The first trimester aneuploidy biochemical markers in IVF/ICSI patients have no additional benefit compared to spontaneous conceptions in the prediction of pregnancy complications
-
Iwona Szymusik
, Katarzyna Kosinska-Kaczynska
Abstract
Objectives:
The aim of this study was to determine if the levels of biochemical aneuploidy markers in in vitro fertilisation (IVF)/intracytoplasmic sperm injection (ICSI) pregnancies differ from those in spontaneous pregnancies and to verify if biochemical markers could predict pregnancy outcome in IVF/ICSI gestations.
Methods:
This was a prospective observational study performed in a group of 551 patients who underwent a combined first trimester prenatal screening (ultrasound scan and serum markers). All patients were divided into two groups according to the mode of conception: IVF/ICSI pregnancies (study group) and spontaneous conceptions (control group). The concentrations of first trimester biochemical markers were presented as multiples of median (MoM) and were compared between the study and control groups. Analysed pregnancy complications included: preterm delivery (PTD), small for gestational age (SGA), gestational hypertension (GH), preeclampsia (PE) and gestational diabetes (GDM).
Results:
The analysis was performed on 183 IVF/ICSI and 368 spontaneously conceived gestations, with complete data regarding obstetric outcome. There were no significant differences in the concentrations of biochemical markers between the analysed groups. Pregnancy-associated plasma protein-A (PAPP-A) levels were lower in hypertensive than in normotensive patients, although the difference was not significant. Twenty-three patients had GDM (12.5%), 16 had GH or PE (8.7%), SGA was diagnosed in 18 (9.8%) and 25 delivered preterm (13.6%).
Conclusions:
The trend for lower PAPP-A MoM was visible in all affected patients, although the results did not reach statistical significance. The first trimester biochemical markers in assisted reproduction technique (ART) pregnancies do not seem to have additional effect on predicting the risk of pregnancy complications.
Introduction
The number of children born after assisted reproduction techniques (ARTs) exceeded 6.5 million according to 2016 estimates, with the proportion of in vitro fertilisation (IVF) ranging from 0.8% to 4.1% of all deliveries in different countries [1]. In Poland, over 10,000 IVF/intracytoplasmic sperm injection (ICSI) procedures were initiated in 2012, with a 95% proportion of ICSI – it means that, with a success rate of over 30%, more than 3000 IVF pregnant women each year seek perinatal surveillance [2].
Since the introduction of IVF, various concerns have been raised regarding the poorer perinatal outcome of ART singletons, especially in terms of preterm delivery (PTD) [1], [3], [4]. However, its causes are not completely clear and seem to be multifactorial. One of the causes is more advanced maternal age. The more advanced age already implicates a greater risk not only for aneuploidies, but also for various pathologies of pregnancy.
The current first trimester prenatal screening program concentrates on identifying women at a high risk of chromosomal abnormalities. It incorporates maternal age, an ultrasound screening at 11+0–13+6 weeks of gestation with the measurement of nuchal translucency (NT) thickness, additional markers of aneuploidy and two serum markers: pregnancy-associated plasma protein-A (PAPP-A) and free β human chorionic gonadotropin (β-hCG). The combination of NT and biochemical markers allows a 90% detection rate with only 5% false-positive rate for most common aneuploidies [5]. It is known that some conditions, such as ART procedures, can alter the results of biochemical markers, leading to overestimation of the risk of aneuploidy. However, the IVF population is more reluctant to undergo invasive procedures; therefore, all risk calculations should be corrected for the mode of conception [6], [7]. PAPP-A plays a role in the development and function of the placenta, therefore it can be used as a marker of complications related to placentation, such as gestational hypertension (GH), preeclampsia (PE) or intrauterine growth restriction (IUGR) [8].
The primary aim of this study was to determine if the levels of biochemical aneuploidy markers in IVF/ICSI pregnancies differ from those in spontaneous pregnancies. The secondary objective was to verify if biochemical markers could predict pregnancy complications in IVF/ICSI gestations.
Methods
This prospective observational study was carried out between July 2013 and December 2016 at the First Department of Obstetrics and Gynecology, Medical University of Warsaw. Pregnant women were recruited during a routine ultrasound scan at 11–13+6 weeks. All patients responded to a questionnaire on maternal age, racial origin (Caucasian, African, Asian or mixed), mode of conception (spontaneous or ART – only fresh IVF/ICSI transfers were taken into account), parity and medical history. Maternal weight and height were measured on the day of the scan. Only women who decided to undergo a combined prenatal screening (ultrasound and two first trimester serum markers: PAPP-A and free β-hCG) were included for further analysis. The exclusion criteria were: multiple pregnancy, major foetal anomalies, a known abnormal karyotype or maternal history of severe medical conditions, pregnancies obtained by oocyte donation or frozen/thawed embryo transfer.
A blood sample for free β-hCG and PAPP-A concentration was taken on the day of the scan upon consent of the patient (the analysis of biochemical markers in Poland is not reimbursed in a healthy population below 35 years of age). These biochemical markers were evaluated using the Delfia Xpress® System by Perkin Elmer Life (Turku, Finland).
