Abstract
Objectives
In patients with non-small cell lung cancer (NSCLC) the pathologic lymph node status N2 is a heterogeneous entity, with different degrees of lymph node involvement representing different prognoses. It is speculated whether extra capsular nodal extension may help to define a subgroup with implications on long-term survival.
Methods
We retrospectively identified 118 patients with non-small cell lung cancer (65 men, 53 women), who were treated between 2013 and 2018 and found to have pathologic N2 lymph node involvement. In all patients lung resection with systematic mediastinal and hilar lymph node dissection was performed with curative intent. In N2 lymph node metastases capsules of affected lymph nodes were examined microscopically as to whether extracapsular extension was present.
Results
51 patients (43 %) had extracapsular extension (ENE). Most of these patients (n=35) only had ENE in a single lymph node (69 %). The overall 5-year survival rate was 24.6 % and progression-free survival rate 17.8 %. In the multivariate analysis OS was worse for patients with multiple affected pN2 stations, concurrent N1 metastases, increasing age, and larger tumor size. For the percentage of lymph nodes affected with ENE (of total examined) only a non-significant trend towards worse OS could be observed (p=0.06).
Conclusions
Although we could not demonstrate significant prognostic differences between N2 extra capsular nodal involvement within our patient population, other analyses may yield different results. However, clinicians should continue performing thorough lymph nodes dissections in order to achieve local complete resection even in patients with extra capsular tumor spread
Introduction
Despite at least 30 years of increasingly aggressive anti-smoking campaigns, as well as advances in early recognition and targeted immunotherapies, non-small cell lung cancer (NSCLC) remains a leading cause of death in the Western world [1, 2]. If discovered early enough, surgery remains a mainstay of treatment [3, 4]. The extent to which adjuvant chemotherapy and radiation should play a role is largely determined by the tumor stage at time of surgery but remains controversial. For patients with pN2 status, according to the TNM staging system, adjuvant treatment is almost universally recommended. And yet pN2 represents a heterogenous group. Although a more precise differentiation of the N indicator has been discussed [5], none of the characteristics considered were incorporated into the most recent 8th edition of the TNM staging system [1]. As it stands, a single, enclosed metastasis to a mediastinal lymph node assigns the patient to the pN2 category. The number of nodes and stations affected is not considered. The morphology of the affected lymph nodes does not play a role in staging either. The presence of extra capsular extension of lymph node metastases (ENE) is considered in the TNM staging system for some types of cancer: in head and neck cancer, for example, ENE (defined as N3b) is suggested to have a significant detrimental effect on prognosis [6]. Although it does not currently affect staging in NSCLC, there is some evidence that ENE may play a significant prognostic role in NSCLC as well [7], [8], [9]. In this monocentric investigation of surgically resected NSCLC patients with pN2 metastases, we seek to determine, whether ENE had a significant negative impact on prognosis.
Patients and methods
Our study included 118 patients, who received curatively intended surgical resection for NSCLC between January 2013 and December 2017. Only patients, who had received complete lymph node dissection and had histologically established metastases of the mediastinal lymph nodes were included. Patients with neoadjuvant treatment, non R0 resections, or death within 30 days of surgery were excluded. Patients with recurrent tumors were also excluded. Patient selection is represented visually in Figure 1.

Patients included in analysis.
Preoperative staging included physical examination, PET-CT of the entire body. Cerebral imaging was only performed preoperatively in cases of clinically suspected metastases.
Surgeries were usually lobectomy or extended lobectomy and, less frequently, pneumonectomy or segment resection. Systematic lymph node dissection [10] included complete removal of hilar, interlobar, lobar, intralobar, and mediastinal lymph nodes – when possible, as complete compartments. As is recommended practice mediastinal nodal resection on the right side included lymph node positions 7, 8, 9, 2, and 4; left sided resection included lymph node positions 7, 8, 9, 5, and 6.
