Dehydrogenation of Propane to Propylene with Highly Stable Catalysts of Pt-Sn Supported Over Mesoporous Silica KIT-6
-
Jose P. Ruelas-Leyva
, Alejandro Mata-Martinez
Abstract
During several reactions, similar to dehydrogenation of propane to propylene, coke is one of the main reasons for the catalyst deactivation. The coke formation and further deactivation of the catalyst are strongly dependent to the active site in the catalyst and/or the properties of the support. KIT-6 with interconnected porous and high surface area can handle with the coke formation, and can disperse easily the deposited Pt nanoparticles. In this sense, a series of Pt-Sn/KIT-6 catalysts were synthesized with distinct Sn loadings and used in the dehydrogenation of propane. The performance of these catalysts during reaction varied with the Sn loading. The specific activities for propylene formation obtained with the catalysts were comparable to the best result reported in the literature. The nanoparticles present in the catalyst through pretreatment and reaction condition was the Pt-Sn alloy (1:1 atomic ratio), and that alloy is suggested to be the active phase. This Pt-Sn alloy was stable during the entire reaction time, that even in two catalysts containing a considerable amount of coke, deactivation was not observed. Also, the support (KIT-6) with high connectivity helped to avoid deactivation by coke.
Acknowledgements
This work was financially supported by the Mexican National Council for Science and Technology (CONACyT; project CB-2015-01 # 256268); and the Autonomous University of Sinaloa (UAS; project PROFAPI-2015/251).
References
Bariås, O.A., A. Holmen, and E.A. Blekkan. 1996. “Propane Dehydrogenation over Supported Pt and Pt–Sn Catalysts: Catalyst Preparation, Characterization, and Activity Measurements.” Journal of Catalysis 158: 1–12.10.1006/jcat.1996.0001Search in Google Scholar
Baronetti, G.T., S.R. De Miguel, O.A. Scelza, and A.A. Castro. 1986. “State of Metallic Phase in PtSn/Al2O3 Catalysts Prepared by Different Deposition Techniques.” Applied Catalysis 24 (1-2): 109–116.10.1016/S0166-9834(00)81261-0Search in Google Scholar
Che-Galicia, G., R. Quintana-Solórzano, R.S. Ruiz-Martínez, J.S. Valente, and C.O. Castillo-Araiza. 2014. “Kinetic Modeling of the Oxidative Dehydrogenation of Ethane to Ethylene over a MoVTeNbO Catalytic System.” Chemical Engineering Journal 252: 75–88.10.1016/j.cej.2014.04.042Search in Google Scholar
Che-Galicia, G., R.S. Ruiz-Martínez, F. López-Isunza, and C.O. Castillo-Araiza. 2015. “Modeling of Oxidative Dehydrogenation of Ethane to Ethylene on a MoVTeNbO/TiO2 Catalyst in an Industrial-Scale Packed Bed Catalytic Reactor.” Chemical Engineering Journal 280: 682–694.10.1016/j.cej.2015.05.128Search in Google Scholar
Chen, D., K. Moljord, and A. Holmen. 2012. “A Methanol to Olefins Review: Diffusion, Coke Formation and Deactivation on SAPO Type Catalysts.” Microporous and Mesoporous Materials 164: 239–250.10.1016/j.micromeso.2012.06.046Search in Google Scholar
Collett, C.H., and J. McGregor. 2016. “Things Go Better with Coke: The Beneficial Role of Carbonaceous Deposits in Heterogeneous Catalysis.” Catalysis Science & Technology 6: 363–378.10.1039/C5CY01236HSearch in Google Scholar
Deng, L., T. Shishido, K. Teramura, and T. Tanaka. 2014. “Effect of Reduction Method on the Activity of Pt–Sn/SiO2 for Dehydrogenation of Propane.” Catalysis Today 232: 33–39.10.1016/j.cattod.2013.10.064Search in Google Scholar
