Abstract
Metal corrosion is a destructive process that must be managed carefully to prevent unintentional material losses. Most organic and inorganic compounds reported as corrosion inhibitors are highly toxic, causing severe environmental pollution. Recently, expired drugs have been tried as green inhibitors for many metals. The effectiveness of expired drugs as corrosion inhibitors was investigated by techniques such as weight loss, linear polarization, potentiodynamic polarization, and electrochemical impedance spectroscopy methods. The surface morphology of specimen metals was tested before and after the addition of drug inhibitors using scanning electron microscopy, atomic force microscopy, X-ray diffraction, etc., to confirm the inhibition behavior of the drugs. Additional parameters by quantum chemical calculations and molecular dynamics study were computed, and the outcomes agreed with the experimental results. These investigations demonstrated the effectiveness of drugs as corrosion inhibitors and opened up possibilities for managing the disposal of expired drugs. The present paper gives an overview of the usage of expired drugs as efficient inhibitors to control the corrosion of carbon steel and mild steel. The comparison of inhibition performances of different expired drugs and the mechanism of corrosion inhibition have been discussed. The existing challenges faced in using expired drugs as inhibitors were highlighted.
1 Introduction
Corrosion is the process by which a metal or its characteristics deteriorate due to interaction with its surroundings; this often happens through electrochemical interactions. Consequently, the metal experiences a change in properties that can frequently result in its loss and negatively impact its regular functioning. Metals corrode because they tend to return to their most stable lower energy oxidized state. Thus, corrosion is the reverse process of extractive metallurgy (Kaesche 2003). The cost of global corrosion was estimated to be over $2.5 trillion yearly, according to the NACE International Institute. This covers the price of replacing and maintaining deteriorated machinery and infrastructure. As per the estimate, globally, corrosion consumes around 3–4 % of industrialized countries’ GDP (Gross Domestic Product) per year (Koch et al. 2016).
Acid descaling, acid cleaning, acidizing oil wells, and acid pickling are among the industrial operations that use mineral acids like sulfuric and hydrochloric acids (Lagrenee et al. 2002). In such processes, the corrosive attack of acid on the metals should be controlled to avoid their undue dissolution. Metals also suffer from localized corrosion in an aggressive chloride medium. Among the several corrosion management techniques, corrosion inhibitors are the most practical, economical, and successful approach. In addition, an inhibitor can be added without disrupting the normal process. Corrosion inhibitors are used extensively in the chemical, water treatment, petroleum refinery, oil/gas production and exploration, and product additive industries. The corrosion inhibitors market was estimated to be worth US$ 8.1 billion globally in 2022 and is expected to rise at a 4.8 % compound annual growth rate (CAGR) from 2023 to 2030, reaching US$ 10.3 billion (Anupama et al. 2020).
Traditional inhibitors suffer from limitations such as high toxicity and low solubility in corrosive media. Adding some organic solvent is necessary to use these conventional inhibitors to increase the solubility in the medium, which naturally aggravates the environmental pollution problems. These limitations of traditional inhibitors can be resolved using green inhibitors such as drug compounds. The usage of expired drugs as inhibitors also provides an alternative method for their disposal. The present paper is a critical review of expired drugs as effective eco-friendly inhibitors against the corrosion of carbon steel and mild steel.
2 Corrosion inhibitors
Chemical compounds known as inhibitors can be introduced to a medium in small amounts to regulate, lessen, or stop interactions between a metal and its surroundings. They are broadly classified as interface inhibitors and scavengers (Uhlig and Revie 2008). Figure 1 provides an overview of the inhibitor classification.
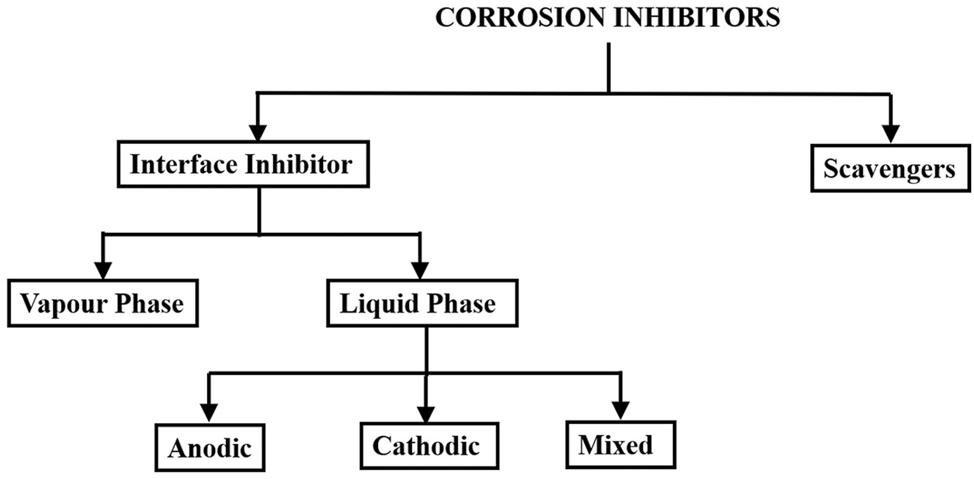
Types of corrosion inhibitors.
Scavengers prevent corrosion by removing corrosive materials from the environment. Although these inhibitors perform poorly in strong acid solutions, they are most effective in solutions where oxygen reduction is the cathodic reaction being regulated. Sodium sulfite and hydrazine are two examples of this inhibitor, which eliminates dissolved oxygen from aqueous solutions.
Interface inhibitors create a barrier at the metal/solution interface to prevent corrosion.
Further, they are divided into two groups: vapor-phase and liquid-phase inhibitors. An inhibitor that is volatilized from a source and transferred to the corrosion site in a confined environment is known as a vapor-phase corrosion inhibitor. They can either vaporize in their molecular form or dissociate first, followed by vaporization. In both situations, vapor phase inhibitors adsorb on the metal surface chemically or physically. For instance, in boilers, steam is used to carry volatile essential substances like morpholine or hydrazine, which neutralize acidic carbon dioxide or change the pH of the surface to less acidic, thus preventing corrosion in condenser tubes (Miksic 1983).
Liquid phase inhibitors are classified as anodic, cathodic, or mixed inhibitors depending on their ability to control one or both of the anodic or cathodic reactions. Anodic inhibitors reduce the anodic area by polarizing the anodic process and affecting the anodic sites. They lower the corrosion rate by slowing the anodic reaction and shifting the corrosion potential positively. Oxyanions such as sodium nitrite, tungstates, molybdates, and chromates are potent anodic inhibitors. The metal ion generated at the anodic area combines with these anodic inhibitors to form insoluble compounds. Later, it is deposited over the anodic sites, creating a protective layer that retards further anodic reactions.
Cathodic inhibitors decrease the cathodic area by polarizing the cathodic reaction and interacting with the cathode sites. The corrosion rate and current are reduced when the corrosion potential is shifted negatively. These inhibitors include, for instance, calcium and magnesium carbonates, silicates or borates, and inorganic phosphates. Two categories of cathodic inhibitors exist. Both cathodic precipitation and cathodic poisoning are examples. While compounds like arsenic, bismuth, and antimony are reduced at the cathode and create a protective layer, cathodic poisoning inhibitors like sulfides and selenides are adsorbed on the metal surface. These inhibitors are effective in acidic solutions because they slow down the rate of hydrogen reduction.
Cathodic precipitators are another form of cathodic inhibitor that preferentially precipitate on cathodic regions to reduce corrosion. For instance, ions like calcium, magnesium, and zinc precipitate on cathodic sites as their corresponding hydroxides. In contrast to anodic inhibitors, which frequently cause severe localized pitting, a cathodic inhibitor’s concentration deficit does not. As the inhibitor concentration lowers, the corrosion rate drops consistently across the surface.
Chemicals known as mixed inhibitors inhibit cathodic as well as anodic reactions. They can protect the metal via Physisorption, Chemisorption, and Film formation (Prakash Shetty 2020). The physicochemical characteristics of the inhibitor substance, the concentration of the corrosive medium, temperature, flow velocity, and metal type affect the effectiveness of the inhibitors (Bardal 2004). Efficient corrosion inhibitors should be nontoxic, soluble, and stable in the medium and perform best at low dosages. Organic compounds as inhibitors usually adsorb onto metal surfaces to prevent corrosion in aggressive environments. The inhibitor can adsorb through various processes, such as replacing water molecules on the metal, the interaction of heteroatoms or multiple bonds in its molecule with the unoccupied metal orbitals, or a protonated inhibitor species’ electrostatic attraction to a charged metal surface or a combination of these processes. During the adsorption process, two different kinds of interactions could occur.
An electrostatic interaction between the inhibitor species and the charged metal surface often causes physisorption. Because of the preadsorbed chloride/sulfate ions, the metal surface may acquire negative charges in an acidic environment. Physisorption can occur due to the inhibitor’s protonated molecules being attracted to the oppositely charged metal. In general, physisorption is a weaker kind of adsorption, and as the temperature rises, its bonding strength decreases (Mansfeld 1987). The chemisorption process often involves transferring electron pairs between inhibitor molecules and metal to produce a coordination bond. Inhibitor molecules’ heteroatoms and π-bonds interact with the metal’s vacant orbital. This interaction is more favorable when the metal has a lower energy vacant orbital. Higher temperatures cause a greater degree of chemisorption, which is correlated with a higher activation energy (Landolt 2007). Chemisorption is often facilitated by organic inhibitors containing heteroatoms, electron-releasing groups, and π-bonds (Morad 2007). Drug compounds showing these structural properties have the potential to be used as adsorption inhibitors because of their large molecular size, ready solubility in water, and safe for use.
3 Corrosion rate measurement techniques
The metal corrodes due to electrochemical reactions at the metal–solution interface. Several techniques have been developed over time to investigate the rate of corrosion. Traditional techniques such as weight loss (WL) and the gasometric method (GM) can be used to measure the corrosion rate. Nevertheless, electrochemical techniques, including electrochemical impedance spectroscopy (EIS), linear polarization (LP), and potentiodynamic polarization (PDP), are faster and more precise (Robert et al. 2003; Uhlig and Revie 2008). A brief account of these techniques is presented below.
The WL method measures the rate of corrosion in terms of metal loss. Metal specimens having a defined surface area are exposed to the corrosive media for a specified time, and the weight difference before and after the exposure is calculated.
Using the GM, the amount of hydrogen gas liberated is monitored at the predefined time intervals under constant temperature. The metal loss is computed as the amount of hydrogen gas released.
Liner polarization is a fast method to monitor the rate of corrosion. An incremental potential of 25 mV is applied above and below the corrosion potential, and the current obtained is plotted against the applied potential.
Using an electrochemical workstation, PDP and EIS techniques were performed in a conventional three-electrode cell. A saturated calomel electrode (SCE) is used as the reference electrode, the platinum electrode as the auxiliary electrode, and the specimen sample as the working electrode. Figure 2 represents the photograph of the electrochemical workstation.
