The Effect of Sn Content in a Pt/KIT-6 Catalyst Over its Performance in the Dehydrogenation of Propane
-
Alejandro Mata-Martinez
Abstract
Propylene is one of the most important commodity chemicals. Its future demand is expected to exceed its production. Alternative routes to obtain this product need to be implemented. Dehydrogenation of propane assisted with catalyst is a promising route to meet demands. The Pt and Cr supported catalysts are amongst the most effective possibilities. However, Pt catalysts are preferred over Cr due to the toxic nature of Cr species. Despite the high performance of the Pt catalysts, they deactivate during reaction, mainly due to coke deposits blocking the active site and/or pores. This effect can be reduced with a support having high connectivity and surface area, like KIT-6. In this work the mesoporous silica KIT-6 was employed as support in a series of Pt-Sn catalysts. The influence of adding or increasing the weight % of Sn to Pt catalyst was studied. There were species of SnO2 and metallic Pt in the fresh catalysts. After reaction, it was found that in the catalysts with the lowest wt % of Sn (0.5), there were metallic Pt and a Pt-Sn alloy. In the rest of the used catalysts (containing 1.0, 1.5 and 2.0 wt % of Sn) the only detected specie was the Pt-Sn alloy. In the two most active catalysts (having 0.5 and 1.5 wt % of Sn), it was observed a difference of three times the quantity of coke deposited on the surface. The catalysts containing the highest coke deposits maintained its activity due to the high connectivity of the support.
Acknowledgements
Authors would like to thank to the Mexican National Council for Science and Technology (CONACyT) for the support of the project CB-2015-01 # 256268 and the Autonomous University of Sinaloa (UAS) with the project (PROFAPI-2015/251).
Appendix
The results of the nitrogen adsorption-desorption isotherms of KIT-6 can be seen in the Figure 6. The isotherm is type IV with a specific surface area, pore volume and pore diameter of 809 m2/g, 0.93 cm3/g and 45.8 Å, respectively. These values are in agreement with those reported in the literature for similar samples (Kleitz, Choi, and Ryoo 2003; Kleitz et al. 2010).
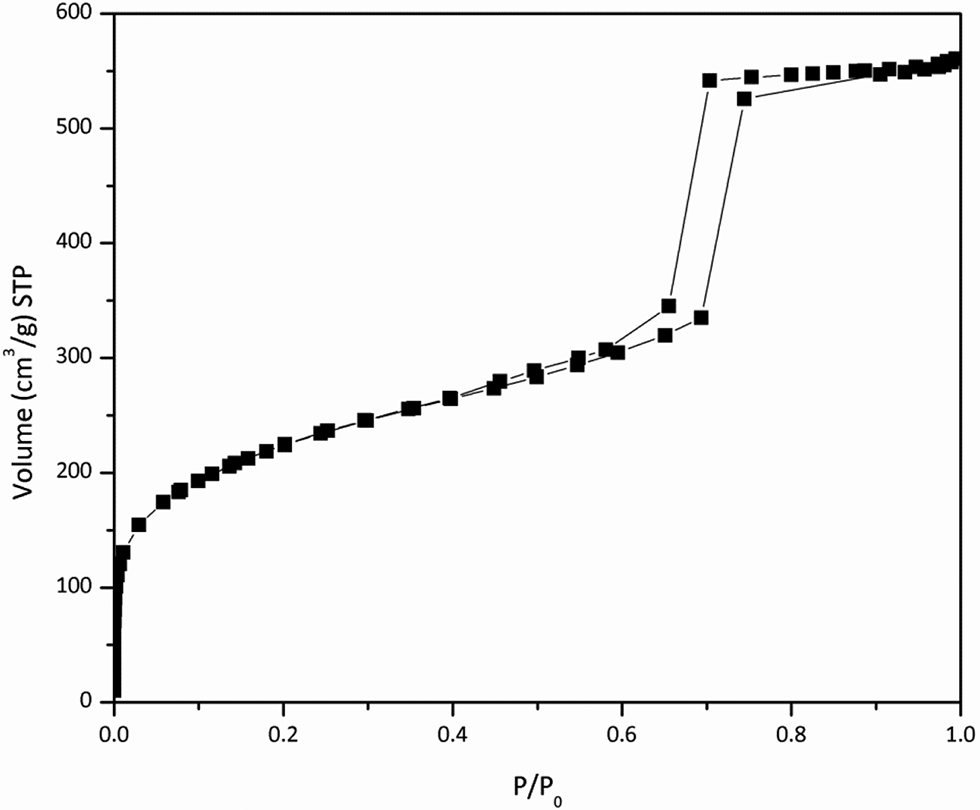
Nitrogen adsorption-desorption isotherms of KIT-6.
