Abstract
Nitric oxide (NO) signaling represents one of the major regulatory pathways for cardiovascular function. After the discovery of NO, awarded with the Nobel Prize in 1998, this signaling cascade was stepwise clarified. We now have a good understanding of NO production and NO downstream targets such as the soluble guanylyl cyclases (sGCs) which catalyze cGMP production. Based on the important role of NO-signaling in the cardiovascular system, intense research and development efforts are currently ongoing to fully exploit the therapeutic potential of cGMP increase. Recently, NO-independent stimulators of sGC (sGC stimulators) were discovered and characterized. This new compound class has a unique mode of action, directly binding to sGC and triggering cGMP production. The first sGC stimulator made available to patients is riociguat, which was approved in 2013 for the treatment of different forms of pulmonary hypertension (PH). Besides riociguat, other sGC stimulators are in clinical development, with vericiguat in phase 3 clinical development for the treatment of chronic heart failure (HF). Based on the broad impact of NO/cGMP signaling, sGC stimulators could have an even broader therapeutic potential beyond PH and HF. Within this review, the NO/sGC/cGMP/PKG/PDE-signaling cascade and the major pharmacological intervention sites are described. In addition, the discovery and mode of action of sGC stimulators and the clinical development in PH and HF is covered. Finally, the preclinical and clinical evidence and treatment approaches for sGC stimulators beyond these indications and the cardiovascular disease space, like in fibrotic diseases as in systemic sclerosis (SSc), are reviewed.
Introduction: the NO/sGC/cGMP signaling cascade and its major pharmacological intervention sites
Besides the discovery of andenylyl cyclases (ACs) and cAMP signal transduction first described by Sutherland and Rall in 1962 which was awarded the Nobel Prize in 1971, the discovery of the NO/soluble guanylyl cyclase signaling by Ignarro, Murad and Furchott – awarded the Nobel Prize in 1998 – were landmarks in elucidating intracellular signal transduction pathways regulated by cyclic nucleotides (Beavo and Brunton, 2002). After it was shown that the so-called endothelium relaxing factor (EDRF) was identical to NO and that NO binds and stimulates sGC (Furchgott and Zawadzki, 1980; Ignarro et al., 1987, 1988; Murad, 1998), a huge amount of research has produced compelling evidence for a pivotal role of NO/sGC/cGMP signaling. NO-driven cGMP production regulates various functions of cells and tissues, and thereby plays a key role in maintaining physiological tissue homeostasis in mammals, especially in the cardiovascular system. In addition, it is well established that impairment of the NO/sGC/cGMP signaling can cause cardiovascular, cardiopulmonary and cardiorenal diseases (Stasch et al., 2009; Umar and van der Laarse, 2010; Leineweber et al., 2017). Thus, NO has important roles beyond the cardiovascular system and was selected as one out of the “6 wonder molecules for a healthy living” (Leslie, 2016) and increasing endogenous cGMP production developed into a fundamental principle of pharmacological intervention. The NO/cGMP pathway and its pharmacological modulation have been extensively reviewed in the literature and Figure 1 schematically summarizes this pathway and the major pharmacological intervention sites. NO is formed out of L-arginine via the activity of NO-synthases of which three iso-enzymes have been identified. These are termed according to their expression, eNOS for endothelium-derived NOS, nNOS for neuronal-dervied NOS and iNOS for the inducible form of NOS. When NO is liberated by nerve fibers or endothelial cells, it can easily diffuse due to its gaseous nature and activates the soluble guanylyl cyclase (sGC). sGC is a heterodimer which consists of an α subunit and a β subunit with α1/β1 or α2/β1 being the predominant and active isoforms. The β-subunit carries a heme-group with a central ferrous atom (Fe2+) which can bind NO with high affinity. After NO-binding, a conformational change of sGC activates the catalytic domain which triggers the formation of cGMP from GTP. Interestingly, in disease states which are associated with oxidative stress this NO-binding is at least partially impaired. However, under physiological conditions, NO-induced sGC stimulation is leading to substantial cGMP production and cGMP regulates the activity of different downstream targets, mainly cGMP-regulated protein kinases (PKGs), cGMP-regulated phosphodiesterases (PDEs), predominantly PDE2 and PDE3 (Bender and Beavo, 2006) and cGMP-regulated ion channels (CNGCs), e.g. potassium channels (Fesenko et al., 1985). These cGMP downstream targets, especially PKG1 (cGK-I) and PKG2 (cGK-II), phosphorylate a broad variety of further downstream molecules, e.g. the regulatory myosin phosphatase (MYPT1), the IP3R and IP3 receptors associated PKC substrate (IRAG), the vasodilator specific protein (VASP) or the cystic fibrosis trans-membrane transporter regulator (CFTR) (Schlossmann and Desch, 2009, 2011). Moreover, new downstream targets and phosphorylation sites for PKG are discovered, e.g. titin, expressed in cardiomyocytes important for systolic and diastolic heart function (Krüger et al., 2009; Kovács et al., 2016). The most prominent physiological effect when intracellular cGMP is increased by NO, especially in vascular smooth muscle cells (VSMCs) and smooth muscle cells (SMCs), is tissue relaxation. Thus, cGMP increase leads to vasorelaxation and a decrease in blood pressure. On the molecular level this effect is mediated by the cGMP/PKG-dependent phosphorylation of potassium channels, leading to hyperporlarization of the cell and closing of voltage gated calcium channels. In addition, cGMP/PKG phosphorylates the IP3 receptor and IP3-receptor associated PKG substrate (IRAG), both causing a decrease in sarcoplasmatic- and endoplasmatic-free calcium. Moreover, cGMP/PKG-dependent regulation of the myosin light chain phosphatase (MLCP) decreases the sensitivity of contractile filaments in smooth muscle cells also leading to vasodilation (Tsai and Kass, 2009). However, due to the broad distribution of the NO/cGMP signaling cascade beyond VSMCs/SMCs, and due to the various different downstream targets, a variety of other effects are well described. Thus, cGMP has shown antiproliferative, antifibrotic, antiinflammatory, proapoptotic, angiogenic and neuroprotective effects. In addition, it was shown that cGMP might interact with ERK pathways rather than with Smad-signaling involved in TGF-β-induced fibrotic remodeling (Matei et al., 2018). In contrast to the vasorelaxation, these pathways are less understood and are intensively investigated as this could also broaden the impact of cGMP-increasing drugs. The NO/cGMP signaling is terminated by the cleavage of cGMP through highly effective PDEs, in particular through PDE5 and PDE9 which are the major cGMP-degrading PDEs.
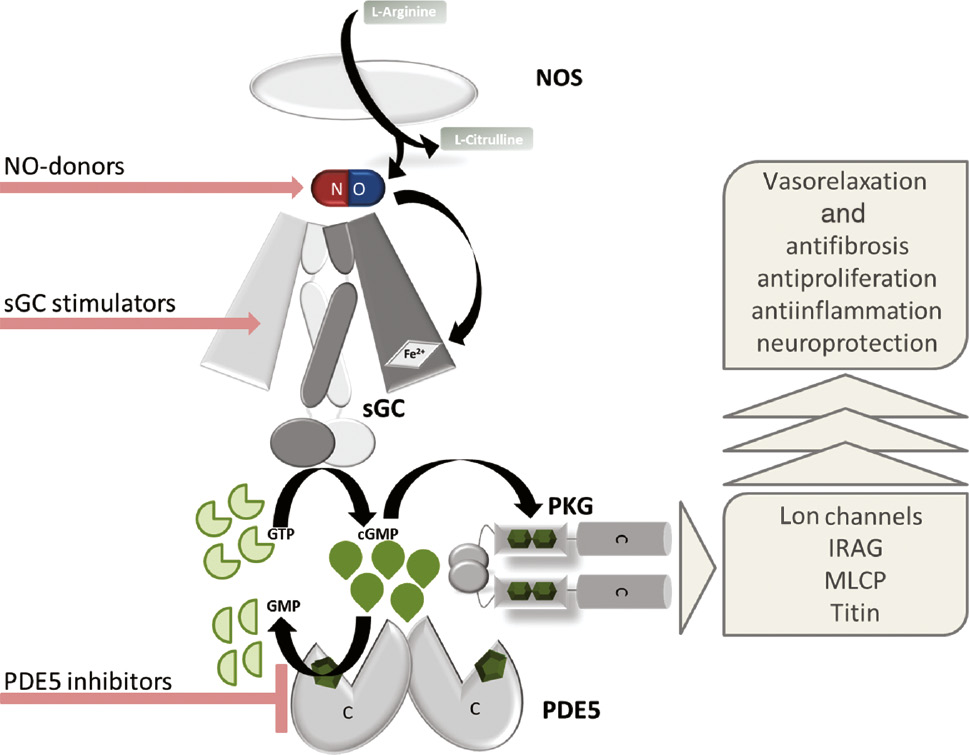
The NO/sGC/PKG/PDE signaling cascade and the major pharmacological intervention sites.
NO binds to the heme group of the sGC and triggers the formation of cGMP which activates PKG. Cleavage of cGMP by PDE5 terminates the signaling. The pathway can be pharmacologically targeted by NO-donors, sGC stimulators and PDE5 inhibitors. It is known to regulate PKG-dependent vasorelaxation, and in addition, it also has an impact on fibrosis, proliferation, inflammation and neuronal function.
