Abstract
Readily available 2-(toluene-4-sulfonylamino)ethylamine is a convenient starting material for a two-step synthesis of 1-[2-(toluene-4-sulfonamido)ethyl]thiourea (2). Heterocyclization of the thiourea moiety in 2 furnished a thiazole derivative 3 which was further functionalized into substituted thiazoles 4–6. A series of 2-(thiazol-5-yl)-[1,3,4]oxadiazoles 8, 10 and 2-(thiazol-5-yl)-[1,3,4]thiadiazoles 9, 11 were obtained.
Introduction
Thiazole, 1,3,4-thiadiazole and 1,3,4-oxadiazole are interesting and important heterocyclic systems, derivatives of which possess a large diversity of biological activity (http://www.alanwood.net/pesticides/class_pesticides.html, [1]). In particular, we have established that thiazolyl-substituted 1,3,4-thiadiazoles and 1,3,4-oxadiazoles are fungicidal and growth-stimulant agents [1].
The aim of this investigation was to develop facile and efficient methods for the synthesis of derivatives mentioned above, containing pharmacophore 2-(toluene-4-sulfonylamino)-ethylamino moiety. It can be predicted that the desired products may show interesting physiological activity.
Results and discussion
Synthesis
1-(Toluene-4-sulfonamidoethyl)-3-benzoylthiourea (1) was synthesized by the reaction of 2-(toluene-4-sulfonylamino)ethylamine with benzoyl isothiocyanate (Scheme 1). Debenzoylation of 1 furnished 1-(toluene-4-sulfonamidoethyl)thiourea (2), heterocyclization of which with ethyl 2-chloro-3-oxobutyrate afforded ethyl 4-methyl-2-[2-(toluene-4-sulfonylamino)ethylamino]thiazole-5-carboxylate (3). Treatment of ester 3 with hydrazine hydrate yielded 4-methyl-2-[2-(toluene-4-sulfonylamino)ethylamino]thiazole-5-carboxylic acid hydrazide (4), which was subsequently transformed into azide 5. The reactions of azide 5 with propylamine and pyrrolidine did not proceed with the expected elimination of nitrogen and formation of 3-ureido derivatives. Instead, the respective product 6 was obtained. The isolation of 6 is fully consistent with 1H NMR and mass spectra. In particular, the mass spectra of 6a (R = C3H7NH) and 6b (R = pyrrolidino) show the respective molecular ion peaks at m/z 396 and m/z 408, as calculated for these structures.
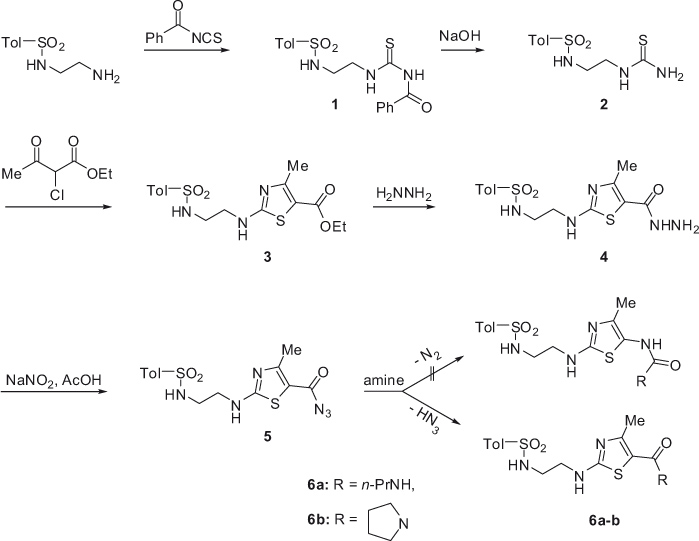
4-Methyl-2-[2-(toluene-4-sulfonylamino)ethylamino]thiazole-5-carboxylic acid hydrazide (4) was also used as an initial compound for the syntheses of noncondensed bicyclic systems, including thiazole ring in combination with [1,3,4]oxadiazole and [1,3,4]thiadiazole heterocycles (Scheme 2). First, the reaction of hydrazide 4 with carbon disulfide and potassium hydroxide in ethanol furnished the potassium salt of 2-{2-[2-(toluene-4-sulfonylamino)ethylamino]-4-methyl-thiazole-5-carbonyl}hydrazinecarbodithioic acid (7). Then, alkaline and acidic hydrolysis of 7 furnished 2-{[2-(toluene-4-sulfonylamino)ethylamino]-4-methylthiazol-5-yl}-5-thioxo-4,5-dihydro[1,3,4]oxadiazole (8) and 2-{[2-(toluene-4-sulfonylamino)ethylamino]-4-methylthiazol-5-yl}-5-thioxo-4,5-dihydro[1,3,4]thiadiazole (9), respectively.