Microscopic examination
All resected lymph nodes were retrospectively examined by board certified pathologists, and patients with one or more metastasis in an N2 position were included in this analysis. The presence or absence of N1 metastases was also documented. All lymph node and tumor specimens were fixed in formalin, sectioned into 3–5 mm slices, stained with hematoxylin-eosin and examined microscopically. After malignancy was established, further characteristics were determined: histological type, differentiation grade (G), tumor size (largest diameter), and when possible vascular or lymphatic invasion. Finally, the capsules of affected lymph nodes were examined microscopically by two board-certified pathologists, and a consensus was reached as to whether ENE was present. We defined ENE of nodal metastasis as continuous extension of metastatic cells through the nodal capsule into the perinodal tissue. Fragmented lymph node tissue with metastasis at the edges but no clearly recognizable capsule was classified as non ENE. Tumor deposits found only in surrounding fat tissue were also classified as non ENE. A lymph node with extracapsular extension is presented in Figure 2.

Lymph node with extracapsular extension.
The number and station of affected lymph nodes was documented, and the number (if >1) of lymph nodes with extracapsular extension was noted. We also noted what percentage of lymph nodes (of the number examined) were affected.
Immunhistology and molecular pathology
All tumors were examined with immunohistochemistry for a precise entity diagnosis. Molecular analysis was performed according to standard diagnostic procedure for adenocarcinomas, sarcomatoid carcinomas and large cell carcinomas, but not for squamous carcinomas. Additional immunohistochemical analysis of lymph node metastasis was not performed.
Follow-up
We retrospectively extracted data on clinical patient characteristics from the data bank of the Lung Clinic. These included whether or not patients had received adjuvant chemotherapy and radiation, as is recommended in cases of mediastinal nodal metastases. We also extracted data on age, gender, type of surgery, tumor histology, tumor size, pV (vascular invasion) and pL (lymphatic invasion) status, and EGFR and KRAS mutations. We also extracted data on tumor stage and reclassified it based on the currently applied 8th edition of the TNM system. Following treatment, patients were followed up with chest CT alternating with chest radiography every three months for the first two postoperative years and twice a year for the three years thereafter. In cases where patients were lost to follow-up we contacted the district resident’s registry to determine whether the patients were still alive or if they were not, when they had died. All patients had preoperatively given their informed consent to have their anonymized data and tissue specimens used for future research projects. For this reason ethics committee approval has been waived.
Statistical analysis
Non-censored patients were followed for up 99 months, with a median follow-up time of 72 months. Overall survival (OS) time was calculated as the difference between date of surgery and date of death from any cause or last known follow-up. Progression-free survival (PFS) was the difference between date of surgery and date of established recurrence or death from any cause. The Kaplan–Maier method was used to generate estimated survival curves for OS and PFS.
The above mentioned histological and clinical characteristics were analyzed to determine their potential influences on progression-free survival (PFS) and overall survival (OS). Initial analyses were performed using the chi-squared test for categorical variables and Student’s t-test and Wilcoxon test for continuous variables. Variables where p<0.1 were considered for the multivariable analysis (Cox proportional hazards).
All statistical analyses were performed using R 3.3.0 software (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
We identified 65 men and 53 women, who fit our study inclusion criteria. See Figure 1. The median age was 64.6 years. The median tumor size was 45 mm. Adenocarcinoma was the most frequently represented histology (n=83; 70.3 %), followed by squamous cell carcinoma (n=31; 26.3 %) and other forms (n=4; 3.3 %). Immunohistochemical analysis was performed in 87 patients (73.7 %). The most frequent mutations were EGFR (n=17) and KRAS (n=19). The majority of surgeries performed were lobectomies or extended lobectomies (n=99), with a much smaller number of pneumonectomies (n=18) and a single segmental resection. In 115 cases the procedure was performed as an open thoracotomy; three were performed as VATS. 114 patients (96.6 %) had adjuvant radiation, chemotherapy, or both.
Lymph nodes
A total of 51 patients (43 %) had extracapsular extension (ENE). Most of these patients (n=35) only had ENE in a single lymph node (69 %), while 9 patients had ENE in 2 lymph nodes (18 %), and 7 patients had ENE in 3 or more nodes (14 %). In 78 of 81 ENE positive lymph nodes, the lymph node was complete. In three cases the lymph node was fragmented, but after careful examination by the pathologists it was possible to reconstruct enough to determine that the affected fragments belonged to a single lymph node so that they were not erroneously counted twice. 67 patients (57 %) were negative for ENE. Here, we counted a total of 157 lymph node specimens; 24 of these were lymph-node fragments (15 %), and the rest were complete lymph nodes. Of the 1800 lymph nodes examined, 81 lymph nodes were ENE positive. Thus, in 95.5 % of examined lymph nodes no ENE was detected. The median number of examined lymph nodes was 14, and the median percentage of affected lymph nodes was 14 %. 24 patients (29 %) had affected lymph nodes in multiple stations. 39 patients (33 %) had skip metastases where no metastases in the N1 lymph nodes were present. These data are summarized in Tables 1 and 2.
