Abstract
The study of a stir cast Al356-Nb2O5)P composite immersed in third-generation microalgal-derived biodiesel blends with enhanced plasma electrolyte oxidation surface modification revealed the corrosion susceptibility and possible by-product formation. The effect of (oxide)P reinforcement and mixed-oxide surface coatings were studied separately and cumulatively. Samples were immersed in different biodiesel and petrodiesel blends for up to 3000 h, and their corrosion and electrochemical behavior was studied. Although some weight change was recorded in all samples, the corrosion rates significantly decreased from 1.8 to 1.3 by 10 wt% Nb2O5)P reinforcement, which further decreased 10 times after surface modification. Electron microscopy revealed primary fine-grained microstructure with low porosity content of fine and needlelike dendritic structures in composites and irregular volcanic with scattered micropores and microcracks in surface-modified composites that changed to corrosion spots and flake-covered microcracks after immersion.
1 Introduction
The highly oxidative, chemically aggressive, and dissociation susceptible nature of most biofuel blends, especially in contact with metals and alloys (Cursaru and Nassreddine 2018), besides high affinity for by-product formation of aldehydes, ketones, catalyst (alkali or acid), alcohol water, alcohols, and carboxylic acids have drastically increased corrosion risks in internal combustion engine (ICE) applications (Geller 2008; Kugelmeier et al. 2021). The main cause of corrosion is due to low pH (less than three) medium via the formation of carboxylic acids (i.e., acetic acid, formic acid, propionic acid, and fatty acids with up to 10 carbons in their formula and fatty acid methyl esters [FAME]) from hydroperoxide radicals (Fazal 2014). The corrosive attacks intensify with hydrolytic reactions of absorbed and formed water contents (hygroscopic nature of biofuel blends with extra absorbed moisture and condensed water) at high pressure and high biofuel temperatures near and above 100 °C (Meenakshi 2021). These corrosion-based reactions have caused less biofuel stability (Silva et al. 2021), higher light sensitivity (Magara-Gomez and Olson 2012), higher temperature dependency (Trost et al. 2021), complex dissociation in the presence of dissolved metal ions (Aquino and Hernandez 2012; Siddharth and Sharma 2014), and microorganism attack (Adegboye et al. 2021). The high corrosiveness of biofuel blends results in metal oxide formation at metal-fuel interface and causes high abrasion and wear (Kumar Kurre 2017), due to lack of lubrication caused by biofuels oxidation (Li and Liu 2019). The corrosion phenomena evolve around pitting corrosion in steels (Sorate and Bhaleh 2018) or high content by-product formation in aluminum alloys (Luís Pereira Elias et al. 2020). Most applications of aluminum alloys (such as hypereutectic aluminum-silicon with high thermal conductivity and low value dimension clearance) in ICEs involves 30–60% mass reduction via proper reinforcement with ceramic oxides and nonoxides to achieve combinations of hardness (wear resistance) (Soares et al. 2020), ductility (impact resistance), with improved strength to weight ratio and stiffness (Akaehomen et al. 2018), and temperature resistance in 100–250 °C range (Jankowski 2017), such as SiCp (Maleque 2012; Posmyk and Filipczyk 2013), Al2O3p (Mahmoud 2012), TiCp (Kumar and Kumar 2019), TiB2 (Xia et al. 2020), SiO2 (Zhang et al. 2018), graphene (Liu and Pu 2020), SiCp/graphite (Ravikumar and Reddapa 2018), and Al2O3p/graphite (Bihari 2018) with subsequent T3 and T6 heat treatments (Mroczkowska and Antończak 2019). These reinforcements with heavy metal oxides including Nb2O5 (niobia) (Bahador et al. 2020) significantly improves physicomechanical properties of widely used Al-Si alloys (i.e., Al356). The corrosion resistance via the formation of severely adhered natural oxide layer of aluminum alloys and further oxidation inhibition (Kramer et al. 2018) deteriorates in the presence of aggressive ions, such as chloride and other halides (Zhang Nie and Han 2010). The localized breakdown of this oxide-passive film leads to the initiation and growth of corrosion via pitting by chemical reaction and formation of Al3+ and Al(OH)3 in aqueous or highly hydrated mediums including biofuel blends (Asrat Mengesha et al. 2020). The improvements on this oxide layer via surface modification techniques, i.e., plasma electrolytic oxidation (PEO), to produce thin and well-adhered protective coatings on aluminum alloys have a significant effect on their enhanced microstructure, morphology, wear, and corrosion resistance (Díaz-Ballote and López-Sansores 2009). The PEO-treated Nb2O5-reinforced aluminum alloys consist of dense inner and outer mixed oxide layers; combinations of α, γ alumina with interoxides