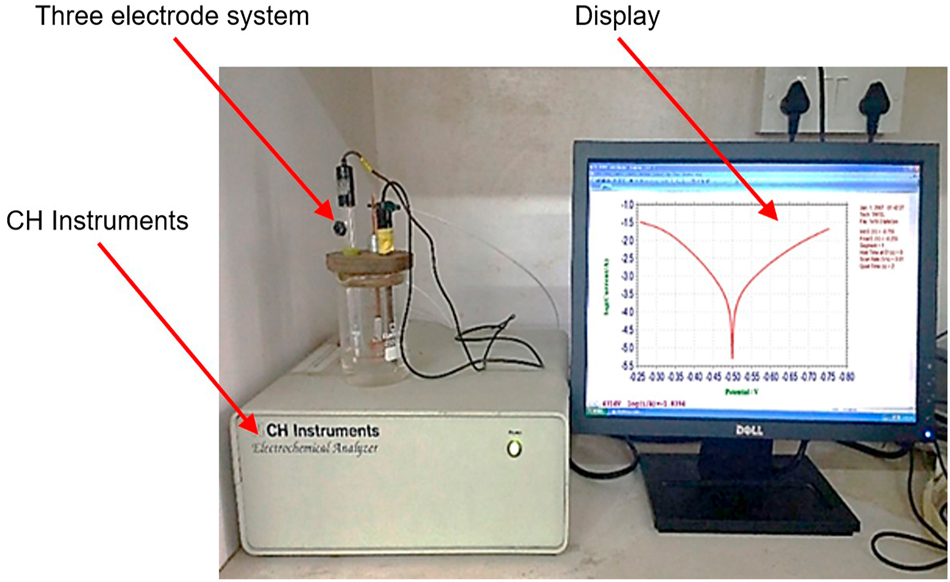
Photograph of the electrochemical workstation.
The Tafel polarization method is another name for the PDP method. At a potential scan rate of 0.1–10 mVs−1, the test material specimen is polarized +250 mV anodically and −250 mV cathodically from the corrosion potential (E corr ). The applied potential is plotted against the log of the current density, resulting in a graph. By extrapolating the linear segments of the anodic and cathodic branches of the polarization curves to the corrosion potential (E corr ), the corrosion current density (I corr ) is calculated from the plot. Supplementary Figure 1 (SF1) shows a typical Tafel plot.
In the EIS technique, a small amplitude sinusoidal potential is applied to the working electrode at several discrete frequencies to measure the alternating current (AC) impedance over a range of applied frequencies. In this technique, a small (10–20 mV) AC signal is superimposed on the electrochemical system of interest, and the system’s responses to this perturbation are measured. The resulting current waveform will have a sinusoidal reaction at each of these frequencies, which is somewhat out of phase with the applied potential signal. The impedance is the relationship between phase ᶲ at various frequencies, possible change, and current (∆E/∆I). The fundamental component (Z′) and imaginary component (Z″) can be used to express the impedance (Z). Plotting actual impedance (Z′) against imaginary impedance (−Z″) gives results in the form of Nyquist plot. In SF2, a typical Nyquist plot is shown.
The Nyquist plot is then analyzed by fitting the experimental curves to an equivalent electrical circuit composed of standard components like resistors, capacitors, and inductors. These circuit components must be physically related to the electrochemical parameters of the reaction. In the model circuit, an equivalent resistor represents the electrochemical characteristics, such as solution resistance and polarization resistance.
4 Drugs as inhibitors for carbon steel corrosion
Carbon steel (CS) contains carbon (up to 1.5 wt%) as the primary alloying element, with other elements (Mn, Si, P, S, etc.) present in smaller amounts (Dwivedi et al. 2017). It is commonly used in manufacturing many industrial products and structures due to its high mechanical strength, availability, and low cost. CS readily corrodes, particularly in an acid medium that can be readily controlled by the addition of a suitable drug inhibitor. A summary of these reported works is given in Table 1.
Drug inhibitors for carbon steel corrosion.
| Drug inhibitor (molecular structure in the Supplementary Material) | Medium, temperature | Dosages, inhibitor type, isotherm, adsorption mode | IE (%) | References |
|---|---|---|---|---|
| Carbamazepine Paracetamol (SF3) |
0.1 M H2SO4 0.25 M CH3COOH +0.25 M CH3COONa 298 K |
5 × 10−3 M Mixed 1 × 10−2 M Cathodic Mixed adsorption |
90 (PDP) 85 (PDP) |
Vaszilcsin et al. (2012) |
| Cefazolin Cefotaxime (SF4) |
0.1 M H2SO4 303 K |
5 × 10−4 M 7 × 10−4 M Mixed Langmuir Mixed adsorption |
99.6 (PDP), 95.8 (EIS), 96.7 (EFM). 90.9 (PDP),88.4 (EIS), 89.8 (EFM) |
Nazeer et al. (2013) |
| Phenytoin sodium (SF5) | 1 M HCl 298 K |
500 ppm Mixed Langmuir Physisorption |
79.1 (WL), 81.78 (PDP), 79 (EIS) | Al-Shafey et al. (2014) |
| Penicillin G Ampicillin Amoxicillin (SF 6) |
1 M HCl 298 K |
10 mM Mixed Langmuir Mixed adsorption |
98.4 (PDP), 95.9 (EIS) 97.5 (PDP), 95.5 (EIS) 93.0 (PDP), 93.7 (EIS) |
Golestani et al. (2014) |
| Salbutamol (SF7) | 1 M HCl 293 K |
20 % (V/V) Mixed Langmuir Mixed adsorption |
95.0 (PDP) | Attia (2015) |
| Melatonin (SF8) | 0.5 M H2SO4 303 K |
500 ppm Mixed Langmuir Physisorption |
94.76 (PDP), 93.90 (EIS) | Al-Fahemi et al. (2016) |
| Moxifloxacin (SF 9) | I M HCl 303 K |
300 ppm Mixed Langmuir Physisorption |
94.1 (WL), 92.0 (PDP), 92.0 (EIS) | Fouda et al. (2016) |
| Domperidone (SF10) | I M HCl 298 K |
1.12 × 10−4M Mixed Langmuir Mixed adsorption |
95.0 (WL), 94.9 (PDP), 94.7 (EIS) | Fouda et al. (2017a) |
| Carvedilol (SF11) | I M HCl 298 K |
1.6 × 10−4 M Mixed Langmuir Physisorption |
98.9 (WL), 98.1 (PDP), 98.6 (EIS) | Fouda et al. (2017b) |
| Podocip (SF12) | I M HCl 308 K |
100 mg L−1 Mixed Langmuir Mixed adsorption |
97.55 (PDP) 97.93 (EIS) | Dohare et al. (2018) |
| Cephapirin (SF13) | 2 M HCl 303 K |
600 ppm Mixed Temkin Mixed adsorption |
83.0 (WL), 85.1 (PDP), 79.4 (EIS) | El-Haddad et al. (2019) |
| Metformin hydrochloride (SF14) | 15 % HCl 298 K |
500 ppm Cathodic Langmuir Mixed adsorption |
77.79 (WL), 83.97 (EIS), 82.33 (PDP) | Haruna et al. (2020) |
| Ceftriaxone sodium, cefuroxime sodium, cefotaxime sodium (SF 15) | 0.1 M H2SO4 298 K |
1 × 10−3M Anodic Mixed adsorption |
80.8 (WL), 85.0 (PDP), 84.4 (EIS) 68.5 (WL), 71.8 (PDP), 71.5 (EIS) 74.7 (WL),79.2 (PDP), 78.2 (EIS) |
Guo et al. (2020) |
| Tobramycin (SF 16) | 2 M HCl 303 K |
500 ppm Mixed Langmuir Chemisorption |
90.5 (PDP), 84.3 (EIS) | Abeng et al. (2020) |
| Indomethacin (SF 17) | 1 M HCl 298 K |
500 ppm Mixed Langmuir Physisorption |
83.91 (WL), 79.61 (PDP), 82.37 (EIS) | Abdel Hameed et al. (2020) |
| Tenoxicam (SF18) | 0.5 M HCl 303 K |
4 × 10−4 M Mixed Temkin Mixed adsorption |
81.0 (PDP), 71.0 (EIS) | Elabbasy and Gadow (2020) |
| Levofloxacin, moxifloxacin Metolazone Nifedipine (SF 19) |
2 M HCl 303 K |
500 ppm Mixed Langmuir, Temkin, El-away, Frumkin and Flory Huggins Physisorption |
90.1 (PDP), 93.1(EIS) 86.7 (PDP), 83.5 (EIS) 89.9 (PDP), 92.8 (EIS) 89.6 (PDP), 89.2(EIS) |
Abeng et al. (2021) |
| Desloratidine (SF20) | 1 M HCl 303K |
19.3 × 10−5M Mixed Langmuir Mixed adsorption |
92.7 (WL), 85.2 (PDP) | Eid (2021) |
| Metformin (SF 21) | 3.5 wt% NaCl | 200 ppm Cathodic Langmuir Mixed adsorption |
89.47 (EIS), 86.31 (PDP) | Onyeachu et al. (2021) |
| Isosorbide dinitrate (SF 22) | 1 M HCl | 250 ppm Mixed Langmuir Mixed adsorption |
87.50 (WL), 81.39 (PDP), 85.43 (EIS). | EL-Etre et al. (2021) |
| Amoxicillin (SF 23) | 0.6 M NaCl 313 K |
6 × 10−3 M Mixed Langmuir Mixed adsorption |
82.15 (PDP) | Mahmoud et al. (2021) |
| Lioresal (SF24) | 1 M H2SO4 303 K |
500 ppm Mixed Langmuir Chemisorption |
94.7 (PDP) | Abdel Hameed et al. (2022) |
| Omeprazole (SF25) | 1 M HCl 1 M H2SO4 Room temperature |
40 mg/L Mixed Langmuir Mixed adsorption |
92 (WL), 84 (PDP) 90 (WL),78 (PDP) |
Tsygankova et al. (2022) |
| Tetracycline (SF 26) | 1 M HCl 298 K |
300 ppm Mixed Langmuir Physisorption |
82 (PDP), 78 (EIS) | Shojaee et al. (2022) |
| Naproxen (SF 27) | 1 M HCl 298 K |
8 × 10−3 M Mixed Langmuir Chemisorption |
94.96 (WL) | Shams et al. (2023) |
| Sulbutiamine (SF 28) | 1 M HCl 298 K |
300 ppm Mixed Temkin and Freundlich Mixed adsorption |
95.8 (PDP), 94.8 (EFM), 94.6 (EIS) | Alghamdi et al. (2023) |
| Glucosamine sulfate, glucosamine hydrochloride (SF 29) | 0.5 M H2SO4 Room temperature |
5 mM 10 mM Mixed Mixed adsorption |
44.5 (PDP) 82.2 (PDP) |
Feng et al. (2023) |
| Anaprilin (SF30) | 1 N HCl 1 N H2SO4 Room temperature |
80 mg/L Anodic Langmuir Phisisorption |
79 (PDP) and 94 (EIS) in 1 N HCl 80 (PDP), 86 (EIS) in 1 N H2SO4 |
Tsygankova et al. (2024) |
| Furosemide (SF 31) | 1 M HCl 298 K |
300 ppm Mixed Temkin Mixed adsorption |
83.2 (WL), 87.6 (PDP), 82.5 (EIS) | Abd El Maksoud et al. (2024) |
| Salbutamol sulfate (SF 32) | 1 M HCl 298 K |
200 ppm Mixed Langmuir Physisorption |
85.32 (WL), 79.13 (PDP), 89.65 (EIS) | Al-Gorair et al. (2024) |
5 Drug inhibitors for mild steel corrosion
Mild steel (MS), sometimes called “low carbon steel,” has carbon content in the 0.05 %–0.25 % range. Due to its low carbon content, MS is more ductile and easier to shape, form, and weld than other steel forms. MS has good machinability and can be easily drilled, cut, and fabricated into various shapes and sizes. MS is among the most widely used in the construction and automotive industries and innumerable other applications because of its good weldability, machinability, and ductility (Shoesmith 1987). However, MS exhibits poor corrosion resistance, particularly in an aggressive acid medium (such as HCl and H2SO4). The corrosion of MS can best controlled using expired drugs as inhibitors, and a summary of such reported research works is presented in Table 2.