Guisnet, M., and P. Magnoux. 2001. “Organic Chemistry of Coke Formation.” Applied Catalysis A-Gen 212: 83–96.10.1016/S0926-860X(00)00845-0Search in Google Scholar
Jiang, W., R. Huang, P. Li, S. Feng, G. Zhou, C. Yu, and Q. Xu. 2016. “Metathesis and Isomerization of N-Butene and Ethylene over WO3/SiO2 and MgO Catalysts: Thermodynamic and Experimental Analysis.” Applied Catalysis A-Gen 517: 227–235.10.1016/j.apcata.2016.03.009Search in Google Scholar
Katsuki, H., E.K. Choi, W.J. Lee, K.T. Hwang, W.S. Cho, W. Huang, and S. Komarneni. 2018. “Ultrafast Microwave-Hydrothermal Synthesis of Hexagonal Plates of Hematite.” Materials Chemistry and Physical 205: 210–216.10.1016/j.matchemphys.2017.10.078Search in Google Scholar
Kleitz, F., F. Berube, R. Guillet-Nicolas, C.M. Yang, and M. Thommes. 2010. “Probing Adsorption, Pore Condensation, and Hysteresis Behavior of Pure Fluids in Three-Dimensional Cubic Mesoporous KIT-6 Silica.” The Journal of Physical Chemistry C 114: 9344–9355.10.1021/jp909836vSearch in Google Scholar
Kleitz, F., S.H. Choi, and R. Ryoo. 2003. “Cubic Ia 3 D Large Mesoporous Silica: Synthesis and Replication to Platinum Nanowires, Carbon Nanorods and Carbon Nanotubes.” Chemical Communications 17: 2136–2137.10.1039/b306504aSearch in Google Scholar PubMed
Kotanjac, Z.S., J.P. Niederer, and G.F. Versteeg. 2007. “Influence of the Pressure on the Product Distribution in the Oxidative Dehydrogenation of Propane over a Ga2O3/MoO3 Catalyst.” International Journal of Chemical Reactor Engineering 5: 1.10.2202/1542-6580.1392Search in Google Scholar
Kumar, B.R. 2006. “Effect of Silica Support on V-Mg-O Catalysts during ODH of Propane: Experimental and Modeling Using Genetic Algorithms.” International Journal of Chemical Reactor Engineering 4: 1.10.2202/1542-6580.1309Search in Google Scholar
Kumar, M.S., D. Chen, A. Holmen, and J.C. Walmsley. 2009. “Dehydrogenation of Propane over Pt-SBA-15 and Pt-Sn-SBA-15: Effect of Sn on the Dispersion of Pt and Catalytic Behavior.” Catalysis Today 142: 17–23.10.1016/j.cattod.2009.01.002Search in Google Scholar
Kumar, M.S., D. Chen, J.C. Walmsley, and A. Holmen. 2008. “Dehydrogenation of Propane over Pt-SBA-15: Effect of Pt Particle Size.” Catalysis Communications 9: 747–750.10.1016/j.catcom.2007.08.015Search in Google Scholar
Liu, Q., J. Li, Z. Zhao, M. Gao, L. Kong, J. Liu, and Y. Wei. 2016. “Design, Synthesis and Catalytic Performance of Vanadium-Incorporated Mesoporous Silica KIT-6 Catalysts for the Oxidative Dehydrogenation of Propane to Propylene.” Catalysis Science & Technology 6: 5927–5941.10.1039/C6CY00404KSearch in Google Scholar
Nava, N., and T. Viveros. 2000. “Effect of the Support on the Structural Characteristics of Tin.” Journal of Radioanalytical and Nuclear Chemistry 243: 689–696.10.1023/A:1010670219739Search in Google Scholar
Sangaletti, L., L.E. Depero, B. Allieri, F. Pioselli, E. Comini, G. Sberveglieri, and M. Zocchi. 1998. “Oxidation of Sn Thin Films to SnO2. Micro-Raman Mapping and X-Ray Diffraction Studies.” Journal of Materials Research 13: 2457–2460.10.1557/JMR.1998.0343Search in Google Scholar
Sattler, J.J., J. Ruiz-Martinez, E. Santillan-Jimenez, and B.M. Weckhuysen. 2014. “Catalytic Dehydrogenation of Light Alkanes on Metals and Metal Oxides.” Chemical Reviews 114: 10613–10653.10.1021/cr5002436Search in Google Scholar PubMed
Sharma, L.D., M. Kumar, A.K. Saxena, D.S. Rawat, and T.P. Rao. 1998. “The Determination of Accessible Pt Metal Fraction in Pt–Sn/Al2O3 Reforming Catalyst.” Applied Catalysis A-Gen 168: 251–259.10.1016/S0926-860X(97)00356-6Search in Google Scholar
