Abstract
An invasive mealybug (Hemiptera: Coccomorpha: Pseudococcidae) was first detected and identified in Florida in 2019 as Phenacoccus sisymbriifolium Granara de Willink. This species was described from Uruguay in 2007 on Solanum sisymbriifolium Lam. (Solanaceae) and Florida specimens largely matched the description. However, new morphological and molecular evidence supports that this species is Phenacoccus miruku Tanaka & Choi, recently described from Japan in 2022. P. miruku is presumed Neotropical or Nearctic in origin and invasive in Japan. We discuss the issues around the taxonomic identities of these species and for each give diagnoses. An available list of host plants, a current distribution map, notes on ecological associates, images of live specimens in the field, and a key to the species of Phenacoccus in Florida also are provided. Since its detection, this mealybug has been widely found throughout 20 Florida counties with a continuously expanding host list. It is currently most common on roadside weeds such as Bidens alba (L.) DC. and Ambrosia artemisiifolia L. (Asteraceae) but it has recently been identified on cultivated crops such as tomato (Solanum lycopersicum L.), eggplant (Solanum melongena L.), naranjilla (Solanum quitoense Lam.), peppers (Capsicum L.) (Solanaceae), and sweet potato (Ipomoea batatas L.) (Convolvulaceae). This paper serves to provide information on this emerging mealybug pest, give resources for its identification, and facilitate detection and management.
Resumen
Una cochinilla invasora (Hemiptera: Coccomorpha: Pseudococcidae) fue detectada e identificada por primera vez en Florida en 2019 como Phenacoccus sisymbriifolium Granara de Willink. Esta especie fue descrita en Uruguay en 2007 en Solanum sisymbriifolium Lam. (Solanaceae) y especímenes de Florida coincidían en gran medida con esta descripción. Sin embargo, nueva evidencia morfológica y molecular respalda que esta especie es Phenacoccus miruku Tanaka & Choi, descrita recientemente en Japón en 2022. Se presume que P. miruku es de origen neotropical o neártico e invasivo en Japón. En este estudio, discutimos temas relacionados con las identidades taxonómicas de estas especies y damos diagnósticos para cada una. También se proporciona una lista disponible de plantas hospedantes, un mapa de distribución actual, notas sobre acompañantes ecológicos, imágenes de especímenes vivos en el campo y una clave para las especies de Phenacoccus en Florida. Desde su detección, esta cochinilla se ha encontrado en 20 condados de Florida con una lista de huéspedes en continua expansión. Actualmente es más común en las malezas al borde de las carreteras con Bidens alba (L.) DC. y Ambrosia artemisiifolia L. (Asteraceae) pero recientemente se ha identificado en cultivos como tomate (Solanum lycopersicum L.), berenjena (Solanum melongena L.), naranjilla (Solanum quitoense Lam.), pimiento (Capsicum L.) (Solanaceae) y batata (Ipomoea batatas L.) (Convolvulaceae). Este documento sirve para proporcionar información sobre esta emergente plaga de cochinilla, brindar antecedentes para su identificación, y facilitar su detección y manejo.
1 Introduction
Numerous pest mealybugs (Hemiptera: Coccomorpha: Pseudococcidae) have invaded Florida in the last two decades. These include species such as the bamboo specialist Palmicultor lumpurensis (Takahashi), pink hibiscus mealybug Maconellicoccus hirsutus (Green), Lebbeck mealybug Nipaecoccus viridis (Newstead), passionvine mealybug Planococcus minor (Maskell), and Phenacoccus multicerarii Granara de Willink (Hodges 2002; Hodges and Hodges 2004; Stocks 2012; Stocks and Hodges 2010; Stocks and Roda 2011). Florida’s many ports of entry, booming agricultural trade, and subtropical climate make the state particularly susceptible to invasive species (Frank and McCoy 1995; Frank and Thomas 2004; Skelley et al. 2024). In 2019, unknown mealybugs were found on the roots of B. alba (L.) DC. (Asteraceae), a common roadside perennial, in Lake County, Florida (Ahmed and Miller 2019) (Figures 1 and 2a). In subsequent months, this mealybug was collected repeatedly, quickly expanding its known Florida range and list of host plants. This Phenacoccus species joins five congenerics in Florida, all of which are polyphagous and can reach economically damaging thresholds including Phenacoccus madeirensis Green, P. multicerarii Granara de Willink, Phenacoccus parvus Morrison, Phenacoccus solani Ferris, and Phenacoccus solenopsis Tinsley. The newly invasive species was originally identified as P. sisymbriifolium Granara de Willink (Ahmed and Miller 2019). Phenacoccus sisymbriifolium was first described in 2007 from Uruguay on Solanum sisymbriifolium Lam. (Solanaceae) from a single series of specimens collected in 1943 (Granara de Willink and Szumik 2007). Virtually nothing is known about its biology in its South American range.

Phenacoccus miruku Tanaka & Choi in-situ. (a) An adult female Phenacoccus miruku Tanaka & Choi on Physalis heterophylla Nees. (b, e) Adult females with ovisacs and nymphs on the roots of Bidens alba (L.) DC. (c) Infestation on Solanum quitoense Lam. fruit and (d) leaves. Photographs by (a) Erin Powell, (b, e) Lyle Buss, (c) Alexander Tasi and Victoria Benjamin, and (d) Tavia Gordon.

The six Phenacoccus species present in Florida in-situ. Note the similarities and how it is necessary to slide-mount specimens for species-level identification. (a) Phenacoccus miruku Tanaka & Choi, (b) Phenacoccus parvus Morrison, (c) Phenacoccus madeirensis Green, (d) Phenacoccus multicerarii Granara de Willink, (e) Phenacoccus solani Ferris, and (f) Phenacoccus solenopsis Tinsley. Photographs (a, c, d, e) by Erin Powell and (b, f) by Lyle Buss.
A newly described species from Japan, P. miruku Tanaka & Choi, prompted us to reevaluate the identification of the specimens previously reported as P. sisymbriifolium in Florida. P miruku is presumed to be adventive to Japan, Neotropical or Nearctic in origin, and at the time of its description was known only from Bidens pilosa var. radiata L. (Asteraceae) (Tanaka et al. 2022). DNA sequences supported that what had been known as P. sisymbriifolium in Florida was a genetic match to P. miruku. Upon borrowing paratype specimens for examination, it became clear that the new Florida Phenacoccus reported in 2019 was also a morphological match to P. miruku. Further complicating matters, the type material of P. sisymbriifolium is unavailable because of strict export regulations in Argentina where the types are deposited. No additional material of the species has been collected in 80 years and collaborators in South America indicate that recollection is difficult or impossible (Pacheco da Silva et al. 2020; pers. comm. Vitor Pacheco da Silva, July 2023). Therefore, our best strategy is to use the information available currently to determine the correct identity of P. miruku and P. sisymbriifolium.