Clinicopathologic characteristics of study population (categorical variable).
| Variable | Number | Percent |
|---|---|---|
| Sex | ||
| Male | 65 | (55 %) |
| Female | 53 | (45 %) |
| Radiation | ||
| No | 9 | (7.1 %) |
| Yes | 109 | (92.9 %) |
| Chemotherapy | ||
| No | 8 | (6.8 %) |
| Yes | 110 | (93.2 %) |
| Adjuvant treatment | ||
| Yes | 114 | (96.6 %) |
| Only surgery | 4 | (3.4 %) |
| Operation | ||
| (Bi)lobectomy | 99 | (83.9 %) |
| Segmentectomy | 1 | (0.8 %) |
| Pneumonectomy | 18 | (15.3 %) |
| Histology | ||
| Adeoncarcinoma | 83 | (70.3 %) |
| Squamous cell | 31 | (26.3 %) |
| Other | 4 | (3.4 %) |
| T-indicator (8th Ed. TNM) | ||
| pT1 | 16 | (13.6 %) |
| pT2 | 43 | (36.4 %) |
| pT3 | 34 | (28.8 %) |
| pT4 | 25 | (21.2 %) |
| Highest nodal station affected | ||
| 3/9 | 5 | (4.3 %) |
| 7/8 | 46 | (39.7 %) |
| 2/4 or 5/6 | 65 | (56 %) |
| ENE | ||
| Positive | 51 | (43 %) |
| Negative | 67 | (57 %) |
| ENE | ||
| No affected nodes | 67 | (57 %) |
| One affected | 35 | (30 %) |
| ≥2 affected | 16 | (13 %) |
| pN1 metastases | ||
| Yes | 79 | (67 %) |
| No | 39 | (33 %) |
| Multiple nodal stations with metastases | ||
| Yes | 24 | (20 %) |
| No | 93 | (80 %) |
| Lymph invasion | ||
| L0 | 47 | (45.6 %) |
| L1 | 56 | (54.4 %) |
| Vascular invasion | ||
| V0 | 67 | (63.8 %) |
| V1 | 38 | (36.2 %) |
| Tumor size | ||
| ≤40 mm | 44 | (39 %) |
| >40 mm | 69 | (61 %) |
| EGFR | ||
| Positive | 17 | (19.5 %) |
| Negative | 70 | (80.5 %) |
| KRAS | ||
| Positive | 19 | (21.8 %) |
| Negative | 68 | (78.2 %) |
-
ENE, extracapsular extension.
Clinicopathologic characteristics (continous variables).
| Variable | |
|---|---|
| OS, months/median | 38.3 (24) |
| PFS, months/median | 29.7 (24.6) |
| Age, years/median | 64.6 (9.6) |
| N2 nodes affected, % | 2 (0–2) |
| Tumor size, mm/mean | 45 (14–76) |
| Number ENE | 0 (0–2) |
| Percent ENE | 0 (0–7.6 %) |
| Number N2 nodes affected/mean | 13.5 (3.5–23.5) |
-
Data presented as mean (+/− SD) or median (IQR). OS, overall survival; PFS, disease free survival; ENE, extracapsular extension (of nodal metastases).
Survival
The median follow-up for non-censored patients was 72 months. The median OS was 38.3 months (SD 24), and the median PFS was 29.7 months (SD 24.6). 62 patients died during the follow-up period, and 80 patients died or had a tumor recurrence. The 5-year survival rate for OS was 24.6 %; for PFS it was 17.8 %. See Figures 3 and 4 for cumulative survival curves.

Cumulative survival curve – overall survival.
