Abstract
Fish oil was encapsulated with whey protein isolate (WPI) as wall material using a Micro-Fluidic Jet Spray Dryer. The effects of core/wall material ratio, drying temperature and total solids content on the properties of microcapsules were studied. Low core/wall material ratios at 1:5 and 1:3 resulted in high encapsulation efficiency (EE) and excellent oxidative stability of microparticles during storage. Reducing the inlet temperature from 160 to 110 °C remarkably decreased EE from around 99 to 64.8%, associated with substantial increases in peroxide value during storage. The total solids content mainly altered the morphology of microcapsules, showing little influence on EE and oxidative stability. We proposed that the different drying conditions impacted on particle formation behavior during spray drying, which could be a crucial factor responsible for the differences in the quality attributes of microparticles. A low core/wall material ratio and high drying temperature facilitated the formation of a rigid protein skin at droplet surface during drying, whereas a high solids fraction in the droplets could limit possible droplet shrinkage. These factors contributed positively to the encapsulation of the lipophilic core material.
1 Introduction
Fish oil contains a high content of omega-3 polyunsaturated fatty acids such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), which are recommended as health-benefiting supplementary in dietary guidelines [1], [2], [3]. Its beneficial effect includes reducing the risk of inflammation, cardiovascular and certain neurological diseases, etc. [4]. DHA and EPA are also positively associated with neurodevelopment and visual function of infants and children [5], [, 6]. Owing to the high degree of unsaturation, fish oil tends to be easily oxidized during storage, leading to deteriorated quality and reduced efficacy. In addition, fish oil is relatively difficult to be consumed directly because of its strong astringent taste and oily nature. To develop functional food products with fish oil, it is crucial to protect the active ingredients in fish oil and to mask the unfavorable smell, which can be achieved with microencapsulation technology [7], [, 8].
Microencapsulation is a technique to embed solid, liquid and gaseous substances in a protective matrix, in order to mask the odor of the entrapped material, protect its activity, and release the core material in a controlled manner after consumption [9], [, 10]. It has been widely used in pharmaceutical and food industries to develop stable products with extended shelf-life for bioactive material that is sensitive to heat, moisture, oxygen and light, such as omega-3 polyunsaturated fatty acids and phytosterols [11], [, 12]. Microencapsulation of lipophilic substances can convert them to a water-dispersible form, thus facilitating the incorporation of the substances in aqueous formula such as functional beverage [13]. Microcapsules in dry powder form also have most advantages of powdered product, for example, ease in transportation and application [7], [, 14].
A number of microencapsulation techniques have been reported in the literature, including spray drying, coacervation, fluidized-bed coating and so forth. Among these techniques, spray drying is the prevailing approach utilized in industries to produce microparticles that encapsulate oils and perfumes, because of its low operating cost and high production capacity [12], [15], [16]. Previous studies reported that wall material composition and emulsification condition are important factors affecting the encapsulation efficiency, physicochemical properties, and storage stability of spray dried microcapsules [17], [18], [19]. Desired characteristics of wall material include high water-solubility, good emulsifying property, good film-forming property, and high encapsulation efficiency of the core material. In addition, materials with low viscosity in the solution form may facilitate atomization during spray drying, and those with high oxidation stability may help improve the storage stability of spray dried microcapsules [8]. Common wall materials for the microencapsulation of food ingredients include gelatin [20], [, 21], maltodextrin [22], amylopectin [23], [, 24], whey protein isolate (WPI) [25], [, 26] and gum Arabic [17], [20], [21].
In reported studies investigating the microencapsulation of lipophilic substances by spray drying, the effects of wall material composition, core/wall material ratio, total solids content and inlet drying temperature on the properties of microcapsules were investigated, to explore the optimum conditions for encapsulating a specific core material [22], [25], [27]. Since microparticles produced with a conventional spray dryer often demonstrate different size, morphology, and properties even in the same batch, it is relatively difficult to quantitatively examine the influence of a single process parameter on the properties of spray dried microcapsules. In addition, the mechanism underlying the formation of particle structure and the relationship between particle structure and particle properties have been scarcely reported, which are crucial to the targeted design and precise control of the properties of spray dried particles.
In this study, microfluidic spray drying technology was utilized to produce uniform fish oil microcapsules with similar particle size and morphology, using WPI as wall material. A series of fish oil microcapsules were produced, with differences in core/wall material ratio (1:5, 1:3, 1:1, and 2:1 by mass), total solids content of precursor emulsion (6, 12, and 18 wt%), and inlet drying temperature (100, 130, and 160 °C). The particle morphology, powder density, moisture content, encapsulation efficiency and oxidative stability of the microcapsules were analyzed, to explore the crucial factors affecting the quality attributes of fish oil microcapsules and to elucidate the mechanisms underlying microparticle structure formation.
2 Materials and methods
2.1 Materials
Fish oil was purchased from Wuxi Schindler Marine Biological Products Co., Ltd. It contained approximately 50% DHA and 20% EPA according to product specification. BiPro® whey protein isolate (WPI) was supplied by Beijing Jiali Kangyuan International Trading Co., Ltd. The composition of WPI was 92.54% protein, 1.29% fat, 2.67% ash and 4.52% moisture on dry basis. Glacial acetic acid (CH3COOH), petroleum ether, chloroform (CHCl3) and methanol (CH3OH) were purchased from Sinopharm Chemical Reagent Suzhou Co., Ltd. Barium chloride (BaCl3·2H2O) was purchased from Shanghai Bailingwei Chemical Technology Co., Ltd. Potassium iodide (KI) and starch were purchased from Sigma-Aldrich (Shanghai) Trading Co., Ltd. Sodium thiosulfate (Na2O3S2) was purchased from Beijing Yinuokai Technology Co., Ltd. Deionized water was used in emulsion preparation and powder characterization experiments.