including AlNbO4 reach hardness up to 1900 HVN with Ecorr of −348 (mV), Icorr of 0.001 (μA cm−2), and polarization resistance of 2.417 (MΩ cm−2) (Bahador et al. 2021) The enhanced corrosion resistance alongside improved physicomechanical properties (most importantly microhardness and as a result, wear resistance) of PEO-treated and oxide-reinforced aluminum matrix (Shi and Wang 2021) indicates their applicability in contact with biofuel blends and combustion in IC block engines. These modifications can minimize the corrosive impacts of aqueous electrolyte, i.e., hydrated biofuels. Among four major categories of biofuel generation, the third-generation microalgal-based (such as cyanobacteria prokaryotic, green/red eukaryotic, and diatoms (Ananthi et al. 2020)) biofuels have gained a growing interest due to the easy and accessible aquatic cultivation media (Zhu et al. 2015). The most advantageous rapid biomass production (rapid exponential growth) and considerable fuel capacity content from their lipids (Wood 2021), carbohydrates, and proteins have made microalgae a considerable source of biodiesels by the formation of alkyl ester from their molecule of glycerol and three fatty acid chains through the transesterification reaction or the thermal cracking (or pyrolysis) for alkanes, alkenes, aromatics, and carboxylic acids formation (Hussain et al. 2021). Simultaneously, the high polyunsaturated fatty acid contents of these biodiesel can cause metal corrosion and deterioration due to high affinity for the oxidation reactions (Akubude et al. 2019).
This study is focused on the compatibility assessment of niobium pentoxide-reinforced Al356 alloy matrix composites with biofuel blends and their chemically induced corrosion susceptibility and the effect of PEO surface modification treatment, via evaluation of the corrosion behavior in the biofuel blends.
2 Materials and methods
2.1 Sample preparation and characterization
The Al356 alloy, with considerable castability, strength to density ratio, heat treatability, and formability, was chosen as the alloy matrix with bonus properties of Si-rich alloys such as high microhardness and oxide-combability through SiO2 formation. The alloy consists of 7.23 wt% of Si with 0.4 wt% and around 0.5 wt% combination of Cu, Fe, and reinforcement wettability agents including manganese. The stir-cast sample with 10 wt% Nb2O5 reinforcement microsized powders were prepared by mechanical and ultrasonic dispersing under pure argon purge during gradual solidification from 1100 °C in steel molds. The details of MMC formation, the applied T6 heat treatment (to induce precipitation of soluble alloying elements from the solid solutions), and characterizations are mentioned in our previous study (Bahador et al. 2020). The monolithic Al alloy (AlM) and composite (Al10Nb) were chosen for corrosion behavior studies. Both samples were PEO treated with anode sample plates and a stainless-steel cathode with 1 and 25 cm2 surface area, respectively. The alkaline electrolyte (pH: 11–12) was prepared with Na2SiO3 and NaOH with 10 and 3 gL−1 in distilled water, respectively. The pulsed DC power supply was used to create dense and thin oxide coating during the 12 min electrical charge of the 1 kHz pulse with a current density ratio of R: ∼2, +400 to −200 V potential range, with a mean of 0.1 A cm−2 current density. The coated monolithic (P−AlM) and composite (P−Al10Nb) samples were prepared accordingly for further characterizations. The mentioned process parameters have assured the formation of a thin well-adhered dense coating layer (Bahador et al. 2021). The samples were analyzed for phase, crystal structure, lattice parameters, and crystallinity via low-angle X-ray diffraction (GI-XRD) Cu Ka using Philips MPD-XPERT monochromate Cu kα = 0.154 nm filtered by Ni at a voltage of 50 kV and 10 mA current (scan range: 2θ = 10–90°). The X’Pert High Score software (version 3.0.0) with ICDD PDF2 (Powder Diffraction File for inorganic materials) database and MATCH-CRYSTAL IMPACT software was used to study the XRD patterns. Electron microscopy enabled the study of surface morphology and cross section of samples via scanning electron microscopy (SEM, JEOL 2100 and FE-SEM, JSM-7600F). The corrosion behavior of the PEO-coated samples was evaluated using electrochemical impedance spectroscopy (EIS) using potentiostat/galvanostat/frequency response analyzer from Metrohm (Model: AutoLab PG STAT30) with a three-electrode cell, a platinum plate as a counter electrode, and a saturated Ag/AgCl as the reference electrode; the coated samples as the working electrode for corrosion studies, and data collection was analyzed via NOVA software. The EIS recorded data were analyzed via Z-view software and fitted (with chi-squared values between 0.01 and 0.001) to appropriate equivalent circuit (EC) models.