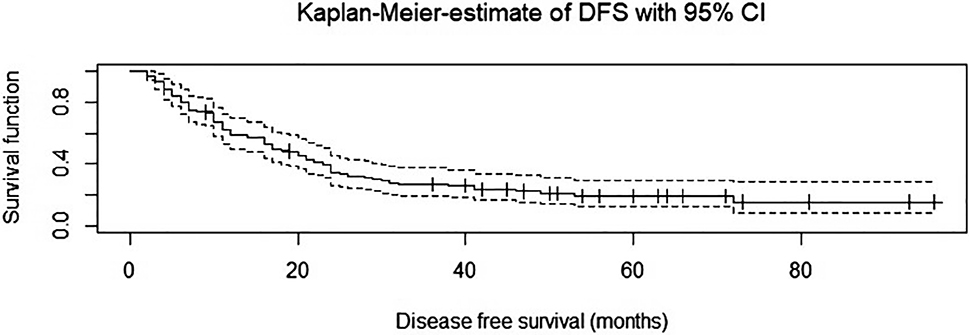
Cumulative survival curve – progression free survival (Disease free survival).
In the univariate analysis metastases in multiple lymph nodes stations, concurrent N1 metastases, no adjuvant treatment, greater age, and larger tumor size were all associated with worse OS and PFS. ENE as a binary category (y/n) was not significant, but the number and percent of lymph nodes affected with ENE was. In a post hoc stratification of ENE into 0, 1, or 2+ (number of affected LN) the effect on OS and PFS was also not significant. The total number and percent of pN2 lymph nodes (with or without ENE) was also associated with worse OS and PFS. See Tables 3 and 4. EGFR and KRAS mutations had no significant effect on OS or PFS in this collective.
Univariate analysis between categorical clinicopathological characteristics and prognosis for categorical variables.
| Variable | p-Value (PFS) | p-Value (OS) |
|---|---|---|
| Chemotherapy, y/n | 0.186 | 0.193 |
| EGFR, y/n | 0.339 | 0.403 |
| ENE, y/n | NS | NS |
| Highest nodal station affected | NS | NS |
| Histology | NS | NS |
| KRAS | 0.568 | 0.44 |
| Multiple nodal stations affected | 0.004 | 0.004 |
| pN1 metastases | 0.045 | 0.08 |
| Uncertain resection | NS | NS |
| Only surgery | 0.022 | 0.0125 |
| Operation type | NS | NS |
| pT status (1,2,3,4) | NS | NS |
| Radiation, y/n | NS | NS |
| Sex, m/f | 0.17 | 0.098 |
| Lymph invasion | NS | NS |
| Vascular invasion | 0.089 | 0.2 |
| ENE (0, 1, 2+) | NS | NS |
-
PFS, disease free survival; OS, overall survival; ENE, extracapsular extension; NS, non-significant.
Univaraite analysis for continous variables.
| Variable | p-Value (PFS) | p-Value (OS) |
|---|---|---|
| Age | 0.05 | <0.001 |
| Tumor size | <0.001 | <0.001 |
| Number ENE | <0.001 | <0.001 |
| Percent ENE | <0.001 | <0.001 |
| Total pN2 nodes | <0.001 | <0.001 |
| Percent pN2 nodes | <0.001 | <0.001 |
-
PFS, disease free survival; OS, overall survival; ENE, extracapsular extension.
Since it was of interest to this study an additional category of “uncertain resection” was created post hoc. This was based on a concept developed by Gagliasso et al. [11] and includes patients with either ENE or an affected lymph node in the highest position (2/4 or 5/6). Uncertain resection, however, was not prognostically significant in our study population.
In the multivariate analysis OS was worse for patients with multiple pN2 stations, concurrent N1 metastases, increasing age, and larger tumor size. For the percentage of lymph nodes affected with ENE (of total examined) only a non-significant trend towards worse OS could be observed (p=0.06). Multiple pN2 stations and concurrent N1 metastases were associated with worse PFS as well. See Tables 5 and 6 as well as Figure 5. 26 patients (of 106 on whom data was available) had local recurrences (24.5 %). Among these patients the median time until recurrence was 22.4 months.
Multivariable analysis for OS.
| Variable | p-Value | Hazard ratio (95 % CI) |
|---|---|---|
| Multiple nodal stations affected | <0.001 | 8.16 (4.2–15.7) |
| No pN1 metastases | 0.003 | 0.45 (0.26–0.77) |
| Age | 0.004 | 1.04 (1.01–1.08) |
| Tumor size | 0.028 | 1.01 (1.001–1.02) |
| Percent ENE | 0.061 | 3.2 (1.1–4.2) |
-
CI, confidence interval; OS, overall survival; ENE, extracapsular extension.