2.2 Emulsion preparation and characterization
The fish oil and WPI powder were weighed and dispersed in deionized water to prepare emulsion of 6 wt% solids content, and the mass ratios of fish oil and WPI were controlled at 1:5, 1:3, 1:1, and 2:1, respectively. For the fish oil/WPI ratio of 1:3, emulsion at higher solids contents, namely 12 and 18 wt%, was prepared (Table 1). The resulting mixture was stirred at room temperature for 40–60 min, and then homogenized with a mechanical homogenizer at 400 rpm for three times. The size distribution of oil droplets in each type of emulsion was examined with a light microscope (Olympus CX31, Olympus Corporation, Tokyo, Japan) and with a laser diffraction particle sizer (Mastersizer 2000, Malvern Instruments, Ltd., Worcestershire, UK). The refractive indices of 1.469 and 1.330 were used for the fish oil and dispersant, respectively, and triplicate measurements were made for each sample.
Spray drying conditions used for preparing fish oil microcapsules, and the corresponding powder properties of the microcapsules.
| Variable | Sample name | wt% fish oil | wt% WPI | Total solids content (wt%) | Drying temperature (°C) | Moisture content (%) | Bulk density (g·cm−3) | Tap density (g·cm−3) | CI (%) | Particle size (μm) |
|---|---|---|---|---|---|---|---|---|---|---|
| Composition | O1W5-6-160 | 1 | 5 | 6 | 160 | 4.82 ± 0.59 | 0.196 ± 0.018 | 0.422 ± 0.049 | 53.19 ± 7.28 | 71.17 ± 3.18 |
| O1W3-6-160 | 1 | 3 | 6 | 160 | 3.16 ± 0.21 | 0.179 ± 0.005 | 0.376 ± 0.004 | 52.49 ± 1.44 | 69.87 ± 5.99 | |
| O1W1-6-160 | 1 | 1 | 6 | 160 | 1.73 ± 0.36 | 0.258 ± 0.011 | 0.496 ± 0.018 | 47.94 ± 0.97 | 70.69 ± 4.42 | |
| O2W1-6-160 | 2 | 1 | 6 | 160 | 1.41 ± 0.20 | 0.249 ± 0.008 | 0.445 ± 0.016 | 43.92 ± 0.34 | 79.01 ± 5.78 | |
| Drying temperature | O1W3-6-130 | 1 | 3 | 6 | 130 | 4.48 ± 0.12 | 0.230 ± 0.005 | 0.502 ± 0.024 | 54.16 ± 1.46 | 62.35 ± 3.58 |
| O1W3-6-100 | 1 | 3 | 6 | 100 | 7.18 ± 0.43 | 0.267 ± 0.013 | 0.528 ± 0.027 | 49.46 ± 0.58 | 58.31 ± 3.76 | |
| Solids content of emulsion | O1W3-12-160 | 1 | 3 | 12 | 160 | 5.46 ± 0.37 | 0.267 ± 0.025 | 0.496 ± 0.071 | 45.87 ± 5.10 | 87.64 ± 5.30 |
| O1W3-18-160 | 1 | 3 | 18 | 160 | 7.35 ± 0.39 | 0.340 ± 0.015 | 0.548 ± 0.014 | 37.97 ± 2.62 | 93.83 ± 6.56 |
2.3 Spray drying experiment
Each type of fish oil emulsion was spray dried with a micro-fluidic jet spray dryer (MFJSD; Model MDSD-III, Nantong Dong Concept New Material Technology Ltd., China) to produce uniform microcapsules. The working principle and experimental procedure of MFJSD were described in detail in previous studies [28], [29], [30]. Briefly, MFJSD was equipped with a micro-fluidic aerosol nozzle as atomizer, which converted emulsion into monodisperse droplets. The droplets were dispersed in concurrent hot air with optimized air dynamics, to be dried and converted to uniform microparticles. In the current study, the orifice of the nozzle was 75 μm and the flow rate of inlet air was 260 L/min. Spray drying was carried out at three inlet temperatures, namely 100, 130, and 160 °C, to study the influence of drying temperature on the properties of fish oil microcapsules. The obtained powder samples were stored in a desiccator at 4 °C before further analysis. Each sample of fish oil microcapsules was denoted by its core/wall material ratio followed by the solids content of emulsion and the drying temperature used (Table 1).
2.4 Scanning electron microscopy (SEM) observation
The spray dried powders were loaded to an aluminum stub using double-sided carbon tape, and then sputter-coated with gold to produce a conductive surface. The particle morphology of each sample was observed with a Hitachi S4700 (Hitachi High Technologies Corporation, Japan) operated at 15 kV. The recorded SEM images were analyzed with a Shineso software (SHINESO, Hangzhou, China) to determine the particle size of fish oil microcapsules. For each sample, more than 100 particles were measured and average values were reported.
2.5 Fourier transform infrared (FTIR) spectrophotometry
The spray dried powders were subjected to Fourier transform infrared spectroscopy (Bruker tensor 27, Brooke, Germany) to detect potential changes in molecular structure and possible interactions between fish oil and proteins during microencapsulation. The commercial fish oil and WPI powder were also analyzed as controls. For each sample, around 1–3 mg of sample was mixed with potassium bromide. Then the mixture was irradiated with an infrared lamp, evenly grounded and pressed, to produce a transparent sheet. The sheet was scanned at a resolution of 2 cm−1 with a wavelength range between 400 and 4000 cm−1, to obtain an infrared transmittance spectrum.
2.6 Characterization of powder properties
2.6.1 Moisture content
The moisture content of spray dried microcapsules was measured gravimetrically. Approximately 0.1 g of powder sample was placed in a forced convection oven at 105 °C for 12 h, and the weight of the sample before (W0) and after oven-drying (W1) was measured accurately using a four-decimal analytical balance (XS204, Mettler-Toledo International Inc., Zurich, Switzerland). The moisture content of the sample was calculated following Eq. (1).
2.6.2 Powder density and flowability
Each spray dried sample was gently transferred to a 5 mL cylinder till 3 mL mark (V1). The weight of the cylinder without and with the sample was accurately measured as M1 and M2, respectively. The cylinder was then shaken up and down for over 200 times till the volume of powder reached constant, which was recorded as V2. The bulk density (ρbulk) and tapped density (ρtap) of the sample were calculated with Eqs. (2) and (3), respectively.
The flowability of each spray dried sample was evaluated using the Carr’s Index (CI) as shown in Eq. (4).
