Abstract
A novel series of triazolothiadiazines has been successfully synthesized with high efficiency through Knoevenagel condensation, utilizing thiocarbamoyl dihydrazine as the starting material. The structures of these compounds were characterized through 1H-NMR, 13C-NMR, high-resolution mass spectrometry. The MTT assay was utilized to evaluate the in vitro cytotoxicity of the synthesized compounds against four human cancer cell lines including SW620, A549, Hela, and MCF-7. Among the tested compounds, (Z)-3-methyl-6-phenyl-7-(3-(trifluoromethyl)benzylidene)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine exhibited notably potent antiproliferative activity against all four human cancer cell lines.
Graphical abstract
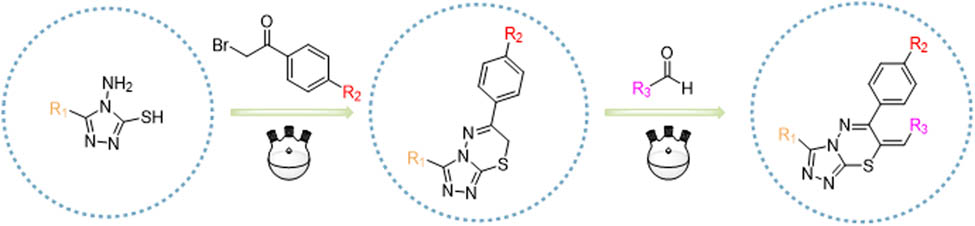
1 Introduction
Heterocyclic compounds are a class of organic compounds characterized by the presence of a heterocyclic ring system within their molecular structure, which includes at least one heteroatom in addition to the carbon atoms. These heterocycles are ubiquitous in both natural and synthetic sources and their significant pharmacological properties are renowned. Considerable interest has been garnered in medicinal chemistry by thiadiazines and triazoles, which are part of the nitrogen-containing heterocyclic class.
Thiadiazines are a class of heterocyclic compounds known for their diverse pharmacological activities, which encompass antibacterial [1], antiparasitic [2], and antidepressant effects [3]. These compounds are frequently integrated into the molecular frameworks of various pharmaceuticals as pharmacodynamic moieties, as illustrated by the examples of chlorothiazide, cyclopentothiazide, and bendroflumethiazide. Two isomeric forms based on the positional arrangement of the nitrogen atom within the ring are exhibited by triazoles, which feature a five-membered heterocyclic ring containing nitrogen: 1,2,3-triazole and 1,2,4-triazole. The 1,2,4-triazole isomer has been associated with a broad spectrum of pharmacological activities, including antimicrobial, anticancer, and antiviral effects, as documented in the literature [4–7]. In oncology, significant potential has been shown by this isomer as an inhibitor of aromatase [8,9], protein kinase [10,11], and carbonic anhydrase [12,13]. A number of pharmacologically relevant drugs, such as trimethoprim, anastrozole, and letrozole, featuring the aforementioned heterocyclic structural motif, have been approved for clinical use.
It has been demonstrated that a range of biological activities, including anti-inflammatory [14,15], antitumour [16–20], antimicrobial [21–24], cholinesterase-inhibitory [25], antioxidant [26], antiviral [27], and anticonvulsant properties, are exhibited by the fusion of the 1,2,4-triazole ring with the thiadiazine ring. On the basis of the above, our interest was aroused by the synthesis of novel triazolothiadiazine analogues.
2 Results and discussion
2.1 Synthesis
Novel triazolothiadiazine compounds 7a–7t were synthesized in accordance with the established methodology, with the synthetic route illustrated in Scheme 1 [28,29]. The intermediates 3a and b were initially obtained through the cyclization of thiocarbamoyl dihydrazide with aliphatic acids. The resulting products were then subjected to the subsequent step without further purification. Subsequently, intermediate 3 was condensed with α-bromoacetophenone/4-hydroxybromoacetophenone in order to obtain the key intermediates 5a–c. Caution must be exercised regarding the amount of solvent used, as either a suboptimal or an excessive quantity can lead to increased by-product formation, thereby impacting the reaction yield. Ultimately, intermediate 5 was condensed with aldehydes possessing diverse substituents through a Knoevenagel condensation reaction, affording the target compounds in a yield range of 55–89%. The structure of the desired compounds was confirmed by 1H and 13C NMR spectroscopy and mass spectrometry.
