Abstract
Objectives
Recently, there is an increased number of reports being published on Methotrexate (MTX) related cutaneous manifestations. We aimed to identify and critically appraise descriptive studies describing the MTX related skin manifestations, treatment approach, and their outcomes.
Methodology
An extensive literature search was performed in the PubMed, Embase, and Scopus databases from inception to April 2021 without any restrictions along with the bibliographic search of included studies, grey literature search, and a snowball search was performed in Google and Google Scholar to identify the relevant literature. Descriptive studies reporting MTX related cutaneous manifestations were considered for the review. The study selection, data extraction, and quality assessment were conducted by two independent reviewers and any disagreements were settled by consensus with the third reviewer.
Results
31 out of 8,365 descriptive studies including 38 patients (22 females and 16 males) aged between 12 and 78 years prescribed for the management of rheumatoid arthritis, ankylosing spondylitis, and psoriasis were included in this review. Toxic epidermal necrolysis (TEN), papular eruption, vasculitis, erosions of psoriasis, ulcerated psoriatic plaques, local reactions, keratinocyte dystrophy, erythema multiforme, drug rash with eosinophilia and systemic symptoms, Steven Johnson syndrome and photosensitive dermatitis were the majority of MTX induced cutaneous reactions. Immediate withdrawal of MTX, providing appropriate care with anti-inflammatory, topical steroids, and supplementation with folic acid were reported to be effective for the management of the MTX related cutaneous manifestations.
Conclusions
Clinicians and healthcare professionals should be aware of possible acute cutaneous drug reactions induced by MTX to avoid further consequences and fatal conditions. Immediate withdrawal of MTX and supportive care were reported as an efficacious therapeutic management of acute cutaneous drug reactions.
PROSPERO Registration number
CRD42020220038.
-
Research funding: We did not receive any funding for conducting this systematic literature review.
-
Author contributions: Conceptualization: Muhammed Rashid (MR), Mohammed Zuber (MZ); Study design: MR, MZ, Harikrishna (H); Literature search: MR, MZ, AH; Study Selection & Data Extraction: MZ, AH, Vidhyashree (V); Quality Assessment: MR, MZ; Manuscript original drafting: MR, MZ, H, V; Editing: MR, Manik Chhabra (MC); Manuscript review and revising: MR, Rajesh Venkataraman; Sathish Kumar; Guarantor: MR. All authors reviewed, discussed, and agreed to their individual contributions ahead of this time. Also all authors agreed their position in author listing.
-
Competing interests: The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
-
Informed consent: Not applicable.
-
Ethical approval: Not applicable.
Appendix 1: Detailed search strategy used in various databases
Appendix 1A: PubMed search strategy
| Search | Query | Items found |
|---|---|---|
| #1 | Methotrexate [MeSH Terms] | 38,119 |
| #2 | ((((((((((((Amethopterin) OR Methotrexate, (D)-Isomer) OR Methotrexate, (DL)-Isomer) OR Mexate) OR Methotrexate Sodium) OR Sodium, Methotrexate) OR Methotrexate, Sodium Salt) OR Methotrexate, Disodium Salt) OR Methotrexate Hydrate) OR Hydrate, Methotrexate) OR Methotrexate, Dicesium Salt) OR Dicesium Salt Methotrexate) | 24,511 |
