Abstract
Mitochondria are essential for cellular metabolism, serving as the primary source of adenosine triphosphate (ATP). This energy is generated by the oxidative phosphorylation (OXPHOS) system located in the inner mitochondrial membrane. Impairments in this machinery are linked to serious human diseases, especially in tissues with high energy demands. Assembly of the OXPHOS system requires the coordinated expression of genes encoded by both the nuclear and mitochondrial genomes. The mitochondrial DNA encodes for 13 protein components, which are synthesized by mitochondrial ribosomes and inserted into the inner membrane during translation. Despite progress, key aspects of how mitochondrial gene expression is regulated remain elusive, largely due to the organelle’s limited genetic accessibility. However, emerging technologies now offer new tools to manipulate various stages of this process. In this review, we explore recent strategies that expand our ability to target mitochondria genetically.
1 Introduction
Mitochondria are essential cellular organelles that fulfill key functions in cellular metabolism. Among these functions, the production of ATP by oxidative phosphorylation is central to driving cellular energy demands. The OXPHOS system in the inner mitochondrial membrane (IMM) is composed of subunits that are either encoded by the nuclear or mitochondrial genome (Isaac et al. 2018; Kummer and Ban 2021; Ott et al. 2015; Priesnitz and Becker 2018; Richter-Dennerlein et al. 2015; Tang et al. 2020). The human mitochondrial genome is a 16,569 bp double-stranded circular DNA molecule that encodes 13 OXPHOS system subunits, 22 transfer RNAs (tRNAs), and two ribosomal RNAs (rRNAs) (Figure 1). The double-stranded genome consists of a guanine-rich heavy (H) strand and a cytosine-rich light (L) strand (Anderson et al. 1981; Falkenberg et al. 2024).
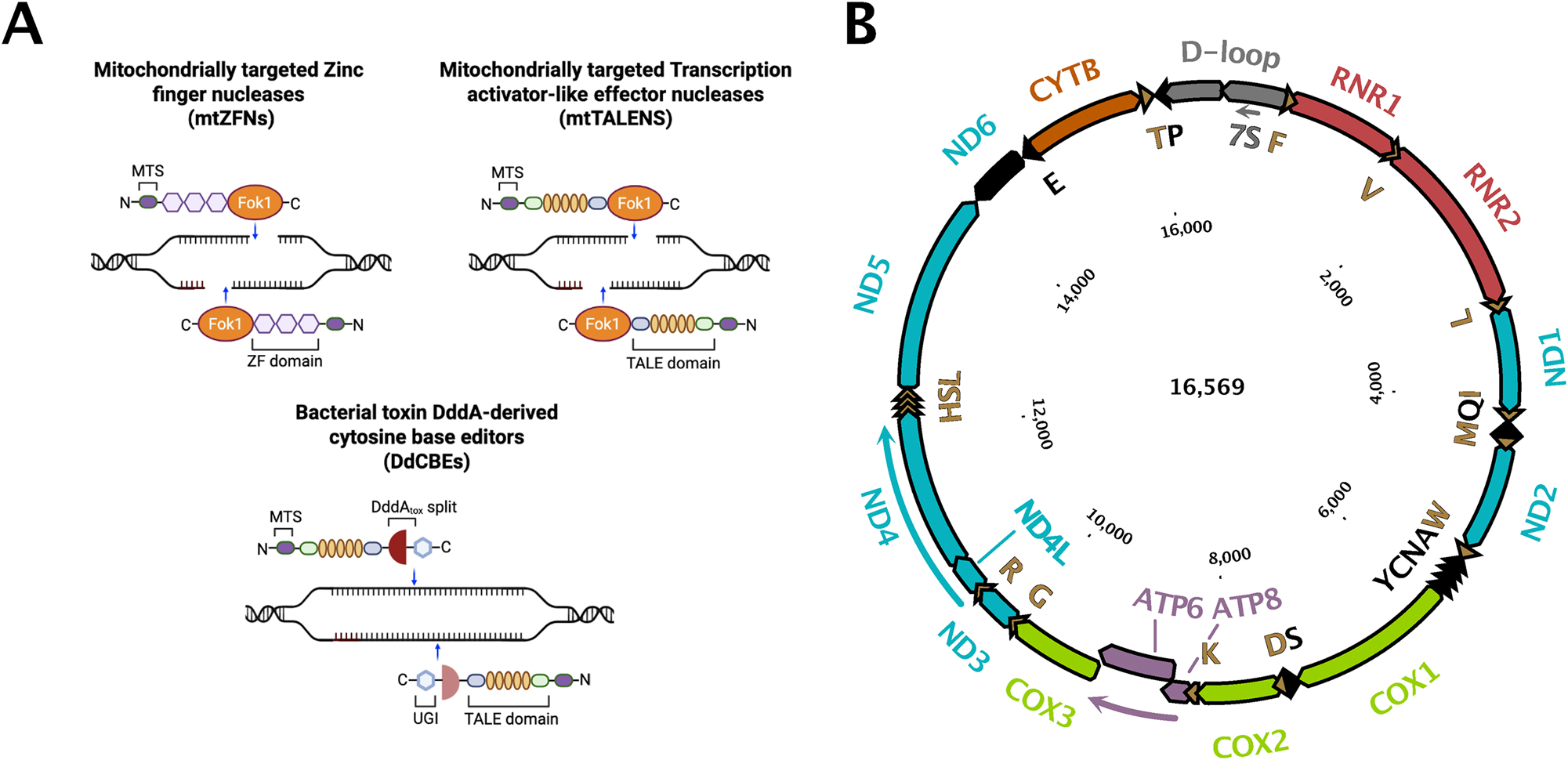
Overview of various tools to manipulate mitochondrial DNA and graphical illustration of mitochondrial genome (A). Mitochondrially targeted nucleases, including mitochondrially targeted zinc finger nucleases (mtZFNs), transcription activator-like effector nucleases (mtTALENs), and DddA-derived cytosine base editors (DdCBEs), can be directed into mitochondria via a mitochondrial targeting sequence (MTS). mtZFNs consist of DNA sequence-specific zinc finger (ZF) domains fused to the FokI endonuclease, enabling targeted double-strand breaks (DSBs) in mtDNA. Similarly, mtTALENs are composed of sequence-specific TALE DNA-binding domains conjugated to FokI, also generating site-specific DSBs. In contrast, DdCBEs employ split DddA cytidine deaminase fused to TALE domains, conferring sequence-specific cytosine deaminase activity that enables precise C- G-to-T- A base editing without introducing DSBs. Importantly, DdCBEs also consist of an uracil glycosylase inhibitor (UGI), which stabilizes the edited base by inhibiting the base excision repair pathway, thereby preventing the removal of uracil and ensuring efficient and permanent C-to-T conversion (B) map of human mitochondrial DNA (mtDNA). Protein-coding genes encoded on the heavy strand (H) are depicted with distinct colors and a black border, while those on the light strand (L) are shown in black. Transfer RNA (tRNA) genes are represented as triangles. Subunits of respiratory complex I are labeled in blue, complex III subunits in orange, and complex IV subunits in green. Ribosomal RNA (rRNA) genes are highlighted in red, and the D-loop region is indicated in grey.