Multivariable analysis for PFS.
| Variable | p-Value | Hazard ratio (95 % CI) |
|---|---|---|
| Multiple nodal stations affected | <0.001 | 3.01 (1.1–4.17) |
| No pN1 metastases | 0.007 | 0.51 (0.30–0.62) |
| Age | NS | |
| Tumor size | NS | |
| Percent ENE | NS |
-
CI, confidence interval; PFS, disease free survival; ENE, extracapsular extension; NS, non-significant.

Results of multivariate analyses: The effects of concurrent N1 metastases (continuous) and multiple affected N2 nodal stations vs. single N2 nodal station on OS and PFS (DFS).
We did not however find any significant relationship between ENE and likelihood of local recurrence.
Discussion
The primary finding of this paper was that ENE did not have a significant independent impact on long-term survival or progression-free survival in surgically resected NSCLC patients with pN2 metastases. This stands in opposition to previous studies [7], [8], [9]. Although ENE was associated with worse OS and PFS in our univariate analyses, none of the measures of ENE were significant in the multivariable analyses. Here we can speculate that the more powerful factors of overall extent of nodal metastatic spread (involvement of multiple nodal stations, concurrent N1 metastases) may have outweighed any effect of ENE. These factors were not considered either in the analysis by Liu and colleagues [9] or Lee and colleagues [7]. Moreover, our study was confined to patients with pN2 metastases, whereas Liu and Lee included patients with only pN1 metastases and, as would be expected, found that these patients had better prognoses. We did find, however, that patients with concurrent pN1 metastases had worse outcomes than those with only pN2 (skip metastases). This is in concurrence with previous findings [12], [13], [14], [15]. Luchini et al. [8] found that the detrimental effects of ENE were more pronounced in cases of pN1 rather than pN2 metastases.
It is worth noting that most of our patients with ENE had only less than 3 nodes with extranodal involvement. This may have affected our results. Liu only considered presence vs. absence of ENE and did not report on the number of affected nodes [9]. Lee differentiated between 0, 1–3 and >3 nodes affected with ENE. Here, of the patients with ENE over half (53.7 %) had >3 nodes [7]. In our collective, in contrast, many patients with ENE had only a single affected node (35.7 %), whereas only three patients (5.9 %) had 3 or more nodes affected with ENE. In contrast to our study, neither of the studies mentioned above give a clear definition of ENE. For example, it is unclear whether specimens with isolated tumor cells in fat tissue and fragmented lymph node tissue were counted as ENE or not. In other studies as well, there seems to be some ambiguity as to what constitutes ENE. In a meta-analysis on the topic Luchini et al. [8] found that in some studies ENE was defined as metastatic cells appearing to extend continuously beyond lymph node capsule, whereas in others it was defined to included tumor deposits and vascular emboli observed in the surrounding adipose tissue. Theunissen et al. [16] have also pointed to some possible difficulties in achieving consistent diagnoses of extra capsular extension but argue that it is possible as long as clearly established criteria is applied. The lack of a consistent definition of ENE makes inter-study comparison difficult. Not only should surgeons and pathologists be encouraged to consider ENE in dissected lymph nodes, but for future, larger studies a consensus on ENE definition would be of utmost importance.
A secondary finding of our study is that metastases to multiple lymph nodes stations – whether ENE was present or not -- were associated with worse OS and PFS. This is in concurrence with several previous studies [17], [18], [19], [20].
It is well established that locoregional metastases are one of the most important prognostic factors in NSCLC [21, 22]. Based on the current TNM classification system (8th edition) a single, circumscribed mediastinal lymph node metastasis determines a status of pN2, usually with a consequent recommendation of sequential chemotherapy and radiation of the mediastinum. A whole range of potential considerations including morphology (i.e. “bulky”), number of affected nodes or nodal stations, vascular invasion, preoperative suspicion vs. occult metastases (cN status), tumor aggressivity (G status), or in this case the presence or absence of ENE are not incorporated into the current TNM staging system. Attempts have been made to better differentiate among pN2 cases [23], [24], [25], in particular to determine which patients will truly benefit from adjuvant therapy. Drake et al. [26], for example, determined that in patients with only microscopic N2 progression adjuvant mediastinal radiation provided no benefit. Interestingly, Moretti et al. found that adjuvant radiation only improved prognosis in pN2 patients without ENE, but not in those with ENE [27].
