Abstract
In this work, the possibility of using ruthenium-based Shvo catalyst for bond cleavage of lignin model compounds and lignin has been investigated. Shvo-catalyzed degradation procedure was applied to four lignin model compounds containing β–O–4 linkages, common for native lignin fractions. The reactions, in general, resulted in Cβ–Cγ and Cγ–Oγ bond cleavage, with the degradation products depending on the exact model compound studied. Distribution of the degradation products can be explained by the range of substituents and structural complexity of the model compounds. The major products of degradation were separated using preparative HPLC and characterized to enable further studies on bond cleavage in lignin. The Shvo-catalyzed degradation protocol was further applied to the lignan hydroxymatairesinol and a model diol with protected primary hydroxyl group. For both compounds, only dehydrogenation of benzylic hydroxyl group in the absence of bond cleavage was observed. With extracted lignin, only thermal degradation of the starting material was evidenced, while detailed characterization of the reaction products was not possible due to their poor solubility and structural complexity.
1 Introduction
Lignin – the Nature’s aromatic polymer – is a potential renewable resource for synthesis of a wide range of aromatic compounds and materials for different industrial and commodity applications (Li et al. 2015). Lignin structure is built from three phenylpropanoid units connected with different linkages (Ralph et al. 2019). The most common linkage in native lignin is the β–O–4 ether linkage (Figure 1) (Adler 1977). Consequently, lignin model compounds containing β–O–4 linkages are widely used for studying the reactivity of lignin and for developing new procedures for lignin modification, including depolymerization (Binder et al. 2009; Gao et al. 2018; Guadix-Montero and Sankar 2018; Parthasarathi et al. 2011).
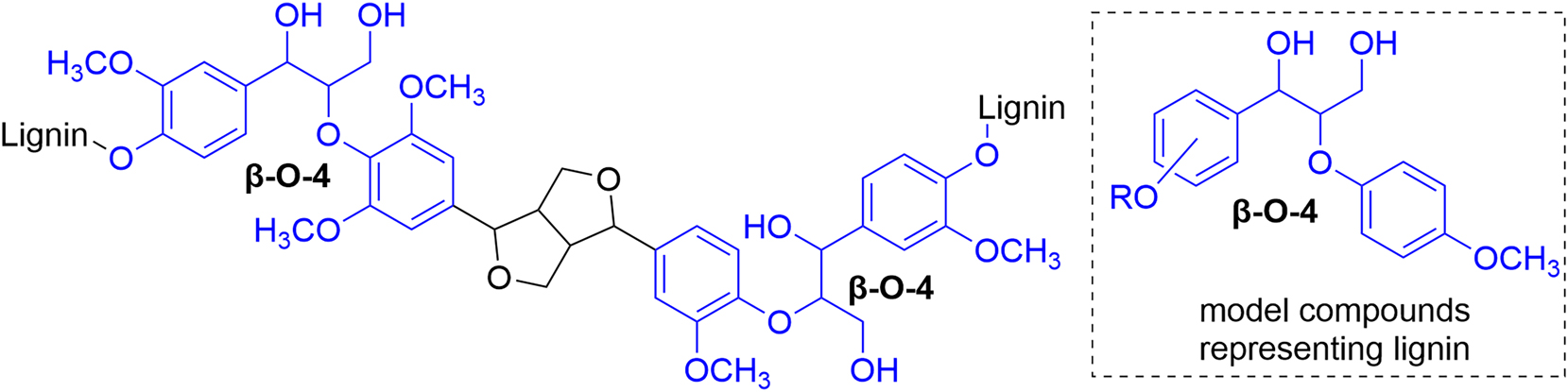
Structures of lignin rich with β–O–4 linkages and the lignin model compounds studied in this work.
Depolymerization is a promising direction for lignin valorization towards the different bio-based products. Developing procedures for lignin defragmentation is, however, complicated, due to the structure complexity and high variety of C–C and C–O linkages in the cross-linked lignin structures (Ragauskas et al. 2014).
Oxidative deconstruction using both heterogeneous and homogeneous metal-based catalysts is one of the approaches for lignin depolymerization described in the literature (Biannic and Bozell 2013; Chmely et al. 2013; Deuss and Barta 2016; Lange et al. 2013; Rahimi et al. 2013; Son and Toste 2010). It was shown that Ni (Klein et al. 2015; Ma et al. 2019a; Sergeev and Hartwig 2011; Wang et al. 2019), Pd (Onwudili and Williams 2014; Shu et al. 2015, 2018), Co (Badamali et al. 2009; Biswas et al. 2023), V (Chan et al. 2013; Mottweiler et al. 2015; Son and Toste 2010), Cu (Barta et al. 2014; McClelland et al. 2019; Mottweiler et al. 2015; Sedai et al. 2011), Pt (Kim et al. 2015; Wang et al. 2020), Ir (Nguyen et al. 2014; Hao et al. 2018), Rh (Liu et al. 2019) and Ru-based (Deng et al. 2023; Kloekhorst and Heeres 2015; Li and Song 2019; Ma et al. 2019b; Yao et al. 2015) catalysts can be used for lignin degradation towards phenolic products (Figure 2).
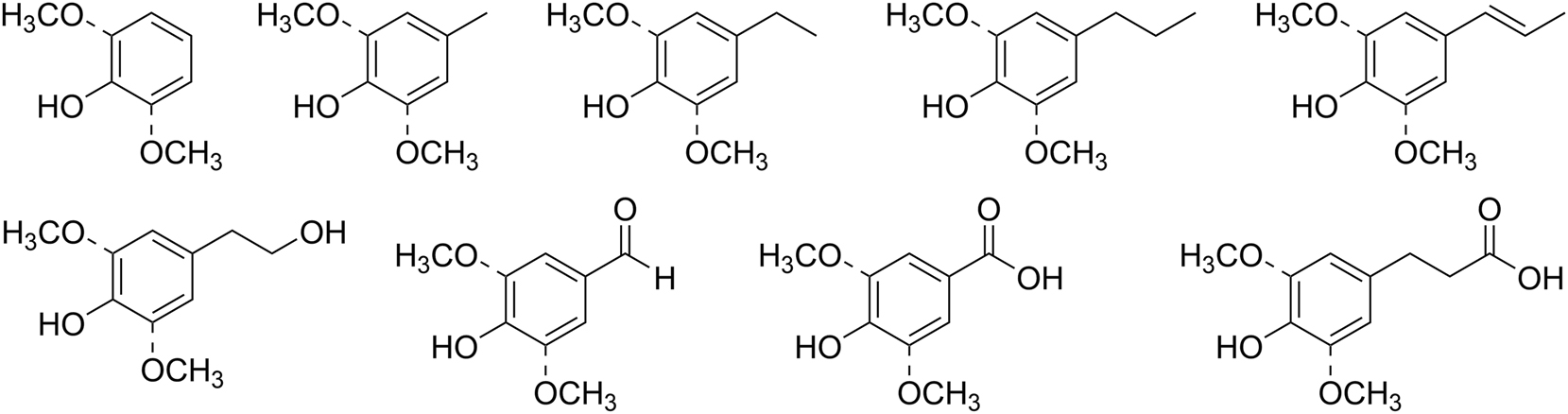
Common phenolic products of catalytic lignin degradation.
It has been shown that homogeneous ruthenium-based catalytic systems can be used for selective C–C and C–O bond degradation in β–O–4 fragments, resulting in the formation of acetophenone in up to 100 % yield (Huo et al. 2014; Nichols et al. 2010; vom Stein et al. 2015; Wu et al. 2012; Zhang et al. 2019). It was earlier demonstrated that Shvo catalyst (Figure 3) can be used for Cα–Cβ and Cγ–Oγ bond cleavage in β–O–4 model compounds leading to formation of arylaldehydes, guaiacol and other products (Kusumoto et al. 2020). It was shown that Shvo catalyst can be used for hydrogenation of lignin-derived monomers in the bio-oils (Busetto et al. 2011). In the studies on catalytic valorization of olive pomace it was shown that Shvo-catalyst allows to obtain various phenolic products from lignin containing feedstock (Cequier et al. 2020).
