The origin of amniotic fluid monocytes/macrophages in women with intra-amniotic inflammation or infection
-
Nardhy Gomez-Lopez
, Roberto Romero
, Yaozhu Leng
Abstract
Background
Monocytes, after neutrophils, are the most abundant white blood cells found in the amniotic cavity of women with intra-amniotic inflammation/infection. However, the origin of such cells has not been fully investigated. Herein, we determined (1) the origin of amniotic fluid monocytes/macrophages from women with intra-amniotic inflammation/infection, (2) the relationship between the origin of amniotic fluid monocytes/macrophages and preterm or term delivery and (3) the localization of monocytes/macrophages in the placental tissues.
Methods
Amniotic fluid samples (n = 16) were collected from women with suspected intra-amniotic inflammation or infection. Amniotic fluid monocytes/macrophages were purified by fluorescence-activated cell sorting, and DNA fingerprinting was performed. Blinded placental histopathological evaluations were conducted. Immunohistochemistry was performed to detect CD14+ monocytes/macrophages in the placental tissues.
Results
DNA fingerprinting revealed that (1) 56.25% (9/16) of amniotic fluid samples had mostly fetal monocytes/macrophages, (2) 37.5% (6/16) had predominantly maternal monocytes/macrophages and (3) one sample (6.25% [1/16]) had a mixture of fetal and maternal monocytes/macrophages. (4) Most samples with predominantly fetal monocytes/macrophages were from women who delivered early preterm neonates (77.8% [7/9]), whereas all samples with mostly maternal monocytes/macrophages or a mixture of both were from women who delivered term or late preterm neonates (100% [7/7]). (5) Most of the women included in this study presented acute maternal and fetal inflammatory responses in the placenta (85.7% [12/14]). (6) Women who had mostly fetal monocytes/macrophages in amniotic fluid had abundant CD14+ cells in the umbilical cord and chorionic plate, whereas women with mostly maternal amniotic fluid monocytes/macrophages had abundant CD14+ cells in the chorioamniotic membranes.
Conclusion
Amniotic fluid monocytes/macrophages can be of either fetal or maternal origin, or a mixture of both, in women with intra-amniotic inflammation or infection. These immune cells could be derived from the fetal and maternal vasculature of the placenta.
Introduction
Intra-amniotic inflammation/infection is a well-established etiology for spontaneous preterm labor [1], [2], [3], [4] and is strongly associated with clinical chorioamnionitis [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]. This clinical condition can result from microbial invasion of the amniotic cavity, also known as microbial-induced intra-amniotic inflammation or intra-amniotic infection [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29]. Intra-amniotic inflammation can also occur in the absence of detectable microorganisms using cultivation and molecular microbiology techniques (i.e. sterile intra-amniotic inflammation) [30], [31], [32], [33], [34], a process that can be mediated by danger signals (also known as alarmins [35], [36], [37]) found in the amniotic fluid [33], [38], [39], [40], [41], [42]. Intra-amniotic inflammation/infection is characterized by an elevated number of white blood cells (WBCs) (i.e. leukocytes) in the amniotic cavity [43], [44], [45], [46], [47], [48] and increased concentrations of inflammatory mediators such as anti-microbial peptides [49], [50], [51], [52], [53], [54], cytokines [10], [33], [38], [39], [55], [56], [57], [58], [59] and lipids [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71]. Yet, the cellular immune responses in intra-amniotic infection and intra-amniotic inflammation are different [72], highlighting the importance of characterizing the specific immune cells in the amniotic cavity.
Neutrophils are the most abundant leukocyte subset in the amniotic cavity of women with inflammation/infection [43], [48]. The functions of amniotic fluid neutrophils include (1) trapping and killing pathogens in the amniotic cavity by forming web-like structures called neutrophil extracellular traps (NETs) [73], (2) phagocytizing bacteria commonly found in the lower genital tract [74] and (3) releasing antimicrobial products [29], [49], [50], [51], [52], [53], [54], [75], [76] and cytokines [48]. The origin of amniotic fluid neutrophils can be either fetal [77], [78], [79] or maternal [79], [80], or a mixture of both [79]. In the former, fetal neutrophils could derive from the chorionic plate [81] or the fetus itself (e.g. fetal lung or gastrointestinal tract) [77], [82]. In contrast, maternal neutrophils could derive from the maternal vasculature of the chorioamniotic membranes (e.g. decidual tissues) [83], [84], as has been shown for other immune cells (e.g. T cells [85]).
Following neutrophils, monocytes/macrophages are the most abundant leukocyte subset in the amniotic fluid of women with intra-amniotic inflammation/infection [48]. The classical function of monocytes is to release pro-inflammatory mediators such as cytokines [86], but these immune cells have complex functions that could vary according to the microenvironment [87], [88], [89]. We have shown that amniotic fluid monocytes/macrophages release specific cytokines in women with intra-amniotic inflammation/infection, which are different from those mediators released by neutrophils [48], indicating that these innate immune cells may display unique functions in the amniotic cavity. Early reports have suggested that monocytes/macrophages in amniotic fluid are derived from the fetus [90], [91], [92]. In line with these observations, the number of macrophages in the amniotic fluid is increased in pregnancies where fetal anomalies such as anencephaly, neural tube defects and gastroschisis are present [93], [94], [95], [96], [97], [98], [99], [100]. These findings have led to the prevailing belief that amniotic fluid monocytes/macrophages are derived from fetal tissues, particularly in the context of intra-amniotic inflammation/infection [77]. Yet, the origin of amniotic fluid monocytes/macrophages in preterm and term gestations with intra-amniotic inflammation or infection have not been fully elucidated.
The aims of this study were to (1) establish the origin of amniotic fluid monocytes/macrophages from women with intra-amniotic inflammation or infection using a highly specific technology, DNA fingerprinting, (2) assess the relationship between the origin of amniotic fluid monocytes/macrophages and preterm or term gestations and (3) associate the origin of amniotic fluid monocytes/macrophages with the localization of monocytes/macrophages in the placenta using immunohistochemistry.
Materials and methods
Study population
This was a cross-sectional study of women who underwent transabdominal amniocentesis due to clinical indications or sampling of amniotic fluid during cesarean delivery. Women were enrolled at Hutzel Women’s Hospital of the Detroit Medical Center. Amniotic fluid samples were acquired using an automatic cell counter (Cellometer Auto 2000, Nexcelom Bioscience, Lawrence, MA, USA) to obtain the viable cell numbers, most of which are WBCs or leukocytes [48]. The inclusion criteria were as follows: (1) amniotic fluid samples without blood contamination and (2) amniotic fluid samples with a large number of viable leukocytes (including mostly monocytes and neutrophils [48]) (>1×105 cells/mL) to perform fluorescence-activated cell sorting (FACS) of amniotic fluid monocytes/macrophages.
All of the women provided written informed consent to donate additional amniotic fluid for research purposes, according to protocols approved by the Institutional Review Boards of the Detroit Medical Center (Detroit, MI, USA), Wayne State University and the Perinatology Research Branch, an intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS).
Clinical definitions
Gestational age was determined by the last menstrual period and confirmed by ultrasound examination. The gestational age derived from sonographic fetal biometry was used when the estimation was inconsistent with menstrual dating. Clinical chorioamnionitis was diagnosed by the presence of maternal fever (temperature >37.8°C) accompanied by two or more of the following criteria: (1) uterine tenderness; (2) malodorous vaginal discharge; (3) fetal tachycardia (heart rate >160 beats/min); (4) maternal tachycardia (heart rate >100 beats/min) and (5) maternal leukocytosis (leukocyte count >15,000 cells/mm3) [7], [101], [102], [103], [104], [105]. Term delivery was defined as birth after 37 weeks of gestation, whereas preterm delivery was defined as birth between 20 and 36 6/7 weeks of gestation. Preterm deliveries were further classified as early (<28 weeks of gestation) or late (between 34 and 36 6/7 weeks of gestation) [106].
Intra-amniotic inflammation was diagnosed when the concentration of interleukin (IL)-6 in the amniotic fluid was ≥2.6 ng/mL [27], [107], [108]. Microbial invasion of the amniotic cavity was defined as a positive amniotic fluid culture [19], [20], [21], [109], [110], [111]. Intra-amniotic infection was defined as the presence of microbial invasion of the amniotic cavity with intra-amniotic inflammation [9], [27], [30], [31], [32], [33], [34], [107], [112], [113], [114], [115].
Sample collection
Amniotic fluid was either retrieved by transabdominal amniocentesis under antiseptic conditions using a 22-gauge needle monitored by ultrasound or sampled during cesarean delivery. Amniotic fluid samples were transported to the clinical laboratory in a capped sterile syringe and were cultured for aerobic and anaerobic bacteria, as well as for genital mycoplasmas [9], [116], [117], [118], [119]. Shortly after collection, a WBC count was determined in each amniotic fluid sample using a hemocytometer chamber, according to methods previously described [43]. Glucose concentration was also determined [120] and a Gram stain [121] was performed for each amniotic fluid sample. Cultures, WBC count, glucose concentration and Gram stain were not performed in all of the amniotic fluid samples collected during cesarean delivery as these samples were collected for research purposes only. However, both IL-6 concentration [48] and the presence of bacteria (bacterial live/dead staining [73], [122]) were assessed in most of the amniotic fluid samples, as previously described.
Fluorescence-activated cell sorting (FACS) of amniotic fluid monocytes/macrophages
Amniotic fluid samples were passed through a sterile 15-μm filter (Cat# 43-50015-03; pluriSelect Life Science, Leipzig, Germany) to remove epithelial cells and centrifuged at 200×g for 5 min at room temperature (n=16). The cell pellet (mostly leukocytes [73]) was washed with 1X phosphate-buffered saline (1X PBS; Life Technologies, Grand Island, NY, USA), resuspended at 1×106 cells in 100 μL of BD FACS stain buffer (Cat# 554656; BD Biosciences, San Jose, CA, USA) containing 20% human Fc receptor (FcR) blocking reagent (Cat# 130-059-901; Miltenyi Biotec, San Diego, CA, USA) and incubated for 10 min at 4°C. Next, amniotic fluid cells were incubated with the following fluorochrome-conjugated anti-human antibodies (BD Biosciences) for 30 min at 4°C in the dark: CD14-APC-Cy7 (clone MϕP9, Cat# 557831, BD Biosciences) and CD15-FITC (clone W6D3, Cat# 562370, BD Biosciences). After washing with 1X PBS, the cells were resuspended in pre-sort buffer (Cat# 563503, BD Biosciences) at a concentration of 5×106 cells/mL. Amniotic fluid monocytes/macrophages (CD14+CD15− cells) were purified using a BD FACSAria cell sorter (BD Biosciences) and BD FACSDiva 6.0 software (BD Biosciences). The purity of amniotic fluid monocytes/macrophages ranged from 71% to 98% (Figure 1). The purified monocytes/macrophages were then resuspended in RLT buffer (Qiagen, Germantown, MD, USA) and stored at −80°C until use.

Origin of amniotic fluid monocytes/macrophages.
Fluorescence-activated cell sorting (FACS) of amniotic fluid monocytes/macrophages. (A) Before cell sorting: representative flow cytometry gating strategy of an amniotic fluid sample from a woman with intra-amniotic inflammation/infection containing different cell populations: monocytes/macrophages (CD14+ cells) and neutrophils (CD15+ cells). After cell sorting: a representative image of the flow cytometry analysis of purified amniotic fluid monocytes/macrophages (CD14+CD15− cells) from a woman with intra-amniotic inflammation/infection; purity, 95.9%. (B) Origin of each sample’s amniotic fluid monocytes/macrophages based on DNA fingerprinting analysis. Red text indicates primarily fetal monocytes, blue indicates mostly maternal and gray shows a sample of mixed origin in amniotic fluid. Two pairs of samples (13&14 and 15&16) were collected from the same two patients at different gestational ages. (C) Pie chart displaying the origin of amniotic fluid monocytes/macrophages. aDetermined with AO/PI on Cellometer Auto 2000 (Nexcelom).