The XRD analyses for the KIT-6 support are given in Figure 7. The low-angle diffraction pattern shows evidence of two reflections at 2 Teta values of 0.94° and 1.72° corresponding to the interplanar spacing (211) and (420), respectively (Kleitz, Choi, and Ryoo 2003; Kleitz et al. 2010).
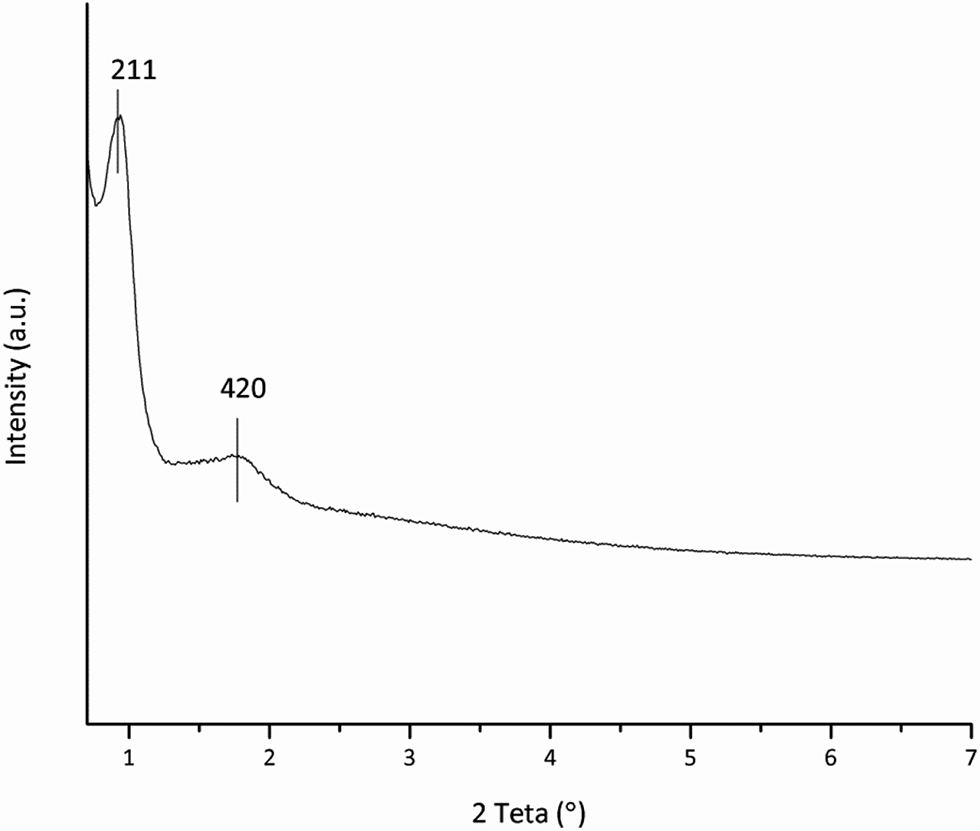
Low-angle XRD analyses of KIT-6.