Mitochondria contain multiple copies of their genome (mtDNA), the number of which differs significantly between different cell types (Stewart and Chinnery 2021). Mutations of the mitochondrial DNA leads to dysfunction of the OXPHOS system and concomitantly severe human disorders (Smeitink et al. 2001). Mitochondrial DNA mutation syndromes encompass a broad range of disorders, each linked to specific genetic variants. For example, MELAS (Mitochondrial Encephalopathy, Lactic Acidosis, and Stroke-like Episodes) is predominantly caused by the m.3243A > G mutation in the mitochondrial tRNALeu gene (MT-TL1), affecting translation and energy production; MERRF (Myoclonic Epilepsy with Ragged Red Fibers) commonly arises from m.8344A > G in the tRNALys gene (MT-TK), impairing mitochondrial protein synthesis; and LHON (Leber Hereditary Optic Neuropathy) is primarily associated with three mutations-m.3460G > A (MT-ND1), m.11778G > A (MT-ND4), and m.14484T > C (MT-ND6)-which affect complex I of the OXPHOS system and cause optic nerve atrophy (Fu et al. 2021; Gusic and Prokisch 2021). Underlying these disorders and impacting their phenotype and progression is the heteroplasmy of mitochondrial DNA mutations (mtDNA that is present in multiple copies displays a mixture of mutant and wild-type molecules within a cell). The clinical manifestations of mitochondrial diseases depend on the mutant-to-wild-type mtDNA ratio, with symptoms emerging when the mutant mtDNA exceeds tissue-specific thresholds. Tissues with high energy demands, such as the brain, heart, and skeletal muscle, are particularly vulnerable due to their reliance on oxidative phosphorylation for energy production (Stewart and Chinnery 2015). Moreover, heteroplasmy levels can vary between cells, organs, and individuals, and contribute to the variable disease severity and tissue involvement observed in mitochondrial disorders (Stewart and Chinnery 2021).
Mitochondrial gene expression is a highly orchestrated process involving DNA replication, transcription, RNA processing, and translation. mtDNA is tightly packed into nucleoids by the mitochondrial transcription factor A (TFAM), thereby regulating DNA availability for replication and transcription (Kaufman et al. 2007; Kukat et al. 2015; Ngo et al. 2014). DNA replication is executed by DNA polymerase γ (POLγ), which binds to the heavy-strand origin (OH) and extends RNA primers synthesized by mitochondrial RNA polymerase (POLRMT) (Falkenberg et al. 2024; Montoya et al. 1982; Robberson et al. 1972). As POLγ synthesizes the new heavy strand, the TWINKLE helicase unwinds the double-stranded DNA ahead of the replication fork, enabling strand separation (Ikeda et al. 2015; Korhonen et al. 2003; Korhonen et al. 2008; Milenkovic et al. 2013; Tyynismaa et al. 2004). The displaced parental heavy strand is stabilized by the mitochondrial single-stranded DNA-binding protein (mtSSB), which prevents secondary structure formation and unscheduled primer synthesis (Curth et al. 1994; Kuznetsov et al. 2006; Montoya et al. 1982; Tiranti et al. 1993). When the replication fork reaches the light-strand origin (OL), the displaced heavy strand forms a stem-loop structure that excludes mtSSB, allowing POLRMT to synthesize a new RNA primer. POLγ then initiates light-strand synthesis using this primer (Fusté et al. 2010; Kühl et al. 2016; Wanrooij et al. 2008). Apart from synthesizing primers for DNA replication, POLRMT recruited by TFAM also performs transcription of mtDNA into two polycistronic primary RNAs from the heavy (HSP) and light strand promoters (LSP) (Fisher and Clayton 1985; Hillen et al. 2017; Kühl et al. 2016; Minczuk et al. 2011; Morozov et al. 2015; Ngo et al. 2014). The heavy strand encodes 12 OXPHOS proteins (subunits of complexes I, III, IV, V), two rRNAs, and 14 tRNAs, while the light strand codes for MT-ND6 and eight tRNAs (Falkenberg et al. 2024; Mercer et al. 2011). These primary transcripts are processed into individual mRNAs, tRNAs, and rRNAs by endonucleases such as RNase P and ELAC2 (Bhatta et al. 2021; Brzezniak et al. 2011; Holzmann et al. 2008; Sanchez et al. 2011). FASTKD5 performs MT-CO1, MT-CO3 and MT-CYB mRNA processing at the non-canonical junctions which lack tRNA punctuation (Antonicka et al. 2025). Mature mRNAs are stabilized by LRPPRC/SLIRP complexes (Chujo et al. 2012; Lagouge et al. 2015; Mootha et al. 2003; Ruzzenente et al. 2012; Sasarman et al. 2010; Singh et al. 2024). Translation of mature mRNAs occurs on membrane-tethered mitoribosomes, which directly dock to the inner membrane, enabling the co-translational insertion of nascent OXPHOS subunits into the lipid bilayer via the OXA1L insertase (Homberg et al. 2023; Itoh et al. 2021; Jia et al. 2003; Ott et al. 2015; Szyrach et al. 2003). Despite the importance of mitochondrial gene expression for cellular energy metabolism and physiology, there still are gaps in our knowledge of the mechanisms and their coordination. A lack of genetic access to mitochondria has hampered experimental access to address these questions. However, in recent years, new technologies have been established that enable us to manipulate each level of mitochondrial gene expression to elucidate mechanistic details of mitochondrial gene expression. In this review, we aim to detail tools to manipulate both mtDNA and mtRNA, which not only provides technologies for studying mitochondrial gene expression but might also pave the way to potential therapeutic options.
2 Targeting mitochondrial DNA
2.1 Biolistic transformation of mitochondria
Biolistic transformation, also known as particle bombardment, is one of the most established and effective methods for introducing genetic modifications into the mitochondrial DNA (mtDNA) of Saccharomyces cerevisiae (Bonnefoy and Fox 2001; Fox et al. 1988; Johnston et al. 1988). In this approach, DNA-coated tungsten or gold microprojectiles are accelerated into yeast cells using a gene gun, physically breaching cellular and mitochondrial membranes to deliver exogenous DNA directly into the organelle (Bonnefoy and Fox 2007) (Figure 2). This method circumvents the natural barriers that typically prevent nucleic acid uptake into mitochondria, making it a valuable tool for mitochondrial genome engineering.
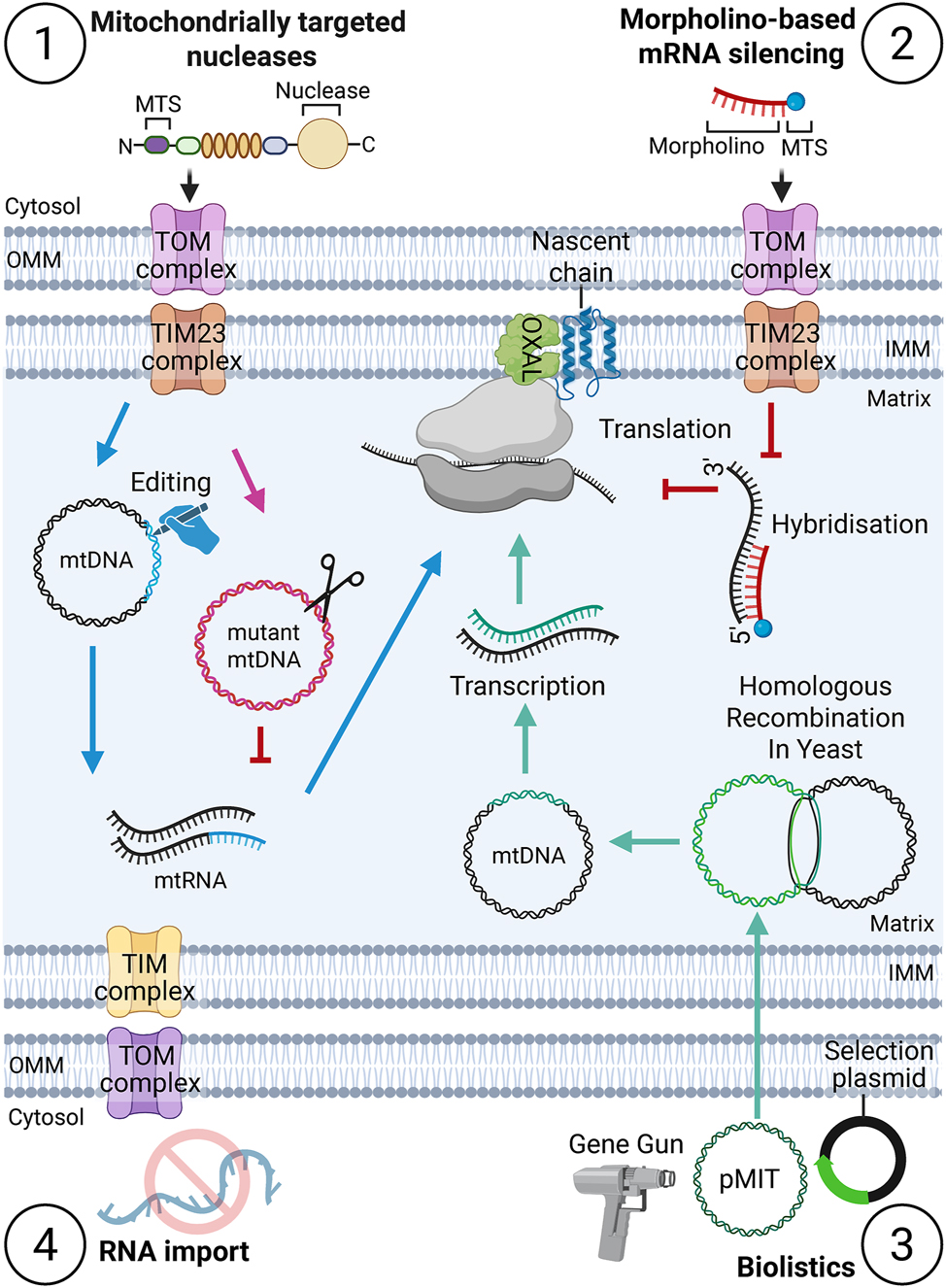
Tools for manipulating mitochondrial gene expression: (1) mitochondria-targeted nucleases are directed to the mitochondrial matrix, enabling targeted degradation of mtDNA or nucleotide base editing. This approach prevents the expression of mutant mitochondrial genes or facilitates the expression of edited gene sequences. (2) morpholino-based mRNA silencing tools utilize mRNA sequence-specific morpholinos targeted to the mitochondrial matrix; these inhibit mitochondrial translation by blocking ribosome-mRNA interactions through hybridization with mRNA. (3) in yeast, the pMIT (mitochondrially targeted plasmid) system allows integration of an exogenous gene of interest alongside a selection plasmid, exploiting homologous recombination within yeast mitochondria for stable genetic manipulation. (4) no efficient tool for enabling RNA import into mitochondria is currently available.
