Model-based learning about structures and properties of chemical elements and compounds via the use of augmented realities
-
Mei-Hung Chiu
, Chin-Cheng Chou
Abstract
Scientific models play a vital role in science learning, representing major characteristics of scientific phenomena. A useful visualization of models that matches target concepts to source objects can facilitate students’ learning of abstract and complex structures of chemical elements and compounds. This report will show the importance of visualization and innovative technology (such as augmented reality), which has the potential of supporting students’ learning of stereochemistry and interactions among molecules. Examples (including organic compounds, chemical elements of 1A and 7A in the periodic table, water polarity, and carbon nanotube) are drawn to illustrate the potential use of augmented reality in chemistry instruction.
Introduction
Science education aims to play various roles to achieve its goals for science learning and teaching, including science learning, learning of science, learning about science, and learning how to do science (Hodson, 1992, 2014). These aspects encompass a wide range of science products (such as theories) and processes (such as hypothesis, testing, revising, and predicting) that intend to help learners understand how scientists do their work, what philosophy and history of science is about, and how to become scientifically literate. Therefore, meaningful learning of constructing conceptual models for scientific phenomena becomes a challenge to students and teachers in science education. The purpose of model-based approach is to facilitate students in constructing internal representations of phenomena that are not observable or tangible at the sensory level. In addition, in chemistry, many concepts are presented two-dimensionally in school learning. Students need to rely on their visual abilities to traverse between two- and three- dimensional representations to understand the complex relations among atoms at the microscopic level. Taking advantage of today’s mobile phone and applications, the use of external representations, such as augmented reality, might be a channel to uncover the molecular world that is not easily accessible to most students.
Model-based learning
A classic research by Grosslight, Unger, and Jay (1991) indicated that students in secondary school (7th and 11th grades) had conceptions of models that are basically consistent with a naïve realist epistemology in which the young students considered models to be replicas of the actual objects or events. While science experts showed their sophisticated thinking by using models to construct, test, and explain ideas or phenomenon. Modelers should play active roles in constructing and evaluating models. Epistemological approaches from naïve to sophisticated understanding of models should be emphasized in school science teaching. How curriculum can be developed based upon these aspects and how teacher competence in teaching chemistry can be enhanced became important research areas in science education. Several researchers pointed out that chemistry learning involves macro, sub-micro, symbolic, and their inter-relationships (Gilbert, 2008; Treagust, Chittleborough, & Mamiala, 2003). Based on Gilbert’s perspective, models can be external (e.g. something tangible) and internal (e.g. individual mentality) representations. In addition, Gilbert and Justi (2016, p. 197) delineated that students need to be able to demonstrate their competence in visualization and linkage between mental models and the aspect of reality that is particularly needed in learning science. The conventions governing the nature of these links are called “modes of representation” for models and sub-models. They pinpointed four aspects to be emphasized, including a full understanding of the modes of representation of all the modes and sub-modes of visualization used in science education, a capacity to “translate” a given model between those modes of visualization that are appropriate, a capacity to construct a visualization in any one of the modes and submodes used in science education, and a capacity to use a suitable visualization to solve novel problems with respect to a given phenomenon (Gilbert & Eilam, 2014).
In chemistry, molecular structure and property, especially complex organic molecular structure, are often found to be difficult concepts to grasp, and therefore, developing appropriate learning assistive tools can decrease students’ cognitive loads. In our study described here, we used model-based instruction as the approach for learning chemistry.
The power of augmented reality and virtual reality
Augmented Reality (AR) products have experienced exponential growth in commercial and gaming industries. However, in education, there is still a need for more research and development. According to existing literature, AR features the following characteristics: 1. It enables 3-D perspectives, 2. It allows ubiquitous, collaborative, and situated learning, 3. It provides learners a sense of presence, immediacy, and immersion, 4. It can visualize concepts or events that cannot be seen, 5. It bridges formal learning with informal learning. These characteristics provide students with opportunities in engagement, contextualized, and authenticity learnings (Wu, Lee, Chang, & Liang, 2013). Research has found that AR can act as a scaffolding tool to assist students to conceptualize and make linkage between micro- and macro-worlds. Also, the AR tool is more effective for low-achieving students than high-achieving ones (Cai, Wang, & Chiang, 2014).
As for VR, in a research study that conducted a meta-analysis of 13 journal articles on VR-based instruction, it was found that gaming, simulation, and virtual world all have positive influences on learning, and student achievements from gaming-styled VR instruction is higher than those of simulations and virtual world. Another interesting find was that students in solo-game play outperformed those in grouped game play (Merchant, Goetz, Cifuentes, Keeney-Kennicutt, and Davis 2014).
To extend our research on students’ learning of models in chemistry, a virtual reality (VR) assessment app was developed to evaluate students’ understanding of chemical compounds.
In the following sections, four apps are introduced, covering topics of organic compounds, elements in 1A and 7A, the polarity of chemical compounds, and carbon nanotubes. Two decks of cards are also made, with one designed for organic molecular structure and AR/VR, and the other for compounds that combined Group 1A and 7A atomic structures with AR. We worked closely with school science teachers to identify the key needs of teaching chemistry.
Organic molecular structure app
The Organic Molecule AR/VR cards the current research developed is the only application in the app market that features both learning and game play. It is available with three different decks of cards. The manual and the QR-code download link for the app can be found on the cards. One can also search for Molecules 1 AR/VR, Molecules 2 AR/VR & Molecules 3 AR/VR in the Apple App Store (10+) or Google Play in Android devices (6.0+). Information can also be found on the website of Chemical Society Located in Taipei (http://www.chemistry.org.tw/app_download.php). Some descriptions of these apps were published in Chinese at www.chemed.chemistry.org.tw. (Please see Chiu, Chou, Hung, & Hsu, 2018).
The Organic Molecule AR/VR cards were designed mainly with the organic compounds found in local high school textbooks in Taiwan. The three decks of cards are based on the functional groups. The first deck is consisted of hydrocarbons, namely alkane, alkene, and alkyne. The second deck includes alcohol, ether, and ketone. The third includes acid, ester, amine, and amide. The chosen organic compounds in each deck are various chemical compounds and their isomers, appeared in the textbooks. For example, with a carbon number of 4, there are 1-butene, cis-2-butene, trans-2-butene, and 2-methylpropene (as shown in Figure 1). Figure 2 shows the chemical compounds with the same functional group (-COOH), there are formic acid and acetic acid (as shown in Figure 2).