The discovery of sGC stimulators and their mode of action
Already in the 19th century, the beneficial effects of nitroglycerin (GTN) for the treatment of angina pectoris were known. There was no mechanistic idea how this compound was acting, but angina pectoris patients described an immediate symptomatic relief (Murrell, 1879). Today, it is obvious that NO-donors and nitrates release NO, which leads to the pharmacological effect via sGC binding and cGMP/PKG signaling. We also know now, that NO released by nitrates binds to the heme group in the β subunit of sGC, triggering the formation of cGMP, leading to relaxation of blood vessels and coronary arteries. However, these drugs – which are still used today for the treatment of angina pectoris – have significant limitations for chronic oral treatment regimens, e.g. a high first pass effect, short half-life time, and can cause tachypylaxis, known as nitrate tolerance. In addition, NO not only binds to sGC, but it also produces reactive oxygen species as superoxide anion (⋅O2−) or peroxynitrite (ONOO−) which is undesired and could even trigger endothelial dysfunction especially in cardiovascular diseases (Pacher et al., 2007). With the identification of selective PDE inhibitors, the 2nd class of drugs which impact on the NO/sGC cGMP signaling, leading to cGMP increase, was introduced. In 1998, with sildenafil, the first selective inhibitor of PDE5 was approved for the treatment of erectile dysfunction (ED) and marketed under the trade name Viagra™, followed by vardenafil (Levitra™) and Tadalafil (Cialis™) in 2003 (Sandner et al., 2009). In the meantime, PDE5 inhibitors are also approved for the treatment of pulmonary arterial hypertension (PAH) and treatment of signs and symptoms of benign prostatic hyperplasia (BPH), supporting the concept that cGMP increase might be a treatment strategy for a broad variety of diseases. However, PDE5 inhibitors are also limited in their efficacy under conditions with low NO production, e.g. in diabetic and obese patients, as cGMP levels are also decreased. Inhibiting the degradation of these low levels of cGMP did not reach the relevant cGMP threshold for treatment effects. Therefore, researchers aimed for identifying new treatment approaches to increase cGMP independently of NO.
The discovery of sGC stimulators started more than 20 years ago, when in 1994, scientists at Bayer in Wuppertal, Germany were searching for compounds which would be able to increase NO synthesis by enhancing NOS activity. By chance compounds were identified which were able to stimulate sGC directly and enhance cGMP production in endothelial cells (Stasch and Hobbs, 2009). Simultaneously, researchers at the Taiwan University and Yung Shin Pharmaceuticals, Taipei, Taiwan reported that a benzyl indazole compound termed YC-1 (Figure 2, Formula 1) was able to inhibit platelet aggregation via stimulation of cGMP synthesis. YC-1 was later characterized as a direct NO-independent, but heme-dependent, sGC stimulator (Mülsch et al., 1997).
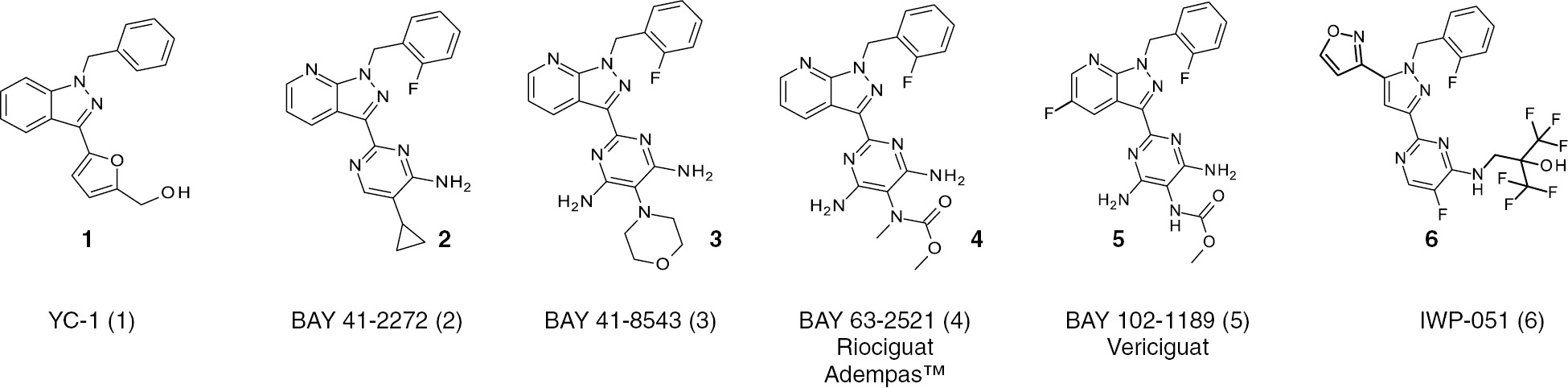
Chemical structures of sGC stimulators.
YC-1, BAY 41-2272 and BAY 41-8543 are early sGC stimulator compounds still used as pharmacological tool compounds for preclinical experiments to explore this pharmacological principle. The new generation sGC stimulator riociguat is approved under the tradename Adempas™ for the treatment of pulmonary hypertension (PAH/CTEPHP) and vericiguat is in phase 3 clinical development for the treatment of chronic heart failure (HFrEF).
YC-1 and the initial compounds identified could not be followed further in clinical development due to low potency and/or selectivity – especially against PDEs – or increased in vitro potency by exposure to daylight (BAY F9317). However, the basic principle, that sGC could be stimulated independently of NO, by compounds directly binding to the sGC enzyme was demonstrated and broad structure–activity relationship (SAR) studies at Bayer were initiated. A first breakthrough in terms of improved potency was the identification of BAY 41-2272 (Figure 2, Formula 2), in which the benzyl indazole moiety of YC-1 was replaced by a (2-fluorobenzyl) pyrazolopyridine moiety and the (hydroxymethyl)furan component was replaced by a 5-substituted 4-aminopyrimidine or 4,6-diaminopyrimidine group. BAY 41-2272 was tested on the sGC overexpressing CHO cell on cGMP production and in the functional organ bath assay on relaxation of pre-contracted blood vessels. BAY 41-2272 exhibited a greatly improved sGC stimulating potency and a minimum effective concentration (MEC) of 0.03 μm for cGMP formation in CHO cells with an IC50 of 0.3 μm for the contraction of pre-contracted rabbit aortic rings compared to YC-1 (IC50=10 μm). Further studies led to the 4,6-diamino-5-morpholino analog BAY 41-8543 (Figure 2, Formula 3), displaying 3-fold higher potency in the phenylephrine-induced contraction of rabbit aorta (IC50=0.10 μm). Unfortunately, both compounds, BAY 41-2272 and BAY 41-8543, had a low metabolic stability, low oral bioavailability, and were strong inducers and inhibitors of cytochrome P450 (CYP) enzymes not allowing further development and use in humans. However, continuous efforts of medicinal chemistry lead to the identification of BAY 63-2521 which received the INN name riociguat (Figure 2, Formula 4). Riociguat stimulated purified, recombinant sGC up to 73-fold, from 0.1 to 100 μm, and showed the typical profile of sGC stimulators. In addition, riociguat showed no relevant CYP interaction and a superior pharmacokinetic profile, including good oral bioavailability. However, riociguat has a relatively short half-life in humans and needs a 3 times daily dosing (Frey et al., 2017). Therefore a further optimization strategy aimed for reduced blood clearance to achieve this longer half-life. In these studies, BAY 102-1189 was identified which later received the INN name vericiguat (Figure 2, Formula 5). Vericiguat demonstrated a typical sGC stimulator profile as well, leading to NO-independent sGC stimulation and cGMP production but also to an enhancement of NO-effects with synergistic efficacy. Vericiguat has a low clearance and long half-life in rats and dogs after intravenous dosing, as well as high oral bio-availability. Most importantly, the pharmacokinetic profile of vericiguat in humans allows for a once-daily dosing in patients (Follmann et al., 2017). More recently, researchers at Ironwood Pharmaceuticals have discovered several novel sGC stimulators, however, the chemical structures of all compounds have not been disclosed yet. Anyway, these efforts led to the bis-heteroaryl pyrazole structure IWP-051 (Figure 2, Formula 6), a pharmacodynamically active compound with low clearance and a long half-life in rats (Nakai et al., 2016). Ironwood has advanced additional sGC stimulators, IW-1973 and IW-1701, into human clinical development studies and IW-1973 is described as a sGC stimulator with a long half-life in preclinical species and its pharmacokinetic half-life in humans is consistent with QD dosing (Hanrahan et al., 2016).
Mode of action of sGC stimulators
Binding of NO to the heme-entity of sGC, the HNOX domain, induces a conformational change which activates the catalytic domain of sGC and triggers the formation of cGMP from GTP. The enzyme is known as the NO-sensitive soluble guanylyl cyclase and consists of a heterodimeric α/β-heme protein (for review see Derbyshire and Marletta 2009, 2012). The aforementioned sGC stimulators are compounds which most likely bind to the α subunit of the sGC, increasing the catalytic activity and cGMP production even in the absence of NO. Therefore, these molecules are described as NO-independent sGC stimulators. In addition, these sGC stimulators show a strong synergistic effect with NO, when NO is already bound to the heme-moiety stabilizing the NO-heme-sGC bond. In summary, sGC stimulators have a dual mode of action, leading to a substantial boost of the cGMP signal in the absence, but also, in the presence of NO. It is important to note that increased oxidative stress renders the sGC enzyme unresponsive to NO but also reduced significantly the efficacy of sGC stimulators. Oxidative stress can cause an oxidation of the central ferrous atom of the sGC HNOX domain (Fe2+ to Fe3+) leading to an untightening of the heme-group and an ultimately heme-free sGC. In addition, NO-dependent thiol oxidation and nitrosylation of cysteins in the sGC, prevents NO-binding and cGMP formation (Beuve, 2017). The mode of action of sGC stimulators binding to the α subunit is covered in Figure 3.
