Abstract
The β-glucosidase properties from one yeast isolate identified as Wickerhamomyces anomalus C4 were characterised. The β-glucosidase activity of W. anomalus C4 was 41.83 ± 0.25 mU/mL, and the optimum temperature and pH were 40 °C and 5.0, respectively. The glucose, 10% v/v of ethanol and 10 mmol/L of Cu2+ inhibited the β-glycosidases activities. The isolate W. anomalus C4 had a stronger alcohol metabolism capacity than commercial Saccharomyces cerevisiae X16. Besides, fermentation with W. anomalus C4 alone and co-fermentations with S. cerevisiae X16 and W. anomalus C4 reduced the volatile acids content and the sourness value compared to S. cerevisiae X16 control. Moreover, inoculation with W. anomalus C4 could enhance volatile aroma richness and complexity of Rosa roxburghii wines, regardless of type or amount thereof. Therefore, the R. roxburghii native yeast isolate W. anomalus C4 may have some application potentials for R. roxburghii wine-making.
1 Introduction
Aroma profile is one of the important quality characteristics of a wine [1]. The volatile compounds derived therefrom are major contributors to the typical flavour of wine; however, in many cases some bound form of non-volatile compounds (usually present as flavourless glycoconjugates) accumulated in higher amounts in fruit. These odourless glycosidic precursors could be hydrolysed by β-glucosidase during the winemaking processes, and released the aromatic components, contributing to the varied flavour complexity of wine [2].
The β-d-glucosidases (βG; EC 3.2.1.21) are ubiquitous in oenological yeasts including Saccharomyces and non-Saccharomyces yeasts [3]. It is believed that the aroma of native fermentations could be enhanced by higher hydrolytic enzyme producing yeasts [4], therefore, the use of indigenous β-d-glucosidase from native yeasts for winemaking is a better choice when trying to improve wine aroma [5]. However, β-d-glucosidase activities from different yeast species exhibit significant variability [6]. Therefore, it is necessary to screen and characterise special glycosidases from indigenous yeast for winemaking in the initiation of fermentation.
Rosa roxburghii Tratt (R. roxburghii) is a plant that is widely distributed in south-western areas of China, such as Guizhou, Chongqing, Sichuan, and Guangxi [7]. The fruit of R. roxburghii is nutritional and contains medicinal ingredients [8]. For example, the vitamin C content is 1700–2900 mg per 100 g of edible part, which is 10 times that of kiwi fruit. The fruit of R. roxburghii tastes bad due to an excess of tannins and organic acids [9], making their comprehensive utilisation difficult when relying thereon for fermentation into wine.
In this study, β-d-glucosidase-producing indigenous yeasts were screened from R. roxburghii fruit in Longli County, Guizhou Province, China. Furthermore, β-d-glucosidase activities were also evaluated under various brewing conditions. Moreover, fermentation was performed using the R. roxburghii native yeast to analyse the effect of β-d-glucosidase on the aroma of R. roxburghii wine. To our knowledge, this is the first screening and description of β-glucosidase activity involved in R. roxburghii indigenous yeast and its utilisation in releasing bound-form aromas.
2 Materials and methods
2.1 Yeast strains
The commercial Saccharomyces cerevisiae (S. cerevisiae) X16 obtained from Laffort Company (France) was used as a reference strain. A total of 65 strains of yeasts isolated from R. roxburghii fruit in Longli County, Guizhou Province were analysed in this study to investigate the β-glucosidase activities. All yeasts cells were cultured on YPD solid medium (1% yeast extract, 2% peptone, 2% glucose, and 2% agar) containing 100 mg/L of chloromycetin at 28 °C for 72 h, and then kept at 4 °C for later use.
2.2 β-glucosidase activity assay
Esculin semi-quantitative colouration analysis was applied to screen β-glucosidase production yeast strains. Yeasts were incubated in 200 μL esculin medium with 105 live cells/mL in 96-well plates, and then incubated at 28 °C for 72 h. β-glucosidase activity was determined according to the colour depth.
To confirm the preliminary screening results, β-glucosidase activity was determined using p-nitrophenyl-β-d-glycoside (pNPG) as the substrate and the amount of p-nitrophenol (pNP) released from pNPG was checked. Firstly, yeasts were incubated in 50 mL YPD liquid medium with 5% (v/v) seed culture, cultured at 28 °C while being shaken at 160 rpm for 72 h, and collection via centrifugation at 3000 g for 10 min. The supernatant was used as an enzyme solution. Enzyme solution (0.1 mL) was mixed with pNPG solution (0.2 mL, 0.002 M) in citrate phosphate buffer (0.1 M, pH 5.0). The reaction was maintained at 40 °C for 30 min, and stopped by adding Na2CO3 (2.0 mL, 0.25 M). The released pNP was measured spectrophotometrically at 405 nm. One unit (U) of enzyme activity was defined as the amount of enzyme that released 1 nmol of pNP per min under the aforementioned experimental conditions.
2.3 Identification of yeast strain
The selected isolate strain was streaked on the WL nutrient agar and incubated at 28 °C for 5 days. The micromorphology of the selected yeast was captured by using a microscope (Olympus, Tokyo, Japan) and classified according to its morphotype.
The selected isolate strain was further identified by amplifying the D1/D2 domain of 26S rDNA via polymerase chain reaction (PCR) with forward primer NL1 (5′GCATATCAATAAGCG GAGGAAAAG-3′), and reverse primer NL4 (5′-GGTCCGTGTTTCAAGACGG-3′). The sequences of the D1/D2 domain of 26S rDNA were compared by BLASTN Homology Search.
2.4 Effect of brewing conditions on β-glucosidase activity
In order to ascertain the effect of temperature or pH on β-glucosidase activity, the enzyme reactions were incubated at different temperatures over the range between 20 and 60 °C (20, 30, 40, 50 and 60 °C) at pH 5, or with citrate-phosphate buffer across the pH range from 3.0 to 7.0 (3.0, 4.0, 5.0, 6.0 and 7.0). The enzyme assay was conducted with pNPG as the substrate as previously described.