DNA fingerprinting
Genomic DNA was isolated from FACS-purified amniotic fluid monocytes/macrophages (n=16) using an AllPrep DNA/RNA Mini Kit (Qiagen), following the manufacturer’s instructions. Fetal or maternal genomic DNA was isolated from frozen samples of either umbilical cord or maternal blood (buffy coat) using a DNeasy Blood & Tissue Kit (Qiagen), according to the manufacturer’s instructions. DNA concentration and purity were assessed using the NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). DNA samples (amniotic fluid, umbilical cord blood and maternal blood) were submitted for DNA fingerprinting to Genetica DNA Laboratories (https://www.celllineauthentication.com, Laboratory Corporation of America/LabCorp, Burlington, NC, USA). Briefly, analytical procedures for polymerase chain reaction (PCR) and capillary electrophoresis were performed on a 3130xl genetic analyzer (Applied Biosystems, Foster City, CA, USA). The 13 core CODIS STR loci plus PENTA E and PENTA D, and the gender-determining locus, amelogenin, were analyzed using the commercially available PowerPlex® 16HS amplification kit (Promega Corporation, Madison, WI, USA) and GeneMapper ID v3.2.1 software (Applied Biosystems). Appropriate positive and negative controls were concurrently used throughout the analysis. The interpretation of the DNA profile of each sample was performed by geneticists from LabCorp and returned as a written report. The reported sensitivity for this method was between 2% and 5%, indicating that this technology is capable of detecting as low as 2–5% of the DNA in question among the total DNA mixture. A cut-off of 90% was used to establish the predominant origin of amniotic fluid monocytes/macrophages.
Placental histopathological examination
Sampling of the placentas was conducted according to protocols established by the Perinatology Research Branch [123]. Five-micrometer-thick sections of formalin-fixed, paraffin-embedded tissue specimens were cut and mounted on SuperFrost Plus microscope slides (Erie Scientific LLC, Portsmouth, NH, USA). After deparaffinization, slides were rehydrated and stained with hematoxylin-eosin. A minimum of five full-thickness sections of chorionic plate, three sections of umbilical cord and three chorioamniotic membrane rolls from each case were examined by a placental pathologist who was blinded to clinical history and additional testing results. Acute inflammatory lesions of the placenta (maternal inflammatory response and fetal inflammatory response) were diagnosed according to established criteria, including staging and grading [84], [124], [125].
Immunohistochemistry
Five-micrometer-thick sections of formalin-fixed, paraffin-embedded chorioamniotic membranes, umbilical cord and chorionic plate samples from the placenta (n=12 each tissue) were cut, mounted on SuperFrost Plus microscope slides and subjected to immunochemistry using rabbit anti-human CD14 antibody (Cat# ab183322; Abcam, Cambridge, MA, USA). The detection of CD14 was used instead of that of CD68 as the former identified both monocytes and macrophages (data not shown). The staining was performed using the Leica Bond Max automatic staining system (Leica Microsystems, Wetzlar, Germany) with the Bond Polymer Refine Detection Kit (Leica Microsystems). Staining with rabbit immunoglobulin G (IgG) (Cat# ab172730; Abcam) was used as a negative control. Following staining, tissue slides were scanned using the Vectra Polaris Multispectral Imaging System (PerkinElmer, Waltham, MA, USA), and images were analyzed using the InForm 2.4.1 image analysis software (PerkinElmer).
Results
Clinical characteristics and placenta pathology of the study population
A total of 16 amniotic fluid samples were included in this study. The clinical characteristics and the placental pathology of the study population are shown in Table 1. The amniotic fluid samples had one or several of the following: (1) a positive microbiological culture, (2) an elevated concentration of IL-6 (≥2.6 ng/mL), (3) an increased WBC (>50 cells/mm3) or a viable cell (i.e. leukocytes; >100 cells/mm3) count and (4) a positive bacterial live/dead staining (Table 1). Most of the amniotic fluid samples with quantifiable glucose had a low glucose concentration (<14 mg/dL) (Table 1). Five of the samples were from women diagnosed with clinical chorioamnionitis (Table 1). Four of the amniotic fluid samples were collected from two women; for each, the first was collected during a transabdominal amniocentesis and the second was sampled during cesarean delivery (Table 1, samples 13 and 14 and samples 15 and 16). The most common microorganisms found in these amniotic fluid samples were Ureaplasma urealyticum and Mycoplasma hominis followed by Prevotella spp. (Table 1). The vast majority of women (12/14) with intra-amniotic inflammation or intra-amniotic infection presented with acute maternal and fetal inflammatory responses in the placenta (Table 1).
Clinical characteristics of amniotic fluid samples utilized for DNA fingerprinting assays.
| Sample | Predominant origin of amniotic fluid monocytes/macrophages | Clinical chorioamnionitis | Viable cell counta, cells/mm3 | Gestational age at amniocentesis, weeks | IL-6, ng/mL | Gram stain | Bacterial live/dead staining | Amniotic fluid culture | WBC, cells/mm3 | Glucose, mg/dL | Gestational age at delivery, weeks | Placental pathology results | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute maternal inflammatory response | Acute fetal inflammatory response | ||||||||||||
| 1 | Fetal | Yes | 786 | 22.1 | 123.7 | Negative | Negative | Negative | 390 | 7 | 23.9 | Stage 3/grade 2 | Stage 2/grade 2 |
| 2 | Fetal | No | 180 | 19 | 55.5 | Negative | Negative | Negative | 43 | 17 | 19.4 | Stage 2/grade 1 | Stage 1/grade 1 |
| 3 | Fetal | No | 286 | 39.3 | 0.5 | NA | Negative | Negative | NA | NA | 39.3 | None | None |
| 4 | Fetal | No | 508 | 23.1 | 121 | Positive | Positive | Bacteroides ureolyticus, Gardnerella vaginalis, Ureaplasma urealyticum | 750 | <1 | 23.6 | Stage 3/grade 2 | Stage 1/grade 1 |
| 5 | Fetal | No | 100 | 18.9 | 121.3 | Positive | Positive | Bacteroides fragilis | 65 | 20 | 19.6 | Stage 3/grade 2 | Stage 2/grade 1 |
| 6 | Fetal | No | 9920 | 25.7 | 27 | Positive | Positive | Enterobacter aerogenes, Enterococcus faecalis, Mycoplasma hominis, Prevotella species, Streptococcus viridans | 6938 | 4 | 25.7 | Stage 3/grade 2 | Stage 1/grade 1 |
| 7 | Fetal | No | 3660 | 21.3 | 118.7 | Negative | Positive | Staphylococcus hominis | 355 | <1 | 21.9 | Stage 3/grade 2 | Stage 2/grade 1 |
| 8 | Fetal | No | 1160 | 22.3 | 125.5 | Positive | Positive | Mycoplasma hominis, Fusobacterium nucleatum | 700 | 10 | 22.7 | Stage 2/grade 1 | Stage 1/grade 1 |
| 9 | Mix of fetal and maternal | No | 125 | 40.6 | 39.9 | NA | Positive | NA | NA | NA | 40.6 | Stage 2/grade 1 | Stage 1/grade 1 |
| 10 | Maternal | No | 9600 | 38.1 | 101.4 | Negative | Positive | Mycoplasma hominis, Ureaplasma urealyticum | NA | NA | 38.1 | Stage 2/grade 1 | Stage 2/grade 1 |
| 11 | Maternal | No | 1650 | 39.1 | 22.1 | NA | Positive | NA | NA | NA | 39.1 | Stage 1/grade 1 | Stage 1/grade 1 |
| 12 | Maternal | No | 3090 | 40.4 | 54.6 | Negative | Positive | Ureaplasma urealyticum | NA | NA | 40.4 | None | None |
| 13 | Maternal | Yes | 2200 | 35.6b | 70.6 | Positive | Positive | Mycoplasma hominis, Ureaplasma urealyticum, Prevotella spp., Streptococcus agalactiae, Streptococcus anginosus | 4000 | <1 | |||
| 14 | Maternal | Yes | 6780 | 35.6b | NA | Positive | Positive | Mycoplasma hominis, Ureaplasma urealyticum, Prevotella species | NA | NA | 35.6 | Stage 3/grade 2 | Stage 2/grade 2 |
| 15 | Fetal | Yes | 860 | 39.9c | 73.6 | Negative | Negative | Negative | 600 | <1 | |||
| 16 | Maternal | Yes | 18,800 | 40c | 47.7 | Negative | Positive | Ureaplasma urealyticum | NA | NA | 40 | Stage 2/grade 1 | Stage 2/grade 1 |
IL, interleukin; NA, not available; WBC, white blood cell. aDetermined with AO/PI on Cellometer Auto 2000 (Nexcelom). bSample 14 obtained 4 h after sample 13. cSample 16 obtained 14 h after sample 15.
Origin of amniotic fluid monocytes/macrophages
Our previous study has shown that neutrophils in the amniotic fluid can be of maternal, fetal, and/or mixed origin [79]. Herein, we sought to investigate the origin of monocytes/macrophages in the amniotic fluid of women with intra-amniotic inflammation/infection. Figure 1A shows the representative images of the flow cytometry gating strategy used to detect monocytes/macrophages in amniotic fluid from women with intra-amniotic inflammation/infection, before and after cell sorting. Amniotic fluid monocytes/macrophages were identified within the total leukocyte population by the expression of CD14 (Figure 1A). Despite neutrophils being the dominant leukocyte population in the amniotic fluid of women with intra-amniotic inflammation/infection, monocytes/macrophages were able to be isolated by cell sorting and their purity ranged from 71% to 98% (Figure 1A).
DNA fingerprinting, a highly specific technique that detects unique genetic patterns, was used to determine the origin of amniotic fluid monocytes/macrophages. As shown in Figure 1B, monocytes/macrophages of maternal and fetal origin were detected in different proportions. Samples with more than 90% of monocytes/macrophages of fetal or maternal origin were considered predominantly of that origin. An overall representation of the origin of amniotic fluid monocytes/macrophages is shown in Figure 1C.
Amniotic fluid monocytes/macrophages in women with intra-amniotic inflammation or infection can be predominantly of fetal origin
A representation of the DNA fingerprinting of amniotic fluid monocytes/macrophages of fetal origin (sample 4) is shown in Figure 2, an electropherogram containing 16 genetic sites: D3S1358, TH01, D21S11, D18S51, Penta_E, D5S818, D13S317, D7S820, D16S539, CSF1PO, Penta_D, X- and Y-specific amelogenin genes, vWA, D8S1179, TPOX and FGA. The electropherogram was separated into three sections: blue, green and black. Each color indicates the dye used for the PCR multiplex: blue represents the genes amplified using fluorescein (FL dye), green represents the genes amplified using 6-carboxy-4′,5′-dichloro-2′,7′-dimethoxyfluorescein (JOE dye) and black represents the genes amplified using tetramethylrhodamine (TMR dye). Each genetic site has STR alleles, which is represented by peaks. The number next to each STR allele (peak) is the number of repeats for each STR allele. Figure 1B, C shows that 56.25% (9/16) of the samples had predominant (90%–100%) fetal monocytes/macrophages in the amniotic fluid (Figure 1B, C, samples 1–8, 15; Table 1). Figure 2 illustrates that the DNA fingerprinting of amniotic fluid monocytes/macrophages is identical to the DNA fingerprinting of the fetus. By contrast, the DNA fingerprinting of amniotic fluid monocytes/macrophages differed from the one displayed by the mother (Figure 2).