| #3 | #1 OR #2 | 47,736 |
| #4 | Skin Manifestations [MeSH Terms] | 72,767 |
| #5 | (((Manifestation, Skin) OR Manifestations, Skin) OR Skin Manifestation) | 97,829 |
| #6 | Exanthema [MeSH Terms] | 7,657 |
| #7 | ((((Skin Rash) OR Rash, Skin) OR Rash) OR Exanthem) | 34,148 |
| #8 | Stevens-Johnson Syndrome [MeSH Terms] | 5,340 |
| #9 | (((((((((((((((((((((((((((((((((Stevens Johnson Syndrome) OR Stevens-Johnson Syndrome Toxic Epidermal Necrolysis Spectrum) OR Stevens Johnson Syndrome Toxic Epidermal Necrolysis Spectrum) OR Toxic Epidermal Necrolysis Stevens-Johnson Syndrome Spectrum) OR Toxic Epidermal Necrolysis Stevens Johnson Syndrome) OR Toxic Epidermal Necrolysis Stevens Johnson Syndrome Spectrum) OR Toxic Epidermal Necrolysis Stevens-Johnson Syndrome) OR Stevens Johnson Syndrome Toxic Epidermal Necrolysis) OR Stevens-Johnson Syndrome Toxic Epidermal Necrolysis) OR Mycoplasma-Induced Stevens-Johnson Syndrome) OR Mycoplasma Induced Stevens Johnson Syndrome) OR Stevens-Johnson Syndrome, Mycoplasma-Induced) OR Syndrome, Mycoplasma-Induced Stevens-Johnson) OR Mycoplasma-Induced Stevens Johnson Syndrome) OR Drug-Induced Stevens Johnson Syndrome) OR Drug Induced Stevens Johnson Syndrome) OR Drug-Induced Stevens-Johnson Syndrome) OR Drug-Induced Stevens-Johnson Syndromes) OR Stevens-Johnson Syndrome, Drug-Induced) OR Stevens-Johnson Syndromes, Drug-Induced) OR Epidermal Necrolysis, Toxic) OR Epidermal Necrolyses, Toxic) OR Necrolyses, Toxic Epidermal) OR Necrolysis, Toxic Epidermal) OR Toxic Epidermal Necrolyses) OR Toxic Epidermal Necrolysis) OR Scalded Skin Syndrome, Nonstaphylococcal) OR Lyell’s Syndrome) OR Lyell Syndrome) OR Lyell’s Syndromes) OR Syndrome, Lyell’s) OR Syndromes, Lyell’s) OR Nonstaphylococcal Scalded Skin Syndrome) | 7,241 |
| #10 | Drug Eruption [MeSH Terms] | 23,746 |
| #11 | (((((((((((((((((((((((((Drug Eruption) OR Eruption, Drug) OR Eruptions, Drug) OR Dermatitis Medicamentosa) OR Dermatitis, Adverse Drug Reaction) OR Morbilliform Drug Reaction) OR Drug Reaction, Morbilliform) OR Drug Reactions, Morbilliform) OR Morbilliform Drug Reactions) OR Reaction, Morbilliform Drug) OR Reactions, Morbilliform Drug) OR Maculopapular Exanthem) OR Exanthem, Maculopapular) OR Exanthems, Maculopapular) OR Maculopapular Exanthems) OR Morbilliform Exanthem) OR Exanthem, Morbilliform) OR Exanthems, Morbilliform) OR Morbilliform Exanthems) OR Maculopapular Drug Eruption) OR Drug Eruption, Maculopapular) OR Drug Eruptions, Maculopapular) OR Eruption, Maculopapular Drug) OR Eruptions, Maculopapular Drug) OR Maculopapular Drug Eruptions) | 30,496 |
| #12 | ((((((((((((Dermatitis, Contact, Allergic) OR Dermatitis, Allergic Eczematous) OR Allergic Eczematous Dermatitides) OR Allergic Eczematous Dermatitis) OR Dermatitides, Allergic Eczematous) OR Eczematous Dermatitides, Allergic) OR Eczematous Dermatitis, Allergic) OR Allergic Contact Dermatitis) OR Allergic Contact Dermatitides) OR Contact Dermatitides, Allergic) OR Contact Dermatitis, Allergic) OR Dermatitides, Allergic Contact) | 17,658 |
| #13 | (((((((Fasciitides, Necrotizing) OR Necrotizing Fasciitides) OR Necrotizing Fasciitis) OR Fascitis, Necrotizing) OR Fascitides, Necrotizing) OR Necrotizing Fascitides) OR Necrotizing Fascitis) | 5,129 |
| #14 | #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 | 1,75,014 |
| #15 | #3 AND #14 | 1063 |
Appendix 1B: Scopus search strategy
| Search | Query | Items found |