Drug inhibitors for mild steel corrosion.
| Drug inhibitor (molecular structure in the Supplementary Material) | Medium, temperature | Dosages, inhibitor type, isotherm, adsorption mode | IE (%) | References |
|---|---|---|---|---|
| Sulfadiazine Sulfamethoxazole Sulfamethazine Sulfaguanidine (SF 33) |
1 M HCl 298 K |
5 mM 5 mM 5 mM 0.75 mM Cathodic |
94.0 (PDP) 92.3 (PDP) 89.5 (PDP) 67.7 (PDP) |
El-Naggar (2007) |
| Penicillin V potassium (SF 34) | 2.5 M H2SO4 303 K |
15 × 10−4 M Langmuir and Frumkin Physisorption |
63.33 (GM) 52.33 (TM) | Eddy and Odoemelam (2008) |
| Sparfloxacin (SF 35) | HCl 303 K |
12 × 10−4 M Langmuir Physisorption |
97.47 (WL) 96.67 (GM) 82.30 (TM) | Eddy et al. (2008) |
| Ceftriaxone (SF36) | 1 N HCl 308 K |
400 ppm Mixed Langmuir Mixed adsorption |
90.10 (WL) 92.59 (PDP) 87.59 (EIS) | Shukla and Quraishi (2009a) |
| Cefotaxime sodium (SF 37) | 1 M HCl 308 K |
300 ppm Mixed Langmuir Mixed adsorption |
95.8 (WL) 93.5 (PDP) 90.0 (EIS) |
Shukla and Quraishi (2009b) |
| Streptomycin (SF 38) | 1 M HCl 308 K |
500 ppm Mixed Chemisorption |
88.5 (WL) 84.5 (PDP) 83.9 (EIS) |
Shukla et al. (2009) |
| Doxycycline (SF 39) | 1 M HCl 308 K |
9.02 × 10−4 M Mixed Langmuir Physisorption |
94.7 (WL) 96.3 (PDP) 95.9 (EIS) |
Shukla and Quraishi (2010) |
| Penicillin G Amoxicillin Penicillin V potassium (SF 40) |
0.1 M HCl 303 K |
3 × 10−4 M Mixed Langmuir Physisorption |
95.26 (GM) 94.29 (GM) 81,47 % (GM) |
Okon Eddy and Ebenso (2010) |
| Pheniramine (SF 41) | 1 M HCl 308 K |
0.833 mM Mixed Langmuir Physisorption |
98.0 (WL) 86.0 (LPR) 91.2 (PDP) 86.4 (EIS) |
Ahamad et al. (2010a) |
| Fexofenadine drug (SF 42) | 1 M HCl | 3.0 × 10-4 M Mixed Langmuir Chemisorption |
97 (WL) 94 (PDP) 98 (EIS) |
Ahamad et al. (2010b) |
| Ciprofloxacin Norfloxacin Ofloxacin (SF 43) |
1 M HCl 303 K |
3.16 × 10−4 M Mixed Mixed adsorption |
92.0 (WL) 91.4 (PDP) 90.9 (EIS) 91.4 (WL) 87.1 (PDP) 87.2 (EIS) 91.0 (WL) 82.1 (PDP) 84.1 (EIS) |
Xuehui et al. (2010) |
| Disulfiram (SF 44) | 1 M HCl 308 K |
0.337 mM Mixed Langmuir Physisorption |
96.0 (PDP) 89.0 (EIS) |
Singh and Quraishi (2011a) |
| Ceftobiprole (SF 45) | 1 M HCl 308 K |
7.45 × 10-4 M Mixed Langmuir Mixed adsorption |
91.60 (WL) 90.50 (PDP) 91.21 (EIS) | Singh and Qurashi (2011b) |
| Cefixime (SF 46) | 1 M HCl 303 K |
8.8 × 10−4 M Mixed Langmuir Mixed adsorption |
90.0 (WL) 96.3 (PDP) 91.6 (EIS) |
Naqvi et al. (2011) |
| Cefadroxil (SF 47) | 1 M HCl 308 K |
11.0 × 10−4 M Mixed Langmuir Physisorption |
95.0 (WL) 95.2 (PDP) 95.3 (EIS) |
Shukla et al. (2011) |
| Cefuroxime (SF 48) | 1 M HCl 303 K |
200 ppm Mixed Langmuir Chemisorption |
93.4 (PDP) 91.8 (EIS) |
Singh et al. (2011a) |
| Ceftazidime (SF 49) | 0.5 M HCl 308 K |
250 ppm Mixed Langmuir Physisorption |
90.2 (PDP) 87.5 (EIS) | Singh et al (2011b) |
| Ranitidine (SF 50) | 1 M HCl 308 K |
400 ppm Mixed Langmuir Chemisorption |
89 (WL) 90 (PDP) 92 (EIS) |
Abdel Hameed (2011) |
| Metformin (SF 21) | 1 M HCl 308 K |
400 ppm Mixed Langmuir Mixed adsorption |
95 (WL), 93 (PDP) 96 (EIS) |
Singh et al. (2012) |
| Rabeprazole sulfide (SF 51) | 0.5 M H2SO4 303 K |
1.0 mM Mixed Langmuir Mixed adsorption |
98.0 (WL) 98.25 (PDP) 95.09 (EIS) | Pavithra et al. (2012) |
| Ciprofloxacin (SF 52) | 0.1 M HCl 308 K |
2.57 × 10-3 M Mixed Langmuir Physisorption |
86 (WL) | Akpan and Offiong (2013) |
| Amoxicillin (SF 53) | 0.1 M HCl 303 K |
0.5 g/L Mixed Langmuir Physisorption |
84.77 (WL) | Siaka et al. (2013) |
| Chloroquine (SF 54) | 1 M HCl 308 K |
3.1 × 10−4M Mixed Langmuir Chemisorption |
98.4 (WL) 97.9 (PDP) 96.9 (EIS) |
Singh et al. (2013a) |
| Cephamycin (SF 55) | 1 M HCl 303 K |
300 ppm Mixed Mixed adsorption |
88.7 (PDP) 90.0 (EIS) | Singh et al. (2013b) |
| Cephalexin (SF 56) | 0.1 M HCl 303 K |
13 × 10−3 M Langmuir Mixed adsorption |
86.8 (WL) 80.0 (PDP) |
Akpan and Offiong (2014) |
| Aspirin (SF 57) | 0.5 M H2SO4 303K |
3 × 10−3 M Langmuir Physisorption |
71.80 (WL) | Kushwah and Pathak (2014) |
| Ambroxol (SF 58) | 1 M HCl 1 M H2SO4 333 K |
9 % (v/v) Mixed Langmuir Mixed adsorption |
94.75 (WL-HCl) 82.52 (WL- H2SO4) |
Geethamani and Kasthuri (2015) |
| Asthalin (SF 59) | 1 M HCl 1 M H2SO4 333 K |
9 % (v/v) Mixed Langmuir Mixed adsorption |
55.53 (PDP) 55.0 (EIS) 75.56 (PDP) 69.2 (EIS) |
Geethamani and Kasthuri (2016) |
| Atenolol (SF 60) | 1 M HCl | 300 ppm Mixed Langmuir Mixed adsorption |
92.8 (WL) 93.8 (PDP) 92.5 (EIS) |
Karthik and Sundaravadivelu. (2016) |
| Clozapine (SF 61) | 1 M HCl 303 K |
10−3 M Mixed Langmuir Mixed adsorption |
97.4 (WL) 95.6 % (PDP) 96.2 (EIS) |
Lgaz et al. (2016) |
| Nebicard (SF 62) | 1 M HCl Room temperature |
100 ppm Mixed Langmuir Chemisorption |
97.0 (PDP) 97.5 (EIS) |
Srivastava et al. (2016) |
| Amoxicillin, cefixime, cephalexin (SF 63) | 1 M H2SO4 303 K |
0.001 M Langmuir Physisorption |
60.95 (WL) 80.06 (WL) 76.94 (WL) |
Raheem Z. A. (2016) |
| Phenylalanine Rutin (SF 64) |
1 M HCl 303 K |
0.018 M Langmuir Chemisorption |
83.78 (WL) 90.40 (WL) |
Ngobiri and Okorosaye-Orubite (2017) |
| Ondansetron hydrochloride (SF 65) | 1 M HCl 303 K |
300 ppm Mixed Langmuir Physiisorption |
90.40 (WL) 90.57 (PDP) 88.56 (EIS) | Vengatesh et al. (2017) |
| Tramadol (SF 66) | 1 M HCl 308 K |
100 ppm Mixed Langmuir Mixed adsorption |
96.12 (WL) 97.10 (WL) 97.20 (WL) |
Dohare et al. (2017) |
| Irbesartan (SF67) | 1 M HCl 0.5 M H2SO4 299 K |
300 ppm Mixed Langmuir Mixed adsorption |
95 (PDP) 91(EIS) -HCl 83 (PDP) 81(EIS)- H2SO4 |
Srivastava et al. (2017) |
| Atenolol nifedipine (SF 68). | 1 M HCl 308 K |
200 ppm Cathodic Mixed adsorption |
91.04 (PDP) 93.29 (EIS) 93.13 (PDP) 95.61 (EIS) |
Gupta et al. (2017) |
| Atorvastatin (SF 69) | 1 M HCl Room temperature |
150 ppm Mixed Langmuir Mixed adsorption |
97.05 (WL) 99.08 (PDP) 96.38 (EIS) |
Singh et al. (2017) |
| Biotin (SF 70) | 15 % HCl 308 K |
500 ppm Mixed Langmuir Mixed adsorption |
95.3 (WL) 97.0 (PDP) 97.0 (EIS) |
Xu et al. (2017) |
| Gentamicin (SF 71) | 1 M HCl 303 K |
0.9 %(v/v) Mixed Langmuir Mixed adsorption |
76.65 (WL) 74.13 (PDP) 78.44 (EIS) | Srinivasulu and Kasturi (2017) |
| Thiamine hydrochloride Biotin (SF 72) |
250 ppm chloride solution | 200 ppm Mixed Mixed adsorption |
91.42 (WL) 90.57 (PDP) 87.96 (EIS) 91.19 (WL) 91.19 (PDP) 86.61 (EIS) |
Aloysius et al. (2018) |
| Ethambutol (SF 73) | 0.5 M HCl 303 K |
1,000 ppm Mixed Langmuir Physisorption |
99.60 (WL) 97.6 (PDP) 93.72 (EIS) | Dahiya et al. (2018) |
| Analgin (SF 74) | 1 M HCl 298 K |
4,000 ppm Mixed Langmuir Physisorption |
96.0 (WL) 96.25 (PDP) 92.47 (EIS) | Bashir et al. (2018) |
| Co-Amoxiclav (SF 75) | 1 N HCl 298K |
15 × 10−4 M Mixed Temkin Physisorption |
88.2 (WL) 88.0 (PDP) 87.8 (EIS) | Jeeva et al. (2019) |
| Rosuvastatin (SF 76) | 1 M HCl 0.5 M H2SO4 298 K |
600 ppm Mixed Langmuir Mixed adsorption |
88 (PDP-HCl) 90 (PDP- H2SO4) |
Gholamhosseinzadeh et al. (2019) |
| Pyrazinamide (a) Isoniazid (b) Rifampicin (c) (SF 77) |
0.5 M HCl 303 K |
1,000 ppm Mixed (a & c) Anodic (b) Langmuir Mixed adsorption |
WL-92.5 (a), 82.2 (b), 94.7 (c) PDP-90.79 (a), 98.08 (b), 97.06 (c) EIS-95.86(a), 97.89 (b), 96.67 (c) |
Dahiya et al. (2019) |
| Sulfaguanidine (SF 78) | 3 % HCl 303 K |
0.008 M Mixed Langmuir Physisorption |
97.61 (WL) | Mahmmod (2019) |
