Abstract
Subepithelial esophageal tumors (SET) are normally benign intramural esophageal lesions of mesenchymal origin. Although rare, the incidence of SET has increased in recent decades due to the more widespread use of endoscopy and diagnostic imaging. The current review aims to provide an overview of the histopathologic spectrum and the most frequent entities including leiomyoma and gastrointestinal stromal tumor (GIST), diagnostic workup, and multidisciplinary treatment options. Staging for SET should include endoscopy, endoscopic ultrasonography (EUS), and tissue sampling. Current consensus guidelines recommend that SET suggestive of gastrointestinal stromal tumor (GIST) larger than 20 mm or lesions with high-risk stigmata should undergo tissue sampling. Most SET have an excellent long-term outcome, but malignancy may be present in certain subtypes. Asymptomatic SET without high-risk stigmata discovered incidentally usually do not require specific treatment. However, depending on the size and location of the lesion symptoms may occur. Therapeutic interventions range from endoscopic interventional resections to major surgical procedures. Enucleation via minimally invasive or robotic-assisted access remains the standard of care for most SET sub-entities.
Introduction
Subepithelial esophageal tumors (SET) are usually benign intramural esophageal lesions of mesenchymal origin covered by normal-looking mucosa and protruding into the lumen. SET are generally rare, occurring in postmortem series with an incidence of 0.5–1 % [1], [2], [3]. SET are much less common than malignant epithelial esophageal tumors [4], [5], [6] and account for less than 5 % of all surgically treated esophageal neoplasms [3], [6], [7], [8]. Of note, the incidence has increased in recent decades, most likely related to a more widespread use of endoscopy and diagnostic imaging [9], 10], while also showing some geographical variation [11].
SET is an umbrella term for several subtypes, and a solid knowledge of the histopathologic spectrum, diagnostic workup, and treatment options is essential. Therefore, treatment planning involving a multidisciplinary tumor board is generally recommended. Small asymptomatic SET usually requires only close surveillance, whereas therapy may be indicated based on symptoms, tumor size and growth, or an unclear diagnosis. Most SET have an excellent long-term outcome, but malignancy may be present in GIST and, less commonly, in Schwannoma, granular cell tumor, or even leiomyoma. Therapeutic interventions range from endoscopic interventional resections to major surgical procedures such as esophagectomy and multimodal approaches. However, enucleation via a minimally invasive or robotic-assisted access remains the standard of care for most SET sub-entities.
Given the increasing incidence and the growing demand for a refined management of SET, this review aims to provide a topical update with a focus on treatment strategies. Esophageal neuroendocrine neoplasms (NEN) and esophageal malformations such as cysts and duplications may also manifest clinically as SET. However, due to the high malignant potential of esophageal NEN and the non-neoplastic nature of esophageal malformations, these entities fall outside the scope of this narrative review.
Pre-therapeutic diagnostic work-up
Staging for SET should include endoscopy, endoscopic ultrasonography (EUS) and tissue sampling. Endoscopy is essential for macroscopic evaluation of the lesion, accurate pretherapeutic determination of the anatomic location, and histopathologic diagnosis. Endoscopically, SET typically appear as roundish luminal bulging lesions of variable size and texture with or without a central umbilication; the consistency usually is soft and may be roughly assessed with biopsy forceps [12], 13]. Magnifying endoscopy or chromoendoscopy is of limited value because of the normal overlying mucosa, but some lesions may show through in whitish-grey or reddish-brown color. Larger lesions, rapid growth, or ulceration are indicators of possible malignant transformation [14].
EUS is the diagnostic tool of choice to characterize important features such as size and shape, exact location, originating layer and echogenicity [12]. The most recent ESGE guidelines recommend EUS-guided fine-needle biopsy for all SET suggestive of GIST, greater than 2 cm in diameter, and with high-risk stigmata. In addition, EUS allows reliable differentiation of extrinsic compression [12], 15] and can determine the intramural layer of origin, the size and the margins of the lesion with high accuracy [15], 16]. However, except for lipomas and hemangiomas, the classic EUS features such as homogeneity, spots, halo, and echogenicity are not specific for most SET subtypes [17], [18], [19].
In our institutional routine, cross-sectional radiologic imaging with thoraco-abdominal 3-phase CT-scan is also performed [20]. In selected cases – particularly GIST (which are often FDG positive), suspected malignancy, or large tumors – PET-scan or MRI can provide valuable additional information for oncological staging and therapeutic planning [21], 22].
Histopathological assessment
Current consensus guidelines recommend that SET suggestive of GIST larger than 20 mm or lesions with high-risk stigmata should undergo tissue sampling to assess risk of progression before any therapeutic decision is made [12]. Likewise, in symptomatic tumors (except for SET with acute hemorrhage), a definitive diagnosis should be made before treatment, regardless of size. The need for a histopathological diagnosis is debated for small asymptomatic SET <20 mm without high-risk features [12], 23], which can also be followed up endoscopically according to the National Comprehensive Cancer Network guidelines [24]. However, because most national and international guidelines [25], [26], [27] recommend surgical resection of small esophageal GIST <20 mm, it is our institutional policy to strive for a definite diagnosis even in these situations [14]. As recommended in the current European Society for Gastrointestinal Endoscopy (ESGE) guideline, tissue sampling for SET ≥20 mm can be performed using either EUS-guided fine-needle biopsy or mucosal incision-assisted biopsy (MIAB), whereas tumors <20 mm should be exclusively biopsied using MIAB [12].
Spectrum of SET and indication for therapy
Leiomyoma
Leiomyomas are usually diagnosed between the 3rd and 5th decades of life [3], 7], 28], 29]. They are the most common benign neoplasms of the esophagus, accounting for approximately two-thirds of all SET [1], 3], 4], 30], 31]. Leiomyoma usually grow in the middle and distal esophageal thirds because they develop from smooth muscle cells and are less common in the upper third, where the muscle layer is primarily striated [6]. Most leiomyomas develop from the muscularis propria of the esophagus and present as a subepithelial mass; however, cases arising from the muscularis mucosae may manifest luminally with a polyp-like growth pattern [32].
Esophageal leiomyomata are often small, reflecting their slow growth, with approximately 50 % <5 cm and 93 % <15 cm7. Symptomatic tumors have usually reached a size of at least 5 cm3. Endoscopically, leiomyomas present as whitish-gray to brown–yellow, rubber-like, encapsulated masses with a smooth surface [6]. An irregular, often dendritic, horseshoe- or dumbbell-shaped growth pattern is typical, and even smaller lesions may occasionally present with clamp-like narrowing of the lumen.
Histologically, spindle-shaped cells arranged in fascicles or whorls are typically found. The cytoplasm is eosinophilic with surrounding hypovascular connective tissue [33]. There are few or no mitoses, with minimal nuclear atypia and general hypocellularity. Definitive diagnosis of leiomyoma is based on positivity of the immunohistochemical markers desmin and smooth muscle antigen (SMA) [33]. Malignant transformation to leiomyosarcoma is extremely rare, with fewer than 200 cases reported [6], 31], 32], 34]. Esophageal leiomyosarcomas tend to grow intraluminally rather than intramurally and have a 5-year survival rate of 20 % [32].
Treatment is indicated in all symptomatic cases. Minimally invasive surgical enucleation is the treatment of choice [35], because it involves fewer pulmonary complications, pain, and hospitalization compared with an open procedure [36]. However, extended resection techniques may be required for giant leiomyomas [37]. In asymptomatic cases, endoscopic surveillance at 6–12-month intervals is recommended.