Several variations of the approach have been reported in the literature. Since the transformation efficiency is low, the approach usually utilizes a selection strategy to identify those cells in which mitochondria have received the exogenous DNA molecule. The original work on this method used a yeast strain that was defective in the mitochondrial COX1 gene. DNA transfer into mutant mitochondria rescued the respiratory defect enabling selection on non-fermentable medium (Johnston et al. 1988). In standard protocols, yeast strains that lack functional mtDNA are transformed with plasmids containing the desired mitochondrial. Further, mating with a rho+ strain is carried out to introduce desired gene into the target mtDNA via homologous recombination. This approach was utilized for the first time to transfer a plasmid borne COX2 (Bonnefoy and Fox 2007). In addition to mitochondria-specific genes, plasmids that harbor reporter genes recoded to the mitochondrial genetic code have been introduced by the bombardment technology and can be used to select for transformed mitochondria (Cohen and Fox 2001; Franco and Barros 2023; Hall et al. 2012; Mireau et al. 2003; Suhm et al. 2018).
Despite its utility, biolistic transformation presents several limitations. The potential for off-target integration of exogenous DNA into the nuclear genome cannot be entirely excluded, raising concerns about unintended genetic alterations. Additionally, the efficiency of transformation and, if desired, recombination between the introduced and endogenous genomes may be insufficient to surpass the heteroplasmy threshold required for phenotypic manifestation of the edited mtDNA, necessitating screening and selection procedures. Moreover, the reliance on homologous recombination restricts the applicability of this method to organisms like yeast, whose mitochondrial genomes are amenable to recombination. In contrast, higher eukaryotes generally lack efficient mitochondrial homologous recombination mechanisms, limiting the broader utility of biolistic transformation for mitochondrial genome editing in complex organisms.
2.2 Mitochondrially targeted nucleases
The development of programmable nucleases, particularly CRISPR-Cas9, has revolutionized genetic engineering by enabling highly specific and efficient editing of nuclear genomes in a wide range of organisms. CRISPR-Cas9 has been instrumental in gene therapy, functional genomics, and disease modeling. However, its application to mitochondrial genomes remains challenging due to the unique structural and functional properties of mitochondria. The double-membrane architecture of mitochondria, coupled with the lack of established mechanisms for importing guide RNAs and other nucleic acids into the organelle, has hindered the direct use of CRISPR-Cas9 for mitochondrial genome editing (Schmiderer et al. 2022). To overcome these obstacles, researchers have developed mitochondrially targeted nucleases, such as mitochondria-targeted zinc finger nucleases (mtZFNs) and transcription activator-like effector nucleases (mtTALENs) (Figure 1). These engineered proteins are equipped with mitochondrial targeting sequences that direct them to the organelle and into the matrix, where they can induce site-specific double-strand breaks in mtDNA. By selectively cleaving mutant or undesired mtDNA molecules, these nucleases facilitate the degradation of targeted genomes, thereby promoting a shift toward wild-type mtDNA and enabling the study of mitochondrial gene function and disease-associated mutations.
Zinc finger proteins are characterized by a distinctive DNA-binding motif in which a zinc ion stabilizes a ββα structural fold. Each zinc finger domain typically recognizes a DNA sequence of 3–4 base pairs, and engineered proteins containing tandem arrays of zinc finger domains can achieve sequence specificity ranging from 9 to 18 base pairs. Zinc finger nucleases (ZFNs) are created by fusing these DNA-binding domains to the FokI endonuclease, thereby enabling the generation of targeted double-strand breaks in mitochondrial DNA. Despite their utility, ZFNs exhibit relatively modest editing efficiencies in mitochondrial DNA, with reported rates ranging from 3 % to 30 %. Additionally, their modular protein interactions can lead to off-target effects, limiting their precision and safety in genome editing applications (Gammage et al. 2016; Willis et al. 2022).
TALENs employ arrays of 33–34 amino acid repeats, known as TALE arrays, which confer single-nucleotide recognition specificity. These arrays are also fused to the FokI nuclease domain. Compared to ZFNs, TALENs demonstrate enhanced specificity due to reduced context-dependent effects on DNA binding. However, their large molecular size poses challenges for viral vector packaging, and the requirement for complete redesign of the TALE array when targeting new sequences adds complexity to their application (Bacman et al. 2013).
Mitochondrially targeted endonucleases, such as restriction endonucleases (REs) like SmaI and XmaI, have historically been utilized to introduce double-strand breaks in mutant mtDNA (Tanaka et al. 2002). Notably, mitochondrially targeted ApaLI-based restriction enzymes (mitoREs) were successfully used to shift heteroplasmy from 60 % to 15 % in cells derived from patients with Leber’s hereditary optic neuropathy (LHON) (Reddy et al. 2015). However, the utility of REs is constrained by their inability to be reprogrammed for novel restriction sites. In contrast, homing endonucleases such as mitoARCUS can selectively cleave mtDNA at specific mutation sites (e.g., m.5024C > T), achieving single-nucleotide resolution in targeting (Zekonyte et al. 2021; Shoop et al. 2023). This represents a significant advancement in the precision of mitochondrial genome editing.
Collectively, these engineered nucleases provide diverse strategies for targeted manipulation of the mitochondrial genome, each with unique advantages and limitations regarding specificity, efficiency, and adaptability to new target sequences (Figure 2). Ongoing innovations in protein engineering and delivery methods are expected to further enhance the therapeutic potential of these genome editing tools for mitochondrial diseases.