2.6.3 Encapsulation efficiency
The surface oil content of each spray dried sample was extracted and measured using the method described by Bae and Lee [31] with appropriate modifications. To 1 g of powder, 25 mL of hexane was added, followed by shaking in a shaking incubator (MaxQ™ 6000, ThermoFisher Scientific, USA) at 200 rpm, room temperature for 10 min. The mixture was then filtered, and the collected powder was washed with another 25 mL of hexane. The filtrate solvent that contained extracted oil from powder was transferred to a clean beaker and left to evaporate in a fume cupboard till a constant weight. The mass of extracted surface oil was determined as the weight difference between the initial clean beaker and that containing the extracted oil, since the fish oil was involatile. The encapsulation efficiency of each spray dried sample was determined by comparing the mass of surface oil and total oil as shown in Eq. (5),
2.6.4 Changes in peroxide value during the storage of microparticles
The oxidative stability of each spray dried sample was analyzed by changes in peroxide value (PV) over 4-week storage at 4 °C. The measurement of PV was carried out following the method described by Wang et al. [26] with slight modifications. At an interval of 7 days, 1 g of spray dried microparticles were dissolved in 15 mL of chloroform/glacial acetic acid solution (3:2, v/v), followed by the addition of 0.5 mL of saturated potassium iodide solution. The resulting mixture was vortexed for 30 s and kept in dark for another 5 min. Then, 37.5 mL distilled water was added. The solution was titrated with 0.02 mol/L sodium thiosulphate till its color changed to light yellow, and then 0.5 mL of starch solution (1 wt%) was added as indicator. The titration was proceeded with vigorous shaking of the container, till the blue color disappeared. The control was prepared using blank chloroform/glacial acetic acid solution without microparticles. The PV value (meq/kg) of each spray dried sample was calculated using Eq. (6).
where C is the concentration of sodium thiosulphate (mol/L), V and V0 (mL) refer to the volume of sodium thiosulphate consumed by the sample and control, respectively, and M (g) is the mass of fish oil contained in each sample.
The analyses of powder properties, including moisture content, bulk and tapped densities, encapsulation efficiency, and peroxide value, were carried out in triplicate, and the results were reported as mean values ± standard deviation.
3 Results and discussion
3.1 The size distribution of oil droplets in the emulsion
Changes in the size and distribution of oil droplets before and after homogenization were visualized with light microscope (Figures. 1A, B). Crude emulsion containing fish oil and WPI at a mass ratio of 1:5 showed relatively large, non-uniform and scattered oil droplets prior to homogenization, whereas the homogenized emulsion was continuous without observable oil droplets under 100x magnification. When analyzed with laser diffraction particle sizer, emulsions with different fish oil/WPI ratios showed similar size distribution curves after homogenization, with the highest volume percentage at around 210 nm (Figure 1C). The results indicated that the emulsification was complete for all fish oil/WPI ratios tested, which may be related to the excellent property of WPI in stabilizing oil droplets [8].

Distribution of oil droplets in the fish oil emulsion. (A, B) Images of the O1W5 emulsion captured with light microscope (100x magnification) before and after homogenization, respectively; (C) the size distribution of oil droplets in different types of emulsion after homogenization.
3.2 The particle size and morphology of spray dried microcapsules
Microparticles produced under a specific drying condition using MFJSD showed uniform size and morphology (Figure 2), and their particle properties could hence be similar. As such, the technique can be used to accurately examine the effect of each emulsion condition and process parameters on the powder properties of spray dried microparticles. The particle morphology of fish oil microcapsules produced with various fish oil/WPI ratios, drying temperatures and total solids contents is shown in Figure 2. With an inlet temperature of 160 °C and solids content of 6 wt%, spray dried WPI particles without fish oil showed spherical shape with a relatively small opening, similar to a jar (Figure 2A1). The particles showed a thin wall with compact texture (Figure 2A2), which may be a result of early surface skin formation during drying of WPI droplets [32], [, 33]. As water was evaporated from droplets, whey protein molecules could quickly enrich at droplet/air interface, forming a semi-solid surface skin [34]. The skin rapidly solidified owing to the relatively high drying temperature, and hence could no longer shrink with the continuous removal of moisture that remained in the semi-dried droplet, producing the final particle morphology in Figure 2A1.