Although KRAS and EGFR mutations did not affect outcome in our investigation, molecular pathological examinations can be expected to play an increasingly significant role in refining adjuvant or neoadjuvant medical treatment in surgical patients. It has been well established that the prognosis of operable NSCLC is to a large extent dependent on whether or not it is truly locally contained. Postoperative examination of resection margins and lymph nodes are the main methods of estimating the chances of non-local spread. The calculation, however, is statistical; nodal metastases are associated with a higher likelihood of systemic spread but do not determine it. We wondered whether ENE status might offer a more precise tool for estimating the chances of systemic spread.
In recent years there has been some interest in liquid biopsies as a more direct method of determining systemic spread. Here, various methods exist for examining patient blood for circulating tumor cells as well as tumor cell fragments. Circulating tumor DNA (ctDNA) can be examined for relevant mutations (such as EGFR), which may aid in determining the appropriateness of immunotherapies. Liquid biopsy has the primary benefit of being virtually non-invasive, and in patients where surgery or tissue biopsy are not tenable, it may be a helpful diagnostic tool. At the moment, however, it is not considered a suitable replacement for histological examination of tissue specimens, which remains the gold standard [28].
The main limitations of this study are its retrospective nature and relatively small cohort size. Our failure to corroborate the findings others regarding the negative prognostic effects of ENE may have been a simple matter of being underpowered. Although our paper did not find that ENE had a significant effect on prognosis in NSCLC, ENE, when considered along with what appear to be other more powerful nodal characteristics, may indeed be yet one more aspect to take into consideration when making decisions about adjuvant treatment.
Acknowledgments
The authors thank Ms. Stephanie Krüger for her assistance in the data management.
-
Research funding: None declared.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Authors state no conflict of interest.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Ethical approval: The local Institutional Review Board deemed the study exempt from review.
References
1. Rami-Porta, R, Asamura, H, Travis, WD, Rusch, VW. Lung cancer - major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA A Cancer J Clin 2017;67:138–55. https://doi.org/10.3322/caac.21390.Search in Google Scholar PubMed
2. Bray, F, Ferlay, J, Soerjomataram, I, Siegel, RL, Torre, LA, Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin 2018;68:394–424. https://doi.org/10.3322/caac.21492.Search in Google Scholar PubMed
3. Neppl, C, Keller, MD, Scherz, A, Dorn, P, Schmid, RA, Zlobec, I, et al.. Comparison of the 7th and 8th edition of the UICC/AJCC TNM staging system in primary resected squamous cell carcinomas of the lung-A single center analysis of 354 cases. Front Med 2019;6:196. https://doi.org/10.3389/fmed.2019.00196.Search in Google Scholar PubMed PubMed Central
4. Al-Shahrabani, F, Vallböhmer, D, Angenendt, S, Knoefel, WT. Surgical strategies in the therapy of non-small cell lung cancer. World J Clin Oncol 2014;5:595–603. https://doi.org/10.5306/wjco.v5.i4.595.Search in Google Scholar PubMed PubMed Central
5. Rami-Porta, R, Bolejack, V, Crowley, J, Ball, D, Kim, J, Lyons, G, et al.. The IASLC lung cancer staging project: proposals for the revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol 2015;10:990–1003. https://doi.org/10.1097/jto.0000000000000559.Search in Google Scholar
6. Amin, MB, Edge, SD, Greene, FL, Byrd, DR, Brookland, RK, Washington, MK, et al.. AJCC cancer staging manual. 8th ed Cham: Springer International Publishing, American Joint Commission on Cancer; 2017.10.1007/978-3-319-40618-3_2Search in Google Scholar