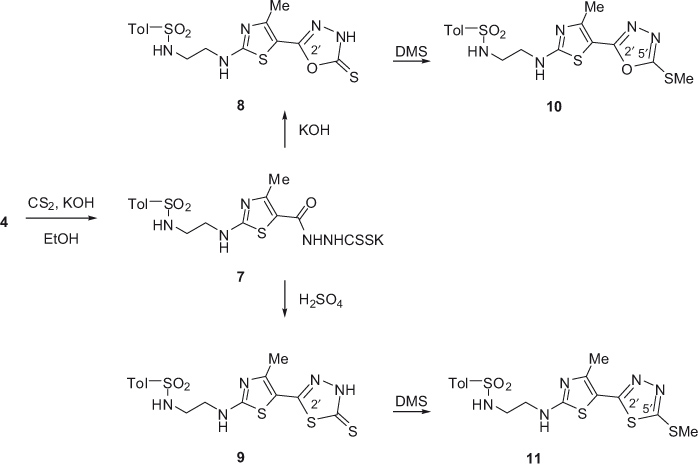
The suggested thione forms of 8 and 9 are consistent with their 13C NMR spectra that show signals at 176 ppm for the C=S function. The reaction of compounds 8 and 9 with dimethyl sulfate yielded their respective S-methylated derivatives 10 and 11. In 13C NMR spectra of these compounds instead of the C=S bond signals, new signals due to C=N bond of 1,3,4-oxadiazole or 1,3,4-thiadiazole system appear.
Biological activity
In preliminary biological tests the growth regulatory properties of synthesized compounds were investigated for their aqueous emulsions (25 mg/L and 50 mg/L) on the germination, growth and survivability of seeds and seedlings of dicotyledonous bean (Phaseolus vulgaris L.). The activities of these compounds were compared with that of heteroauxin. Practically all investigated substances 1–11 have shown strongly pronounced growth stimulant properties and their calculated activities were in the interval of 75–98%.
Experimental section
General
1H NMR (300 MHz) and 13C NMR (75 MHz) spectra were recorded on a Varian Mercury-300 spectrometer, in a mixture of DMSO-d6 and CCl4 (1:3) or in pure DMSO-d6. The reaction progress and purity of products were checked by using TLC on Silufol UV-254 plates with acetone/hexane (2:1) as an eluent. Melting points are uncorrected. 2-(Toluene-4-sulfonylamino)ethylamine was obtained as described previously [2].
3-Benzoyl-1-(toluene-4-sulfonamidoethyl)thiourea (1)
The mixture of 2-(toluene-4-sulfonylamino)-ethylamine (10.7 g, 0.05 mol) and benzoyl isothiocyanate (8.15 g, 0.05 mol) in 50 mL of toluene in the presence of catalytic amounts of pyridine was heated under reflux for 4 h. The precipitate of product 1 was filtered and washed with hexane: yield 17.7 g (94%) of white crystals; mp 148–150°С; 1H NMR: δ 2.36 (s, 3H), 3.08 (m, 2H), 3.75 (m, 2H), 7.23–8.00 (m, 10H), 10.88 (brs, 1H), 10.96 (t, 1H, J = 5.5 Hz). Anal. Calcd for С17Н19N3O3S2: C, 54.09; H, 5.07; N, 11.13; S, 16.99. Found: C, 54.15; H, 4.95; N, 10.82; S, 16.73.
