART | 48 (11.7 %) | 9 (17.0 %) | 0.265 | 17 (10.8 %) | 40 (13.0 %) | 0.480 | 12 (11.9 %) | 45 (12.4 %) | 0.896 | 11 (15.9 %) | 46 (11.6 %) | 0.312 |
| Diabetes mellitus | 23 (5.6 %) | 2 (3.8 %) | 0.755 | 10 (6.3 %) | 15 (4.9 %) | 0.513 | 1 (1.0 %) | 24 (6.6 %) | 0.027 | 3 (4.3 %) | 22 (5.6 %) | 1.000 |
| Hypertension | 231 (56.1 %) | 11 (20.8 %) | <0.0001 | 106 (67.1 %) | 136 (44.3 %) | <0.0001 | 40 (39.6 %) | 202 (55.5 %) | 0.005 | 30 (43.5 %) | 212 (53.5 %) | 0.123 |
| Preeclampsia | 216 (52.4 %) | 9 (17.0 %) | <0.0001 | 104 (65.8 %) | 121 (39.4 %) | <0.0001 | 33 (32.6 %) | 192 (52.7 %) | 0.000 | 26 (37.7 %) | 199 (50.3 %) | 0.054 |
| Doppler findings | ||||||||||||
| Abnormal UA | 127 (30.8 %) | 4 (7.5 %) | 0.003 | 61 (38.6 %) | 70 (22.8 %) | 0.002 | 34 (33.7 %) | 97 (26.6 %) | 0.419 | 19 (27.5 %) | 112 (28.3 %) | 0.846 |
| Abnormal MCA | 251 (60.9 %) | 16 (30.2 %) | 0.000 | 107 (67.7 %) | 160 (52.1 %) | 0.022 | 76 (75.2 %) | 191 (52.5 %) | 0.001 | 49 (70.0 %) | 218 (55.1 %) | 0.001 |
| Perinatal outcomes | ||||||||||||
| Cesarean section | 346 (84.0 %) | 35 (66.0 %) | 0.001 | 139 (88.0 %) | 242 (78.8 %) | 0.015 | 87 (86.1 %) | 294 (80.8 %) | 0.215 | 62 (90.0 %) | 319 (80.6 %) | 0.064 |
| Placental abruption | 32 (7.8 %) | 5 (9.4 %) | 0.596 | 12 (7.6 %) | 25 (8.1 %) | 0.836 | 3 (3.0 %) | 34 (9.3 %) | 0.036 | 2 (2.9 %) | 35 (8.8 %) | 0.093 |
| Gestational age at delivery, days | 222.1 ± 27.8 | 242.7 ± 18.6 | <0.001 | 216.3 ± 28.3 | 228.7 ± 26.5 | <0.001 | 219.8 ± 28.3 | 225.8 ± 27.4 | 0.058 | 214.7 ± 30.4 | 226.2 ± 26.9 | 0.001 |
| Birth weight, g | 1,236.9 ± 562 | 1784.6 ± 431.3 | <0.001 | 1,128.6 ± 562.1 | 1,387.2 ± 563 | <0.001 | 1,144.8 ± 561.8 | 1,342.2 ± 572.4 | 0.002 | 1,086.6 ± 582 | 1,336.4 ± 566.8 | 0.001 |
| Cord pH | 7.2 ± 0.1 | 7.3 ± 0.1 | 0.190 | 7.2 ± 0.1 | 7.3 ± 0.1 | 0.173 | 7.2 ± 0.1 | 7.2 ± 0.1 | 0.704 | 7.2 ± 0.1 | 7.2 ± 0.1 | 0.517 |
| 1 min Apgar score <4 | 47 (11.4 %) | 3 (5.7 %) | 0.204 | 19 (12.0 %) | 31 (10.1 %) | 0.525 | 14 (13.9 %) | 36 (9.9 %) | 0.254 | 12 (17.4 %) | 38 (9.6 %) | 0.054 |
| 5 min Apgar score <7 | 39 (9.5 %) | 4 (7.5 %) | 0.804 | 19 (12.0 %) | 24 (7.8 %) | 0.138 | 16 (15.8 %) | 27 (7.4 %) | 0.010 | 12 (17.4 %) | 31 (7.8 %) | 0.011 |
| Neonatal mortality | 44 (10.7 %) | 2 (3.8 %) | 0.113 | 16 (10.1 %) | 30 (9.8 %) | 0.904 | 16 (15.8 %) | 30 (8.2 %) | 0.024 | 14 (20.3 %) | 32 (8.1 %) | 0.002 |
-
p-Values less than 0.05 are shown in bold. MVM, maternal vascular malperfusion; FVM, fetal vascular malperfusion; BMI, body mass index; ART, assisted reproductive technology; UA umbilical artery; MCA, middle cerebral artery.
Both villous and vascular lesions of MVM were significantly associated with abnormal UA and abnormal MCA Doppler (p<0.05 for all). Villous and vascular lesions of FVM were also associated with abnormal MCA Doppler (both p<0.05) but not with UA Doppler.
