Abstract
The concept of Traditional Chinese Medicine (TCM) emphasizes the intrinsic connection between human beings and nature, positing that the human body undergoes distinct physiological changes in response to various natural environments. Cold, as a primary external factor in cold areas, necessitates the body’s autonomous adaptation to uphold optimal living conditions. The repercussions of cold on the body are both far-reaching and profound, with metabolic equilibrium adjustments playing a pivotal role. This article, rooted in the TCM principle of Yin-Yang balance, delves into the metabolic intricacies and adaptive responses to the human body in cold environments. The effects manifest in heat-producing tissues, systemic substance consumption, the blood substance concentrations, liver function, and metabolic rhythms. The article subsequently presents TCM recommendations for maintaining health in cold climates. It concludes by advocating the exploration of metabolic homeostasis changes as a key avenue for investigating the metabolic traits s of populations in cold regions. We posit that such insights will enhance comprehension of the metabolic shifts in cold region populations and advance the evolution of regional medicine.
Cold presents itself as a fundamental environmental challenge that humanity must confront to ensure survival. With elevating dimensions, temperatures decline, and winters extend, particularly affecting individuals residing in cold regions. For these inhabitants, the harsh cold becomes a primary survival concern, influencing all facets of life and intricately impacting bodily functions. A growing body of research attests to the widespread and enduring effects of cold on the human physiology. Within Traditional Chinese Medicine (TCM), the interplay between the environment and the human body assumes heightened significance. TCM posits that people in different regions exhibit distinct physiological characteristics, a crucial consideration in disease treatment and health maintenance. Chinese medicine has long acknowledged that unique physiological traits and disease susceptibility of individuals residing in cold environments, offering an alternative perspective compared to modern medicine. While these TCM insights may lean towards a more general understanding, we contend that grasping the overarching direction will pave the way for further exploration into the physiological changes occurring in the human body in cold environments.
The metabolism of the body reflects the way the body uses energy substances to sustain its functionality and characteristics. The consequential impact of metabolic changes on the human body is profound and demands attention. Investigating how the external factor of a cold environment affects human metabolism stands as a key research focus. In TCM philosophy, the body continually strives to maintain its balance, a state crucial for human health development. Our inquiry delves into the disruption that cold imposes on the organism’s metabolic equilibrium, exploring whether adaptation leads to a new state of equilibrium, elucidating subsequent effects, and comprehending these dynamics as a cohesive whole. These are the focal points we aim to explore, offering researchers potential insights aligned with TCM principles.
1 Important perspective of TCM on the interplay between the environment and the human body
1.1 Characterization of organisms by the environment
TCM posits a profound interconnectedness between humanity and nature, wherein humans are not merely passive occupants of their surroundings, but integral components within the broader ecosystem. This symbiotic relationship underscores the notion that human existence is intricately intertwined with the natural world. Analogous to the organs and functions within the human body, humans and nature share a profound interdependence (Fig. 1).
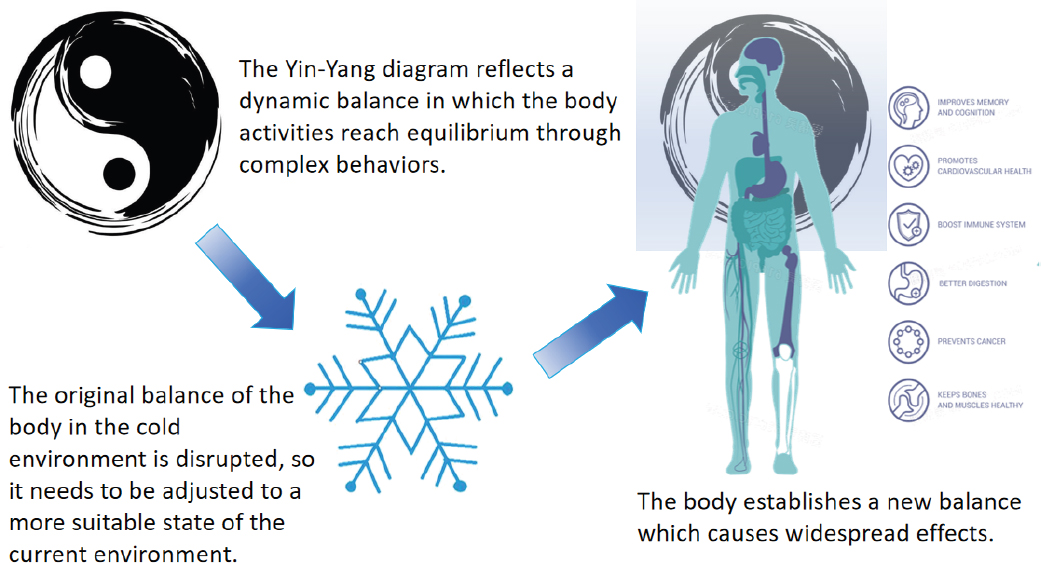
Yin-yang balance and the change of human body balance in cold environments
Just as the same crop cultivated in diverse natural environments manifests distinct characteristics bestowed by its surroundings, individuals inhabiting different regions also bear unique traits influenced by their environments. While the impact of geographical variations on human attributes is multifaceted and complex, humans, unlike plants, possess mobility and agency to adapt their behaviors in response to environmental stimuli and resource availability. Consequently, the confluences of these environmental factors manifest in multifaceted ways, permeating every facet of human physiology. This recognition underscores TCM’s acknowledgment of the environment as a pivotal determinant in shaping human characteristics. Consequently, these environmental influences intricately shape the physiological and psychological states of individuals.
1.2 The significance of Yin-Yang balance in human life activities
In the realm of Chinese philosophy, the concept of Yin and Yang elucidates the dual aspects that influence the stability and development of entities or states. These aspects embody opposing forces: one sustaining and nurturing, while the other purging and transforming. This fundamental duality mirrors the Marxist theory of contradictions, albeit with distinctions. While Marxian philosophy espouses a trajectory of perpetual advancement marked by ups and downs, ancient Chinese wisdom maintains that amidst the flux of appearances, a fundamental balance persists, guiding the evolution of phenomena. Human agency can, to a certain extent, manipulate the interplay of Yin and Yang, perpetuating a state of equilibrium until the inevitable cessation dictated by natural law, akin to the life cycle from birth to demise. The essence of balance lies in safeguarding this process against disruption, thereby facilitating the attainment of an optimal state of existence. The concept of the Yin-Yang equilibrium profoundly the theoretical framework of TCM, providing a lens through which to interpret the metabolic processes of the human body. Indeed, myriad correspondences emerge when scrutinizing bodily functions through the prism of Yin-Yang balance.
Metabolism, the intricate process through which the body assimilates nature substances for sustenance and expels waste, epitomizes the dynamic interplay of Yin and Yang. Initiated by the assimilation of natural resources, metabolism culminates in the expulsion of waste via various physiological pathways, thus preserving the body’s material and energetic equilibrium. Throughout this intricate dance, substances traverse diverse tissues to fulfill their respective physiological roles. Guided by the imperative of survival, the human body continually strives to ascertain optimal equilibrium points, a phenomenon akin to homeostasis. Crucially, the body discerns pivotal molecular cues within these balances, orchestrating regulatory mechanisms to maintain harmony and vitality (Fig. 1)[1].
2 Organism behavior for maintaining temperature homeostasis in cold environments
The hallmark of mammalian survival lies in the steadfast maintenance of body temperature, as even minor deviations can precipitate severe consequences such as frostbite, hypothermia, or even fatality[2].In cold environments, the pronounced temperature gradient between the body and its surroundings accelerates heat loss, posing a formidable challenge to thermal equilibrium. To counteract this, the human body employs a repertoire of behaviors aimed at preserving core temperature stability, primarily through the modulation of heat dissipation and production. In response to cold stimuli, the body orchestrates the constriction of peripheral capillaries, diminishing the transfer of heat from the core to the periphery, thus mitigating heat loss. Central to temperature regulation in cold conditions is the augmentation of heat generation within the body. Muscle shivering and non-shivering thermogenesis, facilitated by brown adipose tissue (BAT) and white adipose tissue, emerge as pivotal mechanisms in this process. Three regulatory modalities—neuromodulation, humoral regulation, and cellular self-regulation—underpin this intricate orchestration (Fig. 2).
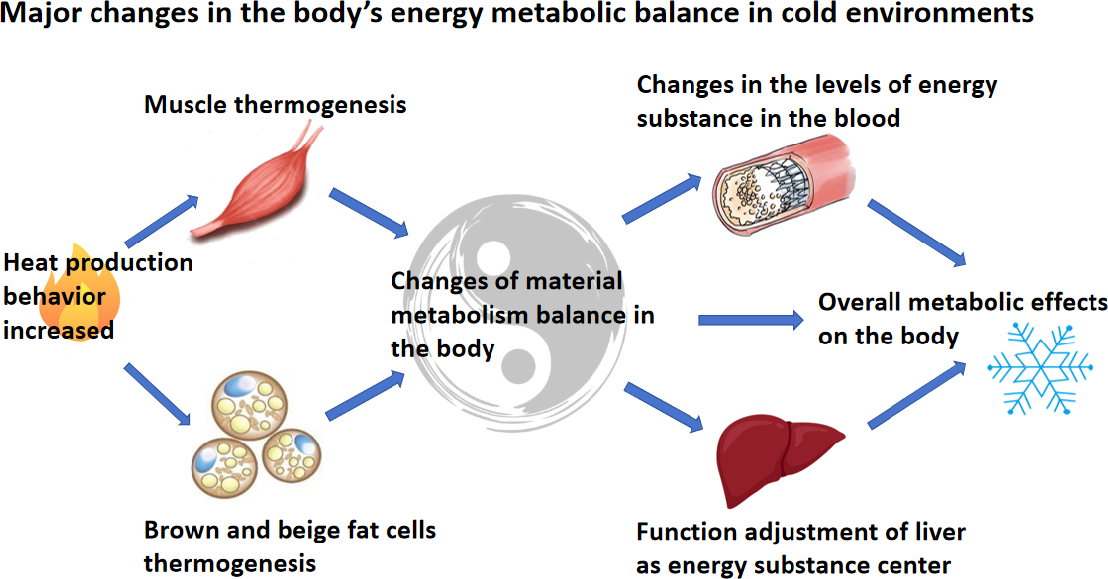
Major changes in the body’s energy metabolic balance in cold environments
In neuromodulation, preoptic anterior hypothalamus (POAH) serve as neural command centers governing thermoregulation. Sensing temperature fluctuations via peripheral thermoreceptors scattered throughout the body, these regions activate thermoeffector organs to modulate H (prod) and dissipation[3-4]. Notably, the presence of temperature-sensitive receptors in visceral tissues suggests a holistic involvement of the entire organism in thermal regulation.
However, it has been demonstrated that peripheral receptors of temperature are also present in the viscera[5], and direct temperature changes in the hypothalamus lead to the activation of thermoregulatory mechanisms[6-7]. This may indicate that the whole-body tissues of the organism play a role in the neural regulation of body temperature. Cold-sensitive receptors are activated everywhere in the cold, and the activated pathways are all aimed primarily at increasing body H (prod) and decreasing body temperature dissipation under normal physiology.
Notably, noradrenaline influences the expression of uncoupling protein 1 (UCP1) in BAT, while glucagon and insulin maintain glycogen availability for thermogenic processes. The thyrotropin system exerts direct influence over thermoregulation by modulating ATP utilization and inducing browning of adipose tissue[7-8]. Hormones such as adrenaline, glucagon, insulin, and leptin intricately regulate energy metabolism and thermogenesis, exhibiting differential expression patterns in response to cold exposure[9], and they are involved in involuntary behavior during cold exposure to varying extents. For example, noradrenaline affects the expression of UCP1 in BAT[10-11], while the reciprocal regulation of glucagon and insulin ensures the glycogen supply in BAT. Similarly, insulin activates the differentiation of BAT[12-13]. Leptin is an adipocyte-secreted hormone, together with hunger hormone (growth hormone-releasing peptide), regulating appetite for long-term energy balance in cold environments and body metabolism[14]. The exact mechanism of which, however, remains unclear and controversial[15-16]. All these hormones are differentially expressed during cold exposure in mammals[17,18,19].
Recent studies highlight the adaptation of white adipocytes to low temperature[20], suggesting a comprehensive involvement of all three regulatory modalities in cold adaptation. Central to these regulatory efforts is the overarching goal of homeostasis—the preservation of the body’s internal equilibrium—amidst fluctuating environmental conditions. In summary, the multifaceted interplay of physiological mechanisms underscores the remarkable capacity of organisms to navigate and thrive in cold environments, guided by the imperative of internal balance and sustained vitality (Fig. 2).
3 Generation of thermogenic behavior and sources of material consumption
In elucidating the intricate dynamics of thermogenesis within the body in response to cold stimuli, it becomes imperative to delineate the specific metabolic processes at play. Adhering to the law of conservation of energy, the generation of calories necessitates the consumption of intrinsic bodily substances. While fats, sugars, and proteins represent primary reservoirs of thermogenic substrates, the questions arise as what is the relative proportion of consumption and what is the major component in fueling thermogenesis. Indeed, exploring this aspect is crucial, as fats, sugars, and proteins, while all capable of serving as thermogenic raw materials, perform distinct roles within the human body. Therefore, elucidating the balance and interplay between these substrates is pivotal in comprehending the nuanced mechanisms governing thermogenesis in cold environments.
3.1 Muscle shivering and non-shivering thermogenesis
The human body has evolved mechanisms for maintaining the core body temperature through enhancing H (prod) and reducing peripheral blood flow to minimize heat loss, in cold environments[21]. Muscle and adipose tissue are generally considered to be the primary contributors to thermogenesis. The mode of thermogenesis can be further divided into shivering thermogenesis (ST) and non-shivering thermogenesis (NST). ST is of the major process for H (prod) in cold conditions[22]. However, NST is also indispensable for mammalian thermogenesis in cold environments[23,24,25,26,27,28], as ST is weakened in individuals unacclimatized or undergoing acclimatization to cold surroundings.