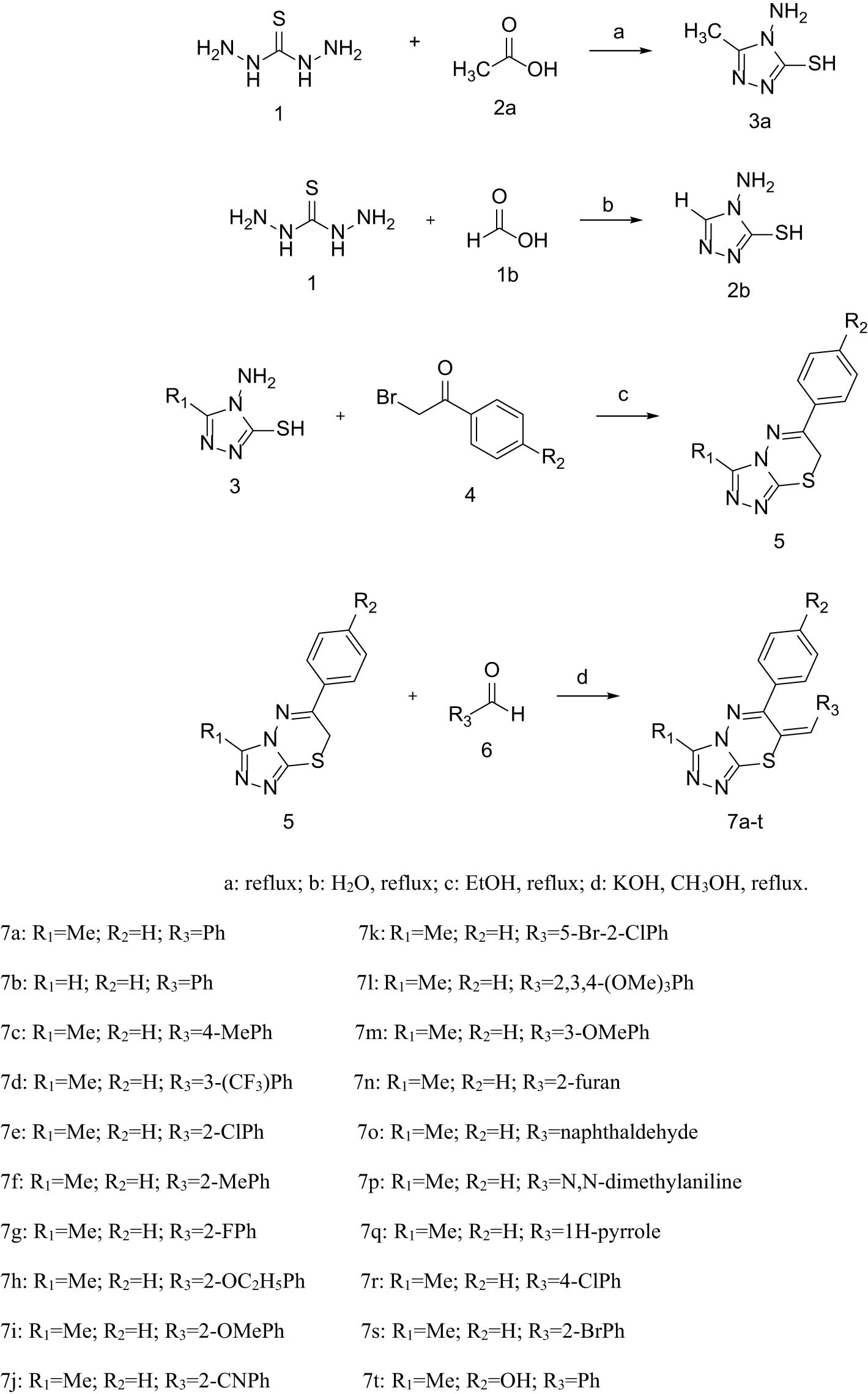
Synthesis of triazolothiadiazine derivatives.
2.2 Bioactivity studies
The in vitro cytotoxic effects of the synthesized compounds were evaluated using the MTT assay on four different human cancer cell lines: SW620, A549, HeLa, and MCF-7. The corresponding IC50 values, expressed in µM, are summarized in Table 1.
Antiproliferative activity against SW620, A549, Hela, and MCF-7 cells IC50 (µM)
| Compd | A549 | Hela | MCF-7 | SW620 |
|---|---|---|---|---|
| Cisplatin | 7.3 | 6.6 | 20.3 | 18.5 |
| 7a | 28.6 | >50 | >50 | 30.5 |
| 7b | 34.5 | >50 | >50 | 42.8 |
| 7c | 32.6 | 18.2 | 33.6 | >50 |
| 7d | 16.9 | 21.6 | 31.7 | 29 |
| 7e | >50 | 40.3 | >50 | >50 |
| 7f | >50 | >50 | >50 | 43.6 |
| 7g | 35.6 | 42.1 | 46.6 | >50 |
| 7h | >50 | >50 | >50 | >50 |
| 7i | >50 | 30.5 | >50 | 24.8 |
| 7j | 38.6 | >50 | >50 | 26.6 |
| 7k | 26.5 | 39.5 | 30.5 | >50 |
| 7l | 38.6 | >50 | >50 | 24.6 |
| 7m | >50 | 42.6 | >50 | 40.8 |
| 7n | 29.1 | >50 | >50 | >50 |
| 7o | >50 | >50 | >50 | >50 |
| 7p | >50 | 18.9 | >50 | >50 |
| 7q | >50 | >50 | >50 | >50 |
| 7r | 39.9 | >50 | >50 | 48.1 |
| 7s | 38.6 | 48.1 | 40.5 | >50 |
| 7t | 19.3 | 28.3 | >50 | >50 |
Encouragingly, when compared to the standard drug cisplatin, the majority of our tested compounds exhibited inhibitory effects on SW620, A549, Hela, and MCF-7 cancer cells. Notably, among the tested compounds, several demonstrated potent antiproliferative activity against SW620 cells, with the following IC50 values: 7d, bearing a trifluoromethyl group at R3, showed an IC50 of 29 µM; 7j, with a cyano substitution, had an IC50 of 26.6 µM; 7i, featuring a monomethyl group, exhibited an IC50 of 24.8 µM; and 7l, with a trimethoxyl substitution, displayed an IC50 of 24.6 µM. For A549 cells, compounds with trifluoromethyl (7d), furanyl (7n), hydroxyl (7t), and bromochlorine substitutions (7k) showed enhanced inhibitory activity. The potency order was as follows: 7d (16.9 µM) was the most potent, followed by 7t (19.3 µM), 7k (26.5 µM), and 7n (29.1 µM). Additionally, against Hela cells, 7c, which contains a methyl group, 7d with a trifluoromethyl substitution, 7p featuring a dimethylamino group, and 7t substituted with a hydroxyl group, all exhibited significant activity. Notably, 7c and 7p demonstrated the strongest inhibitory effects (the structure of the compound is shown in Figure 1), with IC50 values of 18.2 and 18.9 µM, respectively. Concurrently, our findings indicated that compounds adorned with ethoxy, naphthylidene, and pyrrolyl groups were essentially ineffective against MCF-7 cells. However, 7d, which incorporates a trifluoromethyl group, and 7k, which contains chlorine and bromine atoms, demonstrated enhanced activity.