For perinatal outcomes, both villous and vascular lesions of MVM and FVM were associated with lower neonatal birth weight (p<0.01 for all). Villous and vascular lesions of MVM were also related with higher rate of Cesarean section.
Of note, neither villous nor vascular lesions of MVM were associated with neonatal mortality in our study population. However, both villous (15.8 vs. 8.2 %, p=0.024) and vascular (20.3 vs. 8.1 %, p=0.002) lesions of FVM were significantly related to neonatal mortality.
Specific key findings of MVM or FVM in the placenta
Next, we compared maternal characteristics, Doppler findings, and perinatal outcomes according to the specific key findings of MVM and FVM in the placenta, which were considered clinically significant [4], 18]. As shown in Table 2, distal villous hypoplasia, acute atherosis of basal plate arteries and/or decidual arterioles, fetal thrombotic vasculopathy, and intramural fibrin deposition in large fetal vessels were analyzed.
Maternal characteristics, Doppler findings, and perinatal outcomes according to specific key findings of maternal and fetal vascular malperfusion in placenta.
| Distal villous hypoplasia | Acute atherosis of basal plate arteries and/or decidual arterioles | Fetal thrombotic vasculopathy | Intramural fibrin deposition of large fetal vessels | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes (n=13) | No (n=452) | p-Value | Yes (n=130) | No (n=335) | p-Value | Yes (n=16) | No (n=449) | p-Value | Yes (n=48) | No (n=417) | p-Value | |
| Maternal characteristics | ||||||||||||
| Maternal age, year | 34.5 ± 4.1 | 33.3 ± 4 | 0.317 | 33.5 ± 3.9 | 33.3 ± 4 | 0.690 | 33.1 ± 4.5 | 33.4 ± 4 | 0.799 | 33.8 ± 4.4 | 33.3 ± 3.9 | 0.466 |
| Pre-pregnancy BMI, kg/m2 | 22 ± 4.1 | 21.8 ± 3.6 | 0.879 | 22.4 ± 3.6 | 21.6 ± 3.5 | 0.056 | 21.7 ± 3.8 | 21.8 ± 3.6 | 0.854 | 21.8 ± 2.7 | 21.8 ± 3.7 | 0.935 |
| Multiparity | 5 (38.5 %) | 149 (33.0 %) | 0.767 | 45 (34.6 %) | 109 (31.9 %) | 0.669 | 6 (37.5 %) | 148 (33.0 %) | 0.705 | 14 (29.2 %) | 140 (33.6 %) | 0.539 |
| ART | 1 (7.7 %) | 56 (12.4 %) | 1.000 | 14 (10.8 %) | 43 (12.8 %) | 0.542 | 2 (12.5 %) | 55 (12.2 %) | 1.000 | 8 (16.7 %) | 49 (11.8 %) | 0.325 |
| Diabetes mellitus | 0 (0 %) | 25 (5.5 %) | 1.000 | 7 (5.4 %) | 18 (5.4 %) | 0.996 | 0 (0 %) | 25 (5.6 %) | 1.000 | 1 (2.1 %) | 24 (5.8 %) | 0.497 |
| Hypertension | 10 (76.9 %) | 232 (51.3 %) | 0.069 | 90 (69.2 %) | 152 (45.4 %) | <0.0001 | 4 (25.0 %) | 238 (53.0 %) | 0.028 | 22 (45.8 %) | 220 (52.8 %) | 0.363 |
| Preeclampsia | 10 (76.9 %) | 215 (47.6 %) | 0.037 | 88 (67.7 %) | 137 (40.9 %) | <0.0001 | 3 (18.8 %) | 222 (49.4 %) | 0.016 | 19 (39.6 %) | 206 (49.4 %) | 0.197 |
| Doppler findings | ||||||||||||
| Abnormal UA | 7 (53.8 %) | 124 (27.4 %) | 0.017 | 50 (38.5 %) | 81 (24.2 %) | 0.010 | 6 (37.5 %) | 125 (27.8 %) | 0.400 | 16 (33.3 %) | 115 (27.6 %) | 0.435 |
| Abnormal MCA | 11 (84.6 %) | 256 (56.6 %) | 0.038 | 90 (69.2 %) | 177 (52.8 %) | 0.017 | 13 (81.3 %) | 254 (56.6 %) | 0.013 | 33 (68.8 %) | 234 (56.1 %) | 0.041 |
| Perinatal outcomes | ||||||||||||
| Cesarean section | 13 (100.0 %) | 368 (81.4 %) | 0.138 | 118 (90.8 %) | 263 (78.5 %) | 0.002 | 15 (93.8 %) | 366 (81.5 %) | 0.326 | 43 (89.6 %) | 338 (81.1 %) | 0.146 |