In this paper, we discuss the morphology of P. miruku and P. sisymbriifolium, give updated species diagnoses, provide DNA sequence data, and describe the geographic distribution, ecological associations, and host data from observations compiled since the first detection of this mealybug. We also provide a key to slide-mounted adult females of the six species of Phenacoccus present in Florida.
2 Materials and methods
2.1 Morphology
We examined 90 adult female specimens of P. miruku from 13 counties in Florida, USA, from 15 host plants, which are deposited in the Florida State Collection of Arthropods (FSCA). These specimens were previously reported as P. sisymbriifolium in Ahmed and Miller (2019), the Florida Department of Agriculture and Consumer Services, Division of Plant Industry (FDACS-DPI) database (FDACS-DPI 2024), and FDACS-DPI’s Tri-ology reports through 2023.
The following Florida P. miruku material was examined: USA: FLORIDA: Alachua County: Gainesville, 17 July 2023, on Coreopsis sp. (Asteraceae), L. Line (2023-07275) (two adult ♀♀ on two slides); Gainesville, 2 April 2022, on Geranium carolinianum L. (Geraniaceae), J. Brambila (2022-02838) (three adult ♀♀ on three slides); Gainesville, 3 September 2020, on unknown host, G. Ouwinga, J. Allen, L. Deeter, M. Ahmed (2020-3411) (eight adult ♀♀ on eight slides); Brevard County: Melbourne, 29 September 2023, on Solanum quitoense Lamarck (Solanaceae), A. Tasi, V. Benjamin (2023-10088) (two adult ♀♀ on two slides); Melbourne, 5 October 2023, on I. batatas (L.) Lam. (Convolvulaceae), A. Tasi, V. Benjamin (2023-10383) (one adult ♀ on one slide); Melbourne, 5 October 2023, on S. quitoense, A. Tasi, V. Benjamin (2023-10393) (one adult ♀ on one slide); Melbourne, 23 October 2023, on Solanum melongena L. (Solanaceae), A. Tasi, V. Benjamin (2023-11004) (two adult ♀♀ on two slides); Melbourne, 12 April 2024, on Solanum lycopersicum L. (Solanaceae), A. Tasi, V. Benjamin (2024-03643) (one adult ♀ on one slide); Broward County: Hollywood, 13 March 2023, on unknown Compositae, M. Zenoble, R. Tordi (2023-02544) (one adult ♀ on one slide); Dixie County: Old Town, 20 March 2023, on Physalis heterophylla Nees (Solanaceae), A. Barrios (2023-02586) (two adult ♀♀ on two slides); Hernando County: Brooksville, 8 January 2020, on Gomphrena serrata L. (Amaranthaceae), N. Marquez (2020-79) (one adult ♀ on one slide); Brooksville, 29 January 2020, on G. serrata, N. Marquez (2020-417) (one adult ♀ on one slide); Lake County: Eustis, 8 January 2019, on B. alba (L.) DC. (Astaraceae), N. Marquez (2019-93) (two adult ♀♀ on two slides); Eustis, 29 January 2019, on B. alba, N. Marquez (2019-322) (three adult ♀♀ on three slides); Eustis, 12 March 2019, on B. alba, N. Marquez (2019-1068) (one adult ♀ on one slide); Eustis, 12 March 2019, on B. alba, N. Marquez (2019-1069) (one adult ♀ on one slide); Clermont, 26 March 2019, on B. alba, N. Marquez (2019-1410) (two adult ♀♀ on two slides); Eustis, 26 March 2019, on B. alba, N. Marquez (2019-1411) (six adult ♀♀ on six slides); Eustis, 2 April 2019, on B. alba, N. Marquez (2019-1596) (two adult ♀♀ on two slides); Fruitland Park, 3 April 2019, on B. alba, N. Marquez (2019-1625) (three adult ♀♀ on three slides); Tavares, 3 April 2019, on B. alba, N. Marquez (2019-1622) (three adult ♀ on three slides); Tavares, 3 April 2019, on B. alba, N. Marquez (2019-1616) (three adult ♀♀ on three slides); Mount Dora, 8 April 2019, on B. alba, N. Marquez (2019-1748) (three adult ♀♀ on three slides); Grand Island, 11 April 2019, on B. alba, N. Marquez (2019-1841) (two adult ♀♀ on two slides); Clermont, 17 April 2019, on Gamochaeta antillana (Urb.) Anderb. (Asteraceae), N. Marquez (2019-2068) (one adult ♀ on one slide); Tavares, 18 April 2019, on B. alba, N. Marquez (2019-2066) (two adult ♀♀ on two slides); Astatula, 19 April 2019, on A. artemisiifolia L. (Asteraceae), N. Marquez (2019-2142) (one adult ♀ on one slide); Eustis, 22 April 2019, on G. antillana, Marquez (2019-2174) (three adult ♀♀ on three slides); Tavares, 24 April 2019, on B. alba, N. Marquez (2019-2207) (one adult ♀ on one slide); Mount Dora, 6 May 2019, on G. antillana, N. Marquez (2019-2494) (two adult ♀♀ on two slides); Fruitland Park, 17 June 2020, on B. alba, N. Marquez (2020-2369) (three adult ♀♀ on three slides); Clermont, 18 April 2022, on B. alba, N. Marquez (2022-03335) (two adult ♀♀ on two slides); Clermont, 8 March 2022, on Baccharis halimifolia L. (Asteraceae), B. Danner (2022-01954) (two adult ♀♀ on two slides); Eustis, 17 May 2022, on Achillea sp. (Asteraceae), M. Sellers (2022-05621) (two adult ♀♀ on two slides); Manatee County: Parrish, 13 May 2023, on B. alba, C. Poock (2023-05078) (five adult ♀♀ on five slides); Marion County: Dunnellon, 26 August 2022, on unknown host, T. Deuel (2022-07883) (one adult ♀ on one slide); Orange County: Apopka, 3 October 2020, on B. alba, G. Ouwinga, L. Deeter, M. Ahmed (2020-3889) (two adult ♀♀ on two slides); Palm Beach County: Lake Worth, 17 March 2023, on Bidens sp. (Asteraceae), D. Miller (2023-02641) (one adult ♀ on one slide); St. Johns County: St. Augustine, 9 May 2019, on Gnaphalium sp. (Asteraceae), L. Deeter (2019-3077) (two adult ♀♀ on two slides); Sumter County: Sumterville, 5 April 2019, on B. alba, N. Marquez (2019-1744) (one adult ♀ on one slide); Sumterville, 5 April 2019, on A. artemisiifolia, N. Marquez (2019-1747) (two adult ♀♀ on two slides); Volusia County: Tomoka Springs, 17 March 2022, on Ruellia blechum L. (Acanthaceae), N. Marquez (2022-02236) (one adult ♀ on one slide).
