Abstract
The disruption of protein homeostasis leads to the increased un- and misfolding of proteins and the formation of toxic protein aggregates. Their accumulation triggers an unfolded protein response that is characterized by the transcriptional upregulation of molecular chaperones and proteases, and aims to restore proteome integrity, maintain cellular function, suppress the cause of perturbation, and prevent disease and death. In the green microalga Chlamydomonas reinhardtii, the study of this response to proteotoxic stress has provided insights into the function of chaperone and protease systems, which are, though simpler, closely related to those found in land plants. In addition, there has been considerable progress in understanding the triggers and regulation of compartment-specific unfolded protein responses. This review provides an overview on how the dysfunction of protein homeostasis is sensed in the different compartments of Chlamydomonas, and summarizes the current knowledge on the pathways that are triggered to restore equilibrium in the cell, while also highlighting similarities and differences to the unfolded protein responses of other model organisms.
1 Introduction
Cellular protein homeostasis, or proteostasis, is essential for the health of living systems. This dynamic process maintains equilibrium by precisely controlling protein biosynthesis, folding and degradation through various sensing and feedback mechanisms which are hallmarks of homeostasis (Cannon 1929). Different classes of molecular chaperones play a central role in preserving a balanced, functional proteome. With their assistance, proteins that are synthesized de novo on ribosomes or imported into a compartment manage to correctly fold into unique three-dimensional structures and become functionally active. Furthermore, chaperones are responsible for the conformational maintenance of the proteins in the crowded environment of the cell (Balchin et al. 2016).
Abiotic stresses, such as heat and oxidative stress, challenge protein homeostasis by promoting the unfolding of proteins. Due to their exposed hydrophobic amino acid residues, un- and misfolded proteins form protein aggregates that, if not dealt with, cause disease and death (Hipp et al. 2014). The accumulation of unfolded proteins is sensed and triggers an ancient protein quality control mechanism found in many eukaryotic organisms, the so-called unfolded protein response (UPR). It is characterized by the transcriptional upregulation of molecular chaperones, and aims to increase the folding capacity of the cell and suppress the source of the perturbation (Balchin et al. 2016; Howell 2021). These chaperones include heat shock proteins (HSPs) from the HSP90, HSP70 and HSP60 classes that are constitutively present at high levels, as well as small HSPs (sHSPs) and CLPB-type proteins of the HSP100 class, which often only accumulate upon stress. The chaperones cooperate with protein degradation machineries to disassemble toxic protein aggregates, assist in protein refolding and remove terminally misfolded proteins (Cashikar et al. 2005; Hayer-Hartl et al. 2016; Mogk et al. 2015; Nathan et al. 1997; Rosenzweig et al. 2019). When proteotoxic stress affects only a single cellular compartment, a compartment-specific UPR is induced to uphold the function of that compartment and restore proteostasis.
This review summarizes the current knowledge on the UPRs in the unicellular green microalga Chlamydomonas reinhardtii which is used for the study of fundamental plant stress response mechanisms due to its gene families being smaller, yet closely related to those found in land plants (Schroda et al. 2015). The elucidation of gene functions in this alga is facilitated by the existence of an insertional mutant library and the availability of highly advanced techniques such as CRISPR/Cas, the Modular Cloning system and in vivo proximity labeling (Crozet et al. 2018; Kreis et al. 2023b; Lau et al. 2023; Li et al. 2015; Shin et al. 2016). We describe the sensing and signaling pathways of the UPRs in the cytosol, endoplasmic reticulum (ER) and chloroplast in Chlamydomonas and compare them to the corresponding UPRs in other model organisms.
2 Characteristics and triggers of unfolded protein responses
When unfolded proteins accumulate and form proteotoxic aggregates somewhere in the cell, signaling pathways are activated and lead to the strong transcriptional upregulation of genes encoding molecular chaperones and proteases (Lindquist and Craig 1988). This is considered a hallmark of the UPR and occurs if the rate of protein biosynthesis or import exceeds the folding capacity of the cell. Alternatively, an UPR can be caused by the disturbance of protein homeostasis through environmental factors that lead to the unfolding of proteins that are already present and active in the cell (Balchin et al. 2016).
Abiotic stress conditions that can trigger the UPR in plants include elevated temperatures, oxidative stress caused by high light intensities, or nutrient starvation, although these external stimuli elicit more changes in the cell than just the disruption of proteome balance. Heat stress, for instance, also increases membrane fluidity, causes metabolic imbalances by changing enzyme activities, and impairs DNA replication and DNA damage repair (Hemme et al. 2014; Schroda et al. 2015). In Chlamydomonas, the heat shock response is triggered by temperatures between 35 °C and 39 °C (Kobayashi et al. 2014; Rütgers et al. 2017b; Tanaka et al. 2000; Zhang et al. 2022), with temperatures above 43 °C being lethal for the alga (Schroda et al. 2015). The specific threshold temperature can vary due to strain and assay differences and depends on the HSP used as a marker.
Experimentally, the upregulation of HSP genes can also be induced through genetic interference or by various pharmacological treatments that inhibit protein folding, ER or chaperone function. As an example, feeding cells with the arginine analog canavanine perturbs the formation of secondary protein structures due to the incorporation of the non-proteinogenic amino acid into newly synthesized proteins, thereby triggering an UPR in mammalian cells and Chlamydomonas (Hightower and White 1981; Schmollinger et al. 2013). In the following sections, other triggers of the Chlamydomonas UPR and the changes they elicit in the cell are described alongside the regulation mechanisms of the UPR in the respective compartments.
3 Regulation of the unfolded protein response in the cytosol
Heat shock factors (HSFs) are key regulators of the cytosolic UPR in all eukaryotes and mediate the increased transcription of HSP genes during conditions that promote protein un- and misfolding. They act through so-called heat shock elements (HSEs), which are located in the promoter region upstream of HSP genes and contain at least three contiguous repeats of the highly conserved pentameric sequence 5′-nGAAn-3′ in alternating orientation (Åkerfelt et al. 2010). The number of HSFs in different organisms varies, with land plants like Arabidopsis encoding 21 HSFs, while yeast only encodes a single HSF (Scharf et al. 2012). In Chlamydomonas, two HSFs named HSF1 and HSF2 exist (Schulz-Raffelt et al. 2007) (Table 1). Unlike in yeast, the levels of both Chlamydomonas HSFs increase during elevated temperatures – a characteristic shared with other typical land plant class A HSFs (Hübel and Schöffl 1994; Schulz-Raffelt et al. 2007; Wiederrecht et al. 1988). All three HSF classes (A–C) harbor domains for DNA binding, oligomerization and nuclear localization. Yet, where class A HSFs contain short activator peptide (AHA) motifs, class B HSFs have motifs with repressor functions, and class C HSFs possess neither (Scharf et al. 2012). Despite the increase of both Chlamydomonas HSFs during heat stress, the synthesis of major HSPs, as well as the induction of its own and the HSF2 gene, is only mediated by HSF1, which solidifies its role as the alga’s essential master regulator of the heat shock response. Except for the canonical DNA-binding domain, HSF2 lacks all the sequence signatures specific for plant HSFs that are present in HSF1, and could not compensate for the loss of HSF1 in HSF1-RNAi strains that led to the reduced accumulation of HSPs under heat stress. Hence, the role of HSF2 in the heat shock response is unknown (Schmollinger et al. 2010; Schulz-Raffelt et al. 2007).
HSFs are synthesized during non-stress conditions, but do not acquire transcriptional competence until they form trimers and become activated (Figure 1). In mammals, the trimerization is a stress-induced process in which HSP90 functions as a negative regulator. Under ambient conditions, the chaperone binds to HSF1 monomers and represses their activity, while under proteotoxic stress, HSP90 releases HSF1 to bind and stabilize accumulating unfolded proteins, thereby allowing for trimerization of the transcription factor (Zou et al. 1998). Chlamydomonas HSF1 is, like yeast HSF1, constitutively trimeric and forms a complex with HSP90A and HSP70A under ambient and heat stress conditions (Schmollinger et al. 2013; Schulz-Raffelt et al. 2007; Sorger and Nelson 1989). While the inhibition of Chlamydomonas HSP90A with the drugs geldanamycin or radicicol induces HSP gene expression in the absence of heat stress, and increases and prolongs this response under heat stress, it is not clear whether this effect is caused by cytosolic HSP90A serving as a negative regulator of HSF1 in the alga (Schmollinger et al. 2013). HSP90A could simply cooperate with cytosolic HSP70A in the maturation or stabilization of HSF1, so the induction of an UPR by inactivating cellular HSP90s may instead be explained by increased unfolding and misfolding of released HSP90 clients (Schroda et al. 2015).