| Placental abruption | 2 (15.4 %) | 35 (7.7 %) | 0.277 | 9 (6.9 %) | 28 (8.4 %) | 0.608 | 1 (6.3 %) | 36 (9.0 %) | 1.000 | 1 (2.1 %) | 36 (8.6 %) | 0.157 |
| Gestational age at delivery, days | 207.6 ± 35.2 | 225 ± 27.4 | 0.026 | 216.4 ± 27.7 | 227.6 ± 27.1 | 0.000 | 201.1 ± 28 | 225.3 ± 27.4 | 0.001 | 214 ± 31.4 | 225.7 ± 27 | 0.006 |
| Birth weight, g | 975.4 ± 645.7 | 1,308.6 ± 571.3 | 0.039 | 1,118.5 ± 538.1 | 1,369.5 ± 574.8 | 0.000 | 768.1 ± 500 | 1,318.2 ± 569.2 | 0.000 | 1,074.6 ± 611.7 | 1,325.2 ± 566.1 | 0.004 |
| Cord pH | 7.2 ± 0.1 | 7.2 ± 0.1 | 0.038 | 7.2 ± 0.1 | 7.3 ± 0.1 | 0.272 | 7.2 ± 0.2 | 7.2 ± 0.1 | 0.198 | 7.2 ± 0.1 | 7.2 ± 0.1 | 0.550 |
| 1 min Apgar score <4 | 5 (38.5 %) | 45 (10.0 %) | 0.008 | 15 (11.5 %) | 35 (10.4 %) | 0.733 | 6 (37.5 %) | 44 (10.0 %) | 0.004 | 10 (20.8 %) | 40 (9.6 %) | 0.017 |
| 5 min Apgar score <7 | 3 (23.1 %) | 40 (8.8 %) | 0.109 | 15 (11.5 %) | 28 (8.4 %) | 0.288 | 6 (37.5 %) | 37 (8.2 %) | 0.002 | 9 (18.8 %) | 34 (8.2 %) | 0.030 |
| Neonatal mortality | 3 (23.1 %) | 43 (9.5 %) | 0.128 | 14 (10.8 %) | 32 (9.6 %) | 0.693 | 3 (18.8 %) | 43 (9.6 %) | 0.204 | 11 (22.9 %) | 35 (8.4 %) | 0.004 |
-
p-Values less than 0.05 are shown in bold. MVM, maternal vascular malperfusion; FVM, fetal vascular malperfusion; BMI, body mass index; ART, assisted reproductive technology; UA, umbilical artery; MCA, middle cerebral artery.
Distal villous hypoplasia (53.8 vs. 27.4 %, p=0.017) and acute atherosis of basal plate arteries and/or decidual arterioles (38.5 vs. 24.2 %, p=0.01) were associated with abnormal UA Doppler. Similarly, these lesions were significantly associated with abnormal MCA Doppler (84.6 vs. 56.6 %, p=0.038 for distal villous hypoplasia and 69.2 vs. 52.8 %, p=0.017 for acute atherosis of basal plate arteries and/or decidual arterioles). Fetal thrombotic vasculopathy and intramural fibrin deposition of large fetal vessels were associated with abnormal MCA Doppler (p<0.05 for each) but not with UA Doppler.
For perinatal outcomes, four of the specific villous and vascular lesions of MVM or FVM were associated with lower neonatal birth weight (p<0.05 for all). However, only intramural fibrin deposition in large fetal vessels was significantly related to neonatal mortality (22.9 vs. 8.4 %, p=0.004).
Risk of abnormal Doppler and perinatal outcomes according to MVM or FVM of the placenta
We calculated OR by crude and adjusted models (adjusted by gestational age at delivery and preeclampsia) and visualized the results as forest plots. For perinatal outcomes, Cesarean section and neonatal mortality were selected as outcome variables.
Villous MVM was associated with abnormal UA and MCA Doppler (Figure 1). However, this association was attenuated after adjustment for GAD and/or preeclampsia. A similar trend was observed for vascular MVM lesions. However, for villous and vascular changes of FVM, the association with abnormal MCA Doppler remained significant even after adjustment for GAD. The associations between villous and vascular changes of MVM and Cesarean section were attenuated after adjustment for GAD and/or preeclampsia.