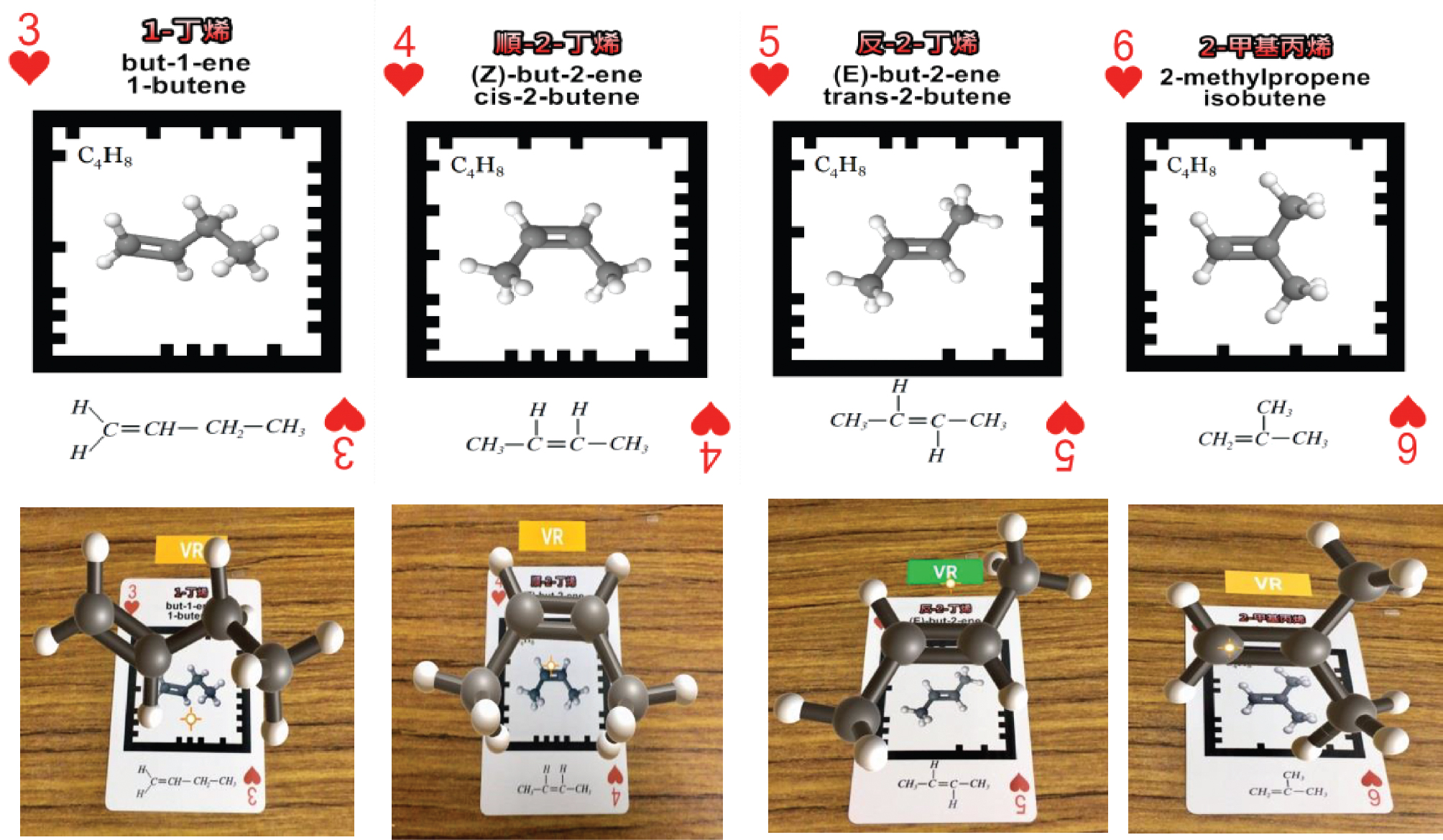
Isomers for C4H8.
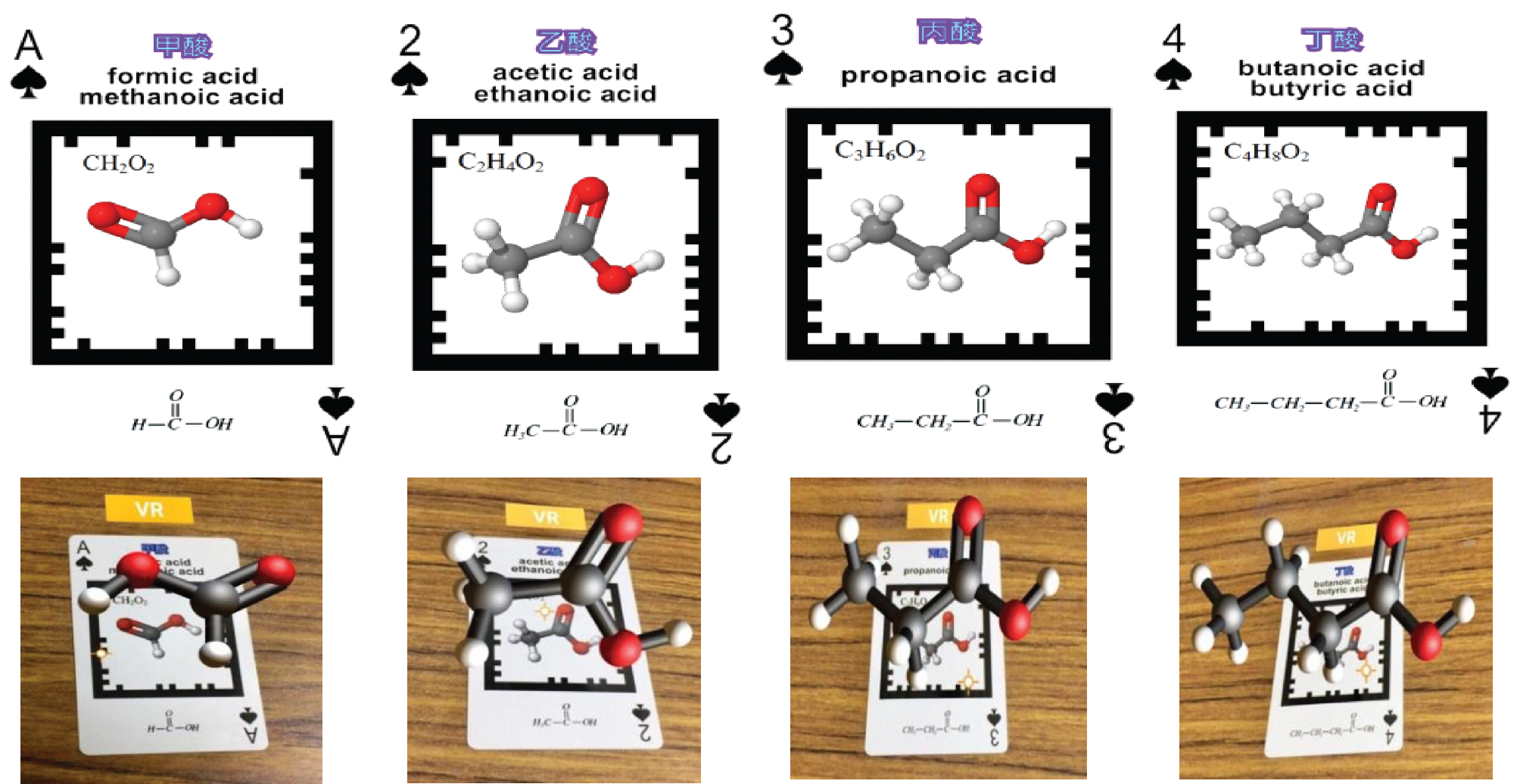
Homologues of the same functional group.