2.2 Biofuel blends immersion evaluation test (IT)B
The monolithic and composite samples were prepared prior to immersion based on general preparation criteria of ASTM E3 – 11(2017), which consist of grinding with subsequent SiC sandpaper (grit #300, #600, and #1200); the residual debris was cleansed ultrasonically in distilled water and degreased in 1:1 ethanol and acetone for 20 min, and air dried at 50 °C for 1 day. The PEO-treated samples were just cleansed with the same procedure. All samples were weighed with a 10−1 mg readability (Analytical Balance ME104) for reference before immersion. All monolithic and composite (base and PEO-treated) immersion test samples were prepared according to specifications of ASTM G1 – 03(2017)e1 as round plates (d: 25.4 mm, t: 4.0 mm, rin: 2.0 mm) and were immersed entirely into the fuel media via Teflon thin wire in a completely sealed glass vial, without stirring/mixing/vibration in a dark box at 27 ± 2 °C. Samples were cleansed from deposits and corrosion products at each IT interval by acidic solution (phosphoric and chromic acid [0.1 N]) for 10 min and nitric acid (0.1 N) for 1 min before reimmersion, according to ASTM G31 – 21 requirements. The distilled water washed and air flow dried samples were weighed periodically for weight loss due to corrosion. The immersions were carried out in different batches for different intervals, and samples were not reimmersed after cleansing. The biodiesel was produced by methanol-assisted transesterification in the presence of KOH alkaline catalyst, according to the specifications of EN 14214 and ASTM D6751 – 20a standards (ARI-IROST labs) with clear and slightly yellowish appearance. The properties of biodiesel and blends were measured according to related standards. The immersion medium blends were mixtures of Chaetoceros gracilis (Bacilariophyceae)-based biodiesel: petroleum-based diesel (Petrodiesel, ASTM-D975-no.2) with 90:10, 50:50, and 10:90 ratio that are coded as B90, B50, and B10, respectively. Fourier-transform infrared spectroscopy (FTIR) was carried out for samples to study the chemical bond and their dependency to immersion conditions, using a Thermo-mattsen IR310 in the range of 500–4000 cm−1. Gas chromatography–mass spectrometry (GC-MS–Shimadzu QP2020 NX) of immersion mediums was carried out for the determination of organic compounds (such as fatty acid and esters) and possible contaminants or by-products. The corrosion behavior of samples was evaluated using EIS via a potentiostat/galvanostat/frequency response analyzer from Metrohm (Model: AutoLab PG STAT30). A three-electrode cell setup (platinum plate as the counter electrode/a saturated Ag/AgCl as the reference electrode/the sample as the working electrode) was used for corrosion studies data collection (with an electrolyte solution of 0.5 mol L−1 sodium sulfate buffered with potassium biphthalate and sodium hydroxide and pH = 4.0) that were analyzed via NOVA software at ambient temperature. The bond dissociation and possible transformation in biofuel blends due to contact with samples were also checked by red–green–blue, hue–saturation–intensity (Pereira Franco dos Santos et al. 2019), and grayscale image analysis, and CIE L * a * b* coordinates change via CIELAB method (Schloss and Lessard 2018), using a CHNspec 610 color spectrophotometer for L as the perceptual lightness and b as the yellow versus blue. The medium images were decomposed into histograms, and pixel statistical distributions were recorded. The reproducibility data and repeatability of results were assured by reporting a mean of three times repeated tests and consequent deviations.