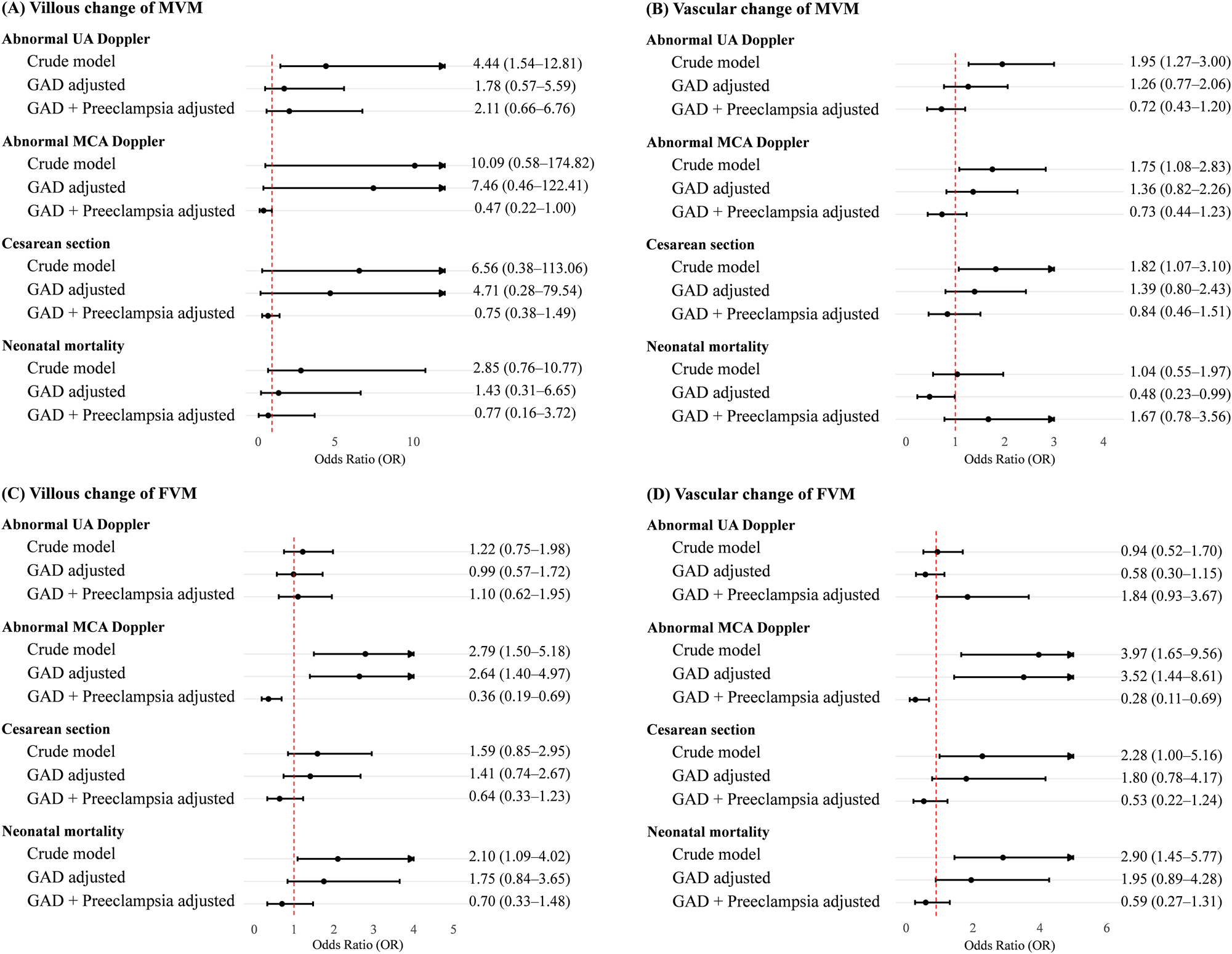
Forest plot of risk of abnormal Doppler and perinatal outcomes according to placental findings of maternal vascular malperfusion (MVM) and fetal vascular malperfusion (FVM). Odds ratio (OR) with 95 % confidence interval (CI) of the crude model in univariable analysis and of the adjusted model in multivariable analysis after controlling for GAD and preeclampsia. UA, umbilical artery; MCA, middle cerebral artery.
Regarding four specific key findings of MVM or FVM in the placenta, distal villous hypoplasia was highly associated with abnormal UA Doppler (crude OR 5.01, 95 % CI 1.27–19.68) (Figure 2). Acute atherosis of basal plate arteries and/or decidual arterioles was associated with Cesarean section (crude OR 2.35, 95 % CI 1.27–4.33). Intramural fibrin deposition was associated with abnormal MCA Doppler (crude OR 2.49, 95 % CI 1.01–6.13) and neonatal mortality (crude OR 3.25, 95 % CI 1.52–6.92). As expected, adjustment for GAD and/or preeclampsia resulted in attenuation of most associations.
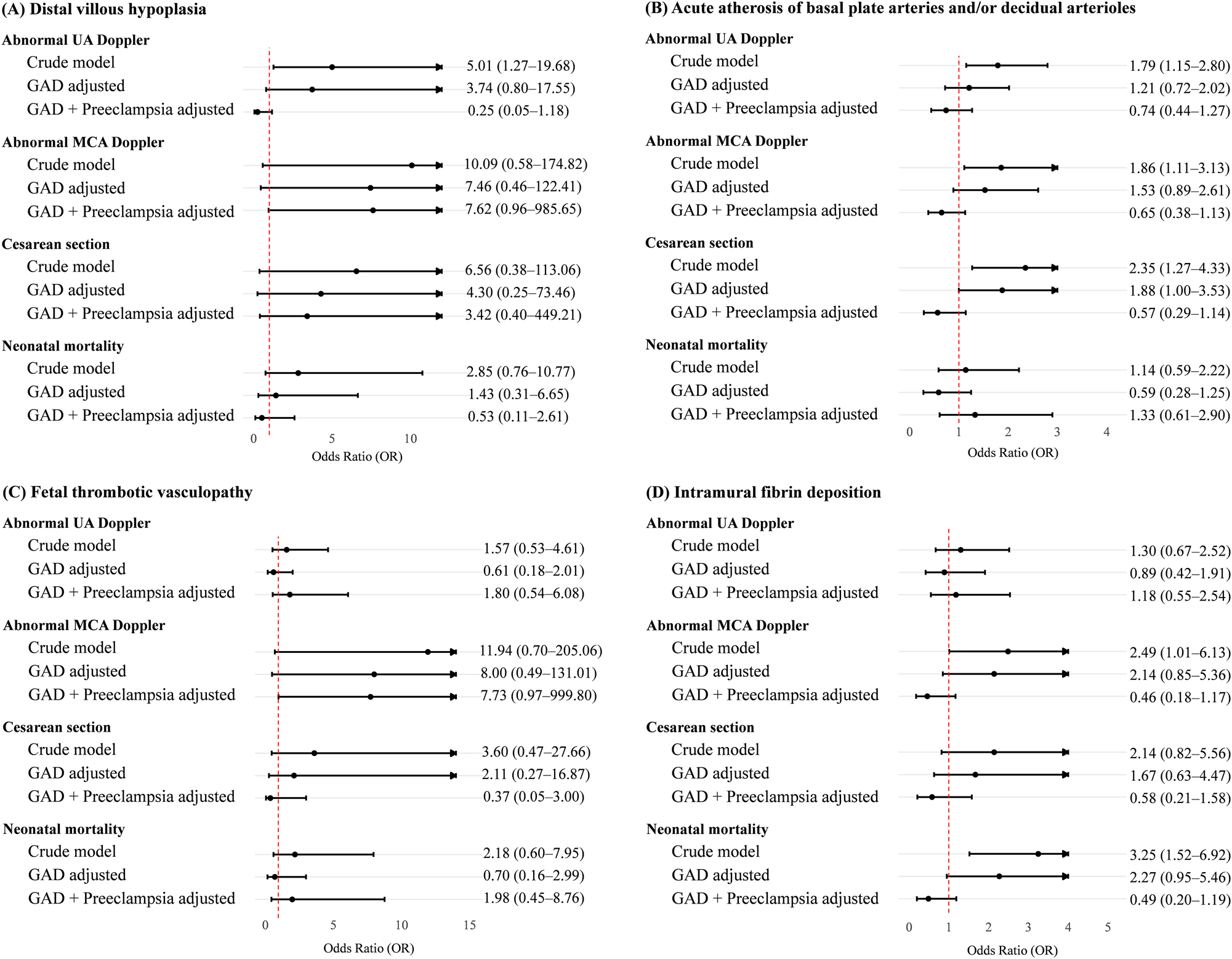
Forest plot of risk of abnormal Doppler and perinatal outcomes according to key findings of maternal and fetal vascular malperfusion in the placenta. Odds ratio (OR) with 95 % confidence interval (CI) of the crude model in univariable analysis and of the adjusted model in multivariable analysis after controlling for GAD and preeclampsia. UA, umbilical artery; MCA, middle cerebral artery.
Discussion
Study summary
Placental MVM lesions (villous or vascular) were observed in the majority of FGR neonates delivered preterm and were significantly related to abnormal UA and MCA Doppler findings. Meanwhile, FVM lesions were observed in about half of our study population and were significantly related to abnormal MCA but not abnormal UA Doppler. Among specific key findings of MVM or FVM, distal villous hypoplasia was highly associated with abnormal UA Doppler (crude OR 5.01), and intramural fibrin deposition of large fetal vessels was significantly associated with abnormal MCA Doppler (crude OR 2.49). Both villous and vascular lesions of MVM and FVM were associated with earlier gestational age at delivery and lower neonatal birth weight. FVM lesions but not