All patients underwent a routine first trimester scan in accordance with The Fetal Medicine Foundation [all ultrasonographers were certified by The Fetal Medicine Foundation (www.fetalmedicine.org)]. Patients were given their adjusted individual risk for trisomy 21, 18 and 13 using the Astraia software GmBH (Germany). Those with high risk were given the option of invasive testing for foetal karyotype. Data on pregnancy outcome were collected from the hospital maternity records or directly from patients.
All the initially included patients were divided into two groups according to the mode of conception: IVF/ICSI pregnancies (study group) and spontaneous conceptions (control group) (Figure 1). The concentrations of first trimester biochemical markers presented as multiples of median (MoM) were compared between the groups. Afterwards, the above-mentioned markers were also compared among patients presenting with selected pregnancy complications.
Analysed pregnancy complications included: PTD, small for gestational age (SGA), GH, PE and gestational diabetes (GDM).
PTD was defined as labour occurring before completion of 37 weeks of gestation. SGA was defined as foetal weight below the 10th percentile for gestational age. PE was diagnosed according to the American College of Obstetricians and Gynecologists (ACOG) guidelines [9]. GDM was diagnosed according to the recommendations of Polish Gynaecological Society [10].
Statistical analysis was performed using the MedCalc software version.17.5. Data were presented as means±standard deviations (SDs) medians or number of subjects and percentages. Statistical analysis was performed using the Fisher exact test for categorical variables and Mann-Whitney U-test for continuous variables. P-values <0.05 were considered significant and all tests were two-tailed.
Results
The final analysis was performed on 183 IVF/ICSI and 368 spontaneously conceived gestations, with complete data regarding obstetric outcome. There were several significant differences in baseline characteristics between the study and the control groups (Table 1). The patients in the study group were significantly older and had higher pre-gravid body mass index (BMI). PTD was found in 13.6% of the patients in the study group (n=25) and 6.5% in the control group (n=25) (P=0.005). Newborns in the study group had a significantly lower birthweight.
Baseline characteristics of the studied groups.
| IVF/ICSI group n=183 | Control group n=368 | P-value | |||||
|---|---|---|---|---|---|---|---|
| Median | Average | SD | Median | Average | SD | ||
| Age (years) | 35 | 35.2 | 3.7 | 33 | 32.4 | 4.1 | <0.001 |
| BMI (kg/m2) | 24.9 | 25.43 | 4.1 | 22.27 | 23.09 | 3.3 | <0.001 |
| Weeks of gestation at delivery | 39 | 37.83 | 3.9 | 39 | 38.76 | 4 | <0.001 |
| Neonatal birthweight | 3299 | 3238 | 421 | 3415 | 3353 | 497 | 0.028 |
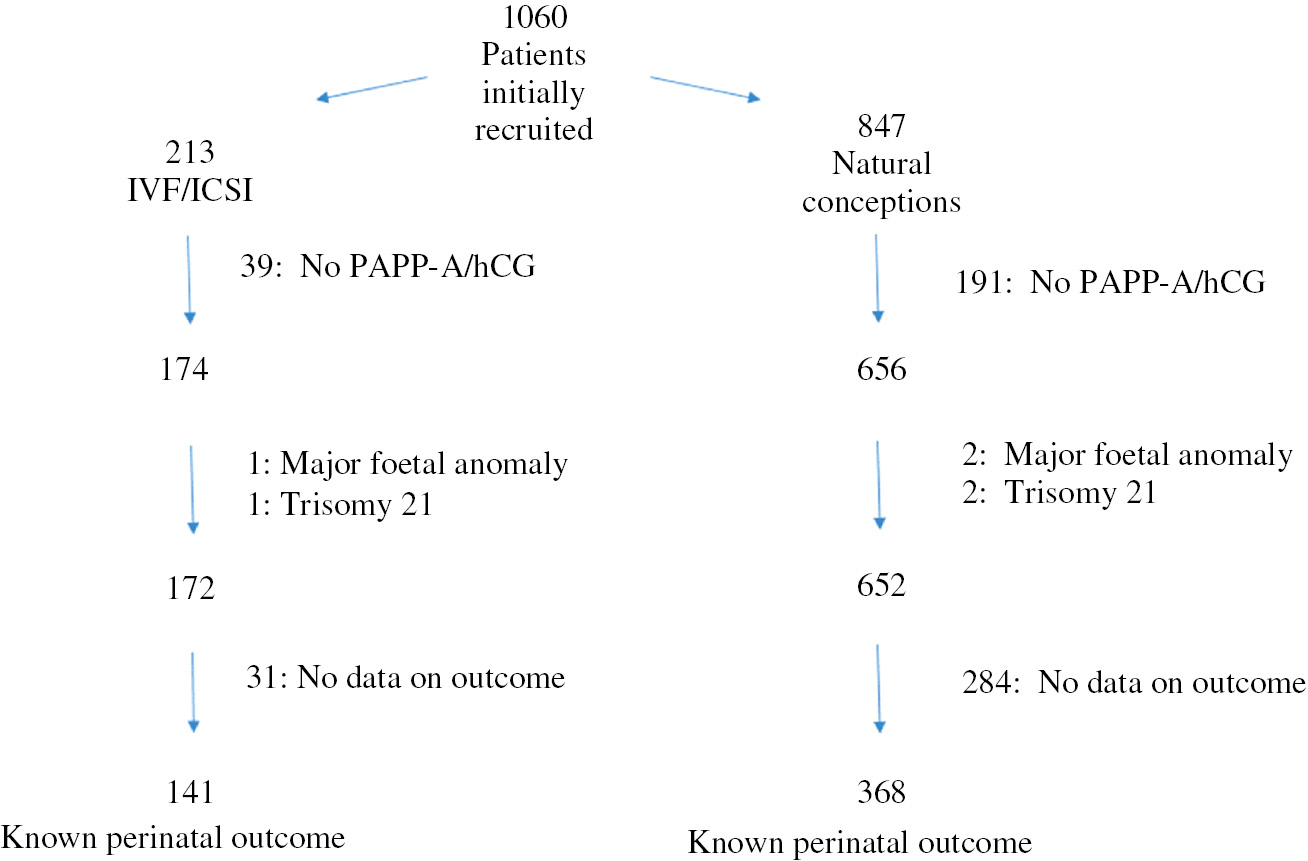
Recruitment of the patients from the IVF/ICSI and control groups.