In addition to showing organic molecules’ three-dimensional structures, the main concepts the cards intend to convey also includes isomers and homologues. Through these cards, the students develop interest in learning and a deeper understanding of symbols, chemical equations, properties, structures, and conversion between 2D and 3D structures (see data analysis in later sessions).
As described above, the app for Organic Molecule AR/VR cards, with its AR technology, can assist teachers to better present and explain the 3D structure and characteristics of organic molecules. Students can also manipulate the molecules through touchscreen or joystick for a better view of the molecule’s structure. Furthermore, with its VR technology, students can be assessed on what they have previously learned in class regarding molecular structures through a virtual computer game scenario. In the game, students have to complete a mission of finding the structures of organic molecules (such as alkanes, alkenes, or alkynes) within a limited time frame (300 s). This scenario features gaming elements such as time limit for the mission, rewards and punishment during game play, and feedback at the end of the mission (see Figure 3). Once a student finds three assigned compounds, they are allowed to get into the next stage for another group of organic compounds. Players need to complete the assigned task within 5 min. The player can gain an extra 10 s if they find a gem in the game. Research indicates that learning through gaming would make learning gaining a wide range of cognitive, perceptual, behavioral, affective and motivational impacts and outcomes (Connolly, Boyle, MacArthur, Hainey, and Boyle 2012).
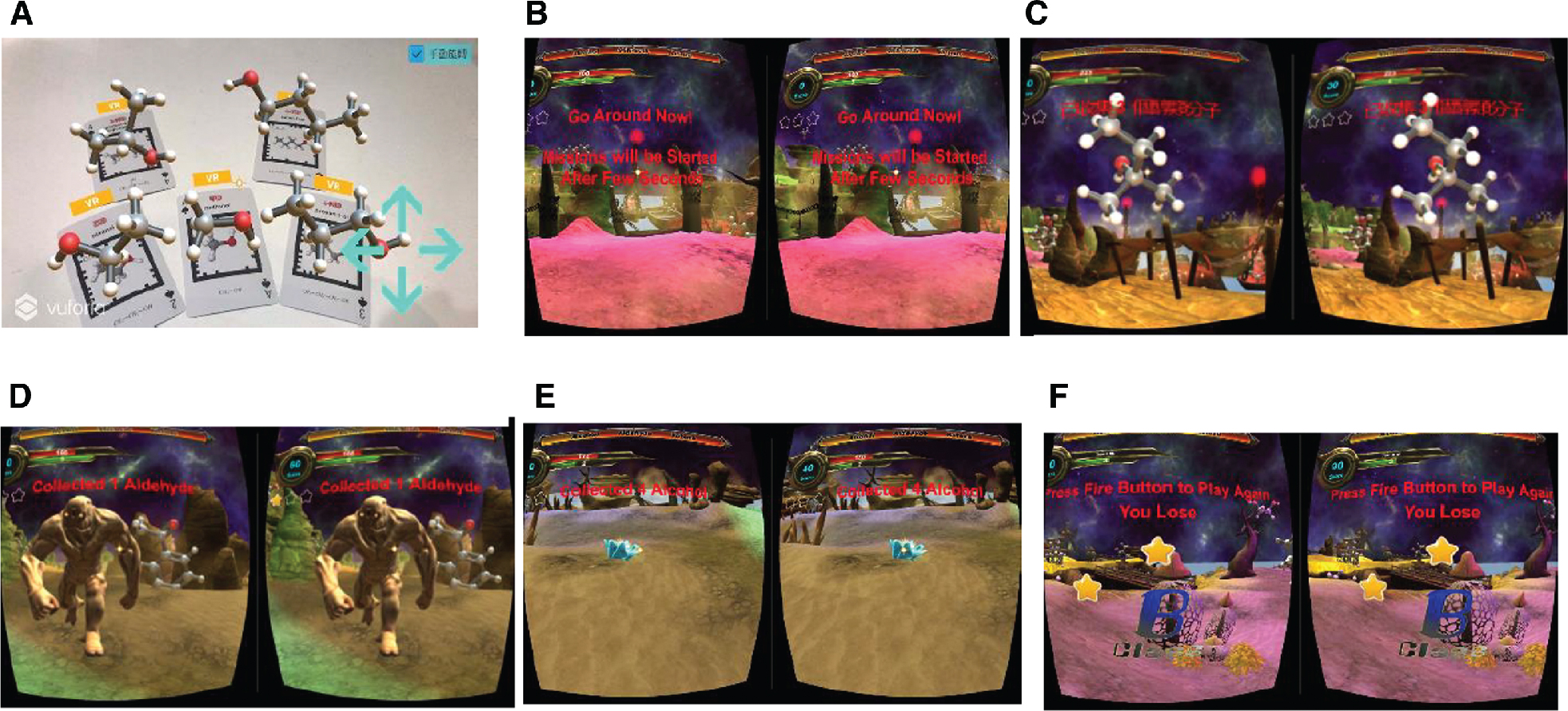
VR game for organic molecules. (A) Molecular Structure AR mode. (B) VR Game Play Scenario. (C) The mission to gather molecules with –OH functional group. (D) Monster appears if the wrong molecule was selected. (E) Gathering jewels to extend working time. (F) Feedback when the mission is accomplished.