1-(Toluene-4-sulfonamido-ethyl)thiourea (2)
A suspension of compound 1 (3.8 g, 0.01 mol) in 12 mL of 10% NaOH solution was heated under reflux with continuous stirring for 3 h. After cooling, 10 mL of water was added and the solution was acidified with acetic acid. In 1 h the compound 2 was separated: yield 1.86 g (68%) of white crystals; mp116–118°С; 1H NMR: δ 2.42 (s, 3H), 2.88 (m, 2H), 3.38 (m, 2H), 6.80 (brs, 2H), 7.23–7.82 (m, 5H), 8.17 (t, 1H, J = 5.5 Hz). Anal. Calcd for С10Н15N3O2S2: C, 43.93; H, 5.53; N, 15.37; S, 23.46. Found: C, 43.76; H, 5.41; N, 15.09; S, 23.21.
4-Methyl-2-[2-(toluene-4-sulfonylamino)ethylamino]thiazole-5-carboxylic acid ethylester (3)
A suspension of compound 2 (2.75 g, 0.01 mol), ethyl 2-chloro-3-oxobutyrate (1.65 g, 0.01 mol) and 94% K2CO3 (0.75 g, 0.005 mol) in 20 mL of absolute ethanol was heated under reflux with continuous stirring for 5 h. After concentration on a rotary evaporator, the solid residue of 3 was treated with 20 mL of water, filtered and washed with 50% ethanol: yield 2.83 g (74%) of white crystals; mp 152–154°С; 1H NMR: δ 1.30 (t, 3H, J = 7.0 Hz), 2.40 (s, 3H), 2.42 (s, 3H), 2.92 (m, 2H), 3.35 (m, 2H), 4.18 (t, 2H, J = 7.0 Hz), 7.22–7.70 (m, 4H) 7.43 (t, 1H, J = 5.5 Hz), 7.95 (t, 1H, J = 5.5 Hz). Anal. Calcd for С16Н21N3O4S2: C, 50.11; H, 5.52; N, 10.96; S, 16.72. Found: C, 49.97; H, 5.40; N, 10.68; S, 16.49.
4-Methyl-2-[2-(toluene-4-sulfonylamino)ethylamino]thiazole-5-carboxylic acid hydrazide (4)
A suspension of compound 3 (3.83 g, 0.01 mol) in 20 mL of hydrazine hydrate (53%) was stirred at 20–25°С for 48 h. The precipitate of 4 was filtered, washed with water and dried: yield 2.9 g (79%) of white crystals; mp 206–208°C; 1H NMR: δ 2.38 (s, 3H), 2.42 (s, 3H), 2.95 (m, 2H), 3.32 (m, 2H), 4.08 (brs, 2H), 7.22–7.72 (m, 4H), 7.45 (t, 1H, J = 5.5 Hz), 7.70 (brs, 1H), 8.50 (brt, 1H). Anal. Calcd for С14Н19N5O3S2: C, 45.51; H, 5.18; N, 18.96; S, 17.36. Found: C, 45.38; H, 5.06; N, 18.67; S, 17.08.
