Abstract
Ghrelin is a 28-amino acid (aa) stomach-derived peptide discovered in 1999 as the endogenous ligand for growth hormone secretagogue-receptor (GHS-R). Ghrelin-producing cells constitute a distinct group of endocrine cells dispersed throughout the gastric mucosa and to a lesser extent in the small intestine and the endocrine pancreas. Ghrelin plasma levels rise during fasting and chronic caloric restriction to stimulate food intake and fat storage and to prevent life-threatening falls in blood glucose. Plasma ghrelin levels decrease after a meal is consumed and in conditions of energy surplus (such as obesity). Ghrelin has emerged as a key player in the regulation of appetite and energy homeostasis. Ghrelin achieves these functions through binding the ghrelin receptor GHS-R in appetite-regulating neurons and in peripheral metabolic organs including the endocrine pancreas. Ghrelin levels are negatively correlated with body mass index (BMI) and insulin resistance. In addition, ghrelin secretion is impaired in obesity and insulin resistance. Several studies highlight an important role for ghrelin in glucose homeostasis. Genetic, immunological, and pharmacological blockade of ghrelin signaling resulted in improved glucose tolerance and insulin sensitivity. Furthermore, exogenous ghrelin administration was shown to decrease glucose-induced insulin release and increase glucose level in both humans and rodents. GHS-R was shown to be expressed in pancreatic β-cells and ghrelin suppressed insulin release via a Ca2+-mediated pathway. In this review, we provide a detailed summary of recent advances in the field that focuses on the role of insulin and insulin resistance in the regulation of ghrelin secretion and on the role of ghrelin in glucose-stimulated insulin secretion (GSIS).
Introduction
Blood glucose is tightly controlled by the pancreatic hormones, insulin, and glucagon. Insulin secreted by pancreatic β-cells in response to a nutrient challenge acts on major target organs, i.e. skeletal muscle, liver, and adipose tissue, to increase glucose uptake and storage. Glucagon is secreted by pancreatic α-cells during the fasting state to increase blood glucose by promoting glycogenolysis in the liver. The most common glucose disorder, type 2 diabetes, is often associated with obesity and results from insufficient insulin production/secretion and insulin resistance [1].
Ghrelin is a peptide hormone that was first discovered in 1999 as the endogenous ligand to the (then orphan) growth hormone secretagogue-receptor (GHS-R) [2]. Centrally, the ligand-receptor interaction is important for increasing hunger and growth hormone secretion [2]. Thus, ghrelin is a fundamental regulator of energy homeostasis.
Although the highest expression of ghrelin occurs in the stomach, ghrelin-producing cells are also present in the pancreatic islets, where ghrelin may play a developmental role, as well as modulating the release of insulin and glucagon [3]. Ghrelin and the GHS-R are both expressed by cells in the pancreatic islets [4–6], raising the possibility of a novel system involved in islet hormone secretion through endocrine or paracrine mechanisms. Acylated ghrelin (AG) inhibits glucose-stimulated insulin secretion (GSIS) in β-cell lines and in animal models [6–8]. In humans, AG administration suppresses insulin secretion, induces peripheral insulin resistance, and impairs glucose tolerance [9–12].
Deficiency of the adipocyte hormone leptin results in hyperphagia, obesity, and insulin resistance. Surprisingly, ablation of ghrelin in leptin-deficient mice did not reverse hyperphagia and obesity. However, there was a reduction in blood glucose and an increase in insulin secretion and action when ghrelin was deleted [13]. These findings raise the possibility that the ghrelin-GHS-R system contributes to the regulation of β-cell function. Ghrelin antagonism may be a new approach for treating type 2 diabetes by increasing insulin secretion and enhancing peripheral insulin action [14].
Ghrelin levels are negatively correlated with body mass index (BMI) and insulin resistance [4, 15]. Glucose and insulin were shown to play an important role in postprandial ghrelin suppression [5]. Interestingly, ghrelin secretion was shown to be impaired in obesity and insulin resistance. Obese people were shown to have reduced fasting ghrelin levels and blunted postprandial suppression [6].
In this review, we will briefly summarize factors affecting ghrelin synthesis and secretion and focus on the role of ghrelin in controlling insulin secretion and glucose homeostasis.
Mechanism of proghrelin processing
Ghrelin is derived from an 114-amino acid (aa) preprohormone precursor, which requires both signal peptide cleavage and endoproteolytic cleavage by proprotein convertase-1 (PC1/3) to produce the active 28-aa peptide [7]. In addition to this processing, ghrelin is also modified by the addition of an oxygen-linked 8-carbon fatty acid (octanoic acid) on the third serine residue (AG) [2]. This unique acylation is produced by the ghrelin O-acyl transferase (GOAT) [8, 9]. Using antibodies specific for the octanoylated ghrelin and mass spectrometry analysis, Zhu et al. demonstrated that ghrelin can be acylated prior to being processed by PC1/3 [7]. In addition, GOAT is localized in the endoplasmic reticulum (ER) membrane, which also suggests that proghrelin is likely to be acylated prior to processing [9]. While only AG is able to bind to the ghrelin receptor, an unacylated ghrelin (UAG) form exists in the circulation at a 5–10-fold higher concentration than its acylated counterpart [10–12]. Some GHS-R independent actions of AG and UAG have been documented and such phenomena are reviewed in [16]. In addition to these two forms of ghrelin, a third peptide can also be produced from the C-terminal end of proghrelin called obestatin (Figure 1). Initially, this hormone was believed to have an opposite effect on appetite to that of ghrelin [17]. However, there are controversies in the role of obestatin as several other groups were not able to confirm its effects on appetite [18, 19].