Effects of glucose or ethanol on enzyme activities were identified by using glucose concentrations over a range of 0–20% w/v or ethanol concentrations over a range of 0–20% v/v, respectively. Stock solutions of glucose or ethanol were prepared in citrate-phosphate buffer (pH 5.0) and were added to the reaction mixture resulting in final concentrations from 0 to 20% w/v or 0–20% v/v, respectively. The enzyme assay was conducted as previously described.
To verify the effect of metal ion on the β-glycosidases activity, K+, Na+, Mg2+, Cu2+ and Al3+ were added to citrate-phosphate buffer (pH 5.0) with the final concentration of 10 mmol/L. The reaction mixtures were incubated at 40 °C for 30 min, and then enzyme activities were measured.
2.5 Kinetic of β-glucosidase enzyme
Kinetic parameters, Km and Vmax, were detected from Lineweaver-Burk plots of β-glucosidase activity at pNPG concentrations ranging from 0.2 to 2.0 mM (at pH 5.0 and 40 °C). Each sample was detected in triplicate.
2.6 Laboratory-scale fermentation of R. roxburghii wine
The R. roxburghii wine fermentation was conducted as described by Huang [10] with some modifications. Fresh R. roxburghii was washed with sterile water and crushed to acquire its R. roxburghii must. The must was filtrated with microporous film, poured into a 2 L bottle and divided into three groups: an S. cerevisiae group, a Wickerhamomyces anomalus group and a mixed group. The initial R. roxburghii must contained 50 mg/L SO2 and 20 mg/L pectinase at pH 3.5. Logarithmic growths of S. cerevisiae X16 (107 CFU/mL) and W. anomalus (108 CFU/mL) were inoculated into the S. cerevisiae and W. anomalus groups, respectively. The mixed group was simultaneously inoculated with 107 CFU/mL of S. cerevisiae X16 and 108 CFU/mL of W. anomalus. Fermentation was performed at 25 °C without agitation until finished. Three replicates for each group were tested.
2.7 Chemical compositions of R. roxburghii wine
pH measurements were conducted using a digital pH meter (Model PT-10, Sartorius AG, Germany). The concentrations of ethanol, residual sugars, total acidity and volatile acids were determined using methods recommended by the International Organization of the Vine and Wine (OIV 2005). Each sample was detected in triplicate.
2.8 Volatile compound analysis of R. roxburghii wine
Fermented R. roxburghii wine was centrifuged at 3000 g for 15 min and supernatant was prepared for volatile compound analysis. Cyclohexanone was added to the R. roxburghii wine as an internal control. A headspace solid-phase micro extraction (HS-SPME) method was performed at 40 °C for 30 min to extract volatile compounds. An Agilent TQ8040 gas chromatograph mass spectrometer was used to analyse each specimen. The flow rate of the carrier gas (helium) was maintained at 1.2 mL/min. The column temperature was programmed as follows: 40 °C for 2 min, increased to 230 °C at a rate of 5 °C/min and then held at 230 °C for 15 min. The temperatures for the injector and detector were set to 250 and 280 °C, respectively. The mass spectrometer was operated in electron-impact mode at 70 eV. Volatile compounds of orange wine were identified by comparing their linear retention indices with those of pure standards, published data or MS fragmentation patterns obtained from databases. The odour active value (OAV), as the ratio of the concentration of a flavour compound to its odour threshold, is a parameter widely used to obtain odour patterns starting from quantitative compositions. In this research, compounds with OAV > 1 are considered to be responsible for aroma, and the higher their OAV, the more they contribute to the aroma profile.
2.9 Statistical analysis
The results were shown as mean ± SD. One-way analysis of variance, Student’s t-test, and principal component analysis (PCA) were conducted using SPSS 25.0 statistical software (SPSS). Differences were considered significant when P < 0.05.
3 Results
3.1 Screening of high β-glucosidase production of yeasts
β-glucosidase production yeasts were screened from “Guinong 5” R. roxburghii fruit (Figure 1), obtained from Longli County, Guizhou Province, China. A total of 65 yeast isolates were chosen from YPD plates and then evaluated for β-glucosidase production using the esculin reaction method. As shown in Figure 2, eight yeast isolates (B3, B9, B11, C4, C5, C9, D8, and E9) were found to exhibit high β-glucosidase activity on account of their dark brown colour in esculin medium.

“Guinong 5” R. roxburghii fruit used in this study.

Detection of β-glucosidase production using the esculin reaction method.
To verify the β-glucosidase activities of these eight candidate yeast isolates, quantitative analysis was undertaken using p-NPG colorimetry. The results demonstrated that the highest β-glucosidase activity was produced by Strain C4 (Table 1). Therefore, the C4 strain was subjected to selected for further studies.
Quantitative analysis of β-glucosidase activities of eight strains of candidate yeast isolates.
| Strain number | β-glucosidase activities (mU/mL) |
|---|---|
| B3 | 38.70 ± 0.40 |
| B9 | 37.33 ± 0.21 |
| B11 | 36.17 ± 0.25 |
| C4 | 41.83 ± 0.25 |
| C5 | 35.60 ± 0.20 |
| C9 | 40.30 ± 0.17 |
| D8 | 36.85 ± 0.23 |
| E9 | 32.50 ± 0.17 |
3.2 Identification of selected yeast strain
Firstly, morphological characteristics were checked to identify the C4 strain. The morphology of the colony on WL (Wallerstein laboratory nutrient agar) identification medium was white on the surface and dark green within, villiform and flat. The cellular microscopic morphology was assessed after staining by crystal violet (Figure 3). These observations indicated that the yeast C4 isolate might belong to the species W. anomalus.

Morphology of cells and colonies of C4 strain. A, Microscopic morphology of C4 strain via crystal violet staining. B-D, Colony morphology of C4 strain on YPD medium (B), WL medium (C), and lysine medium (D).