4-Methyl-2-[2-(toluene-4-sulfonylamino)ethylamino]thiazole-5-carboxylic acid azide (5)
To a suspension of compound 4 (3.7 g 0.01 mol) in 30 mL of water, 1.75 g (0.025 mol) of NaNO2 was added. Then the mixture was cooled to 0°С and treated portion-wise with 1.5 mL (0.025 mol) of acetic acid. After stirring at 20–25°С for 3 h, the resultant precipitate of 5 was filtered, washed with 30 mL of water and dried: yield 3.42 g (90%) of white crystals; mp 135–136°С; 1H NMR: δ 2.42 (s, 3H), 2.45 (s, 3H), 2.95 (m, 2H), 3.36 (m, 2H), 7.24–7.70 (m, 4H) 7.50 (t, 1H, J = 5.5 Hz), 8.43 (brt, 1H). Anal. Calcd for С14Н16N6O3S2: C, 44.20; H, 4.24; N, 22.09; S, 16.86. Found: C, 44.03; H, 4.12; N, 21.80; S, 16.59.
General procedure for 6a,b
A suspension of compound 5 (1.14 g, 0.003 mol) and 0.0033 mol of n-propylamine or pyrrolidine in 20 mL of anhydrous benzene in the presence of a catalytic amount of pyridine was heated under reflux for 2 h. Concentration under reduced pressure was followed by crystallization of the residue from aqueous ethanol (50%).
N-Propyl-2-[2-(toluene-4-sulfonylamino)ethylamino]-4-methylthiazole-5-carboxamide (6a)
Yield 1.1 g (89%) of white crystals; mp 210–212°С; 1H NMR: δ 0.90 (t, 3H, J = 7 Hz), 1.53 (m, 2H), 2.38 (s, 3H), 2.42 (s, 3H), 2.95 (m, 2H), 3.12 (q, 2H, J = 7 Hz), 3.30 (m, 2H), 7.00 (t, 1H, J = 5 Hz), 7.25–7.70 (m, 4H) 7.46 (brt, 1H, J = 5 Hz), 7.60 (t, 1H); MS: m/z 396 (M+). Anal. Calcd for С17Н24N4O3S2: C, 51.49; H, 6.10; N, 14.13; S, 16.17. Found: C, 51.32; H, 6.00; N, 13.88; S, 15.80.
2-[2-(Toluene-4-sulfonylamino)ethylamino]-4-methyl-5-(pyrrolidinocarbonyl)thiazole (6b)
Yield 1.1 g (87%) of white crystals; mp 150–152°С; 1H NMR: δ 1.90 (m, 4H), 2.22 (s, 3H), 2.42 (s, 3H), 2.95 (m, 2H), 3.30 (m, 2H), 3.48 (m, 4H), 7.25–7.70 (m, 4H) 7.46 (brt, 1H), 7.55 (t, 1H, J = 5 Hz); MS: m/z 408 (M+). Anal. Calcd for С18Н24N4O3S2: C, 52.92; H, 5.92; N, 13.71; S, 15.70. Found: C, 52.80; H, 5.82; N, 13.47; S, 15.49.
Potassium salt of 2-{2-[2-(toluene-4-sulfonylamino)ethylamino]-4-methylthiazole-5-carbonyl}hydrazinocarbodithioic acid (7)
A suspension of compound 4 (3.7 g, 0.01 mol) and KОН (0.84 g, 0.015 mol) in 20 mL of absolute ethanol was cooled to 0°С and treated dropwise with 0.9 mL (0.015 mol) of carbon disulfide. The mixture was briefly stirred at 20–25°С and then allowed to stand for 24 h. The precipitate of 7 was filtered and washed with 10 mL of cool ethanol: yield 3.96 g (82%) of white crystals; mp 136–138°С; 1H NMR: δ 2.40 (s, 3H), 2.42 (s, 3H), 2.95 (m, 2H), 3.31 (m, 2H), 7.22–7.72 (m, 4H), 7.15 (brs, 1H), 7.40 (t, 1H, J = 5.5 Hz), 7.65 (brs, 1H), 8.32 (brt, 1H). Anal. Calcd for С15Н18N5O3S4: C, 37.25; H, 3.75; N, 14.48; S, 26.52. Found: C, 37.06; H, 3.58; N, 14.20; S, 26.29.
