Abstract
The final set of equations defining the interdiffusion process in solid state is presented. The model is supplemented by vacancy evolution equation. The competition between the Kirkendall shift, backstress effect and vacancy migration is considered. The proper diffusion flux based on the Nernst–Planck formula is proposed. As a result, the comparison of the experimental and calculated evolution of the void formation in the Fe-Pd diffusion couple is shown.
Introduction
The difference in diffusion coefficient of components causes several effects during mass transport process. Most often Kirkendall and Frenkel effects occur [1, 2]. Kirkendall effect (lattice drift) caused by the vacancy flux divergence leads to dislocation climb and subsequently to the construction of extra planes in accumulation region [3–5]. The drift velocity is then generated. This constraint means zero divergence of overall volume flux density:
When the only driving force is chemical potential gradient,
In this paper the generalized description of the interdiffusion in solid state will be shown. The final set of equations: mass and volume continuity; flux relation and vacancy evolution equation will be formulated. Moreover, the kinetic effects related to the difference of mobilities, namely the stress generation and relaxation [3, 6], vacancy migration and void formation will be discussed. Due to volume constraint, interdiffusion leads to the accumulation of matter, and thus this accumulation must be reduced to zero. This reduction will be realized by the introduction of (1) Kirkendall effect; (2) backstress effect and (3) non-equilibrium vacancy distribution (Frenkel effect). Void evolution during interdiffusion process in Fe-Pd system will be presented.
Mathematical formulation
Consider a multicomponent mixture, where
where
where i = 1,…, r and r denotes the number of components.
During an arbitrary transport process, when volume is affected by the distribution of every component mixture, the volume continuity equation [7] follows:
The final form of eq. (3) can be rewritten in the form that allows to determine the drift velocity:
In one-dimensional space the drift velocity can be expressed by analytical function (after integration):
However, in two or free dimension space eq. (4) should be solved by numerical method, e.g. by replacing the drift velocity by its potential, i.e.
The component flux,
The Kirkendall effect means the movement of lattice from slower diffusant side towards the faster diffusant side with some drift velocity,
The diffusion flux is defined by the Nernst–Planck flux equation [8, 9], which in general form reads:
where
Backstress effect – the diffusion potential is affected by the internal stress effect – stress gradient appearing due to attempt of matter accumulation. Each diffusing atom of both species is affected by common stress force:
Non-equilibrium vacancy distribution – the diffusion potential is a difference of component chemical and common vacancy potentials,
Finally, the diffusion potential is a sum of component chemical, common vacancy and stress potentials,
The internal pressure,
The last equation defining the model is the vacancy exchange equation defined as
where
To calculate void formation, in one-dimensional system, during the interdiffusion process one additional equation should be introduced, mainly, the void radii evolution [10, 11]:
where
Results
In this section the experimental and calculated results of interdiffusion and void formation will be shown. The calculations will be analysed in two-dimensional space. The simulation results will be verified with diffusion couple experiments in Fe-Pd binary system (Figure 1). The void radii will be estimated and the results for different calculation times will be presented. The following set of equations will be solved:
where
The subset
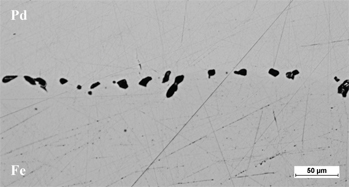
The experimental results of Frenkel effect (voids formation) during diffusion between pure Fe and Pd after 150 h at 1,273 K.
In Fe-Pb system the voids growth on the iron reach side of the diffusion couple. We assume that in Fe-Pd system the mean migration length for vacancy is

The simulation results of the Fe concentration (a)–(b) and voids formation (vacancies evolution) (c)–(d) in Fe-Pd system after annealing at 1,273 K for 100 h.

The simulation results of the Fe concentration (a)–(b) and voids formation (vacancies evolution) (c)–(d) in Fe-Pd system after annealing at 1,273 K for 200 h.