List of important protein factors involved in unfolded protein responses in Chlamydomonas.
| Name | Function | Localization | References for dedicated studies |
|---|---|---|---|
| – Molecular chaperones | |||
|
|
|||
| BIP1 (Binding immunoglobulin protein 1) | HSP70 molecular chaperone | er | Dìaz-Troya et al. (2008, 2011) |
| CLPB3 (Caseinolytic peptidase B protein homolog 3) | Removal of heat-induced protein aggregates | cp | Kreis et al. (2023a) |
| HSP22E/F (Heat shock protein 22 E/F) | Small heat shock proteins; intercalate into protein aggregates | cp | Rütgers et al. (2017a), Perlaza et al. (2019), Theis et al. (2020) |
| HSP70A (Heat shock protein 70 A) | Potential involvement in stabilization or maturation of HSF1 | cyt | Schulz-Raffelt et al. (2007), Schmollinger et al. (2013), Rütgers et al. (2017b) |
| HSP70B/CDJ2 (Heat shock protein 70 B/chloroplast DnaJ homolog 2) | Plastidic chaperone pair; bind to VIPP1/2 | cp | Liu et al. (2005, 2007) |
| HSP90A (Heat shock protein 90 A) | Potential involvement in negative regulation, stabilization or maturation of HSF1 | cyt | Schulz-Raffelt et al. (2007), Schmollinger et al. (2013), Rütgers et al. (2017b) |
|
|
|||
| – Proteases | |||
|
|
|||
| ClpP (Caseinolytic peptidase P) | ATP-dependent serine protease | cp | Ramundo et al. (2014) |
| DEG1C (Degradation of periplasmic proteins 1 C) | ATP-independent serine endopeptidase | cp | Theis et al. (2019) |
| FTSH2 (Filamentous temperature-sensitive 2) | ATP-dependent zinc metallopeptidase | cp | Malnoe et al. (2014), Wang et al. (2017) |
|
|
|||
| – Transcription factors | |||
|
|
|||
| BLZ8 (Basic leucine zipper 1) | Induction of carbon-concentrating mechanism to confer oxidative stress tolerance | nuc | Choi et al. (2022) |
| bZIP1 (Basic leucine zipper 1) | Regulation of ER-stress related gene expression | er/nuc | Yamaoka et al. (2018, 2019) |
| HSF1 (Heat shock factor 1) | Master regulator of Chlamydomonas heat shock response; regulation of HSP gene expression | cyt/nuc | Schulz-Raffelt et al. (2007), Schmollinger et al. (2010) |
|
|
|||
| – Other | |||
|
|
|||
| ALB3.2 (ALBINO3.2) | Membrane protein insertase; important for thylakoid biogenesis | cp | Göhre et al. (2006) |
| ERG5 (C-22 sterol desaturase) | Sterol biosynthesis; maintaining of ER membrane fluidity and permeability | cyt | Je et al. (2024) |
| IRE1 (Inositol-requiring enzyme 1) | Transmembrane endoribonuclease that splices mRNA encoding bZIP1 during proteotoxic ER stress | er | Yamaoka et al. (2018, 2019) |
| MARS1 (Mutant affected in chloroplast-to-nucleus retrograde signaling 1) | Retrograde signaling kinase of cpUPR | cyt | Perlaza et al. (2019) |
| VIPP1/2 (Vesicle-inducing protein in plastids 1/2) | Thylakoid biogenesis (VIPP1); sensors of SCE stress in chloroplast membranes | cp | Nordhues et al. (2012), Theis et al. (2020), Kreis et al. (2023b) |
| VPL1–11 (VIPP proximity labeling 1–11) | Potential VIPP1/2 interaction partners | cp | Kreis et al. (2023b) |
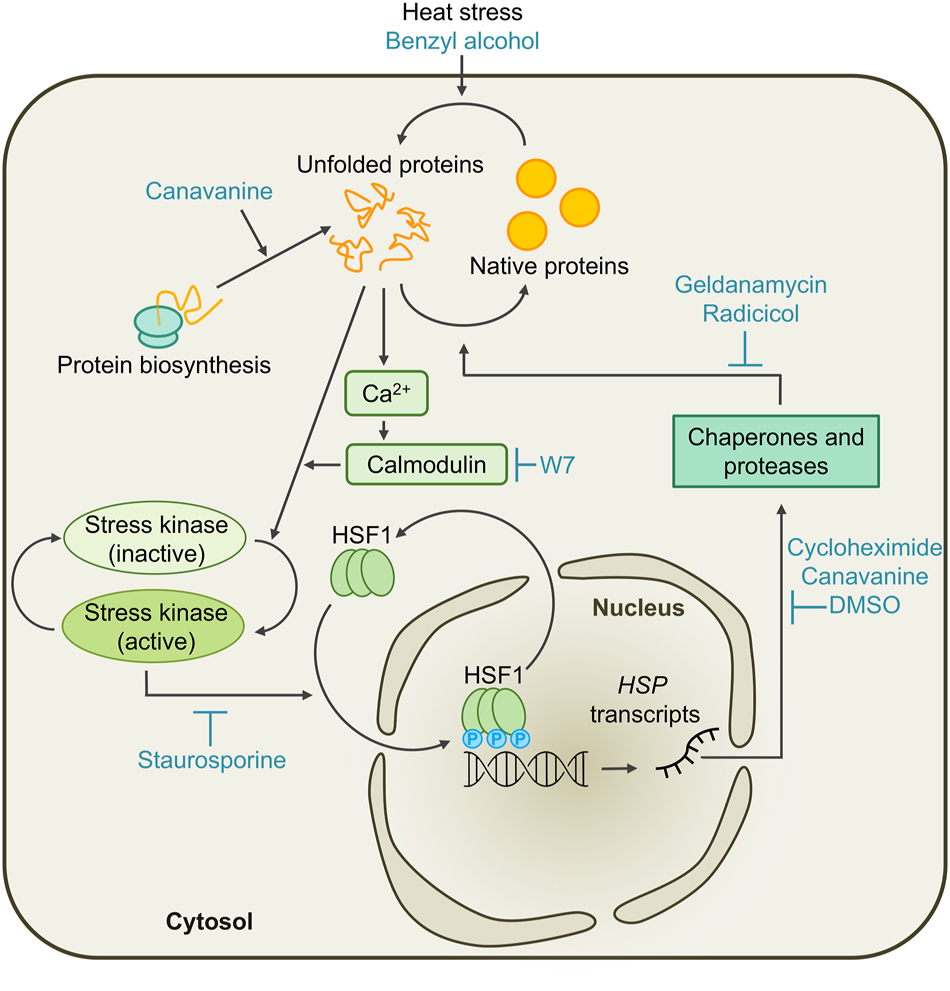
Working model for the cytosolic unfolded protein response (cytUPR) in Chlamydomonas. Un- and misfolded proteins accumulate in the cytosol if the folding capacity of the cell is exceeded by increased protein biosynthesis, heat stress, or by feeding the cells with canavanine or benzyl alcohol. This leads to the activation of stress kinases in a calcium-dependent manner, which is perturbed by the calmodulin antagonist W7. With the consequent hyperphosphorylation of constitutive HSF1 trimers by stress kinases, which can be inhibited by staurosporine, HSF1 obtains transcriptional competence and induces the transcription of HSP genes encoding molecular chaperones and proteases. The production of these HSPs is disturbed/inhibited by cycloheximide, canavanine, or DMSO. The chaperones and proteases assist in protein refolding and degrade terminally misfolded proteins, thereby restoring protein homeostasis. This process is slowed down by inhibition of HSP90 through the pharmacological treatment with geldanamycin or radicicol. Once equilibrium is restored, HSF1 is inactivated in a process termed attenuation.
In both yeast and Chlamydomonas, the constitutively trimeric HSF1 occupies the HSEs of target genes even under ambient conditions, yet, in most cases, their transcription is only activated after HSF1 is hyperphosphorylated during proteotoxic stress, as indicated by the correlation between the accumulation of HSP gene transcripts and the hyperphosphorylation of HSF1 in mammals, yeast, and Chlamydomonas (Cotto et al. 1996; Schulz-Raffelt et al. 2007; Sorger and Pelham 1988). When protein kinase activity and, consequently, HSF1 phosphorylation is inhibited through staurosporine feeding, HSP transcript accumulation is also reduced (Schmollinger et al. 2013). A decrease in HSP levels under elevated temperatures could be observed upon treatment with the calmodulin antagonist W7 as well, which shows that the activation of Chlamydomonas HSF1 by stress kinases occurs in a calcium-dependent manner (Figure 1). Since neither the removal of extracellular calcium by EGTA nor the inhibition of calcium transporters in the plasma membrane affected HSP levels during elevated temperatures, this process appears to rely mostly on calcium stores inside of the Chlamydomonas cell (Rütgers et al. 2017b; Schmollinger et al. 2013).