The median PAPP-A in the study group did not differ from that in controls and equalled (1.02 MoM vs. 1.06 MoM; P=0.8). The free β-hCG serum concentration was also similar in the study and control groups (1.18 MoM vs. 1.17 MoM, respectively; P=0.9). The biochemical markers’ concentrations of both groups are presented in Figure 2 and Table 2.
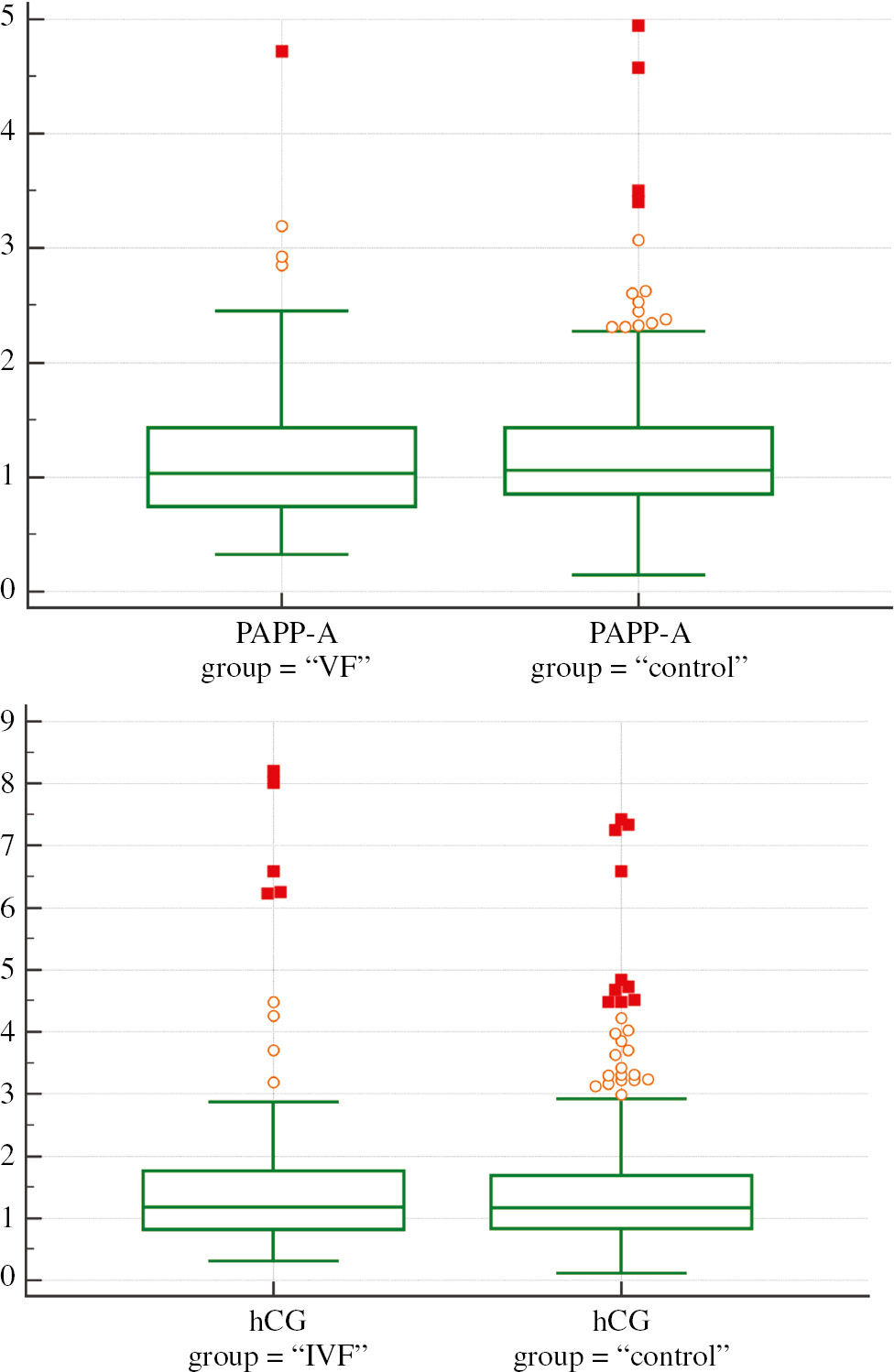
First trimester results of PAPP-A MoM and β-hCG MoM in the studied groups.