2.3 Base editors for mitochondrial genome engineering
The development of base editors represents a significant advancement in the field of mitochondrial genome engineering, offering a robust and precise alternative to traditional genome editing methods. One example is the Cytosine Base Editors (CBEs). DddA-derived cytosine base editors (DdCBEs) utilize a split bacterial deaminase, DddAtox, in conjunction with transcription activator-like effector (TALE) DNA-binding domains to facilitate site-specific cytosine-to-thymine (C-to-T) conversions within the double stranded mitochondrial DNA. These editors have demonstrated editing efficiencies ranging from 5 % to 50 %, making them highly effective tools for targeted mtDNA modifications (Mok et al. 2020). The advent of high-fidelity DdCBEs (HiFi-DdCBEs) has further improved the specificity of these systems by substantially reducing off-target editing events, thereby enhancing their safety profile for potential therapeutic applications (Lee et al. 2023).
A comprehensive collection of DdCBEs has been engineered to create the mitoKO library, which enables the precise ablation of all protein-coding genes in the mouse mitochondrial genome (Silva-Pinheiro et al. 2023). This system operates by introducing premature stop codons into mtDNA, thereby facilitating the knockout of each of the mtDNA-encoded proteins. The approach relies on TALE-based DNA-binding domains fused to split DddA deaminase halves, allowing for highly specific, targeted C-to-T base editing. Through iterative cycles of transfection and selection, researchers have successfully established near-homoplasmic knockout cell lines and generated mouse models characterized by high levels of heteroplasmy and minimal off-target modifications (Silva-Pinheiro et al. 2023). These advances underscore the versatility and precision of DdCBEs for mitochondrial functional genomics.
Adenine base editors, including TALE-linked deaminases (TALEDs) and mitochondrial adenine base editors (mtABEs), have expanded the toolkit for mitochondrial genome editing by enabling targeted adenine-to-guanine (A-to-G) conversions. These editors employ the engineered deaminase TadA8e and are mitochondrially targeted (Cho et al. 2024). Moreover, an advanced tool now combines TALE-fused nickase (MutH or Nt.BspD6I) with adenine or cytosine deaminases to perform single stranded base editing of mitochondrial DNA. The tools has been reported to be up to 77 % efficient and highly specific (Yi et al. 2024) The development of TALEDs and mtABEs has opened new avenues for correcting pathogenic mtDNA mutations and for functional studies of mitochondrial genes, complementing the capabilities of cytosine base editors (Figure 2).
In summary, the development of both cytosine and adenine base editors has enabled new ways of mitochondrial genome engineering, providing researchers with precise, efficient, and versatile tools for studying mitochondrial biology and developing potential therapeutic strategies.
3 Targeting mitochondrial RNA
3.1 RNA import into mitochondria: mechanisms and functional implication
In Trypanosoma brucei, all mitochondrial tRNAs are nuclear-encoded and subsequently imported into the organelle. Accordingly, RNA molecules can in principle pass the inner mitochondrial membrane. Recent studies have elucidated that tandem tRNA precursors are imported into the organelle in a manner dependent on both ATP and the mitochondrial membrane potential (Shikha et al. 2020; Tschopp et al. 2011). Notably, the translocase of the inner membrane (TIM) complex has been identified as essential for tRNA import. Interestingly, this process operates independently of the presequence-associated import motor, supporting the so-called “alternate import model” (Shikha et al. 2020). According to this model, tRNA and protein import utilize common translocation machineries but are mediated through distinct mechanisms, ensuring the specificity and regulation of mitochondrial import pathways.
In the budding yeast S. cerevisiae, most mitochondrial tRNAs are encoded by the mitochondrial genome. However, an exception exists for tRNALys (CUU), which is imported from the cytosol. The import of this tRNA is facilitated by interactions with specific cytosolic proteins, including the precursor of mitochondrial lysyl-tRNA synthetase (preMsk1p) (Entelis et al. 1998). These interactions are critical for the efficient delivery of tRNALys into the mitochondrial matrix. The import machinery responsible for this process involves components of both the TOM and TIM23 complex, suggesting functional overlap with the canonical protein import machinery (Entelis et al. 1998; Sepuri et al. 2012).
Despite these examples, the transport of RNA into mitochondria appears to be an exception (Figure 2). This is supported by the composition of the mitochondrial ribosome in which the RNA content is markedly reduced compared to its cytosolic counterparts and the rRNAs are encoded on mtDNA (Amunts et al. 2015; Itoh et al. 2021; Lavdovskaia et al. 2024; Metodiev et al. 2009; Ott et al. 2015). A second example is the loss of the RNA component in mitochondrial RNase P (Bhatta et al. 2021; Holzmann et al. 2008), highlighting that the mitochondrion reduced the demand for RNA compounds during evolution. It appears that mitochondria have very limited capacity for RNA import. As of today, no robust import system that enables RNA transport into mitochondria has been established.
3.2 Polymorpholino-mediated gene silencing
Small interfering RNA (siRNA)-mediated RNA interference (RNAi) is a widely used tool for silencing cytosolic mRNA that revolutionized molecular biology by enabling sequence-specific gene silencing, yet its application remains constrained to cytosolic targets. While cytosolic siRNA exploits the RNA-induced silencing complex (RISC), mitochondrial transcripts lack comparable endogenous silencing pathways. Moreover, the delivery of custom RNA molecules into the mitochondrial matrix represents a significant challenge (Phan et al. 2023; Silva-Pinheiro and Minczuk 2022). To address this limitation, phosphorodiamidate morpholino oligonucleotides (PMOs) have emerged as a promising and powerful tool for achieving precise mitochondrial gene silencing (Figure 2). PMOs are synthetic antisense molecules characterized by a morpholine ring backbone and neutral phosphorodiamidate linkages, features that confer high resistance to nucleases and high stability within biological systems. Nevertheless, conventional PMOs are unable to passively traverse cellular or mitochondrial membranes, which restricts their utility for targeting mitochondrial transcripts. To overcome this barrier, researchers have engineered mitochondrially targeted protein/peptide-PMO chimeras using click chemistry, thus enabling specific binding to individual mitochondrial mRNAs (mt-mRNAs) (Cruz-Zaragoza et al. 2021, 2025). In a recent study, a mitochondrially targeted mitochondrial matrix protein conjugated to a PMO was successfully imported into isolated mitochondria, where it demonstrated transcript-specific and efficient silencing activity (Cruz-Zaragoza et al. 2021; Koschel and Cruz-Zaragoza 2024). Building on these findings, further optimization efforts focused on reducing the size of the chimera. This was achieved by replacing the full-length protein with only a mitochondrial targeting sequence (MTS, presequence), which was directly linked to the PMO. The improved presequence-PMO chimera exhibited enhanced silencing efficiency in isolated mitochondria. Importantly, the presequence-PMO chimera could be transfected into cells where they facilitate robust and specific mitochondrial mRNA silencing (Cruz-Zaragoza et al. 2025). This marked the first successful demonstration of this approach in living cells, representing a significant advance in the field of mitochondrial gene regulation. The chimeras used in these studies, typically comprising 19–25 nucleotides, are imported into the mitochondrial matrix, where they bind complementary mt-mRNAs and sterically block mitochondrial ribosome interactions at the 5′ end of the target mRNA. Thereby, they effectively inhibit translation and achieve transcript-specific silencing (Cruz-Zaragoza et al. 2021, 2025). This strategy highlights the potential of targeted PMO chimeras as a versatile platform for mitochondrial gene manipulation and functional studies.
4 Conclusions
Despite decades of research in mitochondrial biology, significant gaps remain in our understanding of mitochondrial gene expression processes and their modulation in response to cellular requirements. Progress in this field has been hampered by the lack of suitable techniques for analyzing gene expression within mitochondria. However, the development of new technologies in recent years has opened promising avenues to address key questions regarding the mechanisms and regulation of mitochondrial gene expression. While these technologies have enabled advances in basic research, their application in the treatment of mitochondrial disorders remains limited. Nevertheless, it is anticipated that continued progress in this area will not only deepen our understanding of fundamental biological processes but also shed light on the pathological mechanisms underlying mitochondrial diseases. Ultimately, the ongoing development of innovative techniques holds the promise of paving the way toward future clinical strategies.