We also examined a series of seven adult female paratype specimens of P. miruku borrowed from Ehime University Museum, Matsuyama, Japan with the following verbatim label data: JAPAN,/ Okinawa prefecture,/ Okinawa Is. Ogimi-son,/ on B. pilosa/ var. radiata,/ 26. vi.2021,/ coll. J. Choi (seven adult ♀♀ on seven slides). ‘/’ indicates the positions of the line breaks on the label. Furthermore, we have contacted Dr. Cristina Granara de Willink (pers. comm., November 2023) and she has provided information based on her examination of the type series of P. sisymbriifolium with the following label data: URUGUAY: Montevideo, I-1943, on Solanum sisymbriifolium, A. Silveira (eight adult ♀).
2.2 Molecular methods
Whole specimens of P. miruku (Florida: Alachua: Gainesville, 3 April 2022, on B. alba, D. Miller, 2022-02798; Florida: Lake County: Clermont, 18 April 2022, on B. alba, N. Marquez, 2022-03335) were non-destructively DNA extracted with a Qiagen DNeasy Blood and Tissue Kit (Hilden, Germany). Voucher specimens were slide-mounted and deposited in the FSCA. The polymerase chain reactions (PCRs) were conducted as 25 µl reactions with the Kapa HiFi HotStart ReadyMix Kit (Basel, Switzerland). Primer sets and thermocycler conditions for 18S, 28S D10, 28S D2, and COI-5′ followed the methods in Ahmed et al. (2020), except for an additional COI-3′ fragment. This PCR was conducted with reverse complement of HCO2198 (Folmer et al. 1994) and TL2-N-3014 (Simon et al. 1994). The thermocycler conditions for this primer pair was as follows: 1) 95 °C for 2 min, [32× steps 2–4] 2) 98 °C for 30 s, 3) 50 °C for 30 s, 4) 72 °C for 75 s, 5) 72 °C for 7 min, 6) 4 °C indefinite hold. PCR products were visualized by gel electrophoresis and purified for bidirectional sequencing. All PCRs targeting the COI-5′ barcode region failed in these specimens. Sanger sequencing was conducted on the ABI SeqStudio platform with BigDye Terminator v3.1 chemistry. Sequence chromatograms were trimmed and assembled in Geneious Prime 2023.2.1. New sequences were queried to the GenBank nucleotide database by MegaBLAST searches (Altschul et al. 1990). Newly generated sequences were deposited in GenBank (COI: PP503189, 18S: PP503320, 28S D2: PP532812, 28S D10: PP532813). Highly similar sequences returned from MegaBLAST searches were downloaded for further comparison. Sequences were aligned using the default settings of MUSCLE (Edgar 2004) as implemented in MEGA7 (Kumar et al. 2016) and trimmed to 100 % data coverage. Sequence similarity was then calculated using p-distance for each target in MEGA7 (Kumar et al. 2016).
3 Results
3.1 Morphology
P. miruku diagnosis based on seven adult female paratype specimens from Japan: with 18 pairs of cerarii; nine-segmented antennae; 1–6 dorsal multilocular pores on margins of abdomen; ventral multilocular pores extending to margin of abdomen, on abdominal segments III–VIII; 0–3 quinquelocular pores from widest part of clypeus up to anterior margin of head; quinquelocular pores abundant on thorax, on abdominal segments II–V, present in anterior row on abdominal segment V only; two sizes of ventral oral collar tubular ducts; dorsal oral collar tubular ducts present marginally from abdominal segment VIII to prothorax or head, a few also present medially on thorax; small translucent pores on each hind tibia; and circulus present or absent, highly variable in shape and size, sometimes split into two circular pieces.
P. miruku diagnosis based on 90 adult females from Florida: with 18 pairs of cerarii; nine-segmented antennae; 0–10 dorsal multilocular pores on margins of abdomen; ventral multilocular pores extending to margin of abdomen, on abdominal segments III–VIII; 0–5 quinquelocular pores from widest part of clypeus up to anterior margin of head; quinquelocular pores abundant on thorax, on abdominal segments II–V, present in anterior row on abdominal segment V only; two sizes of ventral oral collar tubular ducts; dorsal oral collar tubular ducts present marginally from abdominal segment VIII to prothorax or head, a few present medially on thorax; small translucent pores on each hind tibia; and circulus present or absent, highly variable in shape and size, sometimes split into two circular pieces.
P. sisymbriifolium diagnosis (translated and adapted from Granara de Willink and Szumik 2007): with 18 pairs of cerarii; nine-segmented antennae; 0–2 dorsal multilocular pores on margins of abdomen; ventral multilocular pores extending to margin of abdomen, on abdominal segments III–VIII; about 32 quinquelocular pores ahead of mouthparts, 0–16 quinquelocular pores from widest part of clypeus up to anterior margin of head (pers. comm. Cristina Granara de Willink, November 2023); quinquelocular pores abundant on thorax, on abdominal segments II–VII; two sizes of ventral oral collar tubular ducts; dorsal oral collar tubular ducts present marginally and submarginally from abdominal segment VIII to prothorax or head, absent medially; small translucent pores on each hind tibia; and with large, anvil-shaped circulus.
`When we examined the paratype specimens of P. miruku, we observed quinquelocular pores on the abdomen as far posteriorly as the anterior area of abdominal segment V; small, inconspicuous translucent pores on the hind tibiae typical of Phenacoccus; a few dorsal oral collar tubular ducts in the medial area of the thorax, and two sizes of ventral oral collar tubular ducts. That is, we found that specimens of P. miruku were morphologically more similar to the description of P. sisymbriifolium in Granara de Willink and Szumik (2007). However, differences remain between P. miruku and P. sisymbriifolium as described in Granara de Willink and Szumik (2007), including in the form of the circulus (variable vs. large anvil-shaped), the distribution of the quinquelocular pores (to the anterior row of abdominal segment V vs. to abdominal segment VII), the number of quinquelocular pores anterior to the widest part of the clypeus (consistently 0–5 in P. miruku and more variable in P. sisymbriifolium (0–16)), and the dorsal oral collar tubular ducts (present on medial area of thorax vs. absent from this area). We found that the shape of the circulus in Florida specimens is highly variable, ranging from small to large oval, or oxbow shaped, or split into two separate circular pieces, or even absent in some specimens, fitting the description of P. miruku. Quinquelocular pores extended from the head as far posteriorly as the anterior area of abdominal segment V in P. miruku paratypes and Florida specimens. Finally, a few dorsal oral collar tubular ducts in the medial area of the thorax were found to be present on the paratypes of P. miruku and on our Florida material.