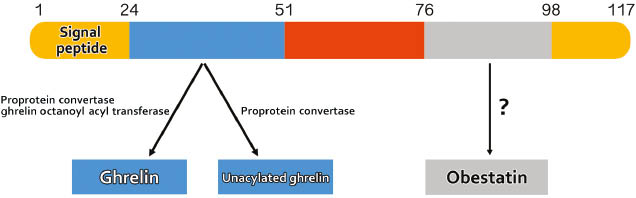
Proghrelin structure and derived peptides.
Preproghrelin is first produced as an 117-polypeptide precursor. During its synthesis or post-translationaly, an octanoic acid moiety is added to the third serine of the ghrelin peptide and the precursor is processed by PCSK1 to release the 28-amino-acid ghrelin peptide. Acylated ghrelin (active form) can become deacylated (inactive form). A third putative hormone, obestatin, may also be produced from the proghrelin precursor.
Localizations of ghrelin-producing cells
Ghrelin is primarily localized in the gastric fundus (upper region) within the oxyntic gland [20]. Ghrelin is not co-localized with other gastric cell types (enterochromaffin-like cells, enterochromaffin cells, and somatostatin cells). Rather, it is observed in the previously uncharacterized endocrine X/A cells [21]. Ghrelin-producing cells represent ~20% of the endocrine cells in the gastric fundus [20]. In addition to the fundus, ghrelin-positive cells are also sparsely localized in the upper region of the small intestine [20]. While the stomach is the major source of ghrelin in the circulation, ghrelin is also produced in other tissues where it may have a paracrine effect. Such production has been observed within the endocrine pancreas where it was shown to regulate insulin and glucagon secretion, in part, through a paracrine effect. In the human fetus (18–22 weeks), and at birth, about 10% of ghrelin-immunoreactive (IR) cells were detected in pancreatic islets [22, 23]. Comparing pancreatic and stomach ghrelin-IR cells at the same gestational age showed a predominance of ghrelin-producing cells in the pancreas. However, in a human adult, the percentage of ghrelin-IR cells is lower (~1% of pancreatic islets), and more prominent in stomach [22, 23].
A study from a human autopsy revealed that ghrelin-IR cells were co-localized with glucagon-producing α-cells [24]. On the contrary, Volante et al. analyzed non-tumoral human pancreatic tissue for ghrelin expression and found co-localization with insulin-producing β-cells, rather than α-cells [25]. Ghrelin was also found in rat pancreatic islets [26, 27]. Unlike human studies, animal studies showed distinctive ghrelin-producing cells in the pancreas, named ε-cells.
Nutrients regulate ghrelin synthesis and secretion
Ghrelin levels were shown to be elevated in rodents and human circulation during the fasting state, and decreased after nutrients were consumed [28, 29]. By sampling ghrelin over a 24-h period, Cummings et al. demonstrated the peaks and troughs of circulating ghrelin that occurred pre-meal and post-meal, respectively [5]. Initial investigations of the mechanism and regulation of ghrelin secretion compared several factors including stomach distention and meal type. A water meal was unable to cause the drop in ghrelin secretion that was observed with carbohydrate, ruling out the effect of stretch signaling [28]. Furthermore, blocking gastric emptying by occluding the pyloric sphincter prevented the effect of carbohydrate on reducing ghrelin levels [30]. This suggested that stomach luminal sensing may not play an important role compared to post gastric nutrient sensing. Indeed, gastric ghrelin-containing X/A cells mostly have no continuity with the lumen (closed type) and therefore are likely to respond to stimuli from the basolateral side. Gastric X/A cells were shown to be closely associated with the capillary network in the lamina propria. However, ghrelin cells in the small intestine were found to be “open” type and therefore are able to sense luminal content [3].
Both the quantity and the type of nutrients consumed can affect the degree and duration of the postprandial drop in ghrelin levels. In humans, Callahan et al. examined the effect of caloric content in liquid meals and identified that higher calorie meals caused a greater drop in ghrelin and prolonged satiety [31]. With regards to the type of macronutrient, meals comprised mainly of carbohydrate caused a greater drop in ghrelin compared to an isocaloric meal comprised primarily of fat in normal weight women [32]. A similar finding was later reported in rodents [33]. As previous reports indicated, the mere presence of nutrients in the stomach was unable to affect ghrelin secretion. Overduin et al. perfused nutrients into the duodenum and jejunum of the small intestine of rats. They determined that while the macronutrient composition did not affect the level of ghrelin suppression, the onset, and duration of ghrelin suppression were highest in the glucose-infused rats [34]. Interestingly, lipid infusions had the lowest effects on plasma ghrelin [34]. While these studies have indicated that carbohydrates may cause a more rapid and longer suppression of ghrelin levels, there may also be a rebound effect. Comparing the levels of ghrelin after an isocaloric meal, made principally of carbohydrate, protein or lipid, it was shown that carbohydrates cause a biphasic (drop then rise) in ghrelin post-prandially [10]. This greater rise in ghrelin may have implications in rebound hunger after a primarily carbohydrate meal. The exact mechanism by which glucose induces ghrelin suppression remains unknown and requires further examination.
Neurotransmitters and hormone-mediated regulation of ghrelin secretion
As stated above, many studies in clinical settings and rodent models have been fundamental in identifying factors involved in ghrelin secretion. However, the information these studies have provided are rather indirect as it is very difficult to control all aspects and factors in a living system given its complexity. In addition, even greater difficulties are met in teasing out which specific factors are involved in regulation and which molecular pathways are involved in the control of ghrelin secretion. Therefore, to clearly understand how ghrelin secretion is regulated and by which intracellular pathways, in vitro cellular models of ghrelin secretion, were required and have since been developed. Such models include primary cultures of dispersed rodent stomach cells [35, 36] and mouse ghrelinoma-derived cell lines [37, 38]. These models have been instrumental in dissecting the cellular signaling mechanism involved in regulating ghrelin secretion by various neurotransmitters and hormones, and few important examples will be summarized in this section.