7. Lee, YC, Wu, CT, Kuo, SW, Tseng, YT, Chang, YL. Significance of extranodal extension of regional lymph nodes in surgically resected non-small cell lung cancer. Chest 2007;131:993–9. https://doi.org/10.1378/chest.06-1810.Search in Google Scholar PubMed
8. Luchini, C, Veronese, N, Nottegar, A, Cheng, M, Kaneko, T, Pilati, C, et al.. Extranodal extension of nodal metastases is a poor prognostic moderator in non-small cell lung cancer: a meta-analysis. Virchows Arch 2018;472:939–47. https://doi.org/10.1007/s00428-018-2309-1.Search in Google Scholar PubMed
9. Liu, W, Shao, Y, Guan, B, Hao, J, Cheng, X, Ji, K, et al.. Extracapsular extension is a powerful prognostic factor in stage IIA-IIIA non-small cell lung cancer patients with completely resection. Int J Clin Exp Pathol 2015;8:11268–77.Search in Google Scholar
10. Dienemann, H, Hoffmann, H, Koebe, HG. Technique and rationale of lymph node dissection in bronchial carcinoma. Chirurg 1998;69:412–7. https://doi.org/10.1007/s001040050431.Search in Google Scholar PubMed
11. Gagliasso, M, Migliaretti, G, Ardissone, F. Assessing the prognostic impact of the international association for the study of lung cancer proposed definitions of complete, uncertain, and incomplete resection in non-small cell lung cancer surgery. Lung Cancer 2017;111:124–30. https://doi.org/10.1016/j.lungcan.2017.07.013.Search in Google Scholar PubMed
12. Wang, X, Guo, H, Hu, Q, Ying, Y, Chen, B. The impact of skip vs. Non-skip N2 lymph node metastasis on the prognosis of non-small-cell lung cancer: a systematic Review and meta-analysis. Front Surg 2021;8:749156. https://doi.org/10.3389/fsurg.2021.749156.Search in Google Scholar PubMed PubMed Central
13. Prenzel, KL, Mönig, SP, Sinning, JM, Baldus, SE, Gutschow, CA, Grass, G, et al.. Role of skip metastasis to mediastinal lymph nodes in non-small cell lung cancer. J Surg Oncol 2003;82:256–60. https://doi.org/10.1002/jso.10219.Search in Google Scholar PubMed
14. Yazgan, S, Ucvet, A, Gursoy, S, Samancilar, O, Yagci, T. Single-station skip-N2 disease: good prognosis in resected non-small-cell lung cancer (long-term results in skip-N2 disease). Int Cardiovasc Thorac Surg 2019;28:247–52. https://doi.org/10.1093/icvts/ivy244.Search in Google Scholar PubMed
15. Maniwa, T, Shintani, Y, Okami, J, Kadota, Y, Takeuchi, Y, Takami, K, et al.. Upfront surgery in patients with clinical skip N2 lung cancer based on results of modern radiological examinations. J Thorac Dis 2018;10:6828–37. https://doi.org/10.21037/jtd.2018.10.115.Search in Google Scholar PubMed PubMed Central
16. Theunissen, PH, Bollen, EC, Koudstaal, J, Thunnissen, FB. Intranodal and extranodal tumour growth in early metastasised non-small cell lung cancer: problems in histological diagnosis. J Clin Pathol 1994;47:920–3. https://doi.org/10.1136/jcp.47.10.920.Search in Google Scholar PubMed PubMed Central
17. Legras, A, Mordant, P, Arame, A, Foucault, C, Dujon, A, Le Pimpec Barthes, F, et al.. Long-term survival of patients with pN2 lung cancer according to the pattern of lymphatic spread. Ann Thorac Surg 2014;97:1156–62. https://doi.org/10.1016/j.athoracsur.2013.12.047.Search in Google Scholar PubMed
18. Mordant, P, Pricopi, C, Legras, A, Arame, A, Foucault, C, Dujon, A, et al.. Prognostic factors after surgical resection of N1 non-small cell lung cancer. Eur J Surg Oncol 2015;41:696–701. https://doi.org/10.1016/j.ejso.2014.10.003.Search in Google Scholar PubMed
19. Zhang, Z, Liu, D, Guo, Y, Shi, B, Song, Z, Tian, Y. Effects of multiple factors on the prognosis of pIIIa/N2 patients with non-small cell lung cancer. Zhongguo Fei Ai Za Zhi 2010;13:781–5. https://doi.org/10.3779/j.issn.1009-3419.2010.08.06.Search in Google Scholar PubMed PubMed Central
20. Iwasaki, A, Shirakusa, T, Miyoshi, T, Hamada, T, Enatsu, S, Maekawa, S, et al.. Prognostic significance of subcarinal station in non-small cell lung cancer with T1-3 N2 disease. Thorac Cardiovasc Surg 2006;54:42–6. https://doi.org/10.1055/s-2005-865828.Search in Google Scholar PubMed