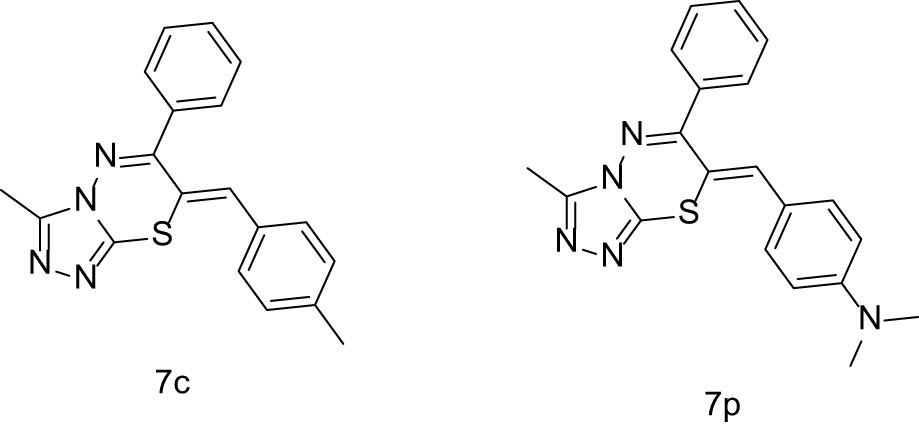
Compounds 7c and 7p.
Preliminary structure–activity relationship analysis revealed that the antiproliferative potency of the target compounds was significantly influenced by the substituents on the triazolothiadiazine ring, with the nature of the R3 substituent exerting a pronounced effect on activity. Moreover, the incorporation of halogenated groups was found to be particularly beneficial in augmenting the biological potency of these compounds. This enhancement was hypothesized to involve two key mechanistic aspects. First, the electronic effects of halogen atoms were identified as critical modulators of activity. Fluorine atoms, owing to their high electronegativity, were shown to exhibit the most pronounced enhancement, while synergistic electronic effects derived from polyhalogen substitutions (e.g., trifluoromethyl groups) were associated with significantly higher activity compared to monosubstituted analogues. Second, halogen atoms were linked to reduced steric hindrance relative to bulky substituents (e.g., naphthyl or ethoxy groups), which was proposed to facilitate ligand–receptor binding. Notably, dihalogen-substituted compounds (e.g., Br/Cl) were observed to demonstrate superior activity over monosubstituted counterparts, further supporting the hypothesis that both the number and type of halogen substituents could synergistically modulate biological activities.
3 Conclusion
A series of triazolothiadiazine derivatives have been synthesized with different substituents at triazolyl ring and thiadiazine. Triazolothiadiazine derivatives were synthesized under mild conditions, which yielded well. The spectroscopic analysis was used to characterize the new compounds. The newly synthesized compounds were tested for their anti-cancer properties against MCF-7 cells, Hela cells, A549 cells, and SW620 cells. IC50 values of 18.2 and 18.9 µM were exhibited by 7c and 7p, respectively, for the strongest inhibition of Hela cells, whereas IC50 values of 16.9 and 19.3 µM were shown by 7d and 7t as the most potent inhibitors against A549 cells. The most active compounds against the SW620 cell line were found to be 7l and 7i, while the most promising ones against the MCF-7 cell line were determined to be 7k and 7d. In addition, certain inhibitory activity was shown by the compound 7d on all four cells. In summary, a leading compound of antitumor drugs has the potential to be developed from the new triazothiodiazine derivative.
4 Experimental
4.1 Experimental details
The melting points were determined using an X-6 microscopic melting point tester. The 1H NMR (400 MHz) and 13C NMR spectra were acquired on a Bruker Avance spectrometer at 298.0 K using CDCl3/DMSO-d 6 as solvent with 16 scans. The mass spectral information of the compounds was obtained using ESI+ mode.
The experimental materials were purchased from Shanghai Xian Ding Biotechnology Co. Ltd and were used directly without any purification.
4.2 Synthesis of 4-amino-5-methyl-4H-1,2,4-triazole-3-thiol (3a)
Thiocarbohydrazide (94.21 mmol) was added to acetic acid (659.46 mmol) and the mixture was stirred for 4 h at reflux. The progress of the reaction was monitored using thin-layer chromatography (TLC). Upon completion of the reaction, the mixture was allowed to cool to room temperature, and the crude product was isolated by filtration. The pure product was subsequently obtained by recrystallization from distilled water. Yield: 78%, white solid.
4.3 Synthesis of 4-amino-4H-1,2,4-triazole-3-thiol (3b)
Thiocarbazide (94.21 mmol) is added to a mixture of formic acid (197.84 mmol) and water (30.6 mL) and stirred for 4 h at reflux temperature. The progress of the reaction was monitored by TLC. After the reaction was complete, the mixture was cooled to room temperature and filtered through a Büchner funnel to obtain the crude product. The pure product was subsequently obtained by recrystallization from distilled water. Yield: 55%, purple solid.
4.4 Synthesis of 3-methyl-6-phenyl-7H-[1,2,4] triazolo [3,4-b][1,3,4] thiadiazine (5a)
In a three-necked round-bottom flask, 3a (76.82 mmol) and α-bromoacetophenone (76.82 mmol) were combined with anhydrous ethanol (180 mL) and the mixture was refluxed for 5 h. The reaction progress was monitored by TLC. After the reaction was complete, the flask was allowed to cool to room temperature, and the pH of the mixture was carefully adjusted to 8 using a 1 M aqueous ammonia solution with continuous stirring, which led to the precipitation of a solid. The solid was isolated by vacuum filtration using a Büchner funnel, and then dried under controlled conditions to afford a red solid product without the need for further purification. Yield: 90%.