P. miruku is also similar morphologically and molecularly to P. parvus, a species that was first detected in Florida in 1983 (Williams and Hamon 1994). P. miruku differs by having (character states of P. parvus are presented in parentheses): zero to two marginal oral collar tubular ducts laterad of the antennae (a cluster of four or more oral collar tubular ducts laterad of the antennae); zero to two marginal oral collar tubular ducts laterad of the anterior spiracle (a cluster of four or more marginal oral collar tubular ducts laterad of the anterior spiracle); quinquelocular pores as far posteriorly as abdominal segment IV or the anterior of V (quinquelocular pores as far posteriorly abdominal segment VI); 0–5 quinquelocular pores on the head (numerous quinquelocular pores on the head); ventral multilocular pores not reaching the body margins (ventral multilocular pores reaching the body margin); and dorsal multilocular pores absent (dorsal multilocular pores present marginally).
P. miruku is also similar morphologically and molecularly to P. montevidensis Pacheco da Silva & Kaydan, a species recently described from Uruguay (Pacheco da Silva et al. 2020). P. miruku differs by having (character states of P. montevidensis are presented in parentheses): dorsal multilocular pores restricted to the margin of the abdomen (dorsal multilocular pores in the medial areas of the posterior abdominal segment VI and VII) and dorsal lanceolate setae with typical acute apices (some dorsal lanceolate setae with a protrusion on the apex).
All of the type specimens of P. sisymbriifolium are currently still in Argentina and could not be examined by the authors. The species description states that P. sisymbriifolium has about 32 quinquelocular pores anterior to the mouthparts (Granara de Willink and Szumik 2007), however it was unclear whether this meant starting at the posterior part of the labrum and everything above it, or only from the anterior of the clypeus to the anterior margin of the head. Direct communication with the author of P. sisymbriifolium shed light on this character and the variability of the number of quinquelocular pores around the mouthparts in this species. From the widest part of the clypeus and anterior to the mouthparts, the holotype has 14 quinquelocular pores, one paratype has 16 quinquelocular pores, and the other six paratypes have 0–3 quinquelocular pores in this area (pers. comm. Cristina Granara de Willink, November 2023).
3.2 Molecular analysis
BLAST searches of COI-3′ of the Florida specimens recovered the highest sequence similarity of 99.68 % to Japanese specimens of P. miruku (ON533756) and P. parvus (GU134696 and KJ530609). These COI sequences differed by a single nucleotide polymorphism. The Florida sample was unique among all available Phenacoccus COI sequences in GenBank. BLAST searches of 18S of the Florida specimens recovered 100 % matches to Phenacoccus manihoti Matile-Ferrero and P. parvus, with P. miruku inexplicably absent from the returned results. The 18S sequence from Japanese P. miruku (ON527953) contains two gap regions that were trimmed out during assembly (pers. comm. Jinyeong Choi, August 2023). Comparison between the untrimmed Japanese 18S sequence demonstrated a 100 % match with the Florida population (pers. comm. Jinyeong Choi, August 2023). BLAST searches of 28S D2 of the Florida specimens recovered 100 % matches to several specimens of P. manihoti, P. miruku, and a Phenacoccus sp. (MW251837). BLAST searches of 28S D10 recovered only one high match to a specimen of P. parvus, from which it differed by a single nucleotide polymorphism.
3.3 Ecological associates and distribution
P. miruku is frequently found in association with the red imported fire ant, Solenopsis invicta Buren (Hymenoptera: Formicidae). On B. alba, P. miruku has been found in mixed infestations with solanum mealybug, P. solani (05152023-05078; 04252024-04239) and in mixed infestations with trochanter mealybug, Pseudococcus sorghiellus (Forbes) (10032022-08824). P. miruku also has been found in mixed infestations with citrus mealybug, Planococcus citri (Risso), on three plant hosts: A. artemisiifolia (2019-2142), S. quitoense (10052023-10393), and S. lycopersicum (04152024-03643). Evidence of parasitism has been noted but the specific wasps attacking this species have not yet been identified. In two instances, predatory flies in the family Chamaemyiidae, which specialize on mealybugs, scales, or aphids, were found with infestations of P. miruku (10262021-5969). Five specimens of chamaemyiid flies that emerged from a mixed infestation of P. miruku and P. citri on S. quitoense were identified as Leucopina bella (Loew) (10052023-10393) (Figure 3). The L. bella specimens are deposited in the FSCA.

Leucopina bella (Loew) (Diptera: Chamaemyiidae) reared from a mixed infestation of Phenacoccus miruku and Planococcus citri on Solanum quitoense Lam. from Brevard County, Florida. Photograph by Erin Powell.
P. miruku has been recorded in the landscape in the following 20 Florida counties: Alachua, Brevard, Broward, Collier, Dixie, Hernando, Hillsborough, Indian River, Lake, Marion, Okaloosa, Orange, Palm Beach, Pasco, Polk, Putnam, St. Johns, St. Lucie, Sumter, and Volusia (Figure 4). P. miruku has been found feeding on 25 host plants belonging to 10 families in Florida (Table 1). To date, the most common host is B. alba.
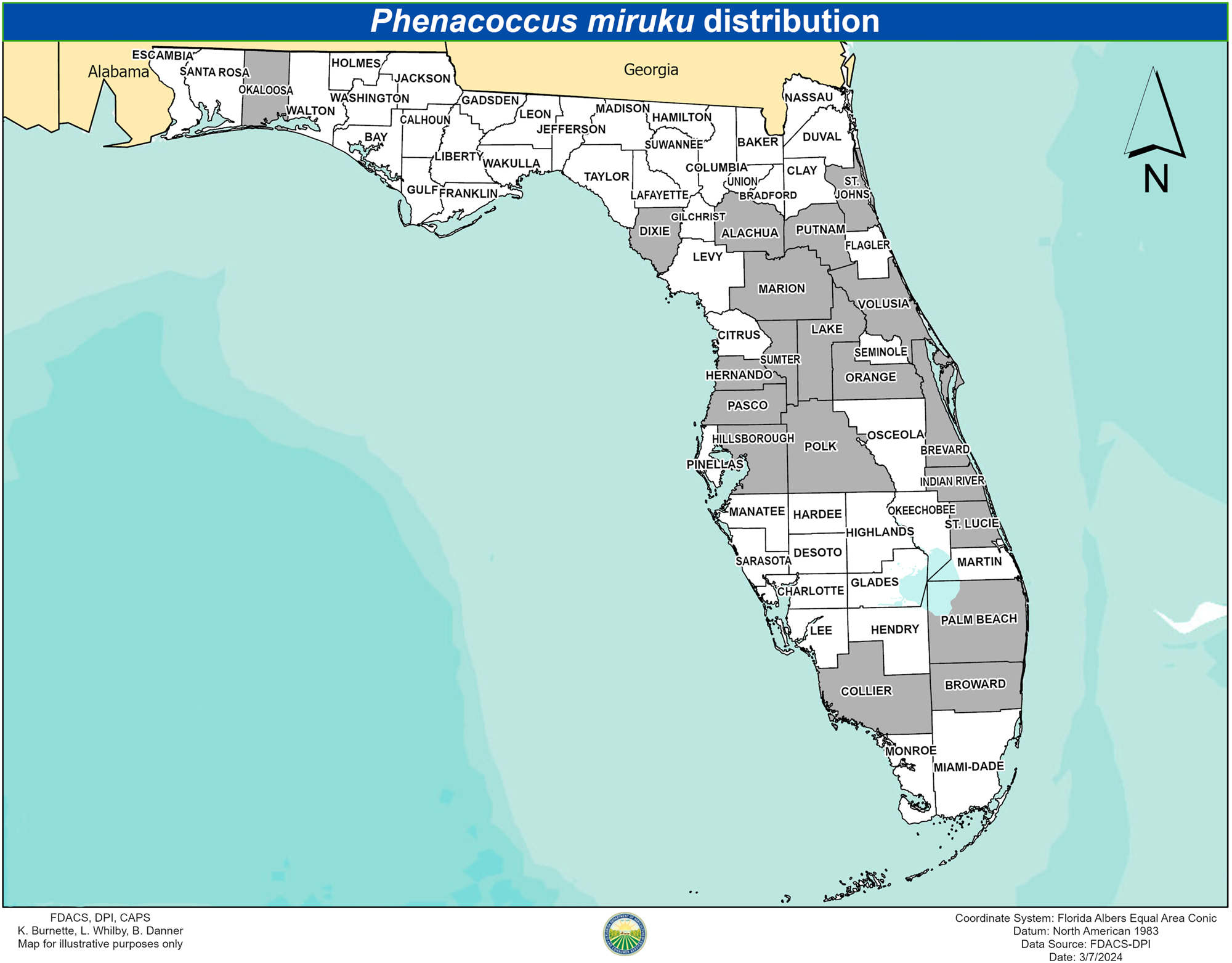
Map of Florida with recorded Phenacoccus miruku distribution (counties shaded in grey) from 2019 to 2024. Map created by K. Burnette.