The app has three modes of game play (see Figure 4), each was designed to be used with a specific deck of cards. There are two modules in each mode. The first module enables the player to observe the 3D structure and characteristics of every organic molecule through the AR module of the software. The second is an assessment of learning outcome in a gaming scenario. This assessment can only be accessed by selecting a VR label on the screen, which is only accessible through the card’s AR mode screen once the player has gained sufficient knowledge about each type of molecules.

Selection for the three modes of game play.
There are three main ways to use the app:
Direct Observation: Teachers can ask students to scan the cards with the app and compare how the structure of the 3D molecule shown on the mobile device is different from those shown on the 2D card. AR would allow students to rotate the 3D molecular structure to a perspective where it would look the same as those shown on the card or books. Students would then be able to take a screenshot and upload it to the cloud storage the instructor had prepared. Finally, students can share their ideas and screenshots with their peers for discussions. It is hoped that students would learn how the 3D organic molecular structure is related to the structural formula and 2D representations. In this process, the instructor may also select specific cards (such as finding isomers or homologues from the same functional group) and ask students to draw or point out the relative spatial position of atoms.
Learning sheets: Instructors may utilize designs similar to those from the table below to conduct their instructional activity (see Figure 5), and ask students to fill in the chemical equations and name the compounds they observe on the app. The learning sheet can be downloaded from the dedicated app instructional material page on the CCS Located in Taipei website (http://www.chemistry.org.tw/app_download.php#pagefive).
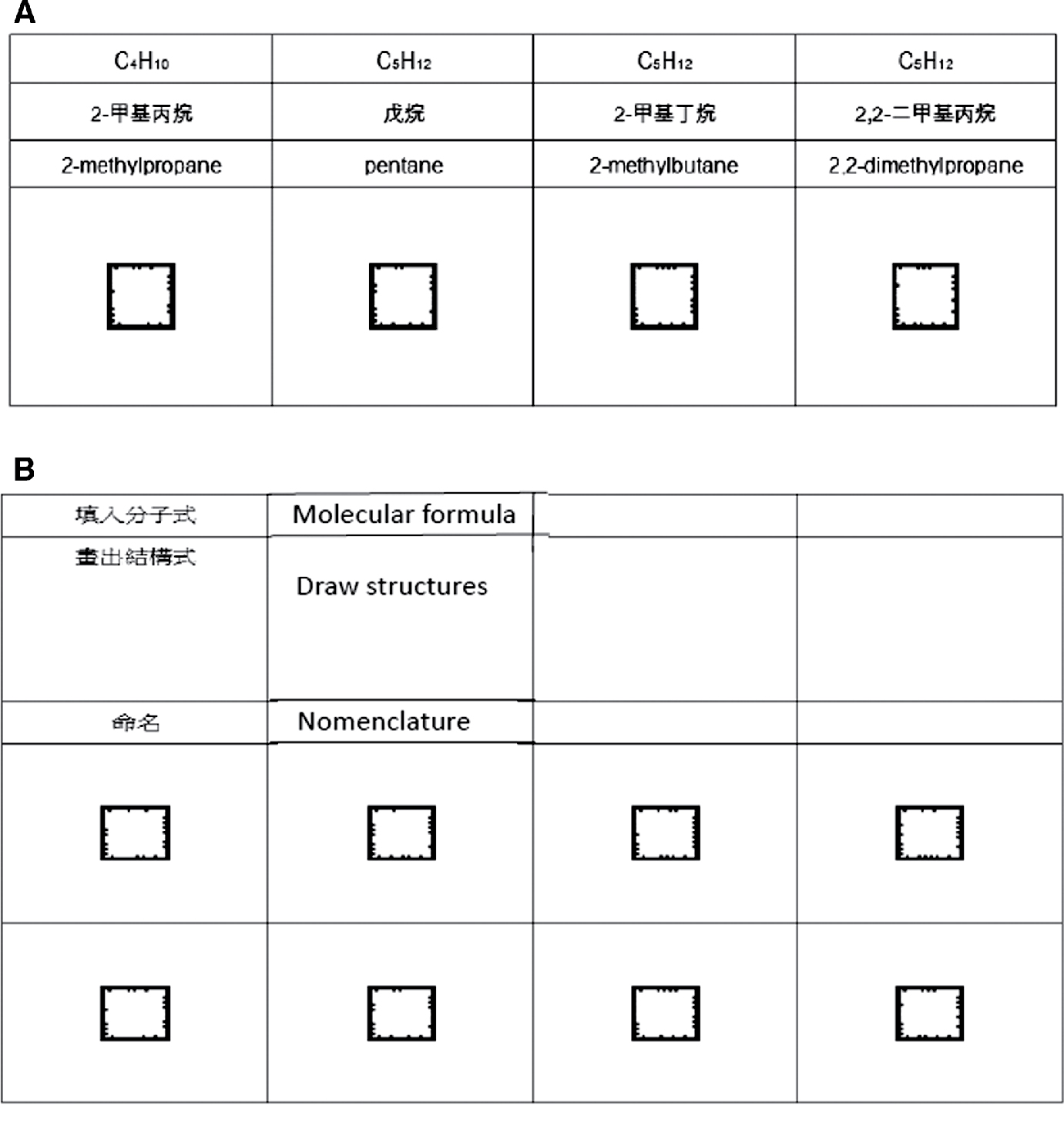
AR Assessment for organic compounds. (a) Original worksheets. (b) Modified version of worksheet for assessment.