Funding source: Funded by the European Research Council (ERC) Advanced Grants MiXpress
Award Identifier / Grant number: ERCAdG No. 101095062
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors contributed to writing of the review.
-
Use of Large Language Models, AI and Machine Learning Tools: Not applicable.
-
Conflict of interest: Not applicable.
-
Research funding: Funded by the European Research Council (ERC) Advanced Grants MiXpress (ERCAdG No. 101095062) to PR, the Deutsche Forschungsgemeinschaft SFB1565 (project number 469281184; P14, PR), the Max Planck Society (PR), supported by the DFG under Germany’s Excellence Strategy - EXC 2067/1- 390729940.
-
Data availability: Not applicable.
References
Amunts, A., Brown, A., Toots, J., Scheres, S.H.W., and Ramakrishnan, V. (2015). The structure of the human mitochondrial ribosome. Science 348: 95–98, https://doi.org/10.1126/science.aaa1193.Search in Google Scholar PubMed PubMed Central
Anderson, S., Bankier, A.T., Barrell, B.G., Bruijn, M.H.L.de, Coulson, A.R., Drouin, J., Eperon, I.C., Nierlich, D.P., Roe, B.A., Sanger, F., et al.. (1981). Sequence and organization of the human mitochondrial genome. Nat. 290: 457–465, https://doi.org/10.1038/290457a0.Search in Google Scholar PubMed
Antonicka, H., Vučković, A., Weraarpachai, W., Hong, S., Brischigliaro, M., Ahn, A., Barrientos, A., Hillen, H.S., and Shoubridge, E.A. (2025). FASTKD5 processes mitochondrial pre-mRNAs at noncanonical cleavage sites. Nucleic Acids Res. 53: gkaf665, https://doi.org/10.1093/nar/gkaf665.Search in Google Scholar PubMed PubMed Central
Bacman, S.R., Williams, S.L., Pinto, M., Peralta, S., and Moraes, C.T. (2013). Specific elimination of mutant mitochondrial genomes in patient-derived cells by mitoTALENs. Nat. Med. 19: 1111–1113, https://doi.org/10.1038/nm.3261.Search in Google Scholar PubMed PubMed Central
Bhatta, A., Dienemann, C., Cramer, P., and Hillen, H.S. (2021). Structural basis of RNA processing by human mitochondrial RNase P. Nat. Struct. Mol. Biol. 28: 713–723, https://doi.org/10.1038/s41594-021-00637-y.Search in Google Scholar PubMed PubMed Central
Bonnefoy, N. and Fox, T.D. (2001). Genetic transformation of Saccharomyces cerevisiae mitochondria. Methods Cell Biol. 65: 381–396, https://doi.org/10.1016/s0091-679x(01)65022-2.Search in Google Scholar PubMed
Bonnefoy, N. and Fox, T.D. (2007). Mitochondria, practical protocols. Methods Mol. Biol. 372: 153–166, https://doi.org/10.1007/978-1-59745-365-3_11.Search in Google Scholar PubMed PubMed Central
Brzezniak, L.K., Bijata, M., Szczesny, R.J., and Stepien, P.P. (2011). Involvement of human ELAC2 gene product in 3’ end processing of mitochondrial tRNAs. RNA Biol. 8: 616–626, https://doi.org/10.4161/rna.8.4.15393.Search in Google Scholar PubMed
Cho, S.-I., Lim, K., Hong, S., Lee, J., Kim, A., Lim, C.J., Ryou, S., Lee, J.M., Mok, Y.G., Chung, E., et al.. (2024). Engineering TALE-linked deaminases to facilitate precision adenine base editing in mitochondrial DNA. Cell 187: 95–109.e26, https://doi.org/10.1016/j.cell.2023.11.035.Search in Google Scholar PubMed
Chujo, T., Ohira, T., Sakaguchi, Y., Goshima, N., Nomura, N., Nagao, A., and Suzuki, T. (2012). LRPPRC/SLIRP suppresses PNPase-mediated mRNA decay and promotes polyadenylation in human mitochondria. Nucleic Acids Res. 40: 8033–8047, https://doi.org/10.1093/nar/gks506.Search in Google Scholar PubMed PubMed Central
Cohen, J.S. and Fox, T.D. (2001). Expression of green fluorescent protein from a recoded gene inserted into Saccharomyces cerevisiae mitochondrial DNA. Mitochondrion 1: 181–189, https://doi.org/10.1016/s1567-7249(01)00012-5.Search in Google Scholar PubMed
Cruz-Zaragoza, L.D., Dahal, D., Koschel, M., Boshnakovska, A., Zheenbekova, A., Yilmaz, M., Morgenstern, M., Dohrke, J.-N., Bender, J., Valpadashi, A., et al.. (2025). Silencing mitochondrial gene expression in living cells. Science: eadr3498, https://doi.org/10.1126/science.adr3498.Search in Google Scholar PubMed PubMed Central
Cruz-Zaragoza, L.D., Dennerlein, S., Linden, A., Yousefi, R., Lavdovskaia, E., Aich, A., Falk, R.R., Gomkale, R., Schöndorf, T., Bohnsack, M.T., et al.. (2021). An in vitro system to silence mitochondrial gene expression. Cell 184: 5824–5837.e15, https://doi.org/10.1016/j.cell.2021.09.033.Search in Google Scholar PubMed
Curth, U., Urbanke, C., Greipel, J., Gerberding, H., Tiranti, V., and Zeviani, M. (1994). Single‐stranded‐DNA‐binding proteins from human mitochondria and Escherichia coli have analogous physicochemical properties. Eur. J. Biochem. 221: 435–443, https://doi.org/10.1111/j.1432-1033.1994.tb18756.x.Search in Google Scholar PubMed
Entelis, N.S., Kieffer, S., Kolesnikova, O.A., Martin, R.P., and Tarassov, I.A. (1998). Structural requirements of tRNALys for its import into yeast mitochondria. Proceedings of the National Academy of Sciences USA 95: 2838–2843, https://doi.org/10.1073/pnas.95.6.2838.Search in Google Scholar PubMed PubMed Central
Falkenberg, M., Larsson, N.-G., and Gustafsson, C.M. (2024). Replication and transcription of human mitochondrial DNA. Annu. Rev. Biochem. 93: 47–77, https://doi.org/10.1146/annurev-biochem-052621-092014.Search in Google Scholar PubMed
Fisher, R.P. and Clayton, D.A. (1985). A transcription factor required for promoter recognition by human mitochondrial RNA polymerase. Accurate initiation at the heavy- and light-strand promoters dissected and reconstituted in vitro. J. Biol. Chem. 260: 11330–11338, https://doi.org/10.1016/s0021-9258(17)39184-6.Search in Google Scholar
Fox, T.D., Sanford, J.C., and McMullin, T.W. (1988). Plasmids can stably transform yeast mitochondria lacking endogenous mtDNA. Proceedings of the National Academy of Sciences USA 85: 7288–7292, https://doi.org/10.1073/pnas.85.19.7288Search in Google Scholar PubMed PubMed Central
Franco, L.V.R. and Barros, M.H. (2023). Biolistic transformation of the yeast Saccharomyces cerevisiae mitochondrial DNA. IUBMB Life 75: 972–982, https://doi.org/10.1002/iub.2769.Search in Google Scholar PubMed
Fu, L., Luo, Y.-X., Liu, Y., Liu, H., Li, H., and Yu, Y. (2021). Potential of mitochondrial genome editing for human fertility health. Front. Genet. 12: 673951, https://doi.org/10.3389/fgene.2021.673951.Search in Google Scholar PubMed PubMed Central
Fusté, J.M., Wanrooij, S., Jemt, E., Granycome, C.E., Cluett, T.J., Shi, Y., Atanassova, N., Holt, I.J., Gustafsson, C.M., and Falkenberg, M. (2010). Mitochondrial RNA polymerase is needed for activation of the origin of light-strand DNA replication. Mol. Cell 37: 67–78, https://doi.org/10.1016/j.molcel.2009.12.021.Search in Google Scholar PubMed