Gastrointestinal stroma tumor (GIST)
Less than 1 % of GIST is found in the esophagus – compared with 50–60 % in the stomach and 30–35 % in the small intestine [38]. GIST arises from the interstitial cells of Cajal, also known as the gastrointestinal motility pacemaker [39]. Most esophageal GIST (eGIST) are diagnosed between the fifth and seventh decade [34] are variable in size and develop primarily in the distal third. Clinically, eGIST may present with gastrointestinal bleeding, but also with dysphagia and weight loss.
The gross appearance of the tumor is usually whitish gray, soft, encapsulated, and fish-flesh-like (Figure 1). Histologically, GIST has an overall basophilic appearance with high cellularity and mild-to-no nuclear pleomorphism on hematoxylin-eosin staining. Total of 70–80 % are composed of spindle cells, while 20–30 % are predominantly epithelioid. On immunohistochemistry, the most common markers are c-kit protein (CD117), DOG-1, and CD34, with CD117 being the most specific [33]. Mitotic activity plays a crucial role in predicting malignant potential. The National Institute of Health (NIH) has developed a classification system for GIST in general with four risk categories based on mitotic activity and size, specifically very low risk, low risk, intermediate risk, and high risk [40]. In 2016, Miettinen from the Armed Forces Institute of Pathology (termed AFIP classification), introduced a classification consisting of six groups further including the anatomic site of the primary tumor as an additional variable [41]. The most recent classification by Joensuu et al., also includes the additional risk of tumor rupture and proposes a continuous risk scale [42]. The used non-linear model accurately predicts the risk of recurrence and can be nicely visualized using prognostic contour maps. All of these risk prediction models distinguish well between high-risk and low-risk patients with resected, non-metastasized GIST [43]. Additionally, it has recently been shown that certain KIT deletion mutations in exon 11 are associated with recurrence and metastasis in eGIST [44].
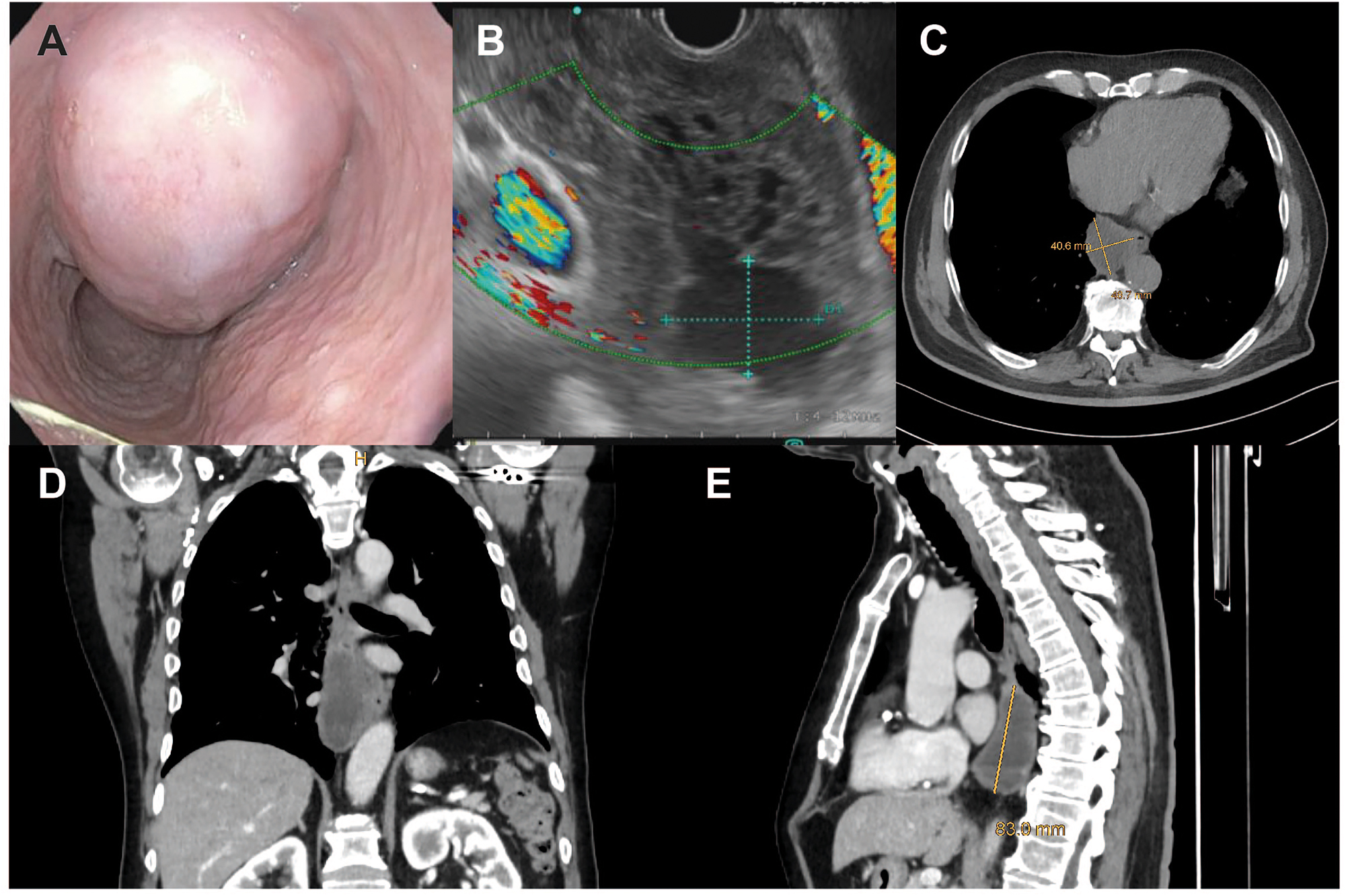
Case of a male patient with a large (9 × 5 cm) esophageal GIST. Endoscopic (A) and endosonographic (B) view, as well as transverse (C), coronal (D) and sagittal (E) computed tomography planes of the tumor.
Because of their inherent malignant potential, eGIST require close clinical monitoring or, if medium- or high-risk, surgical resection. R0 resection remains the aim to strive for during surgery, while lymphadenectomy, as for GIST at other localizations, is usually not required [45]. Imatinib [46] or other tyrosine kinase-inhibitors such as sunitinib, regorafenib [47] (in case of wildtype KIT/PDGFR-A) or avapritinib (in case of wildtype PDGFRA-D842V mutation) [48] can be used as systemic neoadjuvant [49] or adjuvant [50] treatment. According to the latest ESMO guidelines [51], neoadjuvant treatment for downstaging is indicated in case of primary non-resectability, with evaluation of tumor regression in 3–4 monthly intervals. Surgical resection is then scheduled once maximal radiological tumor regression is achieved. Based on data of 3 RCTs, adjuvant therapy for 3 years is recommended for patients with high-risk tumors [50], [52], [53], [54]. In contrast, tyrosine kinase-inhibitors should be continued indefinitely in locally advanced or ruptured GIST due to high-rates of recurrence until disease progression occurs [55], 56], similar as in the palliative setting for metastatic GIST. In cases of irresectability, imatinib has the potential to significantly prolong median survival [57].