2-{[2-(Toluene-4-sulfonylamino)ethylamino]-4-methylthiazol-5-yl}-5-thioxo-4,5-dihydro-[1,3,4]oxadiazole (8)
A mixture of compound 7 (2.42 g, 0.005 mol) and KОН (0.42 g, 0.0075 mol) in 20 mL of water was heated under reflux for 2 h. After cooling the solution was acidified with dilute solution of hydrochloric acid to рН 4–5, allowed to stand for 1 h, and the resultant precipitate of 8 was filtered and crystallized from 50% aqueous ethanol: yield 1.8 g (87%) of white crystals; mp 253–255°С (dec); 1H NMR: δ 2.40 (s, 3H), 2.42 (s, 3H), 2.95 (m, 2H), 3.37 (m, 2H), 7.22–7.70 (m, 4H), 7.50 (t, 1H, J = 5.5 Hz), 8.20 (brt, 1H), 14.05 (brs, 1H); 13C NMR: δ 17.0, 20.9, 41.3, 43.7, 99.2, 126.4, 128.9, 137.5, 141.7, 154.2, 156.6, 168.6, 175.9. Anal. Calcd for С15Н17N5O3S3: C, 43.78; H, 4.16; N, 17.02; S, 23.38. Found: C, 43.62; H, 4.10; N, 16.77; S, 23.11.
2-{[2-(Toluene-4-sulfonylamino)ethylamino]-4-methylthiazol-5-yl}-5-thioxo-4,5-dihydro-[1,3,4]thiadiazole (9)
To 3 mL of concentrated sulfuric acid, 2.42 g (0.005 mol) of compound 7 was added portion-wise and the mixture was briefly stirred at 20–25°С and then allowed to stand overnight. The transparent solution was transferred into a beaker with 50 mL of cold water and neutralized by cooling with 25% solution of ammonium hydroxide. The precipitate of 9 was filtered, washed with water, dried and crystallized from aqueous (50%) ethanol: yield 1.75 g (82%) of white crystals; mp 134–136°С (dec); 1H NMR: δ 2.40 (s, 3H), 2.48 (s, 3H), 2.96 (m, 2H), 3.40 (m, 2H), 7.23–7.72 (m, 4H) 7.50 (brt, 1H), 8.20 (brt, 1H), 14.00 (brs, 1H). Anal. Calcd for С15Н17N5O2S4: C, 42.13; H, 4.01; N, 16.38; S, 30.00. Found: C, 41.95; H, 4.09; N, 16.11; S, 29.77.
General procedure for 10, 11
A solution of KOH (0.01 mol) in 10 mL of water was treated at 0°С with 0.01 mol of compound 8 (or 9) with continuous stirring, and then portion-wise with 1.1 mL (0.011 mol) of dimethyl sulfate. The mixture was briefly stirred at 20°С and then allowed to stand overnight. The precipitate of 10 (or 11) was filtered, washed with water and dried.
2-{[2-(Toluene-4-sulfonylamino)ethylamino]-4-methylthiazol-5-yl}-5-methylsulfanyl-[1,3,4]oxadiazole (10)
Yield 2.72 g (64%) of yellow crystals; mp 176–178°С; 1H NMR: δ 2.40 (s, 3H), 2.43 (s, 3H), 2.75 (s, 3H), 2.95 (m, 2H), 3.38 (m, 2H), 7.25–7.73 (m, 4H), 7.47 (t, 1H, J = 5.3 Hz), 8.05 (t, 1H, J = 5.3 Hz); 13C NMR: δ 14.1, 16.8, 20.8, 41.3, 43.6, 99.7, 126.4, 128.8, 137.5, 141.6, 153.2, 160.7, 161.2, 168.6. Anal. Calcd for С16Н19N5O3S3: C, 45.16; H, 4.50; N, 16.46; S, 22.60. Found: C, 45.03; H, 4.41; N, 16.20; S, 22.29.