4.5 General method for synthesis of target triazole thiadiazine compounds 7a–t
A mixture of triazolothiadiazine 5a–c (8.68 mmol), aldehyde 6 (11.29 mmol) in 25 mL of methanol was at reflux temperature for a period of 8–34 h and monitored by TLC. Once the reaction was complete, the mixture was cooled and diluted with cold water. The crude solid was then purified by column chromatography using a mixture of ethyl acetate and petroleum ether as the eluent.
(Z)-7-benzylidene-3-methyl-6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (7a). Yield: 78%, yellow solid, m.p.: 237–238°C. 1H NMR (400 MHz, DMSO-d 6) δ 7.88–7.81 (m, 2H), 7.65–7.59 (m, 2H), 7.55–7.52 (m, 3H), 7.52–7.48 (m, 3H), 7.32 (s, 1H), 2.50 (s, 3H). 13C NMR (101 MHz, DMSO-d 6) δ 156.13, 150.58, 141.17, 137.68, 135.00, 133.19, 131.38, 130.16, 129.85, 129.11, 128.79, 116.33, 9.75. HRMS (ESI) m/z [M + H]+ calcd for C18H14N4S: 319.1012, found: 319.1014.
(Z)-7-benzylidene-6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (7b). Yield: 74%, yellow solid, m.p.: 184–185°C. 1H NMR (400 MHz, DMSO-d 6) δ 9.18 (s, 1H), 7.85–7.79 (d, 2H), 7.67–7.55 (m, 3H), 7.54–7.47 (m, 5H), 7.31 (s, 1H). 13C NMR (101 MHz, DMSO-d 6) δ 157.29, 143.49, 141.58, 138.19, 135.07, 133.38, 131.70, 130.04, 129.38, 129.10, 117.05. HRMS (ESI) m/z [M + H]+ calcd for C17H12N4S: 305.0856, found: 305.0860.
(Z)-3-methyl-7-(4-methylbenzylidene)-6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (7c). Yield: 70%, yellow solid, m.p.: 156–157°C. 1H NMR (400 MHz, Chloroform-d) δ 7.90–7.82 (m, 2H), 7.67–7.55 (m, 7H), 7.26 (s, 1H), 2.71 (s, 3H), 2.53 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 156.51, 151.10, 140.70, 140.41, 138.61, 135.42, 131.13, 130.55, 130.08, 129.70, 129.54, 129.00, 116.05, 21.65, 10.13. HRMS (ESI) m/z [M + H]+ calcd for C19H16N4S: 333.1169, found: 333.1172.
(Z)-3-methyl-6-phenyl-7-(3-(trifluoromethyl)benzylidene)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (7d). Yield: 89%, yellow solid, m.p.: 177–178°C. 1H NMR (400 MHz, Chloroform-d) δ 7.77–7.74 (m, 2H), 7.70–7.61 (m, 3H), 7.61–7.56 (m, 2H), 7.54–7.51 (m, 2H), 7.19 (s, 1H), 2.59 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 156.00, 151.51, 138.61, 135.17, 134.30, 132.90, 131.71, 129.93, 129.72, 129.49, 127.01, 126.84, 125.39, 122.68, 119.77, 10.42. HRMS (ESI) m/z [M + H]+ calcd for C19H13F3N4S: 387.0886, found: 387.0885.
(Z)-7-(2-chlorobenzylidene)-3-methyl-6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (7e). Yield: 88%, yellow solid, m.p.: 158–159°C. 1H NMR (400 MHz, Chloroform-d) δ 7.81–7.75 (m, 2H), 7.67–7.49 (m, 5H), 7.37–7.34 (m, 2H), 7.30 (s, 1H), 2.60 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 155.92, 151.46, 138.83, 137.72, 135.03, 134.68, 132.14, 131.69, 131.43, 130.42, 130.17, 129.94, 129.38, 127.26, 120.36, 10.49. HRMS (ESI) m/z [M + H]+ calcd for C18H13ClN4S: 353.0623, found: 353.0627.
(Z)-3-methyl-7-(2-methylbenzylidene)-6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (7f). Yield: 84%, yellow solid, m.p.: 185–186°C. 1H NMR (400 MHz, Chloroform-d) δ 7.76–7.75 (m, 2H), 7.59–7.49 (m, 3H), 7.43–7.39 (m, 1H), 7.32–7.21 (m, 4H), 2.59 (s, 3H), 2.14 (s, 3H). 13C NMR (101 MHz, DMSO-d 6) δ 155.63, 150.61, 140.10, 137.88, 136.96, 134.79, 132.50, 131.38, 130.59, 130.04, 129.74, 129.10, 128.87, 126.00, 118.03, 19.51, 9.78. HRMS (ESI) m/z [M + H]+ calcd for C19H16N4S: 333.1169, found: 333.1171.
(Z)-7-(2-fluorobenzylidene)-3-methyl-6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4] thiadiazine (7g). Yield: 85%, yellow solid, m.p.: 167–168°C. 1H NMR (400 MHz, Chloroform-d) δ 7.78–7.75(m, 2H), 7.64–7.48 (m, 4H), 7.45–7.42 (m, 1H), 7.31–7.22 (m, 2H), 7.12–7.10 (m, 1H), 2.60 (s, 3H). 13C NMR (101 MHz, DMSO-d 6) δ 161.06, 158.58, 155.58, 150.98, 137.76, 134.00, 133.46, 132.78, 131.75, 131.00, 130.07 129.43, 128.80, 125.12, 121.37, 119.83, 116.36, 10.02. HRMS (ESI) m/z [M + H]+ calcd for C18H13FN4S: 337.0918, found: 337.0922.