Then, a molecular biological method was applied to determine the C4 strain. The sequence of D1/D2 domain of 26S rDNA was amplified and compared by BLASTN Homology Search. The results revealed that the sequence of yeast isolate, C4, was 99.66% identical to W. anomalus TC2-5. Taken together, it was confirmed that the isolate C4 (GenBank accession: MT705624) was identical to Wickerhamomyces anomalus.
3.3 Effects of brewing conditions on β-glucosidase activity
To identify the effect of temperature on the β-glycosidases activity from the selected yeast strain C4, enzymatic activity was detected in a temperature range from 20 to 60 °C. Data indicated that the β-glycosidases of C4 strain had an optimum temperature of 40 °C (Figure 4A).
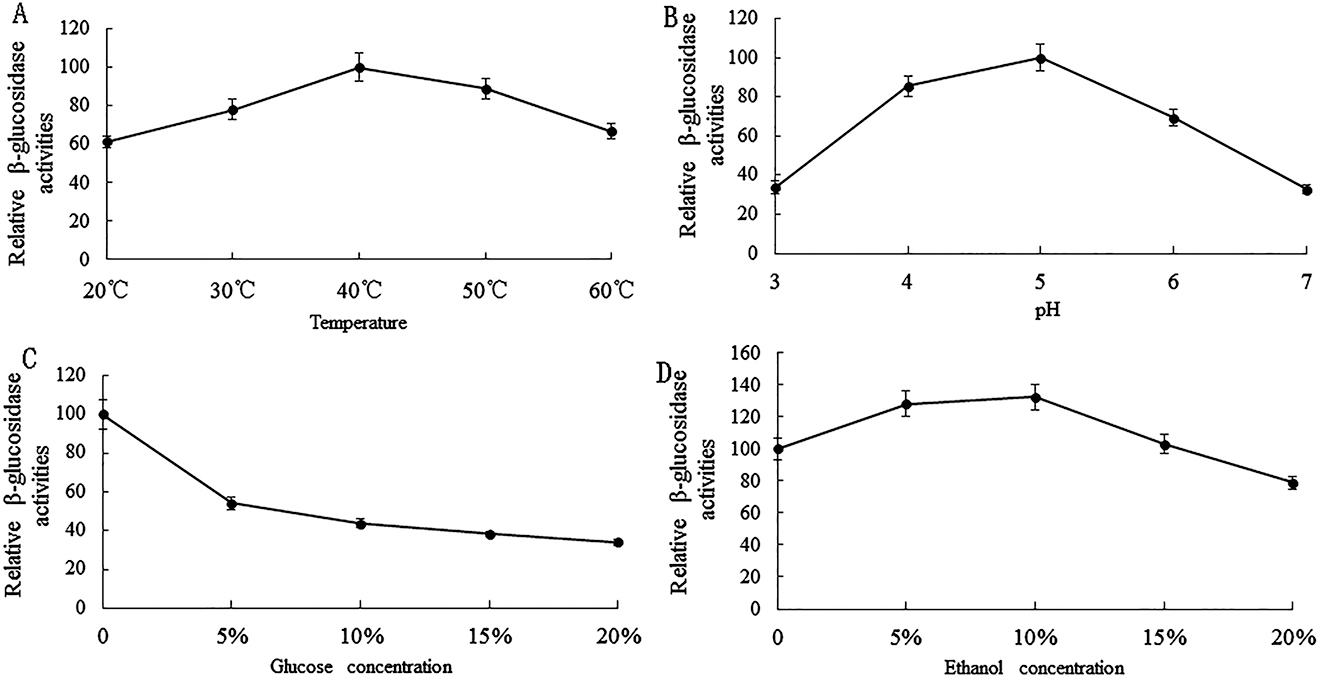
Effects of brewing conditions on β-glucosidase activity. A, Effect of temperature on β-glucosidase activity. B, Effect of pH on β-glucosidase activity. C, Effect of glucose on β-glucosidase activity. D, Effect of ethanol on β-glucosidase activity.
Figure 4B shows the effect of pH on enzymatic activity. The β-glucosidase extracted from W. anomalus C4 was strongly inhibited at pH 3.0. Maximum activity was recorded at a pH of around 5.0, therefore, the optimum pH for W. anomalus C4 was 5.0.
The effects of glucose concentrations in the range from 0–20% w/v on β-glucosidases were determined. The results of glucose inhibition are shown in Figure 4C. The β-glucosidase activity from W. anomalus C4 was strongly inhibited by 5% w/v glucose and the relative activity remained at around 50%. In the presence of 20% w/v glucose, the relative activity of this β-glucosidase was around 30%.
The effect of ethanol concentration in the range of 0–15% v/v on β-glucosidase activity was also investigated (Figure 4D). The activity of β-glucosidase from W. anomalus C4 was enhanced by ethanol concentrations in the range of 0–10% v/v with the maximum activity at a concentration of 10% v/v, however, when the concentration of ethanol exceeded 10% v/v, the β-glucosidase activity of W. anomalus C4 was inhibited.
In addition, the effect of metal ion on the β-glycosidases activity was assessed. As seen in Figure 4, K+, Na+, Mg2+ and Al3+ enhanced β-glycosidases activity, in contrast, Cu2+ inhibited β-glycosidases activity (Figure 5).

Effect of metal ion on β-glucosidase activity.
3.4 Kinetic parameters of β-glucosidase
The kinetic parameters, Km and Vmax of β-glucosidase of W. anomalus C4 were calculated by using p-nitrophenol-β-d-glucopyranoside as a substrate. The results indicated that Km and Vmax were 0.53 ± 0.03 mM and 1.08 U/g, respectively Table 1.