Shi, L., G.M. Deng, W.C. Li, S. Miao, Q.N. Wang, W.P. Zhang, and A.H. Lu. 2015. “Al2O3 Nanosheets Rich in Pentacoordinate Al3+ Ions Stabilize Pt‐Sn Clusters for Propane Dehydrogenation.” Angewandte Chemie International Edition 54: 13994–13998.10.1002/anie.201507119Search in Google Scholar PubMed
Shi, Y., X. Li, X. Rong, B. Gu, H. Wei, and C. Sun. 2017. “Influence of Support on the Catalytic Properties of Pt–Sn–K/θ-Al2O3 for Propane Dehydrogenation.” RSC Advances 7: 19841–19848.10.1039/C7RA02141KSearch in Google Scholar
Srinivasan, R., and B.H. Davis. 1992. “The Structure of Platinum-Tin Reforming Catalysts.” Platinum Metals Reviews 36: 151–163.Search in Google Scholar
Srinivasan, R., R.J. De Angelis, and B.H. Davis. 1990. “Structural Studies of Pt-Sn Catalysts on High and Low Surface Area Alumina Supports.” Catalysis Letters 4: 303–308.10.1007/BF00765315Search in Google Scholar
Tan, S., B. Hu, W.G. Kim, S.H. Pang, J.S. Moore, Y. Liu, … C.W. Jones. 2016. “Propane Dehydrogenation over Alumina-Supported Iron/Phosphorus Catalysts: Structural Evolution of Iron Species Leading to High Activity and Propylene Selectivity.” ACS Catalysis 6: 5673–5683.10.1021/acscatal.6b01286Search in Google Scholar
Wang, X., L. Altmann, J. StöVer, V. Zielasek, M. BäUmer, K. Al-Shamery, and J. Kolny-Olesiak. 2012. “Pt/Sn Intermetallic, Core/Shell and Alloy Nanoparticles: Colloidal Synthesis and Structural Control.” Chemistry of Materials 25: 1400–1407.10.1021/cm302077wSearch in Google Scholar
Xu, G., J. Zhang, S. Wang, Y. Zhao, and X. Ma. 2016. “A Well Fabricated PtSn/SiO2 Catalyst with Enhanced Synergy between Pt and Sn for Acetic Acid Hydrogenation to Ethanol.” RSC Advances 6: 51005–51013.10.1039/C6RA09199GSearch in Google Scholar
Zhang, F., Y. Yan, H. Yang, Y. Meng, C. Yu, B. Tu, and D. Zhao. 2005. “Understanding Effect of Wall Structure on the Hydrothermal Stability of Mesostructured Silica SBA-15.” The Journal of Physical Chemical B 109: 8723–8732.10.1021/jp044632+Search in Google Scholar PubMed
Zhang, Y., Y. Zhou, J. Shi, S. Zhou, X. Sheng, Z. Zhang, and S. Xiang. 2014. “Comparative Study of Bimetallic Pt-Sn Catalysts Supported on Different Supports for Propane Dehydrogenation.” Journal of Molecular Catalysis A-Chem 381: 138–147.10.1016/j.molcata.2013.10.007Search in Google Scholar
Zhang, Y., Y. Zhou, K. Yang, Y. Li, Y. Wang, Y. Xu, and P. Wu. 2006. “Effect of Hydrothermal Treatment on Catalytic Properties of PtSnNa/ZSM-5 Catalyst for Propane Dehydrogenation.” Microporous and Mesoporous Materials 96: 245–254.10.1016/j.micromeso.2006.07.003Search in Google Scholar
Zhao, D., J. Feng, Q. Huo, N. Melosh, G.H. Fredrickson, B.F. Chmelka, and G.D. Stucky. 1998. “Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores.” Science 279: 548–552.10.1126/science.279.5350.548Search in Google Scholar PubMed
Zhao, D., J. Sun, Q. Li, and G.D. Stucky. 2000. “Morphological Control of Highly Ordered Mesoporous Silica SBA-15.” Chemistry of Materials 12: 275–279.10.1021/cm9911363Search in Google Scholar
A Appendix
The results of the nitrogen adsorption-desorption isotherms of KIT-6 can be seen in the Figure 7. The isotherm is type IV with a specific surface area, pore volume and pore diameter of 809 m2/g, 0.93 cm3/g and 45.8 Å, respectively. These values are in agreement with those reported in the literature for similar samples (Kleitz, Choi, and Ryoo 2003; Kleitz et al. 2010).