3.2 Muscle thermogenic pathways
Skeletal muscle, constituting the largest organ in the body and comprising 45% to 55% of total body weight, plays a pivotal role in both ST and NST, thus serving as a significant thermogenic tissue[29-30]. It exhibits the remarkable capacity to generate intensities equivalent to approximately about 40% of maximal oxygen consumption or 5 times resting metabolic rate (RMR)]. Numerous factors influence the intensity of shivering, including the duration and severity of cold exposure, as well as individual characteristics such as age, body fat percentage, gender, surface-to-volume ratio, and exercise habits[22,31,32,33]. The mechanism of underlying shivering is primarily governed by neural modulation: POAH receive neural signals released from peripheral thermoreceptors, either through the modulation of feedforward mechanisms activated by cold stimuli or via direct sensing by the brain[34,35,36,37]. This neural cascade leads to the deinhibition of neurons in the dorsomedial hypothalamic, subsequently enhancing the activity of spinal ventral horn motor neurons and activating fusimotor fibers, ultimately culminating in shivering[36,38,39,40].
During tremors, two distinct types of muscle fibers are recruited: type I and type II. Type I muscle fibers possess a lower threshold for activation and demonstrate resistance to fatigue. These fibers exhibit a slow oxidative metabolism and rely predominantly on fat as an energy source. In contrast, type II muscle fibers have a higher activation threshold and engage in rapid glycolysis. These fibers primarily utilize carbohydrates (CHO) for energy production, and demonstrate a higher rate of thermogenesis[31,41,42,43]. The recruitment of these muscle fiber types underscores the dynamic interplay between oxidative and glycolytic pathways in responding to thermogenic demands during tremors.
Non-shivering H (prod) by muscles represents a vital mechanism of organismal thermogenesis, potentially surpassing the efficacy of shivering[44,45,46]. This assertion finds support in the intriguing adaptation observed in certain deep-sea fish, which have evolved specialized muscles devoid of myogenic fiber structure. These muscles boast densely distributed mitochondria and sarcoplasmic reticulum (SR), effectively serving as heating organs to maintain critical temperatures in vital sites[44,45,46]. This adaptation underscores the remarkable capacity of non-shivering H (prod) by muscles to supplant and complement shivering-induced thermogenesis[47].
Despite its significance, the mechanisms underlying NST in muscles remain relatively unexplored. However, emerging evidence suggests that mitochondrial proton leakage and SR calcium cycling play pivotal roles in driving the NST process within skeletal muscles[26,48-49]. Further research into these mechanisms promises to elucidate the intricate pathways governing nonshivering thermogenesis, thereby deepening our understanding of organismal thermoregulation.
3.2.1 Mitochondrial proton leakage
Mitochondrial uncoupling proteins (UCPs) play a crucial role in facilitating NST through the process of mitochondrial proton leakage. As proton channels, UCPs enable the re-entry of protons ejected from the respiratory chain back into the mitochondrial matrix from the outside membrane. This action disrupts the proton gradient and reduces the membrane potential, allowing the dissipation of proton energy as instead of its conversion into ATP[50-51].
Within muscle tissue, the predominant UCPs are UCP2 and UCP3, both of which are highly expressed[52,53,54]. These proteins are sensitized by elevated levels of mitochondrial reactive oxygen species (ROS)[52,54-55]. While the primary function of UCP2 and UCP3 is believed to be the prevention of excessive ROS production, studies increasingly demonstrate their influence on energy metabolism[52,56,57,58]. Despite conflicting experimental findings[59], it is evident that changes in UCP3 expression occur following cold exposure, fluctuating in tandem with oxidation levels[60]. Notably, Wijers et al. observed that inhibiting BAT thermogenesis led to a modest increase in energy expenditure in individuals exposed to prolonged mild cold temperatures. They hypothesized that skeletal muscle proton leakage may constitute a significant contributor to whole-body NST, although they overlooked the concurrent increase in shivering activity[61]. Further research is required to elucidate the precise role of UCPs and mitochondrial proton leakage in orchestrating non-shivering thermogenesis, thereby advancing our understanding of energy metabolism and thermoregulation in humans.
3.2.2 Sarcoplasmic reticulum calcium cycling
The SR serves as a specialized endoplasmic reticulum within muscle cells, forming an intricate network intersecting longitudinally around muscle fibers, crucially involved in the storage, release, and regulation of calcium ions (Ca2+)[62].
Upon propagation of action potentials, T-tubules undergo allosteric regulation, activating voltage-dependent L-type Ca2+ channels, allowing Ca2+ release from the SR into the cytoplasm via ryanodine receptor 1 (RyR1)[63-64]. This activation allows Ca2+ release from the SR into the cytoplasm via RyR1. Elevated cytoplasmic Ca2+ levels initiate muscle contraction as Ca2+ binds to myofilaments. Subsequently, the sarco/endoplasmic reticulum calcium ATPase (SERCA) pump is activated by the elevated Ca2+ to pump Ca2+ back to the SR lumen. The hydrolysis of ATP during the calcium cycle generates excess heat, contributing significantly to H (prod)[65-66], accounting for 40%-50% of resting skeletal muscle metabolic rate of intact soleus (slow, oxidative) and extensor digitorum longus (fast, glycolytic) muscle in mice and 8%-10% of whole-body metabolic rate, representing the H (prod) capacity of the SERCA pump[67]. Notably, the coupling efficiency between ATP hydrolysis and Ca2+ uptake by SERCA varies[68], ideally 1 ATP hydrolysis can pump 2 Ca2+ into the SR[69], with leakage pathways and slippage increasing the proportion of Ca2+ futile cycling and energy production[69-70], and this process is regulated by several aspects[71]. Pathological conditions, such as excessive or unregulated Ca2+ leakage into the cytoplasm via RYR1, as seen in malignant hyperthermia, underscore the thermogenic capacity of calcium cycling[72]. The interaction of sarcolipin (SLN) with the organism plays a pivotal role in regulating futile calcium cycling and cytoplasmic Ca2+ levels, consequently impacting systemic metabolism, mitochondrial structure, and even muscle fiber type distribution[73-74]. Prolonged cold exposure induces structural adaptations in muscles, including increased mitochondrial content, volume, density of cristae, and microvascularization, enhancing their thermogenic capacity and contributing to overall metabolic homeostasis[75].
Regulation of SERCA calcium cycling by SLN is pivotal in orchestrating thermogenic responses[66,76]. Studies involving mice lacking intra-scapular BAT and myelin, exposed to cold environments (4°C), demonstrated an inability to maintain core temperature. However, the introduction of SLN restored body temperature levels. Furthermore, cold-adapted neonatal mice exhibited elevated levels of SLN in skeletal muscle, suggesting its role in cold adaptation[26,77].
Despite advances, accurately determining the contribution of non-shivering H (prod) remains challenging[48]. The potential involvement of muscle UCP in cold-induced response warrants further investigation. Given muscle’s enormous oxidative capacity, it presents an intriguing avenue for UCP activation during thermogenesis. However, it remains uncertain whether UCP activation correlates with changes in muscle oxidative capacity. Notably, muscle proton leakage diminishes after prolonged mild cold exposure, coinciding with reduced skeletal muscle tremors[23].
The regulatory mechanisms governing SLN expression remain poorly understood. However, its presence of in rodent slow-oxidizing fibers (type I fibers) suggests a physiological link between SLN expression and oxidative metabolism. This notion is reinforced by findings showing that SLN over-expression in fastt-witch glycolytic muscle (type II fibers) promotes mitochondrial organogenesis and oxidative metabolism[78-79]. Notably, SLN appears insensitive to catecholamines and triiodothyronine (T3) hormones[80]. The upstream signaling mechanisms for SLN-mediated thermogenesis during cold exposure remain elusive, representing crucial avenues for future research into human thermogenesis in cold environments.
3.3 Substance consumption for muscle H (prod)
Muscle tissue draws upon both intracellular and extracellular energy sources for H (prod). energy sources for H (prod) glycogen, stored in varying amounts and utilized to fuel muscle activity[81]. Glycogen serves as the principal fuel for high-intensity exercise, playing a critical role in determining muscle endurance[82]. Glycogen stores are distributed across different subcellular regions within muscle cells, strategically located near sites of high energy expenditure[83]. Experimental evidence suggests that glycogen utilization varies among these subcellular regions during activity, indicating potential subtle regulatory relationships between them[84]. In summary, muscle tissue relies on glycogen as a key energy source for H (prod), with its distribution within the cell influencing its utilization during muscle activity. Understanding the dynamics of glycogen utilization within muscle cells sheds light on the complex mechanisms underlying muscle H (prod).
Muscle fibers boast a distinctive structure known as T-tubules, crucial for facilitating their robust demand for external fuel access. Comprising an inward depression of the muscle membrane, T-tubules cover approximately 60% of the muscle’s surface area. Beyond transmitting contractile signals, they serve a pivotal role in enhancing membrane area, thereby optimizing substance transport efficiency[85,86,87]. Within the T-tubules, the fatty acid transport protein 1 (FATP1) is prominently distributed, particularly concentrated in central regions. FATP1 exhibits heightened sensitivity to insulin, reflecting the T-tubules’ insulin responsiveness[88]. This sensitivity extends to glucose transport, as T-tubules contain five times more glucose transport proteins compared to the muscle membrane[89,90,91]. This abundance of transport proteins facilitates robust uptake of external fuels by muscle fibers, ensuring the feasibility of multi-fuel metabolism. In essence, the specialized structure of T-tubules in muscle fibers plays a dual role: facilitating signal transmission and maximizing fuel uptake efficiency, thereby supporting the metabolic demands of muscle tissue.
Different types of muscle fibers exhibit distinct preferences for energy substrate utilization[31], reflecting their specialized metabolic profiles. while Experimental evidence indicates that muscle fibers can adjust metabolic pathways to maintain normal activity without altering recruitment patterns, highlighting the body’s preference for maintaining caloric production consistency[42-43,92]. During short-term cold exposure, the recruitment mode of muscle fibres and the overall thermogenic efficiency during shivering thermogenesis remain unaffected by glycogen store size or degree of glycogen engagement[42,93]. However, the entry of external fuel into the muscle tissue is constrained by transport processes such as transporter proteins, limiting dramatic increases during shivering or exercise[94]. Meanwhile, apparent disparities in metabolic fuel sources, both in terms of biochemical properties and storage size, may influence utilization outcomes[95]. The low shivering H (prod) mode favors lipid utilization, thereby conserving glycogen for potential subsequent exercise demands. This underscores the organism’s rational metabolic pathway arrangement based on fuel characteristics, ensuring efficient energy utilization in varying physiological contexts.
Fuel consumption during shivering is primarily derived from CHO, lipids, with a minimal contribution from protein sources[31]. Given that muscles are involved in both ST and NST, the precise relationship between shivering, mitochondrial uncoupling, and SERCA pumps in these thermogenic modes remains unclear. However, evidence suggests a potential synergistic effect between shivering and mitochondrial thermogenesis[23]. Experiments involving cold-stimulated shivering indicate that fuel consumption associated with NST persists alongside shivering. Thus, it can be tentatively inferred that the fuel consumption observed during shivering thermogenesis may align with the substance consumption characteristic of muscle thermogenesis Overall. Further research is warranted to elucidate the intricate interplay between these thermogenic modes and their respective fuel utilization patterns.
It is worth noting that the fuel consumption patterns during shivering and exercise differ significantly[31,93]. Studies have shown that at equivalent metabolic rates, shivering tends to utilize more CHO and fewer lipids compared to exercise[96]. This disparity in fuel preference may be attributed to various factors, including hormonal and neuromodulatory influences. The precise mechanisms underlying these differential fuel utilization patterns between shivering and exercise remain to be fully elucidated. However, the observed differences underscore the complexity of metabolic regulation in response to distinct physiological stressors, highlighting the need for further investigation into the underlying hormonal and neuromodulatory mechanisms involved.
3.4 Brown adipose tissue and beige adipocytes for H (prod)
BAT is a specialized type of adipose tissue, morphologically characterized by multi-compartmental, intracellular lipid droplets and possess both thermogenic and endocrine functions[97]. In humans, active BAT can account for 2-5% of the resting metabolic rate (RMR)[98]. Regulation of BAT is primarily orchestrated by the hypothalamus, with cold exposure being a potent activator of its thermogenic function[99]. In response to cold stimulation, the hypothalamus regulates sympathetic nerve activity to brown adipocytes, leading to the release of noradrenaline which activates BAT thermogenesis. This process is regulated by various hormones[97,100]. The thermogenic capacity BAT stems from its abundance of mitochondria with high-density cristae structures and the expression of UCP1, which accounts for approximately 10% of mitochondrial proteins. than white adipose tissue[101-102]. UCP1 is predominantly expressed in brown adipocytes and plays a central role in BAT thermogenesis[59]. Unlike other UCPs involved in oxidative stress regulation, UCP1 is specifically implicated in BAT thermogenesis. In the absence of UCP1, noradrenaline fails to induce thermogenesis in brown adipocytes[103]. The mode of thermogenesis mediated by UCP1 involves mitochondrial uncoupling[104], primarily activated by fatty acids (FAs) rather than reactive oxygen species (ROS) as seen in other UCPs[105-106]. Energy dissipation in BAT cells is achieved through various mechanisms, including inefficient cycling of FAs, creatine metabolism, and intracellular calcium cycling[107,108,109,110,111]. These processes collectively contribute to the thermogenic function of BAT, highlighting its significance in regulating energy expenditure and metabolic homeostasis.
BAT constitutes a significant proportion of the body’s mass in newborns, playing a crucial role in thermogenesis and metabolic regulation[112-113]. However, with advancing age, both the number and activity of BAT decline. Despite this decline, BAT persists in adults and retains metabolic functionality[114]. Recent studies have underscored the substantial contribution of BAT to total resting energy expenditure, emphasizing its importance in metabolic homeostasis[115]. Furthermore, research has elucidated the pivotal role of BAT in cold-induced thermogenesis[116], demonstrating a remarkable a two- to three-fold increase in BAT thermogenesis in response to cold adaptation[117-118].