| Dapsone-benzaldehyde (a) Dapsone-salicylaldehyde (b) (SF 79) |
0.5 M H2SO4 298 K |
0.219 mM Mixed Langmuir Mixed adsorption |
WL-95 (a), 94 (b) PDP- 93.14 (a), 86.86 (b) EIS- 95.12 (a), 92.53 (b) |
Singh et al. (2019a) |
| Cefdinir (SF 80) | 1 M HCl 308 K |
5.32 × 10−4 M Mixed Langmuir Mixed adsorption |
97.9 (WL) 95.6 (PDP) 96.9 (EIS) |
Singh et al. (2019b) |
| Rabeprazole sodium (a) Domperidone (b) Benfotiamine (c) (SF 81) |
3.5 % NaCl Room temperature |
0.5 mg Mixed Mixed adsorption |
WL-98.52 (a), 98.81 (b), 98.92 (c) PDP-63.0 (a), 67.0 (b), 58.0 (c) |
Palaniappan et al. (2019) |
| Helicure drug (omeprazole and tinidazole) (SF 82) | 1 M HCl 298 K |
300 ppm Mixed Langmuir Mixed adsorption |
83.2 (WL) 85.8 (PDP) 83.8 (EIS) |
Al-Nami (2020) |
| Metronidazole (SF 83) | 1 M HCl Room temperature |
200 ppm Mixed adsorption |
83.77 (WL) | Manal and Hamzah (2020) |
| d-Penicillamine (a) l-Cysteine (b) (SF 84) |
1 M HCl 328 K |
5 mM Mixed Langmuir Mixed adsorption |
(a)-75 (PDP), 75 (EIS) (b)-91 (PDP), 85 (EIS) |
Farahati et al. (2020) |
| Formoterol (SF 85) | 1 M H2SO4 308 K |
300 ppm Mixed Langmuir Physisorption |
95 (WL) 95 (PDP) 98 (EIS) |
Ma et al. (2020) |
| Ampicillin (a) Flucloxacillin (b) (SF 86) |
1 M H2SO4 293 K |
400 ppm Mixed Langmuir Physisorption |
WL-93.17 (a), 88.20 (b) PDP-93.95 (a), 90.93 (b) EIS-91.02 (a), 88.99 (b) |
Alfakeer et al. (2020) |
| Glibenclamide (a) Glimepiride (b) (SF 87) |
1 M H2SO4 298 K |
500 ppm Mixed Langmuir Physisorption |
WL-86.3 (a), 86.3 (b) PDP-85.1 (a), 88.0 (b) EIS- 86.5 (a), 90.4 (b) |
Abdallah et al. (2020) |
| Dexamethasone (SF 88) | 2 M HCl 333K |
0.4 g/L Mixed Langmuir Physisorption |
80.17 (WL) 83 (PDP) 81.8 (EIS) | Anadebe et al. (2020) |
| Enprofylline (SF 89) | 1 M H2SO4 Room temperature |
200 ppm Mixed Mixed adsorption |
97.0 (PDP) 96.0 (EIS) |
Liangtian et al. (2020) |
| Acarbose (a) Voglibose (b) Miglitol (c) (SF 90) |
700 ppm NaCl solution Room temperature |
100 ppm Anodic Langmuir Physisorption |
WL-86.52 (a), 84.58 (b), 82.52 (c) PDP-91.67 (a), 90.49 (b), 88.54 (c) EIS-82.92 (a), 82.26 (b), 76.80 (c) |
Sundaram et al. (2021) |
| Carbimazole (SF 91) | 0.5 M HCl 303 K |
100 ppm Mixed Langmuir Physisorption |
84.95 (WL) | Al-Abbassi and Shana (2021) |
| Cefalexin (SF 92) | 65 % NaCl solution | 100 pp Mixed Langmuir and Freundlich Physisorption |
65.84 (PDP) | Fayomi et al. (2021) |
| Diphenhydramine hydrochloride (SF 93) | 1 M HCl Room temperature |
1,000 ppm Mixed Langmuir Mixed adsorption |
86.60 (PDP) 93.86 (EIS) |
Ghaderi et al. (2022) |
| Ampicillin (SF 94) | 5 M HCl 328 K |
20 mM Mixed Langmuir Mixed adsorption |
96.7 (WL) 95.5 (PDP) 95.0 (EIS) |
Alamry et al. (2023) |
| Azithromycin (SF 95) | 2 M HCL 303 K |
800 ppm Mixed Langmuir Physisorption |
81.4 (GM) | Ikeuba et al. (2023) |
| Amiodarone (SF 96) | 1 M HCl 303 K |
0.001 M Cathodic Temkin Mixed adsorption |
88.77 (PDP) | Sheit et al. (2024) |
| Rivaroxaban (SF 97) | 1 M HCl 298 K |
40 μM Mixed Langmuir Chemisorption |
93.48 (PDP) 92.85 (EIS) | Ashassi-Sorkhabi et al. (2024) |
| Glucored Forte (SF 98) | 2 M HCl 303 K |
300 ppm Mixed Temkin Physisorption |
85.7 (WL) | Essien et al. (2024) |
| Sertraline (SF 99) | 1 M HCl 303 K |
50 ppm Mixed Langmuir Mixed adsorption |
87.08 (WL) 88.36 (PDP) 93.30 (EIS) | Narang et al. (2024) |
| Prinivil (SF 100) | 1 M HCl 298 K |
500 ppm Mixed Langmuir Mixed adsorption |
93.75 (WL) 87.08 (PDP) 97.35 (EIS) |
Thakur et al. (2024) |
A comparison of inhibition efficiency of drug inhibitors.
| Drug inhibitor | Metal and medium | Inhibition efficiency | References |
|---|---|---|---|
| Cefazolin | CS, 0.5 M H2SO4 | 99.6 % at 5 × 10−4 M concentration and 303 K | Nazeer et al. (2013) |
| Ethambutol | MS, 0.5 M HCl | 99.60 % at 1,000 ppm concentration and 303 K | Dahiya et al. (2018) |
| Atorvastatin | MS, 1 M HCl | 99.08 % at 150 ppm | Singh et al. (2017) |
| Carvedilol | CS, 1 M HCl | 98.9 % at 1.6 × 10−4 M concentration and 298 K | Fouda et al. (2017b) |
| Chloroquine | MS, 1 M HCl | 98.4 % at 3.1 × 10−4 M and 308 K | Singh et al. (2013). |
| Penicillin G | CS, 1 M HCl | 96–98.4 % at 10 mM concentration levels and 298 K. | Golestani et al. (2014) |
| Rabeprazole sulfide | MS 0.5 M H2SO4 | 98.25 % at 1.0 mM and 303 K | Pavithra et al. (2012) |
| Pyrazinamide (a), isoniazid (b), and rifampicin (c) | MS, 0.5 M HCl | (a) (98.08 %) > (c) (97.06 %) > (b) (95.86) at 1,000 ppm concentration and 303 K | Dahiya et al. (2019) |
| Pheniramine | MS, 1 M | 98 % at 0.833 mM concentration and 308 K | Ahamad et al. (2010a) |
| Fexofenadine | MS, 1 M HCl | 98 % at 3.0 × 10−4 M | Ahamad et al. (2010b) |
| Formoterol | MS, 1 M H2SO4 | 98 % at 300 mg/L concentration and 308 K | Ma et al. (2020) |
| Cefdinir | MS, 1 M HCl | 97.9 % at 5.32 × 10−4 M and 308 K | Singh et al. (2019) |
| Sulfaguanidine | MS, 3 % HCl | 97.61 % at 0.008 M and 303 K | Mahmmod (2019) |
| Nebicard | MS, 1 M HCl | 97.5 % at 100 ppm concentration | Srivastava et al. (2016) |
| Clozapine | MS, 1 M HCl | 97.4 % at 10−3 M and 303 K | Lgaz et al. (2016) |
| Sparfloxacin | MS, HCl | 97.4 % at 12 × 10−4 M and 303 K | Eddy et al. (2008) |
| Tramadol | MS, 1 M HCl | 97.2 % 100 mgL−1 concentration and 308 K | Dohare et al. (2017) |
| Biotin | MS, 15 % Hl | 97 % at 500 ppm and 308 K | Xu et al. (2017) |
| Enprofylline | MS, 1 M H2SO4 | 97 % at 200 mg/L concentration | Liangtian et al. (2020) |
| Podocip | CS, 1 M HCl | 96.93 % at 100 mg L−1 concentration and 308 K | Dohare et al. (2018) |
| Ampicillin | MS, 5 M HCl | 96.7 % at 20 mM concentration and 328 K | Alamry et al. (2023) |
| Doxycycline | MS, 1 M HCl | 96.3 % at 9.02 × 10−4 M and 308 K | Shukla and Quraishi (2010) |
| Analgin | MS, 1 M HCl | 96.25 % at 4,000 ppm level and 298 K | Bashir et al. (2018) |
| Metformin | MS, 1 M HCl | 96 % at 400 ppm concentration level and 308 K | Singh et al. (2012) |
| Sulbutiamine | CS, 1 M HCl | 95.8 % 300 ppm and 298 K | Alghamdi et al. (2023) |
| Cefotaxime sodium | MS, 1 M HCl | 95.8 % at 300 ppm and 308 K | Shukla and Quraishi (2009b) |
| Dapsone-benzaldehyde (a) and dapsone-salicylaldehyde (b) | MS, 0.5 M H2SO4 | 95.67 % (a) & 95 % (b) at 0.219 mM and 298 K | Singh et al. (2019) |
| Nifedipine | MS, 1 M HCl | 95.61 % at 200 ppm concentration | Gupta et al. (2017) |
| Cefadroxil | MS, 1 M HCl | 95.3 % at 11.0 × 10−4 M and 308 K | Shukla et al. (2011) |
| Penicillin G | MS, 0.1 M HCl | 95.26 % at 3 × 104 M and 303 K | Okon Eddy and Ebenso (2010) |
| Salbutamol | CS, 1 M HCl | 95 % at 20 % (v/v) concentration and 293 K | Attia (2015) |
6 Mechanism of inhibition
The adsorption of drug molecules at the metal–solution interface is typically the mechanism for inhibiting corrosion in an acidic medium. During inhibition, drug molecules can adsorb at the metal–solution interface in different ways (Negm et al. 2012; Solmaz et al. 2008; Singh 2012) as mentioned below:
By the displacement of water molecules from the metal surface,
The charged metal and protonated drug species can electrostatically attract with each other (physisorption),
Donor–acceptor interaction of unshared electron pairs and π-electrons in the drug molecule with the unoccupied d-orbital of Fe atoms on MS (chemisorption), and
A combination of the above processes.