3.5 Physico-chemical characteristics of R. roxburghii wine fermented with different yeast strains
The physico-chemical characteristics of R. roxburghii wine fermented with different yeast strains, including pH, ethanol content, residual sugar total acidity, and volatile acidity were further evaluated. As shown in Table 2, a lower ethanol concentration and higher residual sugar were detected in R. roxburghii wines produced using S. cerevisiae X16 compared with pure W. anomalus C4, or mixed fermentation with W. anomalus C4 and S. cerevisiae X16. In addition, fermentation with W. anomalus C4 alone and co-fermentations with S. cerevisiae X16 and W. anomalus C4 could decrease the volatile acids content compared to S. cerevisiae X16 control.
Physico-chemical parameters of R. roxburghii wines fermentation with different yeasts.
| Strains | Ethanol (% v/v) | Residual sugar (g/L) | pH | Total acidity (g/L) | Volatile acidity (g/L) |
|---|---|---|---|---|---|
| Saccharomyces cerevisiae X16 | 10.5 ± 0.14 | 12.58 ± 0.54 | 3.20 ± 0.03 | 4.20 ± 0.24 | 0.71 ± 0.01 |
| Wickerhamomyces anomalus C4 | 11.8 ± 0.25* | 9.92 ± 0.35* | 3.43 ± 0.02 | 3.92 ± 0.00 | 0.61 ± 0.04* |
| Mixture | 11.9 ± 0.21# | 9.90 ± 0.41# | 3.41 ± 0.01 | 4.01 ± 0.00 | 0.64 ± 0.02# |
*P < 0.05 versus Saccharomyces cerevisiae X16 group; #P < 0.05 versus S. cerevisiae X16 group.
An electronic tongue assay system (INSENT SS402B, Japan) was also used to compare the sensory characteristics of R. roxburghii wines. The results showed that there were no significant sensory differences between three R. roxburghii wines in bitterness, astringency, aftertaste-A, aftertaste-B, umami, richness, and saltiness except for sourness. The sourness values measured in R. roxburghii wines produced by W. anomalus C4 pure inoculation or mixed inoculation were smaller than that by S. cerevisiae X16 alone (Figure 6).

The R. Roxburghii wine taste attribute radar chart.
3.6 Volatile chemical profiles of finished R. roxburghii wines
A total of 64 volatile chemicals were founded in three different fermented R. roxburghii wines, including five volatile acids, 10 volatile alcohols, 25 volatile esters and 24 other volatile compounds (Table 3). There were 26, 45 and 58 volatile compounds detected in the three types of R. roxburghii wines produced by pure S. cerevisiae X16, W. anomalus C4 or co-inoculation with and S. cerevisiae X16 and W. anomalus C4, respectively. Moreover, wines inoculated with pure W. anomalus C4 or mixed with S. cerevisiae X16 contained lower amounts of volatile acids, increased amounts of volatile alcohols, volatile esters and other volatile compounds, compared to S. cerevisiae control. Taken together, inoculation with W. anomalus C4 could enhance the volatile aroma richness and complexity of R. roxburghii wines, regardless of type or amount.
Volatile compounds (mg/L) in R. roxburghii wines fermented with different yeasts.
| Number | Volatile compound | Saccharomyces cerevisiae X16 | Wickerhamomyces anomalus C4 | Mixture |
|---|---|---|---|---|
| 1 | Acetic acid | 30.61 ± 0.51 | ND | ND |
| 2 | Isobutyric acid | 1.01 ± 0.19 | ND | 0.98 ± 0.37 |
| 3 | Hexanoic acid | 2.74 ± 0.14 | 3.55 ± 0.06 | 2.43 ± 0.11 |
| 4 | Octanoic acid | 20.11 ± 0.28 | 23.23 ± 2.31 | 19.48 ± 0.03 |
| 5 | n-Decanoic acid | 21.95 ± 2.53 | 20.69 ± 0.38 | 8.71 ± 0.23 |
| ∑Acids | 76.42 ± 3.65 | 47.47 ± 2.75* | 31.60 ± 0.74# | |
| 6 | Isobutanol | 9.09 ± 1.07 | 17.59 ± 2.93 | 16.59 ± 0.37 |
| 7 | Linalool | ND | 0.83 ± 0.00 | 0.74 ± 0.01 |
| 8 | Phenylethyl alcohol | 66.11 ± 6.13 | 60.35 ± 1.97 | 81.70 ± 0.66 |
| 9 | 3-Hexen-1-ol, (Z)- | 11.36 ± 0.48 | 13.74 ± 0.28 | 10.99 ± 0.25 |
| 10 | 1-Hexanol | ND | 8.88 ± 0.42 | 7.41 ± 0.47 |
| 11 | 1-Decanol | 3.33 ± 0.72 | ND | 1.72 ± 0.33 |