|---|---|---|
| #1 | ‘methotrexate’ | 2,88,441 |
| #2 | “Amethopterin” OR “Methotrexate Sodium” OR “Sodium, Methotrexate” OR “Methotrexate, Sodium Salt” OR “Methotrexate, Disodium Salt” OR “Methotrexate Hydrate” OR “Hydrate, Methotrexate” OR “Methotrexate, Dicesium Salt” OR “Dicesium Salt Methotrexate” | 3,978 |
| #3 | #1 OR #2 | 2,89,709 |
| #4 | “skin manifestations” OR “exanthema” OR “stevens-johnson syndrome” OR “toxic epidermal necrolysis” OR “SJS” OR “TEN” OR “drug eruption” OR “maculopapular drug eruptions” OR “morbilliform exanthems” OR “dermatitides” OR “necrotizing fasciitis” | 20,71,644 |
| #5 | “cutaneous adverse drug reactions” OR “cutaneous adverse drug effects” OR “adverse cutaneous drug reactions” OR “adverse cutaneous drug effects” OR “cutaneous toxicity” OR “skin toxicity” | 33,280 |
| #6 | #4 OR #5 | 20,95,438 |
| #7 | #3 AND #6 | 30,886 |
| #8 | TITLE-ABS-KEY (“Case Report” OR “Case Series”) | 26,64,768 |
| #9 | #7 AND #8 | 3,211 |
| #10 | #9 AND AND (EXCLUDE (DOCTYPE , “re”) OR EXCLUDE (DOCTYPE , “cp”) OR EXCLUDE (DOCTYPE , “ch”) OR EXCLUDE (DOCTYPE, “sh”) OR EXCLUDE (DOCTYPE, “tb”)) | 2,787 |
Appendix 1C: Embase search strategy
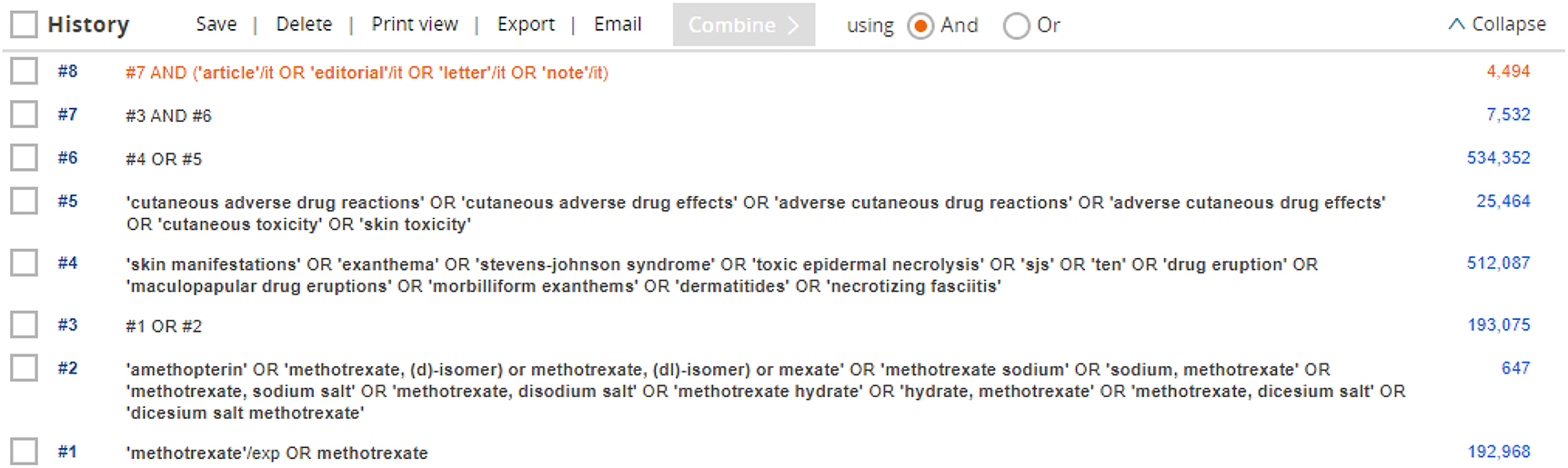
Appendix 2: Quality assessment
| Study Id | Level of evidence (OCEBM) | Methodological quality assessment (Murad et al. [20]) | |
|---|---|---|---|
| Score | Quality | ||
| Goerttler et al. [21] | 4 | 8 | Good |
| Pearce et al. [23] | 4 | 7 | Good |
| Wong et al. [24] | 4 | 5 | Moderate |
| Edward et al. [25] | 5 | 7 | Good |
| Salgüero et al. [26] | 5 | 6 | Good |
| Priego-Recio et al. [27] | 5 | 6 | Good |
| Borman et al. [28] | 5 | 5 | Moderate |
| Simonart et al. [29] | 5 | 5 | Moderate |
| Jeurissen et al. [15] | 5 | 3 | Moderate |
| Mebazaa et al. [30] | 5 | 8 | Good |
| Singal et al. [16] | 5 | 6 | Good |
| Behera et al. [31] | 5 | 5 | Moderate |
| Omoregie et al. [32] | 5 | 6 | Good |
| Teraki et al. [33] | 5 | 8 | Good |
| Thami et al. [34] | 5 | 7 | Good |
| Gupta et al. [35] | 5 | 6 | Good |
| Kurian et al. [36] | 5 | 5 | Moderate |
| Al-Saffar et al. [37] | 5 | 6 | Good |
| Tekur et al. [38] | 5 | 6 | Good |
| Torner et al. [39] | 5 | 5 | Moderate |
| Lewis HA et al. [22] | 4 | 5 | Moderate |
| Kazlow et al. [40] | 5 | 4 | Moderate |
| Hsu et al. [41] | 5 | 4 | Moderate |
| Riquelme-Mc Loughlin et al. [42] | 5 | 6 | Good |
| Skrek et al. [43] | 5 | 5 | Moderate |
| Borcea et al. [44] | 5 | 7 | Good |
| Arora et.al. [45] | 5 | 7 | Good |
| Reed et al. [46] | 5 | 6 | Good |
| Alaya et al. [47] | 5 | 7 | Good |
| Sako et al. [48] | 5 | 7 | Good |
| Blanes et al. [49] | 5 | 6 | Good |
References
1. Czarnecka-Operacz, M, Sadowska-Przytocka, A. The possibilities and principles of methotrexate treatment of psoriasis–the updated knowledge. Postepy Dermatol Alergol 2014;31:392, https://doi.org/10.5114/pdia.2014.47121.Search in Google Scholar PubMed PubMed Central
2. Sheagren, JN. Staphylococcus aureus: the persistent pathogen. N Engl J Med 1984;310:1437–42, https://doi.org/10.1056/nejm198405313102206.Search in Google Scholar PubMed
3. Weinblatt, ME. Methotrexate in rheumatoid arthritis: a quarter century of development. Trans Am Clin Climatol Assoc 2013;124:16.Search in Google Scholar
4. Singh, JA, Saag, KG, Bridges, SLJr, Akl, EA, Bannuru, RR, Sullivan, MC, et al.. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016;68:1–26., https://doi.org/10.1002/art.39480.Search in Google Scholar PubMed