Instructors may reorganize the learning sheet (see Figure 5) according to the organic chemistry content he/she intends to teach. Students may use the app to scan the learning sheet, observe the 3D structures and write down their structural formulae (Figure 5A). Or, the instructor may simply list the compounds’ IUPAC naming and its related codes, such as pentane, 2-methylbutane, 2,2-dimethylpropane, and ask the students to write down their corresponding structural formulae, followed by a grouped discussion on the relationship between the IUPAC naming and the structural formulae (Figure 5B). Figure 5B can be used for various purposes in chemistry teaching through providing different hints or information of the molecules on the cards. By drawing the structures of the compounds, students would be able to practice how to transform 3D representations of the chemical compounds to 2D representations and vice versa.
We have been using these packs of poker cards of organic compounds in a senior high school in Taipei city for many years. In one of the classes, the students expressed positive attitudes toward the use of AR in learning chemistry in their interviews. Their responses are as follows:
82 % (18/22) of the students stated that they appreciated the use of mobile phones in learning science since it allowed them to visualize and manipulate the chemical compounds.
86 % (19/22) of the students stated that their interest in learning science increased after using ARs.
As for the teachers who participated in our workshops or e-questionnaire, they expressed that it was relatively easy to describe the complex and abstract concepts to students via the app compared to textbooks or other resources. The biggest difference between ARs and traditional teaching style is the visualization of the concepts that were difficult to convey to students through 2D representations. However, it was still challenging for teachers as they try to help students convert scientific concepts into 2D and 3D representations. They also suggested to make the game more challenging, providing students opportunities to solve problems by integrating their newly learned knowledge.
Board Game: The first deck of cards: Hydrocarbons, can be used as a card-based board game. Instruction can be found online at: https://goo.gl/E13011.
1A and 7A groups in the periodic table
Another set of AR was developed and titled AR_Elements (1A, 7A). Based upon research in the area of multiple representations, various forms of molecules were considered. This function of the AR app allows students to observe the structures and symbols of the 1A and 7A elements and manipulate the cards to explore the electronic structure of atoms of elements 1A and 7A.
Figure 6 describes the cards, ways to get the app, and the instruction for the operation of the app. The app was created via the software of Jmol. The app included various forms of representations of atoms and molecules. For instance, red and white balls are atom representations with standard atomic radii while the blue dots are valence electrons. In the molecular and ball-and-stick models, the size of these balls does not realistically take into account the spatial distribution of valence electrons for each type of atoms. Only the ball sizes of the van der Waal model accurately describe the three-dimensional electron cloud distribution based upon the electron – electron interaction point of view. As shown in Figure 6F, the size difference between two different atom types can be quite dramatic, making it difficult to visualize.
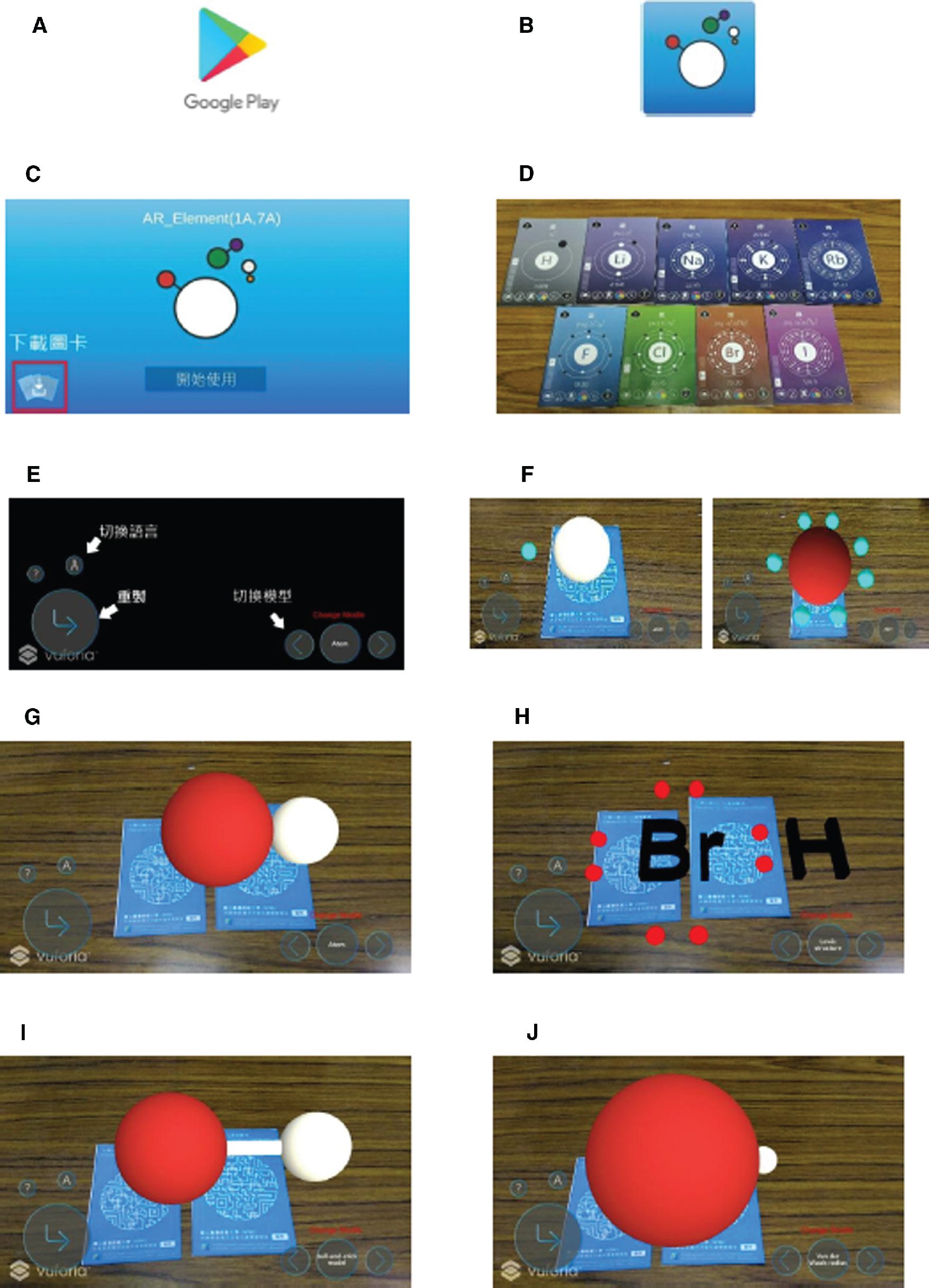
ARs for 1A and 7A chemical elements in the Periodic Table. (A) Search for AR_Element (1A, 7A) at Google Play. (B) Download App and click to start. (C) Select the lower left corner icon to download cards once the app has been started. (D) The front side of the card is the information on 1A, 7A atoms. (E) App Interface Instruction. (F) Using the app to scan the back of card would render the AR model of the atom. (G) By placing two cards that can be combined next to each other, the combined molecular model would be shown. (H) Switching to the Lewis Model. (I) Switching to the ball-and-stick model. (J) Switching to the Van der Waals model.