(Z)-7-(2-methoxybenzylidene)-3-methyl-6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (7h). Yield: 78%, yellow solid, m.p.: 182–183°C. 1H NMR (400 MHz, Chloroform-d) δ 7.80–7.75 (m, 2H), 7.55–7.47 (m, 4H), 7.38–7.35 (m, 2H), 7.04–6.98 (m, 2H), 6.91 (d, J = 1.0 Hz, 1H), 4.03 (d, J = 6.9 Hz, 2H), 2.59 (s, 3H), 1.35 (s, 3H). 13C NMR (101 MHz, DMSO-d 6) δ 156.87, 156.38, 150.82, 138.57, 137.31, 135.30, 132.28, 131.60, 130.04, 129.35, 122.10, 120.62, 117.12, 112.72, 64.24, 14.90, 10.03. HRMS (ESI) m/z [M + H]+ calcd for C20H18N4OS: 363.1275, found: 363.1277.
(Z)-7-(2-methoxybenzylidene)-3-methyl-6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (7i). Yield: 80%, yellow solid, m.p.: 172–173°C. 1H NMR (400 MHz, Chloroform-d) δ 7.83–7.75 (m, 2H), 7.59–7.45 (m, 3H), 7.38–7.37 (m, 2H), 7.34 (s, 1H), 7.04–7.02 (m, 1H), 6.92–6.91 (m, 1H), 3.79 (s, 3H), 2.58 (s, 3H). 13C NMR (101 MHz, DMSO-d 6) δ 157.37, 156.33, 150.79, 138.68, 137.65, 135.27, 132.38, 131.74, 130.58, 130.10, 129.38, 121.91, 120.62, 117.36, 111.83, 56.06, 10.03. HRMS (ESI) m/z [M + H]+ calcd for C19H16N4OS: 349.1118, found: 349.1120.
(Z)-2-((3-methyl-6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-7-ylidene)methyl)benzonitrile (7j). Yield: 88%, yellow solid, m.p.: 247–258°C. 1H NMR (400 MHz, DMSO-d 6) δ 8.21 (d, J = 7.6 Hz, 1H), 7.86 (d, J = 7.4 Hz, 1H), 7.77–7.71 (m 1H), 7.69–7.67 (m, 1H), 7.55–7.49 (m, 2H), 7.45–7.38 (m, 3H), 6.64 (s, 1H), 1.82 (s, 3H). 13C NMR (101 MHz, DMSO-d 6) δ 174.81, 168.85, 163.21, 144.35, 143.31, 137.43, 135.65, 133.56, 131.78, 130.71, 129.32, 128.92, 127.61, 123.82, 122.58, 99.90, 11.50. HRMS (ESI) m/z [M + H]+ calcd for C19H13N5S: 344.0965, found: 344.0971.
(Z)-7-(4-bromo-2-chlorobenzylidene)-3-methyl-6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (7k). Yield: 85%, yellow solid, m.p.: 207–208°C. 1H NMR (400 MHz, Chloroform-d) δ 7.78–7.74 (m, 2H), 7.71–7.70 (m, 1H), 7.58–7.51 (m, 3H), 7.47–7.46 (m, 1H), 7.32–7.30 (m, 1H), 7.19 (s, 1H), 2.61 (s, 3H). 13C NMR (101 MHz, DMSO-d 6) δ 155.02, 151.08, 137.66, 136.35, 134.48, 134.39, 133.99, 133.28, 132.39, 132.03, 131.89, 130.08, 129.46, 121.47, 120.39, 10.05. HRMS (ESI) m/z [M + H]+ calcd for C18H12BrClN4S: 430.9728, found: 430.9730.
(Z)-3-methyl-6-phenyl-7-(2,3,4-trimethoxybenzylidene)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (7l). Yield: 82%, yellow solid, m.p.: 168–169°C. 1H NMR (400 MHz, Chloroform-d) δ 7.75–7.73 (m, 2H), 7.57–7.51 (m, 3H), 7.37–7.26 (m, 2H), 6.77–6.75 (m, 1H), 3.92 (s, 3H), 3.84 (s, 3H), 3.74 (s, 3H), 2.58 (s, 3H). 13C NMR (101 MHz, DMSO-d 6) δ 156.54, 155.91, 152.38, 150.80, 142.09, 138.23, 136.17, 135.50, 131.53, 130.03, 129.32, 125.35, 119.95, 115.71, 108.24, 61.91, 61.03, 56.58, 10.01. HRMS (ESI) m/z [M + H]+ calcd for C21H20N4O3S: 409.1329, found: 409.1335.
(Z)-7-(3-methoxybenzylidene)-3-methyl-6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (7m). Yield: 72%, yellow solid, m.p.: 157–158°C. 1H NMR (400 MHz, Chloroform-d) δ 7.75–7.73 (m, 2H), 7.61–7.47 (m, 3H), 7.37–7.35 (m, 1H), 7.14 (s, 1H), 7.05–6.92 (m, 3H), 3.83 (s, 3H), 2.58 (s, 3H). 13C NMR (101 MHz, DMSO-d 6) δ 159.59, 156.31, 150.83, 141.37, 137.96, 135.28, 134.78, 131.64, 130.14, 129.37, 122.60, 116.95, 116.45, 115.38, 55.77, 10.01. HRMS (ESI) m/z [M + H]+ calcd for C19H16N4OS: 349.1118, found: 349.1119.
(Z)-7-(furan-2-ylmethylene)-3-methyl-6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (7n). Yield: 81%, yellow solid, m.p.: 230–231°C. 1H NMR (400 MHz, DMSO-d 6) δ 8.07 (d, J = 1.8 Hz, 1H), 7.73–7.66 (m, 2H), 7.66–7.52 (m, 3H), 7.09 (d, J = 3.6 Hz, 1H), 6.87 (s, 1H), 6.77–6.76 (m 1H), 2.46 (s, 3H). 13C NMR (101 MHz, DMSO-d 6) δ 155.26, 150.93, 149.63, 147.32, 136.97, 135.30, 131.15, 129.90, 129.36, 124.30, 118.49, 113.93, 112.75, 9.99. HRMS (ESI) m/z [M + H]+ calcd for C16H12N4OS: 309.0805, found: 309.0808.