*In a box-and-whisker plot, the central box represents the values from the lower to upper quartile (25–75 percentile). The middle line represents the median. A line extends from the minimum to the maximum value, excluding “outside” and “far out” values which are displayed as separate points. An outside value is defined as a value that is smaller than the lower quartile minus 1.5 times the interquartile range, or larger than the upper quartile plus 1.5 times the interquartile range (inner fences). A far out value is defined as a value that is smaller than the lower quartile minus 3 times the interquartile range, or larger than the upper quartile plus 3 times the interquartile range (outer fences). These values are plotted with a different marker in the warning colour.
Comparison of first trimester results of PAPP-A MoM and β-hCG MoM in the studied groups.
| PAPP-A MoM | β-hCG MoM | |||
|---|---|---|---|---|
| IVF/ICSI | Controls | IVF/ICSI | Controls | |
| n | 183 | 368 | 183 | 368 |
| Average | 1.14 | 1.93 | 1.51 | 1.45 |
| 95% CI | 1.06–1.24 | 1.14–1.25 | 1.29–1.75 | 1.35–1.56 |
| SD | 0.62 | 0.55 | 1.13 | 1.06 |
| Median | 1.02 | 1.06 | 1.18 | 1.17 |
| Minimum | 0.32 | 0.11 | 0.3 | 0.11 |
| Maximum | 4.72 | 4.95 | 8.2 | 7.42 |
The evaluation of pregnancy complications in the study group was performed and afterwards compared with controls. In the IVF group, 23 (12.5%) patients had GDM in comparison to 31 (8%) in the study group (P=0.08); 16 (8.7%) had GH or PE in comparison to 14 controls (3.6%; P=0.01). SGA was diagnosed in 18 IVF pregnancies (9.8%) and in 31 (8%) controls (P=0.4). The relation between biochemical markers’ concentrations and study outcomes was analysed. The results are presented in Table 3A and B. There were no significant differences in biochemical markers’ concentrations between patients with and without pregnancy complications in the study group. PAPP-A levels were lower in hypertensive than in normotensive patients, although the difference was not significant. In women with GDM, free β-hCG concentration was lower than in healthy pregnant patients in the study group; however, the difference was also not significant. When biochemical markers’ concentrations were compared between complicated/uncomplicated IVF and control pregnancies, no significant differences were found. A detailed analysis is presented in Table 3B.
Comparison of PAPP-A MoM and β-hCG MoM values in various pregnancy complications within the IVF/ICSI group (183 cases).
| PAPP-A | hCG | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes (n) | Median | No (n) | Median | P-value | Yes (n) | Median | No (n) | Median | P-value | |
| GDM | 23 (12.5%) | 0.95 | 160 | 1.09 | 0.3 | 23 | 0.97 | 160 | 1.28 | 0.06 |
| GH and/or PE | 16 (8.7%) | 0.77 | 167 | 1.03 | 0.08 | 16 | 1.42 | 167 | 1.12 | 0.2 |
| SGA | 18 (9.8%) | 1.21 | 165 | 1.07 | 0.5 | 18 | 1.28 | 165 | 1.19 | 0.7 |
| PTD | 25 (13.6%) | 0.86 | 158 | 1.02 | 0.3 | 25 | 1.24 | 158 | 1.22 | 0.9 |
GDM=Gestational diabetes, GH=gestational hypertension, PE=preeclampsia, SGA=small for gestational age, PTD=preterm delivery.
Comparison of PAPP-A MoM and β-hCG MoM values in various pregnancy complications within the control group (386 cases).
| PAPP-A | hCG | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes (n) | Median | No (n) | Median | P-valuea | P-valueb | P-valuec | Yes (n) | Median | No (n) | Median | P-valuea | P-valueb | P-valuec | |
| GDM | 31 (8%) | 1.12 | 355 | 1.06 | 0.6 | 0.7 | 0.9 | 31 | 1.11 | 335 | 1.18 | 0.8 | 0.7 | 0.4 |
| GH and/or PE | 14 (3.6%) | 0.81 | 372 | 1.09 | 0.04 | 0.8 | 0.9 | 14 | 1.2 | 372 | 1.18 | 0.9 | 0.6 | 0.7 |
| SGA | 31 (8%) | 1.18 | 346 | 1.03 | 0.6 | 0.8 | 0.9 | 31 | 1.22 | 346 | 1.2 | 0.9 | 0.8 | 1 |
| PTD | 25 (6.5%) | 0.83 | 358 | 1.02 | 0.3 | 0.9 | 0.9 | 25 | 1.18 | 358 | 1.23 | 0.3 | 0.7 | 0.9 |
aP-value of affected vs. unaffected controls. bP-value of affected controls vs. affected IVF gestations. cP-value of unaffected controls vs. unaffected IVF gestations.