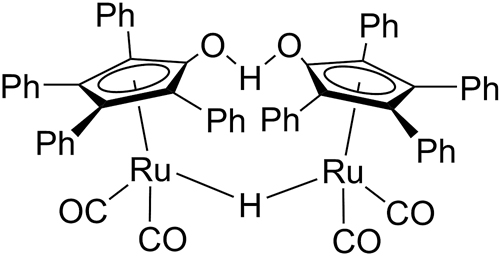
Shvo catalyst structure.
In previous work, a new methodology for selective dehydrogenation of the diol fragments in lignin model compounds, using Shvo-catalyzed hydrogen borrowing procedure was developed (Badazhkova et al. 2022, 2024). During the temperature screening of selective dehydrogenation of 1,3-diol fragments in lignin model compounds, using 2-butanone as both the solvent and the hydrogen acceptor, cleavage of the Cβ–Cγ and Cγ–Oγ bonds in 1-phenyl-1,3-propanediol (1) was observed. At 60 °C, the main product observed was 3-hydroxypropiophenone (2) and only traces of the degradation products were detected.
After increasing the reaction temperature to 140 °C, no 3-hydroxypropiophenone was detected and the reaction products were mainly acetophenone (3) and propiophenone (4) (Scheme 1).
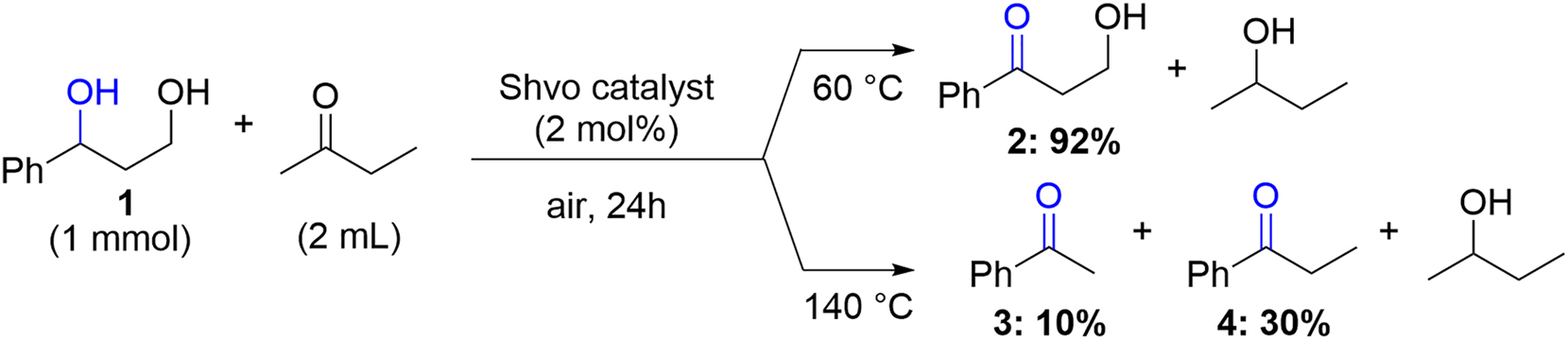
Shvo-catalyzed transfer dehydrogenation of the 1-phenyl-1,3-propanediol model compound, resulting in different main products depending on the reaction temperature.
The objective of this study was to investigate the possibility to apply Shvo-catalyzed dehydrogenation at higher temperatures for degradation of lignin model diols containing β–O–4 fragments, and for degradation of extracted lignin. Compared to previous work by other authors, here new products were obtained from Cβ–Cγ and Cγ–Oγ bond cleavage in the lignin model diols, with the Cβ–Oβ bond remaining intact. New degradation products were separated and characterized to gain understanding on the reactivity and degradation of the lignin model compounds, together with extracted ethanosolve lignin, and for developing more facile analysis of the resulting product fractions.
2 Materials and methods
All chemicals including the Shvo catalyst were purchased from Tokyo Chemical Industry (TCI), abcr GmbH & Co KG or Sigma-Aldrich, and used without further purification unless otherwise indicated. Lignin model compounds were synthesized from the corresponding acetophenones according to literature procedures (Forsythe et al. 2013) and used in the reactions as mixtures of stereoisomers in varying ratios. The NMR spectra were recorded using 500 MHz NMR spectrometer (Bruker) with DMSO-d6 containing internal standard with known concentration. The NMR signal assignments were based on 2D NMR (NOESY, DEPT, COSY, HSQC and HMBC). High resolution mass spectroscopy (HRMS) was carried out with a Waters ACQUITY RDa Detector equipped with a time of flight (TOF) mass analyzer. The product distribution was monitored by both GC/MS and GC/FID. The GC/MS instrument was equipped with an MS detector (EI) and a HP-5MS column (30 m × 250 μm × 0.25 μm) and He was used as the carrier gas with the following temperature program: injector 250 °C, oven Tinitial = 50 °C (4 min), rate 10 °C min−1, Tfinal = 300 °C, and hold 5 min. Preparative HPLC chromatography was carried out by using an automated purification system using Kinetex Phenyl-Hexyl Core-Shell Column. The lignin sample used (MWL) was extracted according to a previously published procedure (Zijlstra et al. 2019) from milled spruce sapwood (particle size approx. below 0.5 mm).
2.1 General procedure for the Shvo-catalyzed degradation reaction
A mixture of the model diol compound (5a–c, 1 mmol), Shvo catalyst (0.02 mmol, 2 mol%) and 2 mL of methyl isobutyl ketone was loaded into a Schlenk reactor. The reactor was sealed with a Teflon plug and heated at 140 °C for 48 h. After 48 h of stirring, the reaction was cooled down, evaporated under vacuum, dissolved in 0.5 mL of DMSO-d6 containing ethyl 3,5-dinitrobenzoate (0.5 mmol/mL) and analyzed by 1H NMR spectroscopy.
2.2 General procedure for preparative HPLC fractionation
To optimize the separation method, the reaction mixture was analyzed with HPLC (Agilent 1100 Series) equipped with Phenomenex Kinetex Phenyl-Hexyl (100 Å, 5 μm, 100 × 4.6 mm) at 27 °C. After Shvo-catalyzed degradation reaction the reaction mixture was evaporated under vacuum and dissolved in MeCN/MeOH mixture 80:20 to obtain the solution with mass concentration 20 mg/mL.
The separation was performed with a CombiFlash EZ Prep purification system (EZ PREP UV/ELSD) equipped with Phenomenex Kinetex Phenyl-Hexyl column (100 Å, 5 μm, 100 × 21.2 mm) at a flow rate 21.0 mL/min. The carrier solvents were ultrapure water and MeCN, each containing 0.1 % formic acid. A gradient of 20 %–100 % acetonitrile over 20 min was used during the purification. Automated fraction collection was triggered by UV detection at 214 nM and 254 nM. Obtained fractions were analyzed by 1H NMR spectroscopy and Waters ACQUITY RDa Detector.
2.3 NMR experiments
All NMR measurements were performed at 298 K on a Bruker Avance III NMR spectrometer operated at 500.13 MHz (1H) and equipped with a BB/1H smartprobe. The diffusion experiments were conducted using Bruker’s ledbpgp2s pulse program (calibrated for sufficient signal decay of lignin samples), with 32 linear field gradient steps (2–98 %). All measurements were processed using TopSpin 4.4.1.
3 Results and discussion
In the present work, to investigate the possibility of Cβ–Cγ and Cγ–Oγ bond cleavage in lignin model compounds, the Shvo-catalyzed reaction was applied to lignin model diols containing β–O–4 linkages at 140 °C. Instead of 2-butatone, methyl-isobutyl ketone was used as the solvent and hydrogen acceptor, due to its higher boiling point. To obtain better understanding about the degradation pathway, the reaction was initially performed in two steps. First, model diol 5a was fully dehydrogenated in the presence of the Shvo-catalyzed at 60 °C to form ketoalcohol 6a with no degradation products observed within 24 h reaction (Scheme 2). Next, the same reaction mixture containing the ketoalcohol 6a was heated at 140 °C for 24 h with the degradation products 7a–12a detected in the reaction mixture. To verify that the degradation is not thermal but catalytic, the model diols 5a–c and the isolated ketoalcohol 6a were heated at 140 °C in methyl-isobutyl ketone without the Shvo-catalyst. After 24 h, changes were only observed on the baseline level of the 1H NMR spectra of all dimeric model compounds tested, confirming that the bond cleavage is not thermal but instead a catalytic process (Scheme 2). In addition, chemical shifts of the new signals on the baseline level do not match with the chemical shifts of the actual degradation products 7 to 12a–c.