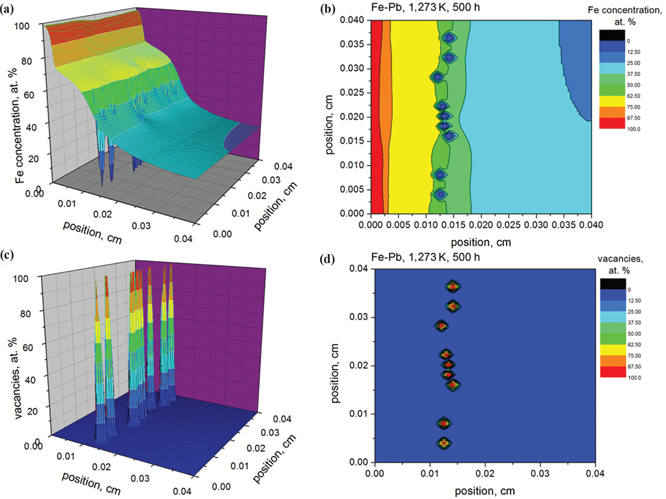
The simulation results of the Fe concentration (a)–(b) and voids formation (vacancies evolution) (c)–(d) in Fe-Pd system after annealing at 1,273 K for 500 h.
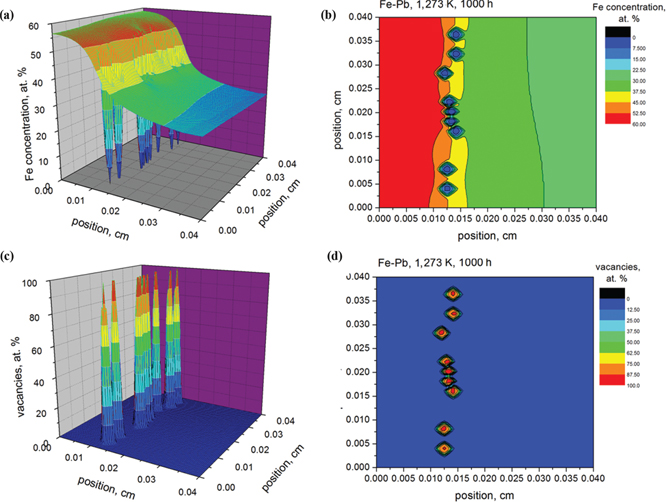
The simulation results of the Fe concentration (a)–(b) and voids formation (vacancies evolution) (c)–(d) in Fe-Pd system after annealing at 1,273 K for 1,000 h.
The estimated voids radii for different times are shown in Figure 6. It is clearly shown that the dependence is a parabolic one.

The estimated void radii for different simulation time.
Conclusions
The presented method allowed the calculation of the interdiffusion process when Kirkendall, Frenkel and backstress effects are introduced. It was shown that the vacancy evolution in all diffusion calculations to approximate the real process should be introduced. The vacancies agglomerate in places where initially void was introduced as in real diffusion experiments. The results of the model were verified with experimental results for Fe-Pb binary system. Moreover, it was presented that the average radii of the void change parabolically with time. In the future the dynamics of the voids should be introduced, mainly simulation should take into account that the voids are moving during the diffusion process.
Funding statement: Funding: This work has been supported by the National Science Centre (NCN) in Poland, decision number 2013/09/B/ST8/00150.