These findings highlight the dynamic nature of BAT and its profound impact on metabolic regulation. Given its metabolic significance, current research endeavors are increasingly focused on strategies to activate BAT as a therapeutic approach for combating metabolic disorders. By harnessing the thermogenic potential of brown adipocytes, researchers aim to develop innovative interventions to promote metabolic health and address metabolic dysregulation.
Beige adipocytes, also known as brite adipocytes, are inducible thermogenic adipocytes dispersed within white adipose tissue (WAT)[119]. Similar to brown adipocytes, beige adipocytes exhibit dense mitochondrial cristae and express UCP1, contributing to their thermogenic capacity. Additionally, they possess multicompartmental lipid droplets characteristic of thermogenic adipocytes[120]. Recent studies propose that beige adipocytes serve as a “reservoir” of brown-like adipocytes within WAT. These cells are believed to differentiate from white adipocytes in response to environmental stimuli, a process known as “browning” or “beiging” of white adipose tissue[121,122,123]. Environmental cues, such as cold exposure or pharmacological agents, can induce the transformation of white adipocytes into beige adipocytes, enhancing their thermogenic function[124-125]. However, when environmental stimuli diminish, beige adipocytes may revert to a white adipocyte phenotype through mitochondrial autophagy, transitioning back to an energy-storing state. This dynamic interconversion between beige and white adipocytes underscores the plasticity of adipose tissue and its responsiveness to environmental cues in regulating metabolic homeostasis.
3.5 The substance sources for BAT and beige adipocytes thermogenesis
The thermogenic activity of BAT and beige adipocytes is predominantly fueled by FAs, sourced from both intracellular and extracellular origins[126]. Brown adipocytes contain multi-compartment lipid droplets, distinct from those found in other cell types, which serve as a readily available reservoir of FAs for fuel. Lipid droplets (LDs) release FAs via lipolysis, subsequently burned by mitochondria to generate heat[119,126]. BAT thermogenesis necessitates a substantial uptake of external FAs to meet energy demands, with triglycerides (TG) serving as the primary energy source, transported in the bloodstream as triglyceride-rich lipoprotein (TRL)[127-128]. Notably, BAT adipocytes do not directly process TRL particles but instead break them down through endothelial cells in neighboring adipose tissue[129]. The released FAs are transported intracellularly via FA transporter protein 1 (FATP1) on the surface of brown adipocytes[130].
In addition to FAs derived from WAT lipolysis, free fatty acids (FFAs) are mainly taken up by the liver for the production of very low-density lipoprotein (VLDL), facilitating FA delivery to activated BAT[131,132,133]. Furthermore, brown adipocytes exhibit the ability to uptake glucose, with glucose transporter proteins GLUT-4 and GLUT-1 present in these cells[134]. While FAs are the primary substrate for BAT thermogenesis, glucose serves as a secondary fuel source (5%-15%), primarily converted to pyruvate and lactate, and a portion can be converted to FAs and triglycerides to supplement fat consumption.
Recent studies have highlighted the critical role of BAT in glucose homeostasis and insulin sensitivity[135,136,137,138,139,140,141]. Insulin plays a pivotal role in regulating BAT function, with insulin deficiency resulting in decreased BAT mass and thermogenic capacity[142,143]. Conversely, insulin administration restores BAT function and mass in insulin-deficient animal models. Insulin receptor knockout rodents also exhibit reduced BAT mass, underscoring the importance of insulin signaling in BAT regulation[144]. In insulin receptor knockout rodents, a decrease in BAT mass was observed[145], and insulin increased BAT glucose uptake by BAT and converted it to lipid droplets rather than oxidizing it in adipose tissue[146].
In summary, while FAs are the dominant substrate for BAT thermogenesis, glucose serves as a significant energy source and plays a crucial role in regulating BAT function and insulin sensitivity. The interplay between these substrates ensures metabolic flexibility and optimal energy utilization in brown and beige adipocytes.
Indeed, research has revealed that beige adipocytes exhibit distinct thermogenic mechanisms compared to brown adipocytes.
While both cell types rely on UCP1 for thermogenesis, beige adipocytes also demonstrate a higher capacity for glycolysis and glucose utilization[107]. Additionally, beige adipocytes appear to rely more on the endoplasmic reticulum calcium null cycle for thermogenesis, highlighting their metabolic flexibility. Despite these insights, the relative contribution of UCP1-dependent thermogenesis to whole-body energy homeostasis in beige adipocytes remains understudied.
Understanding the interplay between UCP1-mediated thermogenesis and other metabolic pathways in beige adipocytes will be crucial for elucidating fuel utilization strategies in these cells. Further research into the specific mechanisms governing thermogenesis in beige adipocytes, as well as their impact on overall energy balance and metabolic homeostasis, will provide valuable insights into the physiological role of these cells and their potential as therapeutic targets for metabolic disorders.
3.6 Rationing of substance consumption in cold environments
In cold environments, the human body adapts to H (prod) mechanisms, leading to changes in fuel utilization ratios[147]. Understanding these patterns can shed light on new H (prod) pathways and provide insights into metabolic responses to cold exposure. Studies have investigated fuel utilization ratios during shivering in cold environments[148,149,150,151,152,153,154,155,156]. Haman et al.[157] quantified the oxidation shares of various fuel substrates, during low-intensity shivering over a prolonged period. They found that lipids accounted for 50% of H (prod), muscle glycogen for 30%, plasma glucose for 10%, and protein for 10%[158]. As shivering intensity increased, the utilization of muscle glycogen increased significantly, leading to a higher proportion of CHO in H (prod). However, blood glucose share did not change significantly, indicating a preference for muscle glycogen utilization during shivering.
Further research explored the effect of glycogen reserves on shivering efficiency[159]. Contrary to expectations, glycogen reserves did not affect thermogenesis during shivering but compensated for glycogen depletion through increased lipid and protein oxidation. Lipids maintained a consistent share of H (prod) at 50% even with varying glycogen reserves. During shivering rewarming after cold exposure, the rate of CHO oxidation decreased while lipid oxidation remained constant, indicating a shift in fuel utilization dynamics[160]. This highlights the importance of shivering behavior in influencing fuel utilization patterns and overall H (prod) in cold environments. These findings underscore the complex interplay between shivering intensity, glycogen reserves, and fuel utilization dynamics during cold exposure, providing valuable insights into the body’s metabolic responses to environmental challenges.
The findings from subsequent exposure experiments lasting more than 3 hours shed further light on fuel utilization dynamics during prolonged cold exposure[161]. It was found that whole-body shivering intensity reached a constant value after 6 h of cold exposure and remained stable thereafter. Despite a significant reduction in CHO utilization during this period, it was entirely compensated by a gradual increase in lipid oxidation, which accounted for up to 80% of total heat production (Hprod). This indicates a high degree of fuel substitutability, suggesting that the body adapts its energy source preferences to sustain thermogenesis over extended cold exposure durations.
While the relevance of these findings for examining cold adaptation is emphasized, it is important to note that the involvement of NST H (prod) cannot be entirely excluded, although its contribution to the rewarming process may be minimal[162]. In another study by Acosta[163], fuel oxidation rates were examined before shivering under cold exposure conditions. It was observed that fat oxidation increased substantially (+72.6%), while CHO oxidation rates remained constant during this process and increased near the onset of shivering, suggesting a potential contribution of NST, particularly through BAT thermogenesis.
However, contrasting findings were noted in Acosta’s study compared to previous research by Haman, wherein no sustained increase in lipid utilization was observed under cold exposure conditions[160,163]. This discrepancy raises questions regarding the maximum extent of NST thermogenesis and its activation rate. Further studies are needed to elucidate the systemic regulatory mechanisms governing the contribution of total H (prod) during cold exposure. Moreover, studies related to cold acclimation have demonstrated that prolonged cold exposure can effectively reduce shivering thermogenesis while substantially increasing NST and BAT quantity[23,117-118,164,165]. Interestingly, these changes did not alter the absolute rate and relative contribution of CHO and lipids to total H (prod), suggesting the presence of a systemic regulatory mechanism maintaining overall thermogenic balance during cold adaptation. Furthermore, the NST pathway in muscle did not show enhancement parallel to BAT thermogenesis, indicating the potential isolation of multiple pathways of NST thermogenesis and providing avenues for future research in this area.
In conclusion, the complex fuel rationing patterns observed during cold exposure suggest a considerable degree of flexibility in H (prod) recruitment and fuel utilization. The overall substance consumption ratios in cold environments reflect the organism’s adaptive responses to various H (prod) modalities and metabolic shifts in cold conditions.
These findings underscore the significant alterations induced by cold on human metabolism, highlighting the dynamic nature of thermoregulation and energy expenditure in response to environmental challenges.
4 Systemic adaptations in response to cold exposure
The effect of cold on the human body extends beyond a mere increase in material consumption. Clear evidence suggests that cold exposure disrupts various aspects of the body’s original equilibrium, prompting it to seek a new state of balance.
4.1 Blood substance alterations
The changes in blood substances under cold conditions largely reflect the body’s response to cold. While these parameters may not directly elucidate the mechanisms and pathways of thermogenesis, they provide insight into the utilization of substances throughout the body during thermogenesis. The concentration of substances in the blood not only meets the metabolic demands of heat-producing tissues but also influences the microenvironment of tissues and organs throughout the body. We are intrigued by whether the cold environment adjusts the metabolic homeostasis of the body.
Studies have suggested that in a cold environment, stimulation of circulating glucose utilization does not necessarily coincide with changes in plasma glucose or insulin concentrations. Earlier studies focused on the detecting blood substances[152,154,157,166,167,168,169], and this consistent finding was verified in subsequent studies[170]. These studies showed no significant changes in plasma glucose and triglyceride concentrations during cold exposure, while FFAs levels increased fold, albeit with varying degrees of oxidation. This phenomenon was also observed in cold-adapted individuals[23]. In the cold condition, insulin levels associated with plasma glucose uptake were not consistently increased and, in some cases, even decreased, despite an increased rate of plasma glucose uptake correlating with increased insulin sensitivity[171-172], a process in which brown adipocytes play an important role[142]. Experiments have illustrated the beneficial effects of cold exposure on insulin resistance[173-174]. The elevation of FFAs is mainly attributed to white adipocytes lipolysis, stimulated by heightened by sympathetic activity in the cold environment[175]. Cold exposure significantly increases circulating FFAs, while triglyceride levels remain largely unchanged. A recent study[176] on human TAG metabolism during cold exposure has proposed a relationship between TAG dynamics and TAG saturation state, highlighting the correlation between TAG uptake in heat-producing tissues and increased hepatic lipogenesis. It suggests that elevated FFAs may be related to the uptake capacity of heat-producing tissues. Although mouse experiments investigating the acute effects of cold exposure on FFA levels (≤ 2 h) are scarce in the literature, numerous human studies have consistently reported an exponential increase in FFAs, even in cases of NST dominance. In conclusion, the changes in blood substances under cold conditions persist despite the body’s adaptation. Moreover, the increased insulin sensitivity and higher rate of energy substance uptake in the blood, while concentrations remain stable except for FFAs, collectively suggest a disruption and adjustment of the organism’s metabolic balance.
4.2 Important role of the liver in cold environments
4.2.1 Energy hubs for thermogenesis
The liver serves as a central hub for energy metabolism in the body, particularly crucial during cold exposure. Studies on rats have shown that in cold environments, there is an increase in hepatic gluconeogenesis, total liver and mitochondrial mass, respiratory capacity of hepatocytes, and liver temperature, alongside a reduction in hepatic glycogen stores[164,177-178]. Consequently, the liver supplies glucose and FAs from very low-density lipoproteins (VLDL) to thermogenic tissues[179]. Notably, BAT exhibits a preference for FAs sourced from hepatic VLDL[180], while cold exposure activates adrenaline-induced hepatic gluconeogenesis to supply glucose[177].
Research by Simcox et al.[133] utilizing non-targeted metabolomics in in cold-exposed mice demonstrated an increase in hepatic synthesis of acylcarnitine during cold exposure. This acylcarnitine serves as a fuel source for peripheral tissues, specifically highlighting its role as a thermogenic fuel for BAT, while liver and WAT decreased uptake of acylcarnitine. Subsequently studies proposed that adipose tissue lipolysis and FFA activation of hepatic Hepatocyte nuclear factor 4α (HNF4a) drove the increase in acylcarnitine, as FFA activation stimulated the expression of genes related to acylcarnitine production. Furthermore, the release of FFAs in cold environments increases substrate concentrations in the liver, supplementing the fraction secreted through VLDL. Hepatocytes esterify these FFAs to form complex lipids such as triglycerides, cholesteryl esters and phospholipids[181]. Research has demonstrated increased hepatic cholesterol synthesis in cold environments[182]. Collectively, these findings underscore the liver’s pivotal role in maintaining blood substance balance in cold environments.
4.2.2 Effects of the liver on metabolism in cold environments
Beyond its role as an energy material transfer station, the liver exerts endocrine functions that can impact H (prod) by generating and releasing thermogenic factors. In cold environments, the conversion of cholesterol to bile acids (BAs) accelerates in mice[183]. These BAs play a pivotal role in systemic metabolic regulation by activating specific BA receptors such as the farnesoid X receptor (FXR) and Takeda G-protein-coupled receptor 5 (TGR5) in various tissues including the intestine, adipose tissue, pancreas, and skeletal muscle[184]. Notably, TGR5 activation induces thermogenesis in BAT, contributing to H (prod)[185]. Research has indicated that increased levels of intestinal BAs during cold exposure lead to distinctive alterations in the gut microbiome[186]. This highlights the far-reaching impact of cold environments on the physiological processes of the human body.
Fibroblast Growth Factor 21 (FGF21) is produced in various tissues, notably in the liver[187]. Circulating FGF21 induces WAT browning process[188] and enhances BAT glucose uptake[189]. Moreover, it stimulates neurons in the hypothalamus, thereby increasing sympathetic activity in adipose tissue[190]. Cold exposure in humans triggers an increase in circulating FGF21 levels, primarily originating from the liver[189,191,192,193].