(Z)-3-methyl-7-(naphthalen-2-ylmethylene)-6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (7o). Yield: 85%, yellow solid, m.p.: 222–223°C. 1H NMR (400 MHz, DMSO-d 6) δ 8.13 (s, 1H), 8.10–8.04 (m, 1H), 7.99–7.97 (m, 2H), 7.88–7.87 (m, 2H), 7.62–7.58 (m, 6H), 7.46 (s, 1H), 2.51 (s, 3H). 13C NMR (101 MHz, DMSO-d 6) δ 156.40, 150.88, 141.11, 137.86, 135.36, 133.48, 132.80, 131.63, 131.07, 130.71, 130.14, 129.41, 129.10, 128.60, 128.19, 128.12, 127.45, 127.15, 116.92, 10.03. HRMS (ESI) m/z [M + H]+ calcd for C22H16N4S: 369.1169, found: 369.1173.
(Z)-N,N-dimethyl-4-((3-methyl-6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-7-ylidene)methyl)aniline (7p). Yield: 65%, yellow solid, m.p.: 198–199°C. 1H NMR (400 MHz, DMSO-d 6) δ 7.79–7.73 (m, 2H), 7.57–7.53 (m, 3H), 7.43 (d, J = 8.5 Hz, 2H), 7.07 (s, 1H), 6.79 (d, J = 8.7 Hz, 2H), 3.00 (s, 6H), 2.47 (s, 3H). 13C NMR (101 MHz, DMSO-d 6) δ 157.57, 151.61, 150.65, 141.45, 138.13, 135.96, 132.71, 131.37, 130.08, 129.28, 120.64, 111.89, 109.27, 40.22, 10.02. HRMS (ESI) m/z [M + H]+ calcd for C20H19N5S: 362.1434, found: 362.1435.
(Z)-7-((1H-pyrrol-2-yl)methylene)-3-methyl-6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (7q). Yield: 79%, yellow solid, m.p.: 183–184°C. 1H NMR (400 MHz, DMSO-d 6) δ 11.54 (s, 1H), 7.76–7.67 (m, 2H), 7.66–7.53 (m, 3H), 7.15–7.14 (m, 1H), 7.10 (s, 1H), 6.95–6.89 (m, 1H), 6.40–6.39 (m, 1H), 2.46 (s, 3H). 13C NMR (101 MHz, DMSO-d 6) δ 156.67, 150.79, 137.38, 135.80, 131.06, 130.04, 129.30, 128.84, 127.09, 124.36, 115.40, 112.16, 107.37, 10.04. HRMS (ESI) m/z [M + H]+ calcd for C16H13N5S: 308.0965, found: 308.0969.
(Z)-7-(4-chlorobenzylidene)-3-methyl-6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (7r). Yield: 64%, yellow solid, m.p.: 215–216°C. 1H NMR (400 MHz, DMSO-d 6) δ 7.91–7.81 (m, 2H), 7.69–7.48 (m, 7H), 7.32 (s, 1H), 2.51 (s, 3H). 13C NMR (101 MHz, DMSO-d 6) δ 156.24, 150.89, 140.22, 137.87, 135.13, 134.97, 132.36, 132.22, 131.71, 130.14, 129.39, 129.09, 117.33, 10.02. HRMS (ESI) m/z [M + H]+ calcd for C18H13ClN4S: 353.0623, found: 353.0627.
(Z)-7-(2-bromobenzylidene)-3-methyl-6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (7s). Yield: 78 %, yellow solid, m.p.: 162–163°C. 1H NMR (400 MHz, DMSO-d 6) δ 7.92–7.85 (m, 2H), 7.77 (d, J = 7.9 Hz, 1H), 7.68 (d, J = 7.2 Hz, 1H), 7.60–7.56 (m, 4H), 7.46–7.38 (m, 1H), 7.21 (s, 1H), 2.51 (s, 3H). 13C NMR (101 MHz, DMSO-d 6) δ 155.23, 151.05, 139.53, 137.55, 134.70, 133.54, 133.39, 132.12, 131.77, 131.01, 130.05, 129.43, 128.34, 123.70, 119.84, 10.06. HRMS (ESI) m/z [M + H]+ calcd for C18H13BrN4S: 397.0118, found: 397.0126.
(Z)-4-(7-benzylidene-3-methyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-6-yl)phenol (7t). Yield: 55%, yellow solid, m.p.: 201–202°C. 1H NMR (400 MHz, DMSO-d 6) δ 7.96–7.94 (m, 1H), 7.73–7.71 (m, 2H), 7.54–7.48 (m, 5H), 7.45 (s, 1H), 6.93 (d, J = 8.6 Hz, 2H), 2.49 (s, 3H). 13C NMR (101 MHz, DMSO-d 6) δ 160.98, 156.71, 150.61, 142.02, 138.52, 133.54, 131.98, 130.54, 130.35, 128.99, 125.32, 116.49, 116.29, 10.04. HRMS (ESI) m/z [M + H]+ calcd for C18H14N4OS: 335.0962, found: 335.0965.
-
Funding information: Project supported by Heilongjiang Provincial Key Laboratory of New Drug Development and Pharmacotoxicological Evaluation (kfkt2023-01), Scientific and technological research project of Heilongjiang provincial science and Technology Department (SZDYF202306), and Natural Science Foundation of Heilongjiang Province of China (LH2022H096).