Norepinephrine
Ghrelin was shown to be increased during fasting and during chronic negative energy balance. Norepinephrine (NE) was found to stimulate ghrelin secretion through the activation of the β1-adrenergic receptor expressed in ghrelin cells via increased cyclic adenosine monophosphate (cAMP) and protein kinase-A activity, whereas acetylcholine had no effect [35].
Insulin
Ghrelin levels were shown to fluctuate in reverse to insulin throughout the day, which is expected given the role of insulin in glucose uptake and promoting satiety [5]. Interestingly, obese individuals who are hyperinsulinemic have lower levels of ghrelin, which suggested potential regulation of ghrelin by insulin [15]. On this note, the use of primary rodent stomach cell culture determined that insulin could act directly on ghrelin cells through the insulin receptors to inhibit ghrelin secretion [35]. Furthermore, insulin inhibited both basal and NE-stimulated ghrelin secretion, caused an increase in phosphorylated serine-threonine kinase (AKT) through the phosphor-inositol-3 kinase-dependent pathway, and reduced intracellular cAMP, but did not alter proghrelin mRNA levels. Interestingly, pre-treating ghrelin cells with high concentrations (100 nM) of insulin for 24 h caused a reduction in insulin receptor expression and prevented the insulin-mediated AKT activation and the suppression of ghrelin secretion with no impact on NE-stimulated ghrelin secretion. Therefore, use of in vitro models found direct evidence that highlight the role of insulin and insulin resistance in the regulation of ghrelin secretion [35].
Glucagon
Ghrelin cells present in the primary rodent stomach cell culture were also found to express the glucagon receptor (GluR). Glucagon significantly stimulated proghrelin mRNA expression and subsequently ghrelin secretion. Glucagon-induced ghrelin secretion and proghrelin mRNA expression were mediated through increased cAMP levels, activation of extracellular signal-related kinases (ERK)1/2 by exchange protein activated by cAMP (EPAC) dependent pathway. These findings show a direct link between glucagon and stomach ghrelin production and secretion and highlight the role of mitogen-activated protein kinases (MAPK), the protein kinase A (PKA)-independent EPAC pathway, and the synergy between NE and glucagon in ghrelin release [36].
Circulating ghrelin levels in metabolic disease states
Ghrelin secretion is tightly regulated based on energy availability. This regulation is important as the downstream targets of ghrelin receptor activation will alter energy intake and metabolism. However, ghrelin levels can be dysregulated in metabolic diseases such as obesity and insulin resistance [39].
Exogenous ghrelin treatment was shown to cause obesity in rats [28]. In addition, individuals with Prader-Willi syndrome who are obese and hyperplasic have elevated plasma ghrelin [40]. Therefore, it was surprising that ghrelin levels were negatively correlated with BMI [4, 15]. In addition to the lower fasting ghrelin levels found in obesity, more studies are suggesting a blunted postprandial ghrelin suppression. Le Roux et al. examined the levels of ghrelin before and after varying calorie meals between obese and normal weight individuals. Strikingly, their results found that obese individuals (while having lower baseline ghrelin levels) had a significantly smaller percentage drop in their circulating ghrelin compared to their lean counterpart [41]. Recently, a study examining the postprandial drop in ghrelin in Hispanic adolescents also found the response to be blunted in obese individuals [6].
As obesity is highly linked with insulin resistance, efforts have been made to separate the effects of obesity and insulin resistance when examining ghrelin levels in humans. McLaughlin et al. examined a group of 40 obese individuals separated into insulin sensitive and insulin resistant groups. The insulin resistant group presented elevated levels of fasting insulin and reduced total ghrelin levels compared to insulin sensitive patients (with no co-founding effect from BMI) [42]. It appears that the hyperinsulinemia associated with insulin resistance, at least in the context of obesity, is an important factor leading to the decreased level of ghrelin. However, most of these studies did not investigate the correlation between insulin resistance and the dynamics of ghrelin secretion, i.e. the postprandial suppression of ghrelin secretion. Bacha et al. investigated the dynamics of ghrelin suppression after an oral glucose tolerance test (OGTT) in normal weight vs. overweight children and the relationship of ghrelin suppression to insulin sensitivity. Fasting ghrelin levels were significantly lower in overweight vs. normal weight youth and were mainly influenced by insulin sensitivity, independent of adiposity. The suppression of ghrelin correlated positively with the whole body insulin sensitivity index and negatively with the change in insulin at 30 min [43]. Collectively, these results suggest that alterations in ghrelin suppression in overweight children may be another manifestation of their insulin resistance. Strikingly, these results are complementary with findings using the primary culture of ghrelin cells that insulin resistance can occur at the level of X/A cells. The mechanisms leading the dysregulation of ghrelin secretion in obesity and insulin resistance require and warrant further investigations.
Ghrelin and the regulation of glucose homeostasis
Ghrelin was demonstrated to cause an increase in plasma glucose and a drop in insulin levels when injected into humans [44]. This corresponded with the discovery of the GHS-R in the islets of the pancreas [45]. Subsequently, most studies analyzing the effects of ghrelin on GSIS have shown an inhibition (reviewed [46]). Noteworthy, the pancreas is also a site of ghrelin production and the effect of ghrelin on the endocrine pancreas may be through a paracrine effect. Blocking endogenous ghrelin was found to increase insulin secretion, suggesting that ghrelin acts directly on pancreatic β-cells to inhibit insulin secretion [46].
Ghrelin was also shown to affect glucose homeostasis through an effect on hepatic glucose production [47]. In cultured hepatocytes, AG was demonstrated to stimulate glucose production while, UAG had the opposite effect [48]. In rats, chronic administration of a nonappetite-stimulating dose of ghrelin led to reduced glycogen synthase kinase and increased PGC1α (activator of gluconeogenesis) protein expression in livers [49]. In support of this, studies measuring hepatic glucose production in mice found a reduced insulin-induced suppression of gluconeogenesis when ghrelin was administered [47].