MVM lesions of the placenta were associated with neonatal mortality. Specifically, intramural fibrin deposition in large fetal vessels was most closely associated with neonatal mortality with a crude OR of 3.25. However, most of these associations were attenuated when adjusted for GAD and/or preeclampsia, emphasizing the potential mediating role of prematurity and maternal hypertensive disorders in these outcomes.
Associations between placental pathology and Doppler
Our observations that placental findings of MVM are closely related to abnormal UA and MCA Doppler are in line with those of previous studies [11], [12], [13], [14, 19], 20]. Our study expands existing knowledge by demonstrating associations with specific lesions. Distal villous hypoplasia, a high-grade MVM lesion, was significantly associated with abnormal UA Doppler, suggesting that villous maldevelopment can substantially increase fetoplacental vascular resistance [21], 22], leading to absent or reversed end-diastolic flow. Intramural fibrin deposition in large fetal vessels, a type of high-grade FVM lesion, was associated with abnormal MCA Doppler, possibly reflecting thrombo-occlusive phenomena compromising cerebral perfusion [4], 23], 24]. These mechanistic interpretations align with pathophysiologic models of fetoplacental circulation and reinforce the diagnostic utility of Doppler surveillance in FGR.
Our results are in agreement with those of a recent study by Spinillo et al. including 250 early-onset FGR neonates [25], which also observed distinct associations between abnormal UA Doppler and severe MVM lesions [14]. However, our study demonstrated a higher prevalence of MVM (90 vs. 42.8 %), likely reflecting differences in study population, as our patients were delivered at earlier gestational ages with lower birth weights, representing a more severe FGR phenotype. Consistent with other studies [26], [27], [28], our data support the hypothesis that MVM lesions are more prevalent and clinically relevant in early-onset compared to late-onset FGR. We observed similar results, as abnormal MCA Doppler was related to both villous and vascular changes of MVM and FVM and, more specifically, to intramural fibrin deposition in large fetal vessels [13].