| 12 | Isoamyl alcohol | ND | 156.94 ± 17.69 | 188.54 ± 9.10 |
| 13 | 1-Propanol | ND | 0.84 ± 0.09 | ND |
| 14 | 1-Octanol | ND | ND | 1.33 ± 0.20 |
| 15 | 3,6-Octadien-1-ol, 3,7-dimethyl-, (Z)- | ND | 2.30 ± 0.05 | 1.98 ± 0.01 |
| ∑Alcohols | 89.89 ± 8.40 | 261.47 ± 23.43** | 311.00 ± 11.40## | |
| 16 | Hexyl acetate | ND | 8.48 ± 0.19 | 9.69 ± 1.74 |
| 17 | 3-hexyl acetate | ND | 8.51 ± 0.22 | 11.66 ± 1.81 |
| 18 | Isobutyl acetate | 1.57 ± 0.10 | 1.98 ± 0.36 | 2.65 ± 0.28 |
| 19 | Isoamyl acetate | 61.17 ± 0.01 | 87.01 ± 6.59 | 91.23 ± 9.24 |
| 20 | Phenethyl acetate | 13.73 ± 1.11 | 10.64 ± 0.20 | 16.30 ± 1.02 |
| 21 | Ethyl acetate | ND | 33.82 ± 1.10 | 30.52 ± 2.97 |
| 22 | Ethyl butyrate | 2.22 ± 0.20 | 2.17 ± 0.09 | 2.66 ± 0.15 |
| 23 | Ethyl hexanoate | 41.33 ± 2.43 | 53.48 ± 3.20 | 47.36 ± 1.01 |
| 24 | Ethyl 2-methylpropionate | 0.38 ± 0.20 | 0.28 ± 0.08 | 0.54 ± 0.10 |
| 25 | Ethyl 3-hexenoate | 0.91 ± 0.03 | 1.63 ± 0.15 | 1.14 ± 0.15 |
| 26 | Ethyl octanoate | 485.82 ± 30.11 | 611.70 ± 7.26 | 540.80 ± 45.71 |
| 27 | Ethyl decanoate | 477.49 ± 8.10 | 366.13 ± 27.95 | 448.68 ± 48.69 |
| 28 | Ethyl laurate | 29.50 ± 3.14 | 26.11 ± 2.58 | 28.52 ± 1.59 |
| 29 | Ethyl cinnamate | 6.12 ± 0.35 | 8.04 ± 0.04 | 7.51 ± 1.17 |
| 30 | Ethenyl formate | ND | 24.07 ± 2.15 | 1.17 ± 0.29 |
| 31 | Ethyl palmitate | ND | ND | 0.72 ± 0.08 |
| 32 | Ethyl myristate | ND | 1.87 ± 0.15 | ND |
| 33 | Ethyl 9-decenoate | ND | 3.45 ± 0.05 | 10.79 ± 0.24 |
| 34 | Ethyl isobutyrate | ND | ND | 2.72 ± 0.18 |
| 35 | Ethyl pelargonate | ND | ND | 1.03 ± 0.07 |
| 36 | Isobutyl caprylate | ND | ND | 1.07 ± 0.26 |
| 37 | Isoamyl caprylate | ND | 8.51 ± 0.23 | 7.19 ± 0.09 |
| 38 | Isoamyl decanoate | ND | 3.50 ± 0.07 | 2.90 ± 0.67 |
| 39 | Ethyl isovalerate | ND | ND | 2.76 ± 0.82 |
| 40 | 2-Propenoic acid, 1,7,7-trimethylbicyclo[2.2.1]hept-2-yl ester, exo- | ND | ND | 0.69 ± 0.08 |
| ∑ Esters | 1120.24 ± 45.78 | 1261.38 ± 52.66* | 1267.58 ± 118.23# | |
| 41 | Styrene | 33.55 ± 2.96 | 36.54 ± 1.00 | 19.53 ± 1.59 |
| 42 | 2,4-Di-tert-butylphenol | 15.96 ± 1.43 | 16.41 ± 0.63 | 12.57 ± 2.19 |
| 43 | Carbamic acid, monoammonium salt | 9.89 ± 1.26 | 27.66 ± 0.11 | 19.97 ± 0.33 |
| 44 | Hydrazinecarboxamide | 73.64 ± 1.18 | 89.28 ± 7.69 | 61.67 ± 1.53 |
| 45 | Borane-methyl sulphide complex | 1.63 ± 0.11 | ND | ND |
| 46 | Dimethyl sulphide | 1.64 ± 0.05 | 8.55 ± 1.52 | 1.24 ± 0.24 |
| 47 | Ammonium acetate | ND | 36.25 ± 2.59 | 20.00 ± 3.00 |
| 48 | Acetaldehyde | ND | 1.77 ± 0.00 | 3.93 ± 0.36 |
| 49 | 1-ethenyl-4-methoxybenzene | ND | 1.82 ± 0.45 | 1.02 ± 0.02 |
| 50 | 2-methyl-1,5-dioxaspiro[5.5]undecane | ND | 19.55 ± 1.60 | 19.57 ± 2.09 |
| 51 | 2,5-dimethyl-, 4-methoxy-, 2,3-dihydro-Furan-3-one | ND | 2.39 ± 0.18 | 1.81 ± 0.13 |
| 52 | Nonacosane | ND | 2.04 ± 0.15 | ND |
| 53 | Naphthalene, 2-methyl- | ND | 0.70 ± 0.02 | ND |
| 54 | Benzene, 4-ethenyl-1,2-dimethoxy- | ND | 2.82 ± 0.23 | 1.45 ± 0.16 |
| 55 | 4-vinyl-Guaiacol | ND | 3.87 ± 0.25 | 1.44 ± 0.39 |
| 56 | Benzofuran, 2,3-dihydro-3-Methyl | ND | 8.50 ± 0.63 | 3.77 ± 0.15 |
| 57 | 3,3-dimethyl-1,5-dioxaspiro[5.5]undecane | ND | ND | 0.99 ± 0.28 |
| 58 | 2-methyl-;2-methyl-5-dioxaspiro[5.5]undecane | ND | ND | 0.63 ± 0.07 |
| 59 | Butylated Hydroxytoluene | ND | ND | 8.29 ± 0.75 |
| 60 | 2,4-Di-tert-butylphenol | ND | ND | 12.57 ± 2.19 |
| 61 | p-Mentha-1(7),8(10)-dien-9-ol | ND | ND | 1.31 ± 0.30 |
| 62 | Furfural | ND | ND | 1.61 ± 0.15 |
| 63 | 5-Hydroxymethylfurfural | ND | ND | 3.46 ± 0.32 |
| 64 | 2,3-dihydro-2,3-Benzofuran | ND | ND | 3.77 ± 0.15 |
| ∑ Other compounds | 136.31 ± 6.99 | 258.15 ± 17.05** | 200.60 ± 16.39## |
*P < 0.05 versus Saccharomyces cerevisiae X16 group; **P < 0.01 versus S. cerevisiae X16 group; #P < 0.05 versus S. cerevisiae X16 group; ##P < 0.01 versus S. cerevisiae X16 group.