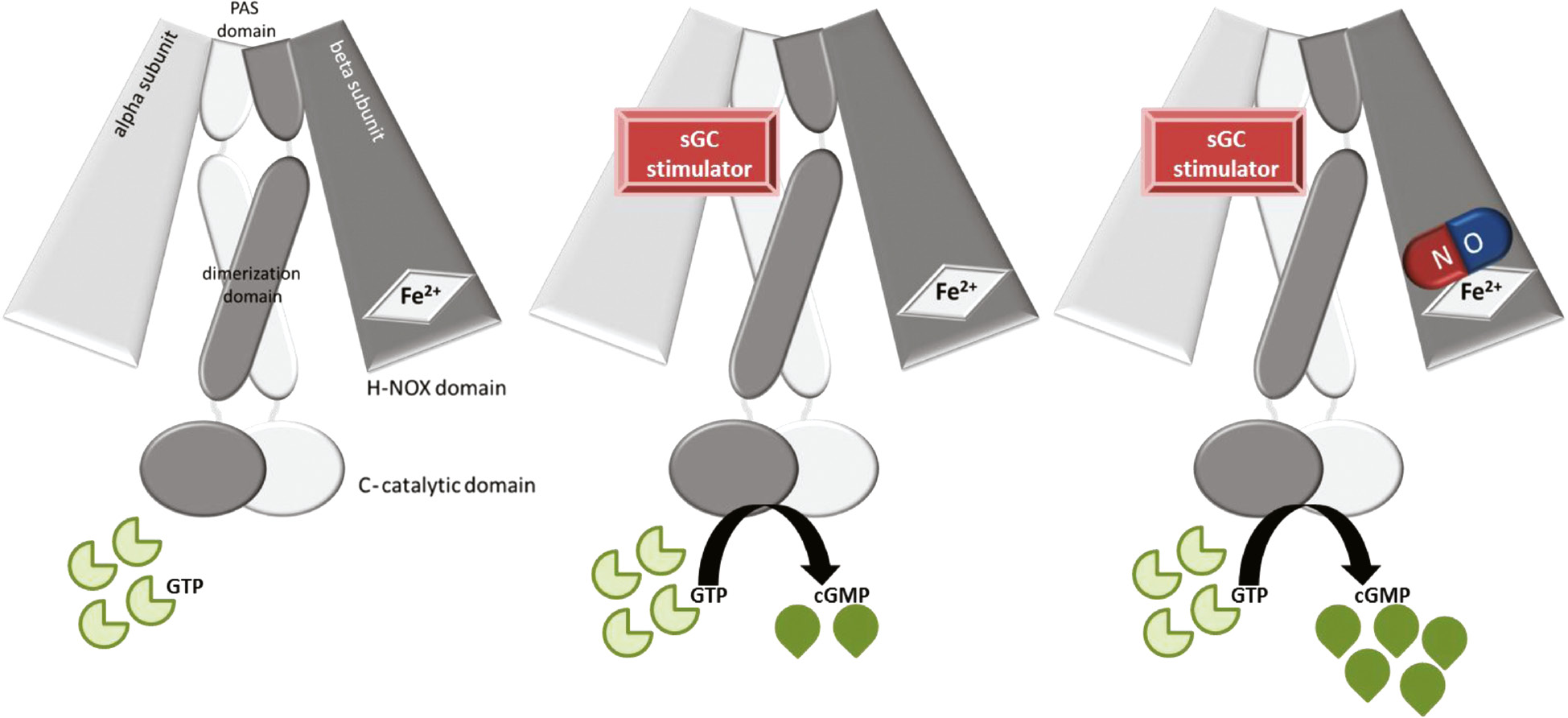
The heterodimeric structure of sGC.
The molecule consists of an α subunit, a β subunit which contains the N-terminal H-NOX (heme-nitric oxide) binding domain, a central dimerization domain and a C-terminal catalytic domain (left). The H-NOX domain binds NO and represents the enzyme activation site. The catalytic domain represents the cGMP producing site. The sGC stimulators most likely bind to the α subunit of sGC and also stimulate cGMP production in the absence of NO (middle). Moreover, the sGC stimulators stimulate sGC activity further when NO is bound and show synergistic effects (right).
Riociguat for the treatment of different forms of pulmonary hypertension
The NO/cGMP system plays an important role in the regulation of the cardiopulmonary system and cGMP significantly decreases pulmonary arterial pressure. Therefore, treatments based on cGMP-increase are beneficial in this disease. In fact, the PDE5 inhibitors sildenafil and tadalafil are approved for the treatment of PAH and distributed as Revatio™ and Adcirca™, respectively. However, in PAH, reduced levels of endogenous NO have been found (Stasch and Evgenov, 2013) which can potentially limit the efficacy of PDE5 inhibitors due to low endogenous cGMP production (Hoeper et al., 2017). Therefore, it was an intriguing concept that sGC stimulators could overcome this limitation of PAH treatment. Broad preclinical profiling in various animal models in different species, reflecting different diseases etiologies were performed clearly demonstrating a beneficial effect of different sGC stimulators including riociguat. Already in 2004 it could be demonstrated that in lambs with acute pulmonary hypertension (PH), the sGC stimulator BAY 41-2272 significantly decreased pulmonary vascular resistance but also prolonged the pulmonary vasodilator response of NO. These data nicely confirmed the mode of action of sGC stimulators in vivo but also suggested that these compounds are effective for PAH-treatment (Evgenov et al., 2004). The reduction of pulmonary artery pressure in preclinical PAH-models was confirmed in other species and was seen in preclinical PAH models in rabbits (Weidenbach et al., 2005), rats (Dumitrascu et al., 2006; Schermuly et al., 2008), dogs (Freitas et al., 2007) and mice (Schermuly et al., 2008). Later on, pig and guinea pig models were also added, consistently demonstrating that sGC stimulators and riociguat treatment leads to a significant and relevant reduction of pulmonary artery pressure in PAH models. Interestingly, these compounds were also effective in a very broad range of PH-etiologies, ranging from solely vasoconstrictive agents like thromboxane A2 agonists (U-46619), over monocrotaline and bleomycin treatment inducing concomitant lung injury, inflammation and lung fibrosis, up to models with pulmonary microembolisms and angioproliferative PAH, caused, e.g. by the VEGF-antagonist SU5416 and hypoxia (Stasch and Evgenov, 2013; Lang et al., 2012). More recently, in a model of pressure overload and RV hypertrophy induced by pulmonary artery banding (PAB) in mice, treatment with riociguat prevented the deterioration of RV function and RV fibrosis (Rai et al., 2018). In addition, also COPD-like phenotypes, induced by chronic smoke exposure were improved when applying sGC stimulators (Weissmann et al., 2014; Paul et al., 2018). Therefore, sGC stimulators might even have a broader treatment potential for other types of PAH and for lung disorders. However, these have to be investigated in the future. Based on the initial preclinical findings, the development of riociguat in PH, started in 2008, when phase 1 clinical trials were initiated to profile riociguat (0.25–5 mg) which was well tolerated (Frey et al., 2008). These data were encouraging phase 2 studies in PAH patients in 2009. In an open label, single dose, phase 2a study, riociguat treatment led to an improvement in all major pulmonary hemodynamic parameters without adversely affecting gas exchange or ventilation/perfusion matching (Grimminger et al., 2009). This phase 2a study also included a few patients with chronic thromboembolic pulmonary hypertension (CTEPH) who demonstrated an increase in cardiac index from baseline after a single dose of riociguat leading to an additional phase 2a open label study of riociguat in patients with CTEPH in which the decrease of pulmonary pressure was confirmed (Ghofrani et al., 2010a,b). In addition, dyspnea and functional class were improved in the 6-min walking distance (6MWD) for both patient groups, PAH and CTEPH (Ghofrani et al., 2010a,b). Therefore, riociguat was tested in two pivotal phase 3 clinical trials, the PATENT-1 and CHEST-1 studies in patients with PAH and CTEPH, respectively (Ghofrani et al., 2013a,b). Patients received placebo or riociguat individually dose-adjusted up to 2.5 mg 3 times daily. In both studies, riociguat was generally well tolerated and significantly improved a range of clinical endpoints, including the 6MWD. Based on these results riociguat was then approved by the Federal Drug Administration (FDA) and European Medicines Agency (EMA) in 2013 and is marketed as Adempas™. The good tolerability and clinical benefit was maintained after 2 years of riociguat treatment in open-label extension studies, the PATENT-2 and CHEST-2 studies (Ghofrani et al., 2016; Simonneau et al., 2016). Riociguat was also investigated in patients with PAH with insufficient response to PDE5 inhibitors. The RESPITE study indicated that replacing PDE5i with riociguat may be a feasible and effective treatment strategy in these patients (Hoeper et al., 2017). Currently, a clinical program is ongoing to confirm the results of the RESPITE study (REPLACE, NCT02891850).
sGC stimulators beyond pulmonary hypertension
As pointed out before, the NO/sGC/cGMP pathway is a key regulator of the cardiovascular system. Therefore sGC stimulators could also become treatment options beyond the cardiopulmonary circulation and could have potential in other indications as well. In addition, the expression of the pathway in other cells and tissues outside the cardiovascular system is triggering preclinical and clinical studies for additional pathologies as fibrotic disorders, systemic sclerosis (SSc) or chronic kidney diseases (CKD) and dementia.