While the effect of ghrelin administration on food intake and fasting blood glucose was shown to be lost in GHS-R knockout (KO) mice, ghrelin administration in a GHS-R KO mouse with selective receptor expression in the brainstem had restored fasting glucose to wild-type levels but not the orexigenic effect [50]. These results suggest that the effect of ghrelin on glucose metabolism is in part occurring through ghrelin signaling in the central nervous system. Interestingly, when submitted to severe chronic calorie restriction, mice lacking components of the ghrelin system are unable to maintain euglycemia [51] suggesting that ghrelin is essential for survival during severe caloric restriction. Overall, these studies clearly implicate ghrelin in the regulation of glucose homeostasis through a direct action on pancreatic β-cells and glucose disposal by peripheral tissues (Figure 2). These finding also suggest that strategies interfering with ghrelin signaling may have beneficial effects in the management of type 2 diabetes [14].
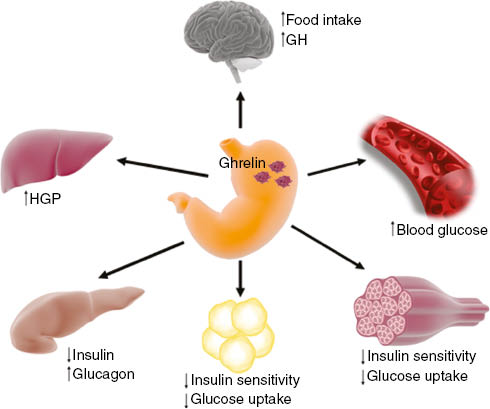
The effect of ghrelin on insulin secretion and glucose homeostasis.
Ghrelin is secreted by distinct stomach endocrine cells named X/A cells to act on the brain to increase food intake and growth hormone (GH) secretion. Ghrelin directly acts on pancreatic α- and β-cells to stimulate glucagon secretion and to inhibit glucose-induced insulin release, respectively. Ghrelin also increases hepatic glucose production (HGP) and decreases glucose uptake and insulin sensitivity in skeletal muscle and adipose tissue, leading to increased blood glucose.
Insulinostatic effect of ghrelin
Several human and animal studies showed that ghrelin exerts an insulinostatic effect. In a study involving 12 healthy non-obese subjects, ghrelin infusion significantly reduced acute insulin and C-peptide response to intravenous glucose in a dose-dependent manner [52]. Similarly, exogenous ghrelin reduced insulin secretion adjusted for insulin sensitivity and decreased the rate of glucose disappearance after the intravenous glucose infusion [52–54]. Comparing AG to UAG, Tong et al. demonstrated the effect of AG but not UAG on raising fasting blood glucose and decreasing insulin sensitivity [54].
In mice studies in vivo, ghrelin KO mice exhibited greater glucose-induced insulin secretion compared to wild-type mice despite normal insulin content per islets [24, 55]. When submitted to a glucose tolerance test, ghrelin KO mice showed enhanced insulin secretion and attenuated glucose responses compared to their wild-type littermates [55].
Intraperitoneal administration of the GHS-R antagonists ([D-Lys3]-GHRP-6) resulted in reducing fasting blood glucose concentrations by 10–30 mg/mL at 30 min and 60 min [56]. These results suggested the involvement of endogenous ghrelin in regulating glucose hemostasis and insulin secretion [56]. Intraperitoneal infusion of ghrelin (1 and 10 nmol/kg) resulted in elevated blood glucose and decreased insulin levels, which were reversed by infusion of a ghrelin antagonist [24, 56].
To test whether the ghrelin effect on insulin secretion is mediated by ghrelin locally produced in the pancreas or by circulating ghrelin originating from the stomach, gastrectomized rats were used. Interestingly, intraperitoneal injection of [D-Lys3]-GHRP-6 (10 μmol/kg) to gastrectomized rats enhanced plasma insulin concentrations to the same degree as normal rats, indicating that ghrelin antagonist effect is attributed to blockade of locally produced ghrelin in the pancreas instead of circulating ghrelin [55]. Similarly, in the perfused rat pancreas model, first and second phases of glucose-induced insulin secretion were enhanced by [D-Lys3]-GHRP-6 anti-ghrelin antiserum [55]. Administration of exogenous ghrelin suppressed both phases [55, 57]. Finally, in isolated rat islets, ghrelin antagonist and anti-ghrelin antiserum enhanced glucose-induced insulin secretion, while inhibited by exogenous ghrelin [24, 56, 58]. All the above studies clearly demonstrate an insulinostatic role of endogenous ghrelin.
GHS-R signaling in the pancreatic β-cell
Ghrelin receptor is expressed in pancreatic β-cells and ghrelin was shown to directly inhibit glucose-stimulating insulin secretion. In isolated rat islets, the effect of ghrelin antagonist on enhanced insulin secretion was suppressed in the absence of extracellular Ca2+ [56]. Using fura-2 microfluorimetry, first-phase of intracellular calcium [Ca2+]i response in islets was enhanced by adding [D-Lys3]-GHRP-6 or anti-ghrelin antiserum and the responses to varying glucose concentrations were higher in the presence of antagonists [56].
In isolated rat β-cells, ghrelin at high concentrations (10 nM) abolishes the peak of the first- and second-phase intracellular calcium ([Ca2+]i) responses to 8.3 mM glucose. The effect of ghrelin on [Ca2+]i oscillations was abolished by GHS-R antagonist [56].