Research using liver-specific Fgf21 knockout (FGF21-KO) mice has demonstrated that hepatic FGF21 plays a pivotal role in maintaining body temperature during short-term hypothermic exposure[194]. Conversely, adipose-specific FGF21-KO mice did not exhibit similar changes in body temperature. The hepatic FGF21-brain-BAT axis regulates sympathetic activity to BAT, as evidenced by reduced sympathetic activity in BAT in liver-specific FGF21-KO mice[194]. However, some experimental studies[193], including those using the Adiponectin-Cre mouse and aP2-Cre transgenic mice, have not shown an increase in hepatic FGF21 secretion during cold-induced thermogenesis. These findings suggest that the role of hepatic FGF21 in cold adaptation requires further investigation and clarification.
4.3 Cold-induced changes in metabolic rhythm
Organisms exhibit circadian periodic rhythms in various physiological activities, including body temperature, which correlates with metabolic rhythms. Typically, animals have higher body temperatures during wakefulness and lower temperatures during sleep, mirroring metabolic fluctuations[195]. Adipocytes also display circadian metabolic rhythms[196]. Numerous factors, including diet[197], environmental conditions[198], and light exposure[199], influence the body’s metabolic rhythm. Cold exposure has also been shown to alter this rhythm, suggesting its impact on metabolic processes. Further research is needed to elucidate the specific mechanisms through which cold affects the metabolic rhythm and to understand its implications for overall metabolic function and health.
The nuclear receptor Rev-erbα, known for its potent transcriptional repression activity, serves as a crucial link between circadian rhythms and metabolic networks[200]. Previous research has highlighted Rev-erbα’s role in regulating glucose and lipid metabolism in various tissues such as skeletal muscle, brown tissue, white has been found to be altered in response to adipocytes, and the liver. Its expression has been found to be altered in response to cold environments[201-202,203,204,205,206,207].
Clock proteins, encoded by clock genes, are central to the formation of circadian rhythm[208]. The expression pattern of the period circadian clock 2 (PER2) rhythm is affected in cold environments, potentially contributing to cold-induced adaptive thermogenesis[209]. Cold exposure can also affect clock gene expression through channels like transient receptor potential melastatin 8 (TRPM8) channel[210] and transient receptor potential vanilloid 1 (TRPV1)[207].
Even a slight decrease in ambient temperature (Ta) can greatly impact the circadian rhythm of FGF21 in humans, which in turn mediates cold-induced metabolic changes[191]. Moreover, there are observed differences in the metabolic responses to cold between men and women[211]. Understanding the intricate interplay between cold exposure, circadian rhythms, and metabolic networks is crucial for unraveling the mechanisms underlying metabolic adaptation to cold environments and may have implications for personalized approaches to metabolic health management.
4.4 Influence of cold environment on human behavior
In cold environments, the body’s increased H (prod) demands a higher caloric intake from the diet[212]. As a result, individuals living in cold regions typically adopt a high-calorie diet, which is both recommended and widely accepted[213]. However, the cold conditions also have specific effects on hormone regulation and appetite. Cold conditions suppress the secretion of leptin[214,215,216], a hormone involved in regulating energy balance and appetite[217]. Additionally, they stimulate the release of pro-appetite hormones, further increasing the body’s desire to eat. Consequently, individuals in cold regions often experience heightened cravings and appetite. Moreover, the pursuit of high-calorie foods is often accompanied by increased fluid intake, including a preference for strong alcoholic beverages. This behavior is influenced by the desire to generate internal heat and maintain warmth in cold climates. Understanding these behavioral responses to cold environments is essential for addressing nutritional needs and promoting overall well-being in populations residing in such regions.
Subtle changes in Ta have complex effects on sleep-wake (S-W) cycle in mammals[218,219]. Cold environments not only affect circadian rhythms but also disrupt sleep patterns in humans[191,209,220], and these effects are also manifested in sleep rhythms[221]. These environmental effects interact with various situational factors encountered during human existence, shaping specific behaviors that, in turn, impact metabolism and overall health. However, analyzing these interactions in a laboratory setting remains challenging.
It is essential to recognize that individuals in frigid zones do not solely inhabit cold environments; they also work, play, eat, and live through prolonged winters. Therefore, the cold environment is more than just the sum of individual cold factors; it represents a unique natural setting intertwined with the lifestyle of their communities.
These factors collectively influence the distinctive metabolic characteristics of populations in cold environments. Effectively understanding and studying the interactions among these complex factors are crucial for guiding clinical practice and addressing the unique health challenges faced by populations living in cold regions.
5 Metabolic adaptations in cold environments: a Yin-Yang perspective
In TCM, the concept of Yin-Yang represents the balance and harmony of opposing forces within the body. When individuals are exposed to cold environments, their metabolic equilibrium is challenged, offering insights into TCM’s rich ideas. While the body may achieve a new equilibrium in response to cold, these adaptations come with consequences. Prolonged exposure to cold environments may lead to depletion of vital energy reserves, exacerbating Yin-Yang imbalances and predisposing individuals to various health issues. Additionally, the stress placed on organs and metabolic pathways during adaptation can impact long-term health outcomes, influencing susceptibility to diseases and disorders. Understanding metabolic changes in cold environments from a Yin-Yang perspective underscores the holistic nature of health in TCM. It emphasizes the interconnectedness of physical, emotional, and environmental factors in shaping overall well-being.
By recognizing the importance of Yin-Yang balance in metabolic adaptations, TCM offers valuable insights into maintaining health and resilience in challenging environments. In conclusion, examining metabolic changes in cold environments through the lens of Yin-Yang balance provides a deeper understanding of the body’s adaptive mechanisms and highlights the importance of holistic health approaches in addressing environmental challenges. Cold temperatures disrupt the body’s natural balance, tipping the equilibrium towards Yin, characterized by coldness, stagnation, and contraction. The body’s metabolic processes, typically regulated to maintain a delicate balance between Yin and Yang, are thrown off by the extreme cold, leading to disruptions in energy flow and organ function. To adapt to the cold environment, the body initiates various metabolic adjustments to restore Yin-Yang balance. Increased thermogenesis, heightened energy consumption, and altered hormonal responses serve to counteract the excess Yin caused by cold exposure. These adaptations aim to restore harmony within the body, ensuring optimal function despite the challenging environmental conditions. Indeed, the human body undergoes significant metabolic changes in frigid environments to maintain temperature balance and ensure survival. These adaptations involve increased H (prod), heightened metabolic intensity, and alterations in the function of key organs like muscle and adipose tissue. However, these adjustments also disrupt certain equilibriums within the body, placing additional strain on organs like the liver to maintain homeostasis. Homeostasis, or the maintenance of internal equilibrium, is a fundamental purpose of metabolism. Understanding how the body’s metabolism responds to cold conditions requires considering both the positive and negative aspects of these adaptations. While increased H (prod) and metabolic intensity are necessary for survival, they can also lead to metabolic imbalances and health issues if not properly regulated.
Therefore, studying the adjustments that occur in the body’s metabolism in response to cold environments is crucial for understanding human physiology in frigid zones. By promoting the concept of balance, we can better comprehend the changes and characteristics of human metabolism in extreme cold, ultimately advancing our knowledge of how the body adapts to challenging environmental conditions.
6 TCM theory on the metabolic homeostasis maintenance in cold environments
TCM views humans as an integral part of nature, highlighting the importance of adapting to local environmental conditions for maintaining health and balance. Throughout history, TCM has recognized the influence of different natural environments on human physiology, offering insights into how to live harmoniously in various climates. While specific effects of cold environments on the body are still being explored, TCM provides valuable principles for coping with cold conditions:
Moderate consumption: In cold environments, it’s essential to reduce excessive consumption to conserve energy and maintain balance within the body.
Sunlight exposure: Despite the cold, exposure to sunlight remains crucial for overall well-being. Sunlight provides essential vitamin D and supports mood regulation, helping individuals adapt to cold climates.
Rhythmic adaptation: Paying attention to changes in body rhythms, such as sleep patterns, can aid in adapting to the cold environment. Embracing these changes in rhythm is believed to enhance survival.
Gratitude and emotional balance: Cultivating gratitude for one’s present circumstances can foster emotional resilience in the face of challenging environments. Maintaining a positive outlook is beneficial for mental and physical health.
Warmth and injury prevention: Rather than confronting the cold directly, TCM advises prioritizing warmth and taking precautions to prevent cold-related injuries.
These principles, rooted in centuries of observation and practice, offer valuable guidance for individuals living in cold regions, helping them navigate the challenges of their environment while promoting overall well-being and balance.
The principles of TCM emphasize maintaining balance within the body as a fundamental aspect of health. In the context of cold environments, achieving equilibrium between H (prod) and dissipation is essential for overall well-being. This balance ensures that the body can replenish energy stores to meet increased caloric demands and restore depleted energy substrates in the blood plasma. TCM recognizes that the body adapts to its environment, evidenced by modern studies on cold adaptation showing higher resting H (prod) and increased tolerance to cold conditions. Moreover, TCM advocates certain behaviors and interventions to promote balance and health in cold environments. These may include consuming foods or herbs known for their warming properties and engaging in exercises that enhance blood circulation throughout the body.
While many of these principles are rooted in ancient Chinese practices and observations, their efficacy and underlying mechanisms require further scientific investigation. Nonetheless, they offer valuable insights and inspiration for future research into cold-related metabolism and health. By combining ancient wisdom with modern scientific inquiry, we may uncover new strategies for maintaining balance and promoting health in cold environments.
7 Conclusion
The article delves into the mechanisms by which the human body regulates temperature and material balance in a cold environment, exploring the sources of energy consumption and the evidence supporting the body’s ability to adapt and rebalance its metabolism in response to cold stress. This exploration is guided by the Yin-Yang balance concept in TCM, which emphasizes harmony between human beings and nature.
Cold’s pervasive and enduring effects on the human body align with TCM’s view of the interconnectedness between humans and their environment. While there remain gaps in our understanding, it is clear that individuals living in cold climates possess unique metabolic traits shaped by their surroundings. Additionally, this paper outlines TCM-based recommendations for promoting health in cold environments, drawing from centuries of empirical knowledge.
Despite limited knowledge about the precise impacts of cold on the body, it is crucial to address key questions: (1) What metabolic changes and new metabolic balances occur in response to cold? (2) How do these changes affect overall health and disease? (3) Base on the above, what strategies do people employ to maintain homeostasis and achieve health in cold environments?
Answers to these questions will form the foundation for the development of frigid zone medicine, guiding future research and interventions to enhance human health and resilience in cold climates.
Funding statement: This work was funded by the National Centre for the Development of TCM Education (TC2023002).
-
Author contributions Zhao T Y and Ma Y F: Conceptualization, writing original draft preparation, writing-reviewing and editing; Zhang J and Zhou X J: Conceptualization supervision; Zhou Y Y, Yan J D: Supervision, project administration.
-
Conflict of interest The authors declare no competing interest.
-
Data availability statement All data generated or analyzed during this study are included in this article.