3 Results and discussion
The compatibility of the as-cast monolithic aluminum alloy, the Nb2O5 reinforced composite, before and after PEO treatments with different third-generation microalgal-based biodiesel and petrodiesels were evaluated after 3000 h exposure. During this immersion period, combinations of interactions between aluminum matrix, reinforcement oxide, and outer oxide layer with biofuel blends are conceivable, and fluctuations in specific parameters such as weight loss and corrosion rate (CR) are proper indicators of biofuel compatibility and corrosion susceptibility of the samples. Due to the electrochemical nature of corrosion, the changes in structure formation bonds of biofuel blends can also affect the biofuel/material interactions. The FAME type and chain length, as a clear indication of biodiesel nature, prior and after contact with samples, can also indicate the chemical interactions and effects of the samples on the fuel blends. The GC-MS graphs of pure biodiesel (B100), standard petrodiesel (D100), and 50:50 blend (B50) consist of concoction of long-chain fatty acid ester with the number of carbon atoms, forming long chains ranging from 15 to 25, which creates building blocks of methyl ester. The GC-MS graphs during 70 min retention time are shown in Figure 1. According to the graphs, the biodiesel is mostly linoleic acid (25.15%) with palmitic acid (30.82%), margaric acid (23.70), oleic acid (11.19%), and traces of others including linoceric acid with suggested overall stoichiometry of C18H32O2 with main properties reported in Table 1, when compared to petrodiesel.

GC-MS chromotogram of B100 (a), D100 (b), and B50 (c) samples gathered at ambient temperature.
Selected properties of fuels.
| Fuel | Density (ASTM D4052) (g cm−3) | Viscosity (ASTM D445) (mm2 s−1) | Heating value (ASTM D420) (kJ g−1) | Cetane number (CN) |
|---|---|---|---|---|
| B100 | 0.87 (±0.02) | 3.96 (±0.12) | 40.051 (±0.002) | 52 |
| D100 | 0.82 (±0.03) | 6.41 (±0.11) | 46.270 (±0.003) | 60 |
-
Measured at 15–40 °C according to standard specifications.
The dominant bond-type study of biodiesel sample shows combinations of C=O, C−O−C, and C−O with C−H stretch vibrations (Figure 2), which is consistent with the nature of biostock-derived fuels and its expected fatty acids. The pattern clearly involves C−H and C−H2 at 2800–2900 cm−1 stretch, C=O bond near ∼1800 cm−1 stretch for fatty acid ester, C−O−C bonds at 1000–1200 cm−1 range, and 1190 vibration correlated with the decreasing ester chain (C−O), in which characteristic aliphatic compounds around 2900 cm−1are a related proof of biodiesel molecules. The compatibility of third-generation biodiesel with petrodiesel can be seen due to similar bonds, especially at 1500–2900 cm−1 area. The total water content (according to Karl Fischer titration (Agatonovic-Kustrin et al. 2020; Zhen et al. 2020)) is measured about 0.08 vol% (155 mg kg−1) in B100 and 0.11 vol% (204 mg kg−1) in D100.

FTIR spectra of B100 (a) and D100 (b) samples gathered at ambient temperature.
The comparative weight changes of base and PEO-coated samples (AlM, Al10Nb, P−AlM, and P−Al10Nb) before, during, and after immersion in B100, D100, B90, B50, and B10 mediums were recorded. The shorter initial and longer final measurement intervals were chosen for more thorough examination of possible weight gains and losses during immersion test. The gradual weight change graphs are shown in Figure 3. As shown, the overall trends consist of minor weight gains (less than 1500 × 10−4 g) over time, which was measured in high petrodiesel concentration mediums (D100 and B10) rather than biodiesel-rich blends (B100 and B90). The trends also include slight fluctuations (gradual increase followed by continuous decrease in pure mediums (B100 and D100) when compared to blend mediums. Most changes are limited to the first month of immersion (measured every 2 days) with a slight increase and decrease (especially in low concentration blends) in trends. The weight change mechanism is directly influenced by the nature of mediums (clear dependence to biodiesel and petrodiesel contents) and water content (main precursor of corrosion aggressiveness of the medium) that is indicated by lower weight change in blends than pure mediums, especially B50 medium with minimum water content of 0.085 vol%). More neutral response of the PEO-coated samples in all mediums can be seen with the linear and semistraight trends for P−AlM and P−Al10Nb samples in pure and blend conditions. The CR (mm y−1) of the samples in different immersion conditions were calculated to facilitate the corrosion behavior condition study of samples, using measured weight loss of samples (w (g)), theoretical density (ρth (g cm−3)), contact cross-section surface area (S (cm2)), and immersion time (t (h)) using equation (1):

Recorded weight changes during immersion for (a) B100, (b) D100, (c) B90, (d) B50, and (e) B50 fuel blends gathered at ambient temperature, for monolithic Al alloy (AlM), composite (Al10Nb), coated monolithic (P−AlM), and composite (P−Al10Nb) samples.