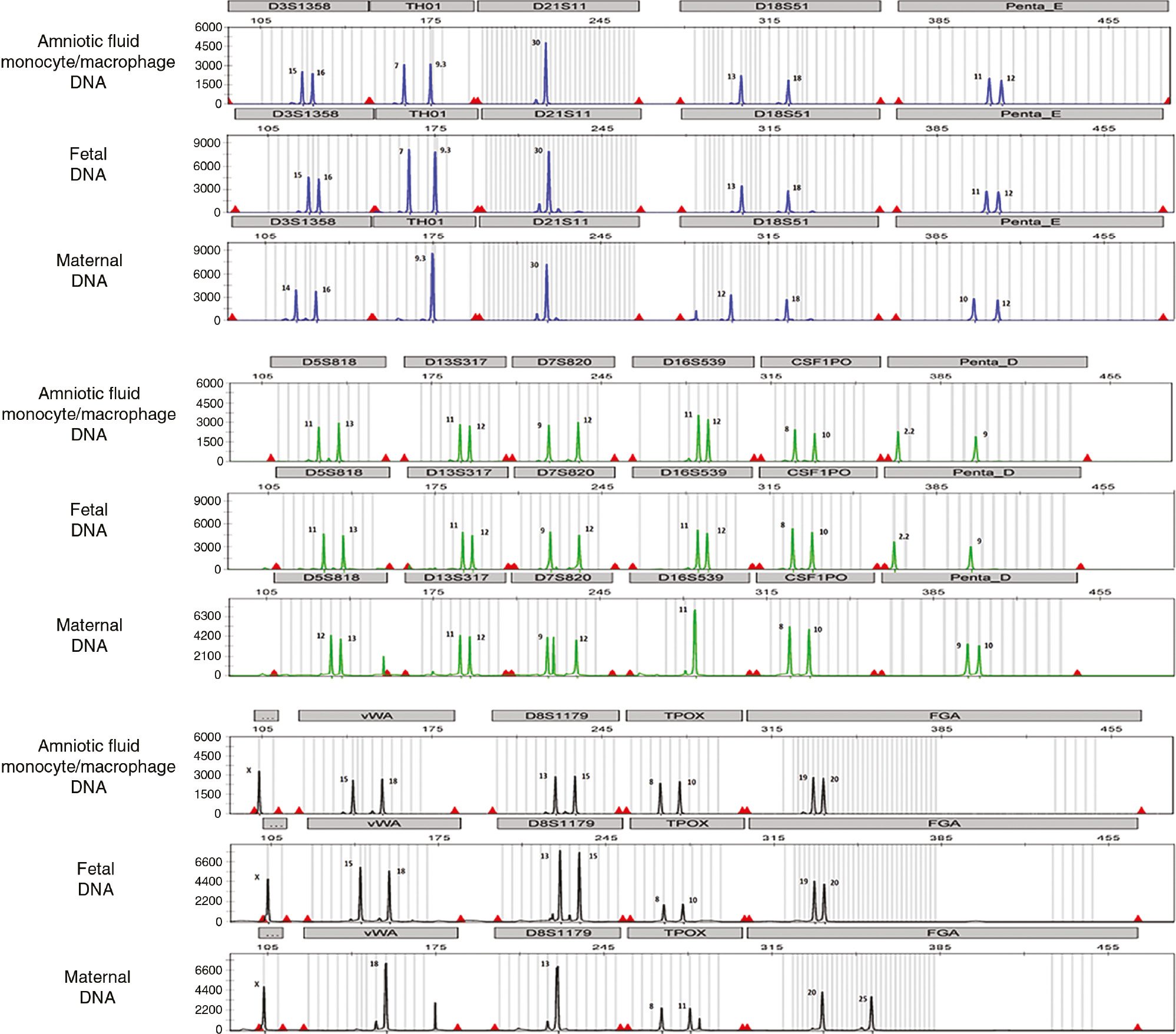
DNA fingerprinting of amniotic fluid monocytes/macrophages that were mostly of fetal origin.
DNA fingerprinting of purified amniotic fluid monocytes/macrophages, the fetus (umbilical cord) and the mother (buffy coat from peripheral blood) is shown in electropherograms. Each electropherogram contains 16 genetic sites: D3S1358, TH01, D21S11, D18S51, Penta_E, D5S818, D13S317, D7S820, D16S539, CSF1PO, Penta_D, X- and Y-specific amelogenin genes, vWA, D8S1179, TPOX and FGA. Each electropherogram was separated into three sections: blue, green and black. Each color indicates the dye used for the PCR multiplex: blue represents the genes amplified using fluorescein (FL dye), green represents the genes amplified using 6-carboxy-4′,5′-dichloro-2′,7′-dimethoxyfluorescein (JOE dye) and black represents the genes amplified using tetramethylrhodamine (TMR dye). Each genetic site has STR alleles, which is represented by peaks. The number next to each STR allele (peak) is the number of repeats for each STR allele. The DNA fingerprinting of the amniotic fluid monocytes/macrophages is identical to the DNA fingerprinting of the fetus.
Amniotic fluid samples in which monocytes/macrophages were predominantly of fetal origin were collected from either women diagnosed with intra-amniotic infection or those with intra-amniotic inflammation without culturable microorganisms. Specifically, four of the samples with monocytes/macrophages predominantly of fetal origin (4/9) had no detectable bacteria using cultivation or microscopy techniques (Table 1). Interestingly, the majority of these samples (7/9) were collected from women who delivered early preterm neonates (<28 weeks of gestation, Table 1).
Amniotic fluid monocytes/macrophages in women with intra-amniotic infection can be predominantly of maternal origin
Figure 3 is also an electropherogram of DNA fingerprinting, which revealed that 37.5% (6/16) of the samples had mostly (96%–100%) maternal monocytes/macrophages in the amniotic fluid as shown in Figure 1B (samples 10–14, 16; Table 1). In Figure 3, the representative DNA fingerprinting of amniotic fluid monocytes/macrophages of maternal origin is shown (sample 13). The DNA fingerprinting of the amniotic fluid monocytes/macrophages is identical to the DNA fingerprinting of the mother (Figure 3). By contrast, the DNA fingerprinting of amniotic fluid monocytes/macrophages differed from the one displayed by the fetus (Figure 3).

DNA fingerprinting of amniotic fluid monocytes/macrophages that were mostly of maternal origin.
DNA fingerprinting of purified amniotic fluid monocytes/macrophages, the fetus (umbilical cord), and the mother (buffy coat from peripheral blood) is shown in electropherograms. Each electropherogram contains 16 genetic sites: D3S1358, TH01, D21S11, D18S51, Penta_E, D5S818, D13S317, D7S820, D16S539, CSF1PO, Penta_D, X- and Y-specific amelogenin genes, vWA, D8S1179, TPOX and FGA. Each electropherogram was separated into three sections: blue, green and black. Each color indicates the dye used for the PCR multiplex: blue represents the genes amplified using fluorescein (FL dye), green represents the genes amplified using 6-carboxy-4′,5′-dichloro-2′,7′-dimethoxyfluorescein (JOE dye) and black represents the genes amplified using tetramethylrhodamine (TMR dye). Each genetic site has STR alleles, which is represented by peaks. The number next to each STR allele (peak) is the number of repeats for each STR allele. The DNA fingerprinting of the amniotic fluid monocytes/macrophages is identical to the DNA fingerprinting of the mother.
Amniotic fluid samples in which monocytes/macrophages were predominantly of maternal origin were collected exclusively from women diagnosed with intra-amniotic infection (Table 1). None of the samples with maternal amniotic fluid monocytes/macrophages came from women with intra-amniotic inflammation without detectable microorganisms (Table 1). Strikingly, all of the women who had predominantly monocytes/macrophages of maternal origin in the amniotic fluid at the time of collection delivered term (≥37 weeks of gestation) or late preterm (34–36 completed weeks of gestation) neonates (Table 1).
A small subset of women with intra-amniotic infection can have a mixture of both fetal and maternal monocytes/macrophages in amniotic fluid
Figure 4 is also an electropherogram of DNA fingerprinting, which revealed that one woman (1/16) who was sampled during cesarean delivery had an evident mixture of fetal and maternal monocytes/macrophages in amniotic fluid as shown in Figure 1B (sample 9; Table 1). In Figure 4, the DNA fingerprinting of such amniotic fluid sample is displayed showing an evident mixture of fetal and maternal monocytes/macrophages in amniotic fluid. Therefore, the DNA fingerprinting of amniotic fluid monocytes/macrophages is a combination of the fetal and maternal STR alleles (Figure 4). The woman with a mixture of both fetal and maternal monocytes/macrophages delivered a term neonate (≥37 weeks of gestation) (Table 1).

DNA fingerprinting of amniotic fluid monocytes/macrophages that were of both fetal and maternal origin.
DNA fingerprinting of purified amniotic fluid monocytes/macrophages, the fetus (umbilical cord), and the mother (buffy coat from peripheral blood) is shown in electropherograms. Each electropherogram contains 16 genetic sites: D3S1358, TH01, D21S11, D18S51, Penta_E, D5S818, D13S317, D7S820, D16S539, CSF1PO, Penta_D, X- and Y-specific amelogenin genes, vWA, D8S1179, TPOX and FGA. Each electropherogram was separated into three sections: blue, green and black. Each color indicates the dye used for the PCR multiplex: blue represents the genes amplified using fluorescein (FL dye), green represents the genes amplified using 6-carboxy-4′,5′-dichloro-2′,7′-dimethoxyfluorescein (JOE dye) and black represents the genes amplified using tetramethylrhodamine (TMR dye). Each genetic site has STR alleles, which is represented by peaks. The number next to each STR allele (peak) is the number of repeats for each STR allele. The DNA fingerprinting of the amniotic fluid monocytes/macrophages included both fetal and maternal STR alleles; therefore, the DNA fingerprinting of amniotic fluid monocytes/macrophages resulted from the combination of the fetal and maternal DNAs.
Evidence that in a subset of cases the origin of amniotic fluid monocytes/macrophages may shift
One woman who was sampled twice had mostly fetal monocytes in her first amniotic fluid sample (sample 15, 860 cells/mm3, 100% of monocytes of fetal origin, Table 1 and Figure 1B). In this sample, the concentration of IL-6 was 73.6 ng/mL but bacteria were not detected by cultivation techniques or bacterial live/dead staining; therefore, this woman was diagnosed with intra-amniotic inflammation (Table 1). Fourteen hours later, a second amniotic fluid sample was collected during a cesarean delivery from the same woman (sample 16), which had a significantly larger number of leukocytes with an approximately 21-fold increase in the number of viable cells, and monocytes were mostly of maternal origin (18,800 cells/mm3, 96% of monocytes/macrophages of maternal origin, Table 1 and Figure 1B). In this second sample, the concentration of IL-6 was 47.7 ng/mL and U. urealyticum was detected by cultivation techniques and bacterial live/dead staining; therefore, this woman was diagnosed as having intra-amniotic infection (Table 1).