Granular cell tumor
Granular cell tumors (GCT) are yellowish polypoid lesions that originate from the submucosa and may show malignant transformation in 1–2 % of cases [58]. Microscopically, GCTs consist of nests of polygonal cells resembling histiocytes with PAS-positive granules separated by collagen bundles. S-100 immunohistochemistry is usually positive. Malignant cases have a poor prognosis and show necrosis, increased mitotic count, large hyperchromatic nucleoli, and a high Ki67 index [59], [60], [61], [62]. Lesions <1 cm can often be surveilled, whereas tumors up to 3 cm may be resected endoscopically, with surgery reserved for larger tumors [58], 63].
Schwannoma
Schwannomas are rare, usually benign, mesenchymal lesions originating from the Schwann cells of the nerve sheath which are found in all human soft tissues [63], [64], [65]. They occur in the 4th – 5th decade of life with a slight predominance in females. Like GCT, Schwannoma originate from the submucosal layer [66] with a yellowish, smooth, firm endoscopic appearance, and show strong expression of S-100 and SOSX10 on immunohistochemistry. Tumors >2 cm should be resected, while malignant transformation is exceedingly rare [67].
Hemangioma
Esophageal hemangioma is a rare and mostly solitary benign lesion with an overall prevalence of 0.04 % in autopsy series [1], 66]. Endoscopically, they appear as blue lesion, while biopsy should not be undertaken due to bleeding risk. Multiple hemangioma are typical for blue rubber bleb nevus (Bean’s) syndrome, a congenital disease associated with multiple skin and gastrointestinal lesions [68]. Most esophageal hemangioma are asymptomatic and found incidentally [69], but intestinal hemorrhage and dysphagia may occur and call for individualized treatment, ranging from iron supplementation and blood transfusions to endoscopic or surgical resection [69], 70].
Lipoma/fibrovascular polyps
Lipomas account for less than 1 % of mesenchymal esophageal tumors. More than 85 % occurs in the proximal third of the esophagus, where they typically present as fibrovascular polyps with a pedunculated intraluminal growth pattern [34], 71], 72]. In contrast, the less common intramural esophageal lipomas are usually small, asymptomatic, and typically located in the lower third of the esophagus. Lipomas are usually discovered incidentally on radiologic imaging or endoscopy, but pedunculated proximal polyps/lipoma carries a risk of asphyxia [73]. Microscopically, esophageal lipomas have mature adipose tissue with fibrovascular septa; given the potential for malignant transformation, MDM2 in situ hybridization is recommended [74], 75]. In a recent systematic review, 63 of 176 cases of esophageal lipomas were ultimately diagnosed as liposarcoma [34].
Glomus tumor
Glomus tumors originate in the glomus apparatus, an arterio-venous shunt of the dermis involved in physiologic thermoregulation of the body. Esophageal glomus tumors are extremely rare, with fewer than 20 cases described in the literature [76], 77]. They arise in the deep mucosa and submucosa and show epithelioid cells with associated proliferation of small blood vessels on histopathological examination. Of note, malignant transformation including distant metastasis has been described [77].
Therapeutic management of SET
Asymptomatic SET without high-risk stigmata discovered incidentally usually do not require specific treatment. However, depending on the size of the lesion and extent of extension into the esophageal lumen, dysphagia, bleeding/ulceration, and thoracic pain may occur. The indication for resection may also be due to compression of extraesophageal organs by large tumors or the risk of asphyxia in pedunculated polyps in the upper esophagus [34]. The decision to resect should be made in a multidisciplinary meeting. The choice of technique should depend on size, location, and local expertise [12]. Treatment options include interventional-endoscopic and surgical procedures up to esophagectomy [6].
Surveillance
According to the current ESGE guidelines, endoscopic surveillance of asymptomatic esophageal leiomyomas, lipomas, granular cell tumors, schwannomas, and glomus tumors is generally not required [12]. In contrast, asymptomatic SET without a clear diagnosis should be followed up endoscopically. In this regard, the recommended frequency of surveillance endoscopies is based on size: 2–3 years for lesions <10 mm and 1–2 years for lesions of 10–20 mm. For asymptomatic SEL >20 mm that are not resected, ESGE recommends surveillance with EGD plus EUS at 6 months and then at 6–12-month intervals [12].
Endoscopic therapy
Endoscopic resections have become a standard procedure to remove superficial/epithelial SET (papillomas, adenomas, inflammatory polyps) as well as deeper submucosal and even transmural tumors. Mucosal lesions are elevated by saline injection, resulting in detachment of deeper tissue layers, and then resected by snare removal, ligation and electrocautery; larger lesions may be removed by piece-by-piece technique [78]. Potential complications of endoscopic mucosal resections include bleeding, perforation and stenosis in up to 88 % [79].
Endoscopic submucosal resections follow a similar principle with opening of the submucosal space and are generally more difficult and associated with higher complication rates than mucosal resections. Relevant bleeding occurs in up to 5 %, especially in lesions >4 cm and patients on anticoagulation, while perforations are reported in up to 10 %, but can directly be treated via application of clips [80].
Another endoscopic modality is peroral endoscopic tumor resection (POET), which is derived from POEM. After incision of the mucosa approximately 5 cm proximal to the lesion, the submucosal space is tunneled for tumor removal. Indications for POET are usually tumors up to 3 cm [81].
Surgical therapy
In the past, open transthoracic enucleation of the SET was associated with significant morbidity. However, with the advent of minimally invasive and robotic procedures, postoperative morbidity and mortality have decreased significantly [36], [82], [83], [84], [85]. Larger tumors >5 cm or lesions encircling the esophagus have been cited as limiting factors of thoracoscopy or laparoscopy [35], but minimally invasive surgery has also been described for tumors of much larger dimensions [84].
Tumor enucleation is an effective treatment for most SET and is technically feasible in up to 97 % [82]. It is the standard surgical approach for esophageal leiomyoma [83]. Because these lesions have a benign clinical course with a very low rate of malignant transformation, recurrence rates after surgery are negligible when appropriate surgical techniques are used [35], 82], 84]. In contrast, recurrence rates can be higher in GIST, especially in larger tumors with high mitotic activity [86], 87]. Therefore, the optimal therapy for GIST remains debated, and current recommendations range from enucleation to partial or subtotal esophagectomy [25], 88], 89]. Nevertheless, in cases with low mitotic index and smaller size (<4 cm), retrospective series indicate that enucleation is a viable treatment option for GIST [87], [90], [91], [92].
From a surgical-technical point of view, intraoperative endoscopy is valuable for exact determination of tumor localization and control of mucosal integrity [93]. In addition, most authors advocate closure of the muscle layer defect after enucleation to prevent the development of pseudodiverticula [83], 94]. Figure 2 shows the main steps of a robot-assisted thoracic enucleation of a distal esophageal GIST.

Steps of a robotic assisted minimally invasive enucleation of the esophageal GIST of the patient presented in Figure 1. (A) initial view, (B) incision of pleura/serosa; (C, D) length incision of esophageal muscle layer, (E, F) circular detachment of the tumor, (G) complete en-bloc removal, (H) checking mucosal integrity by endoscopy, (I) checking mucosal integrity by underwater air leakage test during endoscopy, (J, K) closure of muscle defect by monofilament barbed suture, (L) final view.