Gammage, P.A., Gaude, E., Haute, L.V., Rebelo-Guiomar, P., Jackson, C.B., Rorbach, J., Pekalski, M.L., Robinson, A.J., Charpentier, M., Concordet, J.-P., et al.. (2016). Near-complete elimination of mutant mtDNA by iterative or dynamic dose-controlled treatment with mtZFNs. Nucleic Acids Res. 44: 7804–7816, https://doi.org/10.1093/nar/gkw676.Search in Google Scholar PubMed PubMed Central
Gusic, M. and Prokisch, H. (2021). Genetic basis of mitochondrial diseases. FEBS Lett. 595: 1132–1158, https://doi.org/10.1002/1873-3468.14068.Search in Google Scholar PubMed
Hall, M.P., Unch, J., Binkowski, B.F., Valley, M.P., Butler, B.L., Wood, M.G., Otto, P., Zimmerman, K., Vidugiris, G., Machleidt, T., et al.. (2012). Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem. Biol. 7: 1848–1857, https://doi.org/10.1021/cb3002478.Search in Google Scholar PubMed PubMed Central
Hillen, H.S., Morozov, Y.I., Sarfallah, A., Temiakov, D., and Cramer, P. (2017). Structural basis of mitochondrial transcription initiation. Cell 171: 1072–1081.e10, https://doi.org/10.1016/j.cell.2017.10.036.Search in Google Scholar PubMed PubMed Central
Holzmann, J., Frank, P., Löffler, E., Bennett, K.L., Gerner, C., and Rossmanith, W. (2008). RNase P without RNA: identification and functional reconstitution of the human mitochondrial tRNA processing enzyme. Cell 135: 462–474, https://doi.org/10.1016/j.cell.2008.09.013.Search in Google Scholar PubMed
Homberg, B., Rehling, P., and Cruz-Zaragoza, L.D. (2023). The multifaceted mitochondrial OXA insertase. Trends Cell Biol. 33: 765–772, https://doi.org/10.1016/j.tcb.2023.02.001.Search in Google Scholar PubMed
Ikeda, M., Ide, T., Fujino, T., Arai, S., Saku, K., Kakino, T., Tyynismaa, H., Yamasaki, T., Yamada, K., Kang, D., et al.. (2015). Overexpression of TFAM or twinkle increases mtDNA copy number and facilitates cardioprotection associated with limited mitochondrial oxidative stress. PLoS ONE 10: e0119687, https://doi.org/10.1371/journal.pone.0119687.Search in Google Scholar PubMed PubMed Central
Isaac, R.S., McShane, E., and Churchman, L.S. (2018). The multiple levels of mitonuclear coregulation. Annu. Rev. Genet. 52: 1–23, https://doi.org/10.1146/annurev-genet-120417-031709.Search in Google Scholar PubMed
Itoh, Y., Andréll, J., Choi, A., Richter, U., Maiti, P., Best, R.B., Barrientos, A., Battersby, B.J., and Amunts, A. (2021). Mechanism of membrane-tethered mitochondrial protein synthesis. Science 371: 846–849, https://doi.org/10.1126/science.abe0763.Search in Google Scholar PubMed PubMed Central
Jia, L., Dienhart, M., Schramp, M., McCauley, M., Hell, K., and Stuart, R.A. (2003). Yeast Oxa1 interacts with mitochondrial ribosomes: the importance of the C‐terminal region of Oxa1. EMBO J. 22: 6438–6447, https://doi.org/10.1093/emboj/cdg624.Search in Google Scholar PubMed PubMed Central
Johnston, S.A., Anziano, P.Q., Shark, K., Sanford, J.C., and Butow, R.A. (1988). Mitochondrial transformation in yeast by bombardment with microprojectiles. Science 240: 1538–1541, https://doi.org/10.1126/science.2836954.Search in Google Scholar PubMed
Kaufman, B.A., Durisic, N., Mativetsky, J.M., Costantino, S., Hancock, M.A., Grutter, P., and Shoubridge, E.A. (2007). The mitochondrial transcription factor TFAM coordinates the assembly of multiple DNA molecules into nucleoid-like structures. Mol. Biol. Cell 18: 3225–3236, https://doi.org/10.1091/mbc.e07-05-0404.Search in Google Scholar PubMed PubMed Central
Korhonen, J.A., Gaspari, M., and Falkenberg, M. (2003). TWINKLE has 5’ → 3’ DNA helicase activity and is specifically stimulated by mitochondrial single-stranded DNA-binding protein. J. Biol. Chem. 278: 48627–48632, https://doi.org/10.1074/jbc.m306981200.Search in Google Scholar
Korhonen, J.A., Pande, V., Holmlund, T., Farge, G., Pham, X.H., Nilsson, L., and Falkenberg, M. (2008). Structure–function defects of the TWINKLE linker region in progressive external ophthalmoplegia. J. Mol. Biol. 377: 691–705, https://doi.org/10.1016/j.jmb.2008.01.035.Search in Google Scholar PubMed
Koschel, M. and Cruz-Zaragoza, L.D. (2024). In organello silencing of mitochondrial gene expression. Methods Enzymol. 706: 501–518, https://doi.org/10.1016/bs.mie.2024.07.035.Search in Google Scholar PubMed
Kühl, I., Miranda, M., Posse, V., Milenkovic, D., Mourier, A., Siira, S.J., Bonekamp, N.A., Neumann, U., Filipovska, A., Polosa, P.L., et al.. (2016). POLRMT regulates the switch between replication primer formation and gene expression of mammalian mtDNA. Sci. Adv. 2: e1600963, https://doi.org/10.1126/sciadv.1600963.Search in Google Scholar PubMed PubMed Central
Kukat, C., Davies, K.M., Wurm, C.A., Spåhr, H., Bonekamp, N.A., Kühl, I., Joos, F., Polosa, P.L., Park, C.B., Posse, V., et al.. (2015). Cross-strand binding of TFAM to a single mtDNA molecule forms the mitochondrial nucleoid.Proceedings of the National Academy of Sciences USA 112: 11288–11293, https://doi.org/10.1073/pnas.1512131112Search in Google Scholar PubMed PubMed Central
Kummer, E. and Ban, N. (2021). Mechanisms and regulation of protein synthesis in mitochondria. Nat. Rev. Mol. Cell Biol. 22: 307–325, https://doi.org/10.1038/s41580-021-00332-2.Search in Google Scholar PubMed
Kuznetsov, S.V., Kozlov, A.G., Lohman, T.M., and Ansari, A. (2006). Microsecond dynamics of protein–DNA interactions: direct observation of the wrapping/unwrapping kinetics of single-stranded DNA around the E.coli SSB tetramer. J. Mol. Biol. 359: 55–65, https://doi.org/10.1016/j.jmb.2006.02.070.Search in Google Scholar PubMed
Lagouge, M., Mourier, A., Lee, H.J., Spåhr, H., Wai, T., Kukat, C., Ramos, E.S., Motori, E., Busch, J.D., Siira, S., et al.. (2015). SLIRP regulates the rate of mitochondrial protein synthesis and protects LRPPRC from degradation. PLoS Genet. 11: e1005423, https://doi.org/10.1371/journal.pgen.1005423.Search in Google Scholar PubMed PubMed Central
Lavdovskaia, E., Hanitsch, E., Linden, A., Pašen, M., Challa, V., Horokhovskyi, Y., Roetschke, H.P., Nadler, F., Welp, L., Steube, E., et al.. (2024). A roadmap for ribosome assembly in human mitochondria. Nat. Struct. Mol. Biol. 31: 1898–1908, https://doi.org/10.1038/s41594-024-01356-w.Search in Google Scholar PubMed PubMed Central
Lee, S., Lee, H., Baek, G., and Kim, J.-S. (2023). Precision mitochondrial DNA editing with high-fidelity DddA-derived base editors. Nat. Biotechnol. 41: 378–386, https://doi.org/10.1038/s41587-022-01486-w.Search in Google Scholar PubMed PubMed Central