Host records for Phenacoccus miruku in Florida from 2019 to 2024, Florida Department of Agriculture and Consumer Services, Division of Plant Industry (FDACS-DPI) database (FDACS-DPI 2024).
| Plant family | Plant species | Common name(s) | Number of recordsa |
|---|---|---|---|
| Acanthaceae | Ruellia blechum L. | Green shrimp plant; Browne’s blechum | 1 |
| Amaranthaceae | Gomphrena serrata L. | Prostrate globe amaranth | 3 |
| Asteraceae | Achillea sp. | Yarrow | 1 |
| Ambrosia artemisiifolia L. | Common ragweed; annual ragweed; low ragweed | 12 | |
| Baccharis halimifolia L. | Eastern baccharis; groundsel bush; sea myrtle; saltbush | 1 | |
| Bidens alba (L.) DC. | Shepherd’s needles; beggarticks; Spanish needles | 72 | |
| Coreopsis sp. | Calliopsis; tickseed | 1 | |
| Eupatorium capillifolium (Lam.) Small | Dogfennel | 1 | |
| Gamochaeta antillana (Urb.) Anderb. | The delicate everlasting | 3 | |
| Gnaphalium sp. | Cudweed | 1 | |
| Convolvulaceae | Ipomoea batatas (L.) Lam. | Sweet potato | 1 |
| Euphorbiaceae | Euphorbia cyathophora Murray | Dwarf poinsettia; fire-on-the-mountain; paintedleaf | 1 |
| Geraniaceae | Geranium carolinianum L. | Carolina crane’s-bill; Carolina geranium | 1 |
| Lamiaceae | Plectranthus sp. | Spur-flower | 1 |
| Malvaceae | Sida ulmifolia Mill. | Common wireweed; common fanpetals | 2 |
| Rubiaceae | Gardenia jasminoides J. Ellis | Gardenia; cape jasmine | 1 |
| Solanaceae | Capsicum sp. | Chili pepper; bell pepper | 1 |
| Physalis heterophylla Nees | Clammy groundcherry | 1 | |
| Solanum lycopersicum L. | Tomato | 1 | |
| Solanum melongena L. | Eggplant, aubergine | 1 | |
| Solanum torvum Sw. | Pendejera; Turkey berry; devil’s fig; pea eggplant | 1 | |
| Solanum quitoense Lam. | Naranjilla; lulo | 2 |
-
aNote that specimens were previously identified as Phenacoccus sisymbriifolium in the FDACS-DPI database, Ahmed and Miller (2019), and all Tri-ology reports through 2023.
3.4 A key to the slide-mounted adult females of Phenacoccus species in Florida
*Note that P. solani and P. solenopsis can be difficult to distinguish morphologically and a combination of character states using multiple specimens may be required. We have arranged character states in the couplet in order of diagnostic reliability based on our analysis of Florida specimens and that of Williams (2004), Hodgson et al. (2008) and Zhao et al. (2014).
(0) Dorsum with multilocular pores … 2
Dorsum without multilocular pores … 4
(1) Dorsal multilocular pores forming rows across some abdominal segments … 3
Dorsal multilocular pores restricted to body margins … P. miruku Tanaka & Choi (in part)
(2) Ventral quinquelocular pores absent … P henacoccus multicerarii Granara de Willink
Ventral quinquelocular pores present … Phenacoccus madeirensis Green
(1) Quinquelocular pores present … 5
Quinquelocular pores absent … 6
(4) Ventral multilocular pores absent from body margin; with conspicuous cluster of four or more marginal oral collar tubular ducts laterad of anterior spiracle … Phenacoccus parvus Morrison
Ventral multilocular pores present near body margin; with 0–2 marginal oral collar tubular ducts laterad of anterior spiracle … P. miruku Tanaka & Choi (in part)
(4) Ventral multilocular pores on abdominal segment VII present in row along posterior edge only; circulus usually small, rounded; antennae usually 8-segmented … Phenacoccus solani Ferris
Ventral multilocular pores on segment VII present between anterior and posterior margins; circulus usually large, more flaccid; antennae usually 9-segmented … Phenacoccus solenopsis Tinsley
4 Discussion
Here we report morphological, molecular, and ecological data for P. miruku, an emerging polyphagous pest species in Florida. We were limited in this study by our inability to directly examine the type specimens of P. sisymbriifolium from Uruguay and by highly conserved DNA sequence data. Sequence data for the invasive Phenacoccus species in Florida perfectly matched 18S and 28S D2 of P. miruku from Japan and when type specimens were examined, Florida specimens also matched P. miruku morphologically. The relatedness of the hosts on which both were first detected in their invasive range also is notable. P. miruku was first detected on B. pilosa var. radiata in Japan and the Phenacoccus species in Florida was first detected on B. alba. Bidens alba is treated as a junior synonym of B. pilosa by several authors and this species is native to the neotropics, likely introduced to both Florida and Japan (Ballard 1986; Strother and Weedon 2020; Weakley et al. 2023; Wunderlin et al. 2023).