2-{[2-(Toluene-4-sulfonylamino)ethylamino]-4-methylthiazol-5-yl}-5-methylsulfanyl-[1,3,4]thiadiazole (11)
Yield 3.13 g (71%) of yellow crystals; mp 180–182°С; 1H NMR: δ 2.38 (s, 3H), 2.42 (s, 3H), 2.78 (s, 3H), 2.97 (m, 2H), 3.38 (m, 2H), 7.25–7.72 (m, 4H), 7.46 (t, 1H, J = 5.3 Hz), 8.05 (brt, 1H). Anal. Calcd for С16Н19N5O2S4: C, 43.52; H, 4.34; N, 15.86; S, 29.04. Found: C, 43.39; H, 4.27; N, 15.55; S, 28.76.
References
[1] Knyazyan, A.; Eliazyan, K.; Pivazyan, V.; Ghazaryan, E.; Harutyunyan, S.; Yengoyan, A. Synthesis and growth regulatory activity of novel 5-(3-alkyl-4-methyl-2-thioxo-2,3-dihydro-thiazol-5-yl)-3H-[1,3,4]-thiadiazole(oxadiazole)-2-thiones and their derivatives. Heterocycl. Comm. 2012, 18, 103–108.Search in Google Scholar
[2] Kirsanov, A.; Kirsanov, N. N-Arylsulfanylethylendiamines. Zh. Obsch. Chim. (Russian J. Gener. Chem.)1962, 32, 887–892.Search in Google Scholar
©2013 by Walter de Gruyter Berlin Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Masthead
- Masthead
- Reviews
- A fascinating decade for the synthesis of 1,2-azoles
- Substituted benzothiazoles: synthesis and medicinal characteristics
- Preliminary Communication
- Synthesis of thiazolo[4,5-e][1,2,4]triazolo[1,5-c]pyrimidine derivatives
- Research Articles
- Ultrasonic-assisted Cu-catalyzed multicomponent synthesis of furo[3,4-b]pyrazolo[4,3-f]quinolinones
- Synthesis of dihydropyrrolo[2,1-a]isoquinolines via isocyanide-based four-component reaction
- Efficient oxidative cyclization of N-acylhydrazones for the synthesis of 2,5-disubstituted 1,3,4-oxadiazoles using t-BuOI under neutral conditions
- Synthesis and transformations of 1-[2-(toluene-4-sulfonamido)ethyl]thiourea
- Synthesis of new heterocyclic compounds containing benzimidazole moiety as inhibitors of breast cancer cell growth
- An approach to C-glycosidic conjugates of isoflavones
- Ionic liquid catalyzed one-pot synthesis of spiropyran derivatives via three-component reaction in water
Articles in the same Issue
- Masthead
- Masthead
- Reviews
- A fascinating decade for the synthesis of 1,2-azoles
- Substituted benzothiazoles: synthesis and medicinal characteristics
- Preliminary Communication
- Synthesis of thiazolo[4,5-e][1,2,4]triazolo[1,5-c]pyrimidine derivatives
- Research Articles
- Ultrasonic-assisted Cu-catalyzed multicomponent synthesis of furo[3,4-b]pyrazolo[4,3-f]quinolinones
- Synthesis of dihydropyrrolo[2,1-a]isoquinolines via isocyanide-based four-component reaction
- Efficient oxidative cyclization of N-acylhydrazones for the synthesis of 2,5-disubstituted 1,3,4-oxadiazoles using t-BuOI under neutral conditions
- Synthesis and transformations of 1-[2-(toluene-4-sulfonamido)ethyl]thiourea
- Synthesis of new heterocyclic compounds containing benzimidazole moiety as inhibitors of breast cancer cell growth
- An approach to C-glycosidic conjugates of isoflavones
- Ionic liquid catalyzed one-pot synthesis of spiropyran derivatives via three-component reaction in water