Discussion
To the best of our knowledge, this study is the first regarding the results of the first trimester screening in IVF/ICSI gestations and prediction of pregnancy complications in the Polish population. According to published studies, the concentrations of PAPP-A in IVF/ICSI gestations tend to be lower than in spontaneous pregnancies [6], [7], [11], [12], [13], [14], [15], [16], [17], [18]. However, some authors have shown that PAPP-A concentrations are comparable in ART and spontaneous gestations [19], [20], [21], [22], [23]. We have also demonstrated that the concentrations of PAPP-A in IVF/ICSI pregnancies in the first trimester are similar to those in controls. Regarding the levels of free β-hCG, there were no significant differences between the analysed groups, which is also in accordance with the previously reported data [6], [11], [14], [17]. A recently published meta-analysis points out that the clinicians should be cautious in interpreting first trimester serum results in IVF gestations. Lanes et al. selected 40 articles, of which 28 discussed PAPP-A in ART, but only 14 authors presented their results in MoM – the majority pointed to the lower concentrations in IVF pregnancies. Data regarding free β-hCG are still inconclusive, as the results vary from lower, through similar, to higher concentrations among the included studies [24]. Cavoretto et al. performed another meta-analysis, which sustained the results of Lanes et al. regarding PAPP-A. Nevertheless, they concluded that free β-hCG seems to be significantly higher in ART gestations, but only after ICSI [25]. Our results refer mostly to ICSI, as according to national EIM data ICSI contributes to around 95% of ART procedures in Poland [2].
As a result of lower PAPP-A, all the ART pregnancies may have a higher false-positive rate of genetic screening for trisomies, in particular trisomy 21. Therefore, according to previous suggestions by Nicolaides and his group [7], all the risk estimations should be adjusted for the mode of conception. All the patients included in the presented study were adjusted for that upon risk calculation. The issue of genetic risk calculation in IVF/ICSI pregnancies has been extensively studied [6], [18], [26], [27]. Data suggest that the altered results of the first trimester biochemistry, especially PAPP-A, can be used as additional markers of further perinatal complications [8], [28], [29], [30]. Ong et al. published the results of a large cohort of patients followed up from the first trimester till the delivery. They showed that PAPP-A MoM values were significantly lower in pregnancies resulting in miscarriage, GH, growth restriction and GDM, but not in the group of preterm deliveries [8]. Dugoff et al. [28] reported very similar results, including significant differences also for the PTD. Nevertheless, no such large analyses have been performed so far for IVF/ICSI gestations. As the ART singleton gestations have a proven worse obstetric outcome [1], [3], [4], it is worth verifying if PAPP-A/free β-hCG concentrations could help clinicians predict the outcome.
The infertile population undergoing IVF treatment is a very complex and heterogenic group, with a long list of confounding factors possibly influencing the results of screening markers. Various authors have suggested the influence of underlying infertility, multiple corpora lutea, unknown metabolic disturbances or even an altered foeto-placental development in infertility conditions – however, also of unknown origin [6], [12], [13], [15], [31]. Finally, it is known that ovarian stimulation leads to altered endocrine profiles of the patients during fresh embryo transfer procedures, which may influence implantation and early placentation [1], [32], [33]. It is therefore very difficult to determine which of the above might really be the cause of the problem.
Only single authors followed up the pregnancies from the first trimester to the delivery and compared PAPP-A/free β-hCG with the perinatal outcome. Bender et al. showed a significantly higher rate of SGA in IVF (10%) and ICSI group (8.2%) in comparison to the general population (4.6%) – the rate is comparable to the one obtained in our study (9.8%). The authors further analysed PAPP-A concentrations in SGA and appropriate for gestational age (AGA) groups and they found significantly lower levels of PAPP-A in SGA. However, that result did not depend on the mode of conception. They also underlined that decreased mean levels of PAPP-A in IVF/ICSI pregnancies were not the consequence of impaired foetal growth [15]. Zhong et al. evaluated the impact of PAPP-A and β-hCG on adverse perinatal outcome in a group of 265 ART pregnancies. In contrary, they observed that IVF/ICSI pregnancies with low PAPP-A had a higher risk of a SGA newborn and PTD than controls with low PAPP-A concentrations, suggesting that the mode of conception does matter [34].
In the presented study, the results for SGA did not differ significantly from AGA among IVF/ICSI gestations, but this might be due to the small number of subjects. Nevertheless, PAPP-A concentrations do not seem to be an additional help in predicting SGA in ART with a better accuracy in comparison to general population. As GH, PE and growth restriction have a possible common underlying cause of impaired placentation [35], we decided to combine the above pathologies and verify PAPP-A MoM of affected and non-affected ART gestations. Although the results did not reach significance, probably due to the number of studied subjects, the trend for lower PAPP-A MoM was visible in affected patients (0.72 vs. 1.08; P=0.07). Moreover, there seems to be no additional advantage of biochemical markers in IVF gestations in comparison to spontaneously conceived ones with regard to prediction of pregnancy complications, as there were no differences in PAPP-A and free β-hCG concentrations between affected and unaffected cases between the two groups.