In plants, existing evidence suggests that the calcium signal required for stress kinase activation and subsequent HSF hyperphosphorylation is mediated by the increased fluidity of the plasma membrane under heat stress (Saidi et al. 2009, 2010). While the membrane is highly sensitive for changes in temperature, it is unclear how the protein folding state of the cell reflects on membrane fluidity, especially since protein homeostasis is supposed to be a self-regulating process. In Chlamydomonas, increased un- and misfolding of proteins could be observed when cells grown at ambient temperatures were supplemented with the membrane fluidizer benzyl alcohol, although it is unclear whether this is a result of the increased membrane fluidity or the reagent’s denaturing effect on soluble proteins (Rütgers et al. 2017b). Further experimental evidence suggests that the activation of calcium currents and stress kinases is caused by the accumulation of un- and misfolded proteins (Rütgers et al. 2017b). This might happen through an activation of calcium channels by the direct interaction of non-native proteins with the membrane via exposed hydrophobic surfaces. Alternatively, negative regulators of calcium channels and/or stress kinases could unfold during stress, either because they are intrinsically unstable or require molecular chaperones to be kept in a functional state.
Even under non-stress conditions, HSF1 regulates the expression of some HSP genes, including HSP70A and HSP90A. Accordingly, HSF1 occupies the promoter of HSP70A in the absence of stress (Lodha and Schroda 2005; Strenkert et al. 2011, 2013), and a depletion of HSF1 in Chlamydomonas cells led to lower levels of HSP90A under ambient conditions (Schmollinger et al. 2013). Furthermore, the accumulation of both chaperones increased 1.8-fold and more than 2.5-fold at 30 °C and 35 °C, respectively, compared with 25 °C (Rütgers et al. 2017b). This points to the gradual activation of HSF1 with rising temperature, but the exact mechanism with which this is achieved is unknown. Like in land plants, it might involve H2O2, since H2O2 levels in the cytosol and nucleus of Chlamydomonas also gradually increase with temperature (Niemeyer et al. 2021; Volkov et al. 2006).
Once sufficient amounts of molecular chaperones and proteases have been synthesized, HSF1 is inactivated in a process called attenuation. This can occur while heat stress is still ongoing, but not if protein biosynthesis is disturbed by feeding the cells with the cytosolic protein synthesis inhibitors cycloheximide or DMSO, or with the arginine analog canavanine (Schmollinger et al. 2013). This once again indicates that the accumulation of unfolded proteins and not heat is the trigger of the cytosolic UPR in Chlamydomonas, and that the reestablishment of protein homeostasis by the chaperone network inside the cell feeds back on HSF1 activity.
4 Compartment-specific unfolded protein responses
4.1 The ER-specific UPR in Chlamydomonas is regulated by a highly conserved eukaryotic stress sensor
ER stress occurs if the protein folding ability of the organelle, which is regulated by a variety of molecular chaperones and enzymes, is disrupted by internal factors or adverse environmental conditions. It is characterized by the accumulation of unfolded and misfolded proteins that trigger an ER-specific UPR (erUPR) through intracellular signal transduction between the ER and the nucleus (Ron and Walter 2007). This pathway was first discovered in yeast when the inhibition of protein folding in the ER resulted in the upregulation of several chaperones (Cox et al. 1993). In Chlamydomonas, the erUPR can be triggered by treatment with tunicamycin and dithiothreitol (DTT), inhibitors of N-glycosylation and protein disulfide bond formation, respectively, and results in the specific production of ER-targeted chaperones, including calreticulin 2 (CAL2), J-domain protein PDI6, BIP1 and HSP90B (Pérez-Martín et al. 2014; Traewachiwiphak et al. 2018; Yamaoka et al. 2019).
The erUPR is activated by the transmembrane endoribonuclease inositol-requiring enzyme 1 (IRE1), an ER stress sensor that is highly conserved in many eukaryotes (Cox et al. 1993). Proteotoxic stress in the ER of Chlamydomonas causes this dual-function protein to dimerize and to autophosphorylate, activating its RNase activity. Active IRE1 splices the mRNA encoding the basic leucine zipper 1 (bZIP1) transcription factor. This results in the loss of the transmembrane domain that anchors bZIP1 to the ER membrane, targeting it to the nucleus instead, where it initiates the transcription of ER stress-related genes to reestablish protein homeostasis in the compartment (Figure 2) (Yamaoka et al. 2018).
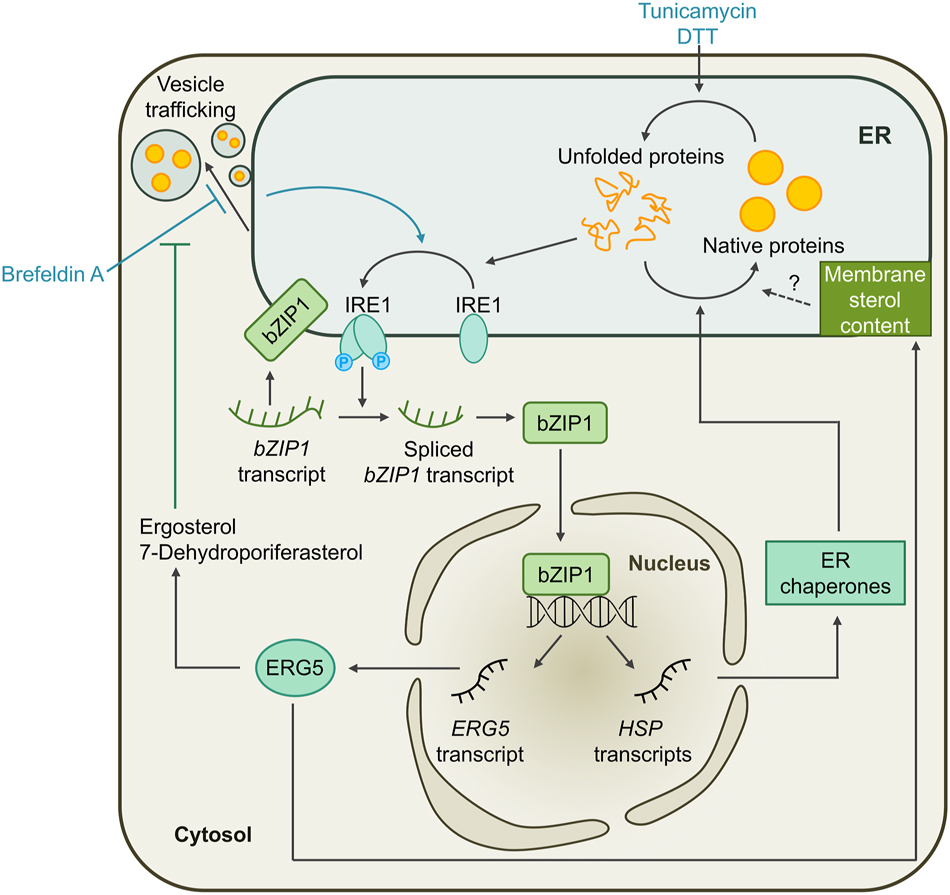
Working model for the unfolded protein response in the ER (erUPR) of Chlamydomonas. Treatment of Chlamydomonas cells with tunicamycin or DTT leads to the accumulation of unfolded proteins in the ER. This induces the dimerization and autophosphorylation of the membrane-localized ER stress sensor IRE1, activating its RNase activity. Active IRE1 splices the mRNA encoding the bZIP1 transcription factor, resulting in the loss of its transmembrane domain and targeting it to the nucleus. There, it initiates the transcription of ER stress-related genes, including molecular chaperones that restore protein homeostasis in the ER. Alternatively, ER stress can be induced by the inhibition of ER-Golgi vesicle trafficking by brefeldin A treatment. Restricted ER-Golgi trafficking also leads to the activation of the IRE1/bZIP1 pathway and the transcription of ERG5, encoding a C-22 sterol desaturase. ERG5 counteracts the effect of brefeldin A by synthesis of ergosterol and 7-dehydroporiferasterol. It also alters the sterol content in the ER membrane which might play a role in reestablishing protein homeostasis.
Under tunicamycin-induced ER stress, the IRE1/bZIP1 pathway in Chlamydomonas also regulates sterol biosynthesis through expression of ERG5, encoding a C-22 sterol desaturase, to maintain ER membrane fluidity and permeability, and to restore homeostasis (Figure 2) (Je et al. 2024). Notably, the same drug treatment had little to no effect on the fatty acid content in Arabidopsis seedlings, despite also inducing ER stress (Je et al. 2023; Yamaoka et al. 2019). The production of the primary sterols in Chlamydomonas, ergosterol and 7-dehydroporiferasterol, was significantly reduced in erg5 mutants. Furthermore, these mutants showed hypersensitivity to the drug brefeldin A which disrupts ER-Golgi trafficking and causes the accumulation of proteins in the lumen of the ER. Induction of ER stress by feeding cells with tunicamycin and dithiothreitol did not have the same effect on the mutants, although sterol biosynthesis was increased under influence of these drugs as well. This indicates that sterols might play an essential role in the coping with ER stress caused specifically by restricted vesicle trafficking (Je et al. 2024).