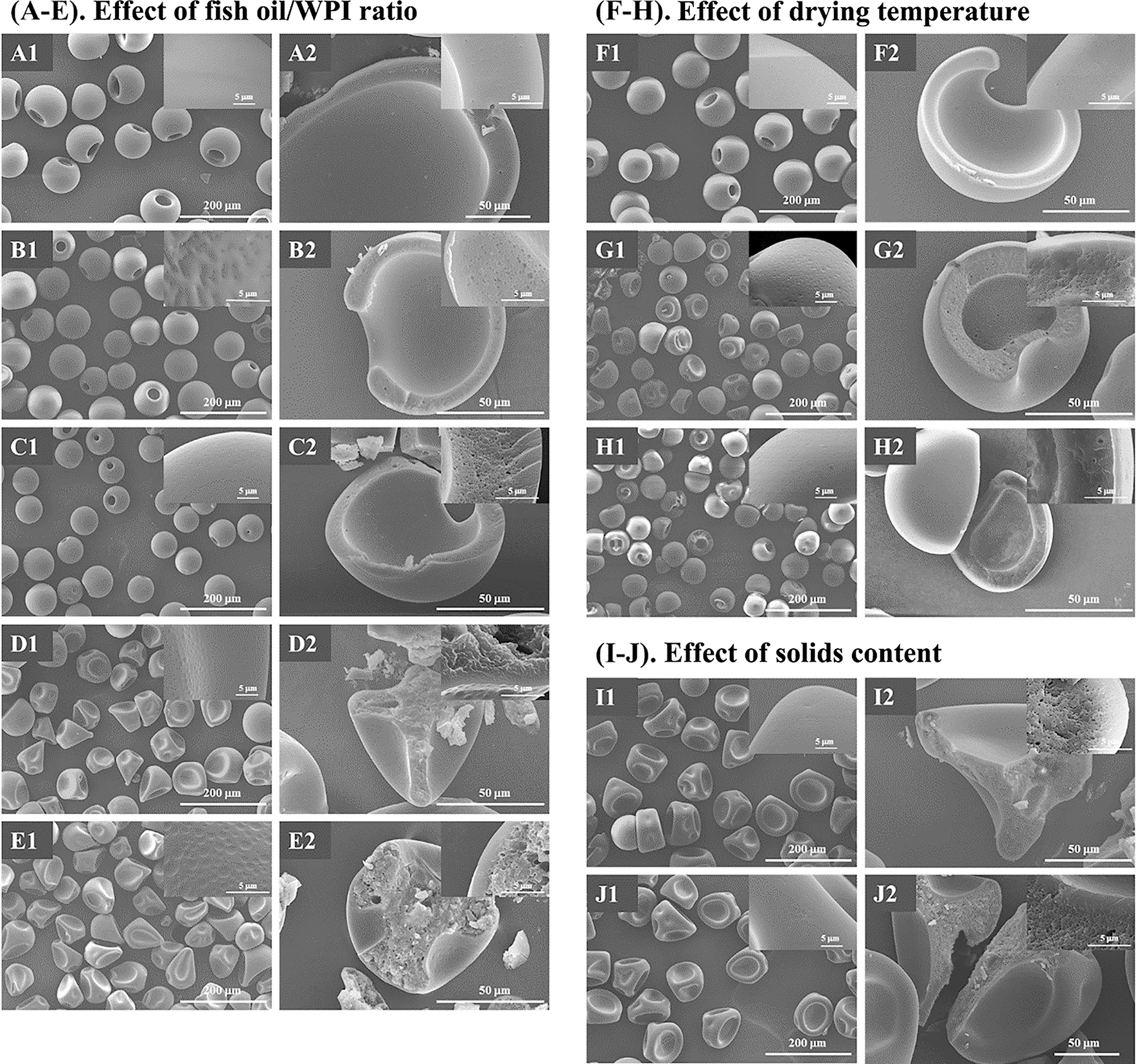
Morphology of fish oil microcapsules produced by the micro-fluidic jet spray dryer. (A–E) SEM images of WPI (O0W6-6-160), O1W5-6-160, O1W3-6-160, O1W1-6-160, and O2W1-6-160 microparticles, respectively; (F–H) SEM images of WPI (O0W6-12-130), O1W3-6-130 and O1W3-6-100 microparticles, respectively; (I, J) SEM images of O1W3-12-160 and O1W3-18-160 microparticles, respectively. The insets in A1–J1 show the surface morphology for each type of microcapsules, and those in A2–I2 show the cross-section of the microcapsules.
Replacing WPI with a low proportion of fish oil hardly altered the morphology of spray dried particles (samples O1W5-6-160 and O1W3-6-160 in Figure 2B1, C1), when the total solids content and inlet temperature were the same. As the ratio of fish oil to WPI was further increased to 1:1 and 1:3, the resulting particles after spray drying demonstrated irregularly spherical shape with a number of large concavities (Figure 2D1, E1). The raisin-like structure was commonly observed for spray dried milk particles [35], [, 36]. It can be seen from Figure 2D2, E2 that with the composition of O1W1 and O2W1, fish oil microparticles showed a solid core, in contrast to the hollow structure of microparticles with lower fish oil contents (Figure 2A2–C2). The dissimilar morphology might indicate that the microparticles underwent different particle formation processes during spray drying. The surface skin formed by the droplets of O1W1 and O2W1 could be less solid than that containing lower fish oil contents, despite the same drying temperature and total solids content. According to Wang et al. [26], the tiny dents observed at the surface of oil-containing microparticles indicated the presence of fish oil during particle surface formation (Figure 2B1–E1). The lipophilic component probably changed the property of the incipient surface skin. As the skin gradually solidified during drying, there might be a couple of unevenly distributed wet spots on it, which sunk inwards along further moisture removal, resulting in the large concavities on dried particles [37], [, 38].
WPI particles produced with increased total solids contents and decreased drying temperatures showed similar particle morphology (12 wt%, 130 °C, Figure 2F1) to that shown in Figure 2A1. By contrast, when drying temperature was decreased from 160 °C to 130 and 100 °C for O1W3 fish oil microparticles, the opening transformed to shallow concavity (Figure 2G1, H1), associated with a decrease in particle size, which was around 69.87, 62.35, and 58.31 µm for O1W3-6-160, O1W3-6-130 and O1W3-6-100 microparticles (Table 1), respectively. The trend indicated that the surface skin formed by atomized droplets at lower drying temperatures might not be as solidified as that at 160 °C, due to comparatively slow evaporation rate at droplet surface. The soft surface skin allowed droplet to shrink further as drying progressed, decreasing the size of final particles and also affecting the formation of final particle morphology in a manner similar to microparticles with a high fish oil/protein ratio (Figure 2D1, E1) [37]. Nevertheless, owing to the relatively low content of fish oil in the composition, the O1W3-6-130 and O1W3-6-100 particles remained a hollow structure (Figure 2G2, H2).
When the initial solids content of emulsion was increased to 12 and 18 wt%, spray dried microparticles demonstrated irregular, raisin-like morphology with solid core (Figure 2I1, J1), similar to the microparticles with a high fish oil/WPI content (Figure 2D1, E1). The increased solids mass might retard water evaporation at the surface of atomized droplets, leading to the occurrence of wet spots on semi-dried surface skin. The size of spray dried microparticles increased with the increased solids mass, reaching 87.64 and 93.83 µm for the solids contents of 12 and 18 wt%, respectively.
A similar characteristic shared by all types of oil-containing microparticles was the fine pores observed at the cross-section of each particle (refer to the insets in Figure 2B2–E2, G2–J2). The feature formed noticeable contrast to the compact texture of the WPI microparticles (Figure 2A2, F2). The analysis of the size distribution of the pores formed by O1W5-6-160 particles showed that the majority of pores had a size around 0.2 µm (Figure 3B), in agreement with the peak diameter of the size distribution curve of oil droplets in the emulsion (Figure 1C). It can be thus inferred that the pores were formed due to the presence of oil droplets, which might be lost under the high vacuum during sputter-coating and SEM processes. At higher fish oil/WPI ratios, the pores tended to coalesce, forming non-uniform distribution at the cross-section of microparticles, which might imply that the emulsifying state had been disturbed (Figure 2D2, E2). The relationship between particle microstructure and powder properties for each type of microparticles was investigated in the following sections.