21. Naruke, T, Tsuchiya, R, Kondo, H, Asamura, H. Prognosis and survival after resection for bronchogenic carcinoma based on the 1997 TNM-staging classification: the Japanese experience. Ann Thorac Surg 2001;71:1759–64. https://doi.org/10.1016/s0003-4975(00)02609-6.Search in Google Scholar PubMed
22. Keller, SM, Adak, S, Wagner, H, Johnson, DH. Mediastinal lymph node dissection improves survival in patients with stages II and IIIa non-small cell lung cancer. Eastern cooperative oncology group. Ann Thorac Surg 2000;70:358–65. https://doi.org/10.1016/s0003-4975(00)01673-8.Search in Google Scholar PubMed
23. Andre, F, Grunenwald, D, Pignon, JP, Dujon, A, Pujol, JL, Brichon, PY, et al.. Survival of patients with resected N2 non-small-cell lung cancer: evidence for a subclassification and implications. J Clin Oncol 2000;18:2981–9. https://doi.org/10.1200/jco.2000.18.16.2981.Search in Google Scholar PubMed
24. Grunenwald, D, Le Chevalier, T. Re: stage IIIA category of non-small-cell lung cancer: a new proposal. J Natl Cancer Inst 1997;89:88–9. https://doi.org/10.1093/jnci/89.1.88-a.Search in Google Scholar PubMed
25. Carter, BW, Lichtenberger, JP, Benveniste, MK, de Groot, PM, Wu, CC, Erasmus, JJ, et al.. Revisions to the TNM staging of lung cancer: rationale, significance, and clinical application. Radiographics 2018;38:374–91. https://doi.org/10.1148/rg.2018170081.Search in Google Scholar PubMed
26. Drake, JA, Portnoy, DC, Tauer, K, Weksler, B. Adding radiotherapy to adjuvant chemotherapy does not improve survival of patients with N2 lung cancer. Ann Thorac Surg 2018;106:959–65. https://doi.org/10.1016/j.athoracsur.2018.04.074.Search in Google Scholar PubMed
27. Moretti, L, Yu, DS, Chen, H, Carbone, DP, Johnson, DH, Keedy, VL, et al.. Prognostic factors for resected non-small cell lung cancer with pN2 status: implications for use of postoperative radiotherapy. Oncol 2009;14:1106–15. https://doi.org/10.1634/theoncologist.2009-0130.Search in Google Scholar PubMed PubMed Central
28. Bonanno, L, Dal Maso, A, Pavan, A, Zulato, E, Calvetti, L, Pasello, G, et al.. Liquid biopsy and non-small cell lung cancer: are we looking at the tip of the iceberg? Br J Cancer 2022;127:383–93. https://doi.org/10.1038/s41416-022-01777-8.Search in Google Scholar PubMed PubMed Central
Supplementary Material
The online version of this article offers reviewer assessments as supplementary material (https://doi.org/10.1515/iss-2022-0023).
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorial
- Impact factor for Innovative Surgical Sciences – heading for the future
- Case Report
- Postoperative intussusception: a rare but critical complication in adult patients with Crohn’s disease – case report and literature review
- Original Articles
- Extracapsular extension of pN2 lymph node metastases is not prognostically significant in surgically resected patients with non-small cell lung cancer
- Presentation, management and outcomes of iliopsoas abscess at a University Teaching Hospital in Nepal
- Female physician and pregnancy- effect of the amended German maternity protection act on female doctors’ careers
- Resection rectopexy as part of the multidisciplinary approach in the management of complex pelvic floor disorders
Articles in the same Issue
- Frontmatter
- Editorial
- Impact factor for Innovative Surgical Sciences – heading for the future
- Case Report
- Postoperative intussusception: a rare but critical complication in adult patients with Crohn’s disease – case report and literature review
- Original Articles
- Extracapsular extension of pN2 lymph node metastases is not prognostically significant in surgically resected patients with non-small cell lung cancer
- Presentation, management and outcomes of iliopsoas abscess at a University Teaching Hospital in Nepal
- Female physician and pregnancy- effect of the amended German maternity protection act on female doctors’ careers
- Resection rectopexy as part of the multidisciplinary approach in the management of complex pelvic floor disorders