To further investigate GHS-R G-protein coupling in β-cells, the effect of ghrelin in pertussis toxin (PTX)-treated rats was evaluated. Interestingly, the effects of endogenous and exogenous ghrelin were blunted, with no effect on ghrelin-induced growth hormone secretion. These results showed distinctive G-protein coupling for ghrelin on insulin and GH release [24]. In perfused pancreas of PTX-treated rats, glucose-induced insulin secretion (both phases) was increased, and ghrelin had no effect on insulin release [57]. These studies indicate that endogenous ghrelin suppresses insulin secretion via PTX-sensitive G-proteins [24]. In primary rat β-cells, treatment with specific antisense for the Gαi2-subunit of G-proteins resulted in ghrelin failure to decrease [Ca2+]i response to glucose and glucose-induced insulin release. Thus, Gαi2-mediated signaling is important for ghrelin suppressive effects on glucose-induced insulin release and attenuation of [Ca2+]i response in β-cells [24].
In MIN6 β-cell line transfected with a fluorescent-translocation biosensor, intracellular cAMP [cAMP]i was induced by raising glucose level from 3 to 11 mM in an oscillatory manner, and ghrelin suppresses it. The effect of ghrelin was reversible by its washout, suggesting that ghrelin inhibition of insulin secretion through reduction of [cAMP]i [57, 59].
Both ghrelin and somatostatin (SST) inhibit GSIS from pancreatic β-cells. Park et al. hypothesized that the mechanism by which ghrelin affects insulin secretion must accommodate noncanonical ghrelin receptor (GHS-R1a)-G-protein coupling to Gα(i/o) instead of Gα(q11) through the formation of a heterodimer with the somatostatin receptor (SST5). The dimerization causes differential signal mechanism and was elegantly elucidated by the use of INS-1SJ cell line, which presents decreased levels of GHSR and SST5 in comparison to rat islets. Thus, INS-1SJ could identify the role of heterodimerization in GSIS by supplementing the missing GPCRs (GHSR and/or SST5) through transfection. Through this cell line, Park et al. showed that in the absence of SST5, the ghrelin-GHSR complex could not inhibit GSIS. In the presence of SST5; however, GHSR-SST5 heterodimers were formed, and GSIS was inhibited in with ghrelin through Gα(i/o)-dependent pathway (Figure 3) [59].
![Figure 3: GHSR signaling in the pancreatic β-cell.In pancreatic β-cells, the ghrelin receptor (GHSR) and the somatostatin receptor (SST5) form a heterodimer when the (ghrelin)/(somatostatin) ratio is high. Ghrelin then activates Gα(i/o)-dependent pathway decreasing cAMP accumulation. Decreased cAMP level enhances Kv2.1 channel conductance, which decreases [Ca2+]i.. This pathway results in attenuation of glucose-stimulating insulin secretion.](/document/doi/10.1515/hmbci-2016-0018/asset/graphic/j_hmbci-2016-0018_fig_003.jpg)
GHSR signaling in the pancreatic β-cell.
In pancreatic β-cells, the ghrelin receptor (GHSR) and the somatostatin receptor (SST5) form a heterodimer when the (ghrelin)/(somatostatin) ratio is high. Ghrelin then activates Gα(i/o)-dependent pathway decreasing cAMP accumulation. Decreased cAMP level enhances Kv2.1 channel conductance, which decreases [Ca2+]i.. This pathway results in attenuation of glucose-stimulating insulin secretion.
Conclusion
It is now evident that ghrelin plays an important role in the regulation of insulin secretion and glucose homeostasis. Exogenous ghrelin administration decreases the glucose-induced insulin release and increases the blood glucose level in humans and rodents. Endogenous ghrelin was shown to suppress insulin release via a Ca2+-mediated pathway. The ratio of the GHS-R to SST receptors in pancreatic β-cells determines whether ghrelin will activate the G-protein αi or αq to modulate insulin release (Figure 3). These results support a key direct role of ghrelin in the regulation of insulin secretion.
Studies using pharmacological blockade, immunoneutralization, and genetic deletion of ghrelin or GOAT all resulted in enhanced insulin sensitivity. These results support a key role of ghrelin in the regulation of insulin secretion and glucose homeostasis. Overall, these studies clearly implicate ghrelin in the regulation of glucose homeostasis and suggest that strategies interfering with ghrelin signaling may have beneficial effects in the management of type 2 diabetes.