Students can try to form molecular models by combining two cards (e.g. by combining the cards of H and Br, the app will display the molecular model of HBr). The instructor may allow students to choose different elements’ cards to form various different molecular models in accordance to the needs of the course. Students may then observe the different ways molecular models can be displayed (e.g. molecular model, Lewis electron models, ball-and-stick model, Van der Waals radius model etc.). This app utilizes emerging technology to enhance students’ learning motivation, provides students with ways to get to know about the structure and representation of materials, and enables the formation of the correct conceptual understanding on material structure through discussions amongst students.
Polarity of water
The third app’s aim is to introduce the concept of polarity between different compounds (such as H2O, CH2O, and CH4). The chemical compounds were chosen purposefully. For example, one of the most valuable properties of water is its ability to dissolve many different ionic or polar substances. Water molecules as a solvent can cause favorable electrostatic interactions with the ionic and polar solute. The surrounding water molecules can form favorable solvation shell to compensate the excluded space of introducing the solute in water through inter-water hydrogen bond interactions. This polarity gives water a greater ability to dissolve compounds. Sanger and Badger (2001) indicated that students who received instructions with computer simulations of electron-density plots were more likely to explain that the intermolecular attractions among water molecules are responsible for the immiscibility of oil and water, and were also more likely to recognize that water molecules are attracted to sodium ions, chloride ions, and each other, but are not strongly attracted to hydrogen molecules.
Our design allows users to observe the structures of each individual compound with multiple representations, and more importantly, to observe how the “positive ends” of the polar compounds (such as water molecules) are attracted to the negatively charged anions to form hydrogen bonds, and that the “negative ends” of the polar compounds to the positively charged anions. This is called hydration in water molecules. Similar phenomena can be seen between ionic substances (such as water and formaldehyde) but not between polar and non-polar molecules (such as water and methane) or between non-polar molecules (such as among methane molecules) (See Figure 7).
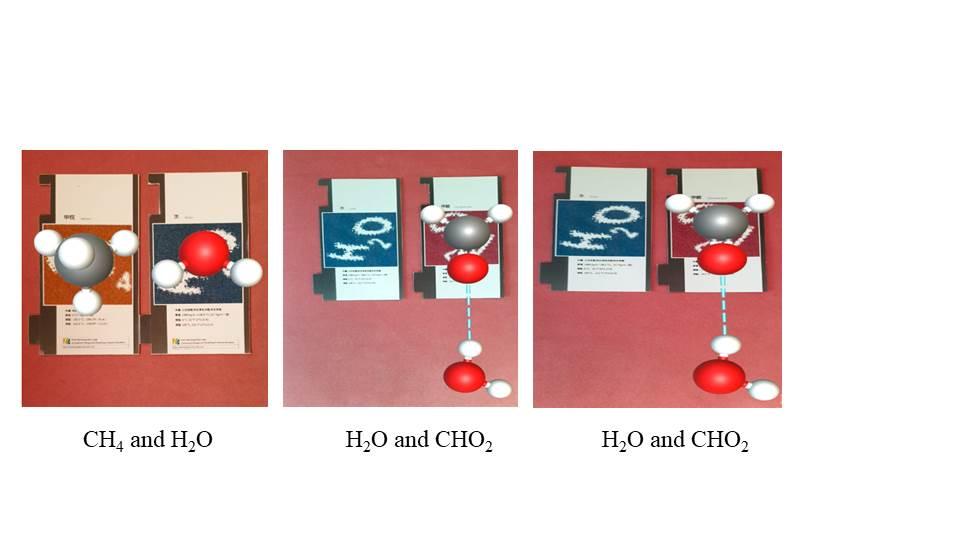
ARs for polar and nonpolar compounds and formation of hydrogen bonds.
Carbon nanotube
The interesting cylindrical shape of nanotubes are composed of carbon atoms linked in hexagonal shape. Carbon nanotubes have several characteristics (such as strength) that attract researchers and companies alike to invest in the research on its application potential. Figure 8 shows the three kinds of carbon nanotube structures, namely, armchair, zigzag, and chiral/helix. This app will show what they look like from a 3D perspective to help students visualize the spatial configuration of the structure. Teachers can ask students to identify the three types of carbon nanotube structures and explain why each type of structure holds different properties.