-
Author contributions: Shujing Zhou conceived and designed the research framework, and established the synthetic routes; Jinjing Li supervised the study, critically reviewed and revised the manuscript, and provided financial support; Jiahui Lv performed data analysis and interpretation, and drafted the manuscript; Xue Luo synthesized the target compounds and conducted biological activity evaluations; Yonghong Du organized and curated experimental datasets; Tiantian He carried out compound purification and characterization.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Nechak R, Bouzroura SA, Benmalek Y, Boufeoua N, Kolli BN, Martini SP, et al. Synthesis, identification and antimicrobial activity of substituted thiazolines and 1,3,4-thiadiazines from dehydroacetic acid. Synth Commun. 2019;49:1895–905.10.1080/00397911.2019.1606918Search in Google Scholar
[2] Fidalgo ML, Álvarez MMA, Geigel FL, Pineiro PR, Navarro MS, Cabrera HR. Effect of thiadiazine derivatives on intracellular amastigotes of Leishmania amazonensis. Mem Inst Oswaldo Cruz. 2004;99:329–30.10.1590/S0074-02762004000300016Search in Google Scholar PubMed
[3] Sarapultsev AP, Chupakhin ON, Sarapultsev PA, Sidorova LP, Tseitler TA. Pharmacologic evaluation of antidepressant activity and synthesis of 2-morpholino-5-phenyl-6H-1,3,4-thiadiazine. Pharmaceuticals. 2016;9:27.10.3390/ph9020027Search in Google Scholar PubMed PubMed Central
[4] Lebouvier N, Pagniez F, Duflos M, Pape PL, Na YM, Baut GL, et al. Synthesis and antifungal activities of new fluconazole analogues with azaheterocycle moiety. Bioorg Med Chem Lett. 2007;17:3686–9.10.1016/j.bmcl.2007.04.038Search in Google Scholar PubMed
[5] Papadopoulou MV, Bloomer WD, Lepesheva GI, Rosenzweig SH, Kaiser M, Aguilera-Venegas B, et al. Novel 3-nitrotriazole-based amides and carbinols as bifunctional antichagasic agents. J Med Chem. 2015;58:1307–19.10.1021/jm5015742Search in Google Scholar PubMed PubMed Central
[6] Akbarzadeh T, TabatabaiS A, Khoshnoud MJ, Shafaghi B, Shafiee A. Design and synthesis of 4H-3-(2-phenoxy)phenyl-1,2,4-triazole derivatives as benzodiazepine receptor agonists. Bioorg Med Chem. 2003;11:769–73.10.1016/S0968-0896(02)00469-8Search in Google Scholar PubMed
[7] Kritsanida M, Mouroutsou A, Marakos P, Pouli N, Papakonstantinou-Garoufalias A, Pannecouque C, et al. Synthesis and antiviral activity evaluation of some new 6-substituted 3-(1-adamantyl)-1,2,4-triazolo[3,4-b][1,3,4]thiadiazoles. Farmaco. 2003;57:253–7.10.1016/S0014-827X(01)01189-2Search in Google Scholar PubMed
[8] Maghraby MT, Almutairi TM, Bräse S, Salem OIA, Youssif BGM, Sheha MM. New 1,2,3-Triazole/1,2,4-triazole hybrids as aromatase inhibitors: design, synthesis, and apoptotic antiproliferative activity. Molecules. 2023;28(20):7092.10.3390/molecules28207092Search in Google Scholar PubMed PubMed Central
[9] Omar AME, AboulWafa OM, Amr ME, El-Shoukrofy MS. Antiproliferative activity, enzymatic inhibition and apoptosis-promoting effects of benzoxazole-based hybrids on human breast cancer cells. Bioorg Chem. 2021;109:104752.10.1016/j.bioorg.2021.104752Search in Google Scholar PubMed
[10] Lin R, Connolly PJ, Huang SL, Wetter SK, Lu YH, Murray WV, et al. 1-Acyl-1H-[1,2,4] triazole-3, 5-diamine analogues as novel and potent anticancer cyclin-dependent kinase inhibitors: synthesis and evaluation of biological activities. J Med Chem. 2005;48(13):4208.10.1021/jm050267eSearch in Google Scholar PubMed
[11] Grieco I, Bissaro M, Tiz DB, Perez DI, Perez C, Martinez A, et al. Developing novel classes of protein kinase CK1δ inhibitors by fusing [1,2,4]triazole with different bicyclic heteroaromatic systems. Eur J Med Chem. 2021;216:113331.10.1016/j.ejmech.2021.113331Search in Google Scholar PubMed
[12] Abdel-Aziz HA, Supuran CT, Eldehna WM. 1,5-diaryl-1,2,4-triazole ureas as new SLC-0111 analogues endowed with dual carbonic anhydrase and VEGFR-2 inhibitory activities. J Med Chem. 2023;66(15):10558.10.1021/acs.jmedchem.3c00721Search in Google Scholar PubMed
[13] Abbas ZK, Naser NH, Atiya RN. In silico study of novel sulfonamide derivatives bearing a 1,2,4-triazole moiety act as carbonic anhydrase inhibitors with promising anti-cancer activity. Pol Merkur Lek. 2023;51(5):527.10.36740/Merkur202305112Search in Google Scholar PubMed
[14] Karegoudar P, Prasad DJ, Ashok M. Synthesis, antimicrobial and anti-inflammatory activities of some 1,2,4-triazolo[3,4-b][1,3,4]thiadiazoles and 1,2,4-triazolo[3,4-b][1,3,4]thiadiazines bearing trichlorophenyl moiety. Eur J Med Chem. 2008;43(4):808–23.10.1016/j.ejmech.2007.06.026Search in Google Scholar PubMed
[15] Sert-Ozgur S, Tel BC, Somuncuoglu EI, Kazkayasi I, Ertan M, Tozkoparan B. Design and synthesis of 1,2,4-triazolo[3,2-b]-1,3,5-thiadiazine derivatives as a novel template for analgesic/anti-inflammatory activity. Arch Pharm. 2017;350:1–10.10.1002/ardp.201700052Search in Google Scholar PubMed