SET above a certain size, specific entities (e.g., malignant GIST with mucosal infiltration), or complications (e.g., central necrosis or abscess formation after biopsy) may not be treatable by enucleation. For oncologic reasons, esophagectomy may also be required for GIST with high-risk stigmata [45]. Figure 3 shows the case of a patient with a large esophageal leiomyoma. Due to the size of the lesion and involvement of the esophagogastric junction with large tumor nodules on both sides of the diaphragm, open surgical thoraco-laparotomy was required for en bloc tumor removal, and continuity was reconstructed with a gastric pull-up procedure and anastomosis at the level of the azygos vein according to Ivor Lewis.
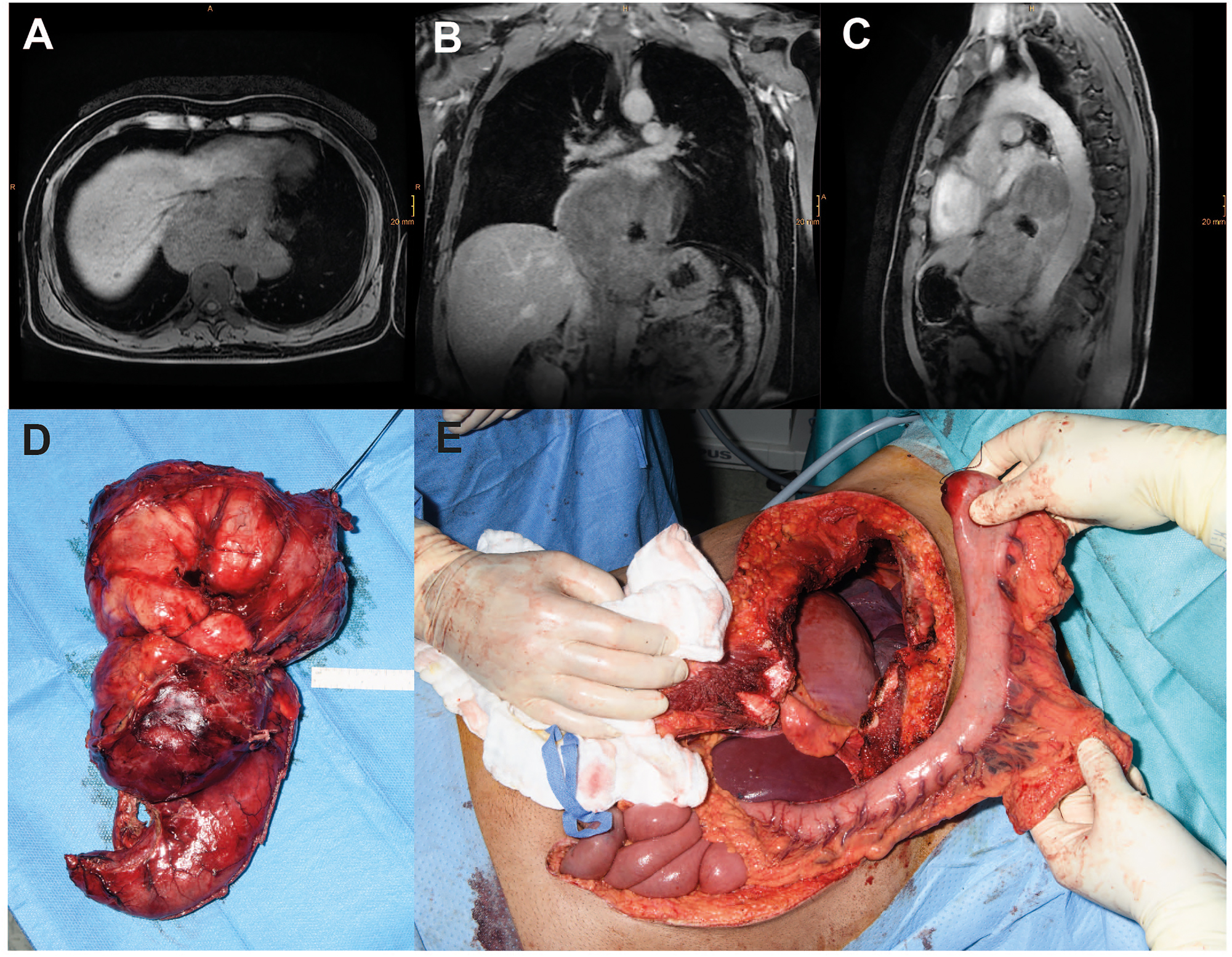
Case of a male patient with a giant (17 × 14 × 8 cm) esophageal leiomyoma with abscess following diagnostic biopsy. Transverse (A), coronal (B) and sagittal (C) magnetic resonance imaging planes of the tumor. (D) Intraoperative images of the resected specimen and (E) the situs after thoraco-laparotomy before reconstruction with interposition of the tubulized stomach.
Conclusions
SET is an umbrella term for various rare sub-entities, and solid knowledge of the histopathological spectrum, the diagnostic work-up and entity-specific treatment options is necessary for an adequate clinical decision-making. A multidisciplinary tumor board should be generally involved in the therapeutic management of SET.
Small asymptomatic SET usually requires only endoscopic surveillance, while therapy is typically indicated based on symptoms, tumor size or growth, or unclear diagnosis. Most SET are benign with excellent long-term outcome, but malignancy may be present in gastrointestinal stroma tumor (GIST), and rarely even in Schwannoma, granular cell tumor, or leiomyoma. For leiomyoma, surgical resection is generally only indicated in symptomatic cases, while intermediate to high-risk GIST should undergo resection. Therapeutic options range from endoscopic interventions to major surgical procedures such as esophagectomy and multimodal strategies. However, enucleation via a minimally invasive or robotic-assisted approach remains the standard of care for most SET sub-entities.
-
Research ethics: Not applicable for a narrative review.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: The authors state no conflict of interest.
-
Research funding: None declared.