Another woman who underwent two amniotic fluid collections had monocytes/macrophages of exclusively maternal origin in both of the samples collected (samples 13 and 14, Figure 1B). In the first collection (sample 13, 2200 cells/mm3, Table 1) this woman presented with M. hominis, U. urealyticum, Prevotella spp., Streptococcus agalactiae and Streptococcus anginosus detected by conventional microbiological techniques and bacterial live/dead staining. She also had an elevated IL-6 concentration of 70.6 ng/mL and was thus diagnosed with intra-amniotic infection. In the second fluid collection done during cesarean delivery 4 h later, this woman still had monocytes exclusively of maternal origin (sample 14, Figure 1B) and had a higher number of leukocytes (6780 cells/mm3) with a 2-fold increase in viable cell counts. The second amniotic fluid sample cultured positive for M. hominis, U. urealyticum and Prevotella spp. as seen in the first collection, but S. agalactiae and S. anginosus were not detected this time.
Associations between the origin of amniotic fluid monocytes/macrophages and their localization in the placental tissues
Immunohistochemistry analysis revealed that monocyte/macrophage localization within the placental tissues of women with intra-amniotic inflammation/infection differs based on their origin. Figure 5A, D and G shows the varying abundance of monocytes/macrophages in the umbilical cord from women with amniotic fluid monocytes/macrophages of mostly fetal, maternal and mixed origin, respectively. The number of black arrows in each staining image corresponds to the relative quantity of CD14+ (brown) cells. A representative umbilical cord sample from a woman with amniotic fluid monocytes/macrophages of fetal origin shows many CD14+ cells spread diffusely throughout the Wharton’s jelly, suggesting a migration pattern from a vessel toward the amnion indicated by the direction of the black arrows (Figure 5A). In contrast, the presence of monocytes/macrophages in the umbilical cord of samples with maternal and mixed origin is minimal (Figure 5D, G). A magnification of these populations can be seen in the top-right corner, which clarifies the cellular patterns in each sample. Figure 5B, E and H shows the presence of monocytes/macrophages in the chorioamniotic membranes. Monocytes/macrophages in the chorioamniotic membranes were more abundant in those cases in which such cells were of maternal origin in the amniotic cavity (Figure 5E). Figure 5C, F and I shows the monocyte presence in the chorionic plate of the placenta from women with amniotic fluid monocytes/macrophages of fetal, maternal and mixed origin, respectively. Monocytes/macrophages in the chorionic plate were more abundant in those cases in which fetal cells were found in the amniotic cavity (Figure 5C) compared to the chorionic plates of both the maternal origin and mixed origin samples, which had very few monocytes/macrophages (Figure 5F, I).

Placental immunohistochemistry examination.
Immunohistochemistry staining of the umbilical cord (A, D, G), chorioamniotic membranes (B, E, H) and chorionic plate (C, F, I) obtained from women who either had mostly fetal (A–C), maternal (D–F) or a mixture of fetal and maternal (G–I) monocytes/macrophages in the amniotic fluid. CD14+ cells can be observed in brown. Magnification of monocyte/macrophage presence in the Wharton’s jelly is also shown in the top-right corner (A, D, G). Images were taken at 200× magnification. Scale bars are shown in all cases.
Discussion
Principal findings of the study
The principal findings of the study are as follows: (1) DNA fingerprinting revealed that more than 50% (56.25%, 9/16) of the amniotic fluid samples had mostly fetal monocytes/macrophages; (2) DNA fingerprinting showed that more than 30% (37.5%, 6/16) of the amniotic fluid samples had predominantly maternal monocytes/macrophages; (3) DNA fingerprinting indicated that one amniotic fluid sample (6.25%, 1/16) had a mixture of fetal and maternal monocytes/macrophages; (4) DNA fingerprinting revealed that a woman from whom two samples were analyzed (samples 15 and 16) had fetal monocytes/macrophages first, and as infection progressed, abundant maternal monocytes/macrophages invaded the amniotic cavity; (5) DNA fingerprinting revealed that another woman from whom two samples were analyzed (samples 13 and 14) had maternal monocytes/macrophages throughout the duration of infection; however, microbial burden was reduced between the first and second sample collections; (6) most samples from women who had predominantly amniotic fluid monocytes/macrophages of fetal origin delivered early preterm neonates (77.8% [7/9]); (7) all samples from women who had predominant amniotic fluid monocytes/macrophages of maternal origin delivered term or late preterm neonates (100% [6/6]); (8) the woman who had an evident mixture of fetal and maternal monocytes/macrophages in the amniotic fluid delivered a term neonate; (9) most of the women presented acute maternal and fetal inflammatory responses in the placenta (85.7% [12/14]); (10) women who had monocytes/macrophages of mostly fetal origin in amniotic fluid had abundant monocytes/macrophages (CD14+ cells) in the umbilical cord (Wharton’s jelly) and chorionic plate; and (11) women who had monocytes/macrophages of mostly maternal origin in amniotic fluid had abundant monocytes/macrophages (CD14+ cells) in the chorioamniotic membranes. Collectively, these data show that amniotic fluid monocytes/macrophages can either be predominantly of fetal or maternal origin, or a mixture of both, in women with intra-amniotic inflammation or infection.
Amniotic fluid monocytes/macrophages can be predominantly of fetal origin in women with intra-amniotic inflammation or infection
Amniotic fluid immune cells were first found to be of fetal origin in cases of intra-amniotic infection, indicating that invading microorganisms evoke a fetal response [77]. A subsequent study showed that fetal cells were predominantly present in amniotic fluid of non-human primates during non-infectious inflammation induced by IL-1β [78]. Recently, we demonstrated that the majority of amniotic fluid neutrophils are fetal in preterm gestations with intra-amniotic inflammation or infection [79]. Consistent with such previous reports, herein we found that monocytes/macrophages in the amniotic fluid of women with intra-amniotic inflammation or infection who delivered preterm neonates are mostly of fetal origin.
Cases in which amniotic fluid monocytes/macrophages were primarily fetal presented with either intra-amniotic infection, in which one or several microorganisms were detected, or intra-amniotic inflammation in the absence of detectable bacteria, a condition that in some cases may be classified as sterile intra-amniotic inflammation [30], [31], [33], [34]. However, this study did not include molecular microbiological techniques required for the diagnosis of such a clinical condition [30]. Sterile intra-amniotic inflammation has been associated with elevated concentrations of danger signals in amniotic fluid [33], [38], [39], [40], [41], [42], which can induce preterm birth in mice [126], [127]. Monocytes and resident macrophages act as sentinels through the detection of signals released by damaged or apoptotic cells [128], [129] and, therefore, are considered to be the primary cells that detect alarmins [130], [131], [132], [133]. Herein, we propose that amniotic fluid monocytes/macrophages could respond to alarmins in the setting of sterile intra-amniotic inflammation. One potential mechanism whereby alarmins induce sterile intra-amniotic inflammation is through the inflammasome [127], [134], [135], which is expressed by monocytes/macrophages [136] and serves as a platform for the caspase-1-mediated cleavage of pro-IL-1β and pro-IL-18 into their mature and bioactive forms [137], [138], [139], [140], [141], [142], [143], [144]. Inflammasome activation often results in pyroptosis, an inflammatory type of cell death first described in macrophages [145], [146], [147]. We recently showed that the effector molecule of pyroptosis, gasdermin D, is increased in amniotic fluid of women with sterile intra-amniotic inflammation who undergo preterm labor [148]. Therefore, it is likely that amniotic fluid monocytes/macrophages undergo inflammasome-mediated pyroptosis in women with sterile intra-amniotic inflammation. Alternatively, fetal macrophages may play central roles in the initiation of parturition [149], [150].
Interestingly, we observed a large number of CD14+ monocytes/macrophages in the Wharton’s jelly and chorionic plate, but few in the chorioamniotic membranes, of women whose amniotic fluid monocytes/macrophages were primarily of fetal origin. Previous studies demonstrated that concentrations of monocyte/macrophage chemoattractants such as monocyte chemoattractant protein-1 (MCP-1) are increased in amniotic fluid of women with preterm labor and intra-amniotic inflammation/infection [33], [59]. Given that the majority of women who had amniotic fluid monocytes/macrophages of fetal origin delivered early preterm neonates, it is tempting to suggest that monocytes/macrophages originating from the fetal vasculature can chemotactically migrate through the Wharton’s jelly and chorionic plate and subsequently infiltrate the amniotic cavity. Yet, further research is required to examine the mechanisms implicated in the recruitment of fetal monocytes/macrophages in this compartment, either in the setting of intra-amniotic infection or sterile intra-amniotic inflammation.
Amniotic fluid monocytes/macrophages can be predominantly of maternal origin in women with intra-amniotic infection
DNA fingerprinting revealed that amniotic fluid monocytes/macrophages can be predominantly of maternal origin in women with intra-amniotic infection. Notably, amniotic fluid monocytes/macrophages of predominantly maternal origin were only observed in samples that (1) were from late preterm or term gestations and (2) had detectable microorganisms. The genital mycoplasma U. urealyticum was found in all of these amniotic fluid samples for which a culture was performed (5/6). Further, U. urealyticum was either found alone or in culture with M. hominis, each of which has been associated with pregnancy complications including preterm labor/birth [21], [70], [109], [117], [118], [151], [152], [153], [154], [155], [156] and clinical chorioamnionitis [9], [12], [157], [158], [159], among others [160], [161], [162], [163], [164]. Genital mycoplasmas in the amniotic cavity and surrounding tissues can initiate a strong host inflammatory response, and thus, the existence of maternal monocytes/macrophages in the amniotic fluid suggests that they are present to participate in the host defense mechanisms against invading microbes as term approaches.
Maternal monocytes/macrophages could potentially migrate from the maternal vasculature of the decidua or the intervillous space and into the amniotic cavity. Indeed, previous studies suggested that neutrophils invade the amniotic cavity through this pathway [79], [80]. In line with this observation, the majority of cases in which amniotic fluid monocytes/macrophages were predominantly of maternal origin came from women whose placentas presented lesions of acute histologic chorioamnionitis (a placental lesion associated with the infiltration of neutrophils and macrophages in the chorioamniotic membranes [84], [165], [166]). Moreover, immunohistochemistry revealed that CD14+ monocytes/macrophages were abundant in the chorioamniotic membranes, but not in the umbilical cord or chorionic plate, in such cases. Together, these results suggest that in cases of intra-amniotic infection resulting in late preterm or term delivery, maternal monocytes/macrophages migrate to the amniotic cavity as part of the host response against microbes. Given that maternal peripheral monocytes are activated during spontaneous preterm labor [167], it is tempting to suggest that the activation status of such immune cells could serve as a readout of intra-amniotic inflammation/infection.
A mixture of fetal and maternal monocytes/macrophages can be found in the amniotic fluid of women with intra-amniotic infection
DNA fingerprinting showed that only one of our study patients (sample 9) had an evident mixture of maternal and fetal monocytes/macrophages in amniotic fluid. Similar to patients in which amniotic fluid monocytes/macrophages were predominantly maternal, this patient delivered at term with a high amniotic fluid IL-6 concentration and viable bacteria detected by live/dead staining, implying an intra-amniotic infection. Moreover, acute maternal and fetal inflammatory responses were observed in the placenta and umbilical cord. One possible explanation for the mixture of maternal and fetal monocytes/macrophages in amniotic fluid may be that the intra-amniotic infection was milder than in those cases at term with intra-amniotic infection, where most of the amniotic fluid monocytes/macrophages were of maternal origin.
It is worth mentioning that only one case (6.25%) included in this study had a mixture of maternal and fetal monocytes/macrophages in amniotic fluid. This is in contrast with our previous study in which we showed that a mixture of fetal and maternal neutrophils is found in 21% of cases with intra-amniotic infection/inflammation [79]. Therefore, it is likely that the immune processes regulating the migration of neutrophils and monocytes/macrophages into the amniotic cavity differ and, therefore, require further investigation.