The (A) morphology and (B) size distribution of pores observed at the cross-section of fish oil microcapsules (O1W5-6-160).
3.3 FT-IR
The infrared transmittance spectra of fish oil microcapsules with different core/wall material ratios were measured, using original fish oil and WPI powders as control (Figure 4). The four curves obtained with fish oil microcapsules showed similar peaks, despite the different composition. The characteristic peak at 3015 cm−1 corresponding to the C–H stretches of olefin (–HC=CH–) was observed for all samples containing fish oil [39], whereas the peak at 3304 cm−1 indicated the carboxylic acid group of whey proteins in the microencapsulated sample [40]. Neither shift in peaks nor new peaks were found, indicating that WPI and fish oil did not react with each other during the microencapsulation process [26].

Infrared transmittance spectrum of fish oil microcapsules with different compositions.
3.4 Moisture content, density and flowability of spray dried microcapsules
While encapsulation efficiency is an important criterion to evaluate the quality of microcapsules, common powder properties such as moisture content, density and flowability are also of high importance, because these properties further impact the stability and packing properties of the product. Dry products with a high bulk density can be stored in containers with small volume, compared to a product with low bulk density. Flowability, which is often examined by CI, reflects the interactions between individual particles, and influences the convenience of handling during further processing of powdered products. The moisture content, density and CI of different types of fish oil microcapsules are shown in Table 1. The moisture content of O1W5 and O1W3 microparticles ranged between 3.1 and 4.9%, and it drastically decreased to around 1.4–1.8% with the increase of the fish oil content in O1W1 and O2W1 microparticles. During spray drying, the surface skin formed by whey proteins at early drying stages might hinder the removal of remaining moisture, whereas with a high content of fish oil at droplet surface, the surface skin could be less solidified, facilitating moisture removal. The trend was in agreement with the different particle formation processes as influenced by microcapsule composition, discussed in Section 3.2. The moisture content of microparticles increased with the decrease in drying temperature and the increase in the solids content of emulsion, which were expected trends. A high drying temperature effectively improves the drying rate. By contrast, although droplets with a high solids content intrinsically contain a smaller water fraction, the high solids mass might retard water diffusion inside semi-dried particles, lowering overall evaporation rate.
The bulk density of O1W5-6-160 and O1W3-6-160 microparticles (0.196 and 0.179 g cm−3, respectively) was perceptibly lower than that of O1W1-6-160 and O2W1-6-160 microparticles (0.258 and 0.249 g cm−3, respectively), despite the higher moisture content. The difference might be linked to the difference in particle morphology, by which the hollow structure of O1W5 and O1W3 microparticles allowed the inclusion of occluded air. At the same composition of O1W3, the decrease of drying temperature and the increase of solids content of emulsion both led to increased bulk density, which was associated with a higher moisture content. All eight types of fish oil microcapsules demonstrated a significance increase in density after being shaken to a constant volume, giving a relatively high CI between 37 and 55%. CI ranged between 32 and 38% and > 40% indicate cohesive powders with poor and very poor flowability, respectively [41]. The flowability of powder is mainly affected by moisture content, particle shape, and the surface chemistry of particles [8]. The O1W5-6-160 and O1W3-6-160 microparticles showed spherical shape with moisture content < 5%, but their CIs were higher than 50%. The specific combination of hollow particle structure and surface chemistry might contribute to the poor flowability of fish oil microcapsules.
3.5 Encapsulation efficiency
Figure 5A–C compare the encapsulation efficiency of fish oil microcapsules by differences in core/wall material ratio, drying temperature, and the solids content of emulsion, respectively. The encapsulation efficiency of O1W5-6-160 and O1W3-6-160 microparticles was around 99%, indicating negligible amount of extractable oil at particle surface. Increasing fish oil/protein ratio to O1W1 and O2W1 substantially decreased the encapsulation efficiency to 69.6 and 55.6%, respectively (Figure 5A). Lower drying temperatures at 100 and 130 °C also led to microparticles with poorer encapsulation efficiency at around 64.8 and 89.8%, respectively (Figure 5B). These trends indicated that microparticles that experienced the early formation of a semi-solid surface skin during spray drying tended to better retain the lipophilic core material. With a high fish oil/protein ratio or at a low drying temperature, the formed surface skin was less solidified than that formed by droplets with a low fish oil/protein ratio dried at a high temperature, which allowed the droplets to shrink further as drying progressed. As such, the receding droplet/air interface may capture increasing amount of oil droplets that were dispersed at the close proximity of droplet surface, because they could barely migrate with the shrinking water phase [34]. The O1W3-12-160 and O1W3-18-160 microparticles also formed relatively soft surface skin as shown by the raisin-like particle shape (Figure 2I1, J1). Nevertheless, the increased solids mass limited the degree of possible droplet shrinkage, helping maintain high encapsulation efficiency for both types of microcapsules (> 90%, Figure 5C). The encapsulation efficiency of microcapsules produced from 18 wt% emulsion was higher than that from 12 wt% emulsion, reaching around 97%. A low encapsulation efficiency indicates a high level of extractable oil at particle surface, which not only adversely affects the flowability and wettability of the microcapsules [8], but could also increase their proneness to oxidation as discussed in the next section.

Comparison of the encapsulation efficiency between fish oil microcapsules (A) with different compositions, (B) produced at different drying temperatures, and (C) produced from emulsions with different initial solids contents.
3.6 Oxidative stability
The stability of fish oil against oxidation is a crucial index governing the stability of microcapsules during storage. In this study, PV was experimentally monitored to examine the degree of fish oil oxidation during storage at 4 °C for 28 days. Figure 6A–C show the changes in PV for fish oil microcapsules with different core/wall material ratios, different drying temperatures, and different solids contents of emulsion, respectively. Raw fish oil as the control sample showed a low PV close to 0 meq/kg when freshly taken from the product container. Its PV gradually increased to around 95 meq/kg during storage in a desiccator at 4 °C for 4 weeks, indicating that some oil had been oxidized, generating peroxides. The PV of freshly spray-dried fish oil microcapsules ranged between 3 and 15 meq/kg, suggesting that the high temperature and rapid dehydration during spray drying caused the oxidation of a small amount of fish oil. Notably, all types of fish oil microcapsules with core/wall material ratio ≤ 1:3 showed PV lower than 10 meq/kg, regardless of drying temperature and the solids content of emulsion. By contrast, the O2W1-6-160 microparticles showed the highest PV among the eight types of microparticles (Figure 6A), suggesting that the protection on fish oil offered by a low content of WPI during spray drying was less effective than that of a high content of WPI.