Mercer, T.R., Neph, S., Dinger, M.E., Crawford, J., Smith, M.A., Shearwood, A.-M.J., Haugen, E., Bracken, C.P., Rackham, O., Stamatoyannopoulos, J.A., et al.. (2011). The human mitochondrial transcriptome. Cell 146: 645–658, https://doi.org/10.1016/j.cell.2011.06.051.Search in Google Scholar PubMed PubMed Central
Metodiev, M.D., Lesko, N., Park, C.B., Cámara, Y., Shi, Y., Wibom, R., Hultenby, K., Gustafsson, C.M., and Larsson, N.-G. (2009). Methylation of 12S rRNA is necessary for in vivo stability of the small subunit of the mammalian mitochondrial ribosome. Cell Metab. 9: 386–397, https://doi.org/10.1016/j.cmet.2009.03.001.Search in Google Scholar PubMed
Milenkovic, D., Matic, S., Kühl, I., Ruzzenente, B., Freyer, C., Jemt, E., Park, C.B., Falkenberg, M., and Larsson, N.-G. (2013). TWINKLE is an essential mitochondrial helicase required for synthesis of nascent D-loop strands and complete mtDNA replication. Hum. Mol. Genet. 22: 1983–1993, https://doi.org/10.1093/hmg/ddt051.Search in Google Scholar PubMed PubMed Central
Minczuk, M., He, J., Duch, A.M., Ettema, T.J., Chlebowski, A., Dzionek, K., Nijtmans, L.G.J., Huynen, M.A., and Holt, I.J. (2011). TEFM (c17orf42) is necessary for transcription of human mtDNA. Nucleic Acids Res. 39: 4284–4299, https://doi.org/10.1093/nar/gkq1224.Search in Google Scholar PubMed PubMed Central
Mireau, H., Arnal, N., and Fox, T.D. (2003). Expression of Barstar as a selectable marker in yeast mitochondria. Mol. Genet. Genomics 270: 1–8, https://doi.org/10.1007/s00438-003-0879-2.Search in Google Scholar PubMed
Mok, B.Y., Moraes, M.H.de, Zeng, J., Bosch, D.E., Kotrys, A.V., Raguram, A., Hsu, F., Radey, M.C., Peterson, S.B., Mootha, V.K., et al.. (2020). A bacterial cytidine deaminase toxin enables CRISPR-free mitochondrial base editing. Nat. 583: 631–637, https://doi.org/10.1038/s41586-020-2477-4.Search in Google Scholar PubMed PubMed Central
Montoya, J., Christianson, T., Levens, D., Rabinowitz, M., and Attardi, G. (1982). Identification of initiation sites for heavy-strand and light-strand transcription in human mitochondrial DNA. Proceedings of the National Academy of Sciences USA 79: 7195–7199, https://doi.org/10.1073/pnas.79.23.7195.Search in Google Scholar PubMed PubMed Central
Mootha, V.K., Lepage, P., Miller, K., Bunkenborg, J., Reich, M., Hjerrild, M., Delmonte, T., Villeneuve, A., Sladek, R., Xu, F., et al.. (2003). Identification of a gene causing human cytochrome c oxidase deficiency by integrative genomics. Proceedings of the National Academy of Sciences USA 100: 605–610, https://doi.org/10.1073/pnas.242716699.Search in Google Scholar PubMed PubMed Central
Morozov, Y.I., Parshin, A.V., Agaronyan, K., Cheung, A.C.M., Anikin, M., Cramer, P., and Temiakov, D. (2015). A model for transcription initiation in human mitochondria. Nucleic Acids Res. 43: 3726–3735, https://doi.org/10.1093/nar/gkv235.Search in Google Scholar PubMed PubMed Central
Ngo, H.B., Lovely, G.A., Phillips, R., and Chan, D.C. (2014). Distinct structural features of TFAM drive mitochondrial DNA packaging versus transcriptional activation. Nat. Commun. 5: 3077, https://doi.org/10.1038/ncomms4077.Search in Google Scholar PubMed PubMed Central
Ott, M., Amunts, A., and Brown, A. (2015). Organization and regulation of mitochondrial protein synthesis. Annu. Rev. Biochem. 85: 1–25, https://doi.org/10.1146/annurev-biochem-060815-014334.Search in Google Scholar PubMed
Phan, H.T.L., Lee, H., and Kim, K. (2023). Trends and prospects in mitochondrial genome editing. Exp. Mol. Med. 55: 871–878, https://doi.org/10.1038/s12276-023-00973-7.Search in Google Scholar PubMed PubMed Central
Priesnitz, C. and Becker, T. (2018). Pathways to balance mitochondrial translation and protein import. Gene Dev. 32: 1285–1296, https://doi.org/10.1101/gad.316547.118.Search in Google Scholar PubMed PubMed Central
Reddy, P., Ocampo, A., Suzuki, K., Luo, J., Bacman, S.R., Williams, S.L., Sugawara, A., Okamura, D., Tsunekawa, Y., Wu, J., et al.. (2015). Selective elimination of mitochondrial mutations in the germline by genome editing. Cell 161: 459–469, https://doi.org/10.1016/j.cell.2015.03.051.Search in Google Scholar PubMed PubMed Central
Richter-Dennerlein, R., Dennerlein, S., and Rehling, P. (2015). Integrating mitochondrial translation into the cellular context. Nat. Rev. Mol. Cell Biol. 16: 586–592, https://doi.org/10.1038/nrm4051.Search in Google Scholar PubMed
Robberson, D.L., Kasamatsu, H., and Vinograd, J. (1972). Replication of mitochondrial DNA. Circular replicative intermediates in mouse L cells. Proceedings of the National Academy of Sciences USA 69: 737–741, https://doi.org/10.1073/pnas.69.3.737.Search in Google Scholar PubMed PubMed Central
Ruzzenente, B., Metodiev, M.D., Wredenberg, A., Bratic, A., Park, C.B., Cámara, Y., Milenkovic, D., Zickermann, V., Wibom, R., Hultenby, K., et al.. (2012). LRPPRC is necessary for polyadenylation and coordination of translation of mitochondrial mRNAs. EMBO J. 31: 443–456, https://doi.org/10.1038/emboj.2011.392.Search in Google Scholar PubMed PubMed Central
Sanchez, M.I.G.L., Mercer, T.R., Davies, S.M.K., Shearwood, A.-M.J., Nygård, K.K.A., Richman, T.R., Mattick, J.S., Rackham, O., and Filipovska, A. (2011). RNA processing in human mitochondria. Cell Cycle 10: 2904–2916, https://doi.org/10.4161/cc.10.17.17060.Search in Google Scholar PubMed
Sasarman, F., Brunel-Guitton, C., Antonicka, H., Wai, T., Shoubridge, E.A., and Consortium, L. (2010). LRPPRC and SLIRP interact in a ribonucleoprotein complex that regulates posttranscriptional gene expression in mitochondria. Mol. Biol. Cell 21: 1315–1323, https://doi.org/10.1091/mbc.e10-01-0047.Search in Google Scholar PubMed PubMed Central
Schmiderer, L., Yudovich, D., Oburoglu, L., Hjort, M., and Larsson, J. (2022). Site-specific CRISPR-based mitochondrial DNA manipulation is limited by gRNA import. Sci. Rep. 12: 18687, https://doi.org/10.1038/s41598-022-21794-0.Search in Google Scholar PubMed PubMed Central
Sepuri, N.B.V., Gorla, M., and King, M.P. (2012). Mitochondrial lysyl-tRNA synthetase independent import of tRNA lysine into yeast mitochondria. PLoS ONE 7: e35321, https://doi.org/10.1371/journal.pone.0035321.Search in Google Scholar PubMed PubMed Central
Shikha, S., Huot, J.L., Schneider, A., and Niemann, M. (2020). tRNA import across the mitochondrial inner membrane in T. brucei requires TIM subunits but is independent of protein import. Nucleic Acids Res. 48: gkaa1098, https://doi.org/10.1093/nar/gkaa1098.Search in Google Scholar PubMed PubMed Central
Shoop, W.K., Lape, J., Trum, M., Powell, A., Sevigny, E., Mischler, A., Bacman, S.R., Fontanesi, F., Smith, J., Jantz, D., et al.. (2023). Efficient elimination of MELAS-associated m.3243G mutant mitochondrial DNA by an engineered mitoARCUS nuclease. Nat. Metab. 5: 2169–2183, https://doi.org/10.1038/s42255-023-00932-6.Search in Google Scholar PubMed PubMed Central