The flavour active values (odour activity value, OAV) can evaluate the contribution of volatile compounds for wine aroma. When OAV > 1, the compound makes a significant contribution to the main aroma: OAV < 1 indicates that the compound does not contribute significantly thereto. The OAV values of the main volatile compounds in R. roxburghii wines are listed in Table 4. There were 21 and 23 compounds with OAV > 1 in pure W. anomalus C4 or in those mixed with S. cerevisiae X16 fermented R. roxburghii wine. In contrast, only 13 compounds had OAV > 1 in S. cerevisiae X16 solar-fermented R. roxburghii wine.
The odour activity values (OAVs) for main compounds of R. roxburghii wines fermentation with different yeasts.
| Number | Volatile compounds | Odour descriptor | Odour threshold (mg/L) | OAV | ||
|---|---|---|---|---|---|---|
| Saccharomyces cerevisiae X16 | Wickerhamomyces anomalus C4 | Mixture | ||||
| A1 | Hexanoic acid | Cheese, fatty | 3 | 0.91 | 1.18 | 0.81 |
| A2 | Octanoic acid | Fatty, rancid | 10 | 2.01 | 2.32 | 1.95 |
| A3 | n-Decanoic acid | Fatty, rancid | 6 | 3.66 | 3.45 | 1.45 |
| B1 | Isobutanol | Alcohol, solvent | 75 | 0.12 | 0.23 | 0.22 |
| B2 | Linalool | Citrus, floral, sweet, grape-like | 0.02 | / | 41.5 | 37 |
| B3 | Phenylethyl alcohol | Rose, honey | 10 | 6.61 | 6.04 | 8.17 |
| B4 | 1-Hexanol | Herbaceous, wood | 1.10 | / | 8.07 | 6.74 |
| B5 | 1-Decanol | Floral, fatty | 0.40 | 8.33 | / | 4.35 |
| B6 | Isoamyl alcohol | Nail Polish, alcohol | 60 | / | 2.62 | 3.14 |
| B7 | 1-Octanol | Orange, rose, sweet herb | 0.80 | / | / | 1.66 |
| C1 | Hexyl acetate | Fruity | 0.67 | / | 12.66 | 14.46 |
| C2 | Isobutyl acetate | Sweet, fruity, apple, banana | 1.60 | 0.98 | 1.24 | 1.66 |
| C3 | Isoamyl acetate | Banana, fruity, sweet | 0.16 | 382.31 | 543.81 | 570.18 |
| C4 | Phenethyl acetate | Floral | 1.80 | 7.63 | 5.91 | 9.06 |
| C5 | Ethyl acetate | Pineapple, fruity, solvent, balsamic | 12 | / | 2.82 | 2.54 |
| C6 | Ethyl butyrate | Fruity | 0.08 | 27.8 | 27.1 | 33.2 |
| C7 | Ethyl hexanoate | Fruity, green apple, banana, brandy, wine-like | 0.08 | 516.63 | 668.5 | 592 |
| C8 | Ethyl octanoate | Sweet, floral, fruity, banana, pear, brandy | 0.58 | 837.62 | 1054.66 | 932.41 |
| C9 | Ethyl decanoate | Brandy, fruity, grape | 0.50 | 954.98 | 732.26 | 897.36 |
| C10 | Ethyl laurate | Fruity, fatty | 3.50 | 8.43 | 7.46 | 8.15 |
| C11 | Ethyl cinnamate | Fruity | 0.01 | 612 | 804 | 751 |
| C12 | Ethyl 9-decenoate | Roses | 0.10 | / | 34.5 | 107.90 |
| C13 | Ethyl isobutyrate | Fruity | 0.06 | / | / | 45.33 |
| D1 | Dimethyl sulphide | Blackcurrant, asparagus | 0.03 | 54.67 | 285 | 41.33 |
| D2 | Acetaldehyde | Pungent | 0.50 | / | 3.54 | 7.86 |
PCA was applied to analyse the chemical data pertaining to the wines obtained with each fermentation. PCA1 accounted for the 55.04% and PCA2 for an additional 44.96% of the variability (Figure 7): as seen in Figure 7A, complex odiferous compounds were clustered in the positive half-axis of PCA1, such as ethyl hexanoate, ethyl octanoate, ethyl cinnamate, isobutanol, linalool and 1-hexanol. 1-decanol and ethyl laurate, with fatty notes, were clustered in the negative half-axis of PCA1. The rose/floral odiferous compounds (phenylethyl alcohol, 1-octanol, and ethyl 9-decenoate) were the main components of the positive half-axis of PCA2. n-Decanoic acid and ethyl butyrate were distributed along the negative half-axis of PCA2.

Principal component analysis of R. roxburghii wines fermentation with different yeasts. A, Principal component load plot of volatile aroma compounds. B, Principal component score of volatile aroma compounds.
This also indicated that the fruity odour (ethyl hexanoate, and ethyl octanoate) and the rose/floral odour (phenylethyl alcohol and 1-octanol) may be the main volatile characteristics of mixture fermented and pure fermented R. roxburghii wine made with W. anomalus C4, respectively (Figure 7B); however, it was difficult to identify the main volatile characteristics of S. cerevisiae X16 fermented R. roxburghii wine.