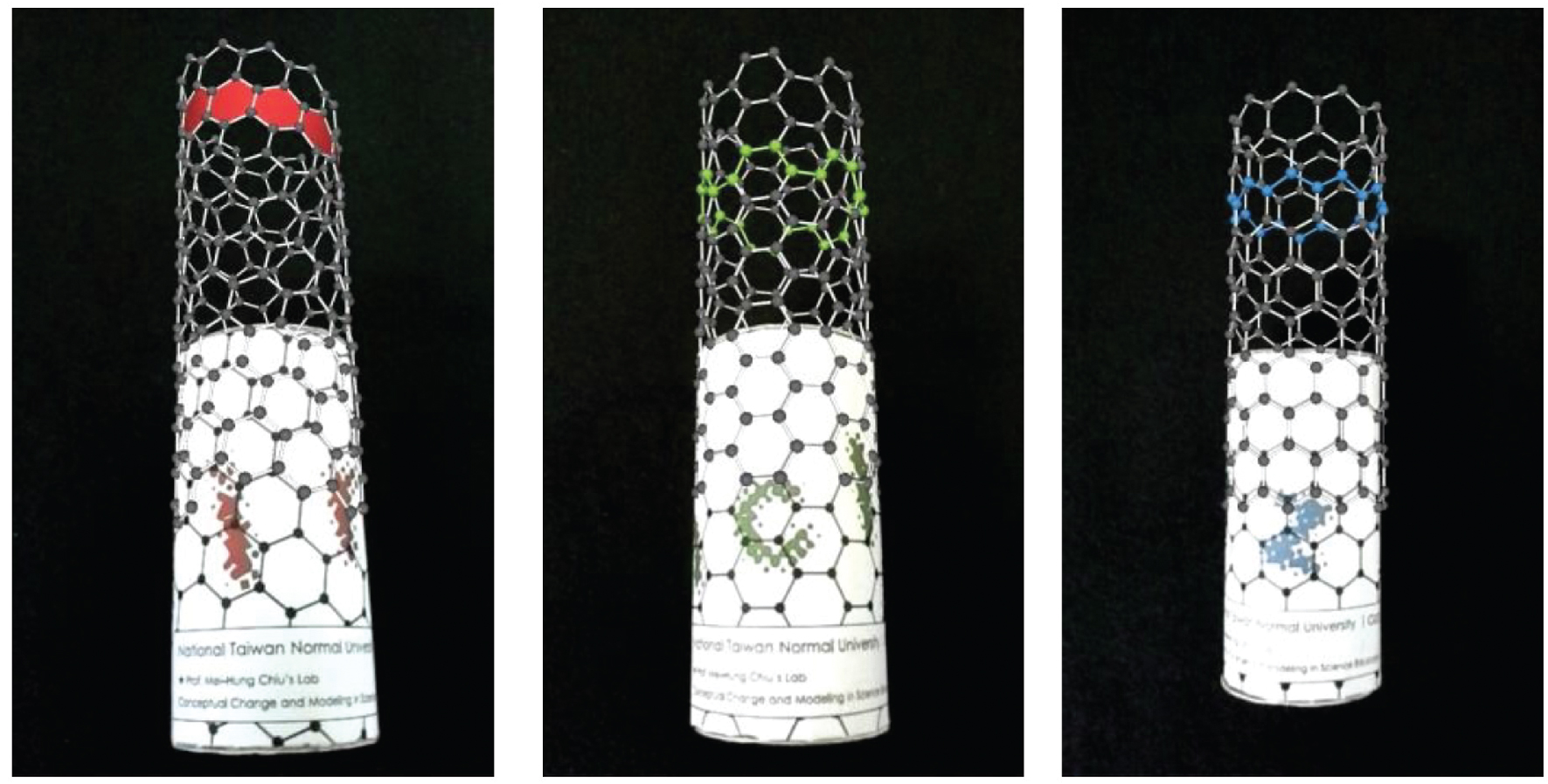
Three kinds of carbon nanotubes.
Conclusions and suggestions
As there is an enormous growth of research in the field of model- and modeling-based approach, educational applications and games are widely used in both formal and informal learning environments. How to make the best use of the app to facilitate science learning, in particular, to help students visualize and construct internal representation of scientific knowledge for deep learning, becomes a challenge to many designers in engineering, technology, and education. In this article, we present potential tools that can act as scaffolds for both teachers in their instruction and students in their knowledge construction and reconstruction processes.
From our experiences of holding workshops for more than 200 teachers (See Figure 9) on the use of ARs and the adoption our products (including apps for chemistry, physics, biology, and geography) in schools, we found that there are three advantages of using ARs: First, ARs can support students to visualize unseen structures and allow them to manipulate compounds from different perspectives; second, it enhances discourses not only between teachers and students but also among students while manipulating the targets and their smart phones; and finally, the students’ perceptions about mobile phones had shifted from a mere communications device to a learning tool. However, there are three challenges as well, namely, first, teachers need to learn how to make the best use of ARs in their classes; second, there is a need for the development of appropriate app-related instructional materials for different levels of students; and finally, alternative assessment of students’ visualization of 3D compounds should be developed according to the learning materials provided by the ARs. More importantly, developers need to work with teachers closely to identify concepts and learning difficulties that can be addressed through the use of AR or VR.

The workshop for teachers professional development
In Taiwan, the 12-year compulsory education has put a great emphasis on the core and cross-discipline concepts. In the field of natural sciences, the “composition and structure of materials” is one of the basic concepts among the core and cross-discipline concepts. As a response to this, for the first time, the natural sciences curriculum has included the “concept of particles” among the contents for elementary stage learning. At the high school level, the cultivation of microscopic views, abstract and critical thinking, and model construction competence have also been greatly increased. In order to help students understand how the complex world functions, assisting students to construct conceptions about structures of molecular compounds and interactions among particles in the microscopic world has become an important task for science teachers. We advocate that enhancing students’ spatial visualization abilities and linking chemistry knowledge between 2D and 3D representations can facilitate students’ learning of abstract concepts in chemistry. Introducing AR and VR assistive tools at this time can also make chemistry learning more meaningful and fun for students. We believe there is a lot of potential for this kind of learning approach in the future.
Funding source: Ministry of Science and Technology in Taiwan
Award Identifier / Grant number: 101-2514-S-003-007102-2514-S-003-007, 103-2514-S-003-003, 104-2514-S-003-004
Funding statement: We would like to express our thanks to the Ministry of Science and Technology in Taiwan for funding our development of Augment Reality and Virtual Reality Assistive Tools through its High Scope Project (Funder Id: 10.13039/501100004663, 101-2514-S-003-007102-2514-S-003-007, 103-2514-S-003-003, 104-2514-S-003-004).
Acknowledgment
We are grateful to Chemical Society Located in Taipei (CSLT) for its assistance on promotion of the poker cards (see information at http://www.chemistry.org.tw/app_download_en.php), which enabled the successful completion of our project. Also, we would like to take this opportunity to thank Hsiang-Kai Chang for their assistance on the development of the apps. Please contact the first author or CSLT at ccs www@gate.sinica.edu.tw if you are interested in the three decks of cards.