Heart failure (HF) with reduced and preserved ejection fraction (HFrEF and HFpEF)
The NO/cGMP pathway was discovered in the cardiovascular system and still represents a pivotal pathway in the maintenance of cardiovascular and heart function. Chronic heart failure (HF) is one of the major health problems worldwide with high morbidity, poor prognosis and unmet medical need (Lewis et al., 2017). The current treatment is based on the blockade of the renin-angiotensin system and the sympathetic system, but their clinical efficacy is limited. The impact of sGC stimulation for treatment of chronic cardiovascular diseases was very much limited due to the pharmacokinetic and pharmacodynamics profiles of available sGC modulators (Breitenstein et al., 2017). In both respects, vericiguat is able to circumvent these limitations. Vericiguat has a once daily kinetic after oral dosing in patients and showed no significant effects on systemic blood pressure in dosages applied for the treatment of chronic HF in the patient. Due to the impact of the NO/sGC/cGMP system for cardiovascular diseases, sGC stimulators are intensively profiled in preclinical models of cardiovascular diseases and HF. The sGC stimulators were shown to limit the hypertrophy of cardiomyocytes, suggesting a direct effect on the level of the failing heart (Irvine et al., 2012). Moreover, left ventricular and vascular fibrosis was reduced in sGC stimulator-treated rats in disease models caused by hypertension via angiotensin II infusion (Masuyama et al., 2006, 2009) or subtotal nephrectomy (Sharkovska et al., 2010) which might be indicative especially of the HFpEF phenotype. Interestingly, these effects were observed in dosages with or without moderate blood pressure lowering efficacy. It was also shown that sGC stimulators could reduce infarct size in a myocardial infarction model but in addition preserved the ejection fraction after MI (Methner et al., 2013; Bice et al., 2014). When the sGC stimulator was given 30 min after onset of ischemia, the ejection fraction was still improved 1 month post event (Methner et al., 2013). HF still has a poor prognosis and therefore, improvement of survival and reduction of cardiovascular and all-cause mortality is one of the major determinates of efficacy. Due to the very broad mode of action of cGMP, sGC stimulators might have beneficial effects on the hearts, kidney and vasculature, and therefore impact on survival. In fact, in animal models of hypertensive-induced heart and kidney damage with concomitant endothelial dysfunction induced by NO-synthase blocking, vericiguat treatment caused a dose-dependent improvement of survival of these rats and improved kidney and heart function (Follmann et al., 2017). These effects were even more pronounced when vericiguat was combined with a mineralocorticoid-receptor antagonist which leads to almost complete survival of these animals (Mathar et al., 2017). Taken together, these preclinical data out of different in vivo models with different HF etiologies, suggested that sGC stimulators and vericiguat could be very beneficial for HF-treatment in patients. Clinically, the trial named SOCRATES (SOluble guanylate Cyclase stimulatoR in heArT failurE Studies) aimed for translation of these beneficial effects in HF patients (Pieske et al., 2014). This program consisted of two randomized, placebo-controlled, double-blind phase 2b clinical studies, investigated the effect of vericiguat in HF patients with reduced ejection fraction (HFrEF), SOCRATES reduced, and with preserved ejection fraction (HFpEF), SOCRATES preserved, respectively. Roughly 500 patients were included in each study and the NT-proBNP levels served as a primary endpoint. In the REDUCED study, a dose–dependent reduction on NT-proBNP and a trend for reduction of CV deaths and HF hospitalizations was observed (Gheorghiade et al., 2015), encouraging further phase 3 clinical investigations. In contrast to these results, the PRESERVED study showed no significant reduction of NT-proBNP (Pieske et al., 2017) but did show an improvement of quality of life scores in HFpEF patients (Filippatos et al., 2017). Currently, a phase 3 confirmatory trial with vericiguat in HFrEF, the VICTORIA trial (VerICiguaT Global Study in Subjects With Heart Failure With Reduced Ejection Fraction), is ongoing (Armstrong et al., 2017). More recently, Ironwood initiated a phase 2 clinical trial, the CAPACITY trial, assessing exercise capacity with the sGC stimulator IW-1973 in HFpEF patients (NCT03254485). If these ongoing phase 2 and phase 3 trials turned out to be successful, HF might be the next indication in which sGC stimulators could get approval.
Hypertension (HTN)
Dilation and vasorelaxation is one of the best understood and most prominent effects of NO/cGMP. Therefore, it seems obvious that sGC stimulators could be used as antihypertensives. The dose-dependent blood pressure lowering effect of sGC stimulators is well established in preclinical models of various species, especially in higher dosages. However, there are no sGC stimulators approved for this indication or in late stage development. As there is a broad range of antihypertensive therapies available, the medical need in this indication might not feel as high as in PAH/CTEPH or HF. Therefore, the already established and mostly generic standard of care (SoC) for HTN might have prevented or delayed the development of sGC stimulators in this indication. However, due to the unique mode of action, actively inducing a cGMP signal and thereby actively relaxing blood vessels, the sGC stimulators might show an even better efficacy compared to currently used antihypertensive therapies which work by mainly blocking vasoconstriction. Ironwood is currently doing clinical studies in hypertensive patients and has very recently shown a relevant blood pressure reduction over placebo with the sGC stimulator IW-1973 in hypertensives. After 2 weeks of IW-1973 treatment, a decrease in mean arterial blood pressure of 6.3 mm Hg from baseline could be demonstrated (Ironwood Pharmaceuticals – press release). These data are suggesting that sGC stimulators could be used effective antihypertensive drugs. However, additional studies are needed to clarify how these effects compare with SoC and if there are situations in which sGC stimulators provide additional benefits, e.g. in resistant hypertension patients.
Fibrotic diseases and systemic sclerosis (SSc)
There is ample of evidence out of different organ systems that cGMP increased by sGC stimulators exhibit a strong antifibrotic effect. It has been shown in vitro, that cGMP could inhibit proliferation and collagen formation of fibroblast from hearts (Abdelaziz et al., 2001), from kidneys (Hewitson et al., 2004) and from lungs (Dunkern et al., 2007). These in vitro observations, translated very well into in vivo, which demonstrate antifibrotic effects of cGMP-increase in hearts (Sharkovska et al., 2010), kidneys (Kalk et al., 2006; Geschka et al., 2011), in lungs (Evgenov et al., 2011) and in the livers (Knorr et al., 2008) of mice and rats (for review see Sandner and Stasch, 2017). Especially for liver fibrosis, comprehensive preclinical data sets are available demonstrating that the orally administered sGC stimulator BAY 41-2272 reduced liver fibrosis in a bile-duct ligation model as in serum-induced liver fibrosis (Nowatzky et al., 2011). Moreover, sGC stimulators also showed antifibrotic effects in animal models for non-alcoholic steatohepatitis (NASH), which is characterized by liver fibrosis, inflammation and steatosis could result in liver cirrhosis. The sGC stimulator, IW-1973, was profiled in vitro and in vivo in animal models of liver fibrosis and NASH (Flores-Costa et al., 2017). Despite the very compelling evidence for antifibrotic effects in liver fibrosis, there is currently no clinical development reported in which the antifibrotic efficacy of sGC stimulators is evaluated. More recently, profiling of antifibrotic efficacy of sGC stimulators especially including BAY 41-2272 and riociguat were focused on skin fibrosis, and especially investigating if sGC stimulators could potentially represent a new treatment option for SSc. SSc is an autoimmune disease affecting the connective tissue and is characterized by excessive skin fibrosis and fibrosis of internal organs, leading to reduced life expectancy. In addition, vascular complications are evident as the development of digital ulcers is very bothersome in the daily lives of SSc patients. There is still a very high medical need with no approved therapy for this rare disease. In a broad spectrum of preclinical experiments, sGC stimulators were profiled in vitro and in vivo in skin fibrosis models. In vitro, sGC stimulators could reduce the collagen production and fibroblast to myofibroblast differentiation of human dermal fibroblasts. Moreover, TGFβ-induced collagen production was also attenuated by sGC stimulators in the dermal fibroblasts of SSc patients (Beyer et al., 2012; Zenzmaier et al., 2015). To further evaluate the treatment potential of riociguat for SSc, sGC stimulators and riociguat were tested in widely accepted mouse models of SSc with different etiologies, which develop significant skin fibrosis (Beyer et al., 2010). This in vivo screening cascade comprised of the bleomycin-induced skin fibrosis model, which resembles an early, inflammatory-driven stage of SSc, the TSK-1 model which resembles a later, non-inflammatory, stage of SSc and finally the sclerodermatous chronic graft versus host disease (cGvHD) model with fibrosis of the intestinal tract and the skin. In the bleomycin-induced skin fibrosis model, a localized dermal fibrosis was induced in mice by intradermal injections of bleomycin every other day for 21 days. To evaluate the potential effects on prevention of fibrosis, treatment was initiated simultaneously with the first bleomycin injection and the effects of riociguat were compared to a placebo. Reduction of dermal thickness, dermal hydroxyproline content and the number of dermal myofibroblasts were measured on day 21. Riociguat showed a dose-dependent and significant reduction of dermal thickness, dermal hydroxyproline-content and the number of dermal myofibroblasts (Beyer et al., 2012; Dees et al., 2015). The TSK-1 mice have a tandem mutation in the fibrillin 1 gene (Fbn1) that leads to progressive skin fibrosis. For baseline measurements, skin fibrosis of untreated TSK-1 mice (5 weeks old) was characterized by quantitative analysis of hypodermal thickness. For the quantification of antifibrotic effects, TSK-1 mice (5 weeks old) were treated for 5 consecutive weeks with either placebo (vehicle) or the sGC stimulator riociguat. Similar to the bleomycin-model, sGC stimulators and riociguat exhibited a dose-dependent and significant prevention of progression of skin fibrosis with a significant reduction of hypodermal thickness, dermal hydroxyproline content and the number of dermal myofibroblasts (Beyer et al., 2012; Dees et al., 2015). Finally, the sclerodermatous cGvHD mouse model was used, inducing skin fibrosis by allogeneic transplantation of splenocytes and bone marrow cells from B10.D2 mice into BALB/c (H-2d) mice. Treatment started 10 days after transplantation, and the outcome was analyzed after 6 weeks. Riociguat showed a dose-dependent and significant prevention of progression of skin fibrosis in the cGVHD-induced skin fibrosis mice and reduced dermal thickness, dermal hydroxyproline content and the number of dermal myofibroblasts. Interestingly, inhibition of the accompanied fibrosis in the gastrointestinal tract in the model was reduced, suggesting a systemic antifibrotic effect of riociguat in SSc (Beyer et al., 2015; Sandner et al., 2017). Based on these preclinical profiling data, a phase 2 trial has been initiated in patients with diffuse cutaneous SSc (RISE-SSc, NCT2283762) and results are expected in 2018.