5. Elmets, CA, Korman, NJ, Prater, EF, Wong, EB, Rupani, RN, Kivelevitch, D, et al.. Joint AAD–NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol 2021;84:432–70, https://doi.org/10.1016/j.jaad.2020.07.087.Search in Google Scholar PubMed
6. Bedoui, Y, Guillot, X, Sélambarom, J, Guiraud, P, Giry, C, Jaffar-Bandjee, MC, et al.. Methotrexate an old drug with new tricks. Int J Mol Sci 2019;20:5023, https://doi.org/10.3390/ijms20205023.Search in Google Scholar PubMed PubMed Central
7. Smith, BJ, Cohen, S, Kremer, JM, Winthrop, K, Fahey, S, Genovese, M. American College of Rheumatology updated guideline for the management of rheumatoid arthritis Project Plan 2018:1–35. Available at: https://www.rheumatology.org/Portals/0/Files/Rheumatoid-Arthritis-Guideline-Project-Plan.pdf.Search in Google Scholar
8. Visser, K, van der Heijde, D. Optimal dosage and route of administration of methotrexate in rheumatoid arthritis: a systematic review of the literature. Ann Rheum Dis 2009;68:1094–9, https://doi.org/10.1136/ard.2008.092668.Search in Google Scholar PubMed PubMed Central
9. Montaudié, H, Sbidian, E, Paul, C, Maza, A, Gallini, A, Aractingi, S, et al.. Methotrexate in psoriasis: a systematic review of treatment modalities, incidence, risk factors and monitoring of liver toxicity. J Eur Acad Dermatol Venereol 2011;25:12–8, https://doi.org/10.1111/j.1468-3083.2011.03991.x.Search in Google Scholar PubMed
10. Henderson, ES, Adamson, RH, Oliverio, VT. The metabolic fate of tritiated methotrexate: II. Absorption and excretion in man. Cancer Res 1965;25:1018–24.Search in Google Scholar
11. Grim, J, Chládek, J, Martínková, J. Pharmacokinetics and pharmacodynamics of methotrexate in non-neoplastic diseases. Clin Pharmacokinet 2003;42:139–51, https://doi.org/10.2165/00003088-200342020-00003.Search in Google Scholar PubMed
12. Robey, RB, Block, CA. Adverse effects of low-dose methotrexate. Ann Intern Med 2020;173:166–7, https://doi.org/10.7326/l20-0524.Search in Google Scholar PubMed
13. Watkins, PB, Seeff, LB. Drug‐induced liver injury: summary of a single topic clinical research conference. Hepatology 2006;43:618–31, https://doi.org/10.1002/hep.21095.Search in Google Scholar PubMed
14. Taylor, P, Balsa Criado, A, Mongey, AB, Avouac, J, Marotte, H, Mueller, RB. How to get the most from methotrexate (MTX) treatment for your rheumatoid arthritis patient?-MTX in the treat-to-target strategy. J Clin Med 2019;8:515, https://doi.org/10.3390/jcm8040515.Search in Google Scholar
15. Jeurissen, ME, Boerbooms, AT, Van de Putte, LB. Eruption of nodulosis and vasculitis during methotrexate therapy for rheumatoid arthritis. Clin rheumatol. 1989;8:417–9, https://doi.org/10.1111/j.1365-4632.2009.04256.x.Search in Google Scholar
16. Singal, A, Sonthalia, S, Pandhi, D. Erythema gyratum repens-like eruption occuring in resolving psoriasis during methotrexate therapy. Int J Dermatol. 2010;49:306. https://doi.org/10.1515/jbcpp-2020-0115.Search in Google Scholar
17. Rashid, M, Kashyap, A, Undela, K. Valproic acid and Stevens‐Johnson syndrome: a systematic review of descriptive studies. Int J Dermatol 2019;58:1014–22, https://doi.org/10.1111/ijd.14411.Search in Google Scholar
18. Rashid, M, Rajan, AK, Chhabra, M, Kashyap, A. Levetiracetam and cutaneous adverse reactions: a systematic review of descriptive studies. Seizure 2020;75:101–9, https://doi.org/10.1016/j.seizure.2020.01.002.Search in Google Scholar