Associations between placental pathology and perinatal outcomes
Consistent with previous studies [29], [30], [31], we found that villous or vascular MVM and FVM lesions were significantly related to earlier gestational age at delivery and lower birth weight. Notably, neonatal mortality itself was associated with FVM but not MVM lesions of the placenta, suggesting that FVM may more directly compromise fetal status or reflect more immediate fetal compromise. Of note, intramural fibrin deposition in large fetal vessels, a high-grade type of FVM lesion, was significantly related to neonatal mortality although the significance decreased when adjusted for gestational age at delivery and preeclampsia, suggesting that perinatal outcomes are partially mediated by these clinical factors.
Associations between placental pathology and preeclampsia
We confirmed that preeclampsia is associated with villous and vascular lesions of MVM including distal villous hypoplasia and acute atherosis of basal plate arteries and/or decidual arterioles. In contrast, FVM was not associated with a higher rate of preeclampsia with FGR. Instead, fetal thrombotic vasculopathy was less frequent in cases of preeclampsia with FGR, suggesting that other mechanisms, such as umbilical cord abnormalities or fetal thrombotic conditions, contribute to FVM independent of maternal hypertensive disease.
Distal villous hypoplasia
Although the actual prevalence of distal villous hypoplasia was low (2.8 %), it was significantly associated with earlier gestational age at delivery and lower neonatal birth weight. Pathophysiologically, distal villous hypoplasia represents villous maldevelopment under chronic hypoxia, leading to increased vascular resistance in the fetal vessels, further causing absent or reversed end-diastolic UA Doppler [22], 32]. Due to increased vascular resistance, the normal villous branching is disrupted, resulting in the formation of narrow and elongated villous structures that restrict transplacental gas transfer [11], 21]. Although the crude OR for abnormal UA Doppler was strong, this was lost after adjustment for GAD and/or preeclampsia, highlighting the influence of gestational age and preeclampsia.
Intramural fibrin deposition
Intramural fibrin deposition in large fetal vessels, observed in 10.3 % of cases, was the only specific lesion consistently associated with neonatal mortality. Its association with abnormal MCA Doppler (crude OR 2.49) suggests a link to altered cerebral hemodynamics and possibly brain injury [13], 23], 31]. Pathophysiologically, such lesions may reflect increased venous pressure from partial cord occlusion, leading to endothelial damage and thrombus formation in large fetal vessels [4], 24]. Although adjusted models weakened the association, the crude effects remain clinically meaningful.
Strengths and limitations
A major strength of this study is the use of centralized and standardized histopathologic evaluation using Amsterdam consensus definitions [18] performed by a single pathologist. A large cohort of preterm FGR neonates was followed for more than a decade, allowing robust subgroup analyses and adjustments for relevant clinical covariates. However, some limitations must be noted. First, the absence of a non-FGR control group restricts comparisons to normal pregnancies. Second, only liveborn preterm FGR cases were included, excluding stillbirths due to incomplete antenatal and outcome data. As a result, the impacts of severe placental lesions on in utero fetal death may have been underestimated. Third, the wide range of gestational age at delivery introduces heterogeneity, although this was partially addressed by subgroup analysis, stratified by early-onset (<32 weeks) and late-onset (≥32 weeks) FGR based on crude and adjusted OR (Supplementary Figures 1 and 2). The results of subgroup analysis suggest gestational age-dependent expression of lesion severity or a higher threshold for clinical decompensation in late gestation [26], [27], [28]. The lack of significant associations in late preterm FGR aligns with the results of prior studies, indicating milder placental pathology and better tolerance to hypoxia later in gestation [13], 20].
Conclusions
We observed distinctive associations between specific pathologic placental findings of villous and vascular lesions of placental malperfusion (MVM and FVM) and abnormal Doppler findings or perinatal outcomes in FGR neonates delivered preterm. Distal villous hypoplasia and acute atherosis of basal plate arteries and/or decidual arterioles were significantly associated with abnormal UA and MCA Doppler. Intramural fibrin deposition in large fetal vessels was associated with abnormal MCA Doppler and had a potentially significant impact on neonatal mortality. Although these associations were attenuated after adjustments for gestational age and preeclampsia, the crude odds ratios suggest underlying mechanistic links between placental lesions and fetal hemodynamic compromise. Given the dynamic nature of placental development and its vulnerability to maternal and fetal insults, specific findings of MVM and FVM associated with abnormal Doppler findings or perinatal outcomes may provide insight to understand the pathophysiology of FGR and to develop individualized monitoring and intervention strategies.
Funding source: the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare
Award Identifier / Grant number: grant number: HI21C1462
-
Research ethics: The study was approved by our Institutional review board (no. 2025-01-044-001) and followed the principles of Declaration of Helsinki.
-
Informed consent: The requirement for written informed consent was waived owing to the retrospective design of our study.
-
Author contributions: Conceptualization and Investigation, review & editing (SYO, JSK). Writing – original draft (YEC). Data curation (JHS). Formal analysis (BP, KK). Funding acquisition (SYO), Methodology, Resources (YRL and SH), Supervision (SJC, CRR, YDK). All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Korea (grant number: HI21C1462).