The drug inhibitor molecule can easily undergo protonation in an acid medium. For example, metformin drugs (Singh et al. 2018) can easily protonate in an acid medium. The protonated drug can electrostatically be attracted toward the chloride/sulfate ions preadsorbed on the metal surface, leading to physisorption (Figure 3). The electron pairs on the N-atom can be transferred to the d-orbital of iron atoms at the metal surface, resulting in chemisorption (Figure 3).
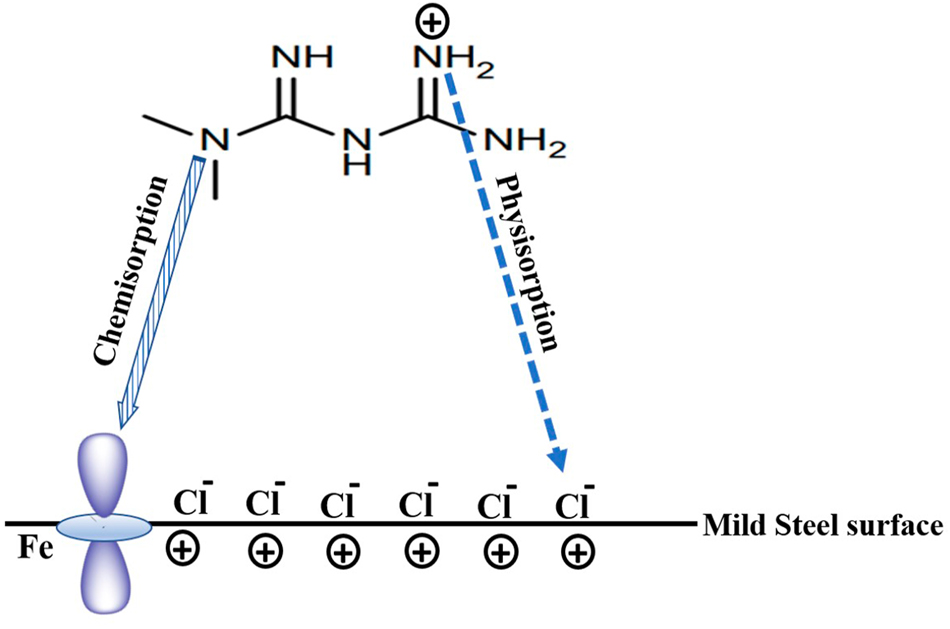
Physical and chemical adsorption mode of metformin drug in HCl medium on mild steel surface.
7 Comparison of inhibition performances
Different researchers have demonstrated the efficient usage of expired drugs as corrosion inhibitors for different metals. A comparison of the inhibition performances shown by some of the excellent drug inhibitors for carbon and mild steel in an acidic environment is summarized in table. Cefazolin drug structure (SF2) contains active functional groups (–NH2, –OH, >C=O) and multiple hetero atoms (N, O, S), which result in its stronger adsorption on CS surface and also cover larger surface area due to its large molecular size. Hence, cefazolin exhibited a maximum IE of 99.6 % at a low dosage of 5 × 10−4 M and 303 K against CS corrosion in 0.5 M H2SO4 medium. A similar inhibition performance (99.60 %) was exhibited by ethambutol drug at 1,000 ppm against MS in 0.5 M HCl medium, which may be due to its stronger adsorption through an active functional group (–OH) and heteroatoms (N, O) as well as its planar structure. Other expired drugs (Table 3) have demonstrated impressive inhibition performances (≥95 %) based on their structural characteristics, such as the presence of active functional groups, heteroatoms, linearity, and large molecular size, leading to stronger adsorption.
8 Existing challenges
Some of the challenges encountered in the usage of expired drugs as corrosion inhibitors are highlighted below:
Since most drugs are insoluble alkaline solutions, the medium such as sodium chloride are rarely employed in the corrosion study experiments.
When a drug comprises multiple active compounds, deciding which contributes the most to corrosion inhibition can be difficult.
The ability and durability of drugs should be examined after 1 month, 6 months, 1 year, or 2 years of expiry date. This is essentially required to confirm the chemical nature of drugs after the expiry date. The change in the chemical nature may affect the inhibition performance exhibited by the expired drug and otherwise lead to environmental pollution if the expired drug finally changes into the toxic form.
9 Conclusions
Many expired drugs have been tested and proven as efficient, eco-friendly carbon and mild steel inhibitors. They have displayed good inhibition efficiency in the 95–99 % range in acid medium against carbon and mild steel corrosion. The discussion outcomes also showed that expired drugs are excellent and environmentally beneficial substitutes for traditional harmful corrosion inhibitors. The corrosion inhibition mechanism involves either physical or chemical adsorption of drug molecules on the metal surface. The chemical composition and molecular structure of drugs significantly influence their inhibition activity. The heteroatoms, aromatic rings, and active functional groups in the drug molecules with large molecular sizes can easily produce a protective film on the metal sample, efficiently controlling corrosion. Most drug materials are soluble in acid medium but showed lower solubility in neutral/ alkaline medium. This made them preferable for use in acidic solutions. The inhibition performance was assessed using different methods such as weight loss, gasometric method, potentiodynamic polarization, and electrochemical impedance spectroscopy. The results obtained by these methods are further supported by SEM, FEM, AFM, UV, FTIR, and DFT analysis. Further studies are needed to ensure the suitability of expired drugs for industrial applications. Using expired drugs as inhibitors alternatively helps in their disposal. Using outdated drugs as inhibitors also encounters difficulties that must be resolved appropriately.
Acknowledgments
The author is indebted to the management of Alva’s Institute of Engineering and Technology, Alva’s Education Foundation, Moodbidri for the support.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: Prakasha Shetty: conceptualization; data curation; formal analysis; resources; supervision; writing – original draft; writing – review & editing. The author has accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: Not applicable.
-
Conflict of interest: The author states no conflict of interest.
-
Research funding: No funding was obtained.
-
Data availability: Not applicable.
References
Abd El Maksoud, S., Abd El Aziz, F., and Badawy, H. (2024). Furosemide drug as a corrosion inhibitor for carbon steel in 1.0 M hydrochloric acid. Sci. Rep. 14: 9052, https://doi.org/10.1038/s41598-024-58713-4.Search in Google Scholar PubMed PubMed Central
Abdallah, M., Fawzy, A., and Alfakeer, M. (2020). Inhibition potentials and adsorption performance of two Sulfonylurea antibiotic expired drugs on the corrosion of mild steel in 0.5 M H2SO4. Int. J. Electrochem. Sci. 15: 10289–10303, https://doi.org/10.20964/2020.10.37.Search in Google Scholar
Abdel Hameed, R.S. (2011). Ranitidine drugs as non-toxic corrosion inhibitors for mild steel in hydrochloric acid medium. Portugaliae Electrochim. Acta. 29: 273–285, https://doi.org/10.4152/pea.201104273.Search in Google Scholar
Abdel Hameed, R.S., Ismail, E.A., Al-Shafey, H.I., and Abbas, M.A. (2020). Expired Indomethacin therapeutics as corrosion inhibitors for carbon steel in 1.0 M hydrochloric acid media. J. Bio- and Tribo-Corros. 6: 114, https://doi.org/10.1007/s40735-020-00403-5.Search in Google Scholar
Abdel Hameed, R.S., Essa, A., Nassar, A., Badr, M., Huwaimel, B., Saedah, R., Al-Mhyawi, S.R., Alshammary, F., Seni, A.A., and Abdallah, M. (2022). Chemical and electrochemical studies on expired Lioresal drugs as corrosion inhibitors for carbon steel in Sulfuric Acid. J. New Mat. Electrochem. Systems. 25: 268–27619.10.14447/jnmes.v25i4.a07Search in Google Scholar
Abeng, F.E., Anadebe, V.C., Idim, V.D., and Edim, M.M. (2020). Anti-corrosion behaviour of expired Tobramycin drug on carbon steel in acidic medium. S. Afr. J. Chem. 73: 125–130, https://doi.org/10.17159/0379-4350/2020/v73a18.Search in Google Scholar
Abeng, F.E., Ikpi, M.E., Ushie, O.A., Anadebe, V.C., Nyong, B.E., Obeten, M.E., Okafor, N.A., Chukwuike, V.I., and Nkom, P.Y. (2021). Insight into corrosion inhibition mechanism of carbon steel in 2 M HCl electrolyte by eco-friendly based pharmaceutical drugs. Chem. Data Coll. 34: 100722, https://doi.org/10.1016/j.cdc.2021.100722.Search in Google Scholar
Ahamad, I., Prasad, R., and Quraishi, M.A. (2010a). Inhibition of mild steel corrosion in acid solution by Pheniramine drug: experimental and theoretical study. Corros. Sci. 52: 3033–3041, https://doi.org/10.1016/j.corsci.2010.05.022.Search in Google Scholar
Ahamad, I., Prasad, R., and Quraishi, M.A. (2010b). Experimental and theoretical investigations of adsorption of fexofenadine at mild steel/hydrochloric acid interface as a corrosion inhibitor. J. Solid State Electrochem. 14: 2095–2105, https://doi.org/10.1007/s10008-010-1041-9.Search in Google Scholar
Akpan, I.A. and Offiong, N.O. (2013). Inhibition of mild steel corrosion in hydrochloric acid solution by Ciprofloxacin drug. Inter. J. Corros. 2013: 301689, https://doi.org/10.1155/2013/301689.Search in Google Scholar
Akpan, I.A. and Offiong, N.O. (2014). Electrochemical and gravimetric studies of thecorrosion inhibition of mild steel in HCl medium by cephalexin drug. American J. Chem. Mater. Sci. 1: 1–6.10.18488/journal.65/2014.1.2/65.2.10.18Search in Google Scholar
Al-abbassi, A.A. and Shana, I.E. (2021). Corrosion inhibition of mild steel in acidic media by expired Carbimazole drug. Sebha University J. Pure. Appl. Sci. 20: 176–180, https://doi.org/10.51984/JOPAS.V20I2.1646.Search in Google Scholar
Alamry, K.A., Khan, A., Aslam, J., Hussein, M.A., and Aslam, R. (2023). Corrosion inhibition of mild steel in hydrochloric acid solution by the expired Ampicillin drug. Sci. Rep. 13: 6724, https://doi.org/10.1038/s41598-023-33519-y.Search in Google Scholar PubMed PubMed Central
Al-Fahemi, J.H., Abdallah, M., and Elshafie, G.A.M. (2016). Experimental and theoretical approach studies for melatonin drug as safe corrosion inhibitors for carbon steel using DFT. J. Mol. Liq. 222: 1157–1163, https://doi.org/10.1016/j.molliq.2016.07.085.Search in Google Scholar
Alfakeer, M., Abdallah, M., and Fawzy, A. (2020). Corrosion inhibition effect of expired Ampicillin and Flucloxacillin drugs for mild steel in aqueous acidic medium. Int. J. Electrochem. Sci. 15: 3283–3297, https://doi.org/10.20964/2020.04.09.Search in Google Scholar