The origin of amniotic fluid monocytes/macrophages may shift as intra-amniotic infection progresses
Two of the patients included in the current study underwent an initial transabdominal amniocentesis and were subsequently sampled again at delivery (samples 13 and 14, 15 and 16). For the first patient (samples 13 and 14), amniotic fluid monocytes/macrophages were consistently of primarily maternal origin, which may be due to the fact that both samples had a positive microbial culture. The first amniotic fluid sample obtained from the second patient (sample 15) was negative for microbiological culture and contained primarily fetal monocytes/macrophages, whereas amniotic fluid obtained at delivery (~14 h later, sample 16) was positive for U. urealyticum and now contained mostly monocytes/macrophages of maternal origin. These findings imply that the progression of intra-amniotic infection may be associated with a maternal response as indicated by the influx of maternal monocytes/macrophages into the amniotic cavity, which is a phenomenon also observed with amniotic fluid neutrophils [79].
Conclusion
In summary, monocytes/macrophages in amniotic fluid can be either predominantly of maternal or fetal origin, or a mixture of both. The detection of CD14+ monocytes/macrophages in the Wharton’s jelly and chorionic plate in cases where the majority of amniotic fluid monocytes/macrophages are of fetal origin provides a possible source for these cells, whereas maternal monocytes/macrophages may be primarily entering the amniotic cavity through the chorioamniotic membranes. Moreover, our findings suggest that the timing, severity and progression of intra-amniotic inflammation/infection may impact the maternal/fetal composition of amniotic fluid monocytes/macrophages. These findings provide evidence that both the fetus and the mother participate in the host defense mechanisms against intra-amniotic inflammation or infection.
Acknowledgments
We thank the physicians and nurses from the Center for Advanced Obstetrical Care and Research and the Intrapartum Unit for their invaluable support in collecting human samples. We also thank staff members of the PRB Clinical Laboratory for their help with processing the placental samples and Dr. Suzanne M. Jacques from the PRB Histology/Pathology Unit for the pathological examination of the histological sections. Finally, we thank Derek Miller for his critical readings of the manuscript.
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: This research was supported, in part, by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS) and, in part, with federal funds from the NICHD/NIH/DHHS under Contract No. HHSN275201300006C. Dr. Romero has contributed to this work as part of his official duties as an employee of the United States Federal Government. This research was also supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal and Child Health.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
1. Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci 1994;734:414–29.10.1111/j.1749-6632.1994.tb21771.xSearch in Google Scholar
2. Berkowitz GS, Blackmore-Prince C, Lapinski RH, SavitzDA. Risk factors for preterm birth subtypes. Epidemiology 1998;9:279–85.10.1097/00001648-199805000-00011Search in Google Scholar
3. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84.10.1016/S0140-6736(08)60074-4Search in Google Scholar
4. Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med 2010;362:529–35.10.1056/NEJMra0904308Search in Google Scholar
5. Gibbs RS. Diagnosis of intra-amniotic infection. Semin Perinatol 1977;1:71–7.Search in Google Scholar
6. Gibbs RS, Castillo MS, Rodgers PJ. Management of acute chorioamnionitis. Am J Obstet Gynecol 1980;136:709–13.10.1016/0002-9378(80)90445-7Search in Google Scholar
7. Gilstrap 3rd LC, Cox SM. Acute chorioamnionitis. Obstet Gynecol Clin North Am 1989;16:373–9.10.1016/S0889-8545(21)00165-0Search in Google Scholar
8. Newton ER. Chorioamnionitis and intraamniotic infection. Clin Obstet Gynecol 1993;36:795–808.10.1097/00003081-199312000-00004Search in Google Scholar PubMed
9. Romero R, Miranda J, Kusanovic JP, Chaiworapongsa T, Chaemsaithong P, Martinez A, et al. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med 2015;43:19–36.10.1515/jpm-2014-0249Search in Google Scholar PubMed PubMed Central
10. Romero R, Chaemsaithong P, Korzeniewski SJ, Tarca AL, Bhatti G, Xu Z, et al. Clinical chorioamnionitis at term II: the intra-amniotic inflammatory response. J Perinat Med 2016;44:5–22.10.1515/jpm-2015-0045Search in Google Scholar PubMed PubMed Central
11. Chaiyasit N, Romero R, Chaemsaithong P, Docheva N, Bhatti G, Kusanovic JP, et al. Clinical chorioamnionitis at term VIII: a rapid MMP-8 test for the identification of intra-amniotic inflammation. J Perinat Med 2017;45:539–50.10.1515/jpm-2016-0344Search in Google Scholar PubMed PubMed Central
12. Oh KJ, Kim SM, Hong JS, Maymon E, Erez O, Panaitescu B, et al. Twenty-four percent of patients with clinical chorioamnionitis in preterm gestations have no evidence of either culture-proven intraamniotic infection or intraamniotic inflammation. Am J Obstet Gynecol 2017;216:604 e1–11.10.1016/j.ajog.2017.02.035Search in Google Scholar
13. Gultekin-Elbir EE, Genc MR. Tinker, tailor, infection, inflammation. J Perinat Med 2019;47:259–61.10.1515/jpm-2019-0082Search in Google Scholar
14. Gultekin-Elbir EE, Ford C, Genc MR. The value of amniotic fluid analysis in patients with suspected clinical chorioamnionitis. J Perinat Med 2019;47:493–9.10.1515/jpm-2018-0306Search in Google Scholar
15. Bobitt JR, Ledger WJ. Unrecognized amnionitis and prematurity: a preliminary report. J Reprod Med 1977;19:8–12.Search in Google Scholar
16. Wallace RL, Herrick CN. Amniocentesis in the evaluation of premature labor. Obstet Gynecol 1981;57:483–6.Search in Google Scholar
17. Bobitt JR, Hayslip CC, Damato JD. Amniotic fluid infection as determined by transabdominal amniocentesis in patients with intact membranes in premature labor. Am J Obstet Gynecol 1981;140:947–52.10.1016/0002-9378(81)90090-9Search in Google Scholar
18. Wahbeh CJ, Hill GB, Eden RD, Gall SA. Intra-amniotic bacterial colonization in premature labor. Am J Obstet Gynecol 1984;148:739–43.10.1016/0002-9378(84)90558-1Search in Google Scholar
19. Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol 1988;31:553–84.10.1097/00003081-198809000-00006Search in Google Scholar
20. Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, et al. Infection in the pathogenesis of preterm labor. Semin Perinatol 1988;12:262–79.Search in Google Scholar
21. Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol 1989;161:817–24.10.1016/0002-9378(89)90409-2Search in Google Scholar
22. Romero R, Avila C, Brekus CA, Morotti R. The role of systemic and intrauterine infection in preterm parturition. Ann N Y Acad Sci 1991;622:355–75.10.1111/j.1749-6632.1991.tb37880.xSearch in Google Scholar
23. Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol 1992;79:351–7.10.1097/00006250-199203000-00005Search in Google Scholar
24. Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol 1992;166:1515–28.10.1016/0002-9378(92)91628-NSearch in Google Scholar
25. Gomez R, Romero R, Edwin SS, David C. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infect Dis Clin North Am 1997;11:135–76.10.1016/S0891-5520(05)70347-0Search in Google Scholar
26. Romero R, Gomez R, Chaiworapongsa T, Conoscenti G, Kim JC, Kim YM. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol 2001;15(Suppl 2): 41–56.10.1046/j.1365-3016.2001.00007.xSearch in Google Scholar PubMed
27. Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2001;185:1130–6.10.1067/mob.2001.117680Search in Google Scholar PubMed
28. Romero R, Espinoza J, Chaiworapongsa T, Kalache K. Infection and prematurity and the role of preventive strategies. Semin Neonatol 2002;7:259–74.10.1053/siny.2002.0121Search in Google Scholar
29. Gravett MG, Novy MJ, Rosenfeld RG, Reddy AP, Jacob T, Turner M, et al. Diagnosis of intra-amniotic infection by proteomic profiling and identification of novel biomarkers. J Am Med Assoc 2004;292:462–9.10.1001/jama.292.4.462Search in Google Scholar PubMed
30. Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol 2014;71:330–58.10.1111/aji.12189Search in Google Scholar PubMed PubMed Central
31. Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol 2014;72:458–74.10.1111/aji.12296Search in Google Scholar PubMed PubMed Central
32. Combs CA, Gravett M, Garite TJ, Hickok DE, Lapidus J, Porreco R, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol 2014;210:125 e1–15.10.1016/j.ajog.2013.11.032Search in Google Scholar PubMed
33. Romero R, Grivel JC, Tarca AL, Chaemsaithong P, Xu Z, Fitzgerald W, et al. Evidence of perturbations of the cytokine network in preterm labor. Am J Obstet Gynecol 2015;213:836 e1–18.10.1016/j.ajog.2015.07.037Search in Google Scholar PubMed PubMed Central
34. Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, et al. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med 2015;28:1343–59.10.3109/14767058.2014.954243Search in Google Scholar PubMed PubMed Central
35. Matzinger P. An innate sense of danger. Semin Immunol 1998;10:399–415.10.1006/smim.1998.0143Search in Google Scholar
36. Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol 2005;17:359–65.10.1016/j.coi.2005.06.002Search in Google Scholar
37. Lotze MT, Deisseroth A, Rubartelli A. Damage associated molecular pattern molecules. Clin Immunol 2007;124:1–4.10.1016/j.clim.2007.02.006Search in Google Scholar
38. Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol 1989;160(5 Pt 1):1117–23.10.1016/0020-7292(90)90214-6Search in Google Scholar
39. Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, et al. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol 1992;27:117–23.10.1111/j.1600-0897.1992.tb00737.xSearch in Google Scholar
40. Friel LA, Romero R, Edwin S, Nien JK, Gomez R, Chaiworapongsa T, et al. The calcium binding protein, S100B, is increased in the amniotic fluid of women with intra-amniotic infection/inflammation and preterm labor with intact or ruptured membranes. J Perinat Med 2007;35:385–93.10.1515/JPM.2007.101Search in Google Scholar
41. Chaiworapongsa T, Erez O, Kusanovic JP, Vaisbuch E, Mazaki-Tovi S, Gotsch F, et al. Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition. J Matern Fetal Neonatal Med 2008;21:449–61.10.1080/14767050802054550Search in Google Scholar
42. Romero R, Chaiworapongsa T, Alpay Savasan Z, Xu Y, Hussein Y, Dong Z, et al. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med 2011;24:1444–55.10.3109/14767058.2011.591460Search in Google Scholar
43. Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, et al. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol 1991;165(4 Pt 1):821–30.10.1016/0002-9378(91)90423-OSearch in Google Scholar
44. Romero R, Yoon BH, Mazor M, Gomez R, Diamond MP, Kenney JS, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol 1993;169:805–16.10.1016/0002-9378(93)90009-8Search in Google Scholar