4.2 Mitochondrial UPR pathways exist in Arabidopsis, but have not been studied in Chlamydomonas yet
The mitochondrial proteome is composed of both nuclear- and mitochondrial-encoded proteins, making mitochondria prone to proteotoxic stress induced by mitonuclear protein imbalances. A mitochondrial UPR (mtUPR) was first observed in mammalian cells when damage to the mitochondrial DNA through exposure to ethidium bromide led to the transcriptional upregulation of nuclear genes encoding a set of mitochondrial chaperones and proteases, while transcript levels of ER-localized chaperones remained unaffected (Zhao et al. 2002). This pathway has been studied extensively in Caenorhabditis elegans, mammalian model systems and yeast, revealing that the eukaryotic kingdoms possesses their own sets of mtUPR regulators that activate nuclear genes to restore mitochondrial proteostasis (reviewed by Tran and Van Aken 2020). However, only little is known about the mtUPR regulation in plants.
In Arabidopsis, an imbalance between nuclear- and mitochondrial-encoded proteins could be induced in ribosomal protein L1 mrpl1 mutant lines or through application of the mitochondrial translation inhibitor doxycycline. The resulting transient oxidative burst activated the mtUPR, leading to the activation of mitogen-activated protein kinases (MAPKs) to restore mitochondrial function. The phytohormones ethylene, auxin and jasmonate appear to serve as essential mediators in this pathway (Wang and Auwerx 2017). Additionally, it has been shown that responses to multiple mitochondrial proteotoxic stresses are regulated by the ER-localized transcription factor Arabidopsis NAC domain containing protein 17 (ANAC017), with resistance or susceptibility to mitochondrial stress-promoting treatments being increased in ANAC017 gain- and loss-of-function mutants, respectively (Kacprzak et al. 2020; Meng et al. 2019). So far, there have been no studies with focus on the mtUPR in Chlamydomonas, but due to the discovery of a conserved plant-specific mtUPR pathway in Arabidopsis, it is likely that mitochondrial proteotoxic stress elicits specific responses in the alga as well.
4.3 The chloroplast UPR: a plant-specific membrane stress sensing pathway
In plants, reduced levels of certain chloroplast-localized chaperones and proteases lead to the transcriptional upregulation of other chloroplast chaperones and proteases, indicating that the loss of compartment-specific proteostasis network components disturbs the folding state and triggers a chloroplast-specific UPR (cpUPR). This was first observed in Arabidopsis mutants with impaired protein degradation due to reduced levels of chloroplast HSP100 members CLPC and CLPD. The consequent accumulation of HSP100 substrates perturbs chloroplast protein homeostasis, which the cell attempts to restore by upregulating chloroplast-targeted chaperones from the HSP60, HSP70 and HSP90 families (Shanklin et al. 1995; Sjögren et al. 2004). In Chlamydomonas, similar observations could be made when levels of chloroplast HSP70B were reduced through expression of an antisense RNA targeting the 5′ UTR of the HSP70B gene. Under heat stress, the synthesis of HSPs was deregulated in these cells, likely due to their constant experiencing mild protein folding stress caused by the lack of HSP70B, which resulted in their desensitization and inability to fully react to a sudden temperature shift (Schmollinger et al. 2013). The inducible depletion of chloroplast-encoded ClpP1 in Chlamydomonas delivered further evidence for the existence of the cpUPR, as these cells showed strong transcriptional upregulation of nuclear genes encoding chloroplast chaperones HSP70B, HSP22E/F, CLPB3 as well as the chloroplast-localized proteases FTSH2 and DEG1C (Ramundo et al. 2014).
How the sensing of un- and misfolded proteins or protein aggregates in the chloroplast is achieved is unclear, but findings from both Arabidopsis and Chlamydomonas suggest that the perception of non-native proteins may involve the chloroplast membranes. In Arabidopsis mutants with impaired ClpP function, precursors and breakdown products of LHCII accumulate in the thylakoid membrane (Kim et al. 2013; Rudella et al. 2006). In Chlamydomonas, the depletion of ClpP1 leads to the upregulation of VIPP1 and ALB3.2, two proteins involved in thylakoid biogenesis, as well as VIPP2 (Göhre et al. 2006; Ramundo et al. 2014). The upregulation of VIPP1 and VIPP2 is also induced by various chloroplast stress triggers, including high light, H2O2, NiCl, inhibition of chloroplast fatty acid synthesis, depletion of chloroplast protease DEG1C, and depletion of thylakoid membrane protein transport components ALB3.2 or SECA (Figure 3) (Blaby-Haas et al. 2016; Blaby et al. 2015; Göhre et al. 2006; Heredia-Martínez et al. 2018; Perlaza et al. 2019; Ramundo et al. 2014; Theis et al. 2019, 2020). VIPP1 is constitutively expressed, while VIPP2 levels are low under ambient conditions and increase strongly during stress conditions. Both VIPPs are unlikely to play a role in protein folding, as they contain an N-terminal amphipathic α-helix (AHa) of 24 amino acids that mediates membrane binding upon oligomerization of the proteins (Gupta et al. 2021; Jovanovic et al. 2014; McDonald et al. 2015, 2017; Otters et al. 2013; Pan et al. 2024). A major prerequisite for membrane binding is stored curvature elastic (SCE) stress, while the presence of anionic lipids plays only a minor role. The accumulation of un- or misfolded membrane proteins are one trigger of SCE stress (McDonald et al. 2015). Under conditions that promote the unfolding or misassembly of membrane proteins, VIPPs might therefore function as sensors of SCE stress in chloroplast membranes and participate in the recruitment of molecular chaperones and proteases to alleviate SCE stress (Figure 3) (Theis et al. 2020). This is supported by the observation that VIPP2 is found in membrane-associated complexes together with VIPP1, HSP22E/F and HSP70B during H2O2 treatment. In vipp2 mutants, the induction of HSP22E/F gene expression was impaired, which is another indication of VIPP2’s role as a SCE stress sensor (Theis et al. 2020). As Arabidopsis lacks VIPP2 and does not upregulate VIPP1 during chloroplast stress conditions, it is unclear if the sensing of non-native proteins or protein aggregates involves other mechanisms, or if it is mediated by constitutively expressed VIPP1. In Chlamydomonas, the proxiomes of VIPP1/2 have been identified through in vivo proximity labeling and comprise 12 proteins whose transcription has been previously found to be upregulated under chloroplast stress conditions. They include HSP70B and CDJ2, as well as 10 proteins without clear functional annotations, named VPL1–11 (VPL2 and VPL8 are two parts of the same protein). As likely interaction partners of the VIPPs, they might be involved in coping with chloroplast membrane stress and mediating retrograde signaling for the cpUPR (Figure 3) (Kreis et al. 2023b).
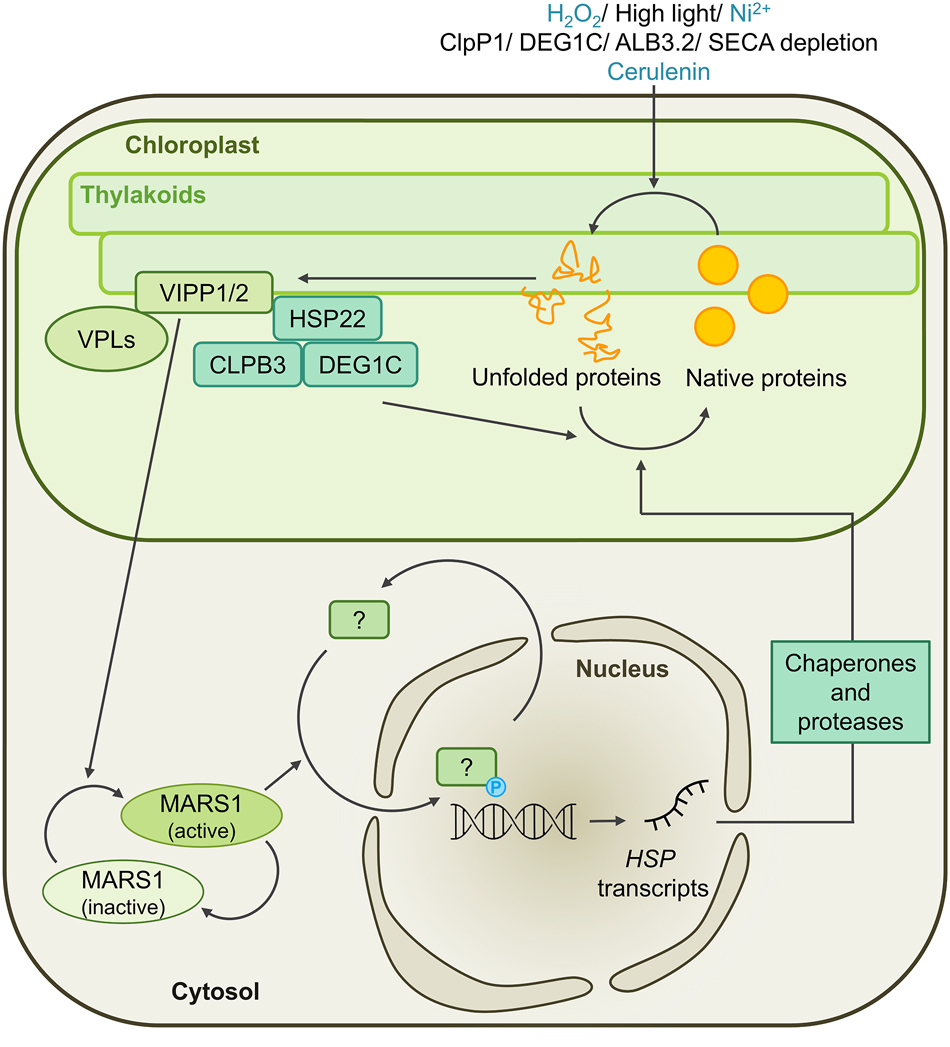
Working model for the unfolded protein response in the chloroplast (cpUPR) of Chlamydomonas. The accumulation of un- and misfolded proteins in or at the chloroplast membranes is triggered by treatment with H2O2, exposure to high light, nickel ions, or chloroplast fatty acid synthase inhibitor cerulenin, or through depletion of the chloroplast chaperones ClpP1 or DEG1C, or the thylakoid membrane protein transport components ALB3.2 or SECA. The resulting stored curvature elastic (SCE) stress in chloroplast membranes is sensed and coped with by VIPP proteins and potentially their interaction candidates, the VPL proteins. A retrograde signaling cascade involving the MARS1 kinase is activated and results in the transcriptional upregulation of chloroplast-targeted chaperones and proteases, such as HSP22E/F, CLPB3 and DEG1C, as well as of other proteins involved in the acclimation to high light.