Changes in the peroxide value of fish oil microcapsules during storage at 4 °C for four weeks. (A) Microcapsules with different compositions, (B) microcapsules produced at different drying temperatures, and (C) microcapsules produced from emulsions with different initial solids contents.
After storage for 7 days, all eight types of fish oil microcapsules showed a rapid increase in PV, which was considerably higher than the increase of PV shown by raw fish oil. The PV either dropped to lower values or maintained at a similar level during the subsequent storage period. It is worthwhile to note that the degree of PV increase shown by the eight types of microcapsules was in line with the trend of encapsulation efficiency. Microcapsules with higher fish oil/protein ratios or dried at lower temperatures showed higher PV during storage (Figure 6A, B), as well as lower encapsulation efficiency (Figure 5A, B). By contrast, the O1W3-12-160 and O1W3-18-160 microparticles showed high encapsulation efficiency > 90% (Figure 5C), and the increase of PV was less significant in Figure 6C compared to that in Figure 6A, B. Previous studies suggested that the autoxidation of core oil in microcapsules mainly occurred at particle surface, and the exposure to high temperature, the formation of surface skin and other interfacial phenomena during spray drying could increase the rate of oxidation of these non-encapsulated surface oil [26], [, 42]. It can be thus inferred that the increase of PV of fish oil microcapsules during storage arose mainly from the oxidation of extractable surface oil. The generated peroxides were unstable, and could further react with the oxidized oil to generate aldehydes and ketones [26], [, 42]. The consequently reduced PV at the later storage period suggested that oxidation reaction in fish oil microcapsules had become less significant, in contrary to the drastically increased PV of raw fish oil. While the non-encapsulated oil at the surface of microcapsules had been fully oxidized, the majority of fish oil, encapsulated at the interior of each microparticle, could be well protected [26]. Nevertheless, the inference warrants in depth investigation, and the relationship between the PV and functionality of fish oil microcapsules still needs further examination.
4 Conclusions
In this study, eight types of fish oil microcapsules with different core/wall material ratios, prepared at different drying temperatures and with different solids contents of emulsion were produced by MFJSD, to investigate the effects of each drying condition on the quality attributes of microcapsules. It was found that the differences in drying conditions influenced particle formation behavior during spray drying, resulting in microparticles with various morphologies. Using WPI as wall material, fish oil microcapsules produced with a relatively low core/wall material ratio at a high drying temperature could experience the rapid formation of a surface skin composed mainly of protein. The rigid surface skin could limit possible droplet shrinkage at the later drying stage, resulting in high encapsulation efficiency and excellent oxidative stability during the storage of fish oil microcapsules. A high core/wall material ratio, low drying temperature and high solids content of emulsion tended to result in soft surface skin that allowed the semi-dried droplets to further shrink. Nevertheless, in drying of high solids emulsion, droplet shrinkage was also limited by the high solids mass, which helped increase the encapsulation efficiency and oxidative stability of microcapsules. The relationship between spray drying conditions, particle formation behavior and the powder properties of fish oil microcapsules discussed in the present study would be useful for producing encapsulated fish oil products with precisely controlled properties.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 21878197, 31601513
Funding source: Natural Science Foundation of Jiangsu Province
Award Identifier / Grant number: BK20180096
Funding source: Jiangsu Higher Education Institutions
Award Identifier / Grant number: 18KJA530004
Funding source: Suzhou Municipal Science and Technology Bureau
Award Identifier / Grant number: SYG201810
Funding source: Priority Academic Program Development of Jiangsu Higher Education Institutions
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: This work is financially supported by the National Natural Science Foundation of China (No. 21878197, No. 31601513), the Natural Science Foundation of Jiangsu Province (No. BK20180096) and Jiangsu Higher Education Institutions (No. 18KJA530004), and the Suzhou Municipal Science and Technology Bureau (No. SYG201810). The support from the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions is also appreciated.
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. U.S. Department of Health and Human Services, U.S. Department of Agriculture. 2015 – 2020 dietary guidelines for Americans. 8th ed.; 2015. Available from: http://health.gov/dietaryguidelines/2015/guidelines/.Suche in Google Scholar
2. Din, JN, Newby, DE, Flapan, AD. Science, medicine, and the future – omega 3 fatty acids and cardiovascular disease – fishing for a natural treatment. Br Med J 2004;328:30–5.10.1136/bmj.328.7430.30Suche in Google Scholar
3. White, MN, Shrubsole, MJ, Cai, Q, Su, T, Hardee, J, Coppola, JA, et al.. Effects of fish oil supplementation on eicosanoid production in patients at higher risk for colorectal cancer. Eur J Canc Prev 2019;28:188–95.10.1097/CEJ.0000000000000455Suche in Google Scholar
4. Fernandes, G, Venkatraman, JT. Role of omega-3 fatty acids in health and disease. Nutr Res 1993;13:S19–45.10.1016/S0271-5317(05)80282-9Suche in Google Scholar