Alghamdi, R.D., Alghamdi, M.D., and Gadow, H.S. (2023). Use of expired Sulbutiamine drug as anticorrosive agent for carbon steel in hydrochloric acid solution. Int. J. Corros. Scale Inhib. 12: 2282–2326, https://doi.org/10.17675/2305-6894-2023-12-4-45.Search in Google Scholar
Al-Gorair, A.S., Felaly, R., Fouad, N., Al-Juaidd, S.S., Seyam, D.F., Saadan, N., El-Etre, A.Y., Mabrouk, E.M., and Abdallah, M. (2024). Corrosion kinetics of carbon steel in hydrochloric acid and its inhibition by recycling Salbutamol extracted from expired Farcolin drug. Green Chem. Lett. Rev. 17: 2413418, https://doi.org/10.1080/17518253.2024.2413418.Search in Google Scholar
Al-Nami, S.Y. (2020). Investigation of adsorption and inhibitive effect of expired Helicure drug on mild steel corrosion in hydrochloric acid solution. Int. J. Electrochem. Sci. 15: 2685–2699, https://doi.org/10.20964/2020.03.34.Search in Google Scholar
Aloysius, A., Ramanathan, R., Christy, A., Baskaran, S., and Antony, N. (2018). Experimental and theoretical studies on the corrosion inhibition of vitamins – Thiamine hydrochloride or biotinin corrosion of mild steel in an aqueous chloride environment. Egypt. J. Petrol. 27: 371–381, http://creativecommons.org/licenses/by-nc-nd/4.0/.10.1016/j.ejpe.2017.06.003Search in Google Scholar
Al-Shafey, H.I., Abdel Hameed, R.S., Ali, F.A., Abd el-Aleem, S., and Aboul-Magd, M.S. (2014). Effect of expired drugs as corrosion inhibitors for carbon steel in 1M HCl solution. Int. J. Pharm. Sci. Rev. Res. 27: 146–152.Search in Google Scholar
Anadebe, V.C., Onukwuli, O.D., Abeng, F.E., Okafor, N.A., Ezeugo, J.O., and Okoye, C.C. (2020). Electrochemical-kinetics, MD-simulation, and multi-input single-output (MISO) modeling using adaptive neuro-fuzzy inference system (ANFIS) prediction for dexamethasone drug as eco-friendly corrosion inhibitor for mild steel in 2M HCl electrolyte. J. Taiwan Inst. Chem. Eng. 115: 251–265, https://doi.org/10.1016/j.jtice.2020.10.004.Search in Google Scholar PubMed PubMed Central
Anupama, R.P., Anupama, K., and Abraham, J. (2020). Corrosion inhibition in oil and gas industry: economic considerations, 1st ed. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.Search in Google Scholar
Ashassi-Sorkhabi, H., Mehralizadeh, M., and Asghari, E. (2024). Inhibiting performance of Rivaroxaban against corrosion of mild steel in 1.0 M HCl solution. Phys. Chem. Res. 12: 515–524, https://doi.org/10.22036/pcr.2023.407143.2376.Search in Google Scholar
Attia, E.M. (2015). Expired Farcolin drug as corrosion inhibitor for carbon steel in 1M HCl solution. J. Basic. Appl. Chem. 5: 1–15.Search in Google Scholar
Bardal, E. (2004). Corrosion and protection. Springer-Verlag, London, UK.10.1007/b97510Search in Google Scholar
Bashir, S., Sharma, V., Lgaz, H., Chung, I-M, Singh, A., and Kumar, A. (2018). The inhibition action of analgin on the corrosion of mild steel in acidic medium: a combined theoretical and experimental, approach. J. Mol. Liq. 263: 454–462, https://doi.org/10.1016/j.molliq.2018.04.143.Search in Google Scholar
Dahiya, S., Saini, N., Dahiya, N., Lgaz, H., Salghi, R., Jodeh, S., and Lata, S. (2018). Corrosion inhibition activity of an expired antibacterial drug in acidic media amid elucidate DFT and MD simulations. Portugaliae Electrochim. Acta 36: 213–230, https://doi.org/10.4152/pea.201803213.Search in Google Scholar
Dahiya, S., Pahuja, P., Lgaz, H., Chung, I-M, and Lata, S. (2019). Advanced quantum chemical and electrochemical analysis of ravage drugs for corrosion inhibition of mild steel. J. Adhes. Sci. Technol. 33: 1066–1089, https://doi.org/10.1080/01694243.2019.1576353.Search in Google Scholar
Dohare, P., Chauhan, D.S., Sorour, A.A., and Quraishi, M.A. (2017). DFT and experimental studies on the inhibition potentials of expired Tramadol drug on mild steel corrosion in hydrochloric acid. Mater. Discov. 9: 30–41, https://doi.org/10.1016/j.md.2017.11.001.Search in Google Scholar
Dohare, P., Chauhan, D.S., and Quraishi, M.A. (2018). Expired Podocip drug as potential corrosion inhibitor for carbon steel in acid chloride solution. Int. J. Corros. Scale Inhib. 7: 25–37, https://doi.org/10.17675/2305-6894-2018-7-1-3.Search in Google Scholar
Dwivedi, D., Lepkova, K., and Becker, T. (2017). Carbon steel corrosion: a review of key surface properties and characterization methods. RSC Adv. 7: 580, https://doi.org/10.1039/c6ra25094g.Search in Google Scholar
Eddy, N.O. and Odoemelam, S.A. (2008). Inhibition of the corrosion of mild steel in acidic medium by Penicillin V Potassium. Adv. Nat. Appl. Sci. 2: 225–232.Search in Google Scholar
Eddy, N.O., Odoemelam, S.A., and Mbaba, A.J. (2008). Inhibition of the corrosion of mild steel in HCl by sparfloxacin. African J Pure Appl. Chem. 2: 132–138.Search in Google Scholar
Eid, S. (2021). Expired Desloratidine drug as inhibitor for corrosion of carbon steel pipeline in hydrochloric acid solution. Int. J. Electrochem. Sci. 16: 150852, https://doi.org/10.20964/2021.01.27.Search in Google Scholar
Elabbasy, H.M. and Gadow, H.S. (2020). Study the effect of expired tenoxicam on the inhibition of carbon steel corrosion in a solution of hydrochloric acid. J. Mol. Liq. 321: 114918, https://doi.org/10.1016/j.molliq.2020.114918.Search in Google Scholar
EL-Etre, A.Y., Seyam, S.M., and Mady, M.A. (2021). Corrosion inhibition of Isosorbidedi nitrate drug on carbon steel in hydrochloric acid solution. Benha J. Appl. Sci. 6: 205–212.10.21608/bjas.2021.169162Search in Google Scholar
El-Haddad, M.N., Fouda, A.S., and Hassan, A.F. (2019). Data from chemical, electrochemical, and quantum chemical studies for interaction between Cephapirin drug as an eco-friendly corrosion inhibitor and carbon steel surface in an acidic medium. Chem. Data Coll. 22: 100251, https://doi.org/10.1016/j.cdc.2019.100251.Search in Google Scholar
El-Naggar, M.M. (2007). Corrosion inhibition of mild steel in acidic medium by some sulfa drugs compounds. Corros. Sci. 49: 2226–2236, https://doi.org/10.1016/j.corsci.2006.10.039.Search in Google Scholar
Essien, K.E., Nelson, I.A., Obosi, E.J., Okon, E.J., and Umana, S.G. (2024). Expired Glucored Forte drug: an eco-friendly corrosion inhibitor for mild steel. J. Mater. Sci. Res. Rev. 7: 302–314, https://www.sdiarticle5.com/review-history/119191.Search in Google Scholar
Farahati, R., Mousavi-Khoshdel, S.M., Ghaffarinejad, A., and Hadi Behzadi, H. (2020). Experimental and computational study of penicillamine drug and cysteine as water-soluble green corrosion inhibitors of mild steel. Prog. Org. Coat. 142: 105567, https://doi.org/10.1016/j.porgcoat.2020.105567.Search in Google Scholar
Fayomi, O.S.I., Akande, I.G., Daramola, D., Oluwadare, G.A., and Popoola, A.P.I. (2021). Inhibitive characteristics of Cefalexin drug addition on corrosion evolution of mild steel in a chloride medium. Portugaliae Electrochim. Acta 39: 149–157.10.4152/pea.202102149Search in Google Scholar
Feng, L., Zhang, S., Zhou, Y., Pan, R., Du, H., Liu, F., and Yang, Y. (2023). Expired Glucosamine drugs as green corrosion inhibitors for carbon steel in H2SO4 solution and synergistic effect of Glucosamine molecules with iodide ions: combined experimental andtheoretical investigations. Crystals 13: 205, https://doi.org/10.3390/cryst13020205.Search in Google Scholar
Fouda, A.S., Shalabi, K., and E-Hossiany, A. (2016). Moxifloxacin antibiotic as green corrosion inhibitor for carbon steel in 1 M HCl. J. Bio. Tribo. Corros. 2: 18, https://doi.org/10.1007/s40735-016-0048-x.Search in Google Scholar
Fouda, A.S., El Morsi, M.A., and El-Mogy, T. (2017a). Investigation of the inhibition of carbon steel corrosion in hydrochloric acid solutions by Domperidone drug. J. Anal. Pharm. Res. 5: 00153, https://doi.org/10.15406/japlr.2017.05.00153.Search in Google Scholar
Fouda, A.S., El Morsi, M.A., and El Mogy, T. (2017b). Studies on the inhibition of carbon steel corrosion in hydrochloric acid solution by expired Carvedilol drug. Green Chem. Lett. Rev. 10: 336–345, https://doi.org/10.1080/17518253.2017.1380236.Search in Google Scholar
Geethamani, P. and Kasthuri, P.K. (2015). Adsorption and corrosion inhibition of mild steel in acidic media by expired pharmaceutical drug. Cogent Chem 1: 1091558, https://doi.org/10.1080/23312009.2015.1091558.Search in Google Scholar
Geethamani, P. and Kasthuri, P.K. (2016). The inhibitory action of expired asthalin drug on the corrosion of mild steel in acidic media: a comparative study. J. Taiwan Inst. Chem. Eng. 63: 490–499, https://doi.org/10.1016/j.jtice.2016.03.008.Search in Google Scholar
Ghaderi, M., Ahmad Ramazani, S.A., Kordzadeh, A., Mahdavian, M., Alibakhshi, E., and Ghaderi, A. (2022). Corrosion inhibition of a novel antihistamine-based compound for mild steel in hydrochloric acid solution: experimental and computational studies. Sci. Rep. 12: 13450, https://doi.org/10.1038/s41598-022-17589-y.Search in Google Scholar PubMed PubMed Central