References
1. Moersch, HJ. Benign tumor of the esophagus. Ann Otol Rhinol Laryngol 1944;53:800–17.Search in Google Scholar
2. Plachta, A. Benign tumors of the esophagus. Review of literature and report of 99 cases. Am J Gastroenterol 1962;38:639–52.Search in Google Scholar
3. Choong, CK, Meyers, BF. Benign esophageal tumors: introduction, incidence, classification, and clinical features. Semin Thoracic Cardiovasc Surg 2003;15:3–8. https://doi.org/10.1016/s1043-0679(03)70035-5.Search in Google Scholar PubMed
4. Attah, EB, Hajdu, SI. Benign and malignant tumors of the esophagus at autopsy. J Thorac Cardiovasc Surg 1968;55:396–404. https://doi.org/10.1016/s0022-5223(19)42966-8.Search in Google Scholar
5. Arnorsson, T, Aberg, C, Aberg, T. Benign tumours of the oesophagus and oesophageal cysts. Scand J Thorac Cardiovasc Surg 1984;18:145–50. https://doi.org/10.3109/14017438409102396.Search in Google Scholar PubMed
6. Seremetis, MG, Lyons, WS, deGuzman, VC, Peabody, JWJr. Leiomyomata of the esophagus. An analysis of 838 cases. Cancer 1976;38:2166–77. https://doi.org/10.1002/1097-0142(197611)38:5<2166::aid-cncr2820380547>3.0.co;2-b.10.1002/1097-0142(197611)38:5<2166::AID-CNCR2820380547>3.0.CO;2-BSearch in Google Scholar
7. Ha, C, Regan, J, Cetindag, IB, Ali, A, Mellinger, JD. Benign esophageal tumors. Surg Clin North Am 2015;95:491–514. https://doi.org/10.1016/j.suc.2015.02.005.Search in Google Scholar
8. Rice, TW. Benign esophageal tumors: esophagoscopy and endoscopic esophageal ultrasound. Semin Thorac Cardiovasc Surg 2003;15:20–6. https://doi.org/10.1016/s1043-0679(03)00035-2.Search in Google Scholar
9. Ma, GL, Murphy, JD, Martinez, ME, Sicklick, JK. Epidemiology of gastrointestinal stromal tumors in the era of histology codes: results of a population-based study. Cancer Epidemiol Biomarkers Prev 2015;24:298–302. https://doi.org/10.1158/1055-9965.epi-14-1002.Search in Google Scholar
10. Ko, WJ, Song, GW, Cho, JY. Evaluation and endoscopic management of esophageal submucosal tumor. Clin Endosc 2017;50:250–3. https://doi.org/10.5946/ce.2016.109.Search in Google Scholar
11. Søreide, K, Sandvik, OM, Søreide, JA, Giljaca, V, Jureckova, A, Bulusu, VR. Global epidemiology of gastrointestinal stromal tumours (GIST): a systematic review of population-based cohort studies. Cancer Epidemiol 2016;40:39–46. https://doi.org/10.1016/j.canep.2015.10.031.Search in Google Scholar
12. Deprez, PH, Moons, LMG, OʼToole, D, Gincul, R, Seicean, A, Pimentel-Nunes, P, et al.. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European society of gastrointestinal endoscopy (ESGE) Guideline. Endoscopy 2022;54:412–29. https://doi.org/10.1055/a-1751-5742.Search in Google Scholar
13. Menon, L, Buscaglia, JM. Endoscopic approach to subepithelial lesions. Therap Adv Gastroenterol 2014;7:123–30. https://doi.org/10.1177/1756283x13513538.Search in Google Scholar PubMed PubMed Central
14. Akahoshi, K, Oya, M, Koga, T, Shiratsuchi, Y. Current clinical management of gastrointestinal stromal tumor. World J Gastroenterol 2018;24:2806–17. https://doi.org/10.3748/wjg.v24.i26.2806.Search in Google Scholar PubMed PubMed Central
15. Rösch, T, Kapfer, B, Will, U, Baronius, W, Strobel, M, Lorenz, R, et al.. Accuracy of endoscopic ultrasonography in upper gastrointestinal submucosal lesions: a prospective multicenter study. Scand J Gastroenterol 2002;37:856–62. https://doi.org/10.1080/713786521.Search in Google Scholar
16. He, G, Wang, J, Chen, B, Xing, X, Wang, J, Chen, J, et al.. Feasibility of endoscopic submucosal dissection for upper gastrointestinal submucosal tumors treatment and value of endoscopic ultrasonography in pre-operation assess and post-operation follow-up: a prospective study of 224 cases in a single medical center. Surg Endosc 2016;30:4206–13. https://doi.org/10.1007/s00464-015-4729-1.Search in Google Scholar PubMed
17. Khan, S, Zhang, R, Fang, W, Wang, T, Li, S, Wang, D, et al.. Reliability of endoscopic ultrasound using miniprobes and grayscale histogram analysis in diagnosing upper gastrointestinal subepithelial lesions. Gastroenterol Res Pract 2020;2020:6591341. https://doi.org/10.1155/2020/6591341.Search in Google Scholar PubMed PubMed Central
18. Minoda, Y, Ihara, E, Komori, K, Ogino, H, Otsuka, Y, Chinen, T, et al.. Efficacy of endoscopic ultrasound with artificial intelligence for the diagnosis of gastrointestinal stromal tumors. J Gastroenterol 2020;55:1119–26. https://doi.org/10.1007/s00535-020-01725-4.Search in Google Scholar PubMed
19. Brand, B, Oesterhelweg, L, Binmoeller, KF, Sriram, PV, Bohnacker, S, Seewald, S, et al.. Impact of endoscopic ultrasound for evaluation of submucosal lesions in gastrointestinal tract. Dig Liver Dis 2002;34:290–7. https://doi.org/10.1016/s1590-8658(02)80150-5.Search in Google Scholar PubMed
20. Levine, MS. Benign tumors of the esophagus: radiologic evaluation. Semin Thorac Cardiovasc Surg 2003;15:9–19. https://doi.org/10.1016/s1043-0679(03)00003-0.Search in Google Scholar PubMed
21. Sakai, M, Kato, H, Saito, K, Tanaka, N, Inose, T, Kimura, H, et al.. Clinical applications of 18F-fluorodeoxyglucose positron emission tomography in gastrointestinal stromal tumor of the esophagus. Int Surg 2008;93:209–13.Search in Google Scholar
22. Winant, AJ, Gollub, MJ, Shia, J, Antonescu, C, Bains, MS, Levine, MS. Imaging and clinicopathologic features of esophageal gastrointestinal stromal tumors. AJR Am J Roentgenol 2014;203:306–14. https://doi.org/10.2214/ajr.13.11841.Search in Google Scholar PubMed PubMed Central
23. Akahoshi, K, Oya, M, Koga, T, Koga, H, Motomura, Y, Kubokawa, M, et al.. Clinical usefulness of endoscopic ultrasound-guided fine needle aspiration for gastric subepithelial lesions smaller than 2 cm. J Gastrointest Liver Dis 2014;23:405–12. https://doi.org/10.15403/jgld.2014.1121.234.eug.Search in Google Scholar PubMed
24. von Mehren, M, Kane, JM, Bui, MM, Choy, E, Connelly, M, Dry, S, et al.. NCCN guidelines insights: soft tissue sarcoma, version 1.2021. J Natl Compr Cancer Netw 2020;18:1604–12. https://doi.org/10.6004/jnccn.2020.0058.Search in Google Scholar PubMed
25. ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumours: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25:iii21–26. https://doi.org/10.1093/annonc/mdu255.Search in Google Scholar PubMed
26. Nishida, T, Hirota, S, Yanagisawa, A, Sugino, Y, Minami, M, Yamamura, Y, et al.. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol 2008;13:416–30. https://doi.org/10.1007/s10147-008-0798-7.Search in Google Scholar PubMed