Funding source: Canadian Institutes of Health Research
Award Identifier / Grant number: MOP-82795
Funding statement: Dr. Younes Anini’s laboratory was supported by grants from the Canadian Institutes of Health Research (MOP-82795), Canada Foundation for Innovation and the IWK Research Foundation, Halifax, NS, Canada. Bader Alamri was sponsored by King Fahad Specialist Hospital in Dammam (KFSH-D), Saudi Arabia. Kyungsoo Shin was supported by a studentship from the Natural Sciences and Engineering Research Council of Canada
Acknowledgments:
Dr. Younes Anini’s laboratory was supported by grants from the Canadian Institutes of Health Research (MOP-82795), Canada Foundation for Innovation and the IWK Research Foundation, Halifax, NS, Canada. Bader Alamri was sponsored by King Fahad Specialist Hospital in Dammam (KFSH-D), Saudi Arabia. Kyungsoo Shin was supported by a studentship from the Natural Sciences and Engineering Research Council of Canada
References
1. Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 2014;383:1068–83.10.1016/S0140-6736(13)62154-6Search in Google Scholar
2. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999;402:656–60.10.1038/45230Search in Google Scholar PubMed
3. Muller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, Batterham RL, Benoit SC, Bowers CY, Broglio F, Casanueva FF, D’Alessio D, Depoortere I, Geliebter A, Ghigo E, Cole PA, Cowley M, Cummings DE, Dagher A, Diano S, Dickson SL, Dieguez C, Granata R, Grill HJ, Grove K, Habegger KM, Heppner K, Heiman ML, Holsen L, Holst B, Inui A, Jansson JO, Kirchner H, Korbonits M, Laferrere B, LeRoux CW, Lopez M, Morin S, Nakazato M, Nass R, Perez-Tilve D, Pfluger PT, Schwartz TW, Seeley RJ, Sleeman M, Sun Y, Sussel L, Tong J, Thorner MO, van der Lely AJ, van der Ploeg LH, Zigman JM, Kojima M, Kangawa K, Smith RG, Horvath T, Tschop MH. Ghrelin. Mol Metab 2015;4:437–60.10.1016/j.molmet.2015.03.005Search in Google Scholar PubMed PubMed Central
4. Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, Nozoe S, Hosoda H, Kangawa K, Matsukura S. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab 2002;87:240–4.10.1210/jcem.87.1.8129Search in Google Scholar PubMed
5. Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001;50:1714–9.10.2337/diabetes.50.8.1714Search in Google Scholar PubMed
6. Mittelman SD, Klier K, Braun S, Azen C, Geffner ME, Buchanan TA. Obese adolescents show impaired meal responses of the appetite-regulating hormones ghrelin and PYY. Obesity (Silver Spring) 2010;18:918–25.10.1038/oby.2009.499Search in Google Scholar PubMed PubMed Central
7. Zhu X, Cao Y, Voogd K, Steiner DF. On the processing of proghrelin to ghrelin. J Biol Chem 2006;281:38867–70.10.1074/jbc.M607955200Search in Google Scholar PubMed
8. Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, Witcher DR, Luo S, Onyia JE, Hale JE. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA 2008;105:6320–5.10.1073/pnas.0800708105Search in Google Scholar PubMed PubMed Central
9. Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 2008;132:387–96.10.1016/j.cell.2008.01.017Search in Google Scholar PubMed
10. Foster-Schubert KE, Overduin J, Prudom CE, Liu J, Callahan HS, Gaylinn BD, Thorner MO, Cummings DE. Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J Clin Endocrinol Metab 2008;93:1971–9.10.1210/jc.2007-2289Search in Google Scholar PubMed PubMed Central
11. Morash MG, Gagnon J, Nelson S, Anini Y. Tissue distribution and effects of fasting and obesity on the ghrelin axis in mice. Regul Pept 2010;163:62–73.10.1016/j.regpep.2010.03.010Search in Google Scholar PubMed
12. Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun 2000;279:909–13.10.1006/bbrc.2000.4039Search in Google Scholar PubMed
13. Sun Y, Asnicar M, Saha PK, Chan L, Smith RG. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab 2006;3:379–86.10.1016/j.cmet.2006.04.004Search in Google Scholar PubMed
14. Gagnon J, Zhu L, Anini Y, Wang Q. Neutralizing circulating ghrelin by expressing a growth hormone secretagogue receptor-based protein protects against high-fat diet-induced obesity in mice. Gene Ther 2015;22:750–7.10.1038/gt.2015.38Search in Google Scholar PubMed
15. Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes 2001;50:707–9.10.2337/diabetes.50.4.707Search in Google Scholar PubMed
16. Baragli A, Lanfranco F, Allasia S, Granata R, Ghigo E. Neuroendocrine and metabolic activities of ghrelin gene products. Peptides 2011;32:2323–32.10.1016/j.peptides.2011.10.024Search in Google Scholar PubMed
17. Zhang JV, Ren PG, Avsian-Kretchmer O, Luo CW, Rauch R, Klein C, Hsueh AJ. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science 2005;310:996–9.10.1126/science.1117255Search in Google Scholar PubMed
18. Nogueiras R, Pfluger P, Tovar S, Arnold M, Mitchell S, Morris A, Perez-Tilve D, Vazquez MJ, Wiedmer P, Castaneda TR, DiMarchi R, Tschop M, Schurmann A, Joost HG, Williams LM, Langhans W, Dieguez C. Effects of obestatin on energy balance and growth hormone secretion in rodents. Endocrinology 2007;148:21–6.10.1210/en.2006-0915Search in Google Scholar PubMed
19. Seoane LM, Al-Massadi O, Pazos Y, Pagotto U, Casanueva FF. Central obestatin administration does not modify either spontaneous or ghrelin-induced food intake in rats. J Endocrinol Invest 2006;29:RC13–5.10.1007/BF03344174Search in Google Scholar PubMed
20. Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 2000;141:4255–61.10.1210/endo.141.11.7757Search in Google Scholar PubMed
21. Stengel A, Tache Y. Ghrelin – a pleiotropic hormone secreted from endocrine x/a-like cells of the stomach. Front Neurosci 2012;6:24.10.3389/fnins.2012.00024Search in Google Scholar