Gholamhosseinzadeh, M.R., Aghaie, H., Shahidi Zandi, M., and Giahi, M. (2019). Rosuvastatin drug as a green and effective inhibitor for corrosion of mild steel in HCl and H2SO4 solutions. J. Mater. Res. Technol. 8: 5314–5324, https://doi.org/10.1016/j.jmrt.2019.08.052.Search in Google Scholar
Golestani, G., Shahidi, M., and Ghazanfari, D. (2014). Electrochemical evaluation of antibacterial drugs as environment-friendly inhibitors for corrosion of carbon steel in HCl solution. Appl. Surf. Sci. 308: 347–362, https://doi.org/10.1016/j.apsusc.2014.04.172.Search in Google Scholar
Guo, W., Umar, A., Zhao, Q., Alsaiari, M.A., Wang, L., and Pei, M. (2020). Corrosion inhibition of carbon steel by three kinds of expired cephalosporins in 0.1 M H2SO4. J. Mol. Liq. 320: 114295, https://doi.org/10.1016/j.molliq.2020.114295.Search in Google Scholar
Gupta, N.K., Gopal, C.S.A., Srivastava, V., and Quraishi, M.A. (2017). Application of expired drugs in corrosion inhibition of mild steel. Int. J. Pharm. Chem. Anal. 4: 8–12, https://doi.org/10.18231/2394-2797.2017.0003.Search in Google Scholar
Haruna, K., Saleh, T.A., and Quraishi, M.A. (2020). Expired metformin drug as green corrosion inhibitor for simulated oil/gas well acidizing environment. J. Mol. Liq. 315: 113716, https://doi.org/10.1016/j.molliq.2020.113716.Search in Google Scholar
Ikeuba, A.I., Ntibi, J.E., Okafor, P.C., Ita, B.I., Agobi, A.U., Asogwa, F.C., Omang, B.J., Eno, E.A., Loius, H., Adalikwu, S.A., et al. (2023). Kinetic and thermodynamic evaluation of azithromycin as a green corrosion inhibitor during acid cleaning process of mild steel using an experimental and theoretical approach. Results Chem. 5: 100909, https://doi.org/10.1016/j.rechem.2023.100909.Search in Google Scholar
Jeeva, P.A., Mali, G.S., Dinakaran, R., Mohanam, K., and Karthikeyan, S. (2019). The influence of Co-Amoxiclav on the corrosion inhibition of mild steel in 1 N hydrochloric acid solution. Int. J. Corros. Scale Inhib. 8: 1–12, https://doi.org/10.17675/2305-6894-2019-8-1-1.Search in Google Scholar
Kaesche, H. (2003). Corrosion of metals: physicochemical principles and current problems. Springer-Verlag, New York.Search in Google Scholar
Karthik, G. and Sundaravadivelu, M. (2016). Studies on inhibiting mild steel corrosion in hydrochloric acid solution by atenolol drug. Egypt. J. Pet. 25: 183–191, http://creativecommons.org/licenses/by-nc-nd/4.0.10.1016/j.ejpe.2015.04.003Search in Google Scholar
Koch, G., Vamey, J., Thompson, N., Moghissi, O., Gould, M., and Payer, J. (2016). International measures of prevention, application, and economics of corrosion technologies study. NACE International, Houston, Texas, USA.Search in Google Scholar
Kushwah, R. and Pathak, R.K. (2014). Inhibition of mild steel corrosion in 0.5 M sulphuric acid solution by Aspirin drug. Inter. J. Emerging Technol. and Advan. Eng. 4: 880–884.Search in Google Scholar
Lagrenée, M., Mernari, B., Bouanis, M., Traisnel, M., and Bentiss, F. (2002). Study of the mechanism and inhibiting efficiency of 3,5-bis(4-methylthiophenyl)-4H-1,2,4-triazole on mild steel corrosion in acidic media. Corros. Sci. 44: 573–588, https://doi.org/10.1016/S0010-938X(01)00075-0.Search in Google Scholar
Landolt, D. (2007). Corrosion and surface chemistry of metals. EPFL Press, Switzerland.10.1201/9781439807880Search in Google Scholar
Lgaz, H., Salghi, R., Jodeh, S., and Hammouti, B. (2016). Effect of clozapine on inhibition of mild steel corrosion in 1.0 M HCl medium. J. Mol. Liq. 225: 271–280, https://doi.org/10.1016/j.molliq.2016.11.039.Search in Google Scholar
Liangtian, Y., Zhang, M., Shidong, C., Yunji, T., Haixia, W. (2020). Investigation of corrosion inhibition effect of Enprofylline drug on mild steel corrosion in sulphuric acid solution. Int. J. Electrochem. Sci. 15: 5102–5114, https://doi.org/10.20964/2020.06.83.Search in Google Scholar
Ma, X., Dang, R., Kang, Y., Gong, Y., Luo, J., Zhang, Y., Fu, J., Li, C., and Ma, Y. (2020). Electrochemical studies of expired drug (Formoterol) as oilfield corrosion inhibitor for mild steel in H2SO4 media. Int. J. Electrochem. Sci. 15: 1964–1981, https://doi.org/10.20964/2020.03.65.Search in Google Scholar
Mahmmod, A.A. (2019). Experimental studies of mild steel corrosion inhibition in hydrochloric using inhibitor type sulfa drugs. Int. J. Corros. Scale Inhib. 8: 1112–1122, https://doi.org/10.17675/2305-6894-2019-8-4-18.Search in Google Scholar
Mahmoud, Z. Sh., Shams, A.K., and Salma, T.A. (2021). Study the inhibition effect of amoxicillin drug for corrosion of carbon steel in saline media. Baghdad Sci. J. 19: 121–131, https://doi.org/10.21123/bsj.2022.19.1.0121.Search in Google Scholar
Manal, O. and Hamzah, M.O. (2020). A corrosion inhibition of mild steel in acid solution using Metronidazole drug. Open J. Sci. Technol. 3: 1–7, https://doi.org/10.31580/ojstv3i1.1312.Search in Google Scholar
Mansfeld, F.B. (1987). Corrosion mechanisms. Marcel Dekker, NY, USA.Search in Google Scholar
Miksic, B.A. (1983). Use of vapor phase inhibitors for corrosion protection of metal products, Paper #308, Corrosion 83. NACE International, Houston, TX.10.5006/C1983-83308Search in Google Scholar
Morad, M.S. (2007). Effect of sulfur-containing amino acids on the corrosion of mild steel in sulfide-polluted sulfuric acid solutions. J. Appl. Electrochem. 37: 1191–1200.10.1007/s10800-007-9386-1Search in Google Scholar
Naqvi, I., Saleemi, A.R., and Naveed, S. (2011). Cefixime: a drug as efficient corrosion inhibitor for mild steel in acidic media. Electrochemical and thermodynamic studies. Int. J. Electrochem. Sci. 6: 146–161.10.1016/S1452-3981(23)14982-0Search in Google Scholar
Narang, R., Vashishth, P., Bairagi, H., Sehrawat, R., Shukla, S.K., and Mangla, B. (2024). Experimental and quantum chemical investigation of corrosion inhibitive action of Sertraline on mild steel in acidic medium. Chem. Afr. 7: 2155–2171, https://doi.org/10.1007/s42250-023-00831-z.Search in Google Scholar
Nazeer, A.A., El-Abbasy, H.M., and Fouda, A.S. (2013). Antibacterial drugs as environmentally-friendly corrosion inhibitors for carbon steel in acid medium. Res. Chem. Intermed. 39: 921–939, https://doi.org/10.1007/s11164-012-0605-y.Search in Google Scholar
Negm, N.A., Kandile, N.G., Badr, B.A., and Mohammed, M.A. (2012). Gravimetric and electrochemical evaluation of environmentally friendly nonionic corrosion inhibitors for carbon steel in 1 M HCl. Corros. Sci. 65: 94–103.10.1016/j.corsci.2012.08.002Search in Google Scholar
Ngobiri, N.C. and Okorosaye-Orubite, K. (2017). Adsorption and corrosion inhibition characteristics of two medicinal molecules. Chem. Int 3: 185–194.Search in Google Scholar
Okon Eddy, N. and Ebenso, E.E. (2010). Quantum chemical studies on the inhibition potentials of some Penicillin compounds for the corrosion of mild steel in 0.1 M HCl. J. Mol. Model. 16: 1291–1306, https://doi.org/10.1007/s00894-009-0635-6.Search in Google Scholar PubMed
Onyeachu, I.B., Abdel-Azeim, S., Singh, D.C., and Quraishi, M.A. (2021). Electrochemical and computational insights on the application of expired metformin drug as a novel inhibitor for the sweet corrosion of C1018 steel. ACS Omega 6: 65–76, https://doi.org/10.1021/acsomega.0c03364.Search in Google Scholar PubMed PubMed Central
Palaniappan, P., Alphonsab, J., Colec, I.S., Balasubramanian, K., I.G., and Bosco, I.G. (2019). Rapid investigation expiry drug green corrosion inhibitor on mild steel in NaCl medium. Mater. Sci. Eng. B 249: 114423, https://doi.org/10.1016/j.mseb.2019.114423.Search in Google Scholar
Pavithra, M.K., Venkatesha, T.V., Punith Kumar, M.K., and Tondan, H.C. (2012). Inhibition of mild steel corrosion by Rabeprazole sulphide. Corros. Sci. 60: 104–111, https://doi.org/10.1016/j.corsci.2012.04.003.Search in Google Scholar
Prakash Shetty (2020). Schiff bases: an overview of their corrosion inhibition activity in acid media against mild steel. Chem. Eng. Comm. 207: 985–1029, https://doi.org/10.1080/00986445.2019.1630387.Search in Google Scholar
Raheem, Z.A. (2016). Study of some drugs as corrosion inhibitors for mild steel in 1 M H2SO4 solution. Int. J. Curr. Res. Chem. Pharm. Sci. 3: 1–7, https://doi.org/10.22192/ijcrcps.2016.03.12.001.Search in Google Scholar
Robert, G.K., John, R.S., David, W.S., and Rudolph, G.B. (2003). Electrochemical techniques in corrosion science and engineering. Marcel Dekker, NY, USA.Search in Google Scholar
Shams, A.K., Hammza, R.A., Salman, E.A., Samawi, K.A., and Salman, T.A. (2023). Experimental and theoretical evaluations of Naproxen drug as a green corrosion inhibitor for carbon steel in an acidic medium. Int. J. Corros. Scale Inhib. 12: 1939–1963, https://doi.org/10.17675/2305-6894-2023-12-4-27.Search in Google Scholar