45. Romero R, Yoon BH, Mazor M, Gomez R, Gonzalez R, Diamond MP, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 1993;169:839–51.10.1016/0002-9378(93)90014-ASearch in Google Scholar
46. Gomez R, Romero R, Galasso M, Behnke E, Insunza A, Cotton DB. The value of amniotic fluid interleukin-6, white blood cell count, and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. Am J Reprod Immunol 1994;32:200–10.10.1111/j.1600-0897.1994.tb01115.xSearch in Google Scholar
47. Yoon BH, Yang SH, Jun JK, Park KH, Kim CJ, Romero R. Maternal blood C-reactive protein, white blood cell count, and temperature in preterm labor: a comparison with amniotic fluid white blood cell count. Obstet Gynecol 1996;87:231–7.10.1016/0029-7844(95)00380-0Search in Google Scholar
48. Martinez-Varea A, Romero R, Xu Y, Miller D, Ahmed AI, Chaemsaithong P, et al. Clinical chorioamnionitis at term VII: the amniotic fluid cellular immune response. J Perinat Med 2017;45:523–38.10.1515/jpm-2016-0225Search in Google Scholar PubMed PubMed Central
49. Heller KA, Greig PC, Heine RP. Amniotic-fluid lactoferrin: a marker for subclinical intraamniotic infection prior to 32 weeks gestation. Infect Dis Obstet Gynecol 1995;3:179–83.10.1155/S1064744995000573Search in Google Scholar PubMed PubMed Central
50. Otsuki K, Yoda A, Saito H, Mitsuhashi Y, Toma Y, Shimizu Y, et al. Amniotic fluid lactoferrin in intrauterine infection. Placenta 1999;20:175–9.10.1053/plac.1998.0368Search in Google Scholar PubMed
51. Pacora P, Maymon E, Gervasi MT, Gomez R, Edwin SS, Yoon BH, et al. Lactoferrin in intrauterine infection, human parturition, and rupture of fetal membranes. Am J Obstet Gynecol 2000;183:904–10.10.1067/mob.2000.108882Search in Google Scholar PubMed
52. Espinoza J, Chaiworapongsa T, Romero R, Edwin S, Rathnasabapathy C, Gomez R, et al. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med 2003;13:2–21.10.1080/jmf.13.1.2.21Search in Google Scholar PubMed
53. Soto E, Espinoza J, Nien JK, Kusanovic JP, Erez O, Richani K, et al. Human beta-defensin-2: a natural antimicrobial peptide present in amniotic fluid participates in the host response to microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med 2007;20:15–22.10.1080/14767050601036212Search in Google Scholar PubMed PubMed Central
54. Para R, Romero R, Miller D, Panaitescu B, Varrey A, Chaiworapongsa T, et al. Human beta-defensin-3 participates in intra-amniotic host defense in women with labor at term, spontaneous preterm labor and intact membranes, and preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2019;202:1–16.Search in Google Scholar
55. Romero R, Parvizi ST, Oyarzun E, Mazor M, Wu YK, Avila C, et al. Amniotic fluid interleukin-1 in spontaneous labor at term. J Reprod Med 1990;35:235–8.Search in Google Scholar
56. Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest 1990;85:1392–400.10.1172/JCI114583Search in Google Scholar PubMed PubMed Central
57. Kacerovsky M, Musilova I, Stepan M, Andrys C, Drahosova M, Jacobsson B. Detection of intraamniotic inflammation in fresh and processed amniotic fluid samples with the interleukin-6 point of care test. Am J Obstet Gynecol 2015;213:435–6.10.1016/j.ajog.2015.05.039Search in Google Scholar
58. Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol 1991;165(4 Pt 1):813–20.10.1016/0002-9378(91)90422-NSearch in Google Scholar
59. Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, et al. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern Fetal Neonatal Med 2005;17:365–73.10.1080/14767050500141329Search in Google Scholar
60. Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mitchell MD. Amniotic fluid prostaglandin levels and intra-amniotic infections. Lancet 1986;1:1380.10.1016/S0140-6736(86)91685-5Search in Google Scholar
61. Romero R, Emamian M, Wan M, Quintero R, Hobbins JC, Mitchell MD. Prostaglandin concentrations in amniotic fluid of women with intra-amniotic infection and preterm labor. Am J Obstet Gynecol 1987;157:1461–7.10.1016/S0002-9378(87)80245-4Search in Google Scholar
62. Romero R, Wu YK, Mazor M, Hobbins JC, Mitchell MD. Amniotic fluid prostaglandin E2 in preterm labor. Prostaglandins Leukot Essent Fatty Acids 1988;34:141–5.10.1016/0952-3278(88)90137-8Search in Google Scholar
63. Bry K, Hallman M. Prostaglandins, inflammation, and preterm labor. J Perinatol 1989;9:60–5.Search in Google Scholar
64. Romero R, Wu YK, Mazor M, Oyarzun E, Hobbins JC, Mitchell MD. Amniotic fluid arachidonate lipoxygenase metabolites in preterm labor. Prostaglandins Leukot Essent Fatty Acids 1989;36:69–75.10.1016/0952-3278(89)90020-3Search in Google Scholar
65. Mazor M, Wiznitzer A, Maymon E, Leiberman JR, Cohen A. Changes in amniotic fluid concentrations of prostaglandins E2 and F2 alpha in women with preterm labor. Isr J Med Sci 1990;26:425–8.Search in Google Scholar
66. Hsu CD, Meaddough E, Aversa K, Hong SF, Lee IS, Bahodo-Singh RO, et al. Dual roles of amniotic fluid nitric oxide and prostaglandin E2 in preterm labor with intra-amniotic infection. Am J Perinatol 1998;15:683–7.10.1055/s-2007-999302Search in Google Scholar PubMed
67. Lee SE, Park IS, Romero R, Yoon BH. Amniotic fluid prostaglandin F2 increases even in sterile amniotic fluid and is an independent predictor of impending delivery in preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2009;22:880–6.10.1080/14767050902994648Search in Google Scholar PubMed PubMed Central
68. Maddipati KR, Romero R, Chaiworapongsa T, Zhou SL, Xu Z, Tarca AL, et al. Eicosanomic profiling reveals dominance of the epoxygenase pathway in human amniotic fluid at term in spontaneous labor. FASEB J 2014;28:4835–46.10.1096/fj.14-254383Search in Google Scholar PubMed PubMed Central
69. Park JY, Ahn TG, Lee J, Yoon BH. Elevated prostaglandin F2a concentration in amniotic fluid is an independent risk factor for intra-amniotic inflammation and adverse pregnancy outcome in patients with preterm labor. Am J Obstet Gynecol 2015;212:S360.10.1016/j.ajog.2014.10.943Search in Google Scholar
70. Park JY, Romero R, Lee J, Chaemsaithong P, Chaiyasit N, Yoon BH. An elevated amniotic fluid prostaglandin F2alpha concentration is associated with intra-amniotic inflammation/infection, and clinical and histologic chorioamnionitis, as well as impending preterm delivery in patients with preterm labor and intact membranes. J Matern Fetal Neonatal Med 2016;29:2563–72.Search in Google Scholar
71. Maddipati KR, Romero R, Chaiworapongsa T, Chaemsaithong P, Zhou SL, Xu Z, et al. Clinical chorioamnionitis at term: the amniotic fluid fatty acyl lipidome. J Lipid Res 2016;57: 1906–16.10.1194/jlr.P069096Search in Google Scholar
72. Gomez-Lopez N, Roberto R, Galaz J, Xu Y, Panaitescu B, Slutsky R, et al. Cellular immune responses in amniotic fluid of women with preterm labor and intra-amniotic infection or intra-amniotic inflammation. Am J Reprod Immunol 2019:e13171.10.1111/aji.13171Search in Google Scholar
73. Gomez-Lopez N, Romero R, Xu Y, Miller D, Unkel R, Shaman M, et al. Neutrophil extracellular traps in the amniotic cavity of women with intra-amniotic infection: a new mechanism of host defense. Reprod Sci 2017;24:1139–53.10.1177/1933719116678690Search in Google Scholar
74. Gomez-Lopez N, Romero R, Garcia-Flores V, Xu Y, Leng Y, Alhousseini A, et al. Amniotic fluid neutrophils can phagocytize bacteria: a mechanism for microbial killing in the amniotic cavity. Am J Reprod Immunol 2017;78:e12723. 10.1111/aji.12723Search in Google Scholar
75. Maymon E, Romero R, Chaiworapongsa T, Kim JC, Berman S, Gomez R, et al. Value of amniotic fluid neutrophil collagenase concentrations in preterm premature rupture of membranes. Am J Obstet Gynecol 2001;185:1143–8.10.1067/mob.2001.118166Search in Google Scholar
76. Helmig BR, Romero R, Espinoza J, Chaiworapongsa T, Bujold E, Gomez R, et al. Neutrophil elastase and secretory leukocyte protease inhibitor in prelabor rupture of membranes, parturition and intra-amniotic infection. J Matern Fetal Neonatal Med 2002;12:237–46.10.1080/jmf.12.4.237.246Search in Google Scholar
77. Sampson JE, Theve RP, Blatman RN, Shipp TD, Bianchi DW, Ward BE, et al. Fetal origin of amniotic fluid polymorphonuclear leukocytes. Am J Obstet Gynecol 1997;176(1 Pt 1):77–81.10.1016/S0002-9378(97)80015-4Search in Google Scholar
78. Macias AE, Wong SW, Sadowsky DW, Luetjens CM, Axthelm MK, Gravett MG, et al. Maternal or fetal origin of rhesus monkey (Macaca mulatta) amniotic fluid leukocytes can be identified by polymerase chain reaction using the zinc finger Y gene. Am J Primatol 2001;55:159–70.10.1002/ajp.1049Search in Google Scholar PubMed
79. Gomez-Lopez N, Romero R, Xu Y, Leng Y, Garcia-Flores V, Miller D, et al. Are amniotic fluid neutrophils in women with intraamniotic infection and/or inflammation of fetal or maternal origin? Am J Obstet Gynecol 2017;217:693 e1–e16.10.1016/j.ajog.2017.09.013Search in Google Scholar PubMed PubMed Central
80. Blanc WA. Pathology of the placenta and cord in ascending and in haematogenous infection. Ciba Found Symp 1979;77:17–38.10.1002/9780470720608.ch3Search in Google Scholar PubMed
81. Lee SD, Kim MR, Hwang PG, Shim SS, Yoon BH, Kim CJ. Chorionic plate vessels as an origin of amniotic fluid neutrophils. Pathol Int 2004;54:516–22.10.1111/j.1440-1827.2004.01659.xSearch in Google Scholar PubMed
82. Marquardt N, Ivarsson MA, Sundstrom E, Akesson E, Martini E, Eidsmo L, et al. Fetal CD103+ IL-17-producing group 3 innate lymphoid cells represent the dominant lymphocyte subset in human amniotic fluid. J Immunol 2016;197:3069–75.10.4049/jimmunol.1502204Search in Google Scholar PubMed
83. Steel JH, O’Donoghue K, Kennea NL, Sullivan MH, Edwards AD. Maternal origin of inflammatory leukocytes in preterm fetal membranes, shown by fluorescence in situ hybridisation. Placenta 2005;26:672–7.10.1016/j.placenta.2004.10.003Search in Google Scholar PubMed
84. Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol 2015;213(4 Suppl):S29–52.10.1016/j.ajog.2015.08.040Search in Google Scholar PubMed PubMed Central
85. Arenas-Hernandez M, Romero R, Xu Y, Panaitescu B, Garcia-Flores V, Miller D, et al. Effector and activated T cells induce preterm labor and birth that is prevented by treatment with progesterone. J Immunol 2019;202:2585–608.10.4049/jimmunol.1801350Search in Google Scholar PubMed PubMed Central
86. Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol 2008;26:421–52.10.1146/annurev.immunol.26.021607.090326Search in Google Scholar PubMed PubMed Central
87. Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity 2010;32:593–604.10.1016/j.immuni.2010.05.007Search in Google Scholar PubMed
88. Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014;40:274–88.10.1016/j.immuni.2014.01.006Search in Google Scholar PubMed PubMed Central