The advantage of the presented study is its prospective character. It is also one of the very few papers referring to the final perinatal outcome in IVF patients with regard to the results of the first trimester biochemical markers.
Based on our study, the first trimester biochemical markers in ART pregnancies do not seem to have any additional effect on predicting the risk of pregnancy complications. Therefore, while trying to predict pathologies resulting from impaired placentation, the clinicians should not underestimate or overestimate the risk based on the mode of conception. It seems that regarding this particular issue IVF/ICSI and spontaneously conceived pregnancies could be treated equally. Future studies should concentrate on large cohorts of ART patients with complete data referring to the first trimester biochemistry and the perinatal outcome.
Author’s statement
Conflict of interest: Authors state no conflict of interest.
Material and methods: Informed consent: Informed consent has been obtained from all individuals included in this study.
Ethical approval: The research related to human subject use has complied with all the relevant national regulations, and institutional policies, and is in accordance with the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee.
References
[1] Pinborg A, Wennerholm UB, Romundstad LB, Loft A, Aittomaki K, Söderström-Anttila V, et al. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum Reprod Update. 2013;19:87–104.10.1093/humupd/dms044Search in Google Scholar PubMed
[2] Calhaz-Jorge C, de Geyter C, Kupka MS, de Mouzon J, Erb K, Mocanu E, et al. Assisted reproductive technology in Europe, 2012: results generated from European registers by ESHRE. European IVF-Monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Hum Reprod. 2016;31:1638–5210.1093/humrep/dew151Search in Google Scholar PubMed
[3] Helmerhorst FM, Perquin DA, Donker D, Keirse MJ. Perinatal outcome of singletons and twins after assisted conception: a systematic review of controlled studies.Br Med J. 2004;328:261.10.1136/bmj.37957.560278.EESearch in Google Scholar PubMed PubMed Central
[4] Jackson RA, Gibson KA, Wu YW, Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol. 2004;103:551–63.10.1097/01.AOG.0000114989.84822.51Search in Google Scholar PubMed
[5] Nicolaides KH. Screening for fetal aneuploidies at 11 to 13 weeks. Prenat Diagn. 2011;31:7–17.10.1002/pd.2637Search in Google Scholar PubMed
[6] Gjerris AC, Loft A, Pinborg A, Christiansen M, Tabor A. First-trimester screening markers are altered in pregnancies conceived after IVF/ICSI. Ultrasound Obstet Gynecol. 2009;33:8–17.10.1002/uog.6254Search in Google Scholar PubMed
[7] Liao AW, Heath V, Kametas N, Spencer K, Nicolaides KH. First-trimester screening for trisomy 21 in singleton pregnancies achieved by assisted reproduction. Hum Reprod. 2001;16:1501–4.10.1093/humrep/16.7.1501Search in Google Scholar PubMed
[8] Ong CY, Liao AW, Spencer K, Munim S, Nicolaides KH. First trimester maternal serum free beta human chorionic gonadotrophin and pregnancy associated plasma protein A as predictors of pregnancy complications. BJOG. 2000;107:1265–70.10.1111/j.1471-0528.2000.tb11618.xSearch in Google Scholar PubMed
[9] American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–31.Search in Google Scholar
[10] Wender-Ozegowska E, Bomba-Opon D, Brazert J, Celewicz Z, Czajkowski K, Karowicz-Bilinska A, et al. Actualization of Polish Gyneacological Society standards of medical care in management of women with diabetes. Ginekol Pol. 2014;85:476–8.Search in Google Scholar
[11] Orlandi F, Rossi C, Allegra A, Krantz D, Hallahan T, Orlandi E, et al. First trimester screening with free beta-hCG, PAPP-A and nuchal translucency in pregnancies conceived with assisted reproduction. Prenat Diagn. 2002;22:718–21.10.1002/pd.390Search in Google Scholar PubMed
[12] Maymon R, Shulman A. Integrated first- and second-trimester Down syndrome screening test among unaffected IVF pregnancies. Prenat Diagn. 2004;24:125–9.10.1002/pd.809Search in Google Scholar PubMed
[13] Hui PW, Lam YH, Tang MH, NG EH, Yeung WS, Ho PC. Maternal serum pregnancy-associated plasma protein-A and free beta-human chorionic gonadotrophin in pregnancies conceived with fresh and frozen-thawed embryos from in vitro fertilization and intracytoplasmic sperm injection. Prenat Diagn. 2005;25:390–3.10.1002/pd.1169Search in Google Scholar
[14] Tul N, Novak-Antolic Z. Serum PAPP-A levels at 10-14 weeks of gestation are altered in women after assisted conception. Prenat Diagn. 2006;26:1206–11.10.1002/pd.1589Search in Google Scholar
[15] Bender F, Hecken J, Reinsberg J, Berg C, van der Ven H, Gembruch U, et al. Altered first-trimester screening markers after IVF/ICSI: no relationship with small-for-gestational-age and number of embryos transferred. Reprod Biomed Online. 2010;20:516–22.10.1016/j.rbmo.2009.12.025Search in Google Scholar