4 Discussion
The effects of β-d-glucosidases have been extensively investigated in many wine yeasts for their important role in the release of volatile aromas [11], [12], [13]. In this study, β-d-glucosidase producing indigenous yeasts was screen from R. roxburghii fruit, and the highest enzyme activity strain (numbered C4) was selected for further evaluation. Morphology and molecular biology data suggested that the isolate C4 was a strain of W. anomalus. To analyse the effect of β-d-glucosidase from W. anomalus C4 on the aroma of R. roxburghii wine, fermentation was conducted using W. anomalus C4 yeast alone or as a mixture. Inoculation W. anomalus C4 could enhance volatile aroma richness and complexity of R. roxburghii wines because more variety of type and higher contents of volatile chemicals in pure W. anomalus C4 and mixed fermented wines. It was reported that more free terpenes are released as a result of the action of β-d-glucosidase [14], [15]; however, only linalool, one type of terpene, was found in W. anomalus-C4-produced R. roxburghii wine. Three reasons might explain this phenomenon: first, the inhibition of β-d-glucosidase by sugars in fruit juices was common. The experimental result suggested that β-glucosidase activity from W. anomalus C4 was strongly inhibited by 5% w/v glucose and the relative activity remained around 50% (Figure 4C). Second, the β-glucosidase activity from W. anomalus C4 was only 1.65 times that of S. cerevisiae X16, so the ability of β-d-glucosidase of W. anomalus C4 to hydrolyse glucosidic bonds was not significantly enhanced. Genetic engineering or mutation breeding methods may be considered in the future to enhance the β-d-glucosidase hydrolysis ability of W. anomalus C4. Third, the β-d-glucosidase location of W. anomalus C4 remained unclear.
W. anomalus is widespread in nature and has been isolated from different environments [16]. W. anomalus exhibits interesting and potentially exploitable physiological and metabolic characteristics and is of considerable importance due to its association with winemaking [17]. It was reported that W. anomalus is a good producer of acetate esters [18]. In this study, no ethyl acetate was detected in S. cerevisiae X16 fermented R. roxburghii wine, and 33.82 ± 1.10 mg/L and 30.52 ± 2.97 mg/L of ethyl acetate were measured in pure W. anomalus C4 or mixture-fermented R. roxburghii wine, respectively. Besides, W. anomalus was also a good producer of fruity acetate esters and other volatiles that exerted a positive effect on wine aroma [19], [20]. Here, the concentrations of isoamyl acetate, ethyl hexanoate, ethyl cinnamate, and ethyl octanoate founded in W. anomalus C4 fermented R. roxburghii wine were higher than that of S. cerevisiae X16 control (Table 3).
In addition, the native yeast W. anomalus C4 may have a better ability to utilise sugar than commercial S. cerevisiae X16 originally obtained from grapes with a lower sugar content and higher ethanol content of R. roxburghii wine fermented with W. anomalus C4 (Table 2). Also, W. anomalus C4 could reduce R. roxburghii wine acidity (Table 2; Figure 6).
In conclusion, the indigenous yeasts that produced β-d-glucosidase were screened from R. roxburghii fruit, and the strain with the highest β-d-glucosidase activities was identified and studied. Morphology and molecular biology data suggested that the isolate C4 was a strain of W. anomalus. The optimum temperature and pH for β-d-glucosidase from W. anomalus C4 were 40 °C and 5.0 respectively. In addition, glucose, 15% v/v ethanol and Cu2+ inhibited β-glycosidase activity. W. anomalus C4 could enhance volatile aroma richness and the complexity of R. roxburghii wines including aroma types and content. Taken together, the R. roxburghii native yeast W. anomalus C4 may have potential for use in R. roxburghii wine-making.
Funding source: Science and Technology Program of Guizhou Province
Funding source: Guizhou Provincial Education Department
Award Identifier / Grant number: KY 2017046
Funding source: Guizhou Institute of Technology
Award Identifier / Grant number: XJGC20190625
Funding source: Guizhou Fruit wine brewing engineering technology innovation talent team
Acknowledgments
This work were supported by the Science and Technology Program of Guizhou Province (Talents of Guizhou Science Cooperation Platform [2017]5789, [2018]5603), Innovation Group Research Project from Guizhou Provincial Education Department (KY 2017046), and High-level Talent Research Funding Project of Guizhou Institute of Technology (XJGC20190625).
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Conflict of interest statement: None declared.
References
1. Wu, Y, Duan, S, Zhao, L, Luo, M, Song, S, Xu, W, et al. Aroma characterization based on aromatic series analysis in table grapes. Sci Rep 2016;6:31116–31116 https://doi.org/10.1038/srep31116.Search in Google Scholar
2. Villena, MA, Iranzo, JF, Perez, AI. β-Glucosidase activity in wine yeasts: application in enology. Enzyme Microb Technol 2007;40:420–5. https://doi.org/10.1016/j.enzmictec.2006.07.013.Search in Google Scholar
3. Monteiro, LMO, Pereira, MG, Vici, AC, Heinen, PR, Buckeridge, MS, Polizeli, MLTM. Efficient hydrolysis of wine and grape juice anthocyanins by Malbranchea pulchella β-glucosidase immobilized on MANAE-agarose and ConA-Sepharose supports. Int J Biol Macromol 2019;136:1133–41. https://doi.org/10.1016/j.ijbiomac.2019.06.106.Search in Google Scholar
4. Thongekkaew, J, Fujii, T, Masaki, K, Koyama, K. Evaluation of Candida easanensis JK8 β-glucosidase with potentially hydrolyse non-volatile glycosides of wine aroma precursors. Nat Prod Res 2019,33:3563–7. https://doi.org/10.1080/14786419.2018.1481845.Search in Google Scholar
5. López, MC, Mateo, JJ, Maicas, S. Screening of β-glucosidase and β-xylosidase activities in four non-Saccharomyces yeast isolates. J Food Sci 2015;80:C1696–704. https://doi.org/10.1111/1750-3841.12954.Search in Google Scholar