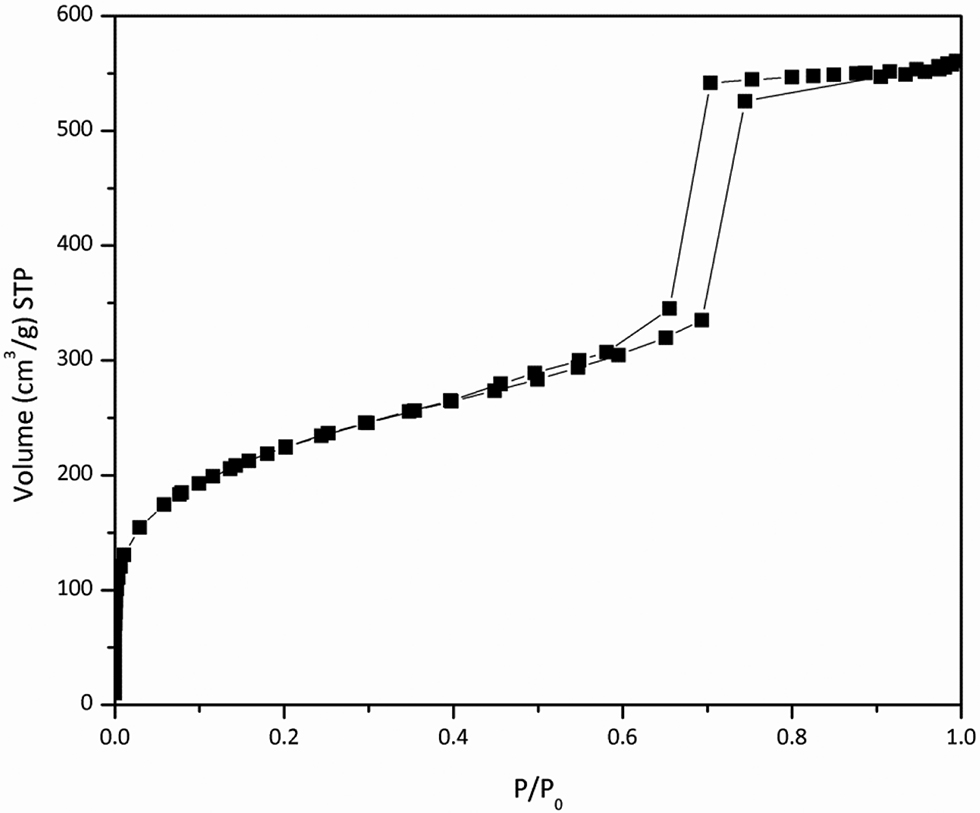
Nitrogen adsorption-desorption isotherms of KIT-6.
The XRD analyses for the KIT-6 support are given in Figure 8. The low-angle diffraction pattern shows evidence of two reflections at 2 Teta values of 0.94° and 1.72° corresponding to the interplanar spacing (211) and (420), respectively (Kleitz, Choi, and Ryoo 2003; Kleitz et al. 2010).
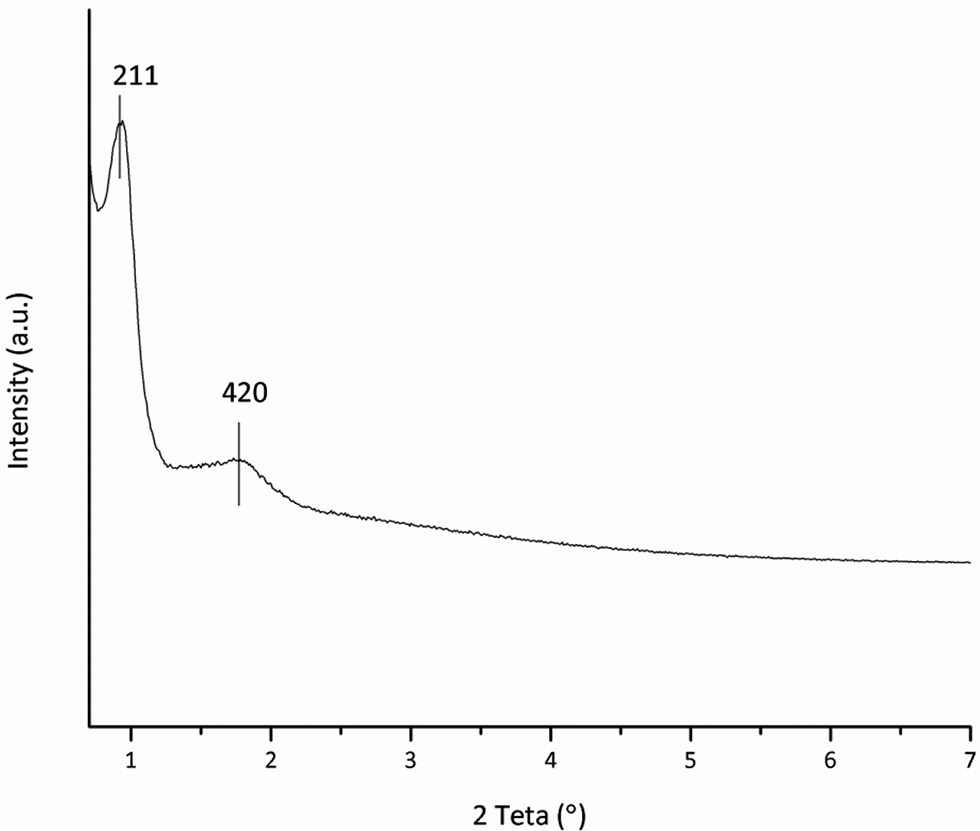
Low-angle XRD analysis of KIT-6.
© 2018 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Preface to the Special Issue Dedicated to the International Energy Conference, IEC 2017: Energy and its Development in the Twenty-First Century: A globalized vision
- Parametric Mathematical Modelling of Cristal Violet Dye Electrochemical Oxidation Using a Flow Electrochemical Reactor with BDD and DSA Anodes in Sulfate Media
- Photocatalytic Degradation of Caffeine in a Solar Reactor System
- Effect of Biomass Concentration on Oxygen Mass Transfer, Power Consumption, Interfacial Tension and Hydrodynamics in a Multiphase Partitioning Bioreactor
- Modeling and Hydraulic Characterization of a Filter-Press-Type Electrochemical Reactor by Using Residence Time Distribution Analysis and Hydraulic Indices
- Waste-to-energy: Coupling Waste Treatment to Highly Efficient CHP
- The Effect of Sn Content in a Pt/KIT-6 Catalyst Over its Performance in the Dehydrogenation of Propane
- Dehydrogenation of Propane to Propylene with Highly Stable Catalysts of Pt-Sn Supported Over Mesoporous Silica KIT-6
- Simultaneous Diesel and Oxygen Transfer Rate on the Production of an Oil-degrading Consortium in an Airlift Bioreactor: High-dispersed Phase Concentration