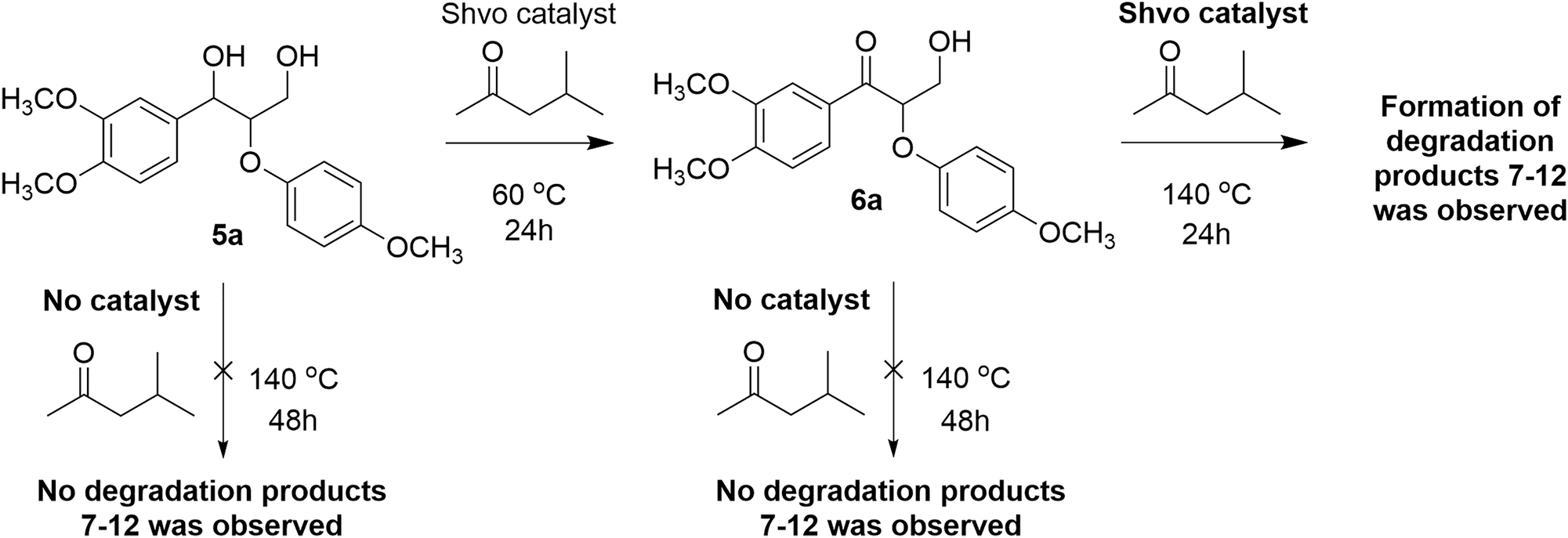
Conformation of catalytic bond cleavage in lignin model compounds in the presence of Shvo catalyst (NMR spectra from stability studies of the model compounds are provided in the Supplementary Material).
Next, the reaction at 140 °C was directly applied to the model diol 5a, resulting in a similar outcome as observed for the two-step dehydrogenation-degradation reaction. After stirring of the model diol 5a in methyl-isobutyl ketone for 24 h at 140 °C, in the presence of the Shvo catalyst, the corresponding ketoalcohol 6a and the degradation products 7a–12a were detected in the reaction mixture. For reaching higher conversion of the model diol 5a into the degradation products, the reaction was then extended. After increasing the reaction time to 48 h, no diol 5a and only traces of the ketoalcohol 6a were observed. Thus, for further experiments the reaction time was set to 48 h. The reaction was applied to three dimeric β–O–4 model diols 5a–c (Scheme 3) and one tetrameric 5-5-bis-β-O-4 model diol 13 (Scheme 4). The products of the Shvo-catalyzed degradation reaction of the dimeric lignin model diols are presented in Table 1. After 48 h of Shvo-catalyzed reactions, the model diols 5a–c were fully consumed and no longer detected in the reaction mixture. The formation of 4-methoxyphenol was observed in all the reaction mixtures for the model diols 5a–c. The products formed from degradation of the starting diols and the product yields varied, most likely because of the different substituents in the aromatic rings of the corresponding model diols. The products 14–16 formed in the degradation reaction of the dimeric model compound 13 containing a 5–5 linkage are presented in Scheme 4. Due to their high molecular weights, the degradation products 14–16 could not be detected by GC-MS. In the thermal studies, after heating the model diol 13 at 140 °C in methyl-isobutyl ketone in the absence of the Shvo-catalyst, 4-methoxyphenol was detected in the reaction mixture, indicating β–O–4 cleavage. However, no dehydrogenation or degradation products 14–16 were observed with the model diol 13 remaining as the major component in the mixture.
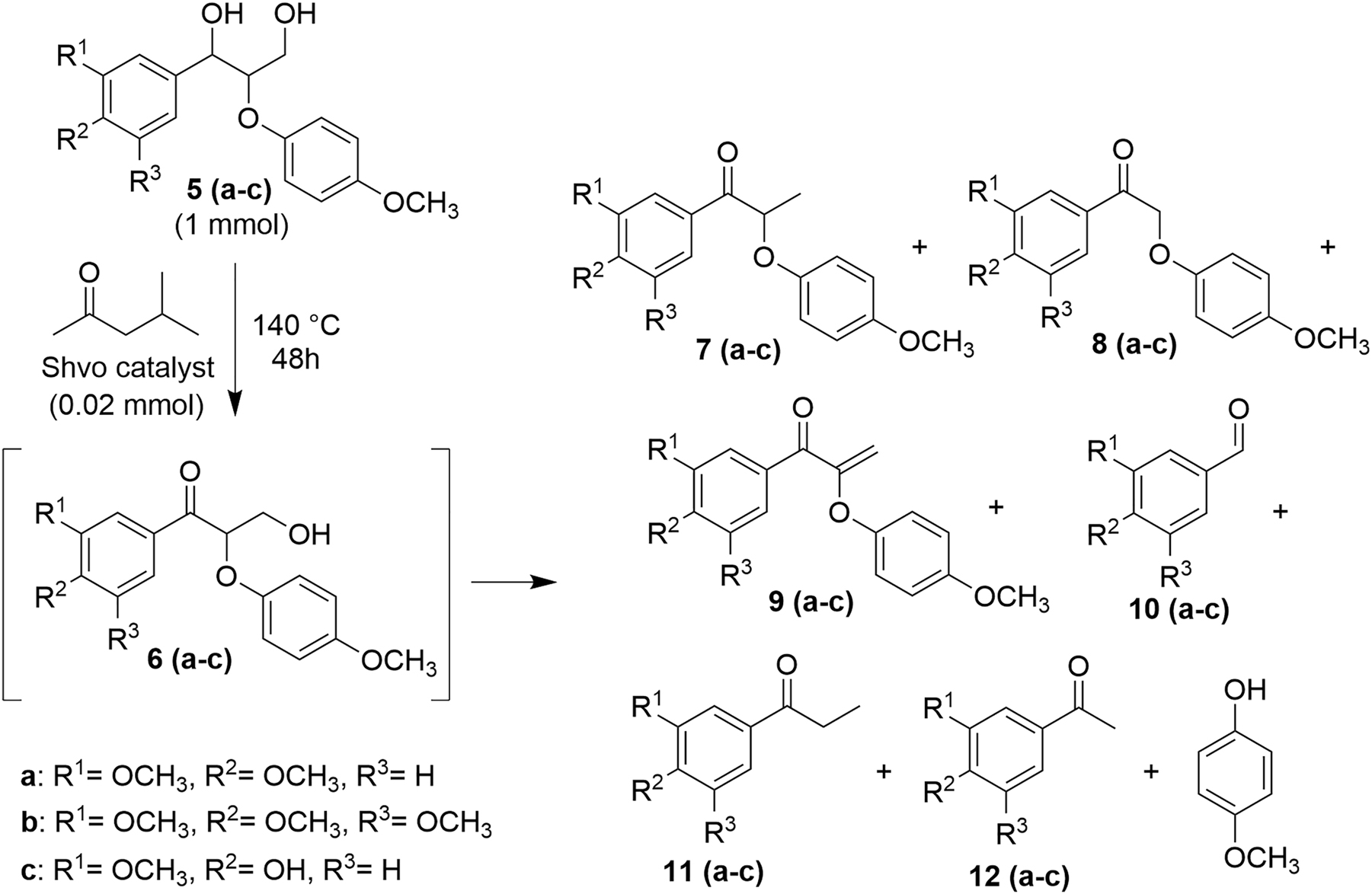
Shvo-catalyzed degradation reaction of the dimeric lignin model diols containing β–O–4 linkages with generalized product scope (a detailed reaction scheme for each model compound is presented in the Supplementary Material).