19. Howick, J, Chalmers, I, Glasziou, P. The oxford 2011 levels of evidence. Oxford, UK: Oxford Centre for Evidence-Based Medicine; 2011. Available at: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/explanation-of-the-2011-ocebm-levels-of-evidence.Search in Google Scholar
20. Murad, MH, Sultan, S, Haffar, S, Bazerbachi, F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med 2018;23:60–3, https://doi.org/10.1136/bmjebm-2017-110853.Search in Google Scholar
21. Goerttler, E, Kutzner, H, Peter, HH, Requena, L. Methotrexate-induced papular eruption in patients with rheumatic diseases: a distinctive adverse cutaneous reaction produced by methotrexate in patients with collagen vascular diseases. J Am Acad Dermatol 1999;40:702–7, https://doi.org/10.1016/s0190-9622(99)70150-7.Search in Google Scholar
22. Lewis, HA, Nemer, KM, Chibnall, RJ, Musiek, AC. Methotrexate-induced cutaneous ulceration in 3 nonpsoriatic patients: report of a rare side effect. JAAD Case Rep 2017;3:236–9, https://doi.org/10.1016/j.jdcr.2017.02.002.Search in Google Scholar
23. Pearce, HP, Wilson, BB. Erosion of psoriatic plaques: an early sign of methotrexate toxicity. J Am Acad Dermatol 1996;35:835–8, https://doi.org/10.1016/s0190-9622(96)90097-3.Search in Google Scholar
24. Wong, SM, Chong, YT, Thevarajah, S, Baba, R. Methotrexate toxicity presenting as ulcerated psoriatic plaques. Australas J Dermatol 2012;53:81–3, https://doi.org/10.1111/j.1440-0960.2011.00779.x.Search in Google Scholar PubMed
25. Primka, EJIII, Camisa, C. Methotrexate-induced toxic epidermal necrolysis in a patient with psoriasis. J Am Acad Dermatol 1997;36:815–8, https://doi.org/10.1016/s0190-9622(97)70029-x.Search in Google Scholar
26. Salgüero, I, Moreno, C, Aguayo-Leiva, I, Harto, A. Papuloerythroderma of Ofuji associated with chronic lymphatic leukaemia. Eur J Dermatol 2009;19:396–7, https://doi.org/10.1684/ejd.2009.0689.Search in Google Scholar PubMed
27. Priego-Recio, CM, Camacho-Martinez, FM. Local reaction after subcutaneous injection of methotrexate: uncommon side effect. J Eur Acad Dermatol Venereol. 2014;30:523–4, https://doi.org/10.1111/jdv.12918.Search in Google Scholar PubMed
28. Borman, P, Bodur, H, Güleç, AT, Ucan, H, Seçkin, Ü, Mocan, G. Atypical methotrexate dermatitis and vasculitis in a patient with ankylosing spondylitis. Rheumatol Int 2000;19:191–3,https://doi.org/10.1007/s002960000047.Search in Google Scholar PubMed
29. Simonart, TH, Durez, P, Margaux, J, Van Geertruyden, J, Goldschmidt, D, Parent, D. Cutaneous necrotizing vasculitis after low dose methotrexate therapy for rheumatoid arthritis: a possible manifestation of methotrexate hypersensitivity. Clin Rheumatol 1997;16:623–5, https://doi.org/10.1007/bf02247805.Search in Google Scholar PubMed
30. Mebazaa, A, Kenani, N, Denguezli, M, SCB, Ziadi, S, Sriha, B, et al.. Methotrexate-induced papular eruption following treatment of psoriasis. Ann Pharmacother 2008;42:138–41, https://doi.org/10.1345/aph.1k271.Search in Google Scholar
31. Behera, B, Kumari, R, Gochhait, D, Thappa, DM. Low dose methotrexate induced asymptomatic keratinocyte dystrophy in a patient of psoriasis vulgaris. J Cutan Pathol 2016;43:1238–41, https://doi.org/10.1111/cup.12810.Search in Google Scholar PubMed
32. Omoregie, FO, Ukpebor, M, Saheeb, BD. Methotrexate-induced erythema multiforme: a case report and review of the literature. West Afr J Med 2011;30:377–9.Search in Google Scholar