Sheit, H.M.K., Musthafa Kani, S., Anwar Sathiq, M., Syed Abuthahir, S.S., Subhapriya, P., Nivedhitha, K.S., Umarfarooq, M.A., Badruddin, I.A., Kamangar, S., and Shaik, A.S. (2024). Experimental studies on the effect of expired Amiodarone drug (EAD) as a corrosion inhibitor on mild steel in 1 M HCl. Materials 17: 751, https://doi.org/10.3390/ma17030751.Search in Google Scholar PubMed PubMed Central
Shoesmith, D.W. (1987). Metals handbook, 9th ed. ASM International, Materials Park, Ohio.Search in Google Scholar
Shojaee, S., Zandi, M.S., and Rastakhiz, N. (2022). The effect of Tetracycline drug as a green corrosion inhibitor for carbon steel in HCl media. J. Indian Chem. Soc. 99: 100700, https://doi.org/10.1016/j.jics.2022.100700.Search in Google Scholar
Shukla, S.K. and Quraishi, M.A. (2009a). Ceftriaxone: a novel corrosion inhibitor for mild steel in hydrochloric acid. J. Appl. Electrochem. 39: 1517–1523, https://doi.org/10.1007/s10800-009-9834-1.Search in Google Scholar
Shukla, S.K. and Quraishi, M.A. (2009b). Cefotaxime sodium: a new and efficient corrosion inhibitor for mild steel in hydrochloric acid solution. Corros. Sci. 51: 1007–1011, https://doi.org/10.1016/j.corsci.2009.02.024.Search in Google Scholar
Shukla, S.K. and Quraishi, M.A. (2010). The effects of pharmaceutically active compound doxycycline on the corrosion of mild steel in hydrochloric acid solution. Corros. Sci. 52: 314–332, https://doi.org/10.1016/j.corsci.2009.09.017.Search in Google Scholar
Shukla, S.K., Singh, A.K., Ahamad, I., and Quraishi, M.A. (2009). Streptomycin: a commercially available drug as corrosion inhibitor for mild steel in hydrochloric acid solution. Mater. Lett. 63: 819–822, https://doi.org/10.1016/j.matlet.2009.01.020.Search in Google Scholar
Shukla, S.K., Quraishi, M.A., and Ebenso, E.E. (2011). Adsorption and corrosion inhibition properties of Cefadroxil on mild steel in hydrochloric acid. Int. J. Electrochem. Sci. 6: 2912–2931.10.1016/S1452-3981(23)18228-9Search in Google Scholar
Siaka, A.A., Eddy, N.O., Idris, S.O., Magaji, L., Garba, Z.N., and Shabanda, I.S. (2013). Quantum chemical studies of corrosion inhibition and adsorption potentials of Amoxicillin on mild steel in HCl solution. Inter. J. Modern Chem. 4: 1–10.Search in Google Scholar
Singh, A.K. (2012). Inhibition of mild steel corrosion in hydrochloric acid solution by 3-(4-((Z)-indolin-3-ylideneamino)phenylimino)indolin-2-one. Ind. Eng. Chem. Res. 51: 3215–3223.10.1021/ie2020476Search in Google Scholar
Singh, A.K. and Quraishi, M.A. (2011a). Investigation of the effect of disulfiram on corrosion of mild steel in hydrochloric acid solution. Corros. Sci. 53: 1288–1297, https://doi.org/10.1016/j.corsci.2011.01.002.Search in Google Scholar
Singh, A.K. and Quraishi, M.A. (2011b). Adsorption properties and inhibition of mild steel corrosion in hydrochloric acid solution by ceftobiprole. J. Appl. Electrochem. 41: 7–18, https://doi.org/10.1007/s10800-010-0202-y.Search in Google Scholar
Singh, A.K., Quraishi, M.A., and Ebenso, E.E. (2011a). Inhibitive effect of Cefuroxime on the corrosion of mild steel in hydrochloric acid solution. Int. J. Electrochem. Sci. 6: 5676–5688.10.1016/S1452-3981(23)18436-7Search in Google Scholar
Singh, A.K., Shukla, S.K., and Quraishi, M.A. (2011b). Corrosion behaviour of mild steel in sulphuric acid solution in presence of Ceftazidime. Int. J. Electrochem. Sci. 6: 5802–5814.10.1016/S1452-3981(23)18446-XSearch in Google Scholar
Singh, A., Ebenso, E.E., and Quraishi, M.A. (2012). Theoretical and electrochemical studies of Metformin as corrosion inhibitor for mild steel in hydrochloric acid solution. Int. J. Electrochem. Sci. 7: 4766–4779.10.1016/S1452-3981(23)19580-0Search in Google Scholar
Singh, A.K., Khan, S., Singh, A., Quraishi, S.M., Quraishi, M.A., and Ebenso, E.E. (2013a). Inhibitive effect of chloroquine towards corrosion of mild steel in hydrochloric acid solution. Res. Chem. Intermed. 39: 1191–1208, https://doi.org/10.1007/s11164-012-0677-8.Search in Google Scholar
Singh, A.K., Ji, G., Prakash, R., Ebenso, E.E., and Singh, A.K. (2013b). Cephamycin: a novel corrosion inhibitor for mild steel corrosion in HCl acid solution. Int. J. Electrochem. Sci. 8: 9442–9448.10.1016/S1452-3981(23)12984-1Search in Google Scholar
Singh, P., Singh, D.C., Srivastava, K., Srivastava, V., and Quraishi, M.A. (2017). Expired Atorvastatin drug as corrosion inhibitor for mild steel in hydrochloric acid solution. Int. J. Ind. Chem. 8: 363–372, https://doi.org/10.1007/s40090-017-0120-5.Search in Google Scholar
Singh, P., Chauhan, D.S., Chauhan, S.S., Singh, G., and Quraishi, M.A. (2019a). Chemically modified expired Dapsone drug as environmentally benign corrosion inhibitor for mild steel in sulphuric acid useful for industrial pickling process. J. Mol. Liq. 286: 110903, https://doi.org/10.1016/j.molliq.2019.110903.Search in Google Scholar
Singh, A.K., Chugh, B., Sahac, S.K, Banerjeec, P., Ebensod, E.E., Thakurb, S., and Panie, B. (2019b). Evaluation of anti-corrosion performance of an expired semi synthetic antibiotic cefdinir for mild steel in 1M HCl medium: an experimental and theoretical study. Results Phys. 14: 102383, https://doi.org/10.1016/j.rinp.2019.102383.Search in Google Scholar
Solmaz, R., Kardas, G., Yazıcı, B., and Erbil, M. (2008). Adsorption and corrosion inhibitive properties of 2-amino-5-mercapto-1,3,4-thiadiazole on mild steel in hydrochloric acid media. Colloids Surf. A. 312: 7–17.10.1016/j.colsurfa.2007.06.035Search in Google Scholar
Srinivasulu, A. and Kasturi, P.K. (2017). Study of inhibition and adsorption properties of mild steel corrosion by expired pharmaceutical Gentamicin drug in hydrochloric acid media. Orient. J. Chem. 33: 2616–2624, https://doi.org/10.13005/ojc/330559.Search in Google Scholar
Srivastava, K., Priyanka Singh, P., and Quraishi, M.A. (2016). Corrosion inhibition behaviour of mild steel in HCl solution in presence of Nebicard. Int. J. Innov. Res. Sci. Eng. Technol. 5: 3401–3405, https://doi.org/10.15680/IJIRSET.2016.0503124.Search in Google Scholar
Srivastava, M., Tiwari, P., Srivastava, S.K., Prakash, R., and Ji, G. (2017). Electrochemical investigation of Irbesartan drug molecules as an inhibitor of mild steel corrosion in 1 M HCl and 0.5 M H2SO4 solution. J. Mol. Liq. 236: 184–197, https://doi.org/10.1016/j.molliq.2017.04.017.Search in Google Scholar
Sundaram, R.G., Vengatesh, G., and Sundaravadivelu, M. (2021). Surface morphological and quantum chemical studies of some expired drug molecules as potential corrosion inhibitors for mild steel in chloride medium. Surf. Interfaces 22: 100841, https://doi.org/10.1016/j.surfin.2020.100841.Search in Google Scholar
Thakur, A., Kumar, A., Dagdag, O., Kim, H., Berisha, A., Sharma, D., and Om, H. (2024). Unraveling the corrosion inhibition behavior of prinivil drug on mild steel in 1M HCl corrosive solution: insights from density functional theory, molecular dynamics, and experimental approaches. Front. Chem. 12: 1403118, https://doi.org/10.3389/fchem.2024.1403118.Search in Google Scholar PubMed PubMed Central
Tsygankova, L.E., Bryksina, V.A., Uryadnikov, A.A., and Abramo, A.E. (2022). Protective efficiency of expired drug against acid corrosion of carbon steel. Int. J. Corros. Scale Inhib 11: 564–576, https://doi.org/10.17675/2305-6894-2022-11-2-7.Search in Google Scholar
Tsygankova, L.E., Bryksina, V.A., Aidemirova, F.A., and Baisheva, U.V. (2024). Anticorrosion protection of carbon steel by expired Anaprilin in acidic media. Int. J. Corros. Scale Inhib. 13: 324–336, https://doi.org/10.17675/2305-6894-2024-13-1-16.Search in Google Scholar
Uhlig, H.H. and Revie, R.W. (2008). Corrosion and corrosion control, 4th ed. John Wiley, Hoboken, New Jersey.Search in Google Scholar
Vaszilcsin, N., Ordodi, V., and Borza, A. (2012). Corrosion inhibitors from expired drugs. Inter. J. Pharm. 431: 241–244, https://doi.org/10.1016/j.ijpharm.2012.04.015.Search in Google Scholar PubMed
Vengatesh, G., Karthik, G., and Sundaravadivelu, M. (2017). A comprehensive study of ondansetron hydrochloride drug as a green corrosion inhibitor for mild steel in 1 M HCl medium. Egypt. J. Pet. 26: 705–719.10.1016/j.ejpe.2016.10.011Search in Google Scholar
Xu, X., Singh, A., Sun, Z., Ansari, K.R., and Lin, Y. (2017). Theoretical, thermodynamic and electrochemical analysis of biotin drug as an impending corrosion inhibitor for mild steel in 15% hydrochloric acid. R. Soc. Open Sci. 4: 170933, https://doi.org/10.1098/rsos.170933.Search in Google Scholar PubMed PubMed Central
Xuehui, P., Xiangbin, R., Fei, K., Jiandong, X., and Baorong, H. (2010). Inhibiting effect of Ciprofloxacin, Norfloxacin and Ofloxacin on corrosion of mild steel in hydrochloric acid. Chin. J. Chem. Eng. 18: 337–345.10.1016/S1004-9541(08)60362-6Search in Google Scholar
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/corrrev-2024-0102).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