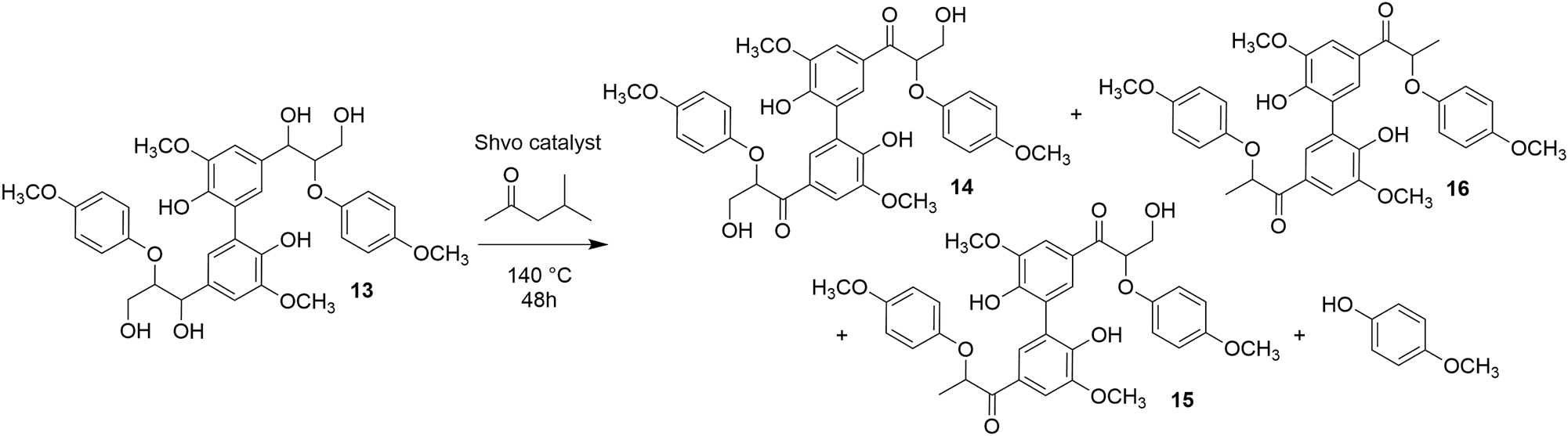
Shvo-catalyzed degradation reaction of the tetrameric lignin model compound containing β–O–4 and 5–5 linkages.
Product scope of the Shvo-catalyzed degradation of lignin model compounds.
| Starting material 5 | Formed products and their NMR yieldsa (%) | ||||||
|---|---|---|---|---|---|---|---|
| 6 | 7 | 8 | 9 | 10 | 11 | 12b | |
| a | Not observed | 27 | 35 | Observedc | 11 | 20 | Observed |
| b | Not observed | 33 | 15 | Not observed | 6 | 15 | Observed |
| c | 18 | 10 | 5 | 9 | 16 | 3 | Observed |
-
aNMR yield based on the analysis with internal standard. bNMR yield calculation was not possible due to signal overlap, formation confirmed by GC-MS method. cThe product 9a was inconsistently formed in the different reactions studied, while isolated and characterized when observed. Characterization data for isolated compounds are presented in the Supplementary Material.
To extend the Shvo-catalyzed degradation studies towards lignans, the reaction was also applied to 7-(S)-hydroxymatairesinol (17), resulting in the formation of 7-oxomatairesinol (18) without any degradation products detected (Scheme 5).
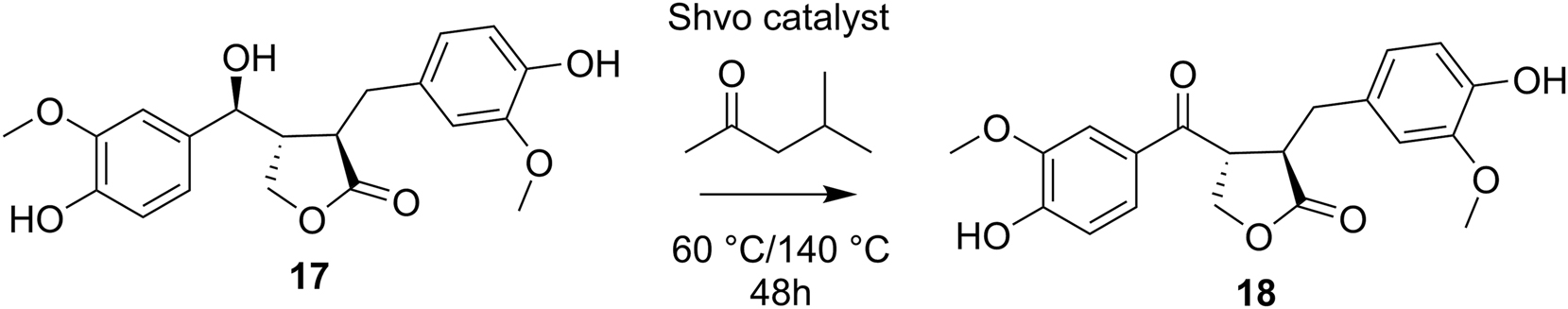
Shvo-catalyzed transfer dehydrogenation of 7-hydroxymatairesinol.
Previously, it has been shown that the 7-hydroxymatairesinol 17 can be selectively oxidized to 7-oxomatairesinol 18 using heterogeneous Au and Pd catalysts (Simakova et al. 2012). Here, however, the Shvo-catalyzed dehydrogenation procedure could offer a better alternative for the preparation of oxomatairesinol, due to the lower temperatures and aerobic conditions used. For better understanding the degradation mechanism, the primary OH group in the model diol 5a was protected as a silyl ether using tert-butyldimethylsilyl chloride. According to the 1H NMR spectra and the GC-MS analysis, the protected compound 19 was isolated as a 9:1 diastereomeric mixture, with the corresponding enantiomers being inseparable by NMR. The protected analogue 19 was then subjected to similar degradation conditions as used for the parent compound (Scheme 6).
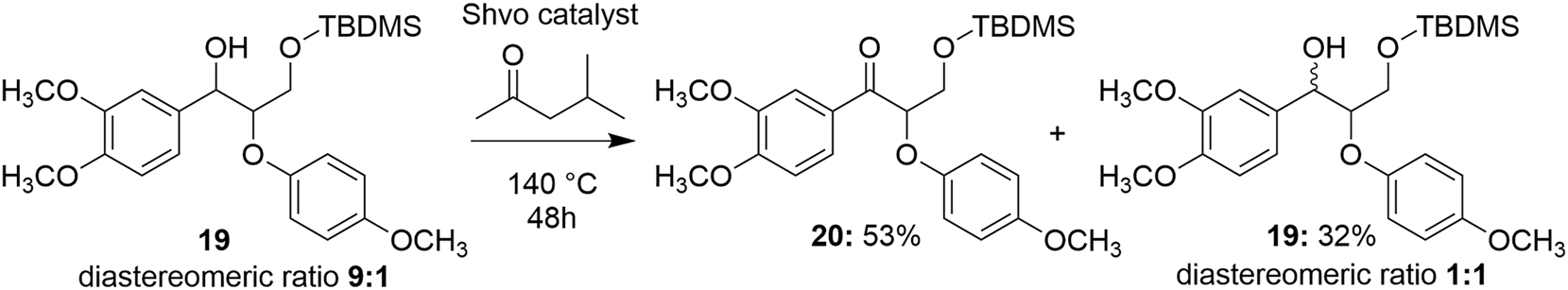
Shvo-catalyzed transfer dehydrogenation of 3-((tert-butyldimethylsilyl)oxy)-1-(3,4-dimethoxyphenyl)-2-(4-methoxyphenoxy)propan-1-ol.