89. Xu Y, Romero R, Miller D, Kadam L, Mial TN, Plazyo O, et al. An M1-like macrophage polarization in decidual tissue during spontaneous preterm labor that is attenuated by Rosiglitazone treatment. J Immunol 2016;196:2476–91.10.4049/jimmunol.1502055Search in Google Scholar PubMed PubMed Central
90. Casadei R, D’Ablaing 3rd G, Kaplan BJ, Schwinn CP. A cytologic study of amniotic fluid. Acta Cytol 1973;17:289–98.10.1097/00006254-197402000-00017Search in Google Scholar
91. Cutz E, Conen PE. Macrophages and epithelial cells in human amniotic fluid: transmission and scanning electron microscopic study. Am J Anat 1978;151:87–101.10.1002/aja.1001510108Search in Google Scholar
92. Tyden O, Bergstrom S, Nilsson BA. Origin of amniotic fluid cells in mid-trimester pregnancies. Br J Obstet Gynaecol 1981;88:278–86.10.1111/j.1471-0528.1981.tb00982.xSearch in Google Scholar
93. Sutherland GR, Brock DJ, Scrimgeour JB. Letter: Amniotic-fluid macrophages and anencephaly. Lancet 1973;2:1098–9.10.1016/S0140-6736(73)92720-7Search in Google Scholar
94. Sutherland GR, Brock DJ, Scrimgeour JB. Amniotic fluid macrophages and the antenatal diagnosis of anencephaly and spina bifida. J Med Genet 1975;12:135–7.10.1136/jmg.12.2.135Search in Google Scholar PubMed PubMed Central
95. Gosden C, Brock DJ. Combined use of alphafetoprotein and amniotic fluid cell morphology in early prenatal diagnosis of fetal abnormalities. J Med Genet 1978;15:262–70.10.1136/jmg.15.4.262Search in Google Scholar PubMed PubMed Central
96. Papp Z, Bell JE. Uncultured cells in amniotic fluid from normal and abnormal foetuses. Clin Genet 1979;16:282–90.10.1111/j.1399-0004.1979.tb01001.xSearch in Google Scholar PubMed
97. Chapman PA, Blenkinsopp WK, Lewis BV. The detection of neural tube closure defects by exfoliative cytology of amniotic fluid. Acta Cytol 1981;25:367–72.10.1097/00006254-198203000-00005Search in Google Scholar
98. Chapman PA. Cytology as a means of detecting neural tube defects. Med Lab Sci 1982;39:215–22.Search in Google Scholar
99. Morrison JJ, Klein N, Chitty LS, Kocjan G, Walshe D, Goulding M, et al. Intra-amniotic inflammation in human gastroschisis: possible aetiology of postnatal bowel dysfunction. Br J Obstet Gynaecol 1998;105:1200–4.10.1111/j.1471-0528.1998.tb09975.xSearch in Google Scholar PubMed
100. Guibourdenche J, Berrebi D, Vuillard E, de Lagausie P, Aigrain Y, Oury JF, et al. Biochemical investigations of bowel inflammation in gastroschisis. Pediatr Res 2006;60:565–8.10.1203/01.pdr.0000242344.22638.94Search in Google Scholar PubMed
101. Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J Infect Dis 1982;145:1–8.10.1093/infdis/145.1.1Search in Google Scholar
102. Gibbs RS, Dinsmoor MJ, Newton ER, Ramamurthy RS. A randomized trial of intrapartum versus immediate postpartum treatment of women with intra-amniotic infection. Obstet Gynecol 1988;72:823–8.10.1097/00006250-198812000-00001Search in Google Scholar
103. Gibbs RS, Duff P. Progress in pathogenesis and management of clinical intraamniotic infection. Am J Obstet Gynecol 1991;164(5 Pt 1):1317–26.10.1016/0002-9378(91)90707-XSearch in Google Scholar
104. Romero R, Chaemsaithong P, Korzeniewski SJ, Kusanovic JP, Docheva N, Martinez-Varea A, et al. Clinical chorioamnionitis at term III: how well do clinical criteria perform in the identification of proven intra-amniotic infection? J Perinat Med 2016;44:23–32.10.1515/jpm-2015-0044Search in Google Scholar
105. Romero R, Gomez-Lopez N, Kusanovic JP, Pacora P, Panaitescu B, Erez O, et al. Clinical chorioamnionitis at term: new insights into the etiology, microbiology, and the fetal, maternal and amniotic cavity inflammatory responses. Nogyogyaszati Szuleszeti Tovabbkepzo Szemle 2018;20:103–12.Search in Google Scholar
106. Frey HA, Klebanoff MA. The epidemiology, etiology, and costs of preterm birth. Semin Fetal Neonatal Med 2016;21:68–73.10.1016/j.siny.2015.12.011Search in Google Scholar
107. Chaemsaithong P, Romero R, Korzeniewski SJ, Dong Z, Yeo L, Hassan SS, et al. A point of care test for the determination of amniotic fluid interleukin-6 and the chemokine CXCL-10/IP-10. J Matern Fetal Neonatal Med 2015;28:1510–9.10.3109/14767058.2014.961417Search in Google Scholar
108. Gomez-Lopez N, Romero R, Maymon E, Kusanovic JP, Panaitescu B, Miller D, et al. Clinical chorioamnionitis at term IX: in vivo evidence of intra-amniotic inflammasome activation. J Perinat Med 2019;47:276–87.10.1515/jpm-2018-0271Search in Google Scholar
109. Romero R, Shamma F, Avila C, Jimenez C, Callahan R, Nores J, et al. Infection and labor. VI. Prevalence, microbiology, and clinical significance of intraamniotic infection in twin gestations with preterm labor. Am J Obstet Gynecol 1990;163:757–61.10.1016/0002-9378(90)91063-ISearch in Google Scholar
110. Romero R, Ghidini A, Mazor M, Behnke E. Microbial invasion of the amniotic cavity in premature rupture of membranes. Clin Obstet Gynecol 1991;34:769–78.10.1097/00003081-199112000-00013Search in Google Scholar PubMed
111. Romero R, Nores J, Mazor M, Sepulveda W, Oyarzun E, Parra M, et al. Microbial invasion of the amniotic cavity during term labor. Prevalence and clinical significance. J Reprod Med 1993;38:543–8.Search in Google Scholar
112. Kacerovsky M, Musilova I, Andrys C, Hornychova H, Pliskova L, Kostal M, et al. Prelabor rupture of membranes between 34 and 37 weeks: the intraamniotic inflammatory response and neonatal outcomes. Am J Obstet Gynecol 2014;210:325 e1–10.10.1016/j.ajog.2013.10.882Search in Google Scholar PubMed
113. Chaemsaithong P, Romero R, Korzeniewski SJ, Martinez-Varea A, Dong Z, Yoon BH, et al. A point of care test for interleukin-6 in amniotic fluid in preterm prelabor rupture of membranes: a step toward the early treatment of acute intra-amniotic inflammation/infection. J Matern Fetal Neonatal Med 2016;29:360–7.10.3109/14767058.2015.1006621Search in Google Scholar
114. Chaemsaithong P, Romero R, Korzeniewski SJ, Martinez-Varea A, Dong Z, Yoon BH, et al. A rapid interleukin-6 bedside test for the identification of intra-amniotic inflammation in preterm labor with intact membranes. J Matern Fetal Neonatal Med 2016;29:349–59.10.3109/14767058.2015.1006620Search in Google Scholar
115. Pacora P, Romero R, Erez O, Maymon E, Panaitescu B, Kusanovic JP, et al. The diagnostic performance of the beta-glucan assay in the detection of intra-amniotic infection with Candida species. J Matern Fetal Neonatal Med 2019;32:1703–20.10.1080/14767058.2017.1416083Search in Google Scholar
116. Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol 1995;172:960–70.10.1016/0002-9378(95)90028-4Search in Google Scholar
117. Yoon BH, Romero R, Lim JH, Shim SS, Hong JS, Shim JY, et al. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am J Obstet Gynecol 2003;189:919–24.10.1067/S0002-9378(03)00839-1Search in Google Scholar
118. DiGiulio DB, Romero R, Amogan HP, Kusanovic JP, Bik EM, Gotsch F, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One 2008;3:e3056.10.1371/journal.pone.0003056Search in Google Scholar
119. DiGiulio DB, Gervasi MT, Romero R, Vaisbuch E, Mazaki-Tovi S, Kusanovic JP, et al. Microbial invasion of the amniotic cavity in pregnancies with small-for-gestational-age fetuses. J Perinat Med 2010;38:495–502.10.1515/jpm.2010.076Search in Google Scholar
120. Romero R, Jimenez C, Lohda AK, Nores J, Hanaoka S, Avila C, et al. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol 1990;163:968–74.10.1016/0002-9378(90)91106-MSearch in Google Scholar
121. Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mazor M, et al. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol 1988;159:114–9.10.1016/0002-9378(88)90503-0Search in Google Scholar
122. Kim MJ, Romero R, Gervasi MT, Kim JS, Yoo W, Lee DC, et al. Widespread microbial invasion of the chorioamniotic membranes is a consequence and not a cause of intra-amniotic infection. Lab Invest 2009;89:924–36.10.1038/labinvest.2009.49Search in Google Scholar PubMed PubMed Central
123. Romero R, Kim YM, Pacora P, Kim CJ, Benshalom-Tirosh N, Jaiman S, et al. The frequency and type of placental histologic lesions in term pregnancies with normal outcome. J Perinat Med 2018;46:613–30.10.1515/jpm-2018-0055Search in Google Scholar PubMed PubMed Central
124. Redline RW. Classification of placental lesions. Am J Obstet Gynecol 2015;213(4 Suppl):S21–8.10.1016/j.ajog.2015.05.056Search in Google Scholar PubMed
125. Khong TY, Mooney EE, Ariel I, Balmus NC, Boyd TK, Brundler MA, et al. Sampling and definitions of placental lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch Pathol Lab Med 2016;140:698–713.10.5858/arpa.2015-0225-CCSearch in Google Scholar PubMed
126. Gomez-Lopez N, Romero R, Plazyo O, Panaitescu B, Furcron AE, Miller D, et al. Intra-amniotic administration of HMGB1 induces spontaneous preterm labor and birth. Am J Reprod Immunol 2016;75:3–7.10.1111/aji.12443Search in Google Scholar PubMed PubMed Central
127. Gomez-Lopez N, Romero R, Garcia-Flores V, Leng Y, Miller D, Hassan SS, et al. Inhibition of the NLRP3 inflammasome can prevent sterile intra-amniotic inflammation, preterm labor/birth and adverse neonatal outcomes. Biol Reprod 2019;100:1306–18.10.1093/biolre/ioy264Search in Google Scholar PubMed PubMed Central
128. Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol 2013;14:986–95.10.1038/ni.2705Search in Google Scholar PubMed PubMed Central
129. Peiseler M, Kubes P. Macrophages play an essential role in trauma-induced sterile inflammation and tissue repair. Eur J Trauma Emerg Surg 2018;44:335–49.10.1007/s00068-018-0956-1Search in Google Scholar PubMed
130. Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006;440:237–41.10.1038/nature04516Search in Google Scholar PubMed
131. Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 2008;320:674–7.10.1126/science.1156995Search in Google Scholar PubMed PubMed Central
132. Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 2008;9:847–56.10.1038/ni.1631Search in Google Scholar PubMed PubMed Central
133. Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010;464:1357–61.10.1038/nature08938Search in Google Scholar
134. Plazyo O, Romero R, Unkel R, Balancio A, Mial TN, Xu Y, et al. HMGB1 induces an inflammatory response in the chorioamniotic membranes that is partially mediated by the inflammasome. Biol Reprod 2016;95:130.10.1095/biolreprod.116.144139Search in Google Scholar
135. Strauss JF, 3rd, Romero R, Gomez-Lopez N, Haymond-Thornburg H, Modi BP, Teves ME, et al. Spontaneous preterm birth: advances toward the discovery of genetic predisposition. Am J Obstet Gynecol 2018;218:294–314 e2.10.1016/j.ajog.2017.12.009Search in Google Scholar
136. Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 2002;10:417–26.10.1016/S1097-2765(02)00599-3Search in Google Scholar
137. Black RA, Kronheim SR, Merriam JE, March CJ, Hopp TP. A pre-aspartate-specific protease from human leukocytes that cleaves pro-interleukin-1 beta. J Biol Chem 1989;264:5323–6.10.1016/S0021-9258(18)83546-3Search in Google Scholar