One important component involved in the retrograde signaling cascade that leads to the upregulation of nuclear genes encoding chloroplast chaperones and proteases is the cytosolic MARS1 kinase (Figure 3). Levels of VIPP2, HSP22E/F, CLPB3 and DEG1C do not increase upon depletion of ClpP1 or high light conditions in mutants lacking MARS1. The expression of a constitutively active MARS1 variant, on the other hand, led to the constant transcription of this gene set and made cells more resistant to high light exposure or increased H2O2 production induced by metronidazole (Perlaza et al. 2019). In mutants lacking one of the effector proteins of the cpUPR, DEG1C, the synthesis of VIPP1, ALB3.2 and the FTSH protease increased. Additionally, even during growth at low light intensities, higher levels of proteins involved in high light acclimation were produced, including components of the carbon concentrating mechanism, photorespiration or antioxidant defense. The misfolded proteins accumulating due to the absence of DEG1C appear to be interpreted as the consequence of high light damage, thus inducing a high light acclimation response (Theis et al. 2019). This response might be mediated by the bZIP-type transcription factor BLZ8 that functions in oxidative stress signaling in the chloroplast of Chlamydomonas and has been shown to induce the carbon concentrating mechanism (Choi et al. 2022). Although chloroplast CLPB3 levels did not increase in the deg1c mutant, DEG1C levels did increase to compensate for the knock-out of CLPB3. CLPB3 is required for conferring thermotolerance under severe heat stress conditions as it plays an important role in resolving heat-induced protein aggregates. Its loss is not only counteracted by increasing protease activity, but also by reducing protein synthesis capacity, as indicated by a lower abundance of the PRPL1 plastid ribosomal subunit (Kreis et al. 2023a).
5 Concluding remarks
In the green model alga Chlamydomonas, the study of the response to proteotoxic stress through different genetic, transcriptomic and proteomic approaches has provided valuable insights into the mechanisms of protein homeostasis, thus elucidating the function of molecular chaperone and protease networks and revealing conserved eukaryotic regulators of the UPR, including the key transcription factor of the cytosolic UPR HSF1 and the IRE1/bZIP1 pathway in the ER. Over the last decade, there has also been notable progress in identifying key sensors, signaling components and effectors of the chloroplast UPR and their interaction partners in this model system. Since the gene families in Chlamydomonas are closely related to those found in land plants, the obtained knowledge can be transferred and conclusions on the evolution of plant UPRs can be drawn. With the availability of genomic editing and in vivo proximity labeling, the research on this topic will progress further in the future, potentially yielding entry points for the genetic editing of crop plants to make them more resilient to environmental stresses.
Funding source: Deutsche Forschungsgemeinschaft
Award Identifier / Grant number: SCHR 617/14-1
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: We would like to thank the DFG for funding (SCHR 617/14-1).
-
Data availability: Not applicable.
References
Åkerfelt, M., Morimoto, R.I., and Sistonen, L. (2010). Heat shock factors: integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 11: 545–555, https://doi.org/10.1038/nrm2938.Search in Google Scholar PubMed PubMed Central
Balchin, D., Hayer-Hartl, M., and Hartl, F.U. (2016). In vivo aspects of protein folding and quality control. Science 353: aac4354, https://doi.org/10.1126/science.aac4354.Search in Google Scholar PubMed
Blaby-Haas, C.E., Castruita, M., Fitz-Gibbon, S.T., Kropat, J., and Merchant, S.S. (2016). Ni induces the CRR1-dependent regulon revealing overlap and distinction between hypoxia and Cu deficiency responses in Chlamydomonas reinhardtii. Metallomics 8: 679–691, https://doi.org/10.1039/c6mt00063k.Search in Google Scholar PubMed PubMed Central
Blaby, I.K., Blaby-Haas, C.E., Pérez-Pérez, M.E., Schmollinger, S., Fitz-Gibbon, S., Lemaire, S.D., and Merchant, S.S. (2015). Genome-wide analysis on Chlamydomonas reinhardtii reveals the impact of hydrogen peroxide on protein stress responses and overlap with other stress transcriptomes. Plant J. 84: 974–988, https://doi.org/10.1111/tpj.13053.Search in Google Scholar PubMed PubMed Central
Cannon, W.B. (1929). Organization for physiological homeostasis. Physiol. Rev. 9: 399–431, https://doi.org/10.1152/physrev.1929.9.3.399.Search in Google Scholar
Cashikar, A.G., Duennwald, M., and Lindquist, S.L. (2005). A chaperone pathway in protein disaggregation: HSP26 alters the nature of protein aggregates to facilitate reactivation by HSP104. J. Biol. Chem. 280: 23869–23875, https://doi.org/10.1074/jbc.m502854200.Search in Google Scholar
Choi, B.Y., Kim, H., Shim, D., Jang, S., Yamaoka, Y., Shin, S., Yamano, T., Kajikawa, M., Jin, E., Fukuzawa, H., et al.. (2022). The Chlamydomonas bZIP transcription factor BLZ8 confers oxidative stress tolerance by inducing the carbon-concentrating mechanism. Plant Cell 34: 910–926, https://doi.org/10.1093/plcell/koab293.Search in Google Scholar PubMed PubMed Central
Cotto, J.J., Kline, M., and Morimoto, R.I. (1996). Activation of heat shock factor 1 DNA binding precedes stress-induced serine phosphorylation: evidence for a multistep pathway of regulation. J. Biol. Chem. 271: 3355–3358, https://doi.org/10.1074/jbc.271.7.3355.Search in Google Scholar PubMed
Cox, J.S., Shamu, C.E., and Walter, P. (1993). Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 73: 1197–1206, https://doi.org/10.1016/0092-8674(93)90648-a.Search in Google Scholar PubMed
Crozet, P., Navarro, F.J., Willmund, F., Mehrshahi, P., Bakowski, K., Lauersen, K.J., Pérez-Pérez, M.E., Auroy, P., Gorchs Rovira, A., Sauret-Gueto, S., et al.. (2018). Birth of a photosynthetic chassis: a MoClo toolkit enabling synthetic biology in the microalga Chlamydomonas reinhardtii. ACS Synth. Biol. 7: 2074–2086, https://doi.org/10.1021/acssynbio.8b00251.Search in Google Scholar PubMed
Dìaz-Troya, S., Florencio, F.J., and Crespo, J.L. (2008). Target of rapamycin and LST8 proteins associate with membranes from the endoplasmic reticulum in the unicellular green alga Chlamydomonas reinhardtii. Eukaryot. Cell 7: 212–222, https://doi.org/10.1128/ec.00361-07.Search in Google Scholar PubMed PubMed Central
Dìaz-Troya, S., Perez-Perez, M.E., Perez-Martin, M., Moes, S., Jenoe, P., Florencio, F.J., and Crespo, J.L. (2011). Inhibition of protein synthesis by TOR inactivation revealed a conserved regulatory mechanism of the BiP chaperone in Chlamydomonas. Plant Physiol. 157: 730–741, https://doi.org/10.1104/pp.111.179861.Search in Google Scholar PubMed PubMed Central
Göhre, V., Ossenbühl, F., Crèvecoeur, M., Eichacker, L.A., and Rochaix, J.D. (2006). One of two Alb3 proteins is essential for the assembly of the photosystems and for cell survival in Chlamydomonas. Plant Cell 18: 1454–1466, https://doi.org/10.1105/tpc.105.038695.Search in Google Scholar PubMed PubMed Central