Silva-Pinheiro, P. and Minczuk, M. (2022). The potential of mitochondrial genome engineering. Nat. Rev. Genet. 23: 199–214, https://doi.org/10.1038/s41576-021-00432-x.Search in Google Scholar PubMed
Silva-Pinheiro, P., Mutti, C.D., Haute, L.V., Powell, C.A., Nash, P.A., Turner, K., and Minczuk, M. (2023). A library of base editors for the precise ablation of all protein-coding genes in the mouse mitochondrial genome. Nat. Biomed. Eng. 7: 692–703, https://doi.org/10.1038/s41551-022-00968-1.Search in Google Scholar PubMed PubMed Central
Singh, V., Moran, J.C., Itoh, Y., Soto, I.C., Fontanesi, F., Couvillion, M., Huynen, M.A., Churchman, L.S., Barrientos, A., and Amunts, A. (2024). Structural basis of LRPPRC–SLIRP-dependent translation by the mitoribosome. Nat. Struct. Mol. Biol. 31: 1838–1847, https://doi.org/10.1038/s41594-024-01365-9.Search in Google Scholar PubMed PubMed Central
Smeitink, J., Heuvel, L.van den, and DiMauro, S. (2001). The genetics and pathology of oxidative phosphorylation. Nat. Rev. Genet. 2: 342–352, https://doi.org/10.1038/35072063.Search in Google Scholar PubMed
Stewart, J.B. and Chinnery, P.F. (2015). The dynamics of mitochondrial DNA heteroplasmy: implications for human health and disease. Nat. Rev. Genet. 16: 530–542, https://doi.org/10.1038/nrg3966.Search in Google Scholar PubMed
Stewart, J.B. and Chinnery, P.F. (2021). Extreme heterogeneity of human mitochondrial DNA from organelles to populations. Nat. Rev. Genet. 22: 106–118, https://doi.org/10.1038/s41576-020-00284-x.Search in Google Scholar PubMed
Suhm, T., Habernig, L., Rzepka, M., Kaimal, J.M., Andréasson, C., Büttner, S., and Ott, M. (2018). A novel system to monitor mitochondrial translation in yeast. Microb. Cell 5: 158, https://doi.org/10.15698/mic2018.03.621.Search in Google Scholar PubMed PubMed Central
Szyrach, G., Ott, M., Bonnefoy, N., Neupert, W., and Herrmann, J.M. (2003). Ribosome binding to the oxa1 complex facilitates co‐translational protein insertion in mitochondria. EMBO J. 22: 6448–6457, https://doi.org/10.1093/emboj/cdg623.Search in Google Scholar PubMed PubMed Central
Tanaka, M., Borgeld, H.-J., Zhang, J., Muramatsu, S., Gong, J.-S., Yoneda, M., Maruyama, W., Naoi, M., Ibi, T., Sahashi, K., et al.. (2002). Gene therapy for mitochondrial disease by delivering restriction endonuclease SmaI into mitochondria. J. Biomed. Sci. 9: 534–541, https://doi.org/10.1159/000064726.Search in Google Scholar PubMed
Tang, J.X., Thompson, K., Taylor, R.W., and Oláhová, M. (2020). Mitochondrial OXPHOS biogenesis: co-regulation of protein synthesis, import, and assembly pathways. Int. J. Mol. Sci. 21: 3820, https://doi.org/10.3390/ijms21113820.Search in Google Scholar PubMed PubMed Central
Tiranti, V., Rocchi, M., DiDonato, S., and Zeviani, M. (1993). Cloning of human and rat cDNAs encoding the mitochondrial single-stranded DNA-binding protein (SSB). Gene 126: 219–225, https://doi.org/10.1016/0378-1119(93)90370-i.Search in Google Scholar PubMed
Tschopp, F., Charrière, F., and Schneider, A. (2011). In vivo study in Trypanosoma brucei links mitochondrial transfer RNA import to mitochondrial protein import. EMBO reports 12: 825–832, https://doi.org/10.1038/embor.2011.111.Search in Google Scholar PubMed PubMed Central
Tyynismaa, H., Sembongi, H., Bokori-Brown, M., Granycome, C., Ashley, N., Poulton, J., Jalanko, A., Spelbrink, J.N., Holt, I.J., and Suomalainen, A. (2004). Twinkle helicase is essential for mtDNA maintenance and regulates mtDNA copy number. Hum. Mol. Genet. 13: 3219–3227, https://doi.org/10.1093/hmg/ddh342.Search in Google Scholar PubMed
Wanrooij, S., Fusté, J.M., Farge, G., Shi, Y., Gustafsson, C.M., and Falkenberg, M. (2008). Human mitochondrial RNA polymerase primes lagging-strand DNA synthesis in vitro. Proceedings of the National Academy of Sciences USA 105: 11122–11127, https://doi.org/10.1073/pnas.0805399105.Search in Google Scholar PubMed PubMed Central
Willis, J.C.W., Silva-Pinheiro, P., Widdup, L., Minczuk, M., and Liu, D.R. (2022). Compact zinc finger base editors that edit mitochondrial or nuclear DNA in vitro and in vivo. Nat. Commun. 13: 7204, https://doi.org/10.1038/s41467-022-34784-7.Search in Google Scholar PubMed PubMed Central
Yi, Z., Zhang, Xiaoxue, Tang, W., Yu, Y., Wei, X., Zhang, Xue, and Wei, W. (2024). Strand-selective base editing of human mitochondrial DNA using mitoBEs. Nat. Biotechnol. 42: 498–509, https://doi.org/10.1038/s41587-023-01791-y.Search in Google Scholar PubMed PubMed Central
Zekonyte, U., Bacman, S.R., Smith, J., Shoop, W., Pereira, C.V., Tomberlin, G., Stewart, J., Jantz, D., and Moraes, C.T. (2021). Mitochondrial targeted meganuclease as a platform to eliminate mutant mtDNA in vivo. Nat. Commun. 12: 3210, https://doi.org/10.1038/s41467-021-23561-7.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Highlight: organelles on and off the map: diversity, specialization and subdomains
- Emerging dimensions of mitochondrial specialization
- Manipulating mitochondrial gene expression
- Conserved function, divergent evolution: mitochondrial outer membrane insertases across eukaryotes
- There and back again: a cell biologist’s journey from organelles to molecules
- Recent advances in glycosome biogenesis and its implications for drug discovery
- Jack of all trades – the lipid droplet organization (LDO) proteins are multifunctional organelle surface receptors
- Update on VAP, a ubiquitous signpost for the ER
- Biogenesis and function of the mitochondrial solute carrier (SLC25) family in yeast
- Getting to the right place at the right time – membrane trafficking and maturation in the endolysosomal system
Articles in the same Issue
- Frontmatter
- Highlight: organelles on and off the map: diversity, specialization and subdomains
- Emerging dimensions of mitochondrial specialization
- Manipulating mitochondrial gene expression
- Conserved function, divergent evolution: mitochondrial outer membrane insertases across eukaryotes
- There and back again: a cell biologist’s journey from organelles to molecules
- Recent advances in glycosome biogenesis and its implications for drug discovery
- Jack of all trades – the lipid droplet organization (LDO) proteins are multifunctional organelle surface receptors
- Update on VAP, a ubiquitous signpost for the ER
- Biogenesis and function of the mitochondrial solute carrier (SLC25) family in yeast
- Getting to the right place at the right time – membrane trafficking and maturation in the endolysosomal system