33. Teraki, Y, Izaki, S. Salazosulfapyridine-induced hypersensitivity syndrome triggered by methotrexate. Clin Exp Dermatol 2009;34:e287–8, https://doi.org/10.1111/j.1365-2230.2009.03212.x.Search in Google Scholar PubMed
34. Thami, GP, Kaur, S, Kanwar, AJ. Delayed reactivation of haloperidol induced photosensitive dermatitis by methotrexate. Postgrad Med 2002;78:116–7, https://doi.org/10.1136/pmj.78.916.116.Search in Google Scholar PubMed PubMed Central
35. Gupta, A, Sardana, K, Bhardwaj, M, Singh, A. Methotrexate cutaneous toxicity following a single dose of 10 mg in a case of chronic plaque psoriasis: a possible idiosyncratic reaction. Indian Dermatol Online J 2018;9:328, https://doi.org/10.4103/idoj.IDOJ_316_17.Search in Google Scholar PubMed PubMed Central
36. Kurian, A, Haber, R. Methotrexate-induced cutaneous ulcers in a nonpsoriatic patient: case report and review of the literature. J Cutan Med Surg 2011;15:275–9, https://doi.org/10.2310/7750.2011.10078.Search in Google Scholar PubMed
37. Al-Saffar, F, Ibrahim, S, Patel, P, Jacob, R, Palacio, C, Cury, J. Skin rash in the intensive care unit: Stevens-Johnson syndrome, toxic epidermal necrolysis, or a rare manifestation of a hidden cutaneous malignancy: a case report. Mol Clin Oncol 2016;4:413–5, https://doi.org/10.3892/mco.2015.713.Search in Google Scholar PubMed PubMed Central
38. Tekur, VK. Methotrexate-induced nonhealing cutaneous ulcers in a nonpsoriatic patient without pancytopenia. Indian Dermatol Online J 2016;7:418, https://doi.org/10.4103/2229-5178.190509.Search in Google Scholar PubMed PubMed Central
39. Torner, O, Ruber, C, Olive, A, Tena, X. Methotrexate related cutaneous vasculitis. Clin Rheumatol 1997;16:108, https://doi.org/10.1007/bf02238776.Search in Google Scholar
40. Kazlow, DW, Federgrun, D, Kurtin, S, Lebwohl, MG. Cutaneous ulceration caused by methotrexate. J Am Acad Dermatol 2003;49:197–8, https://doi.org/10.1067/mjd.2003.388.Search in Google Scholar PubMed
41. Hsu, MC, Chen, CC. Low-dose methotrexate-induced ulcerated psoriatic plaques: a rare case. JAAD Case Rep 2015;1:264–6, https://doi.org/10.1016/j.jdcr.2015.06.003.Search in Google Scholar PubMed PubMed Central
42. Riquelme-Mc Loughlin, C, Giavedoni, P, Mascaró, JMJr. More than skin deep. Am J Emerg Med 2018;36:1719–e3, https://doi.org/10.1016/j.ajem.2018.05.069.Search in Google Scholar PubMed
43. Skrek, SV, Timoshchuk, EA, Sidikov, AA, Zaslavsky, DV, Megna, M. Kaposi varicelliform eruption induced by methotrexate in an adult atopic dermatitis patient. Dermatol Ther 2019;32:e12826, https://doi.org/10.1111/dth.12826.Search in Google Scholar PubMed
44. Borcea, A, Greaves, MW. Methotrexate‐induced exacerbation of urticarial vasculitis: an unusual adverse reaction. Br J Dermatol 2000;143:203–4, https://doi.org/10.1046/j.1365-2133.2000.03624.x.Search in Google Scholar PubMed
45. Arora, S, Abidi, A, Fatima, F, Hasan, R, Rizvi, D, Thadani, A. Methotrexate induced Stevens Johnson syndrome: a rare case report. Era’s J Med Res 2018;5:194–6, https://doi.org/10.24041/ejmr2018.95.Search in Google Scholar
46. Reed, KM, Sober, AJ. Methotrexate-induced necrolysis. J Am Acad Dermatol 1983;8:677–9, https://doi.org/10.1016/s0190-9622(83)70079-4.Search in Google Scholar
47. Alaya, Z, Mokni, S, Guerfala, M, Salem, CB, Sriha, B, Nouira, R, et al.. Acute severe cutaneous methotrexate toxicity in a patient with rheumatoid arthritis: report of a rare side effect. Egypt Rheumatol 2018;40:281–4,https://doi.org/10.1016/j.ejr.2017.08.004.Search in Google Scholar