References
[1] Z. Grzesik and S. Mrowec, High Temp. Mater. Processes, 31 (2012) 539–551.10.1515/htmp-2012-0091Search in Google Scholar
[2] Z. Grzesik and M. Migdalska, High Temp. Mater. Processes, 30 (2011) 277–287.10.1515/htmp.2011.046Search in Google Scholar
[3] L. S. Darken, Trans. AIME., 174 (1948) 184–201.Search in Google Scholar
[4] A. D. Smigelskas and E. O. Kirkendall, Trans. AIME., 171 (1947) 130–142.Search in Google Scholar
[5] J. Bardeen and C. Herring, Diffusion in Alloys and the Kirkendall Effect in Imperfections in Nearly Perfect Crystals edited by W., Shockley, Wiley, New York (1952) 261–288.Search in Google Scholar
[6] R. W. Balluffi, S. M. Allen, W. C. Carter and R. A. Kemper, Kinetics of Materials, Wiley, New York (2005).10.1002/0471749311Search in Google Scholar
[7] B. Wierzba and M. Danielewski, Phys A., 390 (2011) 2325–2332.10.1016/j.physa.2011.02.047Search in Google Scholar
[8] W. Nernst, Z Phys Chem., 4 (1889) 129–181.10.1515/zpch-1889-0412Search in Google Scholar
[9] M. Planck, Ann Rev Phys Chem., 40 (1890) 561–576.10.1002/andp.18902760802Search in Google Scholar
[10] B. Wierzba, Phys A., 403 (2014) 29–34.10.1016/j.physa.2014.02.014Search in Google Scholar
[11] A. M. Gusak and N. V. Storozhuk, Phys. Metals Metall., 114 (2013) 197–206.10.1134/S0031918X13030071Search in Google Scholar
©2016 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Research Articles
- Effect of Activating Agent on the Preparation of Bamboo-Based High Surface Area Activated Carbon by Microwave Heating
- Research on the Microstructure and Mechanical Property of Ti-7Cu Alloy after Semi-Solid Forging
- Synthesis and Characterization of BiFeO3 Ceramic by Simple and Novel Methods
- Synthesis and Characterization of Lead Sulfide Nanostructures with Different Morphologies via Simple Hydrothermal Method
- Comparison of Brazed Residual Stress and Thermal Deformation between X-Type and Pyramidal Lattice Truss Sandwich Structure: Neutron Diffraction Measurement and Simulation Study
- Influence of V–N Microalloying on the High-Temperature Mechanical Behavior and Net Crack Defect of High Strength Weathering Steel
- Numerical Simulation on Optimization of Center Segregation for 50CrMo Structural Alloy Steel
- Direct Extraction of Ti and Ti Alloy from Ti-Bearing Dust Slag in Molten CaCl2
- Constitutive Equation for 3104 Alloy at High Temperatures in Consideration of Strain
- Microstructural Properties and Four-Point Bend Fatigue Behavior of Ti-6.5Al-2Zr-1Mo-1V Welded Joints by Electron Beam Welding
- Oxidizing Roasting Performances of Coke Fines Bearing Brazilian Specularite Pellets
- Purification of Niobium by Electron Beam Melting
- Void Formation during Diffusion – Two-Dimensional Approach
Articles in the same Issue
- Frontmatter
- Research Articles
- Effect of Activating Agent on the Preparation of Bamboo-Based High Surface Area Activated Carbon by Microwave Heating
- Research on the Microstructure and Mechanical Property of Ti-7Cu Alloy after Semi-Solid Forging
- Synthesis and Characterization of BiFeO3 Ceramic by Simple and Novel Methods
- Synthesis and Characterization of Lead Sulfide Nanostructures with Different Morphologies via Simple Hydrothermal Method
- Comparison of Brazed Residual Stress and Thermal Deformation between X-Type and Pyramidal Lattice Truss Sandwich Structure: Neutron Diffraction Measurement and Simulation Study
- Influence of V–N Microalloying on the High-Temperature Mechanical Behavior and Net Crack Defect of High Strength Weathering Steel
- Numerical Simulation on Optimization of Center Segregation for 50CrMo Structural Alloy Steel
- Direct Extraction of Ti and Ti Alloy from Ti-Bearing Dust Slag in Molten CaCl2
- Constitutive Equation for 3104 Alloy at High Temperatures in Consideration of Strain
- Microstructural Properties and Four-Point Bend Fatigue Behavior of Ti-6.5Al-2Zr-1Mo-1V Welded Joints by Electron Beam Welding
- Oxidizing Roasting Performances of Coke Fines Bearing Brazilian Specularite Pellets
- Purification of Niobium by Electron Beam Melting
- Void Formation during Diffusion – Two-Dimensional Approach