References
Aparicio-Mauricio, G., R.S. Ruiz, F. López-Isunza, and C.O. Castillo-Araiza. 2017. “A Simple Approach to Describe Hydrodynamics and Its Effect on Heat and Mass Transport in an Industrial Wall-Cooled Fixed Bed Catalytic Reactor: ODH of Ethane on A MoVNbTeO Formulation.” Chemical Engineering Journal 321: 584–599.10.1016/j.cej.2017.03.043Search in Google Scholar
Argyle, M.D., and C.H. Bartholomew. 2015. “Heterogeneous Catalyst Deactivation and Regeneration: A Review.” Catalysts 5: 145–269.10.3390/catal5010145Search in Google Scholar
Bai, L., Y. Zhou, Y. Zhang, H. Liu, and X. Sheng. 2009. “Effect of Magnesium Addition to PtSnNa/ZSM-5 on the Catalytic Properties in the Dehydrogenation of Propane.” Industrial & Engineering Chemistry Research 48: 9885–9891.10.1021/ie900534mSearch in Google Scholar
Bariås, O.A., A. Holmen, and E.A. Blekkan. 1996. “Propane Dehydrogenation over Supported Pt and Pt-Sn Catalysts: Catalyst Preparation, Characterization, and Activity Measurements.” Journal of Catalysis 158: 1–12.10.1006/jcat.1996.0001Search in Google Scholar
Che-Galicia, G., R.S. Ruiz-Martínez, D. Rios-Morales, J.A. Ayala-Romero, and C.O. Castillo-Araiza. 2018. “Kinetic and Reactor Performance of a Ni-Based Catalyst during the Production of Ethene.” Chemical Engineering Communications 205: 1–15.10.1080/00986445.2017.1396538Search in Google Scholar
Chin, S., A. Hisyam, and H. Prasetiawan. 2015. “Modeling and Simulation Study of an Industrial Radial Moving Bed Reactor for Propane Dehydrogenation Process.” International Journal of Chemical Reactor Engineering 14: 33–44.10.1515/ijcre-2014-0148Search in Google Scholar
Corma, A., F.V. Melo, L. Sauvanaud, and F. Ortega. 2005. “Light Cracked Naphtha Processing: Controlling Chemistry for Maximum Propylene Production.” Catalysis today 107: 699–706.10.1016/j.cattod.2005.07.109Search in Google Scholar
Cortright, R.D., and J.A. Dumesic. 1995. “Effects of Potassium on Silica-Supported Pt and Pt/Sn Catalysts for Isobutane Dehydrogenation.” Journal of Catalysis 157: 576–583.10.1006/jcat.1995.1322Search in Google Scholar
Guo, Y.H., C. Xia, and B.S. Liu. 2014. “Catalytic Properties and Stability of Cubic Mesoporous LaxNiyOz/KIT-6 Catalysts for CO2 Reforming of CH4.” Chemical Engineering Journal 237: 421–429.10.1016/j.cej.2013.09.108Search in Google Scholar
Han, Z., S. Li, F. Jiang, T. Wang, X. Ma, and J. Gong. 2014. “Propane Dehydrogenation over Pt-Cu Bimetallic Catalysts: The Nature of Coke Deposition and the Role of Copper.” Nanoscale 6: 10000–10008.10.1039/C4NR02143FSearch in Google Scholar
Kleitz, F., F. Bérubé, R. Guillet-Nicolas, C.M. Yang, and M. Thommes. 2010. “Probing Adsorption, Pore Condensation, and Hysteresis Behaviour of Pure Fluids in Three-Dimensional Cubic Mesoporous KIT-6 Silica.” The Journal of Physical Chemistry C 114: 9344–9355.10.1021/jp909836vSearch in Google Scholar
Kleitz, F., S.H. Choi, and R. Ryoo. 2003. “Cubic Ia3d Large Mesoporous Silica: Synthesis and Replication to Platinum Nanowires, Carbon Nanorods and Carbon Nanotubes.” Chemical Communications 17: 2136–2137.10.1039/b306504aSearch in Google Scholar PubMed
Korzyński, M.D., and M. Dincӑ. 2017. “Oxidative Dehydrogenation of Propane in the Realm of Metal-Organic Frameworks.” ACS Central Science 3: 10–12.10.1021/acscentsci.7b00013Search in Google Scholar PubMed PubMed Central
Lei, Y., B. Liu, J. Lu, R.J. Lobo-Lapidus, T. Wu, H. Feng, and J.T. Miller. 2012. “Synthesis of Pt–Pd Core–Shell Nanostructures by Atomic Layer Deposition: Application in Propane Oxidative Dehydrogenation to Propylene.” Chemistry of Materials 24: 3525–3533.10.1021/cm300080wSearch in Google Scholar