Chronic kidney diseases (CKD), neuroprotection, dementia and beyond
It became obvious in the last International Conference on cGMP, held at Bamberg, Germany from 23 to 25 June 2017, that enhancement of the sGC signaling with sGC stimulators could become a broad treatment potential even outside the cardiovascular system (Friebe et al., 2017). There is ample of evidence that sGC stimulators improve kidney function. The NO/sGC/cGMP system which regulates renal blood flow, can modify renin release but can also modulate fluid and electrolyte transport. In addition, depending on the animal models, this system can also have antifibrotic, antihypertrophic and anti-inflammatory effects. In summary, the mode of action of sGC stimulators might be multifactorial leading to improved kidney function in CKD and diabetic kidney disease. Recently, the preclinical studies with sGC stimulators are summarized suggesting therapeutic benefits in CKD (Stasch et al., 2015). It could be shown that in a broad range of preclinical models, sGC stimulators are effective, including different etiologies of CKD, like diabetes, obesity, partial renal ablation or hypertension. In renin transgenic rats which are concomitantly treated with sGC stimulators, kidney function was improved significantly with sGC stimulators like riociguat and vericiguat (Sharkovska et al., 2010; Follmann et al., 2017). In addition, also in diabetic kidney disease reflected in ZSF-1 rats which are diabetic, obese and have hypertension, sGC stimulators like IW-1973 improved kidney function. Currently IW-1973 is also being studied in patients with diabetic nephropathy (NCT03217591).
The impact of cGMP elevation for cognitive function has also become evident in recent years. Injection of stable cGMP analogs improved learning and memory of rats (Bernabeu et al., 1996). Later on, PDE5 inhibitors were used and improved learning and memory in various preclinical models in different species, including rat, mice and monkeys (Prickaerts et al., 2002; Rutten et al., 2005, 2008). Besides PDE5 inhibitors, PDE9 inhibitors were shown to increase long-term potentiation and PDE9 inhibitors are in clinical development for Alzheimer’s disease. Preclinical data demonstrating improved learning with sGC stimulators are currently missing and no clinical development is reported. However, as this could be one future direction in which sGC stimulators could be beneficial, preclinical testing is warranted for these indications. The spectrum of diseases in which cGMP signaling plays a role and sGC stimulators could have a beneficial benefit is constantly increasing. There is consistent evidence that sGC stimulators could be beneficial in sickle cell disease (SCD) (Potoka et al., 2017) and IW-1701 and riociguat are currently in phase 2 trials in sickle cell disease patients.
Outlook
The substantial progress in whole genome sequencing and genome wide association studies revealed that the NO/sGC/cGMP signaling is impaired in a variety of diseases and disorders not in the current scope of pharmacological therapy with sGC stimulators (Leineweber et al., 2017; Emdin et al., 2018). Therefore, in the future, these data sources could provide valuable hints for further applications of sGC stimulators, especially in rare diseases and could broaden the potential treatment applications. In parallel to sGC stimulators, the so-called sGC activators were discovered also almost 25 years ago (Stasch et al., 2002; Schmidt et al., 2009). These compounds like cinaciguat, bind to the HNOX domain of the sGC and stimulate the oxidized and heme-free form of sGC. A variety of diseases are linked to increased oxidative stress, rendering the sGC unresponsive to NO but also sGC stimulators. Therefore, it will become very interesting in the future if and how these sGC activators and their treatment effects are differentiated within different pathologies. This will not only broaden our theoretical knowledge of NO/sGC/cGMP signaling under oxidative stress, but could also help to improve and tailor therapies based on sGC stimulation and sGC activation to the benefit of patients.
Acknowledgments
The author wishes to thank Prof. Dr. Johannes-Peter Stasch for his very valuable hints and proof-reading of the manuscript and Dr. Shalini Murali and Dimyana Neufeldt for editorial help. Johannes-Peter Stasch and Peter Sandner wish to thank the whole Bayer AG Research and Development organization and the sGC stimulator Global Project Teams as the MSD Teams for their constant support of the research and development programs.
Conflict of interest statement: Peter Sandner is a full-time employee of Bayer AG.
References
Abdelaziz, N., Colombo, F., Mercier, I., and Calderone, A. (2001). Nitric oxide attenuates the expression of transforming growth factor-β(3) mRNA in rat cardiac fibroblasts via destabilization. Hypertension 38, 261–266.10.1161/01.HYP.38.2.261Search in Google Scholar PubMed
Armstrong, P.W., Roessig, L., Patel, M.J., Anstrom, K.J., Butler, J., Voors, A.A., Lam, C.S.P., Ponikowski, P., Temple, T., Pieske, B., et al. (2018). A multicenter, randomized, double-blind, placebo-controlled trial of the efficacy and safety of the oral soluble guanylate cyclase stimulator: the VICTORIA trial. JACC Heart Fail. 6, 96–104.10.1016/j.jchf.2017.08.013Search in Google Scholar PubMed
Beavo, J.A. and Brunton, L.L. (2002). Cyclic nucleotide research – still expanding after half a century. Nat. Rev. Mol. Cell Biol. 3, 710–718.10.1038/nrm911Search in Google Scholar PubMed
Bender, A.T. and Beavo, J.A. (2006). Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol. Rev. 58, 488–520.10.1124/pr.58.3.5Search in Google Scholar PubMed
Bernabeu, R., Schmitz, P., Faillace, M.P., Izquierdo, I., and Medina, J.H. (1996). Hippocampal cGMP and cAMP are differentially involved in memory processing of inhibitory avoidance learning. Neuroreport 7, 585–588.10.1097/00001756-199601310-00050Search in Google Scholar PubMed
Beuve, A. (2017). Thiol-based redox modulation of soluble guanylyl cyclase, the nitric oxide receptor. Antioxid. Redox. Signal. 26, 137–149.10.1089/ars.2015.6591Search in Google Scholar PubMed PubMed Central
Beyer, C., Schett, G., Distler, O., and Distler, J.H. (2010). Animal models of systemic sclerosis: prospects and limitations. Arthritis Rheum. 62, 2831–2844.10.1002/art.27647Search in Google Scholar PubMed
Beyer, C., Reich, N., Schindler, S.C., Akhmetshina, A., Dees, C., Tomcik, M., Hirth-Dietrich, C., von Degenfeld, G., Sandner, P., Distler, O., et al. (2012). Stimulation of soluble guanylate cyclase reduces experimental dermal fibrosis. Ann. Rheum. Dis. 71, 1019–1026.10.1136/annrheumdis-2011-200862Search in Google Scholar PubMed
Beyer, C., Zenzmaier, C., Palumbo-Zerr, K., Mancuso, R., Distler, A., Dees, C., Zerr, P., Huang, J., Maier, C., Pachowsky, M.L., et al. (2015). Stimulation of the soluble guanylate cyclase (sGC) inhibits fibrosis by blocking non-canonical TGFβ signalling. Ann. Rheum. Dis. 74, 1408–1416.10.1136/annrheumdis-2013-204508Search in Google Scholar PubMed
Bice, J.S., Keim, Y., Stasch, J.P., and Baxter, G.F. (2014). NO-independent stimulation or activation of soluble guanylyl cyclase during early reperfusion limits infarct size. Cardiovasc. Res. 101, 220–228.10.1093/cvr/cvt257Search in Google Scholar PubMed PubMed Central
Breitenstein, S., Roessig, L., Sandner, P., and Lewis, K.S. (2017). Novel sGC stimulators and sGC activators for the treatment of heart failure. Handb. Exp. Pharmacol. 243, 225–247.10.1007/164_2016_100Search in Google Scholar PubMed
Dees, C., Beyer, C., Distler, A., Soare, A., Zhang, Y., Palumbo-Zerr, K., Distler, O., Schett, G., Sandner, P., and Distler, J.H. (2015). Stimulators of soluble guanylate cyclase (sGC) inhibit experimental skin fibrosis of different aetiologies. Ann. Rheum. Dis. 74, 1621–1625.10.1136/annrheumdis-2014-206809Search in Google Scholar PubMed
Derbyshire, E.R. and Marletta, M.A. (2009). Biochemistry of soluble guanylate cyclase. Handb. Exp. Pharmacol. 191, 17–31.10.1007/978-3-540-68964-5_2Search in Google Scholar PubMed
Derbyshire, E.R. and Marletta, M.A. (2012). Structure and regulation of soluble guanylate cyclase. Annu. Rev. Biochem. 81, 533–559.10.1146/annurev-biochem-050410-100030Search in Google Scholar PubMed
Dumitrascu, R., Weissmann, N., Ghofrani, H.A., Dony, E., Beuerlein, K., Schmidt, H., Stasch, J.P., Gnoth, M.J., Seeger, W., Grimminger, F., et al. (2006). Activation of soluble guanylate cyclase reverses experimental pulmonary hypertension and vascular remodeling. Circulation 113, 286–295.10.1161/CIRCULATIONAHA.105.581405Search in Google Scholar PubMed