[16] Kamal A, Khan MNA, Srikanth YVV. Synthesis, structural characterization and biological evaluation of novel [1,2,4]triazolo [1,5-b][1,2,4]benzothiadiazine-benzothiazole conjugates as potential anticancer agents. Chem Biol Drug Des. 2008;16(16):7804.10.1111/j.1747-0285.2007.00609.xSearch in Google Scholar PubMed
[17] Puthiyapurayil P, Poojary B, Chikkanna C, Buridipad SK. Synthesis, spectral characterization and biological evaluation of a novel series of 6-arylsubstituted-3-[2-(4-substitutedphenyl)propan-2-yl]-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazines. Eur J Med Chem. 2012;57:407–16.10.1016/j.ejmech.2012.06.059Search in Google Scholar PubMed
[18] Miao R, Wei J, Lv MH, Cai Y, Du YP, Hui XP, et al. Conjugation of substituted ferrocenyl to thiadiazine as apoptosis-inducing agents targeting the bax/bcl-2 pathway. Eur J Med Chem. 2011;46:5000–9.10.1016/j.ejmech.2011.08.007Search in Google Scholar PubMed
[19] Ansari M, Shokrzadeh M, Karima S, Rajaei S, Hashemi SM, Mirzaei H, et al. Design, synthesis and biological evaluation of flexible and rigid analogs of 4H-1,2,4-triazoles bearing 3,4,5-trimethoxyphenyl moiety as new antiproliferative agents. Bioorg Chem. 2019;93:103300–12.10.1016/j.bioorg.2019.103300Search in Google Scholar PubMed
[20] Radwan RR, Zaher NH, El-Gazzar MG. Novel 1,2,4-triazole derivatives as antitumor agents against hepatocellular carcinoma. Chem-Biol Interact. 2017;274:68–79.10.1016/j.cbi.2017.07.008Search in Google Scholar PubMed
[21] Iradyan MA, Iradyan NS, Minasyan NS, Paronikyan RV, Stepanyan GM. Synthesis and antibacterial activity of 3,6-diaryl-7H-[1,2,4] triazolo [3,4-b][1,3,4]thiadiazines. Pharm Chem J. 2016;50:10–5.10.1007/s11094-016-1389-ySearch in Google Scholar
[22] Almajan GL, Barbuceanu SF, Saramet I, Draghici C. New 6-amino-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazines and [1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-6-ones: synthesis, characterization and antibacterial activity evaluation. Eur J Med Chem. 2010;41:3191–5.10.1016/j.ejmech.2010.02.057Search in Google Scholar PubMed
[23] Bhatt TD, Kalavadiya PL, Joshi HS. Microwave-assisted synthesis, antimicrobial activity, and SAR study of 3-(2-chlorobenzyl)-6-(substituted phenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazines. Russ J Bioorg Chem. 2021;47:1105–13.10.1134/S1068162021050216Search in Google Scholar
[24] Kooi-Mow S, Kah-Cheng T. Synthesis, characterization and antibacterial evaluation of some new 1,2,4-triazolo[3,4-b][1,3,4]thiadiazines as potential antibacterial agents. Lett Drug Des Discov. 2018;15:733–43.10.2174/1570180814666170922165933Search in Google Scholar
[25] Khan I, Ibrar A, Zaib S, Ahmad S, Furtmann N, Hameed S, et al. Active compounds from a diverse library of triazolothiadiazole and triazolothiadiazine scaffolds: synthesis, crystal structure determination, cytotoxicity, cholinesterase inhibitory activity, and binding mode analysis. Bioorg Med Chem. 2014;22(21):6163–73.10.1016/j.bmc.2014.08.026Search in Google Scholar PubMed
[26] Sathyanarayana R, Poojary B, Chandrashekarappa RB, Kumar H, Merugumoiu VKJ. Novel [1,2,4]triazolo[3,4-b][1,3,4]thiadiazine derivatives embedded with benzimidazole moiety as potent antioxidants. Chin Chem Soc. 2020;67:1501–16.10.1002/jccs.201900452Search in Google Scholar
[27] Khramchikhin AV, Skryl’nikova MA, Esaulkova IL, Sinegubova EO, Zarubaev VV, Gureev MA, et al. Novel [1,2,4]triazolo[3,4-b][1,3,4]thiadiazine and [1,2,4]triazolo[3,4-b][1,3,4]thiadiazepine derivatives: synthesis, anti-viral in vitro study and target validation activity. Molecules. 2022;27:7940–64.10.3390/molecules27227940Search in Google Scholar PubMed PubMed Central
[28] Song MX, Zhang CB, Deng XQ, Sun ZG, Quan ZS. Synthesis and anticonvulsant activity evaluation of 6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazines. Lett Drug Des Discov. 2011;8(8):769–73.10.2174/157018011796575962Search in Google Scholar
[29] Olson ME, Li M, Harris RS, Harki DA. Small-molecule APOBEC3G DNA cytosine deaminase inhibitors based on a 4-amino-1,2,4-triazole-3-thiol scaffold. ChemMedChem. 2013;8(1):112–7.10.1002/cmdc.201200411Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Synthesis, activity exploration, molecular docking, and affinity assessment of theophylline derivatives as inhibitors targeting IDO1
- Synthesis of novel triazole hybrids with pyrene, fluorene, and biphenyl groups and evaluation of their antimycobacterial activity
- Synthesis and antiproliferative evaluation of novel triazolothiadiazine compounds
- Review Article
- Development of heterocyclic-based anticancer agents: A comprehensive review
Articles in the same Issue
- Research Articles
- Synthesis, activity exploration, molecular docking, and affinity assessment of theophylline derivatives as inhibitors targeting IDO1
- Synthesis of novel triazole hybrids with pyrene, fluorene, and biphenyl groups and evaluation of their antimycobacterial activity
- Synthesis and antiproliferative evaluation of novel triazolothiadiazine compounds
- Review Article
- Development of heterocyclic-based anticancer agents: A comprehensive review