References
[1] McKinley M J, Yao S T, Uschakov A, et al. The median preoptic nucleus: front and centre for the regulation of body fluid, sodium, temperature, sleep and cardiovascular homeostasis. Acta Physiol (Oxf), 2015; 214(1): 8-32.10.1111/apha.12487Search in Google Scholar PubMed
[2] McInnis K, Haman F, Doucet É. Humans in the cold: Regulating energy balance. Obes Rev, 2020; 21(3): e12978.10.1111/obr.12978Search in Google Scholar PubMed
[3] Morrison S F. Central control of body temperature. F1000Res, 2016; 5: F1000 Faculty Rev-880.10.12688/f1000research.7958.1Search in Google Scholar PubMed PubMed Central
[4] Morrison S F, Madden C J, Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab, 2014; 19(5): 741-756.10.1016/j.cmet.2014.02.007Search in Google Scholar PubMed PubMed Central
[5] Morris N B, Filingeri D, Halaki M, et al. Evidence of viscerally-mediated cold-defence thermoeffector responses in man. J Physiol, 2017; 595(4): 1201-1212.10.1113/JP273052Search in Google Scholar PubMed PubMed Central
[6] Carlisle H J. Behavioural significance of hypothalamic temperature-sensitive cells. Nature, 1966; 209(5030): 1324-1325.10.1038/2091324a0Search in Google Scholar PubMed
[7] Hammel H T, Hardy J D, Fusco M M. Thermoregulatory responses to hypothalamic cooling in unanesthetized dogs. Am J Physiol, 1960; 198: 481-486.Search in Google Scholar
[8] Frare C, Williams C T, Drew K L. Thermoregulation in hibernating mammals: The role of the “thyroid hormones system”. Mol Cell Endocrinol, 2021; 519: 111054.10.1016/j.mce.2020.111054Search in Google Scholar PubMed PubMed Central
[9] Silva J E. Thermogenic mechanisms and their hormonal regulation. Physiol Rev, 2006; 86(2): 435-464.10.1152/physrev.00009.2005Search in Google Scholar PubMed
[10] Weiner J, Hankir M, Heiker J T, et al. Thyroid hormones and browning of adipose tissue. Mol Cell Endocrinol, 2017; 458: 156-159.10.1016/j.mce.2017.01.011Search in Google Scholar PubMed
[11] Commins S P, Marsh D J, Thomas S A, et al. noradrenaline is required for leptin effects on gene expression in brown and white adipose tissue. Endocrinology, 1999; 140(10): 4772-4778.10.1210/en.140.10.4772Search in Google Scholar
[12] Townsend K L, Tseng Y H. Brown fat fuel utilization and thermogenesis. Trends Endocrinol Metab, 2014; 25(4): 168-177.10.1016/j.tem.2013.12.004Search in Google Scholar PubMed PubMed Central
[13] Park K, Li Q, Lynes M D, et al. Endothelial Cells Induced Progenitors Into Brown Fat to Reduce Atherosclerosis. Circ Res, 2022; 131(2): 168-183.10.1161/CIRCRESAHA.121.319582Search in Google Scholar PubMed PubMed Central
[14] Cui H, López M, Rahmouni K. The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat Rev Endocrinol, 2017; 13(6): 338-351.10.1038/nrendo.2016.222Search in Google Scholar PubMed PubMed Central
[15] Fischer A W, Cannon B, Nedergaard J. Leptin: Is it thermogenic? Endocr Rev, 2020; 41(2): 232-260.10.1210/endrev/bnz016Search in Google Scholar PubMed PubMed Central
[16] Seoane-Collazo P, Martínez-Sánchez N, Milbank E, et al. Incendiary leptin. Nutrients, 2020; 12(2): 472.10.3390/nu12020472Search in Google Scholar PubMed PubMed Central
[17] Tokizawa K, Onoue Y, Uchida Y, et al. Ghrelin induces time-dependent modulation of thermoregulation in the cold. Chronobiol Int, 2012; 29(6): 736-746.10.3109/07420528.2012.678452Search in Google Scholar PubMed
[18] Hammel H T, Hardy J D, Fusco M M. Thermoregulatory responses to hypothalamic cooling in unanesthetized dogs. Am J Physiol, 1960; 198: 481-486.10.1152/ajplegacy.1960.198.3.481Search in Google Scholar PubMed
[19] Zhang Y, Zhao Y, Li C, et al. Physiological, immune response, antioxidant capacity and lipid metabolism changes in grazing sheep during the cold season. Animals (Basel), 2022; 12(18): 2332.10.3390/ani12182332Search in Google Scholar PubMed PubMed Central
[20] Mori H, Dugan C E, Nishii A, et al. The molecular and metabolic program by which white adipocytes adapt to cool physiologic temperatures. PLoS Biol, 2021; 19(5): e3000988.10.1371/journal.pbio.3000988Search in Google Scholar PubMed PubMed Central
[21] Zhang D, Chang S, Jing B, et al. Reactive oxygen species are essential for vasoconstriction upon cold exposure. Oxid Med Cell Longev, 2021; 2021: 8578452.10.1155/2021/8578452Search in Google Scholar PubMed PubMed Central
[22] Eyolfson D A, Tikuisis P, Xu X, et al. Measurement and prediction of peak shivering intensity in humans. Eur J Appl Physiol, 2001; 84(1–2): 100-106.10.1007/s004210000329Search in Google Scholar PubMed
[23] Blondin D P, Daoud A, Taylor T, et al. Four-week cold acclimation in adult humans shifts uncoupling thermogenesis from skeletal muscles to brown adipose tissue. J Physiol, 2017; 595(6): 2099-2113.10.1113/JP273395Search in Google Scholar PubMed PubMed Central
[24] Anunciado-Koza R P, Zhang J, Ukropec J, et al. Inactivation of the mitochondrial carrier SLC25A25 (ATP-Mg2+/Pi transporter) reduces physical endurance and metabolic efficiency in mice. J Biol Chem, 2011; 286(13): 11659-11671.10.1074/jbc.M110.203000Search in Google Scholar PubMed PubMed Central
[25] Aydin J, Shabalina I G, Place N, et al. Nonshivering thermogenesis protects against defective calcium handling in muscle. FASEB J, 2008; 22(11): 3919-3924.10.1096/fj.08-113712Search in Google Scholar PubMed PubMed Central
[26] Bal N C, Maurya S K, Sopariwala D H, et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat Med, 2012; 18(10): 1575-1579.10.1038/nm.2897Search in Google Scholar PubMed PubMed Central
[27] Bachman E S, Dhillon H, Zhang C Y, et al. BetaAR signaling required for diet-induced thermogenesis and obesity resistance. Science, 2002; 297(5582): 843-845.10.1126/science.1073160Search in Google Scholar PubMed
[28] Thomas S A, Palmiter R D. Thermoregulatory and metabolic phenotypes of mice lacking noradrenaline and adrenaline. Nature, 1997; 387(6628): 94-97.10.1038/387094a0Search in Google Scholar PubMed
[29] Janssen I, Heymsfield S B, Wang Z M, et al. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol (1985), 2000; 89(1): 81-88.10.1152/jappl.2000.89.1.81Search in Google Scholar PubMed
[30] Zurlo F, Larson K, Bogardus C, et al. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest, 1990; 86(5): 1423-1427.10.1172/JCI114857Search in Google Scholar PubMed PubMed Central
[31] Haman F, Legault S R, Weber J M. Fuel selection during intense shivering in humans: EMG pattern reflects carbohydrate oxidation. J Physiol, 2004; 556(Pt 1): 305-313.Search in Google Scholar
[32] Tikuisis P, Giesbrecht G G. Prediction of shivering heat production from core and mean skin temperatures. Eur J Appl Physiol Occup Physiol, 1999; 79(3): 221-229.10.1007/s004210050499Search in Google Scholar PubMed
[33] Dumont L, Lessard R, Semeniuk K, et al. Thermogenic responses to different clamped skin temperatures in cold-exposed men and women. Am J Physiol Regul Integr Comp Physiol, 2022; 323(1): R149-160.10.1152/ajpregu.00268.2021Search in Google Scholar PubMed
[34] McAllen R M, Tanaka M, Ootsuka Y, et al. Multiple thermoregulatory effectors with independent central controls. Eur J Appl Physiol, 2010; 109(1): 27-33.10.1007/s00421-009-1295-zSearch in Google Scholar PubMed
[35] Nakamura K, Morrison S F. A thermosensory pathway that controls body temperature. Nat Neurosci, 2008; 11(1): 62-71.10.1038/nn2027Search in Google Scholar PubMed PubMed Central
[36] Tanaka M, Owens N C, Nagashima K, et al. Reflex activation of rat fusimotor neurons by body surface cooling, and its dependence on the medullary raphe. J Physiol, 2006; 572(Pt 2): 569-583.10.1113/jphysiol.2005.102400Search in Google Scholar PubMed PubMed Central
[37] Nakamura K, Morrison S F. Central efferent pathways for cold-defensive and febrile shivering. J Physiol, 2011; 589(Pt 14): 3641-3658.10.1113/jphysiol.2011.210047Search in Google Scholar PubMed PubMed Central
[38] Perkins J F The role of the proprioceptors in shivering. Am J Physiol, 1945; 145: 264-271.10.1152/ajplegacy.1945.145.2.264Search in Google Scholar PubMed
[39] Sato H. Fusimotor modulation by spinal and skin temperature changes and its significance in cold shivering. Exp Neurol, 1981; 74(1): 21-32.10.1016/0014-4886(81)90146-1Search in Google Scholar PubMed
[40] Sato H, Hashitani T, Isobe Y, et al. Descending influences from nucleus raphe magnus on fusimotor neurone activity in rats. Journal of Thermal Biology, 1990; 15(3): 259-265.10.1016/0306-4565(90)90012-7Search in Google Scholar
[41] Bell D G, Tikuisis P, Jacobs I. Relative intensity of muscular contraction during shivering. J Appl Physiol (1985), 1992; 72(6): 2336-2342.10.1152/jappl.1992.72.6.2336Search in Google Scholar PubMed
[42] Haman F Shivering in the cold: from mechanisms of fuel selection to survival. J Appl Physiol (1985), 2006; 100(5): 1702-1708.10.1152/japplphysiol.01088.2005Search in Google Scholar PubMed
[43] Haman F, Péronnet F, Kenny G P, et al. Partitioning oxidative fuels during cold exposure in humans: muscle glycogen becomes dominant as shivering intensifies. J Physiol, 2005; 566(Pt 1): 247-256.Search in Google Scholar
[44] Block B A. Structure of the brain and eye heater tissue in marlins, sailfish, and spearfishes. J Morphol, 1986; 190(2): 169-189.10.1002/jmor.1051900203Search in Google Scholar PubMed
[45] Block B A. Thermogenesis in muscle. Annu Rev Physiol, 1994; 56: 535-577.10.1146/annurev.ph.56.030194.002535Search in Google Scholar PubMed
[46] Dickson K A, Graham J B. Evolution and consequences of endothermy in fishes. Physiol Biochem Zool, 2004; 77(6): 998-1018.10.1086/423743Search in Google Scholar PubMed
[47] Periasamy M, Herrera J L, Reis F C G. Skeletal muscle thermogenesis and its role in whole body energy metabolism. Diabetes Metab J, 2017; 41(5): 327-336.10.4093/dmj.2017.41.5.327Search in Google Scholar PubMed PubMed Central
[48] Blondin D P, Haman F. Shivering and nonshivering thermogenesis in skeletal muscles. Handb Clin Neurol, 2018; 156: 153-173.10.1016/B978-0-444-63912-7.00010-2Search in Google Scholar PubMed
[49] Bal N C, Maurya S K, Singh S, et al. Increased Reliance on muscle-based thermogenesis upon acute minimization of brown adipose tissue function. J Biol Chem, 2016; 291(33): 17247-17257.10.1074/jbc.M116.728188Search in Google Scholar PubMed PubMed Central
[50] Mailloux R J, Harper M E. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic Biol Med, 2011; 51(6): 1106-1115.10.1016/j.freeradbiomed.2011.06.022Search in Google Scholar PubMed
[51] Rolfe D F, Brand M D. Contribution of mitochondrial proton leak to skeletal muscle respiration and to standard metabolic rate. Am J Physiol, 1996; 271(4 Pt 1): C1380-1389.10.1152/ajpcell.1996.271.4.C1380Search in Google Scholar PubMed
[52] Boss O, Samec S, Paoloni-Giacobino A, et al. Uncoupling protein-3: a new member of the mitochondrial carrier family with tissue-specific expression. FEBS Lett, 1997; 408(1): 39-42.10.1016/S0014-5793(97)00384-0Search in Google Scholar PubMed
[53] Fleury C, Neverova M, Collins S, et al. Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat Genet, 1997; 15(3): 269-272.10.1038/ng0397-269Search in Google Scholar PubMed
[54] Chan C B, De Leo D, Joseph J W, et al. Increased uncoupling protein-2 levels in beta-cells are associated with impaired glucose-stimulated insulin secretion: mechanism of action. Diabetes, 2001; 50(6): 1302-1310.10.2337/diabetes.50.6.1302Search in Google Scholar PubMed
[55] Cadenas S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim Biophys Acta Bioenerg, 2018; 1859(9): 940-950.10.1016/j.bbabio.2018.05.019Search in Google Scholar PubMed
[56] Schrauwen P, Hesselink M. UCP2 and UCP3 in muscle controlling body metabolism. J Exp Biol, 2002; 205(Pt 15): 2275-2285.10.1242/jeb.205.15.2275Search in Google Scholar PubMed
[57] Esterbauer H, Schneitler C, Oberkofler H, et al. A common polymorphism in the promoter of UCP2 is associated with decreased risk of obesity in middle-aged humans. Nat Genet, 2001; 28(2): 178-183.10.1038/88911Search in Google Scholar PubMed
[58] Aguer C, Fiehn O, Seifert E L, et al. Muscle uncoupling protein 3 overexpression mimics endurance training and reduces circulating biomarkers of incomplete β-oxidation. FASEB J, 2013; 27(10): 4213-4225.10.1096/fj.13-234302Search in Google Scholar PubMed PubMed Central
[59] Monteiro B S, Freire-Brito L, Carrageta D F, et al. Mitochondrial Uncoupling Proteins (UCPs) as key modulators of ROS homeostasis: a crosstalk between diabesity and male infertility? Antioxidants (Basel), 2021; 10(11): 1746.10.3390/antiox10111746Search in Google Scholar PubMed PubMed Central
[60] Lin B, Coughlin S, Pilch P F. Bidirectional regulation of uncoupling protein-3 and GLUT-4 mRNA in skeletal muscle by cold. Am J Physiol, 1998; 275(3): E386-391.10.1152/ajpendo.1998.275.3.E386Search in Google Scholar PubMed