Dunkern, T.R., Feurstein, D., Rossi, G.A., Sabatini, F., and Hatzelmann, A. (2007). Inhibition of TGF-β induced lung fibroblast to myofibroblast conversion by phosphodiesterase inhibiting drugs and activators of soluble guanylyl cyclase. Eur. J. Pharmacol. 572, 12–22.10.1016/j.ejphar.2007.06.036Search in Google Scholar PubMed
Emdin, C.A., Khera, A.V., Klarin, D., Natarajan, P., Zekavat, S.M., Nomura, A., Haas, M., Aragam, K., Ardissino, D., Wilson, J.G., et al. (2018). Phenotypic consequences of a genetic predisposition to enhanced nitric oxide signaling. Circulation. 16, 137, 222–232.10.1161/CIRCULATIONAHA.117.028021Search in Google Scholar PubMed PubMed Central
Evgenov, O.V., Ichinose, F., Evgenov, N.V., Gnoth, M.J., Falkowski, G.E., Chang, Y., Bloch, K.D., and Zapol, W.M. (2004). Soluble guanylate cyclase activator reverses acute pulmonary hypertension and augments the pulmonary vasodilator response to inhaled nitric oxide in awake lambs. Circulation 110, 2253–2259.10.1161/01.CIR.0000144469.01521.8ASearch in Google Scholar PubMed
Evgenov, O.V., Zou, L., Zhang, M., Mino-Kenudson, M., Mark, E.J., Buys, E.S., Raher, M.J., Li, Y., Feng, Y., Jones, R.C., et al. (2011). Nitric oxide-independent stimulation of soluble guanylate cyclase attenuates pulmonary fibrosis. BMC Pharmacol. 11, O9. doi:10.1186/1471-2210-11-s1-o9.10.1186/1471-2210-11-s1-o9Search in Google Scholar
Fesenko, E.E., Kolesnikov, S.S., and Lyubarsky, A.L. (1985). Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature 313, 310–313.10.1038/313310a0Search in Google Scholar PubMed
Filippatos, G., Maggioni, A.P., Lam, C.S.P., Pieske-Kraigher, E., Butler, J., Spertus, J., Ponikowski, P., Shah, S.J., Solomon, S.D., Scalise, A.V., et al. (2017). Patient-reported outcomes in the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED ejection fraction (SOCRATES-PRESERVED) study. Eur. J. Heart Fail. 19, 782–791.10.1002/ejhf.800Search in Google Scholar PubMed
Flores-Costa, R., Alcaraz-Quiles, J., Titos, E., López-Vicario, C., Casulleras, M., Duran-Güell, M., Rius, B., Diaz, A., Hall, K., Shea, C., et al. (2017). The soluble guanylate cyclase stimulator IW-1973 prevents inflammation and fibrosis in experimental non-alcoholic steatohepatitis. Br. J. Pharmacol. 175, 953–967.10.1111/bph.14137Search in Google Scholar PubMed PubMed Central
Follmann, M., Ackerstaff, J., Redlich, G., Wunder, F., Lang, D., Kern, A., Fey, P., Griebenow, N., Kroh, W., Becker-Pelster, E.M., et al. (2017). Discovery of the soluble guanylate cyclase stimulator vericiguat (BAY 1021189) for the treatment of chronic heart failure. J. Med. Chem. 60, 5146–5161.10.1021/acs.jmedchem.7b00449Search in Google Scholar PubMed
Freitas, C.F., Morganti, R.P., Annichino-Bizzacchi, J.M., De Nucci, G., and Antunes, E. (2007). Effect of BAY 41–2272 in the pulmonary hypertension induced by heparin-protamine complex in anaesthetized dogs. Clin. Exp. Pharmacol. Physiol. 34, 10–14.10.1111/j.1440-1681.2007.04524.xSearch in Google Scholar PubMed
Frey, R., Muck, W., Unger, S., Artmeier-Brandt, U., Weimann, G., and Wensing, G. (2008). Single-dose pharmacokinetics, tolerability and safety of the soluble guanylate cyclase stimulator BAY 63–2521; an ascending-dose study in healthy male volunteers. J. Clin. Pharmacol. 48, 926–934.10.1177/0091270008319793Search in Google Scholar PubMed
Frey, R., Becker, C., Saleh, S., Unger, S., van der Mey, D., and Mück, W. (2017). Clinical pharmacokinetic and pharmacodynamic profile of Riociguat. Clin. Pharmacokinet. doi: 10.1007/s40262-017-0604-7. [Epub ahead of print].10.1007/s40262-017-0604-7Search in Google Scholar PubMed PubMed Central
Friebe, A., Sandner, P., and Schmidtko, A. (2017). Meeting report of the 8th International Conference on cGMP “cGMP: generators, effectors and therapeutic implications” at Bamberg, Germany from June 23rd to 25th 2017. Naunyn-Schmiedeberg’s Arch. Pharmacol. 390, 1177–1188.10.1007/s00210-017-1429-5Search in Google Scholar PubMed PubMed Central
Furchgott, R.F. and Zawadzki, J.V. (1980). The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288, 373–376.10.1038/288373a0Search in Google Scholar PubMed
Geschka, S., Kretschmer, A., Sharkovska, Y., Evgenov, O.V., Lawrenz, B., Hucke, A., Hocher, B., and Stasch, J.P. (2011). Soluble guanylate cyclase stimulation prevents fibrotic tissue remodeling and improves survival in salt-sensitive Dahl rats. PLoS One 6, e21853.10.1371/journal.pone.0021853Search in Google Scholar PubMed PubMed Central
Gheorghiade, M., Greene, S.J., Butler, J., Filippatos, G., Lam, C.S., Maggioni, A.P., Ponikowski, P., Shah, S.J., Solomon, S.D., Kraigher-Krainer, E., et al. (2015). Effect of vericiguat, a soluble guanylate cyclase stimulator, on natriuretic peptide levels in Patients with Worsening Chronic Heart Failure and Reduced Ejection Fraction: the SOCRATES-REDUCED Randomized Trial. J. Am. Med. Assoc. 314, 2251–2262.10.1001/jama.2015.15734Search in Google Scholar PubMed
Ghofrani, H.A., Hoeper, M.M., Halank, M., Meyer, F.J., Staehler, G., Behr, J., Ewert, R., Weimann, G., and Grimminger, F. (2010a). Riociguat for chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension: a phase II study. Eur. Respir. J. 36, 792–799.10.1183/09031936.00182909Search in Google Scholar
Ghofrani, H.A., Hoeper, M.M., Halank, M., Meyer, F.J., Staehler, G., Behr, J., Ewert, R., Binnen, T., Weimann, G., and Grimminger, F. (2010b). Riociguat for chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension: first long-term extension data from a phase II study. Am. J. Respir. Crit. Care Med. 181, A6770.10.1164/ajrccm-conference.2010.181.1_MeetingAbstracts.A6770Search in Google Scholar
Ghofrani, H.A., D’Armini, A.M., Grimminger, F., Hoeper, M.M., Jansa, P., Kim, N.H., Mayer, E., Simonneau, G., Wilkins, M.R., Fritsch, A., et al. (2013a). Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N. Engl. J. Med. 369, 319–329.10.1056/NEJMoa1209657Search in Google Scholar
Ghofrani, H.A., Galiè, N., Grimminger, F., Grunig, E., Humbert, M., Jing, Z.C., Keogh, A.M., Langleben, D., Kilama, M.O., Fritsch, A., et al. (2013b). Riociguat for the treatment of pulmonary arterial hypertension. N. Engl. J. Med. 369, 330–340.10.1056/NEJMoa1209655Search in Google Scholar
Ghofrani, H.A., Grimminger, F., Grunig, E., Huang, Y., Jansa, P., Jing, Z.C., Kilpatrick, D., Langleben, D., Rosenkranz, S., Menezes, F., et al. (2016). Predictors of long-term outcomes in patients treated with riociguat for pulmonary arterial hypertension: data from the PATENT-2 open-label, randomised, long-term extension trial. Lancet Respir. Med. 4, 361–371.10.1016/S2213-2600(16)30019-4Search in Google Scholar
Grimminger, F., Weimann, G., Frey, R., Voswinckel, R., Thamm, M., Bölkow, D., Weissmann, N., Muck, W., Unger, S., Wensing, G., et al. (2009). First acute haemodynamic study of soluble guanylate cyclase stimulator riociguat in pulmonary hypertension. Eur. Respir. J. 33, 785–792.10.1183/09031936.00039808Search in Google Scholar
Hanrahan, J., Profy, A.T., Lavins, B.J., Ruff, D., Poirier, G., Wakefield, J., Wilson, P., Hall, M., and Currie, M.G. (2016). First-in-human single-ascending-dose study of IW-1973, a new soluble guanylate cyclase stimulator. JACC 67, 1284.10.1016/S0735-1097(16)31285-2Search in Google Scholar
Hewitson, T.D., Martic, M., Darby, I.A., Kelynack, K.J., Bisucci, T., Tait, M.G., and Becker, G.J. (2004). Intracellular cyclic nucleotide analogues inhibit in vitro mitogenesis and activation of fibroblasts derived from obstructed rat kidneys. Nephron. Exp. Nephrol. 96, e59–66.10.1159/000076405Search in Google Scholar PubMed
Hoeper, M.M., Simonneau, G., Corris, P.A., Ghofrani, H.A., Klinger, J.R., Langleben, D., Naeije, R., Jansa, P., Rosenkranz, S., Scelsi, L., et al. (2017). RESPITE: switching to riociguat in pulmonary arterial hypertension patients with inadequate response to phosphodiesterase-5 inhibitors. Eur. Respir. J. 50, 1602425.10.1183/13993003.02425-2016Search in Google Scholar PubMed PubMed Central
Ignarro, L.J., Buga, G.M., Wood, K.S., Byrns, R.E., and Chaudhuri, G. (1987). Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. USA 84, 9265–9269.10.1073/pnas.84.24.9265Search in Google Scholar PubMed PubMed Central