This reaction predominantly resulted in dehydrogenation of the benzylic hydroxyl group to provide the ketone 20 in admixture with the epimerized protected diol 19 in 1:0.6 ratio. For remaining unreacted compound 19, the presence of two diastereomers in 1:1 ratio was confirmed by 1H and 29Si NMR spectroscopy, as well as by GC-MS analysis. In addition, the degradation products 7a and 9a were observed on the baseline level in the 1H NMR spectrum.
Based on the results obtained from hydroxymatairesinol 17 and the model compound with protected primary hydroxyl group 19, it can be concluded that the primary hydroxyl group plays a crucial role in the overall degradation process.
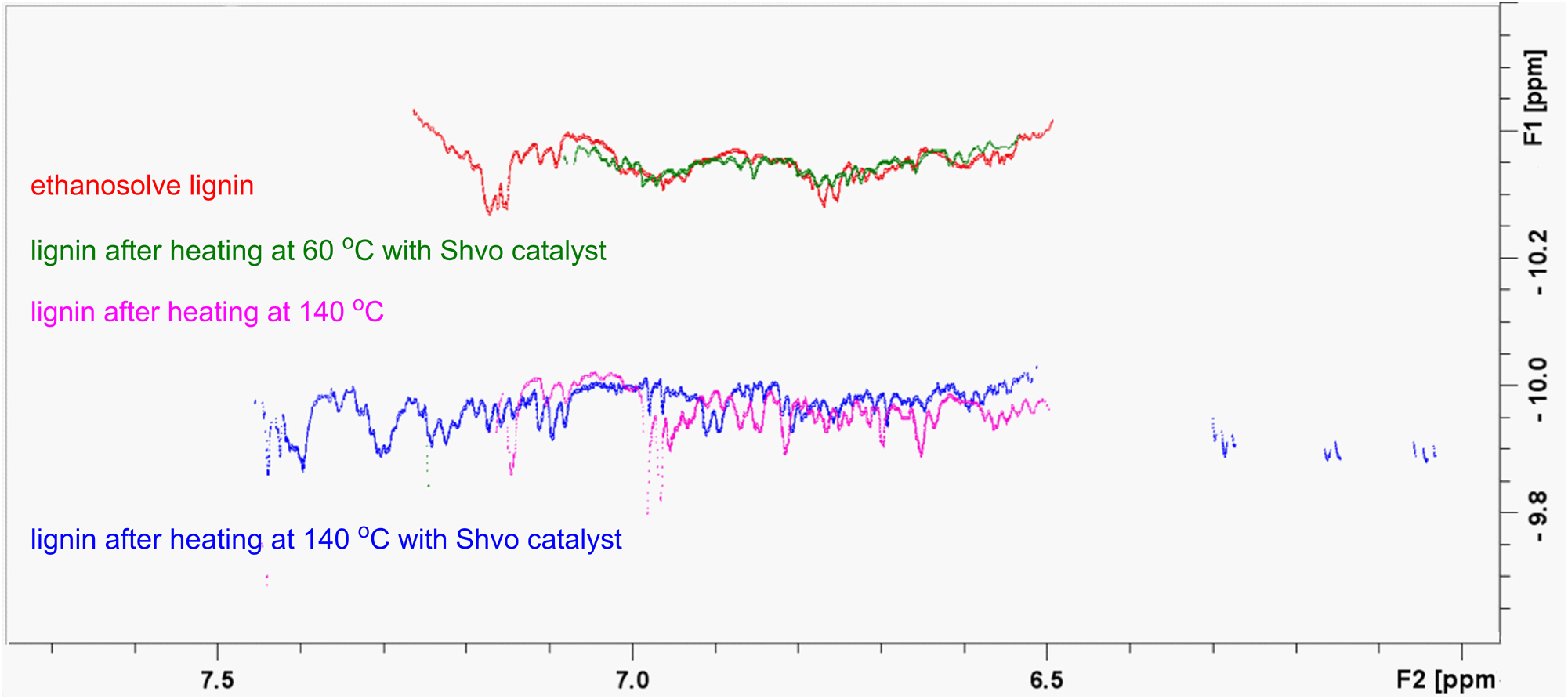
Finally, the Shvo catalyzed degradation protocol was also applied to ethanosolve lignin extracted from spruce milled wood (Zijlstra et al. 2019). After 24 h, 48 h and 72 h stirring, the reaction mixtures were analyzed by NMR spectroscopy. In all cases, formation of new peaks was observed in the 1H, HMBC and HSQC NMR spectra. To evaluate the temperature effect, ethanosolve lignin was heated at 140 °C in methyl-isobutyl ketone without added Shvo catalyst for 48 h and analyzed by NMR spectroscopy. According to the data obtained, not only catalytic but also thermal changes take place in the lignin structure. After 48 h heating at 140 °C, in the absence of the Shvo catalyst, no signals corresponding to α and β protons of the β–O–4 units were found in the HSQC spectra. The signal of the proton in the β-position of the lignin oxidized in the presence of the Shvo catalyst at 60 °C is, likewise, missing from the HSQC spectrum of the corresponding lignin sample heated at 140 °C. However, in the presence of the Shvo catalyst the appearance of new peaks was observed, which were absent in the spectrum recorded from the sample subjected to thermal conditions only (Figure 4). According to DOSY, the molecular weight of the lignin oxidized in the presence of the Shvo catalyst at 60 °C is similar to the molecular weight of ethanosolve lignin. The molecular weight of lignin after heating at 140 °C in the presence of the Shvo catalyst was found to be the same as the molecular weight of lignin after heating without Shvo catalyst, and lower than molecular weight of ethanosolve lignin (Figure 5).