-
Data availability: Data can be shared upon the approval of our Institutional review board according to reasonable request.
References
1. Visentin, S, Grumolato, F, Nardelli, GB, Di Camillo, B, Grisan, E, Cosmi, E. Early origins of adult disease: low birth weight and vascular remodeling. Atherosclerosis 2014;237:391–9. https://doi.org/10.1016/j.atherosclerosis.2014.09.027.Search in Google Scholar PubMed
2. Ozturk, HNO, Turker, PF. Fetal programming: could intrauterin life affect health status in adulthood? Obstet Gynecol Sci 2021;64:473–83. https://doi.org/10.5468/ogs.21154.Search in Google Scholar PubMed PubMed Central
3. Mifsud, W, Sebire, NJ. Placental pathology in early-onset and late-onset fetal growth restriction. Fetal Diagn Ther 2014;36:117–28. https://doi.org/10.1159/000359969.Search in Google Scholar PubMed
4. Redline, RW, Ravishankar, S, Bagby, CM, Saab, ST, Zarei, S. Four major patterns of placental injury: a stepwise guide for understanding and implementing the 2016 amsterdam consensus. Mod Pathol 2021;34:1074–92. https://doi.org/10.1038/s41379-021-00747-4.Search in Google Scholar PubMed
5. Fetal Growth Restriction: ACOG Practice Bulletin. Number 227. Obstet Gynecol 2021;137:e16–28.10.1097/AOG.0000000000004251Search in Google Scholar PubMed
6. Vollgraff Heidweiller-Schreurs, CA, De Boer, MA, Heymans, MW, Schoonmade, LJ, Bossuyt, PMM, Mol, BWJ, et al.. Prognostic accuracy of cerebroplacental ratio and middle cerebral artery Doppler for adverse perinatal outcome: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2018;51:313–22. https://doi.org/10.1002/uog.18809.Search in Google Scholar PubMed PubMed Central
7. Spinillo, A, Montanari, L, Bergante, C, Gaia, G, Chiara, A, Fazzi, E. Prognostic value of umbilical artery Doppler studies in unselected preterm deliveries. Obstet Gynecol 2005;105:613–20. https://doi.org/10.1097/01.aog.0000152382.13490.18.Search in Google Scholar PubMed
8. Meler, E, Martinez, J, Boada, D, Mazarico, E, Figueras, F. Doppler studies of placental function. Placenta 2021;108:91–6. https://doi.org/10.1016/j.placenta.2021.03.014.Search in Google Scholar PubMed
9. Arslan, HC, Corbacioglu Esmer, A, Akca, A, Arslan, K. The effect of middle cerebral artery peak systolic velocity on prognosis in early and late-onset fetal growth restriction. J Perinat Med 2023;51:559–63. https://doi.org/10.1515/jpm-2022-0305.Search in Google Scholar PubMed
10. Spinillo, A, Gardella, B, Bariselli, S, Alfei, A, Silini, E, Dal Bello, B. Placental histopathological correlates of umbilical artery Doppler velocimetry in pregnancies complicated by fetal growth restriction. Prenat Diagn 2012;32:1263–72. https://doi.org/10.1002/pd.3988.Search in Google Scholar PubMed
11. Zur, RL, Kingdom, JC, Parks, WT, Hobson, SR. The placental basis of fetal growth restriction. Obstet Gynecol Clin North Am 2020;47:81–98. https://doi.org/10.1016/j.ogc.2019.10.008.Search in Google Scholar PubMed
12. Ganer Herman, H, Barber, E, Gasnier, R, Gindes, L, Bar, J, Schreiber, L, et al.. Placental pathology and neonatal outcome in small for gestational age pregnancies with and without abnormal umbilical artery Doppler flow. Eur J Obstet Gynecol Reprod Biol 2018;222:52–6. https://doi.org/10.1016/j.ejogrb.2018.01.009.Search in Google Scholar PubMed
13. Shmueli, A, Mor, L, Blickstein, O, Sela, R, Weiner, E, Gonen, N, et al.. Placental pathology in pregnancies with late fetal growth restriction and abnormal cerebroplacental ratio. Placenta 2023;138:83–7. https://doi.org/10.1016/j.placenta.2023.05.010.Search in Google Scholar PubMed
14. Spinillo, A, Meroni, A, Melito, C, Scatigno, AL, Tzialla, C, Fiandrino, G, et al.. Clinical correlates of placental pathologic features in early-onset fetal growth restriction. Fetal Diagn Ther 2022;49:215–24. https://doi.org/10.1159/000522202.Search in Google Scholar PubMed
15. Lee, JK, Jang, HL, Kang, BH, Lee, KS, Choi, YS, Shim, KS, et al.. Percentile distributions of birth weight according to gestational ages in Korea (2010-2012). J Korean Med Sci. 2016;31:939–49. https://doi.org/10.3346/jkms.2016.31.6.939.Search in Google Scholar PubMed PubMed Central
16. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin. Number 222. Obstet Gynecol 2020;135:e237–60.10.1097/AOG.0000000000003891Search in Google Scholar PubMed