Appendix
Information for Molecules 1, 2, & 3
1. (a) Apple IOS
https://itunes.apple.com/ca/app/molecules-1-ar-vr/id1300987004?mt=8
https://itunes.apple.com/ca/app/molecules-2-ar-vr/id1301780738?mt=8
https://itunes.apple.com/ca/app/molecules-3-ar-vr/id1301796224?mt=8
1. (b) Google Play
https://play.google.com/store/apps/details?id=com.rse.molecules1arvr
https://play.google.com/store/apps/details?id=com.rainbow.molecules2arvr
https://play.google.com/store/apps/details?id=com.rainbow.molecules3arvr
2. Introduction of Molecules AR/VRhttp://www.chemistry.org.tw/app_download_en.php
Or http://www.chemistry.org.tw/app_download.php#pagetwo for Chinese
http://www.chemistry.org.tw/app_download_en.php#pagetwo for English
3. Youtube:https://youtu.be/pSM5B76oxIs
4. DNA can be found at
https://play.google.com/store/apps/details?id=com.rse.dna
5. Carbon Nanotube (CNT) can be found athttps://play.google.com/store/apps/details?id=com.rse.cnt
6. Polarity can be found at
https://play.google.com/store/apps/details?id=com.rse.molecules
References
Cai, S., Wang, X., & Chiang, F. K. (2014). A Case Study of Augmented Reality Simulation System Application in a Chemistry Course. Computers in Human Behavior, 37, 31–40.10.1016/j.chb.2014.04.018Search in Google Scholar
Chiu, M. H., Chou, C. C., Hung, T. M. & Hsu, C. W. (2018). Learning elements and organic molecular structure through augmented and virtual realities. Chemistry Education in Taiwan.Search in Google Scholar
Connolly, T. M., Boyle, E. A., MacArthur, E., Hainey, T., & Boyle, J. M. (2012). A systematic literature review of empirical evidence on computer games and serious games. Computers & Education, 59(2), 661–686.10.1016/j.compedu.2012.03.004Search in Google Scholar
Gilbert, J. K. (2008). Visualization: An emergent field of practice and enquiry in science education. In J. Gilbert, M. Reiner & M. Nakhleh (Eds.), Visualization: Theory and practice in science education (pp. 3–24). The Netherlands: Springer Publishers.10.1007/978-1-4020-5267-5_1Search in Google Scholar
Gilbert, J. K., & Eilam, B. (2014). Developing science teachers’ representational competence and its impact on their teaching. In B. Eilam & J. K. Gilbert (Eds.), Science teachers’ use of visual representations (pp. 315–329). Dordrecht, The Netherlands: Springer.10.1007/978-3-319-06526-7_14Search in Google Scholar
Gilbert, J. K., & Justi, R. (2016). Modeling-based teaching in science education. The Netherlands: Springer Publishers.10.1007/978-3-319-29039-3Search in Google Scholar
Grosslight, L., Unger, C., & Jay, E. (1991). Understanding models and their use in science: Conceptions of middle and high school students and experts. Journal of Research in Science Teaching, 28(9), 799–822.10.1002/tea.3660280907Search in Google Scholar
Hodson, D. (1992). In search of a meaningful relationship: an exploration of some issues relating to integration in science and science education. International Journal of Science Education, 14(5), 541–562.10.1080/0950069920140506Search in Google Scholar
Hodson, D. (2014). Learning Science, Learning about Science, Doing Science: Different goals demand different learning methods. International Journal of Science Education, 36(15), 2534–2553.10.1080/09500693.2014.899722Search in Google Scholar
Merchant, Z., Goetz, E., Cifuentes, L., Keeney-Kennicutt, W., & Davis, T. J. (2014). Effectiveness of virtual reality-based instruction on students’ learning outcomes in K-12 and higher education: A meta-analysis. Computers & Education, 70, 29–40.10.1016/j.compedu.2013.07.033Search in Google Scholar
Sanger, M. J., & Badger, S. M. II. (2001). Using computer-based visualization strategies to improve students’ understanding of molecular polarity and miscibility. Journal of Chemical Education, 78(10), 1412–1416.10.1021/ed078p1412Search in Google Scholar
Treagust, D., Chittleborough, G., & Mamiala, T. (2003). The role of submicroscopic and symbolic representations in chemical explanations. International Journal of Science Education, 25(11), 1353–1368.10.1080/0950069032000070306Search in Google Scholar
Wu, H. K., Lee, S. W. Y., Chang, H. Y., & Liang, J. C. (2013). Current status, opportunities and challenges of augmented reality in education, Computers and Education, 62, 41–49.10.1016/j.compedu.2012.10.024Search in Google Scholar
©2019 IUPAC & De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Preface
- From the editor, Jan Apotheker
- Good practice report
- Assistance in handling large-enrolment, introductory General Chemistry courses
- Proceedings Paper
- Using data-driven activities with ChemEd X Data to practice structure-property relationships in General Chemistry
- Other
- Stoichiometry of equations through the Inverse de Donder relation
- Good practice report
- Model-based learning about structures and properties of chemical elements and compounds via the use of augmented realities
- Research article
- Flipping the class – University chemistry students’ experiences from a new teaching and learning approach
- Proceedings Paper
- How can we effectively improve the mathematical capabilities of students of chemistry?
- Research article
- Eye tracking student strategies for solving stoichiometry problems involving particulate nature of matter diagrams
- Good practice report
- Introducing spectrophotometry in the school lab employing LEGO bricks and LEDs
- Proceedings Papers
- Promoting mathematical reasoning and problem solving through inquiry-based relevance focused computer simulations: a stoichiometry lab
- A tribute to Professor Alex H Johnstone (1930–2017)
Articles in the same Issue
- Preface
- From the editor, Jan Apotheker
- Good practice report
- Assistance in handling large-enrolment, introductory General Chemistry courses
- Proceedings Paper
- Using data-driven activities with ChemEd X Data to practice structure-property relationships in General Chemistry
- Other
- Stoichiometry of equations through the Inverse de Donder relation
- Good practice report
- Model-based learning about structures and properties of chemical elements and compounds via the use of augmented realities
- Research article
- Flipping the class – University chemistry students’ experiences from a new teaching and learning approach
- Proceedings Paper
- How can we effectively improve the mathematical capabilities of students of chemistry?
- Research article
- Eye tracking student strategies for solving stoichiometry problems involving particulate nature of matter diagrams
- Good practice report
- Introducing spectrophotometry in the school lab employing LEGO bricks and LEDs
- Proceedings Papers
- Promoting mathematical reasoning and problem solving through inquiry-based relevance focused computer simulations: a stoichiometry lab
- A tribute to Professor Alex H Johnstone (1930–2017)