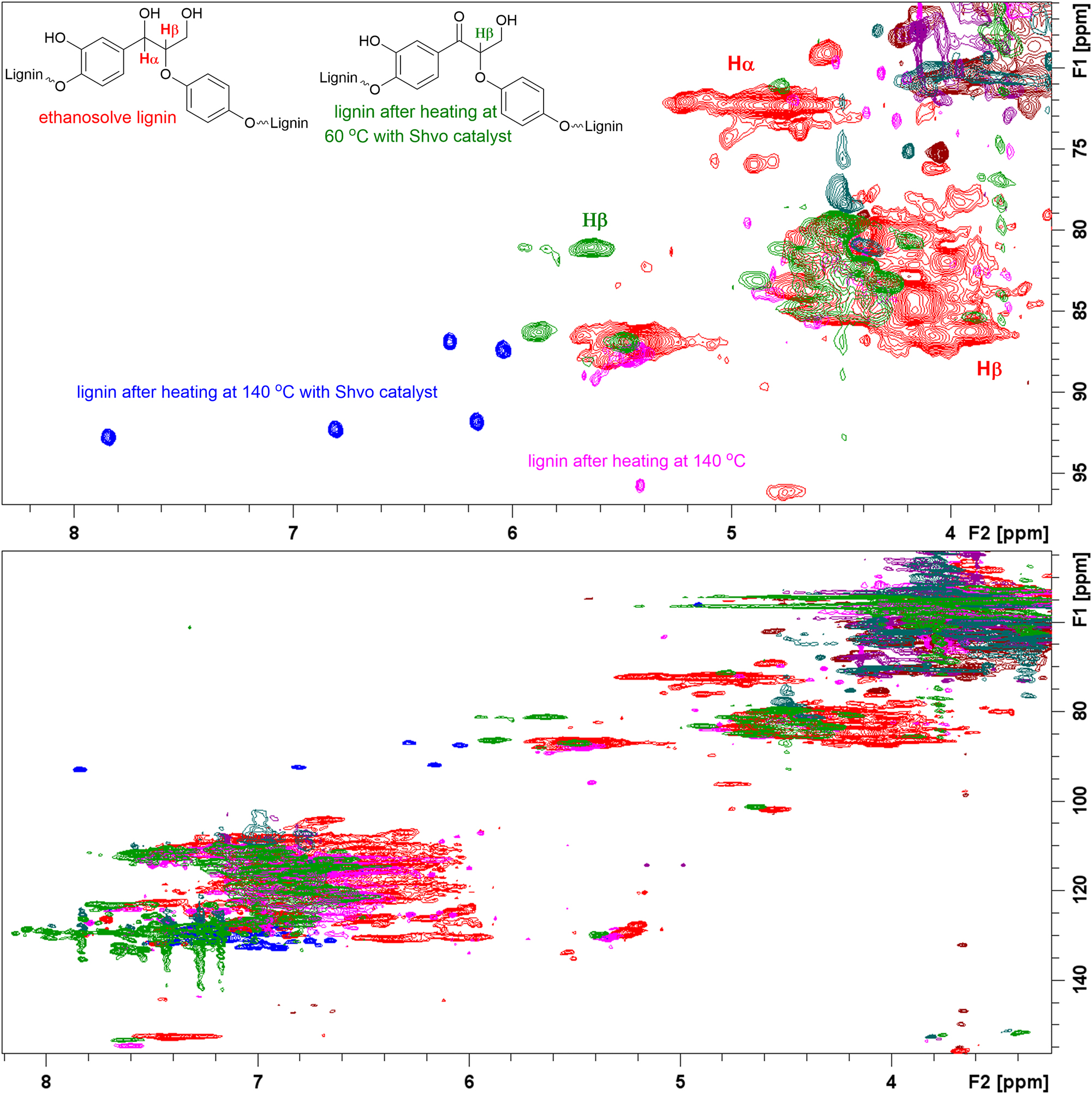
Comparison of the HSQC spectra of ethanosolve lignin (red), oxidized lignin (green), lignin heated at 140 °C (purple), and lignin heated at 140 °C in the presence of the Shvo catalyst (blue). Detailed spectra are provided in the Supplementary Material.
Comparison of the DOSY spectra of ethanosolve lignin (red), oxidized lignin (green), lignin heated at 140 °C (purple), and lignin heated at 140 °C in the presence of Shvo catalyst (blue).
Thus, after applying the Shvo catalyzed degradation procedure to ethanosolve lignin, degradation products similar to those obtained during the degradation of the model compounds 5a–c and 13 were neither isolated nor fully characterized. The decrease of the molecular weight of lignin after the reaction was found to result from thermal but not catalytic degradation. In the HMBC NMR spectra, changes were also observed in the carbon-proton correlations for the cross peaks at 5.3–5.7/85–90 ppm, 4.2–4.9/76–88 ppm, or 7.3–7.7/151–155 ppm, corresponding to carbon-proton correlations in other than β–O–4 lignin units, including β–5, β–β and cinnamyl units. Based on the data obtained, it can be suggested that subsequent transformations of the β–O–4 and other lignin units in lignin take place by possible condensation reactions or other reaction pathways not observed in the model compounds which only contain β–O–4 and 5–5 linkages. Considering that changes in the lignin structure observed after the Shvo catalyzed degradation procedure result from chemical transformations of different lignin units, it is impossible to determine the exact reaction pathway and define the configuration of the new substructures with the cross peaks at 6.0–7.8/86–94 ppm. To obtain more information, H2BC spectrum (shown in the Supplementary Material) was recorded, allowing to suggest about the cyclic composition of the new substructure. Notably, structures with similar chemical shifts were not found in earlier literature.
4 Conclusions
In this work, the possibility to use Shvo-catalyzed hydrogen transfer approach for degradation of lignin model diols containing β–O–4 linkages and ethanosolv lignin was investigated. Reactions of the lignin model diols resulted in Cβ–Cγ, Cγ–Oγ and Cβ–Oβ bond cleavage and the formation of several products, of which the major degradation products were isolated and characterized. The results obtained provide information about possible degradation pathways in lignin, potentially facilitating further synthetic and analytical work in lignin degradation research. Variations in the product scope for the different model diols studied are likely due to effects from the different substituents in these compounds. In each reaction, γCH3 derivatives were, however, detected. It was also shown that the primary hydroxyl group plays a crucial role in the bond cleavage process. While the Shvo-catalyzed procedure proved inefficient for hydroxymatairesinol lignan degradation, it could be successfully utilized for oxomatairesinol generation. In ethanosolve lignin, only thermal degradation was observed under the reaction conditions studied. Formation of degradation products similar to the products formed from the model diols were not observed. However, the formation of new fragments in lignin structure was observed in the presence of the Shvo catalyst. Due to poor solubility and structural complexity of the extracted lignin, the possible degradation products could not be isolated and characterized. To conclude, Shvo-catalyzed procedure can be used for degradation of small lignin-derived dimers and tetramers. However, as a lignin degradation procedure under the reaction conditions investigated, it proved insufficient, resulting in indetermined transformations in the lignin structure.
Funding source: The Finnish Natural Resources Research Foundation
Award Identifier / Grant number: 20210053
Funding source: Alfred Kordelinin Säätiö
Award Identifier / Grant number: Gust. Komppa fund
Funding source: Fortum and Neste Foundation
Award Identifier / Grant number: 20240200
Acknowledgments
We thank Dr. Patrik Eklund, Teddie Strandberg and Rupali Bhadane from Åbo Akademi University for their help with analysis. We are grateful to the reviewers of this manuscript for helpful and insightful comments.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: This work was supported by the Finnish Natural Resources Research Foundation (grant #20210053), Alfred Kordelin Foundation (Gust. Kompa fund), Fortum and Neste Foundation (grant #20240200) and Åbo Akademi University.
-
Data availability: The datasets supporting this article (i.e. the chemical characterization of the described compounds) have been uploaded as Supplementary Material.