Ignarro, L.J., Byrns, R.E., Buga, G.M., Wood, K.S., and Chaudhuri, G. (1988). Pharmacological evidence that endothelium-derived relaxing factor is nitric oxide: use of pyrogallol and superoxide dismutase to study endothelium-dependent and nitric oxide-elicited vascular smooth muscle relaxation. J. Pharmacol. Exp. Ther. 244, 181–189.10.1016/S0022-3565(25)24231-8Search in Google Scholar
Ironwood Pharmaceuticals. (2017). Top-line Phase IIa Data for IW-1973 Demonstrating Positive Cardiovascular, Metabolic and Endothelial Effects (Cambridge, MA: Press Release IW-Pharmaceuticals), Dec 4.Search in Google Scholar
Irvine, J.C., Ganthavee, V., Love, J.E., Alexander, A.E., Horowitz, J.D., Stasch, J.P., Kemp-Harper, B.K., and Ritchie, R.H. (2012). The soluble guanylyl cyclase activator Bay 58-2667 selectively limits cardiomyocyte hypertrophy. PLoS One 7, e44481.10.1371/journal.pone.0044481Search in Google Scholar PubMed PubMed Central
Kalk, P., Godes, M., Relle, K., Rothkegel, C., Hucke, A., Stasch, J.P., and Hocher, B. (2006). NO-independent activation of soluble guanylate cyclase prevents disease progression in rats with 5/6 nephrectomy. Br. J. Pharmacol. 148, 853–859.10.1038/sj.bjp.0706792Search in Google Scholar PubMed PubMed Central
Knorr, A., Hirth-Dietrich, C., Alonso-Alija, C., Härter, M., Hahn, M., Keim, Y., Wunder, F., and Stasch, J.P. (2008). Nitric oxide-independent activation of soluble guanylate cyclase by BAY 60-2770 in experimental liver fibrosis. Arzneimittelforschung 58, 71–80.10.1055/s-0031-1296471Search in Google Scholar PubMed
Kovács, Á., Alogna, A., Post, H., and Hamdani, N. (2016). Is enhancing cGMP-PKG signalling a promising therapeutic target for heart failure with preserved ejection fraction? Neth. Heart J. 24, 268–274.10.1007/s12471-016-0814-xSearch in Google Scholar PubMed PubMed Central
Krüger, M., Kötter, S., Grützner, A., Lang, P., Andresen, C., Redfield, M.M., Butt, E., dos Remedios, C.G., and Linke, W.A. (2009). Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ. Res. 104, 87–94.10.1186/1471-2210-9-S1-P37Search in Google Scholar
Lang, M., Kojonazarov, B., Tian, X., Kalymbetov, A., Weissmann, N., Grimminger, F., Kretschmer, A., Stasch, J.P., Seeger, W., Ghofrani, H.A., et al. (2012). The soluble guanylate cyclase stimulator riociguat ameliorates pulmonary hypertension induced by hypoxia and SU5416 in rats. PLoS One 7, e43433.10.1371/journal.pone.0043433Search in Google Scholar PubMed PubMed Central
Leineweber, K., Moosmang, S., and Paulson, D. (2017). Genetics of no deficiency. Am. J. Cardiol. 120 (Suppl.), S80–S88.10.1016/j.amjcard.2017.06.013Search in Google Scholar PubMed
Leslie, M. (2016). Celebrity molecules promised to transform our health, but haven’t always lived up to their billing. Science 353, 1198–1201.10.1126/science.353.6305.1198Search in Google Scholar PubMed
Lewis, K.S., Butler, J., Bauersachs, J., and Sandner, P. (2017). The three-decade long journey in heart failure drug development. Handb. Exp. Pharmacol. 243, 1–14.10.1007/164_2016_101Search in Google Scholar PubMed
Masuyama, H., Tsuruda, T., Kato, J., Imamura, T., Asada, Y., Stasch, J.P., Kitamura, K., and Eto, T. (2006). Soluble guanylate cyclase stimulation on cardiovascular remodeling in angiotensin II-induced hypertensive rats. Hypertension 48, 972–978.10.1161/01.HYP.0000241087.12492.47Search in Google Scholar PubMed
Masuyama, H., Tsuruda, T., Sekita, Y., Hatakeyama, K., Imamura, T., Kato, J., Asada, Y., Stasch, J.P., and Kitamura, K. (2009). Pressure-independent effects of pharmacological stimulation of soluble guanylate cyclase on fibrosis in pressure-overloaded rat heart. Hypertens. Res. 32, 597–603.10.1038/hr.2009.64Search in Google Scholar
Matei, A.E., Beyer, C., Györfi, A.H., Soare, A., Chen, C.W., Dees, C., Bergmann, C., Ramming, A., Friebe, A., Hofmann, F., et al. (2018). Protein kinases G are essential downstream mediators of the antifibrotic effects of sGC stimulators. Ann. Rheum. Dis. 77, 459. doi:10.1136. [EPub ahead of print].10.1136Search in Google Scholar
Mathar, I., Kretschmer, A., Hartmann, E., Kolkhof, P., and Sandner, P. (2017). Combination of soluble guanylate cyclase stimulation and mineralocorticoid receptor antagonism as new treatment option for heart failure with preserved ejection fraction (HFpEF): results from a preclinical study with vericiguat and finerenone. Circulation 136, A17778.10.1161/circ.136.suppl_1.21000Search in Google Scholar
Methner, C., Buonincontri, G., Hu, C.H., Vujic, A., Kretschmer, A., Sawiak, S., Carpenter, A., Stasch, J.P., and Krieg, T. (2013). Riociguat reduces infarct size and post-infarct heart failure in mouse hearts: insights from MRI/PET imaging. PLoS One 8, e83910.10.1371/journal.pone.0083910Search in Google Scholar
Mülsch, A., Bauersachs, J., Schäfer, A., Stasch, J.P., Kast, R., and Busse, R. (1997). Effect of YC-1, an NO-independent, superoxide-sensitive stimulator of soluble guanylyl cyclase, on smooth muscle responsiveness to nitrovasodilators. Br. J. Pharmacol. 120, 681–689.10.1038/sj.bjp.0700982Search in Google Scholar
Murad, F. (1998). Nitric oxide signaling: would you believe that a simple free radical could be a second messenger, autacoid, paracrine substance, neurotransmitter, and hormone? Recent Prog. Horm. Res. 53, 43–59.Search in Google Scholar
Murrell, W. (1879). Nitro-glycerin as a remedy for angina pectoris. Lancet 113, 80–81.10.1016/S0140-6736(02)46032-1Search in Google Scholar
Nakai, T., Perl, N.R., Barden, T.C., Carvalho, A., Fretzen, A., Germano, P., Im G.Y., Jin, H., Kim, C., Lee, T.W., et al. (2016). Discovery of IWP-051, a novel orally bioavailable sGC stimulator with once-daily dosing potential in humans. ACS Med. Chem. Lett. 7, 465–469.10.1021/acsmedchemlett.5b00479Search in Google Scholar PubMed PubMed Central
Nowatzky, J., Wintermeyer, P., von Degenfeld, G., Hirth-Dietrich, C., and Sandner, P. (2011). Antifibrotic effect of the SGC-stimulator bay 41-2272 in the bile duct ligation liver fibrosis model in rats. Hepatology 54 (4 Suppl.), 755A 838.Search in Google Scholar
Pacher, P., Beckman, J.S., and Liaudet, L. (2007). Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 87, 315–424.10.1152/physrev.00029.2006Search in Google Scholar PubMed PubMed Central
Paul, T., Salazar-Degracia, A., Peinado, V.I., Tura-Ceide, O., Blanco, I., Barreiro, E., and Barberà, J.A. (2018). Soluble guanylate cyclase stimulation reduces oxidative stress in experimental chronic obstructive pulmonary disease. PLoS One 5, e0190628.10.1371/journal.pone.0190628Search in Google Scholar PubMed PubMed Central
Pieske, B., Butler, J., Filippatos, G., Lam, C., Maggioni, A.P., Ponikowski, P., Shah, S., Solomon, S., Kraigher-Krainer, E., Samano, E.T., et al. (2014). Rationale and design of the SOluble guanylate Cyclase stimulatoR in heArT failurE Studies (SOCRATES). Eur. J. Heart Fail. 16, 1026–1038.10.1002/ejhf.135Search in Google Scholar PubMed
Pieske, B., Maggioni, A.P., Lam, C.S.P., Pieske-Kraigher, E., Filippatos, G., Butler, J., Ponikowski, P., Shah, S.J., Solomon, S.D., Scalise, A.V., et al. (2017). Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heart failure patientS with PRESERVED EF (SOCRATES-PRESERVED) study. Eur. Heart J. 38, 1119–1127.10.1093/eurheartj/ehw593Search in Google Scholar PubMed PubMed Central
Prickaerts, J., van Staveren, W.C., Sik, A., Markerink-van Ittersum, M., Niewöhner, U., van der Staay, F.J., Blokland, A., and de Vente, J. (2002). Effects of two selective phosphodiesterase type 5 inhibitors, sildenafil and vardenafil, on object recognition memory and hippocampal cyclic GMP levels in the rat. Neuroscience 113, 351–361.10.1016/S0306-4522(02)00199-9Search in Google Scholar
Potoka, K.P., Wood, K.C., Baust, J.J., Bueno, M., Hahn, S.A., Vanderpool, R.R., Bachman, T., Mallampalli, G.M., Osei-Hwedieh, D.O., Schrott, V., et al. (2017). NO-independent sGC activation improves vascular function and cardiac remodeling in sickle cell disease. Am J Respir Cell Mol Biol. doi: 10.1165/rcmb.2017-0292OC. [Epub ahead of print].10.1165/rcmb.2017-0292OCSearch in Google Scholar PubMed PubMed Central
Rai, N., Veeroju, S., Schymura, Y., Janssen, W., Wietelmann, A., Kojonazarov, B., Weissmann, N., Stasch, J.P., Ghofrani, H.A., Seeger, W., et al. (2018). Effect of riociguat and sildenafil on right heart remodeling and function in pressure overload induced model of pulmonary arterial banding. BioMed. Res. Int. 2018. Article ID 3293584 (in press).10.1155/2018/3293584Search in Google Scholar