[61] Wijers S L J, Schrauwen P, van Baak M A, et al. Beta-adrenergic receptor blockade does not inhibit cold-induced thermogenesis in humans: possible involvement of brown adipose tissue. J Clin Endocrinol Metab, 2011; 96(4): E598-605.10.1210/jc.2010-1957Search in Google Scholar PubMed
[62] Rubtsov A M, Batrukova M A. Ca-release channels (ryanodine receptors) of sarcoplasmic reticulum: structure and properties. A review. Biochemistry (Mosc), 1997; 62(9): 933-945.Search in Google Scholar
[63] Meissner G, Lu X. Dihydropyridine receptor-ryanodine receptor interactions in skeletal muscle excitation-contraction coupling. Biosci Rep, 1995; 15(5): 399-408.10.1007/BF01788371Search in Google Scholar PubMed
[64] Nakai J, Ogura T, Protasi F, et al. Functional nonequality of the cardiac and skeletal ryanodine receptors. Proc Natl Acad Sci U S A, 1997; 94(3): 1019-1022.10.1073/pnas.94.3.1019Search in Google Scholar PubMed PubMed Central
[65] Lyfenko A D, Goonasekera S A, Dirksen R T. Dynamic alterations in myoplasmic Ca2+ in malignant hyperthermia and central core disease. Biochem Biophys Res Commun, 2004; 322(4): 1256-1266.10.1016/j.bbrc.2004.08.031Search in Google Scholar PubMed
[66] Mall S, Broadbridge R, Harrison S L, et al. The presence of sarcolipin results in increased heat production by Ca2+-ATPase. J Biol Chem, 2006; 281(48): 36597-36602.10.1074/jbc.M606869200Search in Google Scholar PubMed
[67] Smith I C, Bombardier E, Vigna C, et al. ATP consumption by sarcoplasmic reticulum Ca2+ pumps accounts for 40-50% of resting metabolic rate in mouse fast and slow twitch skeletal muscle. PLoS One, 2013; 8(7): e68924.10.1371/journal.pone.0068924Search in Google Scholar PubMed PubMed Central
[68] Kjelstrup S, de Meis L, Bedeaux D, et al. Is the Ca2+-ATPase from sarcoplasmic reticulum also a heat pump? Eur Biophys J, 2008; 38(1): 59-67.10.1007/s00249-008-0358-0Search in Google Scholar PubMed
[69] Inesi G, de Meis L. Regulation of steady state filling in sarcoplasmic reticulum. Roles of back-inhibition, leakage, and slippage of the calcium pump. J Biol Chem, 1989; 264(10): 5929-5936.10.1016/S0021-9258(18)83639-0Search in Google Scholar
[70] Lervik A, Bresme F, Kjelstrup S, et al. On the thermodynamic efficiency of Ca2+-ATPase molecular machines. Biophys J, 2012; 103(6): 1218-1226.10.1016/j.bpj.2012.07.057Search in Google Scholar PubMed PubMed Central
[71] Meltzer S, Berman M C. Effects of pH, temperature, and calcium concentration on the stoichiometry of the calcium pump of sarcoplasmic reticulum. J Biol Chem, 1984; 259(7): 4244-4253.10.1016/S0021-9258(17)43036-5Search in Google Scholar
[72] Gamu D, Bombardier E, Smith I C, et al. Sarcolipin provides a novel muscle-based mechanism for adaptive thermogenesis. Exerc Sport Sci Rev, 2014; 42(3): 136-142.10.1249/JES.0000000000000016Search in Google Scholar PubMed
[73] Bal N C, Periasamy M. Uncoupling of sarcoendoplasmic reticulum calcium ATPase pump activity by sarcolipin as the basis for muscle non-shivering thermogenesis. Philos Trans R Soc Lond B Biol Sci, 2020; 375(1793): 20190135.10.1098/rstb.2019.0135Search in Google Scholar PubMed PubMed Central
[74] Sepa-Kishi D M, Sotoudeh-Nia Y, Iqbal A, et al. Cold acclimation causes fiber type-specific responses in glucose and fat metabolism in rat skeletal muscles. Sci Rep, 2017; 7(1): 15430.10.1038/s41598-017-15842-3Search in Google Scholar PubMed PubMed Central
[75] Bal N C, Singh S, Reis F C G, et al. Both brown adipose tissue and skeletal muscle thermogenesis processes are activated during mild to severe cold adaptation in mice. J Biol Chem, 2017; 292(40): 16616-166125.10.1074/jbc.M117.790451Search in Google Scholar PubMed PubMed Central
[76] Smith W S, Broadbridge R, East JM, et al. Sarcolipin uncouples hydrolysis of ATP from accumulation of Ca2+ by the Ca2+-ATPase of skeletal-muscle sarcoplasmic reticulum. Biochem J, 2002; 361(Pt 2): 277-286.10.1042/bj3610277Search in Google Scholar
[77] Pant M, Bal N C, Periasamy M. Cold adaptation overrides developmental regulation of sarcolipin expression in mice skeletal muscle: SOS for muscle-based thermogenesis? J Exp Biol, 2015; 218(Pt 15): 2321-2325.10.1242/jeb.119164Search in Google Scholar PubMed PubMed Central
[78] Maurya S K, Bal N C, Sopariwala D H, et al. Sarcolipin is a key determinant of the basal metabolic rate, and its overexpression enhances energy expenditure and resistance against diet-induced obesity. J Biol Chem, 2015; 290(17): 10840-10849.10.1074/jbc.M115.636878Search in Google Scholar PubMed PubMed Central
[79] Pant M, Bal N C, Periasamy M. Sarcolipin: a key thermogenic and metabolic regulator in skeletal muscle. Trends Endocrinol Metab, 2016; 27(12): 881-892.10.1016/j.tem.2016.08.006Search in Google Scholar PubMed PubMed Central
[80] Kaspari R R, Reyna-Neyra A, Jung L, et al. The paradoxical lean phenotype of hypothyroid mice is marked by increased adaptive thermogenesis in the skeletal muscle. Proc Natl Acad Sci U S A, 2020; 117(36): 22544-22551.10.1073/pnas.2008919117Search in Google Scholar PubMed PubMed Central
[81] Gollnick P D, Armstrong R B, Saubert C W, et al. Glycogen depletion patterns in human skeletal muscle fibers during prolonged work. Pflugers Arch, 1973; 344(1): 1-12.10.1007/BF00587437Search in Google Scholar PubMed
[82] Gejl K D, Ørtenblad N, Andersson E, et al. Local depletion of glycogen with supramaximal exercise in human skeletal muscle fibres. J Physiol, 2017; 595(9): 2809-2821.10.1113/JP273109Search in Google Scholar PubMed PubMed Central
[83] Vigh-Larsen J F, Ørtenblad N, Emil Andersen O, et al. Fibre type-and localisation-specific muscle glycogen utilisation during repeated high-intensity intermittent exercise. J Physiol, 2022; 600(21): 4713-4730.10.1113/JP283225Search in Google Scholar PubMed PubMed Central
[84] Jensen R, 0rtenblad N, Stausholm M L H, et al. Heterogeneity in subcellular muscle glycogen utilisation during exercise impacts endurance capacity in men. J Physiol, 2020; 598(19): 4271-4292.10.1113/JP280247Search in Google Scholar PubMed
[85] Amsellem J, Delorme R, Souchier C, et al. Transverse-axial tubular system in guinea pig ventricular cardiomyocyte: 3D reconstruction, quantification and its possible role in K+ accumulation-depletion phenomenon in single cells. Biol Cell, 1995; 85(1): 43-54.10.1111/j.1768-322X.1995.tb00941.xSearch in Google Scholar
[86] Balijepalli R C, Lokuta A J, Maertz N A, et al. Depletion of T-tubules and specific subcellular changes in sarcolemmal proteins in tachycardia-induced heart failure. Cardiovasc Res, 2003; 59(1): 67-77.10.1016/S0008-6363(03)00325-0Search in Google Scholar PubMed
[87] Dombrowski L, Roy D, Marcotte B, et al. A new procedure for the isolation of plasma membranes, T tubules, and internal membranes from skeletal muscle. Am J Physiol, 1996; 270(4 Pt 1): E667-676.10.1152/ajpendo.1996.270.4.E667Search in Google Scholar PubMed
[88] Stefanyk L E, Bonen A, Dyck D J. Insulin and contraction-induced movement of fatty acid transport proteins to skeletal muscle transverse-tubules is distinctly different than to the sarcolemma. Metabolism, 2012; 61(11): 1518-1522.10.1016/j.metabol.2012.04.002Search in Google Scholar PubMed
[89] Zorzano A, Muñoz P, Camps M, et al. Insulin-induced redistribution of GLUT4 glucose carriers in the muscle fiber. In search of GLUT4 trafficking pathways. Diabetes, 1996; 45 Suppl 1: S70-81.10.2337/diab.45.1.S70Search in Google Scholar
[90] Dohm G L, Dolan P L, Frisell W R, et al. Role of transverse tubules in insulin stimulated muscle glucose transport. J Cell Biochem, 1993; 52(1): 1-7.10.1002/jcb.240520102Search in Google Scholar PubMed
[91] Dohm G L, Dudek R W. Role of transverse tubules (T-tubules) in muscle glucose transport. Adv Exp Med Biol, 1998; 441: 27-34.10.1007/978-1-4899-1928-1_3Search in Google Scholar PubMed
[92] Haman F, Legault S R, Weber J M. Fuel selection during intense shivering in humans: EMG pattern reflects carbohydrate oxidation. J Physiol, 2004; 556(Pt 1): 305-313.10.1113/jphysiol.2003.055152Search in Google Scholar PubMed PubMed Central
[93] Weber J M, Haman F. Fuel selection in shivering humans. Acta Physiol Scand, 2005; 184(4): 319-329.10.1111/j.1365-201X.2005.01465.xSearch in Google Scholar PubMed
[94] Roepstorff C, Helge J W, Vistisen B, et al. Studies of plasma membrane fatty acid-binding protein and other lipid-binding proteins in human skeletal muscle. Proc Nutr Soc, 2004; 63(2): 239-244.10.1079/PNS2004332Search in Google Scholar PubMed
[95] Weber J M, Haman F. Oxidative fuel selection: adjusting mix and flux to stay alive. International Congress Series, 2004; 1275: 22-31.10.1016/j.ics.2004.09.043Search in Google Scholar
[96] Mentel T, Duch C, Stypa H, et al. Central modulatory neurons control fuel selection in flight muscle of migratory locust. J Neurosci, 2003; 23(4): 1109-1113.10.1523/JNEUROSCI.23-04-01109.2003Search in Google Scholar PubMed PubMed Central
[97] Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev, 2004; 84(1): 277-359.10.1152/physrev.00015.2003Search in Google Scholar PubMed
[98] van Marken Lichtenbelt W D, Schrauwen P. Implications of nonshivering thermogenesis for energy balance regulation in humans. Am J Physiol Regul Integr Comp Physiol, 2011; 301(2): R285-296.10.1152/ajpregu.00652.2010Search in Google Scholar PubMed
[99] Tran L T, Park S, Kim S K, et al. Hypothalamic control of energy expenditure and thermogenesis. Exp Mol Med, 2022; 54(4): 358-369.10.1038/s12276-022-00741-zSearch in Google Scholar PubMed PubMed Central
[100] Li L, Li B, Li M, et al. Switching on the furnace: regulation of heat production in brown adipose tissue. Mol Aspects Med, 2019; 68: 60-73.10.1016/j.mam.2019.07.005Search in Google Scholar PubMed
[101] Ko E Y, Sabanegh E S, Agarwal A. Male infertility testing: reactive oxygen species and antioxidant capacity. Fertil Steril, 2014; 102(6): 1518-1527.10.1016/j.fertnstert.2014.10.020Search in Google Scholar PubMed
[102] Virtanen K A, Lidell M E, Orava J, et al. Functional brown adipose tissue in healthy adults. N Engl J Med, 2009; 360(15): 1518-1525.10.1056/NEJMoa0808949Search in Google Scholar PubMed
[103] Matthias A, Ohlson K B, Fredriksson J M, et al. Thermogenic responses in brown fat cells are fully UCP1-dependent. UCP2 or UCP3 do not substitute for UCP1 in adrenergically or fatty scid-induced thermogenesis. J Biol Chem, 2000; 275(33): 25073-25081.10.1074/jbc.M000547200Search in Google Scholar PubMed
[104] Crichton P G, Lee Y, Kunji E R S. The molecular features of uncoupling protein 1 support a conventional mitochondrial carrier-like mechanism. Biochimie, 2017; 134: 35-50.10.1016/j.biochi.2016.12.016Search in Google Scholar PubMed PubMed Central
[105] Klingenberg M. UCP1 - A sophisticated energy valve. Biochimie, 2017; 134: 19-27.10.1016/j.biochi.2016.10.012Search in Google Scholar PubMed
[106] Nicholls D G. The hunt for the molecular mechanism of brown fat thermogenesis. Biochimie, 2017; 134: 9-18.10.1016/j.biochi.2016.09.003Search in Google Scholar PubMed
[107] Ikeda K, Kang Q, Yoneshiro T, et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat Med, 2017; 23(12): 1454-1465.10.1038/nm.4429Search in Google Scholar PubMed PubMed Central
[108] Irshad Z, Dimitri F, Christian M, et al. Diacylglycerol acyltransferase 2 links glucose utilization to fatty acid oxidation in the brown adipocytes. J Lipid Res, 2017; 58(1): 15-30.10.1194/jlr.M068197Search in Google Scholar PubMed PubMed Central
[109] Kazak L, Chouchani E T, Lu G Z, et al. Genetic depletion of adipocyte creatine metabolism inhibits diet-induced thermogenesis and drives obesity. Cell Metab, 2017; 26(4): 693.10.1016/j.cmet.2017.09.007Search in Google Scholar PubMed PubMed Central
[110] Lee Y H, Kim S N, Kwon H J, et al. Metabolic heterogeneity of activated beige/brite adipocytes in inguinal adipose tissue. Sci Rep, 2017; 7: 39794.10.1038/srep39794Search in Google Scholar PubMed PubMed Central
[111] Müller S, Balaz M, Stefanicka P, et al. Proteomic analysis of human brown adipose tissue reveals utilization of coupled and uncoupled energy expenditure pathways. Sci Rep, 2016; 6: 30030.10.1038/srep30030Search in Google Scholar PubMed PubMed Central
[112] Liu J, Zhang C, Zhang B, et al. Comprehensive analysis of the characteristics and differences in adult and newborn Brown Adipose Tissue (BAT): Newborn BAT Is a More Active/Dynamic BAT. Cells, 2020; 9(1): 201.10.3390/cells9010201Search in Google Scholar PubMed PubMed Central
[113] Yeung H W D, Grewal R K, Gonen M, et al. Patterns of (18)F-FDG uptake in adipose tissue and muscle: a potential source of false-positives for PET. J Nucl Med, 2003; 44(11): 1789-1796.Search in Google Scholar
[114] Cypess A M, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med, 2009; 360(15): 1509-1517.10.1056/NEJMoa0810780Search in Google Scholar PubMed PubMed Central