Species of Phenacoccus often differ in seemingly minor ways morphologically as exemplified by the differences between P. gossypii Townsend & Cockerell and P. madeirensis (P. gossypii with more than 70 multilocular pores on the pro- and mesothorax and P. madeirensis with five or fewer) and by P. manihoti and P. herreni Cox & Williams. P. manihoti, which in life is pink in color and parthenogenetic, has 32–68 quinquelocular pores on the head while P. herreni, which is yellow in life and reproduces sexually, has only 0–20 (Cox and Williams 1981). The case for P. manihoti and P. herreni being distinct species is supported by the fact that parasitoid wasps that attack one fail to develop in the other (Cox and Williams 1981). The presence of three distinct differences, quinquelocular pore distribution, dorsomedial oral collars, and the form of the circulus, between P. sisymbriifolium and P. miruku provides evidence that they are separate species given our current understanding of this genus.
Though there are few differences between the description of P. sisymbriifolium and the paratype slides of P. miruku, these differences are significant provided they are consistent and hold true when the P. sisymbriifolium type material is examined. Overall, the polyphyletic Phenacoccinae requires revision using a combination of morphological and molecular tools given the importance of this group as worldwide pests (Choi and Lee 2022; Tanaka et al. 2022). COI, 18S, and 28S alone may not be enough to untangle the relationships amongst pseudococcids, or even reliably diagnose the species in certain sections of Phenacoccus diversity. Additional fast-evolving genes, such as primary endosymbiont sequences, should be evaluated from members of the P. madeirensis + P. parvus + P. solani + P. solenopsis clade recovered by Choi and Lee (2022).
P. miruku has been found on tomato (S. lycopersicum), eggplant (S. melongena), naranjilla (S. quitoense) (Figure 1d), cultivated peppers (Capsicum sp.), and sweet potato (I. batatas) in both outdoor gardens and greenhouse settings. Given its preferences for Solanaceae and Asteraceae, there is potential for this species to become a pest on additional plants cultivated for food, as well as plants used as ornamentals. In Florida in 2022, solanaceous crop (tomatoes, peppers, and potatoes) production was valued at over 700 million dollars, surpassing Florida’s hallmark citrus industry (NASS 2022). Currently, P. miruku is most common on native roadside weeds that serve as resources for pollinators and birds. The flowers of B. alba are visited by a large suite of pollinators across several insect orders (Bennington and May 2020; Grombone-Guaratini et al. 2004) but it is generally regarded as a problematic weed (Grombone-Guaratini et al. 2004). A. artemisiifolia provides fodder to birds but is highly invasive and its spread is facilitated by its frequent inclusion in commercial bird seed (Vitalos and Karrer 2008).
In Florida, P. miruku is most often found in the subterranean strata on the roots and sometimes on the crown of its host (Figure 1b–e), though the mealybugs may be present on the stem and leaves of the host plant, especially when infestations become heavy (Figure 1c and d). Presence on the external parts of the plant both perpetuates plant damage and further facilitates first-instar crawlers dispersing by wind (Barrass et al. 1994; Hoelscher 1967; Jahn and Beardsley 2000; Wright et al. 2023). Given its frequent association with ant nests, ant-facilitated dispersal is also possible (Klein et al. 1992) as is phoretic dispersal via other more mobile insects (Magsig-Castillo et al. 2010; Poinar 2004; Powell 2023; Wright et al. 2023).
While it is possible that the mealybug found in Florida in 2019 is a native species that went undetected, morphological evidence and host preferences (which overlap little with the other Phenacoccus species in Florida), combined with the sheer abundance, rapid expansion, and the absence of any matching specimens, collected in the last hundred years at the FSCA – which houses one of the largest Florida mealybug collections in the United States – suggest that this is a new introduction. The similar species, P. parvus, was first detected in Florida in 1983 (Williams and Hamon 1994) and is now relatively infrequently collected with only 85 identifications in 40 years (FDACS-DPI 2024). In contrast, we have already identified P. miruku 117 times in five years (FDACS-DPI 2024), in part due to its abundance and in part due to increased collection efforts to ascertain the geographical distribution and host range of this species. Mealybugs on roots are largely ignored by collectors despite the biodiversity of insects that live in the subterranean strata, and we urge that roots should receive more attention in surveys for early detection of invasive species. This leaves the possibility that P. miruku was present before 2019 in Florida and went undetected until then.
P. miruku should continue to be monitored for potential damage to commercial plants. Given its propensity to inconspicuously infest the roots of its host plant, it will be important to carefully check the base of the plant for wax, pull up plants to look for infestations, as well as use ant nests as potential indicators of mealybug presence. P. miruku may be easily mistaken for other species of Phenacoccus (Figure 2), or other species that look similar in the field (e.g., papaya mealybug Paracoccus marginatus Williams & Granara de Willink), if slide mounts are not made. With records in the panhandle of Florida, it is possible that this mealybug is already present in Georgia, Alabama, and other neighboring US states.
Acknowledgments
We thank Andy Levy and Lynn Combee for pre-publication reviews of the manuscript and Trudi Deuel for transcribing the label data for the specimens examined. We are grateful to Cristina Granara de Willink for her help with reexamining characters of P. sisymbriifolium and to Jinyeong Choi and Hirotaka Tanaka for kindly answering questions about their molecular data. We thank Lyle Buss, Tavia Gordon, Alec Tasi, and Victoria Benjamin for providing images. Alex de la Paz provided helpful discussion and references on the Bidens taxonomy and Kevin Burnette created the map for Figure 4. Cheryl Roberts and Lynn Combee provided laboratory support in gathering sequence data. Stephen Gaimari is thanked for identifying the Leucopina bella. We appreciate the collecting efforts of DPI inspectors, particularly Nora Marquez. We thank the Florida Department of Agriculture and Consumer Services, Division of Plant Industry (FDACS-DPI), for support of this work. The opinions expressed in this report do not necessarily represent the policies of the US Department of Agriculture. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
-
Research ethics: Not applicable.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: The authors state no conflict of interest.
-
Research funding: The Florida Department of Agriculture and Consumer Services, Division of Plant Industry (FDACS-DPI), supported this work.
-
Data availability: The raw data (character matrices) can be obtained on request from the corresponding author. Specimens are deposited at the Florida State Collection of Arthropods (FSCA) in Gainesville, Florida, USA.