Gupta, T.K., Klumpe, S., Gries, K., Heinz, S., Wietrzynski, W., Ohnishi, N., Niemeyer, J., Spaniol, B., Schaffer, M., Rast, A., et al.. (2021). Structural basis for VIPP1 oligomerization and maintenance of thylakoid membrane integrity. Cell 184: 3643–3659, https://doi.org/10.1016/j.cell.2021.05.011.Search in Google Scholar PubMed
Hayer-Hartl, M., Bracher, A., and Hartl, F.U. (2016). The GroEL-GroES chaperonin machine: a nano-cage for protein folding. Trends Biochem. Sci. 41: 62–76, https://doi.org/10.1016/j.tibs.2015.07.009.Search in Google Scholar PubMed
Hemme, D., Veyel, D., Mühlhaus, T., Sommer, F., Jüppner, J., Unger, A.K., Sandmann, M., Fehrle, I., Schönfelder, S., Steup, M., et al.. (2014). Systems-wide analysis of acclimation responses to long-term heat stress and recovery in the photosynthetic model organism Chlamydomonas reinhardtii. Plant Cell 26: 4270–4297, https://doi.org/10.1105/tpc.114.130997.Search in Google Scholar PubMed PubMed Central
Heredia-Martínez, L.G., Andrés-Garrido, A., Martínez-Force, E., Pérez-Pérez, M.E., and Crespo, J.L. (2018). Chloroplast damage induced by the inhibition of fatty acid synthesis triggers autophagy in Chlamydomonas. Plant Physiol. 178: 1112–1129, https://doi.org/10.1104/pp.18.00630.Search in Google Scholar PubMed PubMed Central
Hightower, L.E. and White, F.P. (1981). Cellular responses to stress: comparison of a family of 71–73‐kilodalton proteins rapidly synthesized in rat tissue slices and canavanine‐treated cells in culture. J. Cell. Physiol. 108: 261–275, https://doi.org/10.1002/jcp.1041080216.Search in Google Scholar PubMed
Hipp, M.S., Park, S.H., and Hartl, U.U. (2014). Proteostasis impairment in protein-misfolding and -aggregation diseases. Trends Cell Biol. 24: 506–514, https://doi.org/10.1016/j.tcb.2014.05.003.Search in Google Scholar PubMed
Howell, S.H. (2021). Evolution of the unfolded protein response in plants. Plant Cell Environ. 44: 2625–2635, https://doi.org/10.1111/pce.14063.Search in Google Scholar PubMed
Hübel, A. and Schöffl, F. (1994). Arabidopsis heat shock factor: isolation and characterization of the gene and the recombinant protein. Plant Mol. Biol. 26: 353–362, https://doi.org/10.1007/bf00039545.Search in Google Scholar PubMed
Je, S., Choi, B.Y., Kim, E., Kim, K., Lee, Y., and Yamaoka, Y. (2024). Sterol biosynthesis contributes to brefeldin-A-induced endoplasmic reticulum stress resistance in Chlamydomonas reinhardtii. Plant Cell Physiol. 65: 916–927, https://doi.org/10.1093/pcp/pcad131.Search in Google Scholar PubMed
Je, S., Lee, Y., and Yamaoka, Y. (2023). Effect of common ER stress-inducing drugs on the growth and lipid phenotypes of chlamydomonas and Arabidopsis. Plant Cell Physiol. 64: 392–404, https://doi.org/10.1093/pcp/pcac154.Search in Google Scholar PubMed
Jovanovic, G., Mehta, P., McDonald, C., Davidson, A.C., Uzdavinys, P., Ying, L., and Buck, M. (2014). The N-terminal amphipathic helices determine regulatory and effector functions of phage shock protein A (PspA) in Escherichia coli. J. Mol. Biol. 426: 1498–1511, https://doi.org/10.1016/j.jmb.2013.12.016.Search in Google Scholar PubMed
Kacprzak, S.M., Dahlqvist, A., and Van Aken, O. (2020). The transcription factor ANAC017 is a key regulator of mitochondrial proteotoxic stress responses in plants. Philos. Trans. R Soc. Lond. B Biol. Sci. 375: 20190411, https://doi.org/10.1098/rstb.2019.0411.Search in Google Scholar PubMed PubMed Central
Kim, J., Olinares, P.D., Oh, S.H., Ghisaura, S., Poliakov, A., Ponnala, L., and van Wijk, K.J. (2013). Modified Clp protease complex in the ClpP3 null mutant and consequences for chloroplast development and function in Arabidopsis. Plant Physiol. 162: 157–179, https://doi.org/10.1104/pp.113.215699.Search in Google Scholar PubMed PubMed Central
Kobayashi, Y., Harada, N., Nishimura, Y., Saito, T., Nakamura, M., Fujiwara, T., Kuroiwa, T., and Misumi, O. (2014). Algae sense exact temperatures: small heat shock proteins are expressed at the survival threshold temperature in Cyanidioschyzon merolae and Chlamydomonas reinhardtii. Genome Biol. Evol. 6: 2731–2740, https://doi.org/10.1093/gbe/evu216.Search in Google Scholar PubMed PubMed Central
Kreis, E., Niemeyer, J., Merz, M., Scheuring, D., and Schroda, M. (2023a). CLPB3 is required for the removal of chloroplast protein aggregates and thermotolerance in Chlamydomonas. J. Exp. Bot. 74: 3714–3728, https://doi.org/10.1093/jxb/erad109.Search in Google Scholar PubMed PubMed Central
Kreis, E., König, K., Misir, M., Niemeyer, J., Sommer, F., and Schroda, M. (2023b). TurboID reveals the proxiomes of Chlamydomonas proteins involved in thylakoid biogenesis and stress response. Plant Physiol. 193: 1772–1796, https://doi.org/10.1093/plphys/kiad335.Search in Google Scholar PubMed PubMed Central
Lau, C.S., Dowle, A., Thomas, G.H., Girr, P., and Mackinder, L.C.M. (2023). A phase-separated CO2-fixing pyrenoid proteome determined by TurboID in Chlamydomonas reinhardtii. Plant Cell 35: 3260–3279, https://doi.org/10.1093/plcell/koad131.Search in Google Scholar PubMed PubMed Central
Li, X., Zhang, R., Patena, W., Gang, S.S., Blum, S.R., Ivanova, N., Yue, R., Robertson, J.M., Lefebvre, P.A., Fitz-Gibbon, S.T., et al.. (2015). An indexed, mapped mutant library enables reverse genetics studies of biological processes in Chlamydomonas reinhardtii. Plant Cell 28: 367–387, https://doi.org/10.1105/tpc.15.00465.Search in Google Scholar PubMed PubMed Central
Lindquist, S.L. and Craig, E.A. (1988). The heat-shock proteins. Annu. Rev. Genet. 22: 631–677, https://doi.org/10.1146/annurev.ge.22.120188.003215.Search in Google Scholar PubMed
Liu, C., Willmund, F., Golecki, J.R., Cacace, S., Hess, B., Markert, C., and Schroda, M. (2007). The chloroplast HSP70B-CDJ2-CGE1 chaperones catalyse assembly and disassembly of VIPP1 oligomers in Chlamydomonas. Plant J. 50: 265–277, https://doi.org/10.1111/j.1365-313x.2007.03047.x.Search in Google Scholar PubMed
Liu, C., Willmund, F., Whitelegge, J.P., Hawat, S., Knapp, B., Lodha, M., and Schroda, M. (2005). J-domain protein CDJ2 and HSP70B are a plastidic chaperone pair that interacts with vesicle-inducing protein in plastids 1. Mol. Biol. Cell 16: 1165–1177, https://doi.org/10.1091/mbc.e04-08-0736.Search in Google Scholar PubMed PubMed Central
Lodha, M. and Schroda, M. (2005). Analysis of chromatin structure in the control regions of the Chlamydomonas HSP70A and RBCS2 genes. Plant Mol. Biol. 59: 501–513, https://doi.org/10.1007/s11103-005-0450-0.Search in Google Scholar PubMed
Malnoe, A., Wang, F., Girard-Bascou, J., Wollman, F.A., and de Vitry, C. (2014). Thylakoid FtsH protease contributes to photosystem II and cytochrome b6f remodeling in Chlamydomonas reinhardtii under stress conditions. Plant Cell 26: 373–390, https://doi.org/10.1105/tpc.113.120113.Search in Google Scholar PubMed PubMed Central
McDonald, C., Jovanovic, G., Ces, O., and Buck, M. (2015). Membrane stored curvature elastic stress modulates recruitment of maintenance proteins PspA and Vipp1. mBio 6: e01188-15, https://doi.org/10.1128/mbio.01188-15.Search in Google Scholar
McDonald, C., Jovanovic, G., Wallace, B.A., Ces, O., and Buck, M. (2017). Structure and function of PspA and Vipp1 N-terminal peptides: insights into the membrane stress sensing and mitigation. Biochim. Biophys. Acta Biomembr. 1859: 28–39, https://doi.org/10.1016/j.bbamem.2016.10.018.Search in Google Scholar PubMed