[16] Engels MA, Kooij M, Schats R, Twisk JW, Blankenstein MA, van Vugt JM. First-trimester serum marker distribution in singleton pregnancies conceived with assisted reproduction. Prenat Diagn. 2010;30:372–7.10.1002/pd.2495Search in Google Scholar
[17] Amor DJ, Xu JX, Halliday JL, Francis I, Healy DL, Breheny S, et al. Pregnancies conceived using assisted reproductive technologies (ART) have low levels of pregnancy-associated plasma protein-A (PAPP-A) leading to a high rate of false-positive results in first trimester screening for Down syndrome. Hum Reprod. 2009;24:1330–8.10.1093/humrep/dep046Search in Google Scholar
[18] Bonne S, Sauleau E, Sananes N, Akaladios C, Rongières C, Pirrello O. Influence of medically assisted reproduction techniques on crown-rump length and biochemical markers of trisomy 21 in the first trimester of pregnancy. Fertil Steril. 2016;105:410–6.10.1016/j.fertnstert.2015.10.031Search in Google Scholar
[19] Wøjdemann KR, Larsen SO, Shalmi A, Sundberg K, Christiansen M, Tabor A. First trimester screening for Down syndrome and assisted reproduction: no basis for concern. Prenat Diagn. 2001;21:563–5.10.1002/pd.124Search in Google Scholar
[20] Bellver J, Lara C, Soares SR, Ramírez A, Pellicer A, Remohí J, et al. First trimester biochemical screening for Down’s syndrome in singleton pregnancies conceived by assisted reproduction. Hum Reprod. 2005;20:2623–7.10.1093/humrep/dei107Search in Google Scholar
[21] Ghisoni L, Ferrazzi E, Castagna C, Levi Setti PE, Masini AC, Pigni A. Prenatal diagnosis after ART success: the role of early combined screening tests in counselling pregnant patients. Placenta. 2003;24:S99–103.10.1016/S0143-4004(03)00178-4Search in Google Scholar
[22] Lambert-Messerlian G, Dugoff L, Vidaver J, Canick JA, Malone FD, Ball RH, et al. First- and second-trimester Down syndrome screening markers in pregnancies achieved through assisted reproductive technologies (ART): a FASTER trial study. Prenat Diagn. 2006;26:672–8.10.1002/pd.1469Search in Google Scholar PubMed
[23] Gong M, Shi H, Zhang Y, Ming L. Prenatal screening at 11–13+6 weeks in assisted reproductive technology singleton pregnancies and those conceived naturally. J Obstet Gynaecol Res. 2015;41:1514–9.10.1111/jog.12752Search in Google Scholar
[24] Lanes A, Huang T, Sprague AE, Leader A, Potter B, Walker M. Maternal serum screening markers and nuchal translucency measurements in in vitro fertilization pregnancies: a systematic review. Fertil Steril. 2016;106:1463–9.10.1016/j.fertnstert.2016.07.1120Search in Google Scholar
[25] Cavoretto P, Giorgione V, Cipriani S, Viganò P, Candiani M, Inversetti A, et al. Review. Nuchal translucency measurement, free β-hCG and PAPP-A concentrations in IVF/ICSI pregnancies: systematic review and meta-analysis. Prenat Diagn. 2017;37:540–55.10.1002/pd.5052Search in Google Scholar
[26] Gjerris AC, Tabor A, Loft A, Christiansen M, Pinborg A. First trimester prenatal screening among women pregnant after IVF/ICSI. Hum Reprod Update. 2012;18:350–9.10.1093/humupd/dms010Search in Google Scholar
[27] Engels MA, Pajkrt E, Groot DT, Schats R, Twisk JW, van Vugt JM. Validation of correction factors for serum markers for first-trimester Down syndrome screening in singleton pregnancies conceived with assisted reproduction. Fetal Diagn Ther. 2013;34:217–24.10.1159/000355527Search in Google Scholar
[28] Dugoff L, Hobbins JC, Malone FD, Porter TF, Luthy D, Comstock CH, et al. First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: a population-based screening study (the FASTER Trial). Am J Obstet Gynecol. 2004;191:1446–51.10.1016/j.ajog.2004.06.052Search in Google Scholar
[29] Smith GC, Stenhouse EJ, Crossley JA, Aitken DA, Cameron AD, Connor JM. Early pregnancy levels of pregnancy-associated plasma protein a and the risk of intrauterine growth restriction, premature birth, preeclampsia, and stillbirth. J Clin Endocrinol Metab. 2002;87:1762–7.10.1210/jcem.87.4.8430Search in Google Scholar
[30] Pihl K, Sørensen TL, Nørgaard-Pedersen B, Larsen SO, Nguyen TH, Krebs L, et al. First-trimester combined screening for Down syndrome: prediction of low birth weight, small for gestational age and pre-term delivery in a cohort of non-selected women. Prenat Diagn. 2008;28:247–53.10.1002/pd.1946Search in Google Scholar
[31] Weisz B, Rodeck CH. An update on antenatal screening for Down’s syndrome and specific implications for assisted reproduction pregnancies. Hum Reprod Update. 2006; 12:513–8.10.1093/humupd/dml021Search in Google Scholar
[32] Romundstad LB, Romundstad PR, Sunde A, von Düring V, Skjaerven R, Gunnell D, et al. Effects of technology or maternal factors on perinatal outcome after assisted fertilisation: a population-based cohort study. Lancet. 2008;372:737–43.10.1016/S0140-6736(08)61041-7Search in Google Scholar
[33] Pandey S, Shetty A, Hamilton M, Bhattacharya S, Maheshwari A. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: a systematic review and meta-analysis. Hum Reprod Update. 2012;18:485–503.10.1093/humupd/dms018Search in Google Scholar PubMed