6. Wu, Q, Zhang, Y, Tang, H, Chen, YS, Xie, BJ, Wang, C. Separation and identification of anthocyanins extracted from blueberry wine lees and pigment binding properties toward β-glucosidase. J Agric Food Chem 2017;65:216–23. https://doi.org/10.1021/acs.jafc.6b04244.Search in Google Scholar
7. Xu, SJ, Zhang, F, Wang, LJ, Hao, MH, Yang, XJ, Li, NN, et al. Flavonoids of Rosa roxburghii Tratt offers protection against radiation induced apoptosis and inflammation in mouse thymus. Apoptosis 2018,23:470–83. https://doi.org/10.1007/s10495-018-1466-7.Search in Google Scholar
8. Xu, P, Liu, XX, Xiong, XW, Zhang, WB, Cai, XH, Qiu, PY, et al. Flavonoids of Rosa roxburghii Tratt exhibit anti-apoptosis properties by regulating PARP-1/AIF. J Cell Biochem 2017;118:3943–52. https://doi.org/10.1002/jcb.26049.Search in Google Scholar
9. Liu, XZ, Zhao, HB, Li, YF, Yu, ZH, Liu, XH, Huang, MZ. Identification and oenological properties analysis of a strain of Hanseniaspora uvarum from Rosa roxburghii. Food Ferment Industries 2020;46:97–104. https://doi.org/10.13995/j.cnki.11-1802/ts.022942.Search in Google Scholar
10. Huang, C, Yin, H. Hong, Y. Optimization of juice production and fruit wine fermentation from Rosa roxburghii tratt fruits wildly grown in Western Hunan. Food Sci 2012;33:16–20.Search in Google Scholar
11. Pi, HW, Anandharaj, M, Kao, YY, Lin, YJ, Chang, JJ, Li, WH. Engineering the oleaginous red yeast Rhodotorula glutinis for simultaneous β-carotene and cellulase production. Sci Rep 2018;8:10850. https://doi.org/10.1038/s41598-018-29194-z.Search in Google Scholar
12. Meng, DD, Wang, B, Ma, XQ, Ji, SQ, Lu, M, Li, FL. Characterization of a thermostable endo-1,3(4)-β-glucanase from Caldicellulosiruptor sp. strain F32 and its application for yeast lysis. Appl Microbiol Biotechnol 2016;100:4923–34. https://doi.org/10.1007/s00253-016-7334-x.Search in Google Scholar
13. Bonciani, T, De Vero, L, Giannuzzi, E, Verspohl, A, Giudici, P. Qualitative and quantitative screening of the β-glucosidase activity in Saccharomyces cerevisiae and Saccharomyces uvarum strains isolated from refrigerated must. Lett Appl Microbiol 2018;67:72–8. https://doi.org/10.1111/lam.12891.Search in Google Scholar
14. Whitener, ME, Stanstrup, J, Carlin, S, Divol, B, Du Toit, M, Vrhovsek, U. Effect of non-Saccharomyces yeasts on the volatile chemical profile of Shiraz wine. Aust J Grape Wine Res 2017;23:179–92. https://doi.org/10.1111/ajgw.12269.Search in Google Scholar
15. Liu, S, Laaksonen, O, Kortesniemi, M, Kalpio, M, Yang, B. Chemical composition of bilberry wine fermented with non-Saccharomyces yeasts (Torulaspora delbrueckii and Schizosaccharomyces pombe) and Saccharomyces cerevisiae in pure, sequential and mixed fermentations. Food Chem 2018;266:262–74. https://doi.org/10.1016/j.foodchem.2018.06.003.Search in Google Scholar
16. Padilla, B, Gil, JV, Manzanares, P. Challenges of the non-conventional yeast Wickerhamomyces anomalus in winemaking. Fermentation 2018;4:1–14. https://doi.org/10.3390/fermentation 4030068.10.3390/fermentation4030068Search in Google Scholar
17. Canas, PMI, García-Romero, E, Manso, JMH, Fernández-González, M. Influence of sequential inoculation of Wickerhamomyces anomalus and Saccharomyces cerevisiae in the quality of red wines. Eur Food Res Tech 2014;239:279–86. https://doi.org/10.1007/s00217-014-2220-1.Search in Google Scholar
18. Viana, F, Gil, JV, Genovés, S, Valles, S, Manzanares, P. Rational selection of non-Saccharomyces wine yeasts for mixed starters based on ester formation and enological traits. Food Microbiol 2008;25:778–85. https://doi.org/10.1016/j.fm.2008.04.015.Search in Google Scholar
19. Domizio, P, Romani, C, Lencioni, L, Comitini, F, Gobbi, M, Mannazzu, I. Outlining a future for non-Saccharomyces yeasts: selection of putative spoilage wine strains to be used in association with Saccharomyces cerevisiae for grape juice fermentation. Int J Food Microbiol 2011;147:170–80. https://doi.org/10.1016/j.ijfoodmicro.2011.03.020.Search in Google Scholar
20. Lambrechts, MG, Pretorius, IS. Yeast and its importance to wine aroma-a review. South Afr J Enol Vitic 2000;21:97–129. https://doi.org/10.21548/21-1-3560.Search in Google Scholar
© 2020 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Articles
- Screening and characterisation of β-glucosidase production strains from Rosa roxburghii Tratt
- Correction of residence time distribution measurements for short holding times in pasteurization processes
- Effects of particle formation behavior on the properties of fish oil microcapsules fabricated using a micro-fluidic jet spray dryer
- Predicting the moisture content of Daqu with hyperspectral imaging
- The effects of reaction parameters on the non-enzymatic browning reaction between l-ascorbic acid and glycine
- Internal quality evaluation of chestnut using nuclear magnetic resonance
- Effect of microwave-drying on the quality and antioxidant properties of Ganoderma lucidum fermented sea-buckthorn tea
- The use of beetroot extract and extract powder in sausages as natural food colorant
Articles in the same Issue
- Frontmatter
- Articles
- Screening and characterisation of β-glucosidase production strains from Rosa roxburghii Tratt
- Correction of residence time distribution measurements for short holding times in pasteurization processes
- Effects of particle formation behavior on the properties of fish oil microcapsules fabricated using a micro-fluidic jet spray dryer
- Predicting the moisture content of Daqu with hyperspectral imaging
- The effects of reaction parameters on the non-enzymatic browning reaction between l-ascorbic acid and glycine
- Internal quality evaluation of chestnut using nuclear magnetic resonance
- Effect of microwave-drying on the quality and antioxidant properties of Ganoderma lucidum fermented sea-buckthorn tea
- The use of beetroot extract and extract powder in sausages as natural food colorant