Meng, X., Li, L., De Clercq, I., Narsai, R., Xu, Y., Hartmann, A., Claros, D.L., Custovic, E., Lewsey, M.G., Whelan, J., et al.. (2019). ANAC017 coordinates organellar functions and stress responses by reprogramming retrograde signaling. Plant Physiol. 180: 634–653, https://doi.org/10.1104/pp.18.01603.Search in Google Scholar PubMed PubMed Central
Mogk, A., Kummer, E., and Bukau, B. (2015). Cooperation of Hsp70 and Hsp100 chaperone machines in protein disaggregation. Front. Mol. Biosci. 2: 22, https://doi.org/10.3389/fmolb.2015.00022.Search in Google Scholar PubMed PubMed Central
Nathan, D.F., Vos, M.H., and Lindquist, S. (1997). In vivo functions of the Saccharomyces cerevisiae Hsp90 chaperone. Proc. Natl. Acad. Sci. U. S. A. 94: 12949–12956, https://doi.org/10.1073/pnas.94.24.12949.Search in Google Scholar PubMed PubMed Central
Niemeyer, J., Scheuring, D., Oestreicher, J., Morgan, B., and Schroda, M. (2021). Real-time monitoring of subcellular H2O2 distribution in Chlamydomonas reinhardtii. Plant Cell 33: 2935–2949, https://doi.org/10.1093/plcell/koab176.Search in Google Scholar PubMed PubMed Central
Nordhues, A., Schöttler, M.A., Unger, A.K., Geimer, S., Schönfelder, S., Schmollinger, S., Rütgers, M., Finazzi, G., Soppa, B., Sommer, F., et al.. (2012). Evidence for a role of VIPP1 in the structural organization of the photosynthetic apparatus in Chlamydomonas. Plant Cell 24: 637–659, https://doi.org/10.1105/tpc.111.092692.Search in Google Scholar PubMed PubMed Central
Otters, S., Braun, P., Hubner, J., Wanner, G., Vothknecht, U.C., and Chigri, F. (2013). The first α-helical domain of the vesicle-inducing protein in plastids 1 promotes oligomerization and lipid binding. Planta 237: 529–540, https://doi.org/10.1007/s00425-012-1772-1.Search in Google Scholar PubMed
Pan, S., Gries, K., Engel, B.D., Schroda, M., Haselwandter, C.A., and Scheuring, S. (2024). The cyanobacterial protein VIPP1 forms ESCRT-III-like structures on lipid bilayers. Nat. Struct. Mol. Biol., https://doi.org/10.1038/s41594-024-01367-7.Search in Google Scholar PubMed PubMed Central
Pérez-Martín, M., Pérez-Pérez, M.E., Lemaire, S.D., and Crespo, J.L. (2014). Oxidative stress contributes to autophagy induction in response to endoplasmic reticulum stress in Chlamydomonas reinhardtii. Plant Physiol. 166: 997–1008, https://doi.org/10.1104/pp.114.243659.Search in Google Scholar PubMed PubMed Central
Perlaza, K., Toutkoushian, H., Boone, M., Lam, M., Iwai, M., Jonikas, M.C., Walter, P., and Ramundo, S. (2019). The Mars1 kinase confers photoprotection through signaling in the chloroplast unfolded protein response. eLlife 8: e49577, https://doi.org/10.7554/elife.49577.Search in Google Scholar
Ramundo, S., Casero, D., Mühlhaus, T., Hemme, D., Sommer, F., Crèvecoeur, M., Rahire, M., Schroda, M., Rusch, J., Goodenough, U., et al.. (2014). Conditional depletion of the Chlamydomonas chloroplast ClpP protease activates nuclear genes involved in autophagy and plastid protein quality control. Plant Cell 26: 2201–2222, https://doi.org/10.1105/tpc.114.124842.Search in Google Scholar PubMed PubMed Central
Ron, D. and Walter, P. (2007). Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8: 519–529, https://doi.org/10.1038/nrm2199.Search in Google Scholar PubMed
Rosenzweig, R., Nillegoda, N.B., Mayer, M.P., and Bukau, B. (2019). The Hsp70 chaperone network. Nat. Rev. Mol. Cell Biol. 20: 665–680, https://doi.org/10.1038/s41580-019-0133-3.Search in Google Scholar PubMed
Rudella, A., Friso, G., Alonso, J.M., Ecker, J.R., and van Wijk, K.J. (2006). Downregulation of ClpR2 leads to reduced accumulation of the ClpPRS protease complex and defects in chloroplast biogenesis in Arabidopsis. Plant Cell 18: 1704–1721, https://doi.org/10.1105/tpc.106.042861.Search in Google Scholar PubMed PubMed Central
Rütgers, M., Muranakam, L.S., Mühlhaus, T., Sommer, F., Thoms, S., Schurig, J., Willmund, F., Schulz-Raffelt, M., and Schroda, M. (2017a). Substrates of the chloroplast small heat shock proteins 22E/F point to thermolability as a regulative switch for heat acclimation in Chlamydomonas reinhardtii. Plant Mol. Biol. 95: 579–591, https://doi.org/10.1007/s11103-017-0672-y.Search in Google Scholar PubMed PubMed Central
Rütgers, M., Muranaka, L.S., Schulz-Raffelt, M., Thoms, S., Schurig, J., Willmund, F., and Schroda, M. (2017b). Not changes in membrane fluidity but proteotoxic stress triggers heat shock protein expression in Chlamydomonas reinhardtii. Plant Cell Environ. 40: 2987–3001, https://doi.org/10.1111/pce.13060.Search in Google Scholar PubMed
Saidi, Y., Finka, A., Muriset, M., Bromberg, Z., Weiss, Y.G., Maathuis, F.J.M., and Goloubinoff, P. (2009). The heat shock response in moss plants is regulated by specific calcium-permeable channels in the plasma membrane. Plant Cell 21: 2829–2843, https://doi.org/10.1105/tpc.108.065318.Search in Google Scholar PubMed PubMed Central
Saidi, Y., Peter, M., Fink, A., Cicekli, C., Vigh, L., and Goloubinoff, P. (2010). Membrane lipid composition affects plant heat sensing and modulates Ca2+-dependent heat shock response. Plant Signal Behav. 5: 1530–1533, https://doi.org/10.4161/psb.5.12.13163.Search in Google Scholar PubMed PubMed Central
Scharf, K.D., Berberich, T., Ebersberger, I., and Nover, L. (2012). The plant heat stress transcription factor (Hsf) family: structure, function and evolution. Biochim. Biophys. Acta 1819: 104–119, https://doi.org/10.1016/j.bbagrm.2011.10.002.Search in Google Scholar PubMed
Schmollinger, S., Schulz-Raffelt, M., Strenkert, D., Veyel, D., Vallon, O., and Schroda, M. (2013). Dissecting the heat stress response in Chlamydomonas by pharmaceutical and RNAi approaches reveals conserved and novel aspects. Mol. Plant 6: 1795–1813, https://doi.org/10.1093/mp/sst086.Search in Google Scholar PubMed
Schmollinger, S., Strenkert, D., and Schroda, M. (2010). An inducible artificial microRNA system for Chlamydomonas reinhardtii confirms a key role for heat shock factor 1 in regulating thermotolerance. Curr. Genet. 56: 383–389, https://doi.org/10.1007/s00294-010-0304-4.Search in Google Scholar PubMed
Schroda, M., Hemme, D., and Mühlhaus, T. (2015). The Chlamydomonas heat stress response. Plant J. 82: 466–480, https://doi.org/10.1111/tpj.12816.Search in Google Scholar PubMed
Schulz-Raffelt, M., Lodha, M., and Schroda, M. (2007). Heat shock factor 1 is a key regulator of the stress response in Chlamydomonas. Plant J. 52: 286–295, https://doi.org/10.1111/j.1365-313x.2007.03228.x.Search in Google Scholar
Shanklin, J., DeWitt, N.D., and Flanagan, J.M. (1995). The stroma of higher plant plastids contain ClpP and ClpC, functional homologs of Escherichia coli ClpP and ClpA: an archetypal two-component ATP-dependent protease. Plant Cell 7: 1713–1722, https://doi.org/10.1105/tpc.7.10.1713.Search in Google Scholar PubMed PubMed Central
Shin, S.E., Lim, J.M., Koh, H.G., Kim, E.K., Kang, N.K., Jeon, S., Kwon, S., Shin, W.S., Lee, B., Hwangbo, K., et al.. (2016). CRISPR/Cas9-induced knockout and knock-in mutations in Chlamydomonas reinhardtii. Sci. Rep. 6: 27810, https://doi.org/10.1038/srep27810.Search in Google Scholar PubMed PubMed Central