[115] Rothwell N J, Stock M J. Luxuskonsumption, diet-induced thermogenesis and brown fat: the case in favour. Clin Sci (Lond), 1983; 64(1): 19-23.10.1042/cs0640019Search in Google Scholar PubMed
[116] Ouellet V, Labbé S M, Blondin D P, et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest, 2012; 122(2): 545-552.10.1172/JCI60433Search in Google Scholar PubMed PubMed Central
[117] Blondin D P, Labbé S M, Tingelstad H C, et al. Increased brown adipose tissue oxidative capacity in cold-acclimated humans. J Clin Endocrinol Metab, 2014; 99(3): E438-446.10.1210/jc.2013-3901Search in Google Scholar PubMed PubMed Central
[118] Blondin D P, Tingelstad H C, Noll C, et al. Dietary fatty acid metabolism of brown adipose tissue in cold-acclimated men. Nat Commun, 2017; 8: 14146.10.1038/ncomms14146Search in Google Scholar PubMed PubMed Central
[119] Cohen P, Kajimura S. The cellular and functional complexity of thermogenic fat. Nat Rev Mol Cell Biol, 2021; 22(6): 393-409.10.1038/s41580-021-00350-0Search in Google Scholar PubMed PubMed Central
[120] Cousin B, Cinti S, Morroni M, et al. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci, 1992; 103 ( Pt 4): 931-942.10.1242/jcs.103.4.931Search in Google Scholar PubMed
[121] Barbatelli G, Murano I, Madsen L, et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab, 2010; 298(6): E1244-1253.10.1152/ajpendo.00600.2009Search in Google Scholar PubMed
[122] Cinti S. Transdifferentiation properties of adipocytes in the adipose organ. Am J Physiol Endocrinol Metab, 2009; 297(5): E977-986.10.1152/ajpendo.00183.2009Search in Google Scholar PubMed
[123] Lee Y H, Petkova A P, Konkar A A, et al. Cellular origins of cold-induced brown adipocytes in adult mice. FASEB J, 2015; 29(1): 286-299.10.1096/fj.14-263038Search in Google Scholar PubMed PubMed Central
[124] Loncar D. Convertible adipose tissue in mice. Cell Tissue Res, 1991; 266(1): 149-161.10.1007/BF00678721Search in Google Scholar PubMed
[125] Ikeda K, Maretich P, Kajimura S. The common and distinct features of brown and beige adipocytes. Trends Endocrinol Metab, 2018; 29(3): 191-200.10.1016/j.tem.2018.01.001Search in Google Scholar PubMed PubMed Central
[126] Chen L, Jin Y, Wu J, et al. Lipid droplets: a cellular organelle vital for thermogenesis. Int J Biol Sci, 2022; 18(16): 6176-6188.10.7150/ijbs.77051Search in Google Scholar PubMed PubMed Central
[127] Bartelt A, Bruns O T, Reimer R, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med, 2011; 17(2): 200-205.10.1038/nm.2297Search in Google Scholar PubMed
[128] Sponton C H, de Lima-Junior J C, Leiria L O. What puts the heat on thermogenic fat: metabolism of fuel substrates. Trends Endocrinol Metab, 2022; 33(8): 587-599.10.1016/j.tem.2022.05.003Search in Google Scholar PubMed
[129] Fischer A W, Jaeckstein M Y, Gottschling K, et al. Lysosomal lipoprotein processing in endothelial cells stimulates adipose tissue thermogenic adaptation. Cell Metab, 2021; 33(3): 547-564.e7.10.1016/j.cmet.2020.12.001Search in Google Scholar PubMed
[130] Wu Q, Kazantzis M, Doege H, et al. Fatty acid transport protein 1 is required for nonshivering thermogenesis in brown adipose tissue. Diabetes, 2006; 55(12): 3229-3237.10.2337/db06-0749Search in Google Scholar PubMed
[131] Heeren J, Scheja L. Brown adipose tissue and lipid metabolism. Curr Opin Lipidol, 2018; 29(3): 180-185.10.1097/MOL.0000000000000504Search in Google Scholar PubMed
[132] Abumrad N A. The liver as a hub in thermogenesis. Cell Metab, 2017; 26(3): 454-455.10.1016/j.cmet.2017.08.018Search in Google Scholar PubMed
[133] Simcox J, Geoghegan G, Maschek J A, et al. Global analysis of plasma lipids identifies liver-derived acylcarnitines as a fuel source for brown fat thermogenesis. Cell Metab, 2017; 26(3): 509-522.e6.10.1016/j.cmet.2017.08.006Search in Google Scholar PubMed PubMed Central
[134] Orava J, Nuutila P, Lidell M E, et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab, 2011; 14(2): 272-279.10.1016/j.cmet.2011.06.012Search in Google Scholar PubMed
[135] Ma S W, Foster D O. Uptake of glucose and release of fatty acids and glycerol by rat brown adipose tissue in vivo. Can J Physiol Pharmacol, 1986; 64(5): 609-614.10.1139/y86-101Search in Google Scholar PubMed
[136] Choung S, Kim J M, Joung K H, et al. Mig-6 is essential for glucose homeostasis and thermogenesis in brown adipose tissue. Biochem Biophys Res Commun, 2021; 572: 92-97.10.1016/j.bbrc.2021.07.088Search in Google Scholar PubMed
[137] DiStefano M T, Roth Flach R J, Senol-Cosar O, et al. Adipocytespecific Hypoxia-inducible gene 2 promotes fat deposition and diet-induced insulin resistance. Mol Metab, 2016; 5(12): 1149-1161.10.1016/j.molmet.2016.09.009Search in Google Scholar PubMed PubMed Central
[138] Fernandes G W, Ueta C B, Fonseca T L, et al. Inactivation of the adrenergic receptor β2 disrupts glucose homeostasis in mice. J Endocrinol, 2014; 221(3): 381-390.10.1530/JOE-13-0526Search in Google Scholar PubMed PubMed Central
[139] van Beek S M M, Kalinovich A, Schaart G, et al. Prolonged β2-adrenergic agonist treatment improves glucose homeostasis in diet-induced obese UCP1-/- mice. Am J Physiol Endocrinol Metab, 2021; 320(3): E619-628.10.1152/ajpendo.00324.2020Search in Google Scholar PubMed
[140] van Schaik L, Kettle C, Green R, et al. Both caffeine and Capsicum annuum fruit powder lower blood glucose levels and increase brown adipose tissue temperature in healthy adult males. Front Physiol, 2022; 13: 870154.10.3389/fphys.2022.870154Search in Google Scholar PubMed PubMed Central
[141] Wang L, Qiu Y, Gu H, et al. Regulation of adipose thermogenesis and its critical role in glucose and lipid metabolism. Int J Biol Sci, 2022; 18(13): 4950-4962.10.7150/ijbs.75488Search in Google Scholar PubMed PubMed Central
[142] Chondronikola M, Volpi E, Børsheim E, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes, 2014; 63(12): 4089-4099.10.2337/db14-0746Search in Google Scholar PubMed PubMed Central
[143] Stanford K I, Middelbeek R J W, Townsend K L, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest, 2013; 123(1): 215-223.10.1172/JCI62308Search in Google Scholar PubMed PubMed Central
[144] Guerra C, Navarro P, Valverde A M, et al. Brown adipose tissue-specific insulin receptor knockout shows diabetic phenotype without insulin resistance. J Clin Invest, 2019; 129(1): 437.10.1172/JCI126191Search in Google Scholar PubMed PubMed Central
[145] Muntzel M S, Anderson E A, Johnson A K, et al. Mechanisms of insulin action on sympathetic nerve activity. Clin Exp Hypertens, 1995; 17(1–2): 39-50.10.3109/10641969509087053Search in Google Scholar PubMed
[146] Maliszewska K, Kretowski A. Brown adipose tissue and its role in insulin and glucose homeostasis. Int J Mol Sci, 2021; 22(4): 1530.10.3390/ijms22041530Search in Google Scholar PubMed PubMed Central
[147] Vallerand A L, Pérusse F, Bukowiecki L J. Stimulatory effects of cold exposure and cold acclimation on glucose uptake in rat peripheral tissues. Am J Physiol, 1990; 259(5 Pt 2): R1043-1049.10.1152/ajpregu.1990.259.5.R1043Search in Google Scholar PubMed
[148] Glickman-Weiss E L, Nelson A G, Hearon C M, et al. Does feeding regime affect physiologic and thermal responses during exposure to 8, 20, and 27 degrees C? Eur J Appl Physiol Occup Physiol, 1993; 67(1): 30-34.10.1007/BF00377700Search in Google Scholar PubMed
[149] Glickman-Weiss E L, Nelson A G, Hearon C M, et al. The thermogenic effect of a carbohydrate feeding during exposure to 8, 12 and 27 degrees C. Eur J Appl Physiol Occup Physiol, 1994; 68(4): 291-297.10.1007/BF00571445Search in Google Scholar PubMed
[150] MacNaughton K W, Sathasivam P, Vallerand A L, et al. Influence of caffeine on metabolic responses of men at rest in 28 and 5 degrees C. J Appl Physiol (1985), 1990; 68(5): 1889-1895.10.1152/jappl.1990.68.5.1889Search in Google Scholar PubMed
[151] Martineau L, Jacobs I. Muscle glycogen availability and temperature regulation in humans. J Appl Physiol (1985), 1989; 66(1): 72-78.10.1152/jappl.1989.66.1.72Search in Google Scholar PubMed
[152] Martineau L, Jacobs I. Free fatty acid availability and temperature regulation in cold water. J Appl Physiol (1985), 1989; 67(6): 2466-2472.10.1152/jappl.1989.67.6.2466Search in Google Scholar PubMed
[153] Tikuisis P, Jacobs I, Moroz D, et al. Comparison of thermoregulatory responses between men and women immersed in cold water. J Appl Physiol (1985), 2000; 89(4): 1403-1411.10.1152/jappl.2000.89.4.1403Search in Google Scholar PubMed
[154] Vallerand A L, Zamecnik J, Jones P J, et al. FFA Ra and TG/FFA cycling in humans. Aviat Space Environ Med, 1999; 70(1): 42-50.Search in Google Scholar
[155] Vallerand A L, Jacobs I. Rates of energy substrates utilization during human cold exposure. Eur J Appl Physiol Occup Physiol, 1989; 58(8): 873-878.10.1007/BF02332221Search in Google Scholar PubMed
[156] Vallerand A L, Jacobs I. Influence of cold exposure on plasma triglyceride clearance in humans. Metabolism, 1990; 39(11): 1211-1218.10.1016/0026-0495(90)90097-VSearch in Google Scholar
[157] Haman F, Péronnet F, Kenny G P, et al. Effect of cold exposure on fuel utilization in humans: plasma glucose, muscle glycogen, and lipids. J Appl Physiol (1985), 2002; 93(1): 77-84.10.1152/japplphysiol.00773.2001Search in Google Scholar PubMed
[158] Haman F, Péronnet F, Kenny G P, et al. Partitioning oxidative fuels during cold exposure in humans: muscle glycogen becomes dominant as shivering intensifies. J Physiol, 2005; 566(Pt 1): 247-256.10.1113/jphysiol.2005.086272Search in Google Scholar PubMed PubMed Central
[159] Haman F, Peronnet F, Kenny G P, et al. Effects of carbohydrate availability on sustained shivering I. Oxidation of plasma glucose, muscle glycogen, and proteins. J Appl Physiol (1985), 2004; 96(1): 32-40.10.1152/japplphysiol.00427.2003Search in Google Scholar PubMed
[160] Haman F, Scott C G, Kenny G P. Fueling shivering thermogenesis during passive hypothermic recovery. J Appl Physiol (1985), 2007; 103(4): 1346-1351.10.1152/japplphysiol.00931.2006Search in Google Scholar PubMed
[161] Haman F, Mantha O L, Cheung S S, et al. Oxidative fuel selection and shivering thermogenesis during a 12- and 24-h cold-survival simulation. J Appl Physiol, 2016; 120(6): 640-648.10.1152/japplphysiol.00540.2015Search in Google Scholar PubMed
[162] Neufer P D, Young A J, Sawka M N, et al. Influence of skeletal muscle glycogen on passive rewarming after hypothermia. J Appl Physiol (1985), 1988; 65(2): 805-810.10.1152/jappl.1988.65.2.805Search in Google Scholar PubMed
[163] Acosta F M, Martinez-Tellez B, Sanchez-Delgado G, et al. Physiological responses to acute cold exposure in young lean men. PLoS One, 2018; 13(5): e0196543.10.1371/journal.pone.0196543Search in Google Scholar PubMed PubMed Central
[164] Rolfe D F, Brown G C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev, 1997; 77(3): 731-758.10.1152/physrev.1997.77.3.731Search in Google Scholar PubMed
[165] Gordon K, Blondin D P, Friesen B J, et al. Seven days of cold acclimation substantially reduces shivering intensity and increases nonshivering thermogenesis in adult humans. J Appl Physiol (1985), 2019; 126(6): 1598-1606.10.1152/japplphysiol.01133.2018Search in Google Scholar PubMed PubMed Central
[166] Himms-Hagen J. Lipid metabolism during cold-exposure and during cold-acclimation. Lipids, 1972; 7(5): 310-323.10.1007/BF02532649Search in Google Scholar PubMed
[167] Thompson G E. Physiological effects of cold exposure. Int Rev Physiol, 1977; 15: 29-69.Search in Google Scholar
[168] Tipton M J, Franks G M, Meneilly G S, et al. Substrate utilisation during exercise and shivering. Eur J Appl Physiol Occup Physiol, 1997; 76(1): 103-108.10.1007/s004210050220Search in Google Scholar PubMed
[169] Vallerand A L, Zamecnik J, Jacobs I. Plasma glucose turnover during cold stress in humans. J Appl Physiol (1985), 1995; 78(4): 1296-1302.10.1152/jappl.1995.78.4.1296Search in Google Scholar PubMed
[170] Blondin D P, Labbé S M, Phoenix S, et al. Contributions of white and brown adipose tissues and skeletal muscles to acute cold-induced metabolic responses in healthy men. J Physiol, 2015; 593(3): 701-714.10.1113/jphysiol.2014.283598Search in Google Scholar PubMed PubMed Central
[171] Cunningham J J, Gulino M A, Meara P A, et al. Enhanced hepatic insulin sensitivity and peripheral glucose uptake in cold acclimating rats. Endocrinology, 1985; 117(4): 1585-1589.10.1210/endo-117-4-1585Search in Google Scholar PubMed
[172] Hanssen M J W, Hoeks J, Brans B, et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med, 2015; 21(8): 863-865.10.1038/nm.3891Search in Google Scholar PubMed
[173] Poher A L, Altirriba J, Veyrat-Durebex C, et al. Brown adipose tissue activity as a target for the treatment of obesity/insulin resistance. Front Physiol, 2015; 6: 4.10.3389/fphys.2015.00004Search in Google Scholar PubMed PubMed Central