- Green Practices with Renewable Distributed Generation Technology in India
- Carcinogenic Hydrocarbon Pollution in Quintana Roo’s Sinkholes: Biotechnology for Remediation and Social Participation for Prevention
Articles in the same Issue
- Preface to the Special Issue Dedicated to the International Energy Conference, IEC 2017: Energy and its Development in the Twenty-First Century: A globalized vision
- Parametric Mathematical Modelling of Cristal Violet Dye Electrochemical Oxidation Using a Flow Electrochemical Reactor with BDD and DSA Anodes in Sulfate Media
- Photocatalytic Degradation of Caffeine in a Solar Reactor System
- Effect of Biomass Concentration on Oxygen Mass Transfer, Power Consumption, Interfacial Tension and Hydrodynamics in a Multiphase Partitioning Bioreactor
- Modeling and Hydraulic Characterization of a Filter-Press-Type Electrochemical Reactor by Using Residence Time Distribution Analysis and Hydraulic Indices
- Waste-to-energy: Coupling Waste Treatment to Highly Efficient CHP
- The Effect of Sn Content in a Pt/KIT-6 Catalyst Over its Performance in the Dehydrogenation of Propane
- Dehydrogenation of Propane to Propylene with Highly Stable Catalysts of Pt-Sn Supported Over Mesoporous Silica KIT-6
- Simultaneous Diesel and Oxygen Transfer Rate on the Production of an Oil-degrading Consortium in an Airlift Bioreactor: High-dispersed Phase Concentration
- Green Practices with Renewable Distributed Generation Technology in India
- Carcinogenic Hydrocarbon Pollution in Quintana Roo’s Sinkholes: Biotechnology for Remediation and Social Participation for Prevention