References
Ahmed, Z. and Miller, D.R. (2019). Phenacoccus sisymbriifolium Granara de Willink (Pseudococcidae: Coccomorpha: Hemiptera), a new U.S. continental record in Florida and potential pest. Circular, Florida Department of Agriculture and Consumer Services, Division of Plant Industry, FDACS-P-02125.Search in Google Scholar
Ahmed, M.Z., Ray, C.H., Moore, M.R., and Miller, D.R. (2020). The Matsucoccus Cockerell, 1909 of Florida (Hemiptera: Coccomorpha: Matsucoccidae): potential pests of Florida pines. Insecta Mundi 0810: 1–31.Search in Google Scholar
Altschul, S.F., Gish, W., Myers, E.W., and Lipman, D.J. (1990). Basic local alignment search tool. J. Mol. Biol. 215: 403–410, https://doi.org/10.1006/jmbi.1990.9999.Search in Google Scholar
Ballard, R. (1986). Bidens pilosa complex (Asteraceae) in North and Central America. Am. J. Bot. 73: 1452–1465, https://doi.org/10.2307/2443850.Search in Google Scholar
Barrass, I.C., Jerie, P., and Ward, S.A. (1994). Aerial dispersal of first-and second-instar longtailed mealybug, Pseudococcus longispinus (Targioni Tozzetti) (Pseudococcidae: Hemiptera). Aus. J. Exp. Agric. 34: 1205–1208, https://doi.org/10.1071/ea9941205.Search in Google Scholar
Bennington, C. and May, P. (2020). Pollinator communities of restored sandhills: a comparison of insect visitation rates to generalist and specialist flowering plants in sandhill ecosystems of central Florida. Nat. Areas J. 40: 168–178, https://doi.org/10.3375/043.040.0208.Search in Google Scholar
Choi, J. and Lee, S. (2022). Higher classification of mealybugs (Hemiptera: Coccomorpha) inferred from molecular phylogeny and their endosymbionts. Syst. Entomol. 47: 354–370, https://doi.org/10.1111/syen.12534.Search in Google Scholar
Cox, J.M. and Williams, D.J. (1981). An account of cassava mealybugs (Hemiptera: Pseudococcidae) with a description of a new species. Bull. Entomol. Res. 71: 247–258, https://doi.org/10.1017/s0007485300008270.Search in Google Scholar
Edgar, R.C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic. Acids. Res. 32: 1792–1797.10.1093/nar/gkh340Search in Google Scholar PubMed PubMed Central
FDACS-DPI (Florida Department of Agriculture and Consumer Services Division of Plant Industry) (2024). FDACS-DPI Database. Florida Department of Agriculture and Consumer Services Division of Plant Industry, Gainesville, FL.Search in Google Scholar
Folmer, O., Black, M., Hoeh, W., Lutz, R., and Vrijenhoek, R. (1994). DNA primers for amplification of mitochondrial cyctochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 3: 294–299.Search in Google Scholar
Frank, J.H. and McCoy, E.D. (1995). Introduction to insect behavioral ecology: the good, the bad, and the beautiful: non-indigenous species in Florida. Invasive adventive insects and other organisms in Florida. Fla. Entomol. 78: 1–15, https://doi.org/10.2307/3495661.Search in Google Scholar
Frank, J.H. and Thomas, M.C. (2004). Invasive insects (adventive pest insects) in Florida. EDIS 827/IN503: 1–7, https://doi.org/10.32473/edis-in503-2004.Search in Google Scholar
Granara de Willink, M.C. and Szumik, C. (2007). Phenacoccinae de centro y sudamérica (Hemiptera: Coccoidea: Pseudococcidae): Sistemática y filogenia. Revista de la Sociedad Entomológica Argentina 66: 29–129.Search in Google Scholar
Grombone-Guaratini, M.T., Solferini, V.N., and Semir, J. (2004). Reproductive biology in species of Bidens L. (Asteraceae). Scientia Agricola 61: 185–189, https://doi.org/10.1590/s0103-90162004000200010.Search in Google Scholar
Hodges, G. (2002). Pink hibiscus mealybug, Maconellicoccus hirsutus (Green). Pest Alert, Florida Department of Agriculture and Consumer Services, Division of Plant Industry, DACS-P-01652.Search in Google Scholar
Hodges, G.S. and Hodges, A.C. (2004). New invasive species of mealybugs, Palmicultor lumpurensis and Chaetococcus bambusae (Hemiptera: Coccoidea: Pseudococcidae), on bamboo in Florida. Fla. Entomol. 87: 396–397, https://doi.org/10.1653/0015-4040(2004)087[0396:nisomp]2.0.co;2.10.1653/0015-4040(2004)087[0396:NISOMP]2.0.CO;2Search in Google Scholar
Hodgson, C.J., Abbas, G., Arif, M.J., Saeed, S., and Karar, H. (2008). Phenacoccus solenopsis Tinsley (Sternorrhyncha: Coccoidea: Pseudococcidae), an invasive mealybug damaging cotton in Pakistan and India, with a discussion on seasonal morphological variation. Zootaxa 1913: 1–35, https://doi.org/10.11646/zootaxa.1913.1.1.Search in Google Scholar
Hoelscher, C.E. (1967). Wind dispersal of brown soft scale crawlers, Coccus hesperidum (Homoptera: Coccidae), and Texas citrus mites, Eutetranychus banksi (Acarina: Tetranychidae) from Texas citrus. Ann. Entomol. Soc. Am. 60: 673–678, https://doi.org/10.1093/aesa/60.3.673.Search in Google Scholar
Jahn, G.C. and Beardsley, J.W. (2000). Interactions of ants (Hymenoptera: Formicidae) and mealybugs (Homoptera: Pseudococcidae) on pineapple. Proc. Hawaiian Entomol. Soc. 34: 161–165.Search in Google Scholar
Klein, R.W., Kovac, D., Schellerich, A., and Maschwitz, U. (1992). Mealybug-carrying by swarming queens of a Southeast Asian bamboo-inhabiting ant. Naturwissenschaften 79: 422–423, https://doi.org/10.1007/bf01138577.Search in Google Scholar
Kumar, S., Stecher, G., and Tamura, K. (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33: 1870–1874, https://doi.org/10.1093/molbev/msw054.Search in Google Scholar
Magsig-Castillo, J., Morse, J.G., Walker, G.P., Bi, J.L., Rugman-Jones, P.F., and Stouthamer, R. (2010). Phoretic dispersal of armored scale crawlers (Hemiptera: Diaspididae). J. Econ. Entomol. 103: 1172–1179, https://doi.org/10.1603/ec10030.Search in Google Scholar
NASS (United States Department of Agriculture National Agriculture Statistics Service) (2022). 2022 state agriculture overview: Florida, https://www.nass.usda.gov/Quick_Stats/Ag_Overview/stateOverview.php?state=FLORIDA (Accessed 15 February 2024).Search in Google Scholar
Pacheco da Silva, V.C., Kaydan, M.B., and Basso, C. (2020). Pseudococcidae (Hemiptera: Coccomorpha) in Uruguay: morphological identification and molecular characterization, with descriptions of two new species. Zootaxa 4894: 501–520, https://doi.org/10.11646/zootaxa.4894.4.1.Search in Google Scholar
Poinar, G.O.Jr. (2004). Fossil evidence of scale phoresy on spiders. Fossil spiders in amber and copal. Straubenhardt, Beiträgezur Araneologie 3: 1878–1880.Search in Google Scholar