[174] Sugimoto S, Mena H A, Sansbury B E, et al. Brown adipose tissue-derived MaR2 contributes to cold-induced resolution of inflammation. Nat Metab, 2022; 4(6): 775-790.10.1038/s42255-022-00590-0Search in Google Scholar PubMed PubMed Central
[175] Young S G, Zechner R. Biochemistry and pathophysiology of intravascular and intracellular lipolysis. Genes Dev, 2013; 27(5): 459-484.10.1101/gad.209296.112Search in Google Scholar PubMed PubMed Central
[176] Straat M E, Jurado-Fasoli L, Ying Z, et al. Cold exposure induces dynamic changes in circulating triacylglycerol species, which is dependent on intracellular lipolysis: a randomized cross-over trial. EBioMedicine, 2022; 86: 104349.10.1016/j.ebiom.2022.104349Search in Google Scholar PubMed PubMed Central
[177] Shiota M, Tanaka T, Sugano T. Effect of noradrenaline on gluconeogenesis in perfused livers of cold-exposed rats. Am J Physiol, 1985; 249(3 Pt 1): E281-286.10.1152/ajpendo.1985.249.3.E281Search in Google Scholar PubMed
[178] Shi H, Yao R, Lian S, et al. Regulating glycolysis, the TLR4 signal pathway and expression of RBM3 in mouse liver in response to acute cold exposure. Stress, 2019; 22(3): 366-376.10.1080/10253890.2019.1568987Search in Google Scholar PubMed
[179] Stoner H B. The role of the liver in non-shivering thermogenesis in the rat. J Physiol, 1973; 232(2): 285-296.10.1113/jphysiol.1973.sp010270Search in Google Scholar PubMed PubMed Central
[180] Khedoe P P S J, Hoeke G, Kooijman S, et al. Brown adipose tissue takes up plasma triglycerides mostly after lipolysis. J Lipid Res, 2015; 56(1): 51-59.10.1194/jlr.M052746Search in Google Scholar PubMed PubMed Central
[181] Scheja L, Heeren J. Metabolic interplay between white, beige, brown adipocytes and the liver. J Hepatol, 2016; 64(5): 1176-1186.10.1016/j.jhep.2016.01.025Search in Google Scholar PubMed
[182] Evangelakos I, Kuhl A, Baguhl M, et al. Cold-induced lipoprotein clearance in CYP7B1-deficient mice. Front Cell Dev Biol, 2022; 10: 836741.10.3389/fcell.2022.836741Search in Google Scholar PubMed PubMed Central
[183] Worthmann A, John C, Rühlemann M C, et al. Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat Med, 2017; 23(7): 839-849.10.1038/nm.4357Search in Google Scholar PubMed
[184] Perino A, Schoonjans K. Metabolic messengers: bile acids. Nat Metab, 2022; 4(4): 416-423.10.1038/s42255-022-00559-zSearch in Google Scholar PubMed
[185] Ockenga J, Valentini L, Schuetz T, et al. Plasma bile acids are associated with energy expenditure and thyroid function in humans. J Clin Endocrinol Metab, 2012; 97(2): 535-542.10.1210/jc.2011-2329Search in Google Scholar PubMed
[186] Soufi N, Kohli R. What’s cold got to do with it? Cold-induced thermogenesis and microbiome modification may be regulated by bile acid physiology. Hepatology, 2018; 68(4): 1644-1646.10.1002/hep.29899Search in Google Scholar PubMed PubMed Central
[187] Spann R A, Morrison C D, den Hartigh L J. The nuanced metabolic functions of endogenous FGF21 depend on the nature of the stimulus, tissue source, and experimental model. Front Endocrinol (Lausanne), 2021; 12: 802541.10.3389/fendo.2021.802541Search in Google Scholar PubMed PubMed Central
[188] Piao Z, Zhai B, Jiang X, et al. Reduced adiposity by compensatory WAT browning upon iBAT removal in mice. Biochem Biophys Res Commun, 2018; 501(3): 807-813.10.1016/j.bbrc.2018.05.089Search in Google Scholar PubMed
[189] Markan K R, Naber M C, Ameka M K, et al. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes, 2014; 63(12): 4057-4063.10.2337/db14-0595Search in Google Scholar PubMed PubMed Central
[190] Owen B M, Ding X, Morgan D A, et al. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab, 2014; 20(4): 670-677.10.1016/j.cmet.2014.07.012Search in Google Scholar PubMed PubMed Central
[191] Lee P, Brychta R J, Linderman J, et al. Mild cold exposure modulates fibroblast growth factor 21 (FGF21) diurnal rhythm in humans: relationship between FGF21 levels, lipolysis, and cold-induced thermogenesis. J Clin Endocrinol Metab, 2013; 98(1): E98-102.10.1210/jc.2012-3107Search in Google Scholar PubMed PubMed Central
[192] Lee P, Linderman J D, Smith S, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab, 2014; 19(2): 302-309.10.1016/j.cmet.2013.12.017Search in Google Scholar PubMed PubMed Central
[193] Huang Z, Zhong L, Lee J T H, et al. The FGF21-CCL11 axis mediates beiging of white adipose tissues by coupling sympathetic nervous system to type 2 immunity. Cell Metab, 2017; 26(3): 493-508.e4.10.1016/j.cmet.2017.08.003Search in Google Scholar PubMed
[194] Ameka M, Markan K R, Morgan D A, et al. Liver derived FGF21 maintains core body temperature during acute cold exposure. Sci Rep, 2019; 9(1): 630.10.1038/s41598-018-37198-ySearch in Google Scholar PubMed PubMed Central
[195] Kinouchi K, Mikami Y, Kanai T, et al. Circadian rhythms in the tissue-specificity from metabolism to immunity: insights from omics studies. Mol Aspects Med, 2021; 80: 100984.10.1016/j.mam.2021.100984Search in Google Scholar PubMed
[196] Matsushita M, Nirengi S, Hibi M, et al. Diurnal variations of brown fat thermogenesis and fat oxidation in humans. Int J Obes (Lond), 2021; 45(11): 2499-2505.10.1038/s41366-021-00927-xSearch in Google Scholar PubMed PubMed Central
[197] Guan D, Xiong Y, Borck P C, et al. Diet-induced circadian enhancer remodeling synchronizes opposing hepatic lipid metabolic processes. Cell, 2018; 174(4): 831-842.e12.10.1016/j.cell.2018.06.031Search in Google Scholar PubMed PubMed Central
[198] Billings M E, Hale L, Johnson D A. Physical and social environment relationship with sleep health and disorders. Chest, 2020; 157(5): 1304-1312.10.1016/j.chest.2019.12.002Search in Google Scholar PubMed PubMed Central
[199] Kraneburg A, Franke S, Methling R, et al. Effect of color temperature on melatonin production for illumination of working environments. Appl Ergon, 2017; 58: 446-453.10.1016/j.apergo.2016.08.006Search in Google Scholar PubMed
[200] Everett L J, Lazar M A. Nuclear receptor Rev-erbα: up, down, and all around. Trends Endocrinol Metab, 2014; 25(11): 586-592.10.1016/j.tem.2014.06.011Search in Google Scholar PubMed PubMed Central
[201] Le Martelot G, Claudel T, Gatfield D, et al. REV-ERBalpha participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol, 2009; 7(9): e1000181.10.1371/journal.pbio.1000181Search in Google Scholar PubMed PubMed Central
[202] Bugge A, Feng D, Everett L J, et al. Rev-erbα and Rev-erbβ coordinately protect the circadian clock and normal metabolic function. Genes Dev, 2012; 26(7): 657-667.10.1101/gad.186858.112Search in Google Scholar PubMed PubMed Central
[203] Delezie J, Dumont S, Dardente H, et al. The nuclear receptor REV-ERBα is required for the daily balance of carbohydrate and lipid metabolism. FASEB J, 2012; 26(8): 3321-3335.10.1096/fj.12-208751Search in Google Scholar PubMed
[204] Chappuis S, Ripperger J A, Schnell A, et al. Role of the circadian clock gene Per2 in adaptation to cold temperature. Mol Metab, 2013; 2(3): 184-193.10.1016/j.molmet.2013.05.002Search in Google Scholar PubMed PubMed Central
[205] Gerhart-Hines Z, Feng D, Emmett M J, et al. The nuclear receptor Rev-erbα controls circadian thermogenic plasticity. Nature, 2013; 503(7476): 410-413.10.1038/nature12642Search in Google Scholar PubMed PubMed Central
[206] Woldt E, Sebti Y, Solt L A, et al. Rev-erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat Med, 2013; 19(8): 1039-1046.10.1038/nm.3213Search in Google Scholar PubMed PubMed Central
[207] Moraes M N, Mezzalira N, de Assis L V M, et al. TRPV1 participates in the activation of clock molecular machinery in the brown adipose tissue in response to light-dark cycle. Biochim Biophys Acta Mol Cell Res, 2017; 1864(2): 324-335.10.1016/j.bbamcr.2016.11.010Search in Google Scholar PubMed
[208] Zhu Y, Liu Y, Escames G, et al. Deciphering clock genes as emerging targets against aging. Ageing Res Rev, 2022; 81: 101725.10.1016/j.arr.2022.101725Search in Google Scholar PubMed
[209] Zhang Z, Cheng L, Ma J, et al. Chronic cold exposure leads to daytime preference in the circadian expression of hepatic metabolic genes. Front Physiol, 2022; 13: 865627.10.3389/fphys.2022.865627Search in Google Scholar PubMed PubMed Central
[210] Ma S, Yu H, Zhao Z, et al. Activation of the cold-sensing TRPM8 channel triggers UCP1-dependent thermogenesis and prevents obesity. J Mol Cell Biol, 2012; 4(2): 88-96.10.1093/jmcb/mjs001Search in Google Scholar PubMed
[211] Straat M E, Martinez-Tellez B, Sardjoe Mishre A, et al. Cold-induced thermogenesis shows a diurnal variation that unfolds differently in males and females. J Clin Endocrinol Metab, 2022; 107(6): 1626-1635.10.1210/clinem/dgac094Search in Google Scholar PubMed PubMed Central
[212] Sellers A J, Khovalyg D, Plasqui G, et al. High daily energy expenditure of Tuvan nomadic pastoralists living in an extreme cold environment. Sci Rep, 2022; 12(1): 20127.10.1038/s41598-022-23975-3Search in Google Scholar PubMed PubMed Central
[213] Kaznacheev V P, Panin L E, Kovalenko L A. Urgent problems of rational nutrition for the immigrant population in Arctic regions and for the natives of the North. Vopr Pitan, 1980; (1): 23-27.Search in Google Scholar
[214] Gibas-Dorna M, Checinska Z, Korek E, et al. Variations in leptin and insulin levels within one swimming season in non-obese female cold water swimmers. Scand J Clin Lab Invest, 2016; 76(6): 486-491.10.1080/00365513.2016.1201851Search in Google Scholar PubMed
[215] Mengel L A, Seidl H, Brandl B, et al. Gender differences in the response to short-term cold exposure in young adults. J Clin Endocrinol Metab, 2020; 105(5): dgaa110.10.1210/clinem/dgaa110Search in Google Scholar PubMed
[216] Sun L, Yan J, Goh H J, et al. Fibroblast growth factor-21, leptin, and adiponectin responses to acute cold-induced brown adipose tissue activation. J Clin Endocrinol Metab, 2020; 105(3): e520-531.10.1210/clinem/dgaa005Search in Google Scholar PubMed PubMed Central
[217] Tomasik P J, Sztefko K, Pizon M. The effect of short-term cold and hot exposure on total plasma ghrelin concentrations in humans. Horm Metab Res, 2005; 37(3): 189-190.10.1055/s-2005-861296Search in Google Scholar PubMed
[218] Schmidek W R, Hoshino K, Schmidek M, et al. Influence of environmental temperature on the sleep-wakefulness cycle in the rat. Physiol Behav, 1972; 8(2): 363-371.10.1016/0031-9384(72)90384-8Search in Google Scholar PubMed
[219] Valatx JL, Roussel B, Curé M. Sleep and cerebral temperature in rat during chronic heat exposure. Brain Res, 1973; 55(1): 107-122.10.1016/0006-8993(73)90491-5Search in Google Scholar PubMed
[220] Fischl H, McManus D, Oldenkamp R, et al. Cold-induced chromatin compaction and nuclear retention of clock mRNAs resets the circadian rhythm. EMBO J, 2020; 39(22): e105604.10.15252/embj.2020105604Search in Google Scholar PubMed PubMed Central
[221] Mahapatra A P K, Mallick H N, Kumar V M. Changes in sleep on chronic exposure to warm and cold ambient temperatures. Physiol Behav, 2005; 84(2): 287-294.10.1016/j.physbeh.2004.12.003Search in Google Scholar PubMed
© 2024 Tengyu Zhao, Yifu Ma, Jian Zhang, Xiaojie Zhou, Yanyan Zhou, Jingdong Yan, published by De Gruyter on behalf of Heilongjiang Health Development Research Center
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review
- Impacts of cold exposure on energy metabolism
- Cryoablation techniques in bladder cancer: A review
- Cold environments and human metabolism: A traditional chinese medicine perspective
- Original Article
- Toll-like receptors 2 polymorphism is associated with psoriasis: A case-control study in the northern Chinese population
- Vitamin D deficiency and increased inflammatory factor intercellular cell adhesion molecule-1 indicate severe leukoaraiosis in northern China
- PLA2G2D and CHIT1: Potential biomarkers for immune infiltration and prognosis in cervical squamous cell carcinoma
- Characteristics of gut microbiota in anastomotic leakage patients in cold zones post-colorectal cancer surgery: A high-throughput sequencing and propensity-score matching study
Articles in the same Issue
- Frontmatter
- Review
- Impacts of cold exposure on energy metabolism
- Cryoablation techniques in bladder cancer: A review
- Cold environments and human metabolism: A traditional chinese medicine perspective
- Original Article
- Toll-like receptors 2 polymorphism is associated with psoriasis: A case-control study in the northern Chinese population
- Vitamin D deficiency and increased inflammatory factor intercellular cell adhesion molecule-1 indicate severe leukoaraiosis in northern China
- PLA2G2D and CHIT1: Potential biomarkers for immune infiltration and prognosis in cervical squamous cell carcinoma
- Characteristics of gut microbiota in anastomotic leakage patients in cold zones post-colorectal cancer surgery: A high-throughput sequencing and propensity-score matching study