22. Wierup N, Sundler F, Heller RS. The islet ghrelin cell. J Mol Endocrinol 2014;52:R35–49.10.1530/JME-13-0122Search in Google Scholar
23. Wierup N, Svensson H, Mulder H, Sundler F. The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regul Pept 2002;107:63–9.10.1016/S0167-0115(02)00067-8Search in Google Scholar
24. Dezaki K, Kakei M, Yada T. Ghrelin uses Galphai2 and activates voltage-dependent K+ channels to attenuate glucose-induced Ca2+ signaling and insulin release in islet beta-cells: novel signal transduction of ghrelin. Diabetes 2007;56:2319–27.10.2337/db07-0345Search in Google Scholar PubMed
25. Volante M, Allia E, Gugliotta P, Funaro A, Broglio F, Deghenghi R, Muccioli G, Ghigo E, Papotti M. Expression of ghrelin and of the GH secretagogue receptor by pancreatic islet cells and related endocrine tumors. J Clin Endocrinol Metab 2002;87: 1300–8.10.1210/jcem.87.3.8279Search in Google Scholar PubMed
26. Adeghate E, Ponery AS. Ghrelin stimulates insulin secretion from the pancreas of normal and diabetic rats. J Neuroendocrinol 2002;14:555–60.10.1046/j.1365-2826.2002.00811.xSearch in Google Scholar PubMed
27. Wierup N, Yang S, McEvilly RJ, Mulder H, Sundler F. Ghrelin is expressed in a novel endocrine cell type in developing rat islets and inhibits insulin secretion from INS-1 (832/13) cells. J Histochem Cytochem 2004;52:301–10.10.1177/002215540405200301Search in Google Scholar PubMed
28. Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature 2000;407:908–13.10.1038/35038090Search in Google Scholar PubMed
29. Tschop M, Wawarta R, Riepl RL, Friedrich S, Bidlingmaier M, Landgraf R, Folwaczny C. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest 2001;24:RC19–21.10.1007/BF03351037Search in Google Scholar PubMed
30. Williams DL, Cummings DE, Grill HJ, Kaplan JM. Meal-related ghrelin suppression requires postgastric feedback. Endocrinology 2003;144:2765–7.10.1210/en.2003-0381Search in Google Scholar PubMed
31. Callahan HS, Cummings DE, Pepe MS, Breen PA, Matthys CC, Weigle DS. Postprandial suppression of plasma ghrelin level is proportional to ingested caloric load but does not predict intermeal interval in humans. J Clin Endocrinol Metab 2004;89: 1319–24.10.1210/jc.2003-031267Search in Google Scholar PubMed
32. Monteleone P, Bencivenga R, Longobardi N, Serritella C, Maj M. Differential responses of circulating ghrelin to high-fat or high-carbohydrate meal in healthy women. J Clin Endocrinol Metab 2003;88:5510–4.10.1210/jc.2003-030797Search in Google Scholar PubMed
33. Sanchez J, Oliver P, Palou A, Pico C. The inhibition of gastric ghrelin production by food intake in rats is dependent on the type of macronutrient. Endocrinology 2004;145:5049–55.10.1210/en.2004-0493Search in Google Scholar PubMed
34. Overduin J, Frayo RS, Grill HJ, Kaplan JM, Cummings DE. Role of the duodenum and macronutrient type in ghrelin regulation. Endocrinology 2005;146:845–50.10.1210/en.2004-0609Search in Google Scholar PubMed
35. Gagnon J, Anini Y. Insulin and norepinephrine regulate ghrelin secretion from a rat primary stomach cell culture. Endocrinology 2012;153:3646–56.10.1210/en.2012-1040Search in Google Scholar PubMed
36. Gagnon J, Sheppard E, Anini Y. Metformin directly inhibits ghrelin secretion through AMP-activated protein kinase in rat primary gastric cells. Diabetes Obes Metab 2013;15:276–9.10.1111/dom.12021Search in Google Scholar PubMed
37. Iwakura H, Li Y, Ariyasu H, Hosoda H, Kanamoto N, Bando M, Yamada G, Hosoda K, Nakao K, Kangawa K, Akamizu T. Establishment of a novel ghrelin-producing cell line. Endocrinology 2010;151:2940–5.10.1210/en.2010-0090Search in Google Scholar PubMed
38. Zhao TJ, Sakata I, Li RL, Liang G, Richardson JA, Brown MS. Goldstein JL, Zigman JM, Ghrelin secretion stimulated by {beta}1-adrenergic receptors in cultured ghrelinoma cells and in fasted mice. Proc Natl Acad Sci USA 2010;107:15868–73.10.1073/pnas.1011116107Search in Google Scholar PubMed PubMed Central
39. Zigman JM, Bouret SG, Andrews ZB. Obesity Impairs the Action of the Neuroendocrine Ghrelin System. Trends Endocrinol Metab 2016;27:54–63.10.1016/j.tem.2015.09.010Search in Google Scholar PubMed PubMed Central
40. Cummings DE, Clement K, Purnell JQ, Vaisse C, Foster KE, Frayo RS, Schwartz MW, Basdevant A, Weigle DS. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat Med 2002;8:643–4.10.1038/nm0702-643Search in Google Scholar PubMed
41. Le Roux CW, Patterson M, Vincent RP, Hunt C, Ghatei MA, Bloom SR. Postprandial plasma ghrelin is suppressed proportional to meal calorie content in normal-weight but not obese subjects. J Clin Endocrinol Metab 2005;90:1068–71.10.1210/jc.2004-1216Search in Google Scholar
42. McLaughlin T, Abbasi F, Lamendola C, Frayo RS, Cummings DE. Plasma ghrelin concentrations are decreased in insulin-resistant obese adults relative to equally obese insulin-sensitive controls. J Clin Endocrinol Metab 2004;89:1630–5.10.1210/jc.2003-031572Search in Google Scholar
43. Bacha F, Arslanian SA. Ghrelin suppression in overweight children: a manifestation of insulin resistance? J Clin Endocrinol Metab 2005;90:2725–30.10.1210/jc.2004-1582Search in Google Scholar
44. Broglio F, Arvat E, Benso A, Gottero C, Muccioli G, Papotti M, van der Lely AJ, Deghenghi R, Ghigo E. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab 2001;86:5083–6.10.1210/jcem.86.10.8098Search in Google Scholar
45. Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res 1997;48:23–9.10.1016/S0169-328X(97)00071-5Search in Google Scholar