Liu, M., W. Tang, Z. Xie, H. Yu, H. Yin, Y. Xu, and S. Zhou. 2017. “Design of Highly Efficient Pt-SnO2 Hydrogenation Nanocatalysts Using Pt@ Sn Core–Shell Nanoparticles.” ACS Catalysis 7: 1583–1591.10.1021/acscatal.6b03109Search in Google Scholar
Nawaz, Z. 2015. “Dynamic Modeling of CATOFIN® Fixed-Bed Iso-Butane Dehydrogenation Reactor for Operational Optimization.” International Journal of Chemical Reactor Engineering 14: 491–515.10.1515/ijcre-2015-0087Search in Google Scholar
Nawaz, Z., X. Tang, Y. Wang, and F. Wei. 2009. “Parametric Characterization and Influence of Tin on the Performance of Pt− Sn/SAPO-34 Catalyst for Selective Propane Dehydrogenation to Propylene.” Industrial & Engineering Chemistry Research 49: 1274–1280.10.1021/ie901465sSearch in Google Scholar
Parlett, C.M.A., A. Aydin, L.J. Durndell, L. Frattini, M.A. Isaacs, A. F. Lee, and C. Wu. 2017. “Tailored Mesoporous Silica Support for Ni Catalyzed Hydrogen Production from Ethanol Steam Reforming.” Catalysis Communications 91: 76–79.10.1016/j.catcom.2016.12.021Search in Google Scholar
Roth, C., N. Martz, and H. Fuess. 2001. “Characterization of Different Pt–Ru Catalysts by X-Ray Diffraction and Transmission Electron Microscopy.” Physical Chemistry Chemical Physics 3: 315–319.10.1039/b004887iSearch in Google Scholar
Ruelas-Leyva, J.P., and G.A. Fuentes. 2017. “Chiral Catalyst Deactivation during the Asymmetric Hydrogenation of Acetophenone.” Catalysts 7: 193–203.10.3390/catal7070193Search in Google Scholar
Searles, K., G. Siddiqi, O.V. Safanova, and C. Copéret. 2017. “Silica-Supported Isolated Galium Sites as Highly Active, Selective and Stable Propane Dehydrogenation Catalysts.” Chemical Science 8: 2661–2666.10.1039/C6SC05178BSearch in Google Scholar
Shao, C.T., W.Z. Lang, X. Yan, and Y.J. Guo. 2017. “Catalytic Performance of Gallium Oxide Based-Catalysts for the Propane Dehydrogenation Reaction: Effects of Support and Loading Amount.” RSC Advances 7: 4710–4723.10.1039/C6RA27204ESearch in Google Scholar
Sharma, L.D., M. Kumar, A.K. Saxena, M. Chand, and J.K. Gupta. 2002. “Influence of Pore Size Distribution on Pt Dispersion in Pt-Sn/Al2O3 Reforming Catalyst.” Journal of Molecular Catalysis A: Chemical 185: 135–141.10.1016/S1381-1169(01)00438-1Search in Google Scholar
Soni, K., K. C. Mouli, and Dalai. 2010. “Influence of Frame Connectivity of SBA-15 and KIT-6 Supported NiMo Catalyst for Hydrotreating of Gas Oil.” Catalysis Letters 136: 116–125.10.1007/s10562-010-0317-0Search in Google Scholar
Sun, P., G. Siddiqi, W.C. Vinning, M. Chi, and A.T. Bell. 2011. “Novel Pt/Mg(in)(Al)O Catalyst for Ethane and Propane Dehydrogenation.” Journal of Catalysis 282: 165–174.10.1016/j.jcat.2011.06.008Search in Google Scholar
Vu, B.K., M.B. Song, I.Y. Ahn, Y.W. Suh, D.J. Suh, W.I. Kim, and E.W. Shin. 2011a. “Pt–Sn Alloy Phases and Coke Mobility over Pt–Sn/Al2O3 and Pt–Sn/ZnAl2O4 Catalysts for Propane Dehydrogenation.” Applied Catalysis A: General 400: 25–33.10.1016/j.apcata.2011.03.057Search in Google Scholar
Vu, B.K., M.B. Song, I.Y. Ahn, Y.W. Suh, D.J. Suh, W.I. Kim, and E.W. Shin. 2011b. “Propane Dehydrogenation over Pt–Sn/Rare-earth-doped Al2O3: Influence of La, Ce, or Y on the Formation and Stability of Pt–Sn Alloys.” Catalysis Today 164: 214–220.10.1016/j.cattod.2010.10.007Search in Google Scholar
Wang, C., Y. Zhou, M. Ge, X. Xu, Z. Zhang, and J.Z. Jiang. 2010. “Large-Scale Synthesis of SnO2 Nanosheets with High Lithium Storage Capacity.” Journal of the American Chemical Society 132: 46–47.10.1021/ja909321dSearch in Google Scholar PubMed