27. Li, J, Ye, Y, Wang, J, Zhang, B, Qin, S, Shi, Y, et al.. Chinese consensus guidelines for diagnosis and management of gastrointestinal stromal tumor. Chin J Cancer Res 2017;29:281–93. https://doi.org/10.21147/j.issn.1000-9604.2017.04.01.Search in Google Scholar PubMed PubMed Central
28. Khalaileh, A, Savetsky, I, Adileh, M, Elazary, R, Abu-Gazala, M, Abu Gazala, S, et al.. Robotic-assisted enucleation of a large lower esophageal leiomyoma and review of literature. Int J Med Robot 2013;9:253–7. https://doi.org/10.1002/rcs.1484.Search in Google Scholar PubMed
29. Mathew, G, Osueni, A, Carter, YM. Esophageal leiomyoma. In: StatPearls. Treasure Island (FL): StatPearls Publishing LLC; 2022.Search in Google Scholar
30. Schmidt, HW, Clagett, OT, Harrison, EGJr. Benign tumors and cysts of the esophagus. J Thorac Cardiovasc Surg 1961;41:717–32. https://doi.org/10.1016/s0022-5223(20)31637-8.Search in Google Scholar
31. Lee, LS, Singhal, S, Brinster, CJ, Marshall, B, Kochman, ML, Kaiser, LR, et al.. Current management of esophageal leiomyoma. J Am Coll Surg 2004;198:136–46. https://doi.org/10.1016/j.jamcollsurg.2003.08.015.Search in Google Scholar PubMed
32. Hatch, GF3rd, Wertheimer-Hatch, L, Hatch, KF, Davis, GB, Blanchard, DK, Foster, RSJr, et al.. Tumors of the esophagus. World J Surg 2000;24:401–11. https://doi.org/10.1007/s002689910065.Search in Google Scholar PubMed
33. Miettinen, M, Sarlomo-Rikala, M, Sobin, LH, Lasota, J. Esophageal stromal tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 17 cases and comparison with esophageal leiomyomas and leiomyosarcomas. Am J Surg Pathol 2000;24:211–22. https://doi.org/10.1097/00000478-200002000-00007.Search in Google Scholar PubMed
34. Ferrari, D, Bernardi, D, Siboni, S, Lazzari, V, Asti, E, Bonavina, L. Esophageal lipoma and liposarcoma: a systematic review. World J Surg 2021;45:225–34. https://doi.org/10.1007/s00268-020-05789-4.Search in Google Scholar PubMed PubMed Central
35. Jiang, G, Zhao, H, Yang, F, Li, J, Li, Y, Liu, Y, et al.. Thoracoscopic enucleation of esophageal leiomyoma: a retrospective study on 40 cases. Dis Esophagus 2009;22:279–83. https://doi.org/10.1111/j.1442-2050.2008.00883.x.Search in Google Scholar PubMed
36. von, RBH, Stein, HJ, Feussner, H, Siewert, JR. Enucleation of submucosal tumors of the esophagus: minimally invasive versus open approach. Surg Endosc 2004;18:924–30. https://doi.org/10.1007/s00464-003-9130-9.Search in Google Scholar PubMed
37. De Giacomo, T, Bruschini, P, Arcieri, S, Ruberto, F, Venuta, F, Diso, D, et al.. Partial oesophagectomy for giant leiomyoma of the oesophagus: report of 7 cases. Eur J Cardio Thorac Surg 2015;47:143–5. https://doi.org/10.1093/ejcts/ezu146.Search in Google Scholar PubMed
38. Tran, T, Davila, JA, El-Serag, HB. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol 2005;100:162–8. https://doi.org/10.1111/j.1572-0241.2005.40709.x.Search in Google Scholar PubMed
39. Kindblom, LG, Remotti, HE, Aldenborg, F, Meis-Kindblom, JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 1998;152:1259–69.Search in Google Scholar
40. Fletcher, CD, Berman, JJ, Corless, C, Gorstein, F, Lasota, J, Longley, BJ, et al.. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Human Pathol 2002;33:459–65. https://doi.org/10.1053/hupa.2002.123545.Search in Google Scholar PubMed
41. Miettinen, M, Lasota, J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70–83. https://doi.org/10.1053/j.semdp.2006.09.001.Search in Google Scholar PubMed
42. Joensuu, H, Vehtari, A, Riihimäki, J, Nishida, T, Steigen, SE, Brabec, P, et al.. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 2012;13:265–74. https://doi.org/10.1016/s1470-2045(11)70299-6.Search in Google Scholar PubMed
43. Schmieder, M, Henne-Bruns, D, Mayer, B, Knippschild, U, Rolke, C, Schwab, M, et al.. Comparison of different risk classification systems in 558 patients with gastrointestinal stromal tumors after R0-resection. Front Pharmacol 2016;7:504. https://doi.org/10.3389/fphar.2016.00504.Search in Google Scholar PubMed PubMed Central
44. Kang, G, Kang, Y, Kim, KH, Ha, SY, Kim, JY, Shim, YM, et al.. Gastrointestinal stromal tumours of the oesophagus: a clinicopathological and molecular analysis of 27 cases. Histopathology 2017;71:805–12. https://doi.org/10.1111/his.13292.Search in Google Scholar PubMed
45. Blum, MG, Bilimoria, KY, Wayne, JD, de Hoyos, AL, Talamonti, MS, Adley, B. Surgical considerations for the management and resection of esophageal gastrointestinal stromal tumors. Ann Thoracic Surg 2007;84:1717–23. https://doi.org/10.1016/j.athoracsur.2007.05.071.Search in Google Scholar PubMed
46. Demetri, GD, von Mehren, M, Blanke, CD, Van den Abbeele, AD, Eisenberg, B, Roberts, PJ, et al.. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472–80. https://doi.org/10.1056/nejmoa020461.Search in Google Scholar PubMed
47. Demetri, GD, van Oosterom, AT, Garrett, CR, Blackstein, ME, Shah, MH, Verweij, J, et al.. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 2006;368:1329–38. https://doi.org/10.1016/s0140-6736(06)69446-4.Search in Google Scholar
48. Heinrich, MC, Jones, RL, von Mehren, M, Schöffski, P, Serrano, C, Kang, Y-K, et al.. Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): a multicentre, open-label, phase 1 trial. Lancet Oncol 2020;21:935–46. https://doi.org/10.1016/s1470-2045(20)30269-2.Search in Google Scholar
49. Yip, D, Zalcberg, J, Ackland, S, Barbour, AP, Desai, J, Fox, S, et al.. Controversies in the management of gastrointestinal stromal tumors. Asia Pac J Clin Oncol 2014;10:216–27. https://doi.org/10.1111/ajco.12187.Search in Google Scholar PubMed
50. Dematteo, RP, Ballman, KV, Antonescu, CR, Maki, RG, Pisters, PW, Demetri, GD, et al.. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373:1097–104. https://doi.org/10.1016/s0140-6736(09)60500-6.Search in Google Scholar
51. Casali, PG, Blay, JY, Abecassis, N, Bajpai, J, Bauer, S, Biagini, R, et al.. Gastrointestinal stromal tumours: ESMO–EURACAN–GENTURIS clinical practice guidelines for diagnosis, treatment and follow-up 2606. Ann Oncol 2022;33:20–33. https://doi.org/10.1016/j.annonc.2021.09.005.Search in Google Scholar PubMed
52. Joensuu, H, Eriksson, M, Sundby Hall, K, Reichardt, A, Hermes, B, Schütte, J, et al.. Survival outcomes associated with 3 years vs. 1 year of adjuvant imatinib for patients with high-risk gastrointestinal stromal tumors: an analysis of a randomized clinical trial after 10-year follow-up. JAMA Oncol 2020;6:1241–6. https://doi.org/10.1001/jamaoncol.2020.2091.Search in Google Scholar PubMed PubMed Central