5. Arab-Tehrany, E, Jacquot, M, Gaiani, C, Imran, M, Desobry, S, Linder, M. Beneficial effects and oxidative stability of omega-3 long-chain polyunsaturated fatty acids. Trends Food Sci Technol 2012;25:24–33.10.1016/j.tifs.2011.12.002Suche in Google Scholar
6. Ryan, AS, Astwood, JD, Gautier, S, Kuratko, CN, Nelson, EB, Salem, N. Effects of long-chain polyunsaturated fatty acid supplementation on neurodevelopment in childhood: a review of human studies. Prostagl Leukot Essent Fat Acids 2010;82:305–14.10.1016/j.plefa.2010.02.007Suche in Google Scholar PubMed
7. Kaushik, P, Dowling, K, Barrow, CJ, Adhikari, B. Microencapsulation of omega-3 fatty acids: a review of microencapsulation and characterization methods. J Funct Foods 2015;19:868–81.10.1016/j.jff.2014.06.029Suche in Google Scholar
8. Fu, N, You, Y-J, Quek, SY, Wu, WD, Chen, XD. Interplaying effects of wall and core materials on the property and functionality of microparticles for co-encapsulation of vitamin E with coenzyme Q10. Food Bioprocess Technol 2020;13:705–21.10.1007/s11947-020-02431-ySuche in Google Scholar
9. Eratte, D, Dowling, K, Barrow, CJ, Adhikari, B. Recent advances in the microencapsulation of omega-3 oil and probiotic bacteria through complex coacervation: a review. Trends Food Sci Technol 2018;71:121–31.10.1016/j.tifs.2017.10.014Suche in Google Scholar
10. Zaghari, L, Basiri, A, Rahimi, S. Preparation and characterization of double-coated probiotic bacteria via a fluid-bed process: a case study on Lactobacillus reuteri. Int J Food Eng 2020;16:20190384.10.1515/ijfe-2019-0384Suche in Google Scholar
11. Mastromatteo, M, Mastromatteo, M, Conte, A, Nobile, MAD. Advances in controlled release devices for food packaging applications. Trends Food Sci Technol 2010;21:591–8.10.1016/j.tifs.2010.07.010Suche in Google Scholar
12. Comunian, TA, Favaro-Trindade, CS. Microencapsulation using biopolymers as an alternative to produce food enhanced with phytosterols and omega-3 fatty acids: a review. Food Hydrocolloids 2016;61:442–57.10.1016/j.foodhyd.2016.06.003Suche in Google Scholar
13. Sukri, N, Multisona, RR, Zaida, Saputra, RA, Mahani, Nurhadi, B. Effect of maltodextrin and Arabic gum ratio to physicochemical characteristic of spray dried propolis microcapsules. Int J Food Eng 2020;20190050. https://doi.org/10.1515/ijfe-2019-0050.Suche in Google Scholar
14. Su, Y, Zheng, X, Zhao, Q, Fu, N, Xiong, H, Wu, WD, et al.. Spray drying of Lactobacillus rhamnosus GG with calcium-containing protectant for enhanced viability. Powder Technol 2019;358:87–94.10.1016/j.powtec.2018.09.082Suche in Google Scholar
15. Koc, M, Gungor, O, Zungur, A, Yalcin, B, Selek, I, Ertekin, FK, et al.. Microencapsulation of extra virgin olive oil by spray drying: effect of wall materials composition, process conditions, and emulsification method. Food Bioprocess Technol 2015;8:301–18.10.1007/s11947-014-1404-9Suche in Google Scholar
16. Vishnu, KV, Chatterjee, NS, Ajeeshkumar, KK, Lekshmi, RGK, Tejpal, CS, Mathew, S, et al.. Microencapsulation of sardine oil: application of vanillic acid grafted chitosan as a bio-functional wall material. Carbohydr Polym 2017;174:540–8.10.1016/j.carbpol.2017.06.076Suche in Google Scholar PubMed
17. Li, JJ, Xiong, SB, Wang, F, Regenstein, JM, Liu, R. Optimization of microencapsulation of fish oil with gum Arabic/casein/beta-cyclodextrin mixtures by spray drying. J Food Sci 2015;80:C1445–52.10.1111/1750-3841.12928Suche in Google Scholar PubMed
18. Melgosa, R, Benito-Roman, O, Sanz, MT, de Paz, E, Beltran, S. Omega-3 encapsulation by PGSS-drying and conventional drying methods: particle characterization and oxidative stability. Food Chem 2019;270:138–48.10.1016/j.foodchem.2018.07.082Suche in Google Scholar PubMed
19. Zuanon, LAC, Fuzari, NC, Ferreira, S, Freitas, MLF, Moser, P, Nicoletti, VR. Production and storage properties of spray-dried red beet extract using polysaccharide-based carrier systems. Int J Food Eng 2019;15:20180371.10.1515/ijfe-2018-0371Suche in Google Scholar
20. Radivojev, S, Pinto, JT, Frohlich, E, Paudel, A. Insights into DPI sensitivity to humidity: an integrated in-vitro-in-silico risk-assessment. J Drug Deliv Sci Technol 2019;52:803–17.10.1016/j.jddst.2019.05.047Suche in Google Scholar
21. Pieczykolan, E, Kurek, MA. Use of guar gum, gum Arabic, pectin, beta-glucan and inulin for microencapsulation of anthocyanins from chokeberry. Int J Biol Macromol 2019;129:665–71.10.1016/j.ijbiomac.2019.02.073Suche in Google Scholar PubMed
22. Abd Ghani, A, Adachi, S, Shiga, H, Neoh, TL, Adachi, S, Yoshii, H. Effect of different dextrose equivalents of maltodextrin on oxidation stability in encapsulated fish oil by spray drying. Biosci Biotechnol Biochem 2017;81:705–11.10.1080/09168451.2017.1281721Suche in Google Scholar PubMed
23. Jiang, M, Hong, Y, Gu, ZB, Cheng, L, Li, ZF, Li, CM. Preparation of a starch-based carrier for oral delivery of vitamin E to the small intestine. Food Hydrocolloids 2019;91:26–33.10.1016/j.foodhyd.2019.01.021Suche in Google Scholar
24. Zhang, C, Xie, Y, Zou, J. Effect of the viscoelastic properties of modified starch as a wall material on the surface of morphology of microcapsules. J Sci Food Agric 2019;99:4725–30.10.1002/jsfa.9713Suche in Google Scholar PubMed
25. Zhang, ZH, Peng, HD, Ma, HL, Zeng, XA. Effect of inlet air drying temperatures on the physicochemical properties and antioxidant activity of whey protein isolate-kale leaves chlorophyll (WPI-CH) microcapsules. J Food Eng 2019;245:149–56.10.1016/j.jfoodeng.2018.10.011Suche in Google Scholar
26. Wang, Y, Liu, WJ, Chen, XD, Selomulya, C. Micro-encapsulation and stabilization of DHA containing fish oil in protein-based emulsion through mono-disperse droplet spray dryer. J Food Eng 2016;175:74–84.10.1016/j.jfoodeng.2015.12.007Suche in Google Scholar