Won, W., K.S. Lee, S. Lee, and C. Jung. 2010. “Repetitive Control and Online Optimization Propane Process.” Computers & Chemical Engineering 34: 508–517.10.1016/j.compchemeng.2009.12.011Search in Google Scholar
Yang, M., Y. Zhu, X. Zhou, Z. Sui, and D. Chen. 2012. “First-Principles Calculations of Propane Dehydrogenation over PtSn Catalysts.” ACS Catalysis 2: 1247–1258.10.1021/cs300031dSearch in Google Scholar
Yu, C., Q. Ge, H. Xu, and W. Li. 2006. “Effects of Ce Addition on the Pt-Sn/γ-Al2O3 Catalyst for Propane Dehydrogenation to Propylene.” Applied Catalysis A: General 315: 58–67.10.1016/j.apcata.2006.08.038Search in Google Scholar
Zhang, Y., Y. Zhou, H. Liu, Y. Wang, Y. Xu, and P. Wu. 2007. “Effect of La Addition on Catalytic Performance of PtSnNa/ZSM-5 Catalyst for Propane Dehydrogenation.” Applied Catalysis A: General 333: 202–210.10.1016/j.apcata.2007.07.049Search in Google Scholar
© 2018 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Preface to the Special Issue Dedicated to the International Energy Conference, IEC 2017: Energy and its Development in the Twenty-First Century: A globalized vision
- Parametric Mathematical Modelling of Cristal Violet Dye Electrochemical Oxidation Using a Flow Electrochemical Reactor with BDD and DSA Anodes in Sulfate Media
- Photocatalytic Degradation of Caffeine in a Solar Reactor System
- Effect of Biomass Concentration on Oxygen Mass Transfer, Power Consumption, Interfacial Tension and Hydrodynamics in a Multiphase Partitioning Bioreactor
- Modeling and Hydraulic Characterization of a Filter-Press-Type Electrochemical Reactor by Using Residence Time Distribution Analysis and Hydraulic Indices
- Waste-to-energy: Coupling Waste Treatment to Highly Efficient CHP
- The Effect of Sn Content in a Pt/KIT-6 Catalyst Over its Performance in the Dehydrogenation of Propane
- Dehydrogenation of Propane to Propylene with Highly Stable Catalysts of Pt-Sn Supported Over Mesoporous Silica KIT-6
- Simultaneous Diesel and Oxygen Transfer Rate on the Production of an Oil-degrading Consortium in an Airlift Bioreactor: High-dispersed Phase Concentration
- Green Practices with Renewable Distributed Generation Technology in India
- Carcinogenic Hydrocarbon Pollution in Quintana Roo’s Sinkholes: Biotechnology for Remediation and Social Participation for Prevention
Articles in the same Issue
- Preface to the Special Issue Dedicated to the International Energy Conference, IEC 2017: Energy and its Development in the Twenty-First Century: A globalized vision
- Parametric Mathematical Modelling of Cristal Violet Dye Electrochemical Oxidation Using a Flow Electrochemical Reactor with BDD and DSA Anodes in Sulfate Media
- Photocatalytic Degradation of Caffeine in a Solar Reactor System
- Effect of Biomass Concentration on Oxygen Mass Transfer, Power Consumption, Interfacial Tension and Hydrodynamics in a Multiphase Partitioning Bioreactor
- Modeling and Hydraulic Characterization of a Filter-Press-Type Electrochemical Reactor by Using Residence Time Distribution Analysis and Hydraulic Indices
- Waste-to-energy: Coupling Waste Treatment to Highly Efficient CHP
- The Effect of Sn Content in a Pt/KIT-6 Catalyst Over its Performance in the Dehydrogenation of Propane
- Dehydrogenation of Propane to Propylene with Highly Stable Catalysts of Pt-Sn Supported Over Mesoporous Silica KIT-6
- Simultaneous Diesel and Oxygen Transfer Rate on the Production of an Oil-degrading Consortium in an Airlift Bioreactor: High-dispersed Phase Concentration
- Green Practices with Renewable Distributed Generation Technology in India
- Carcinogenic Hydrocarbon Pollution in Quintana Roo’s Sinkholes: Biotechnology for Remediation and Social Participation for Prevention