48. Sako, EY, Famenini, S, Wu, JJ. Bullous drug eruption in a patient with psoriasis after a test dose of methotrexate. J Am Acad Dermatol 2013;69:e264–5, https://doi.org/10.1016/j.jaad.2013.07.012.Search in Google Scholar
49. Blanes, M, Silvestre, JF, Albares, MP, Pascual, JC, Pastor, N. Erythema multiforme due to methotrexate reproduced with patch test. Contact Dermatitis 2005;52:164–5, https://doi.org/10.1111/j.0105-1873.2005.0548f.x.Search in Google Scholar
50. Rajan, AK, Kashyap, A, Chhabra, M, Rashid, M. Linezolid induced skin reactions in a multi drug resistant infective endocarditis patient: a rare case. Curr Drug Saf 2020;15:222–6, https://doi.org/10.2174/1574886315666200516175053.Search in Google Scholar
51. Kearsley-Fleet, L, Vicente González, L, Steinke, D, Davies, R, De Cock, D, Baildam, E, et al.. Methotrexate persistence and adverse drug reactions in patients with juvenile idiopathic arthritis. Rheumatology 2019;58:1453–8, https://doi.org/10.1093/rheumatology/kez048.Search in Google Scholar
52. Lalani, R, Lyu, H, Vanni, K, Solomon, DH. Low‐dose methotrexate and mucocutaneous adverse events: results of a systematic literature review and meta‐analysis of randomized controlled trials. Arthritis Care Res 2020;72:1140–6, https://doi.org/10.1002/acr.23999.Search in Google Scholar
53. Frikha, R, Jemaa, MB, Frikha, F, Turki, I, Elloumi, M, Keskes, L, et al.. Involvement of C677T MTHFR variant but not A1298C in methotrexate-induced toxicity in acute lymphoblastic leukemia. J Oncol Pharm Pract 2021;27:1382–7, https://doi.org/10.1177/1078155220951898.Search in Google Scholar
54. McKendry, RJ, Dale, P. Adverse effects of low dose methotrexate therapy in rheumatoid arthritis. J Rheumatol 1993;20:1850–6.Search in Google Scholar
55. Del Campo, M, Kosaki, K, Bennett, FC, Jones, KL. Developmental delay in fetal aminopterin/methotrexate syndrome. Teratology 1999;60:10–2, https://doi.org/10.1002/(sici)1096-9926(199907)60:1<10::aid-tera5>3.0.co;2-h.10.1002/(SICI)1096-9926(199907)60:1<10::AID-TERA5>3.0.CO;2-HSearch in Google Scholar
56. Liu, L, Liu, S, Wang, C, Guan, W, Zhang, Y, Hu, W, et al.. Folate supplementation for methotrexate therapy in patients with rheumatoid arthritis: a systematic review. J Clin Rheumatol 2019;25:197–202, https://doi.org/10.1097/rhu.0000000000000810.Search in Google Scholar
57. Destiani, DP, Naja, S, Dewi, S, Rahmadi, AR, Sulaiman, SA, Abdulah, R. Efficacy of methotrexate in reducing the risk of bone erosion in patients with rheumatoid arthritis: a systematic review of randomized controlled trials. Osteoporos Int 2020:1–2, https://doi.org/10.1007/s00198-020-05743-z.Search in Google Scholar PubMed
© 2021 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial
- Circulating tumor cells in bladder cancer: a new horizon of liquid biopsy for precision medicine
- Minireview
- PCSK9 and LRP6: potential combination targets to prevent and reduce atherosclerosis
- Reviews
- The complex relationship between physical activity and diabetes: an overview
- Methotrexate related cutaneous adverse drug reactions: a systematic literature review
- Long-term use of proton pump inhibitors adversely affects minerals and vitamin metabolism, bone turnover, bone mass, and bone strength