46. Dezaki K, Sone H, Yada T. Ghrelin is a physiological regulator of insulin release in pancreatic islets and glucose homeostasis. Pharmacol Ther 2008;118:239–49.10.1016/j.pharmthera.2008.02.008Search in Google Scholar PubMed
47. Heijboer AC, van den Hoek AM, Parlevliet ET, Havekes LM, Romijn JA, Pijl H, Corssmit EP. Ghrelin differentially affects hepatic and peripheral insulin sensitivity in mice. Diabetologia 2006;49:732–8.10.1007/s00125-006-0138-2Search in Google Scholar PubMed
48. Gauna C, Delhanty PJ, Hofland LJ, Janssen JA, Broglio F, Ross RJ, Ghigo E, van der Lely AJ. Ghrelin stimulates, whereas des-octanoyl ghrelin inhibits, glucose output by primary hepatocytes. J Clin Endocrinol Metab 2005;90:1055–60.10.1210/jc.2004-1069Search in Google Scholar PubMed
49. Barazzoni R, Zanetti M, Cattin MR, Visintin L, Vinci P, Cattin L, Stebel M, Guarnieri G. Ghrelin enhances in vivo skeletal muscle but not liver AKT signaling in rats. Obesity (Silver Spring) 2007;15:2614–23.10.1038/oby.2007.313Search in Google Scholar PubMed
50. Scott MM, Perello M, Chuang JC, Sakata I, Gautron L, Lee CE, Lauzon D, Elmquist JK, Zigman JM. Hindbrain ghrelin receptor signaling is sufficient to maintain fasting glucose. PLoS One 2012;7:e44089.10.1371/journal.pone.0044089Search in Google Scholar PubMed PubMed Central
51. Zhao TJ, Liang G, Li RL, Xie X, Sleeman MW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Goldstein JL, Brown MS. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci USA 2010;107:7467–72.10.1073/pnas.1002271107Search in Google Scholar PubMed PubMed Central
52. Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Kahn SE, Cummings DE, Tschop MH, D’Alessio D. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes 2010;59:2145–51.10.2337/db10-0504Search in Google Scholar PubMed PubMed Central
53. Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Tschop MH, D’Alessio D. Physiologic concentrations of exogenously infused ghrelin reduces insulin secretion without affecting insulin sensitivity in healthy humans. J Clin Endocrinol Metab 2013;98: 2536–43.10.1210/jc.2012-4162Search in Google Scholar PubMed PubMed Central
54. Tong J, Davis HW, Summer S, Benoit SC, Haque A, Bidlingmaier M, Tschop MH, D’Alessio D. Acute administration of unacylated ghrelin has no effect on Basal or stimulated insulin secretion in healthy humans. Diabetes 2014;63:2309–19.10.2337/db13-1598Search in Google Scholar PubMed PubMed Central
55. Dezaki K, Sone H, Koizumi M, Nakata M, Kakei M, Nagai H, Hosoda H, Kangawa K, Yada T. Blockade of pancreatic islet-derived ghrelin enhances insulin secretion to prevent high-fat diet-induced glucose intolerance. Diabetes 2006;55:3486–93.10.2337/db06-0878Search in Google Scholar PubMed
56. Dezaki K, Hosoda H, Kakei M, Hashiguchi S, Watanabe M, Kangawa K, Yada T. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in beta-cells: implication in the glycemic control in rodents. Diabetes 2004;53:3142–51.10.2337/diabetes.53.12.3142Search in Google Scholar PubMed
57. Dezaki K, Damdindorj B, Sone H, Dyachok O, Tengholm A, Gylfe E, Kurashina T, Yoshida M, Kakei M, Yada T. Ghrelin attenuates cAMP-PKA signaling to evoke insulinostatic cascade in islet beta-cells. Diabetes 2011;60:2315–24.10.2337/db11-0368Search in Google Scholar PubMed PubMed Central
58. Damdindorj B, Dezaki K, Kurashina T, Sone H, Rita R, Kakei M, Yada T. Exogenous and endogenous ghrelin counteracts GLP-1 action to stimulate cAMP signaling and insulin secretion in islet beta-cells. FEBS Lett 2012;586:2555–62.10.1016/j.febslet.2012.06.034Search in Google Scholar PubMed
59. Park S, Jiang H, Zhang H, Smith RG. Modification of ghrelin receptor signaling by somatostatin receptor-5 regulates insulin release. Proc Natl Acad Sci USA 2012;109:19003–8.10.1073/pnas.1209590109Search in Google Scholar PubMed PubMed Central
©2016 by De Gruyter
Articles in the same Issue
- Frontmatter
- Editorial Preface
- Preface to special issue on “Hormones and Diabetes”
- Topic A: Insulin Resistance, Pre-Diabetes and Diabetes Status: Endocrine and Molecular Mechanisms
- Mini-Review Article
- The role of ghrelin in the regulation of glucose homeostasis
- Review Articles
- Insulin-stimulated glucose uptake in healthy and insulin-resistant skeletal muscle
- Adipose tissue: an endocrine organ playing a role in metabolic regulation
- Intramyocellular fat storage in metabolic diseases
- Aldosterone and type 2 diabetes mellitus
- 1,25-Dihydroxyvitamin D3 and type 2 diabetes: Ca2+-dependent molecular mechanisms and the role of vitamin D status
- Original Article
- Suppressor of cytokine signaling 2 (SOCS2) deletion protects against multiple low dose streptozotocin-induced type 1 diabetes in adult male mice
Articles in the same Issue
- Frontmatter
- Editorial Preface
- Preface to special issue on “Hormones and Diabetes”
- Topic A: Insulin Resistance, Pre-Diabetes and Diabetes Status: Endocrine and Molecular Mechanisms
- Mini-Review Article
- The role of ghrelin in the regulation of glucose homeostasis
- Review Articles
- Insulin-stimulated glucose uptake in healthy and insulin-resistant skeletal muscle
- Adipose tissue: an endocrine organ playing a role in metabolic regulation
- Intramyocellular fat storage in metabolic diseases
- Aldosterone and type 2 diabetes mellitus
- 1,25-Dihydroxyvitamin D3 and type 2 diabetes: Ca2+-dependent molecular mechanisms and the role of vitamin D status
- Original Article
- Suppressor of cytokine signaling 2 (SOCS2) deletion protects against multiple low dose streptozotocin-induced type 1 diabetes in adult male mice