53. Casali, PG, Cesne, AL, Velasco, AP, Kotasek, D, Rutkowski, P, Hohenberger, P, et al.. Time to definitive failure to the first tyrosine kinase inhibitor in localized gi stromal tumors treated with imatinib as an adjuvant: a European organisation for research and treatment of cancer soft tissue and bone sarcoma group intergroup randomized trial in collaboration with the australasian gastro-intestinal trials group, UNICANCER, French Sarcoma Group, Italian Sarcoma Group, and Spanish Group for Research on Sarcomas. J Clin Oncol 2015;33:4276–83. https://doi.org/10.1200/jco.2015.62.4304.Search in Google Scholar PubMed
54. Joensuu, H. Adjuvant treatment of GIST: patient selection and treatment strategies. Nat Rev Clin Oncol 2012;9:351–8. https://doi.org/10.1038/nrclinonc.2012.74.Search in Google Scholar PubMed
55. Hohenberger, P, Ronellenfitsch, U, Oladeji, O, Pink, D, Ströbel, P, Wardelmann, E, et al.. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumour. British J Surgery 2010;97:1854–9. https://doi.org/10.1002/bjs.7222.Search in Google Scholar PubMed
56. Lu, J, Dai, Y, Zheng, HL, Xie, JW, Wang, JB, Lin, JX, et al.. What is the appropriate duration of adjuvant imatinib mesylate treatment for primary gastrointestinal stromal tumors classified according to the strict definition of tumor rupture? Medicine (Baltimore) 2019;98:e14177. https://doi.org/10.1097/md.0000000000014177.Search in Google Scholar
57. Blanke, CD, Demetri, GD, von Mehren, M, Heinrich, MC, Eisenberg, B, Fletcher, JA, et al.. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 2008;26:620–5. https://doi.org/10.1200/jco.2007.13.4403.Search in Google Scholar
58. Zhong, N, Katzka, DA, Smyrk, TC, Wang, KK, Topazian, M. Endoscopic diagnosis and resection of esophageal granular cell tumors. Dis Esophagus: Off J Int Soc Dis Esophagus/ISDE 2011;24:538–43. https://doi.org/10.1111/j.1442-2050.2011.01197.x.Search in Google Scholar PubMed
59. David, O, Jakate, S. Multifocal granular cell tumor of the esophagus and proximal stomach with infiltrative pattern: a case report and review of the literature. Arch Pathol Lab Med 1999;123:967–73. https://doi.org/10.5858/1999-123-0967-mgctot.Search in Google Scholar PubMed
60. Fanburg-Smith, JC, Meis-Kindblom, JM, Fante, R, Kindblom, LG. Malignant granular cell tumor of soft tissue: diagnostic criteria and clinicopathologic correlation. Am J Surg Pathol 1998;22:779–94. https://doi.org/10.1097/00000478-199807000-00001.Search in Google Scholar PubMed
61. Stašek, M, Aujeský, R, Škarda, J, Švébišová, H, Vrba, R, Szkorupa, M, et al.. Malignant granular cell tumor of the esophagus: a case report. Ann Thorac Cardiovasc Surg 2020;26:359–64. https://doi.org/10.5761/atcs.cr.20-00117.Search in Google Scholar PubMed PubMed Central
62. Wyatt, MG, O’Donoghue, DS, Clarke, TJ, Teasdale, C. Malignant granular cell tumour of the oesophagus. Eur J Surg Oncol 1991;17:388–91.Search in Google Scholar
63. Chen, WS, Zheng, XL, Jin, L, Pan, XJ, Ye, MF. Novel diagnosis and treatment of esophageal granular cell tumor: report of 14 cases and review of the literature. Ann Thoracic Surg 2014;97:296–302. https://doi.org/10.1016/j.athoracsur.2013.08.042.Search in Google Scholar PubMed
64. Reddi, D, Chandler, C, Cardona, D, Schild, M, Westerhoff, M, McMullen, E, et al.. Esophageal granular cell tumor and eosinophils: a multicenter experience. Diagn Pathol 2021;16:49. https://doi.org/10.1186/s13000-021-01113-3.Search in Google Scholar PubMed PubMed Central
65. Thumallapally, N, Ibrahim, U, Kesavan, M, Chang, Q, Opitz, L, Dhar, M, et al.. Esophageal granular cell tumor: a case report and review of literature. Cureus 2016;8:e782. https://doi.org/10.7759/cureus.782.Search in Google Scholar PubMed PubMed Central
66. Tsai, SJ, Lin, CC, Chang, CW, Hung, CY, Shieh, TY, Wang, HY, et al.. Benign esophageal lesions: endoscopic and pathologic features. World J Gastroenterol 2015;21:1091–8. https://doi.org/10.3748/wjg.v21.i4.1091.Search in Google Scholar PubMed PubMed Central
67. Hou, YY, Tan, YS, Xu, JF, Wang, XN, Lu, SH, Ji, Y, et al.. Schwannoma of the gastrointestinal tract: a clinicopathological, immunohistochemical and ultrastructural study of 33 cases. Histopathology 2006;48:536–45. https://doi.org/10.1111/j.1365-2559.2006.02370.x.Search in Google Scholar PubMed
68. Lenarcik, M, Pachlewski, J, Mróz, A. Bean’s syndrome (blue rubber bleb nevus syndrome – BRBNS) as a gastrointestinal bleeding – case report and review of the literature. Pol J Pathol 2021;72:190–4. https://doi.org/10.5114/pjp.2021.109524.Search in Google Scholar PubMed
69. Kim, JH, Jung, SW, Song, JG, Choe, JW, Kim, SY, Hyun, JJ, et al.. [Esophageal hemangioma treated by endoscopic mucosal resection: a case report and review of the literature]. Korean J Gastroenterol 2015;66:277–81. https://doi.org/10.4166/kjg.2015.66.5.277.Search in Google Scholar PubMed
70. Sogabe, M, Taniki, T, Fukui, Y, Yoshida, T, Okamoto, K, Okita, Y, et al.. A patient with esophageal hemangioma treated by endoscopic mucosal resection: a case report and review of the literature. J Med Invest 2006;53:177–82. https://doi.org/10.2152/jmi.53.177.Search in Google Scholar PubMed
71. Mayo, CW, Pagtalunan, RJ, Brown, DJ. Lipoma of the alimentary tract. Surgery 1963;53:598–603.Search in Google Scholar
72. Mehdorn, AS, Schmidt, F, Steinestel, K, Wardelmann, E, Greulich, B, Palmes, D, et al.. Pedunculated, well differentiated liposarcoma of the oesophagus mimicking giant fibrovascular polyp. Ann R Coll Surg Engl 2017;99:e209–12. https://doi.org/10.1308/rcsann.2017.0117.Search in Google Scholar PubMed PubMed Central
73. Taylor, AJ, Stewart, ET, Dodds, WJ. Gastrointestinal lipomas: a radiologic and pathologic review. Am J Roentgenol 1990;155:1205–10. https://doi.org/10.2214/ajr.155.6.2122666.Search in Google Scholar PubMed
74. Graham, RP, Yasir, S, Fritchie, KJ, Reid, MD, Greipp, PT, Folpe, AL. Polypoid fibroadipose tumors of the esophagus: ’giant fibrovascular polyp’ or liposarcoma? A clinicopathological and molecular cytogenetic study of 13 cases. Mod Pathol 2018;31:337–42. https://doi.org/10.1038/modpathol.2017.140.Search in Google Scholar PubMed
75. Boni, A, Lisovsky, M, Dal Cin, P, Rosenberg, AE, Srivastava, A. Atypical lipomatous tumor mimicking giant fibrovascular polyp of the esophagus: report of a case and a critical review of literature. Hum Pathol 2013;44:1165–70. https://doi.org/10.1016/j.humpath.2012.10.023.Search in Google Scholar PubMed