Powell, E.C. (2023). Defensive behaviors of the mealybug Nipaecoccus nipae (Maskell, 1893) (Hemiptera: Pseudococcidae) and the green lacewing Ceraeochrysa claveri (Navás, 1911) (Neuroptera: Chrysopidae), with videos of dorsal packet loading and mealybug ostiole function. Insecta Mundi 1019: 1–11.Search in Google Scholar
Simon, C., Frati, F., Beckenbach, A., Crespi, B., Liu, H., and Flook, P. (1994). Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 87: 651–701, https://doi.org/10.1093/aesa/87.6.651.Search in Google Scholar
Skelley, P., Buss, E., Allen, J., Bolton, S., Deeter, L.A., Fulton, J.C., Greer, D., Halbert, S.E., Hayden, J., Moore, M.R., et al.. (2024). Non-native arthropods in Florida, 1990–2023. Fla. Entomol, In review.Search in Google Scholar
Stocks, I.C. (2012). The mealybug Phenacoccus multicerarii Granara de Willink (Hemiptera: Pseudococcidae) in Florida. Circular, Florida Department of Agriculture and Consumer Services, Division of Plant Industry, FDACS-P-01830.10.32473/edis-in993-2013Search in Google Scholar
Stocks, I.C. and Hodges, G. (2010). Nipaecoccus viridis (Newstead), a new exotic mealybug in South Florida (Coccoidea: Pseudococcidae). Pest Alert, Florida Department of Agriculture and Consumer Services, Division of Plant Industry, DACS-P-01716.Search in Google Scholar
Stocks, I.C. and Roda, A.L. (2011). The passionvine mealybug, Planococcus minor (Maskell), a new exotic mealybug in South Florida (Hemiptera: Pseudococcidae). Pest Alert, Florida Department of Agriculture and Consumer Services, Division of Plant Industry, DACS-P-01769.Search in Google Scholar
Strother, J.L. and Weedon, R.R. (2020). Bidens pilosa. In: Flora of North America Editorial Committee, Vol. 3. Flora of North America North of Mexico [Online], New York and Oxford, http://beta.floranorthamerica.org/Fumaria (Accessed 15 December 2023).Search in Google Scholar
Tanaka, H., Sasaki, D., Choi, J., Husnik, F., and Kamitani, S. (2022). Two new species of mealybugs (Hemiptera: Coccomorpha: Pseudococcidae) from Japan. Zootaxa 5168: 306–318, https://doi.org/10.11646/zootaxa.5168.3.3.Search in Google Scholar
Vitalos, M. and Karrer, G. (2008). Distribution of Ambrosia artemisiifolia L.–is birdseed a relevant vector? J. Plant Dis. Protect. 21: 345–348.Search in Google Scholar
Weakley, A.S. and the Southeastern Flora Team (2023). Flora of the Southeastern United States Web App. University of North Carolina Herbarium, North Carolina Botanical Garden, Chapel Hill, https://fsus.ncbg.unc.edu/ (Accessed 15 December 2023).Search in Google Scholar
Williams, D.J. (2004). Mealybugs of Southern Asia. The Natural History Museum, Southdene SDN. BHD. Kuala Lumpur, Malaysia, pp. 896.Search in Google Scholar
Williams, D.J. and Hamon, A.B. (1994). Phenacoccus parvus Morrison, a possible injurious mealybug recorded for the first time from Florida (Homoptera: Coccoidea: Pseudococcidae). Insecta Mundi 8: 16.Search in Google Scholar
Wright, E.R., Chase, K.D., and Ward, S.F. (2023). Quantifying the potential for wind and phoresy to drive off-plant movement of crapemyrtle bark scale, Acanthococcus lagerstroemiae (Kuwana) (Hemiptera: Eriococcidae): implications for spread in urban landscapes. Agric. Forest Entomol. 26: 210–217, https://doi.org/10.1111/afe.12608.Search in Google Scholar
Wunderlin, R.P., Hansen, B.F., Franck, A.R., and Essig, F.B. (2023). Atlas of Florida plants. [S. M. Landry and K. N. Campbell (application development), USF Water Institute.] Institute for Systematic Botany, University of South Florida, Tampa, http://florida.plantatlas.usf.edu/ (Last accessed 14 February 2024).Search in Google Scholar
Zhao, J., Watson, G.W., Sun, Y., Tan, Y., Xiao, L., and Bai, L. (2014). Phenotypic variation and identification of Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae) in China. Zootaxa 3802: 109–121, https://doi.org/10.11646/zootaxa.3802.1.9.Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter on behalf of the Florida Entomological Society
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Implementing sanitation practices against the hibiscus bud weevil Anthonomus testaceosquamosus (Coleoptera: Curculionidae)
- Evaluation of potential natural enemies of hibiscus mealybug, Nipaecoccus viridis (Hemiptera: Pseudococcidae) in Florida citrus
- Phenology of Nipaecoccus viridis (Hemiptera: Pseudococcidae) in Florida citrus groves
- Florida’s agricultural inspection stations: focus on intercepting invasive Hemiptera in interstate truck shipments
- Taxonomy, hosts, and distribution of an emerging invasive mealybug, Phenacoccus miruku (Hemiptera: Coccomorpha: Pseudococcidae), in Florida
- New invasion or expansion: evaluating the genetic relationships of Bactrocera dorsalis (Diptera: Tephritidae) among detections in Florida
- Ant community composition in a citrus grove reveals eastern expansion in Florida of the South American big-headed ant (Hymenoptera: Formicidae)
- Scientific Notes
- Invasive Chinese tallow tree serves as a new host for four scale insect species
- First report of a predatory mite in association with Floracarus perrepae (Acariformes: Eriophyidae), a biological control agent for Old World climbing fern in Florida
Articles in the same Issue
- Research Articles
- Implementing sanitation practices against the hibiscus bud weevil Anthonomus testaceosquamosus (Coleoptera: Curculionidae)
- Evaluation of potential natural enemies of hibiscus mealybug, Nipaecoccus viridis (Hemiptera: Pseudococcidae) in Florida citrus
- Phenology of Nipaecoccus viridis (Hemiptera: Pseudococcidae) in Florida citrus groves
- Florida’s agricultural inspection stations: focus on intercepting invasive Hemiptera in interstate truck shipments
- Taxonomy, hosts, and distribution of an emerging invasive mealybug, Phenacoccus miruku (Hemiptera: Coccomorpha: Pseudococcidae), in Florida
- New invasion or expansion: evaluating the genetic relationships of Bactrocera dorsalis (Diptera: Tephritidae) among detections in Florida
- Ant community composition in a citrus grove reveals eastern expansion in Florida of the South American big-headed ant (Hymenoptera: Formicidae)
- Scientific Notes
- Invasive Chinese tallow tree serves as a new host for four scale insect species
- First report of a predatory mite in association with Floracarus perrepae (Acariformes: Eriophyidae), a biological control agent for Old World climbing fern in Florida