References
Adler, E. (1977). Lignin chemistry-past, present and future. Wood Sci. Technol. 11: 169–218, https://doi.org/10.1007/BF00365615.Search in Google Scholar
Badamali, S.K., Luque, R., Clark, J.H., and Breeden, S.W. (2009). Microwave assisted oxidation of a lignin model phenolic monomer using Co(salen)/SBA-15. Catal. Commun. 10: 1010–1013, https://doi.org/10.1016/j.catcom.2008.12.051.Search in Google Scholar
Badazhkova, V.D., Savela, R., and Leino, R. (2022). Selective modification of hydroxyl groups in lignin model compounds by ruthenium-catalyzed transfer hydrogenation. Dalton Trans. 51: 6587–6596, https://doi.org/10.1039/D2DT00267A.Search in Google Scholar PubMed
Badazhkova, V.D., Savela, R., Wärnå, J., Murzin, D.Y., and Leino, R. (2024). Reaction kinetics of the Shvo-catalyzed dehydrogenation of 1-phenyl-1,3-propanediol-derived lignin model compound. Mol. Catal. 553: 113780, https://doi.org/10.1016/j.mcat.2023.113780.Search in Google Scholar
Barta, K., Warner, G.R., Beach, E.S., and Anastas, P.T. (2014). Depolymerization of organosolv lignin to aromatic compounds over Cu-doped porous metal oxides. Green Chem. 16: 191–196, https://doi.org/10.1039/C3GC41184B.Search in Google Scholar
Biannic, B. and Bozell, J.J. (2013). Efficient cobalt-catalyzed oxidative conversion of lignin models to benzoquinones. Org. Lett. 15: 2730–2733, https://doi.org/10.1021/ol401065r.Search in Google Scholar PubMed
Binder, J.B., Gray, M.J., White, J.F., Zhang, Z.C., and Holladay, J.E. (2009). Reactions of lignin model compounds in ionic liquids. Biomass Bioenergy 33: 1122–1130, https://doi.org/10.1016/j.biombioe.2009.03.006.Search in Google Scholar
Biswas, B., Kumar, A., Krishna, B.B., Baltrusaitis, J., Adhikari, S., and Bhaskar, T. (2023). Catalytic depolymerization of lignin for the selective production of phenolic monomers over cobalt-supported calcium catalysts. Energy Fuels 37: 3813–3824, https://doi.org/10.1021/acs.energyfuels.2c04193.Search in Google Scholar
Busetto, L., Fabbri, D., Mazzoni, R., Salmi, M., Torri, C., and Zanotti, V. (2011). Application of the Shvo catalyst in homogeneous hydrogenation of bio-oil obtained from pyrolysis of white poplar: new mild upgrading conditions. Fuel 90: 1197–1207, https://doi.org/10.1016/j.fuel.2010.10.036.Search in Google Scholar
Cequier, E., Balcells, M., and Canela-Garayoa, R. (2020). First insights of SHVO catalyst activity for monomeric phenol production from olive pomace. J. Chem. Technol. Biotechnol. 95: 621–630, https://doi.org/10.1002/jctb.6243.Search in Google Scholar
Chan, J.M.W., Bauer, S., Sorek, H., Sreekumar, S., Wang, K., and Toste, F.D. (2013). Studies on the vanadium-catalyzed nonoxidative depolymerization of Miscanthus giganteus-derived lignin. ACS Catal. 3: 1369–1377, https://doi.org/10.1021/cs400333q.Search in Google Scholar
Chmely, S.C., Kim, S., Ciesielski, P.N., Jiménez-Osés, G., Paton, R.S., and Beckham, G.T. (2013). Mechanistic study of a Ru-Xantphos catalyst for tandem alcohol dehydrogenation and reductive aryl-ether cleavage. ACS Catal. 3: 963–974, https://doi.org/10.1021/cs400110r.Search in Google Scholar
Deng, J., Zhou, C., Yang, Y., Nan, B., Dong, L., Cai, L., Li, L., Wang, Z.-J., Yang, X., and Chen, Z. (2023). Visible-light-driven selective cleavage of C–C bonds in lignin model substrates using carbon nitride-supported ruthenium single-atom catalyst. Chem. Eng. J. 462: 142282, https://doi.org/10.1016/j.cej.2023.142282.Search in Google Scholar
Deuss, P.J. and Barta, K. (2016). From models to lignin: transition metal catalysis for selective bond cleavage reactions. Coord. Chem. Rev. 306: 510–532, https://doi.org/10.1016/j.ccr.2015.02.004.Search in Google Scholar
Forsythe, W.G., Garrett, M.D., Hardacre, C., Nieuwenhuyzen, M., and Sheldrake, G.N. (2013). An efficient and flexible synthesis of model lignin oligomers. Green Chem. 15: 3031, https://doi.org/10.1039/c3gc41110a.Search in Google Scholar
Gao, R., Li, Y., Kim, H., Mobley, J.K., and Ralph, J. (2018). Selective oxidation of lignin model compounds. ChemSusChem 11: 2045–2050, https://doi.org/10.1002/cssc.201800598.Search in Google Scholar PubMed
Guadix-Montero, S. and Sankar, M. (2018). Review on catalytic cleavage of C–C inter-unit linkages in lignin model compounds: towards lignin depolymerisation. Top. Catal. 61: 183–198, https://doi.org/10.1007/s11244-018-0909-2.Search in Google Scholar
Hao, Z., Li, S., Sun, J., Li, S., and Zhang, F. (2018). Efficient visible-light-driven depolymerization of oxidized lignin to aromatics catalyzed by an iridium complex immobilized on mesocellular silica foams. Appl. Catal. B Environ. 237: 366–372, https://doi.org/10.1016/j.apcatb.2018.05.072.Search in Google Scholar
Huo, W., Li, W., Zhang, M., Fan, W., Chang, H., and Jameel, H. (2014). Effective C–O bond cleavage of lignin β-O-4 model compounds: a new RuHCl(CO)(PPh3)3/KOH catalytic system. Catal. Lett. 144: 1159–1163, https://doi.org/10.1007/s10562-014-1264-y.Search in Google Scholar
Kim, J.-Y., Park, J., Hwang, H., Kim, J.K., Song, I.K., and Choi, J.W. (2015). Catalytic depolymerization of lignin macromolecule to alkylated phenols over various metal catalysts in supercritical tert-butanol. J. Anal. Appl. Pyrol. 113: 99–106, https://doi.org/10.1016/j.jaap.2014.11.011.Search in Google Scholar
Klein, I., Saha, B., and Abu-Omar, M.M. (2015). Lignin depolymerization over Ni/C catalyst in methanol, a continuation: effect of substrate and catalyst loading. Catal. Sci. Technol. 5: 3242–3245, https://doi.org/10.1039/C5CY00490J.Search in Google Scholar
Kloekhorst, A. and Heeres, H.J. (2015). Catalytic hydrotreatment of alcell lignin using supported Ru, Pd, and Cu catalysts. ACS Sustain. Chem. Eng. 3: 1905–1914, https://doi.org/10.1021/acssuschemeng.5b00041.Search in Google Scholar
Kusumoto, S., Kishino, M., and Nozaki, K. (2020). Cleavage of C–C and C–O bonds in β-O-4 linkage of lignin model compound by cyclopentadienone group 8 and 9 metal complexes. Chem. Lett. 49: 477–480, https://doi.org/10.1246/cl.200037.Search in Google Scholar
Lange, H., Decina, S., and Crestini, C. (2013). Oxidative upgrade of lignin – recent routes reviewed. Eur. Polym. J. 49: 1151–1173, https://doi.org/10.1016/j.eurpolymj.2013.03.002.Search in Google Scholar
Li, H. and Song, G. (2019). Ru-catalyzed hydrogenolysis of lignin: base-dependent tunability of monomeric phenols and mechanistic study. ACS Catal. 9: 4054–4064, https://doi.org/10.1021/acscatal.9b00556.Search in Google Scholar
Li, C., Zhao, X., Wang, A., Huber, G.W., and Zhang, T. (2015). Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 115: 11559–11624, https://doi.org/10.1021/acs.chemrev.5b00155.Search in Google Scholar PubMed
Liu, Y., Li, C., Miao, W., Tang, W., Xue, D., Li, C., Zhang, B., Xiao, J., Wang, A., Zhang, T., et al.. (2019). Mild redox-neutral depolymerization of lignin with a binuclear Rh complex in water. ACS Catal. 9: 4441–4447, https://doi.org/10.1021/acscatal.9b00669.Search in Google Scholar
Ma, D., Lu, S., Liu, X., Guo, Y., and Wang, Y. (2019a). Depolymerization and hydrodeoxygenation of lignin to aromatic hydrocarbons with a Ru catalyst on a variety of Nb-based supports. Chin. J. Catal. 40: 609–617, https://doi.org/10.1016/S1872-2067(19)63317-6.Search in Google Scholar
Ma, H., Li, H., Zhao, W., Li, L., Liu, S., Long, J., and Li, X. (2019b). Selective depolymerization of lignin catalyzed by nickel supported on zirconium phosphate. Green Chem. 21: 658–668, https://doi.org/10.1039/C8GC03617A.Search in Google Scholar
McClelland, D.J., Galebach, P.H., Motagamwala, A.H., Wittrig, A.M., Karlen, S.D., Buchanan, J.S., Dumesic, J.A., and Huber, G.W. (2019). Supercritical methanol depolymerization and hydrodeoxygenation of lignin and biomass over reduced copper porous metal oxides. Green Chem. 21: 2988–3005, https://doi.org/10.1039/C9GC00589G.Search in Google Scholar