- An update on the physiologic changes during pregnancy and their impact on drug pharmacokinetics and pharmacogenomics
- Original Articles
- Apelin-13 protects human neuroblastoma SH-SY5Y cells against amyloid-beta induced neurotoxicity: Involvement of anti oxidant and anti apoptotic properties
- Are hypertensive patients with history of coronary artery disease at risk for silent lower extremity artery disease?
- The effect of long-term ketogenic diet on serum adiponectin and insulin-like growth factor-1 levels in mice
- Increase in cardioprotective SUR2A does not alter heart rate and heart rate regulation by physical activity and diurnal rhythm
- Effect of ascorbic acid supplementation on pulmonary functions in healthy adults: a randomized controlled pilot study
- Alpha2-antiplasmin deficiency affects depression and anxiety-like behavior and apoptosis induced by stress in mice
- Modern gold standard of cardiac output measurement – A simplified bedside measurement of individual oxygen uptake in the cath lab
- Electrical vestibular nerve stimulation (VeNS): a follow-up safety assessment of long-term usage
- Psychological impact of abnormally invasive placenta: an underestimated and hidden morbidity
- Limited diagnostic value of questionnaire-based pre-participation screening algorithms: a “risk-exposed” approach to sports activity
- Predictors of abdominal pain severity in patients with constipation-prevalent irritable bowel syndrome
Articles in the same Issue
- Frontmatter
- Editorial
- Circulating tumor cells in bladder cancer: a new horizon of liquid biopsy for precision medicine
- Minireview
- PCSK9 and LRP6: potential combination targets to prevent and reduce atherosclerosis
- Reviews
- The complex relationship between physical activity and diabetes: an overview
- Methotrexate related cutaneous adverse drug reactions: a systematic literature review
- Long-term use of proton pump inhibitors adversely affects minerals and vitamin metabolism, bone turnover, bone mass, and bone strength
- An update on the physiologic changes during pregnancy and their impact on drug pharmacokinetics and pharmacogenomics
- Original Articles
- Apelin-13 protects human neuroblastoma SH-SY5Y cells against amyloid-beta induced neurotoxicity: Involvement of anti oxidant and anti apoptotic properties
- Are hypertensive patients with history of coronary artery disease at risk for silent lower extremity artery disease?
- The effect of long-term ketogenic diet on serum adiponectin and insulin-like growth factor-1 levels in mice
- Increase in cardioprotective SUR2A does not alter heart rate and heart rate regulation by physical activity and diurnal rhythm
- Effect of ascorbic acid supplementation on pulmonary functions in healthy adults: a randomized controlled pilot study
- Alpha2-antiplasmin deficiency affects depression and anxiety-like behavior and apoptosis induced by stress in mice
- Modern gold standard of cardiac output measurement – A simplified bedside measurement of individual oxygen uptake in the cath lab
- Electrical vestibular nerve stimulation (VeNS): a follow-up safety assessment of long-term usage
- Psychological impact of abnormally invasive placenta: an underestimated and hidden morbidity
- Limited diagnostic value of questionnaire-based pre-participation screening algorithms: a “risk-exposed” approach to sports activity
- Predictors of abdominal pain severity in patients with constipation-prevalent irritable bowel syndrome