[34] Zhong Y, Bradshaw R, Stanley AP, Odibo AO. The impact of assisted reproductive technology on the association between first-trimester pregnancy-associated plasma protein a and human chorionic gonadotropin and adverse pregnancy outcomes. Am J Perinatol. 2011;28:347–54.10.1055/s-0030-1268707Search in Google Scholar PubMed
[35] Kosinski P, Bomba-Opon D, Brawura Biskupski Samaha R, Wielgos M. Suitable application of selected biochemical and biophysical markers during the first trimester screening. Neuro Endocrinol Lett. 2014;35:440–4.Search in Google Scholar
©2018 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial
- Fetal anomalies – From prenatal diagnosis to therapy
- Corner of Academy
- The first trimester aneuploidy biochemical markers in IVF/ICSI patients have no additional benefit compared to spontaneous conceptions in the prediction of pregnancy complications
- Research articles – Obstetrics
- Assessment of strain and dyssynchrony in normal fetuses using speckle tracking echocardiography – comparison of three different ultrasound probes
- Inborn errors of metabolism in a cohort of pregnancies with non-immune hydrops fetalis: a single center experience
- Cytogenetic analysis in fetuses with late onset abnormal sonographic findings
- Fetal MRI, lower acceptance by women in research vs. clinical setting
- Neurological complications after therapy for fetal-fetal transfusion syndrome: a systematic review of the outcomes at 24 months
- Evaluation of management and surgical outcomes in pregnancies complicated by acute cholecystitis
- Pentaerythrityltetranitrate (PETN) improves utero- and feto-placental Doppler parameters in pregnancies with impaired utero-placental perfusion in mid-gestation – a secondary analysis of the PETN-pilot trial
- Early onset preeclampsia is associated with an elevated mean platelet volume (MPV) and a greater rise in MPV from time of booking compared with pregnant controls: results of the CAPE study
- Effect of maternal age, height, BMI and ethnicity on birth weight: an Italian multicenter study
- Risk factors and classification of stillbirth in a Middle Eastern population: a retrospective study
- Selective IUGR in dichorionic twins: what can Doppler assessment and growth discordancy say about neonatal outcomes?
- Early fetal megacystis: Is it possible to predict the prognosis in the first trimester?
- Research articles – Fetus
- A poor long-term neurological prognosis is associated with abnormal cord insertion in severe growth-restricted fetuses
- Birth-weight centiles and the risk of serious adverse neonatal outcomes at term
- Short communication
- Maternal and neonatal vitamin D deficiency and transient tachypnea of the newborn in full term neonates
- Acknowledgment
Articles in the same Issue
- Frontmatter
- Editorial
- Fetal anomalies – From prenatal diagnosis to therapy
- Corner of Academy
- The first trimester aneuploidy biochemical markers in IVF/ICSI patients have no additional benefit compared to spontaneous conceptions in the prediction of pregnancy complications
- Research articles – Obstetrics
- Assessment of strain and dyssynchrony in normal fetuses using speckle tracking echocardiography – comparison of three different ultrasound probes
- Inborn errors of metabolism in a cohort of pregnancies with non-immune hydrops fetalis: a single center experience
- Cytogenetic analysis in fetuses with late onset abnormal sonographic findings
- Fetal MRI, lower acceptance by women in research vs. clinical setting
- Neurological complications after therapy for fetal-fetal transfusion syndrome: a systematic review of the outcomes at 24 months
- Evaluation of management and surgical outcomes in pregnancies complicated by acute cholecystitis
- Pentaerythrityltetranitrate (PETN) improves utero- and feto-placental Doppler parameters in pregnancies with impaired utero-placental perfusion in mid-gestation – a secondary analysis of the PETN-pilot trial
- Early onset preeclampsia is associated with an elevated mean platelet volume (MPV) and a greater rise in MPV from time of booking compared with pregnant controls: results of the CAPE study
- Effect of maternal age, height, BMI and ethnicity on birth weight: an Italian multicenter study
- Risk factors and classification of stillbirth in a Middle Eastern population: a retrospective study
- Selective IUGR in dichorionic twins: what can Doppler assessment and growth discordancy say about neonatal outcomes?
- Early fetal megacystis: Is it possible to predict the prognosis in the first trimester?
- Research articles – Fetus
- A poor long-term neurological prognosis is associated with abnormal cord insertion in severe growth-restricted fetuses
- Birth-weight centiles and the risk of serious adverse neonatal outcomes at term
- Short communication
- Maternal and neonatal vitamin D deficiency and transient tachypnea of the newborn in full term neonates
- Acknowledgment