Mottweiler, J., Puche, M., Räuber, C., Schmidt, T., Concepción, P., Corma, A., and Bolm, C. (2015). Copper- and vanadium-catalyzed oxidative cleavage of lignin using dioxygen. ChemSusChem 8: 2106–2113, https://doi.org/10.1002/cssc.201500131.Search in Google Scholar PubMed
Nguyen, J.D., Matsuura, B.S., and Stephenson, C.R.J. (2014). A photochemical strategy for lignin degradation at room temperature. J. Am. Chem. Soc. 136: 1218–1221, https://doi.org/10.1021/ja4113462.Search in Google Scholar PubMed
Nichols, J.M., Bishop, L.M., Bergman, R.G., and Ellman, J.A. (2010). Catalytic C−O bond cleavage of 2-aryloxy-1-arylethanols and its application to the depolymerization of lignin-related polymers. J. Am. Chem. Soc. 132: 12554–12555, https://doi.org/10.1021/ja106101f.Search in Google Scholar PubMed PubMed Central
Onwudili, J.A. and Williams, P.T. (2014). Catalytic depolymerization of alkali lignin in subcritical water: influence of formic acid and Pd/C catalyst on the yields of liquid monomeric aromatic products. Green Chem. 16: 4740–4748, https://doi.org/10.1039/C4GC00854E.Search in Google Scholar
Parthasarathi, R., Romero, R.A., Redondo, A., and Gnanakaran, S. (2011). Theoretical study of the remarkably diverse linkages in lignin. J. Phys. Chem. Lett. 2: 2660–2666, https://doi.org/10.1021/jz201201q.Search in Google Scholar
Ragauskas, A.J., Beckham, G.T., Biddy, M.J., Chandra, R., Chen, F., Davis, M.F., Davison, B.H., Dixon, R.A., Gilna, P., Keller, M., et al.. (2014). Lignin valorization: improving lignin processing in the biorefinery. Science 344: 1246843, https://doi.org/10.1126/science.1246843.Search in Google Scholar PubMed
Rahimi, A., Azarpira, A., Kim, H., Ralph, J., and Stahl, S.S. (2013). Chemoselective metal-free aerobic alcohol oxidation in lignin. J. Am. Chem. Soc. 135: 6415–6418, https://doi.org/10.1021/ja401793n.Search in Google Scholar PubMed
Ralph, J., Lapierre, C., and Boerjan, W. (2019). Lignin structure and its engineering. Curr. Opin. Biotechnol. 56: 240–249, https://doi.org/10.1016/j.copbio.2019.02.019.Search in Google Scholar PubMed
Sedai, B., Díaz-Urrutia, C., Baker, R.T., Wu, R., “Pete” Silks, L.A., and Hanson, S.K. (2011). Comparison of copper and vanadium homogeneous catalysts for aerobic oxidation of lignin models. ACS Catal. 1: 794–804, https://doi.org/10.1021/cs200149v.Search in Google Scholar
Sergeev, A.G. and Hartwig, J.F. (2011). Selective, nickel-catalyzed hydrogenolysis of aryl ethers. Science 332: 439–443, https://doi.org/10.1126/science.1200437.Search in Google Scholar PubMed
Shu, R., Long, J., Yuan, Z., Zhang, Q., Wang, T., Wang, C., and Ma, L. (2015). Efficient and product-controlled depolymerization of lignin oriented by metal chloride cooperated with Pd/C. Bioresour. Technol. 179: 84–90, https://doi.org/10.1016/j.biortech.2014.12.021.Search in Google Scholar PubMed
Shu, R., Xu, Y., Ma, L., Zhang, Q., Wang, C., and Chen, Y. (2018). Controllable production of guaiacols and phenols from lignin depolymerization using Pd/C catalyst cooperated with metal chloride. Chem. Eng. J. 338: 457–464, https://doi.org/10.1016/j.cej.2018.01.002.Search in Google Scholar
Simakova, O.A., Smolentseva, E., Estrada, M., Murzina, E.V., Beloshapkin, S., Willför, S.M., Simakov, A.V., and Murzin, D.Y. (2012). From woody biomass extractives to health-promoting substances: selective oxidation of the lignan hydroxymatairesinol to oxomatairesinol over Au, Pd, and Au–Pd heterogeneous catalysts. J. Catal. 291: 95–103, https://doi.org/10.1016/j.jcat.2012.04.012.Search in Google Scholar
Son, S. and Toste, F.D. (2010). Non-oxidative vanadium-catalyzed C–O bond cleavage: application to degradation of lignin model compounds. Angew. Chem., Int. Ed. 49: 3791–3794, https://doi.org/10.1002/anie.201001293.Search in Google Scholar PubMed PubMed Central
vom Stein, T., den Hartog, T., Buendia, J., Stoychev, S., Mottweiler, J., Bolm, C., Klankermayer, J., and Leitner, W. (2015). Ruthenium-catalyzed C–C bond cleavage in lignin model substrates. Angew. Chem. 127: 5957–5961, https://doi.org/10.1002/ange.201410620.Search in Google Scholar
Wang, D., Li, G., Zhang, C., Wang, Z., and Li, X. (2019). Nickel nanoparticles inlaid in lignin-derived carbon as high effective catalyst for lignin depolymerization. Bioresour. Technol. 289: 121629, https://doi.org/10.1016/j.biortech.2019.121629.Search in Google Scholar PubMed
Wang, X., Luo, Y., Qian, M., and Qian, E.W. (2020). Catalytic depolymerization of alkali lignin in ionic liquids on Pt-supported La2O3–SO42−/ZrO2 catalysts. Sustain. Energy Fuels 4: 1409–1416, https://doi.org/10.1039/C9SE00682F.Search in Google Scholar
Wu, A., Patrick, B.O., Chung, E., and James, B.R. (2012). Hydrogenolysis of β-O-4 lignin model dimers by a ruthenium-xantphos catalyst. Dalton Trans. 41: 11093, https://doi.org/10.1039/c2dt31065a.Search in Google Scholar PubMed
Yao, G., Wu, G., Dai, W., Guan, N., and Li, L. (2015). Hydrodeoxygenation of lignin-derived phenolic compounds over bi-functional Ru/H-beta under mild conditions. Fuel 150: 175–183, https://doi.org/10.1016/j.fuel.2015.02.035.Search in Google Scholar
Zhang, Z., Lahive, C.W., Zijlstra, D.S., Wang, Z., and Deuss, P.J. (2019). Sequential catalytic modification of the lignin α-ethoxylated β-O-4 motif to facilitate C–O bond cleavage by ruthenium-Xantphos catalyzed hydrogen transfer. ACS Sustain. Chem. Eng. 7: 12105–12116, https://doi.org/10.1021/acssuschemeng.9b01193.Search in Google Scholar
Zijlstra, D.S., De Santi, A., Oldenburger, B., De Vries, J., Barta, K., and Deuss, P.J. (2019). Extraction of lignin with high β-O-4 content by mild ethanol extraction and its effect on the depolymerization yield. JoVE 58575, https://doi.org/10.3791/58575.Search in Google Scholar PubMed
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/hf-2025-0037).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Wood Growth/Morphology
- Anatomical, physical, and chemical characterization of native Caatinga wood species using integrated microscopic and spectroscopic techniques
- Cell wall structure of tensile flexure wood fibers in Populus x euramericana
- Wood Biochemistry
- Biological resistance and chemical properties of wood from an agroforestry system
- Wood Chemistry
- Decay resistance and chemical properties of the heartwood in Larix sibirica naturally growing in Mongolia
- Ruthenium-catalyzed bond cleavage in lignin model compounds containing β–O–4 linkages
- Wood Technology/Products
- Towards more accurate grading: non-destructive methods for assessing low-quality sawlogs
- Surface splits in outdoor undercover unfinished glued laminated timber
- Eco-friendly densified wood obtained by combining urea and hot-pressing treatment
Articles in the same Issue
- Frontmatter
- Wood Growth/Morphology
- Anatomical, physical, and chemical characterization of native Caatinga wood species using integrated microscopic and spectroscopic techniques
- Cell wall structure of tensile flexure wood fibers in Populus x euramericana
- Wood Biochemistry
- Biological resistance and chemical properties of wood from an agroforestry system
- Wood Chemistry
- Decay resistance and chemical properties of the heartwood in Larix sibirica naturally growing in Mongolia
- Ruthenium-catalyzed bond cleavage in lignin model compounds containing β–O–4 linkages
- Wood Technology/Products
- Towards more accurate grading: non-destructive methods for assessing low-quality sawlogs
- Surface splits in outdoor undercover unfinished glued laminated timber
- Eco-friendly densified wood obtained by combining urea and hot-pressing treatment