17. Redline, RW, Heller, D, Keating, S, Kingdom, J. Placental diagnostic criteria and clinical correlation – a workshop report. Placenta 2005;26:S114–7. https://doi.org/10.1016/j.placenta.2005.02.009.Search in Google Scholar PubMed
18. Khong, TY, Mooney, EE, Ariel, I, Balmus, NC, Boyd, TK, Brundler, MA, et al.. Sampling and definitions of placental lesions: Amsterdam placental workshop group consensus statement. Arch Pathol Lab Med 2016;140:698–713. https://doi.org/10.5858/arpa.2015-0225-cc.Search in Google Scholar PubMed
19. Paules, C, Youssef, L, Rovira, C, Crovetto, F, Nadal, A, Peguero, A, et al.. Distinctive patterns of placental lesions in pre-eclampsia vs small-for-gestational age and their association with fetoplacental Doppler. Ultrasound Obstet Gynecol 2019;54:609–16. https://doi.org/10.1002/uog.20350.Search in Google Scholar PubMed
20. Parra-Saavedra, M, Simeone, S, Triunfo, S, Crovetto, F, Botet, F, Nadal, A, et al.. Correlation between histological signs of placental underperfusion and perinatal morbidity in late-onset small-for-gestational-age fetuses. Ultrasound Obstet Gynecol 2015;45:149–55. https://doi.org/10.1002/uog.13415.Search in Google Scholar PubMed
21. Parks, WT. Manifestations of hypoxia in the second and third trimester placenta. Birth Defects Res 2017;109:1345–57. https://doi.org/10.1002/bdr2.1143.Search in Google Scholar PubMed
22. Parks, WT, Catov, JM. The placenta as a window to maternal vascular health. Obstet Gynecol Clin North Am 2020;47:17–28. https://doi.org/10.1016/j.ogc.2019.10.001.Search in Google Scholar PubMed
23. Ravikumar, G, Mascarenhas, D, Suman Rao, PN, Crasta, J. Fetal vascular malperfusion (FVM): diagnostic implications and clinical associations. J Matern Fetal Neonatal Med 2022;35:4526–33. https://doi.org/10.1080/14767058.2020.1854215.Search in Google Scholar PubMed
24. Redline, RW, Ravishankar, S. Fetal vascular malperfusion, an update. APMIS 2018;126:561–9. https://doi.org/10.1111/apm.12849.Search in Google Scholar PubMed
25. Gordijn, SJ, Beune, IM, Thilaganathan, B, Papageorghiou, A, Baschat, AA, Baker, PN, et al.. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol 2016;48:333–9. https://doi.org/10.1002/uog.15884.Search in Google Scholar PubMed
26. Aviram, A, Sherman, C, Kingdom, J, Zaltz, A, Barrett, J, Melamed, N. Defining early vs late fetal growth restriction by placental pathology. Acta Obstet Gynecol Scand 2019;98:365–73. https://doi.org/10.1111/aogs.13499.Search in Google Scholar PubMed
27. Apel-Sarid, L, Levy, A, Holcberg, G, Sheiner, E. Term and preterm (<34 and <37 weeks gestation) placental pathologies associated with fetal growth restriction. Arch Gynecol Obstet 2010;282:487–92. https://doi.org/10.1007/s00404-009-1255-1.Search in Google Scholar PubMed
28. Bujorescu, DL, Ratiu, AC, Motoc, AGM, Citu, IC, Sas, I, Gorun, IF, et al.. Placental pathology in early-onset fetal growth restriction: insights into fetal growth restriction mechanisms. Rom J Morphol Embryol 2023;64:215–24. https://doi.org/10.47162/rjme.64.2.12.Search in Google Scholar PubMed PubMed Central
29. Jo, YS, Jang, DG, Lee, GS. Analysis of placental pathological findings contributing to intrauterine fetal death. Korean J Obstet Gynecol 2010;53:602–7. https://doi.org/10.5468/kjog.2010.53.7.602.Search in Google Scholar
30. Arts, N, Schiffer, V, Severens-Rijvers, C, Bons, J, Spaanderman, M, Al-Nasiry, S. Cumulative effect of maternal vascular malperfusion types in the placenta on adverse pregnancy outcomes. Placenta 2022;129:43–50. https://doi.org/10.1016/j.placenta.2022.09.007.Search in Google Scholar PubMed
31. Spinillo, A, Dominoni, M, Caporali, C, Olivieri, I, La Piana, R, Longo, S, et al.. Placental histological features and neurodevelopmental outcomes at two years in very-low-birth-weight infants. Pediatr Neurol 2021;120:63–70. https://doi.org/10.1016/j.pediatrneurol.2021.04.007.Search in Google Scholar PubMed
32. Jaiman, S, Romero, R, Pacora, P, Jung, E, Bhatti, G, Yeo, L, et al.. Disorders of placental villous maturation in fetal death. J Perinat Med 2020;48:345–68. https://doi.org/10.1515/jpm-2020-0030.Search in Google Scholar PubMed PubMed Central
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/jpm-2025-0140).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.