Rutten, K., Vente, J.D., Sik, A., Ittersum, M.M., Prickaerts, J., and Blokland, A. (2005). The selective PDE5 inhibitor, sildenafil, improves object memory in Swiss mice and increases cGMP levels in hippocampal slices. Behav. Brain Res. 164, 11–16.10.1016/j.bbr.2005.04.021Search in Google Scholar PubMed
Rutten, K., Basile, J.L., Prickaerts, J., Blokland, A., and Vivian, J.A. (2007). Selective PDE inhibitors rolipram and sildenafil improve object retrieval performance in adult cynomolgus macaques. Psychopharmacology (Berl). 196, 643–648.10.1007/s00213-007-0999-1Search in Google Scholar PubMed PubMed Central
Sandner, P. and Stasch, J.P. (2017). Anti-fibrotic effects of soluble guanylate cyclase stimulators and activators: a review of the preclinical evidence. Respir. Med. 122 (Suppl. 1), S1–S9.10.1016/j.rmed.2016.08.022Search in Google Scholar PubMed
Sandner, P., Neuser, D., and Bischoff, E. (2009). Erectile dysfunction and lower urinary tract. Handb. Exp. Pharmacol. 191, 507–531.10.1007/978-3-540-68964-5_22Search in Google Scholar PubMed
Sandner, P., Berger, P., and Zenzmaier, C. (2017). The Potential of sGC modulators for the treatment of age-related fibrosis: a mini-review. Gerontology 63, 216–227.10.1159/000450946Search in Google Scholar PubMed
Schermuly, R., Stasch, J.P., Pullamsetti, S.S., Middendorff, R., Mueller, D., Schlueter, K.D., Dingendorf, A., Hackemack, S., Kolosionek, E., Kaulen, C., et al. (2008). Expression and function of soluble guanylate cyclase in pulmonary arterial hypertension. Eur. Respir. J. 32, 881–891.10.1183/09031936.00114407Search in Google Scholar PubMed
Schlossmann, J. and Desch, M. (2009). cGK substrates. Handb. Exp. Pharmacol. 191, 163–193.10.1007/978-3-540-68964-5_9Search in Google Scholar PubMed
Schlossmann, J. and Desch, M. (2011). IRAG and novel PKG targeting in the cardiovascular system. Am. J. Physiol. Heart Circ. Physiol. 301, H672–H682.10.1152/ajpheart.00198.2011Search in Google Scholar PubMed
Schmidt, H.H., Schmidt, P.M., and Stasch, J.P. (2009). NO- and haem-independent soluble guanylate cyclase activators. Handb. Exp. Pharmacol. 191, 309–339.10.1007/978-3-540-68964-5_14Search in Google Scholar
Sharkovska, Y., Kalk, P., Lawrenz, B., Godes, M., Hoffmann, L.S., Wellkisch, K., Geschka, S., Relle, K., Hocher, B., and Stasch, J.P. (2010). Nitric oxide-independent stimulation of soluble guanylate cyclase reduces organ damage in experimental low-renin and high-renin models. J. Hypertens. 28, 1666–1675.10.1097/HJH.0b013e32833b558cSearch in Google Scholar
Simonneau, G., D’Armini, A.M., Ghofrani, H.A., Grimminger, F., Jansa, P., Kim, N.H., Mayer, E., Pulido, T., Wang, C., Colorado, P., et al. (2016). Predictors of long-term outcomes in patients treated with riociguat for chronic thromboembolic pulmonary hypertension: data from the CHEST-2 open-label, randomised, long-term extension trial. Lancet Respir. Med. 4, 372–380.10.1016/S2213-2600(16)30022-4Search in Google Scholar
Stasch, J.P. and Hobbs, A.J. (2009). NO-independent, haem-dependent soluble guanylate cyclase stimulators. Handb. Exp. Pharmacol. 191, 277–308.10.1007/978-3-540-68964-5_13Search in Google Scholar PubMed
Stasch, J.P. and Evgenov, O.V. (2013). Soluble guanylate cyclase stimulators in pulmonary hypertension. Handb. Exp. Pharmacol. 218, 279–313.10.1007/978-3-662-45805-1_12Search in Google Scholar
Stasch, J.P., Schmidt, P., Alonso-Alija, C., Apeler, H., Dembowsky, K., Haerter, M., Heil, M., Minuth, T., Perzborn, E., Pleiss, U., et al. (2002). NO- and haem-independent activation of soluble guanylyl cyclase: molecular basis and cardiovascular implications of a new pharmacological principle. Br. J. Pharmacol. 136, 773–783.10.1038/sj.bjp.0704778Search in Google Scholar PubMed PubMed Central
Stasch, J.P., Pacher, P., and Evgenov, O.V. (2009). Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 123, 2263–2273.10.1161/CIRCULATIONAHA.110.981738Search in Google Scholar PubMed PubMed Central
Stasch, J.P., Schlossmann, J., and Hocher, B. (2015). Renal effects of soluble guanylate cyclase stimulators and activators: a review of the preclinical evidence. Curr. Opin. Pharmacol. 21, 95–104.10.1016/j.coph.2014.12.014Search in Google Scholar PubMed
Tsai, E.J. and Kass, D.A. (2009). Cyclic GMP signaling in cardiovascular pathophysiology and therapeutics. Pharmacol. Ther. 122, 216–238.10.1016/j.pharmthera.2009.02.009Search in Google Scholar PubMed PubMed Central
Umar, S. and van der Laarse, A. (2010). Nitric oxide and nitric oxide synthase isoforms in the normal, hypertrophic, and failing heart. Mol. Cell Biochem. 333, 191–201.10.1007/s11010-009-0219-xSearch in Google Scholar PubMed
Weidenbach, A., Stasch, J.P., Ghofrani, H.A., Weissmann, N., Grimminger, F., Seeger, W., and Schermuly, R.T. (2005). Inhaled NO and the guanylate cyclase stimulator BAY 41–2272 in oleic acid induced acute lung injury in rabbits. BMC Pharmacol. 5, P61.10.1186/1471-2210-5-S1-P61Search in Google Scholar
Weissmann, N., Lobo, B., Pichl, A., Parajuli, N., Seimetz, M., Puig-Pey, R., Ferrer, E., Peinado, V.I., Domínguez-Fandos, D., Fysikopoulos, A., et al. (2014). Stimulation of soluble guanylate cyclase prevents cigarette smoke-induced pulmonary hypertension and emphysema. Am. J. Respir. Crit. Care Med. 189, 1359–1373.10.1164/rccm.201311-2037OCSearch in Google Scholar PubMed
Zenzmaier, C., Kern, J., Heitz, M., Plas, E., Zwerschke, W., Mattesich, M., Sandner, P., and Berger, P. (2015). Activators and stimulators of soluble guanylate cyclase counteract myofibroblast differentiation of prostatic and dermal stromal cells. Exp. Cell Res. 338, 162–169.10.1016/j.yexcr.2015.08.014Search in Google Scholar PubMed
©2018 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Highlight Issue ‘Molecular Basis of Life 2017’
- HIGHLIGHT: GBM Fall Meeting “Molecular Basis of Life 2017”
- Neuronal RNP granules: from physiological to pathological assemblies
- Regulation of LRRK2: insights from structural and biochemical analysis
- The role of (auto)-phosphorylation in the complex activation mechanism of LRRK2
- Oncogenic BRAFV600E drives expression of MGL ligands in the colorectal cancer cell line HT29 through N-acetylgalactosamine-transferase 3
- Hypoxia and serum deprivation induces glycan alterations in triple negative breast cancer cells
- Targeting autophagy for the treatment of cancer
- From molecules to patients: exploring the therapeutic role of soluble guanylate cyclase stimulators
- DNA-encoded libraries – an efficient small molecule discovery technology for the biomedical sciences
- Transcytosis of payloads that are non-covalently complexed to bispecific antibodies across the hCMEC/D3 blood-brain barrier model
- Mitochondrial contributions to neuronal development and function
- Intracellular communication between lipid droplets and peroxisomes: the Janus face of PEX19
- Protein crystallization in living cells
- Synthetic DNA filaments: from design to applications
- Spectroscopic characterization of the Co-substituted C-terminal domain of rubredoxin-2
- Twitch or swim: towards the understanding of prokaryotic motion based on the type IV pilus blueprint
Articles in the same Issue
- Frontmatter
- Highlight Issue ‘Molecular Basis of Life 2017’
- HIGHLIGHT: GBM Fall Meeting “Molecular Basis of Life 2017”
- Neuronal RNP granules: from physiological to pathological assemblies
- Regulation of LRRK2: insights from structural and biochemical analysis
- The role of (auto)-phosphorylation in the complex activation mechanism of LRRK2
- Oncogenic BRAFV600E drives expression of MGL ligands in the colorectal cancer cell line HT29 through N-acetylgalactosamine-transferase 3
- Hypoxia and serum deprivation induces glycan alterations in triple negative breast cancer cells
- Targeting autophagy for the treatment of cancer
- From molecules to patients: exploring the therapeutic role of soluble guanylate cyclase stimulators
- DNA-encoded libraries – an efficient small molecule discovery technology for the biomedical sciences
- Transcytosis of payloads that are non-covalently complexed to bispecific antibodies across the hCMEC/D3 blood-brain barrier model
- Mitochondrial contributions to neuronal development and function
- Intracellular communication between lipid droplets and peroxisomes: the Janus face of PEX19
- Protein crystallization in living cells
- Synthetic DNA filaments: from design to applications
- Spectroscopic characterization of the Co-substituted C-terminal domain of rubredoxin-2
- Twitch or swim: towards the understanding of prokaryotic motion based on the type IV pilus blueprint