Sjögren, L.L.E., MacDonald, T.M., Sutinen, S., and Clarke, A.K. (2004). Inactivation of the clpC1 gene encoding a chloroplast Hsp100 molecular chaperone causes growth retardation, leaf chlorosis, lower photosynthetic activity, and a specific reduction in photosystem content. Plant Physiol. 136: 4114–4126, https://doi.org/10.1104/pp.104.053835.Search in Google Scholar PubMed PubMed Central
Sorger, P.K. and Nelson, H.C.M. (1989). Trimerization of a yeast transcriptional activator via a coiled-coil motif. Cell 59: 807–813, https://doi.org/10.1016/0092-8674(89)90604-1.Search in Google Scholar PubMed
Sorger, P.K. and Pelham, H.R. (1988). Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell 54: 855–864, https://doi.org/10.1016/s0092-8674(88)91219-6.Search in Google Scholar PubMed
Strenkert, D., Schmollinger, S., and Schroda, M. (2013). Heat shock factor 1 counteracts epigenetic silencing of nuclear transgenes in Chlamydomonas reinhardtii. Nucleic Acids Res. 41: 5273–5289, https://doi.org/10.1093/nar/gkt224.Search in Google Scholar PubMed PubMed Central
Strenkert, D., Schmollinger, S., Sommer, F., Schulz-Raffelt, M., and Schroda, M. (2011). Transcription factor-dependent chromatin remodeling at heat shock and copper-responsive promoters in Chlamydomonas reinhardtii. Plant Cell 23: 2285–2301, https://doi.org/10.1105/tpc.111.085266.Search in Google Scholar PubMed PubMed Central
Tanaka, Y., Nishiyama, Y., and Murata, N. (2000). Acclimation of the photosynthetic machinery to high temperature in Chlamydomonas reinhardtii requires synthesis de novo of proteins encoded by the nuclear and chloroplast genomes. Plant Physiol. 124: 441–449, https://doi.org/10.1104/pp.124.1.441.Search in Google Scholar PubMed PubMed Central
Theis, J., Lang, J., Spaniol, B., Ferté, S., Niemeyer, J., Sommer, F., Zimmer, D., Venn, B., Mehr, S.F., Mühlhaus, T., et al.. (2019). The Chlamydomonas deg1c mutant accumulates proteins involved in high light acclimation. Plant Physiol. 181: 1480–1497, https://doi.org/10.1104/pp.19.01052.Search in Google Scholar PubMed PubMed Central
Theis, J., Niemeyer, J., Schmollinger, S., Ries, F., Rütgers, M., Gupta, T.K., Sommer, F., Muranaka, L.S., Venn, B., Schulz-Raffelt, M., et al.. (2020). VIPP2 interacts with VIPP1 and HSP22E/F at chloroplast membranes and modulates a retrograde signal for HSP22E/F gene expression. Plant Cell Environ. 43: 1212–1229, https://doi.org/10.1111/pce.13732.Search in Google Scholar PubMed
Traewachiwiphak, S., Yokthongwattana, C., Ves-Urai, P., Charoensawan, V., and Yokthongwattana, K. (2018). Gene expression and promoter characterization of heat-shock protein 90B gene (HSP90B) in the model unicellular green alga Chlamydomonas reinhardtii. Plant Sci. 272: 107–116, https://doi.org/10.1016/j.plantsci.2018.04.010.Search in Google Scholar PubMed
Tran, H.C. and Van Aken, O. (2020). Mitochondrial unfolded protein-related responses across kingdoms: similar problems, different regulators. Mitochondrion 53: 166–177, https://doi.org/10.1016/j.mito.2020.05.009.Search in Google Scholar PubMed
Volkov, R.A., Panchuk, I.I., Mullineaux, P.M., and Schöffl, F. (2006). Heat stress-induced H2O2 is required for effective expression of heat shock genes in Arabidopsis. Plant Mol. Biol. 61: 733–746, https://doi.org/10.1007/s11103-006-0045-4.Search in Google Scholar PubMed
Wang, F., Qi, Y., Malnoe, A., Choquet, Y., Wollman, F.A., and de Vitry, C. (2017). The high light response and redox control of thylakoid FtsH protease in Chlamydomonas reinhardtii. Mol. Plant 10: 99–114, https://doi.org/10.1016/j.molp.2016.09.012.Search in Google Scholar PubMed
Wang, X. and Auwerx, J. (2017). Systems phytohormone responses to mitochondrial proteotoxic stress. Mol. Cell 68: 540–551, https://doi.org/10.1016/j.molcel.2017.10.006.Search in Google Scholar PubMed
Wiederrecht, G., Seto, D., and Parker, C.S. (1988). Isolation of the gene encoding the S. cerevisiae heat shock transcription factor. Cell 54: 841–853, https://doi.org/10.1016/s0092-8674(88)91197-x.Search in Google Scholar PubMed
Yamaoka, Y., Choi, B.Y., Kim, H., Shin, S., Kim, Y., Jang, S., Song, W.Y., Cho, C.H., Yoon, H.S., Kohno, K., et al.. (2018). Identification and functional study of the endoplasmic reticulum stress sensor IRE1 in Chlamydomonas reinhardtii. Plant J. 94: 91–104, https://doi.org/10.1111/tpj.13844.Search in Google Scholar PubMed
Yamaoka, Y., Shin, S., Choi, B.Y., Kim, H., Jang, S., Kajikawa, M., Yamano, T., Kong, F., Légeret, B., Fukuzawa, H., et al.. (2019). The BZIP1 transcription factor regulates lipid remodeling and contributes to ER stress management in Chlamydomonas reinhardtii. Plant Cell 31: 1127–1140, https://doi.org/10.1105/tpc.18.00723.Search in Google Scholar PubMed PubMed Central
Zhang, N., Mattoon, E.M., McHargue, W., Venn, B., Zimmer, D., Pecani, K., Jeong, J., Anderson, C.M., Chen, C., Berry, J.C., et al.. (2022). Systems-wide analysis revealed shared and unique responses to moderate and acute high temperatures in the green alga Chlamydomonas reinhardtii. Commun. Biol. 5: 460, https://doi.org/10.1038/s42003-022-03359-z.Search in Google Scholar PubMed PubMed Central
Zhao, Q., Wang, J., Levichkin, I.V., Stasinopoulos, S., Ryan, M.T., and Hoogenraad, N.J. (2002). A mitochondrial specific stress response in mammalian cells. EMBO J. 21: 4411–4419, https://doi.org/10.1093/emboj/cdf445.Search in Google Scholar PubMed PubMed Central
Zou, J., Guo, Y., Guettouche, T., Smith, D.F., and Voellmy, R. (1998). Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell 94: 471–480, https://doi.org/10.1016/s0092-8674(00)81588-3.Search in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Stress response pathways: machineries and mechanisms
- Computational strategies in systems-level stress response data analysis
- Back to the basics: the molecular blueprint of plant heat stress transcription factors
- Unfolded protein responses in Chlamydomonas reinhardtii
- Diversification of glutathione transferases in plants and their role in oxidative stress defense
- How neurons cope with oxidative stress
- The mitochondrial unfolded protein response: acting near and far
- MitoStores: stress-induced aggregation of mitochondrial proteins
- Unclogging of the TOM complex under import stress
- The mitochondrial intermembrane space – a permanently proteostasis-challenged compartment
- The nascent polypeptide-associated complex (NAC) as regulatory hub on ribosomes
- The evolution and diversification of the Hsp90 co-chaperone system
- The proteostasis burden of aneuploidy
Articles in the same Issue
- Frontmatter
- Stress response pathways: machineries and mechanisms
- Computational strategies in systems-level stress response data analysis
- Back to the basics: the molecular blueprint of plant heat stress transcription factors
- Unfolded protein responses in Chlamydomonas reinhardtii
- Diversification of glutathione transferases in plants and their role in oxidative stress defense
- How neurons cope with oxidative stress
- The mitochondrial unfolded protein response: acting near and far
- MitoStores: stress-induced aggregation of mitochondrial proteins
- Unclogging of the TOM complex under import stress
- The mitochondrial intermembrane space – a permanently proteostasis-challenged compartment
- The nascent polypeptide-associated complex (NAC) as regulatory hub on ribosomes
- The evolution and diversification of the Hsp90 co-chaperone system
- The proteostasis burden of aneuploidy