138. Kostura MJ, Tocci MJ, Limjuco G, Chin J, Cameron P, Hillman AG, et al. Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proc Natl Acad Sci U S A 1989;86:5227–31.10.1073/pnas.86.14.5227Search in Google Scholar PubMed PubMed Central
139. Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 1992;356:768–74.10.1038/356768a0Search in Google Scholar PubMed
140. Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, Greenstreet TA, et al. Molecular cloning of the interleukin-1 beta converting enzyme. Science 1992;256:97–100.10.1126/science.1373520Search in Google Scholar PubMed
141. Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, et al. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science 1997;275:206–9.10.1126/science.275.5297.206Search in Google Scholar PubMed
142. Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, et al. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature 1997;386:619–23.10.1038/386619a0Search in Google Scholar PubMed
143. Dinarello CA. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann N Y Acad Sci 1998;856:1–11.10.1111/j.1749-6632.1998.tb08307.xSearch in Google Scholar
144. Fantuzzi G, Dinarello CA. Interleukin-18 and interleukin-1 beta: two cytokine substrates for ICE (caspase-1). J Clin Immunol 1999;19:1–11.10.1023/A:1020506300324Search in Google Scholar
145. Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol 2001;9:113–4.10.1016/S0966-842X(00)01936-3Search in Google Scholar
146. Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun 2005;73:1907–16.10.1128/IAI.73.4.1907-1916.2005Search in Google Scholar PubMed PubMed Central
147. Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 2009;7:99–109.10.1038/nrmicro2070Search in Google Scholar PubMed PubMed Central
148. Gomez-Lopez N, Romero R, Tarca AL, Miller D, Panaitescu B, Schwenkel G, et al. Gasdermin D: evidence of pyroptosis in spontaneous preterm labor with sterile intra-amniotic inflammation or intra-amniotic infection. Am J Reprod Immunol 2019:e13184. doi:10.1111/aji.13184.10.1111/aji.13184Search in Google Scholar PubMed PubMed Central
149. Condon JC, Jeyasuria P, Faust JM, Mendelson CR. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci U S A 2004;101:4978–83.10.1073/pnas.0401124101Search in Google Scholar PubMed PubMed Central
150. Montalbano AP, Hawgood S, Mendelson CR. Mice deficient in surfactant protein A (SP-A) and SP-D or in TLR2 manifest delayed parturition and decreased expression of inflammatory and contractile genes. Endocrinology 2013;154:483–98.10.1210/en.2012-1797Search in Google Scholar PubMed PubMed Central
151. Bashiri A, Horowitz S, Huleihel M, Hackmon R, Dukler D, Mazor M. Elevated concentrations of interleukin-6 in intra-amniotic infection with Ureaplasma urealyticum in asymptomatic women during genetic amniocentesis. Acta Obstet Gynecol Scand 1999;78:379–82.Search in Google Scholar
152. Gerber S, Vial Y, Hohlfeld P, Witkin SS. Detection of Ureaplasma urealyticum in second-trimester amniotic fluid by polymerase chain reaction correlates with subsequent preterm labor and delivery. J Infect Dis 2003;187:518–21.10.1086/368205Search in Google Scholar PubMed
153. Jacobsson B, Mattsby-Baltzer I, Andersch B, Bokstrom H, Holst RM, Nikolaitchouk N, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women with preterm prelabor rupture of membranes. Acta Obstet Gynecol Scand 2003;82:423–31.10.1034/j.1600-0412.2003.00157.xSearch in Google Scholar PubMed
154. Allen-Daniels MJ, Serrano MG, Pflugner LP, Fettweis JM, Prestosa MA, Koparde VN, et al. Identification of a gene in Mycoplasma hominis associated with preterm birth and microbial burden in intraamniotic infection. Am J Obstet Gynecol 2015;212:779 e1–13.10.1016/j.ajog.2015.01.032Search in Google Scholar PubMed PubMed Central
155. Yoneda N, Yoneda S, Niimi H, Ueno T, Hayashi S, Ito M, et al. Polymicrobial amniotic fluid infection with Mycoplasma/Ureaplasma and other bacteria induces severe intra-amniotic inflammation associated with poor perinatal prognosis in preterm labor. Am J Reprod Immunol 2016;75:112–25.10.1111/aji.12456Search in Google Scholar PubMed
156. Oh KJ, Romero R, Park JY, Kang J, Hong JS, Yoon BH. A high concentration of fetal fibronectin in cervical secretions increases the risk of intra-amniotic infection and inflammation in patients with preterm labor and intact membranes. J Perinat Med 2019;47:288–303.10.1515/jpm-2018-0351Search in Google Scholar PubMed PubMed Central
157. Kwak DW, Cho HY, Kwon JY, Park YW, Kim YH. Usefulness of maternal serum C-reactive protein with vaginal Ureaplasma urealyticum as a marker for prediction of imminent preterm delivery and chorioamnionitis in patients with preterm labor or preterm premature rupture of membranes. J Perinat Med 2015;43:409–15.10.1515/jpm-2014-0142Search in Google Scholar PubMed
158. Feng L, Allen TK, Marinello WP, Murtha AP. Infection-induced thrombin production: a potential novel mechanism for preterm premature rupture of membranes (PPROM). Am J Obstet Gynecol 2018;219:101 e1–12.10.1016/j.ajog.2018.04.014Search in Google Scholar PubMed PubMed Central
159. Oh KJ, Romero R, Park JY, Hong JS, Yoon BH. The earlier the gestational age, the greater the intensity of the intra-amniotic inflammatory response in women with preterm premature rupture of membranes and amniotic fluid infection by Ureaplasma species. J Perinat Med 2019;47:516–27.10.1515/jpm-2019-0003Search in Google Scholar PubMed PubMed Central
160. Donders GGG, Ruban K, Bellen G, Petricevic L. Mycoplasma/Ureaplasma infection in pregnancy: to screen or not to screen. J Perinat Med 2017;45:505–15.10.1515/jpm-2016-0111Search in Google Scholar PubMed
161. Vallely LM, Egli-Gany D, Pomat W, Homer CS, Guy R, Wand H, et al. Adverse pregnancy and neonatal outcomes associated with Neisseria gonorrhoeae, Mycoplasma genitalium, M. hominis, Ureaplasma urealyticum and U. parvum: a systematic review and meta-analysis protocol. Br Med J Open 2018;8:e024175.10.1136/bmjopen-2018-024175Search in Google Scholar PubMed PubMed Central
162. Latino MA, Botta G, Badino C, Maria D, Petrozziello A, Sensini A, et al. Association between genital mycoplasmas, acute chorioamnionitis and fetal pneumonia in spontaneous abortions. J Perinat Med 2018;46:503–8.10.1515/jpm-2016-0305Search in Google Scholar PubMed
163. Cao CJ, Wang YF, Fang DM, Hu Y. Relation between mycoplasma infection and recurrent spontaneous abortion. Eur Rev Med Pharmacol Sci 2018;22:2207–11.Search in Google Scholar
164. Randis TM, Rice MM, Myatt L, Tita ATN, Leveno KJ, Reddy UM, et al. Incidence of early-onset sepsis in infants born to women with clinical chorioamnionitis. J Perinat Med 2018;46:926–33.10.1515/jpm-2017-0192Search in Google Scholar PubMed PubMed Central
165. Bae GE, Hong JS, Kim JS, Park HY, Jang JY, Kim YS, et al. Differential immunophenotype of macrophages in acute and chronic chorioamnionitis. J Perinat Med 2017;45:483–91.10.1515/jpm-2015-0353Search in Google Scholar PubMed
166. Doster RS, Sutton JA, Rogers LM, Aronoff DM, Gaddy JA. Streptococcus agalactiae induces placental macrophages to release extracellular traps loaded with tissue remodeling enzymes via an oxidative burst-dependent mechanism. MBio 2018;9. pii: e02084-18. doi: 10.1128/mBio.02084-18.10.1128/mBio.02084-18Search in Google Scholar PubMed PubMed Central
167. Paquette AG, Shynlova O, Kibschull M, Price ND, Lye SJ. Global Alliance to Prevent Prematurity and Stillbirth Systems Biology of Preterm Birth Team. Comparative analysis of gene expression in maternal peripheral blood and monocytes during spontaneous preterm labor. Am J Obstet Gynecol 2018;218:345 e1–30.10.1016/j.ajog.2017.12.234Search in Google Scholar PubMed
©2019 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Review
- The role of complement in preterm birth and prematurity
- Original Articles – Obstetrics
- Variation in C-reactive protein at 1 month post-partum by etiology of preterm birth: selective identification of those at risk for both poor pregnancy outcome and future health complications
- Procedure related risk of premature delivery and fetal growth reduction following amniocentesis, transcervical and transabdominal chorionic villus sampling: a retrospective study
- Cervical length at 31–34 weeks of gestation: transvaginal vs. transperineal ultrasonographic approach
- The origin of amniotic fluid monocytes/macrophages in women with intra-amniotic inflammation or infection
- Placental elasticity assessment by point shear wave elastography in pregnancies with intrauterine growth restriction
- A 17-years analysis of terminations of pregnancy ≥14 weeks of gestation in a German level 1 perinatal center
- Simulation of an impacted fetal head extraction during cesarean section: description of the creation and evaluation of a new training program
- Academic tweeting in #ObGyn. Where do we stand?
- Original Articles – Fetus
- Fetal heart examination at the time of 13 weeks scan: a 5 years’ prospective study
- Comparison of fetal cardiac functions between small-for-gestational age fetuses and late-onset growth-restricted fetuses
- Original Article – Newborn
- Protocols for early discharging of premature infants: an empirical assessment on safety and savings
- Letter to the Editor
- Maternal blood pressure levels prepartum correlate with neonatal birth weight in preeclampsia
Articles in the same Issue
- Frontmatter
- Review
- The role of complement in preterm birth and prematurity
- Original Articles – Obstetrics
- Variation in C-reactive protein at 1 month post-partum by etiology of preterm birth: selective identification of those at risk for both poor pregnancy outcome and future health complications
- Procedure related risk of premature delivery and fetal growth reduction following amniocentesis, transcervical and transabdominal chorionic villus sampling: a retrospective study
- Cervical length at 31–34 weeks of gestation: transvaginal vs. transperineal ultrasonographic approach
- The origin of amniotic fluid monocytes/macrophages in women with intra-amniotic inflammation or infection
- Placental elasticity assessment by point shear wave elastography in pregnancies with intrauterine growth restriction
- A 17-years analysis of terminations of pregnancy ≥14 weeks of gestation in a German level 1 perinatal center
- Simulation of an impacted fetal head extraction during cesarean section: description of the creation and evaluation of a new training program
- Academic tweeting in #ObGyn. Where do we stand?
- Original Articles – Fetus
- Fetal heart examination at the time of 13 weeks scan: a 5 years’ prospective study
- Comparison of fetal cardiac functions between small-for-gestational age fetuses and late-onset growth-restricted fetuses
- Original Article – Newborn
- Protocols for early discharging of premature infants: an empirical assessment on safety and savings
- Letter to the Editor
- Maternal blood pressure levels prepartum correlate with neonatal birth weight in preeclampsia