27. Huang, E, Quek, SY, Fu, N, Wu, WD, Chen, XD. Co-encapsulation of coenzyme Q10 and vitamin E: a study of microcapsule formation and its relation to structure and functionalities using single droplet drying and micro-fluidic-jet spray drying. J Food Eng 2019;247:45–55.10.1016/j.jfoodeng.2018.11.017Suche in Google Scholar
28. Wu, WD, Amelia, R, Hao, N, Selomulya, C, Zhao, D, Chiu, YL, et al.. Assembly of uniform photoluminescent microcomposites using a novel micro-fluidic-jet-spray-dryer. AIChE J 2011;57:2726–37.10.1002/aic.12489Suche in Google Scholar
29. Liu, W, Wu, W, Selomulya, C, Chen, XD. A single step assembly of uniform microparticles for controlled release applications. Soft Matter 2011;7:3323–30.10.1039/c0sm01371dSuche in Google Scholar
30. Zhang, S, Lei, H, Gao, X, Xiong, X, Wu, WD, Wu, Z, et al.. Fabrication of uniform enzyme-immobilized carbohydrate microparticles with high enzymatic activity and stability via spray drying and spray freeze drying. Powder Technol 2018;330:40–9.10.1016/j.powtec.2018.02.020Suche in Google Scholar
31. Bae, EK, Lee, SJ. Microencapsulation of avocado oil by spray drying using whey protein and maltodextrin. J Microencapsul 2008;25:549–60.10.1080/02652040802075682Suche in Google Scholar PubMed
32. Sadek, C, Tabuteau, H, Schuck, P, Fallourd, Y, Pradeau, N, Le Floch-Fouéré, C, et al.. Shape, shell and vacuole formation during the drying of a single concentrated whey protein droplet. Langmuir 2013;29:15606–13.10.1021/la404108vSuche in Google Scholar PubMed
33. Both, EM, Karlina, AM, Boom, RM, Schutyser, MAI. Morphology development during sessile single droplet drying of mixed maltodextrin and whey protein solutions. Food Hydrocolloids 2018;75:202–10.10.1016/j.foodhyd.2017.08.022Suche in Google Scholar
34. Fu, N, Wu, WD, Wu, Z, Moo, FT, Woo, MW, Selomulya, C, et al.. Formation process of core-shell microparticles by solute migration during drying of homogenous composite droplets. AIChE J 2017;63:3297–310.10.1002/aic.15713Suche in Google Scholar
35. Rogers, S, Wu, WD, Lin, SXQ, Chen, XD. Particle shrinkage and morphology of milk powder made with a monodisperse spray dryer. Biochem Eng J 2012;62:92–100.10.1016/j.bej.2011.11.002Suche in Google Scholar
36. Tian, Y, Fu, N, Wu, WD, Zhu, D, Huang, J, Yun, S, et al.. Effects of co-spray drying of surfactants with high solids milk on milk powder wettability. Food Bioprocess Technol 2014;7:3121–35.10.1007/s11947-014-1323-9Suche in Google Scholar
37. Fu, N, Woo, MW, Selomulya, C, Chen, XD. Shrinkage behaviour of skim milk droplets during air drying. J Food Eng 2013;116:37–44.10.1016/j.jfoodeng.2012.11.005Suche in Google Scholar
38. Wu, WD, Liu, W, Selomulya, C, Chen, XD. On spray drying of uniform silica-based microencapsulates for controlled release. Soft Matter 2011;7:11416–24.10.1039/c1sm05879gSuche in Google Scholar
39. Vongsvivut, J, Heraud, P, Zhang, W, Kralovec, JA, McNaughton, D, Barrow, CJ. Quantitative determination of fatty acid compositions in micro-encapsulated fish-oil supplements using Fourier transform infrared (FTIR) spectroscopy. Food Chem 2012;135:603–9.10.1016/j.foodchem.2012.05.012Suche in Google Scholar PubMed
40. Zhang, LY, Dudhani, A, Lundin, L, Kosaraju, SL. Macromolecular conjugate based particulates: preparation, characterisation and evaluation of controlled release properties. Eur Polym J 2009;45:1960–9.10.1016/j.eurpolymj.2009.04.019Suche in Google Scholar
41. Seville, PC, Learoyd, TP, Li, HY, Williamson, IJ, Birchall, JC. Amino acid-modified spray-dried powders with enhanced aerosolisation properties for pulmonary drug delivery. Powder Technol 2007;178:40–50.10.1016/j.powtec.2007.03.046Suche in Google Scholar
42. Drusch, S, Serfert, Y, Van Den Heuvel, A, Schwarz, K. Physicochemical characterization and oxidative stability of fish oil encapsulated in an amorphous matrix containing trehalose. Food Res Int 2006;39:807–15.10.1016/j.foodres.2006.03.003Suche in Google Scholar
© 2020 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Articles
- Screening and characterisation of β-glucosidase production strains from Rosa roxburghii Tratt
- Correction of residence time distribution measurements for short holding times in pasteurization processes
- Effects of particle formation behavior on the properties of fish oil microcapsules fabricated using a micro-fluidic jet spray dryer
- Predicting the moisture content of Daqu with hyperspectral imaging
- The effects of reaction parameters on the non-enzymatic browning reaction between l-ascorbic acid and glycine
- Internal quality evaluation of chestnut using nuclear magnetic resonance
- Effect of microwave-drying on the quality and antioxidant properties of Ganoderma lucidum fermented sea-buckthorn tea
- The use of beetroot extract and extract powder in sausages as natural food colorant
Artikel in diesem Heft
- Frontmatter
- Articles
- Screening and characterisation of β-glucosidase production strains from Rosa roxburghii Tratt
- Correction of residence time distribution measurements for short holding times in pasteurization processes
- Effects of particle formation behavior on the properties of fish oil microcapsules fabricated using a micro-fluidic jet spray dryer
- Predicting the moisture content of Daqu with hyperspectral imaging
- The effects of reaction parameters on the non-enzymatic browning reaction between l-ascorbic acid and glycine
- Internal quality evaluation of chestnut using nuclear magnetic resonance
- Effect of microwave-drying on the quality and antioxidant properties of Ganoderma lucidum fermented sea-buckthorn tea
- The use of beetroot extract and extract powder in sausages as natural food colorant