76. Segura, S, Mansoor, S, Gorelick, AB, Sieber, S, El-Fanek, H. Glomus tumor of the esophagus: a case report and review of the literature. Conn Med 2015;79:93–5.Search in Google Scholar
77. Xiao, A, Ahlers, M, Dry, SM, Weber, AT, Chiu, VY, Pessegueiro, AM. Rare malignant glomus tumor of the esophagus with pulmonary metastasis: a case report. AME Case Rep 2022;6:20. https://doi.org/10.21037/acr-21-72.Search in Google Scholar PubMed PubMed Central
78. Zhang, YM, Boerwinkel, DF, Qin, X, He, S, Xue, L, Weusten, BL, et al.. A randomized trial comparing multiband mucosectomy and cap-assisted endoscopic resection for endoscopic piecemeal resection of early squamous neoplasia of the esophagus. Endoscopy 2016;48:330–8. https://doi.org/10.1055/s-0034-1393358.Search in Google Scholar PubMed PubMed Central
79. Hwang, JH, Konda, V, Abu Dayyeh, BK, Chauhan, SS, Enestvedt, BK, Fujii-Lau, LL, et al.. Endoscopic mucosal resection. Gastrointest Endosc 2015;82:215–26. https://doi.org/10.1016/j.gie.2015.05.001.Search in Google Scholar PubMed
80. Maple, JT, Abu Dayyeh, BK, Chauhan, SS, Hwang, JH, Komanduri, S, Manfredi, M, et al.. Endoscopic submucosal dissection. Gastrointest Endosc 2015;81:1311–25. https://doi.org/10.1016/j.gie.2014.12.010.Search in Google Scholar PubMed
81. Bechara, R, Inoue, H. Recent advancement of therapeutic endoscopy in the esophageal benign diseases. World J Gastrointest Endosc 2015;7:481–95. https://doi.org/10.4253/wjge.v7.i5.481.Search in Google Scholar PubMed PubMed Central
82. Mutrie, CJ, Donahue, DM, Wain, JC, Wright, CD, Gaissert, HA, Grillo, HC, et al.. Esophageal leiomyoma: a 40-year experience. Ann Thorac Surg 2005;79:1122–5. https://doi.org/10.1016/j.athoracsur.2004.08.029.Search in Google Scholar PubMed
83. Zaninotto, G, Portale, G, Costantini, M, Rizzetto, C, Salvador, R, Rampado, S, et al.. Minimally invasive enucleation of esophageal leiomyoma. Surg Endosc 2006;20:1904–8. https://doi.org/10.1007/s00464-005-0838-6.Search in Google Scholar PubMed
84. Chen, YH, Chen, KC, Huang, PM, Kuo, SW, Lin, MW, Lee, JM. Robotic-assisted thoracoscopic (RATS) enucleation of esophageal mesenchymal tumors and foregut cysts. J Clin Med 2022;11. https://doi.org/10.3390/jcm11216471.Search in Google Scholar PubMed PubMed Central
85. Froiio, C, Berlth, F, Capovilla, G, Tagkalos, E, Hadzijusufovic, E, Mann, C, et al.. Robotic-assisted surgery for esophageal submucosal tumors: a single-center case series. Updates Surg 2022;74:1043–54. https://doi.org/10.1007/s13304-022-01247-z.Search in Google Scholar PubMed PubMed Central
86. Lee, HJ, Park, S-I, Kim, DK, Kim, YH. Surgical resection of esophageal gastrointestinal stromal tumors. Ann Thorac Surg 2009;87:1569–71. https://doi.org/10.1016/j.athoracsur.2009.01.051.Search in Google Scholar PubMed
87. Pence, K, Correa, AM, Chan, E, Khaitan, P, Hofstetter, W, Kim, MP. Management of esophageal gastrointestinal stromal tumor: review of one hundred seven patients. Dis Esophagus 2017;30:1–5. https://doi.org/10.1093/dote/dox064.Search in Google Scholar PubMed
88. Demetri, GD, Benjamin, RS, Blanke, CD, Blay, JY, Casali, P, Choi, H, et al.. NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)--update of the NCCN clinical practice guidelines. J Natl Compr Cancer Netw 2007;5:S1–29. https://doi.org/10.6004/jnccn.2007.2002. quiz S30.Search in Google Scholar
89. Landi, B, Blay, J-Y, Bonvalot, S, Brasseur, M, Coindre, JM, Emile, JF, et al.. Gastrointestinal stromal tumours (GISTs): French Intergroup Clinical Practice Guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO). Digest Liver Dis 2019;51:1223–31. https://doi.org/10.1016/j.dld.2019.07.006.Search in Google Scholar PubMed
90. Cohen, C, Pop, D, Icard, P, Berthet, JP, Venissac, N, Mouroux, J. Is there a place for thoracoscopic enucleation of esophageal gastrointestinal stromal tumors? Thorac Cardiovasc Surg 2019;67:585–8. https://doi.org/10.1055/s-0038-1670662.Search in Google Scholar PubMed
91. Robb, WB, Bruyere, E, Amielh, D, Vinatier, E, Mabrut, JY, Perniceni, T, et al.. Esophageal gastrointestinal stromal tumor: is tumoral enucleation a viable therapeutic option? Ann Surg 2015;261:117–24. https://doi.org/10.1097/sla.0000000000000505.Search in Google Scholar
92. Mohammadi, M, Ijzerman, NS, Hohenberger, P, Rutkowski, P, Jones, RL, Martin-Broto, J, et al.. Clinicopathological features and treatment outcome of oesophageal gastrointestinal stromal tumour (GIST): a large, retrospective multicenter European study. Eur J Surg Oncol 2021;47:2173–81. https://doi.org/10.1016/j.ejso.2021.03.234.Search in Google Scholar PubMed
93. Jeon, HW, Choi, MG, Lim, CH, Park, JK, Sung, SW. Intraoperative esophagoscopy provides accuracy and safety in video-assisted thoracoscopic enucleation of benign esophageal submucosal tumors. Dis Esophagus 2015;28:437–41. https://doi.org/10.1111/dote.12220.Search in Google Scholar PubMed
94. Macke, RA, Nason, KS. Minimally invasive resection of benign esophageal lesions. Oper Tech Thorac Cardiovasc Surg 2014;19:396–413. https://doi.org/10.1053/j.optechstcvs.2014.12.002.Search in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorial
- The evolving role of esophageal surgery – advancements, challenges, and the path forward
- Reviews
- Neoadjuvant treatment of esophageal cancer: chemotherapy, chemoradiation, immunotherapy, and future trends of therapy
- Active surveillance in patients with a complete clinical response after neoadjuvant chemoradiotherapy for esophageal- and gastroesophageal junction cancer
- Management of subepithelial esophageal tumors
- What is the best surgical approach for esophageal cancer?
- Immunotherapy as a new perspective for the therapy of esophageal cancer
- Voluminous hiatal hernias – the role of robotic surgery
- Oligometastatic disease – a renaissance for surgery?
Articles in the same Issue
- Frontmatter
- Editorial
- The evolving role of esophageal surgery – advancements, challenges, and the path forward
- Reviews
- Neoadjuvant treatment of esophageal cancer: chemotherapy, chemoradiation, immunotherapy, and future trends of therapy
- Active surveillance in patients with a complete clinical response after neoadjuvant chemoradiotherapy for esophageal- and gastroesophageal junction cancer
- Management of subepithelial esophageal tumors
- What is the best surgical approach for esophageal cancer?
- Immunotherapy as a new perspective for the therapy of esophageal cancer
- Voluminous hiatal hernias – the role of robotic surgery
- Oligometastatic disease – a renaissance for surgery?