The CR trends of samples in 500 h immersion intervals for each substrate were calculated individually and plotted for 3000 h immersion time, as shown in Figure 4a–d. All trends consist of clear diminish in corrosion over time, which is consistent with the weight change measurements. As expected, the CR in higher quantities of biodiesel is more than high fuel content blends due to the aggressiveness of fatty acids, oxygen, and water amounts. Overall, the CR for monolithic and composite samples are near five times the respective for PEO-coated samples. A comparison between calculated CRs shows that the high water content in B50 and B10 mediums has caused more corrosion for all substrates, whereas a significant decrease in corrosion after first 1000 h is detectable in all plots.

Calculated CR as a function of the immersion period time (500 h intervals) for (a) AlM, (b) Al10Nb, (c) P−AlM, and (d) P−Al10Nb in fuel blends gathered at ambient temperature.
The water-induced corrosion has resulted in the formation of hydroxide and, consequently, oxide layers with the controlling reaction such as following, which with increasing the layer thickness can be controlled and even hindered.
Beyond this mechanism, the remaining corrosion is related to the microgalvanic couples between the Al-rich matrix and Si-rich zones (such as semicircular dots on the linear stretch such as rosary beadlike Mg3FeSi6Al8 and needlelike Si2Fe2Al9 intermetallic compounds) in the Al−Si matrix. The presence of niobium as an alloying element or as a reinforcement phase, similar to other refractory elements, derives its corrosion resistance from an already formed adherent and passive oxide outer layer. The existing Nb can form lower and suboxides, such as NbO and NbO2, with different stoichiometrics. At any oxidative condition, the oxide can be composed of each or combinations of three, NbO, NbO2, and Nb2O5, compounds. The presence of oxidizing agents in biodiesel and petrodiesel blends will improve the corrosion resistance of Nb and its intermetallic phases with aluminum. The enhanced corrosion resistance can be achieved even with few nanometers thick oxide layer that can be achieved at low water contents according to
with continuous decomposition and release of hydrogen, which further oxidizes the compound to higher oxide states. The reactions show that free Nb and suboxides has reacted with water, formed thin and compact film, and preserved the oxide from dissolution in medium (Asselin et al. 2007). The phase formation between aluminum-based matrix and Nb2O5 particle during the as-cast process develops mixed-oxide and intermetallic compounds in the microstructure, which are prone to further oxidations (according to following reactions with oxide phase as the major by-product).
Formation of aluminum oxide:
intermetallic and oxide:
The initiation of these typical reactions and the formation of mixed intermetallic and oxide compounds was detected in XRD patterns by comparing monolithic and reinforced samples (Figure 5a). The presence of (Nb2O5)P, as expected, has accelerated the alumina and mixed oxides (such as AlNbO4 or Al2O3·xNb2O5 (x: 10–30)) formation in aluminum matrix and at interfaces. This internal oxide layer and oxide particle formation constitute limitations in the connectivity and contiguity in the microstructure of the composite by highly resistive compounds. The high dielectric constant of aluminum and niobium oxides with large electrochemical potentials can result in a significant decrease in CR from less than 2 in AlM sample to near 0.15 mm y−1 with 10 wt% (Oxide)P reinforcement in Al10Nb sample, which indicates the accumulative effect of oxides on increasing the electrical resistivity.
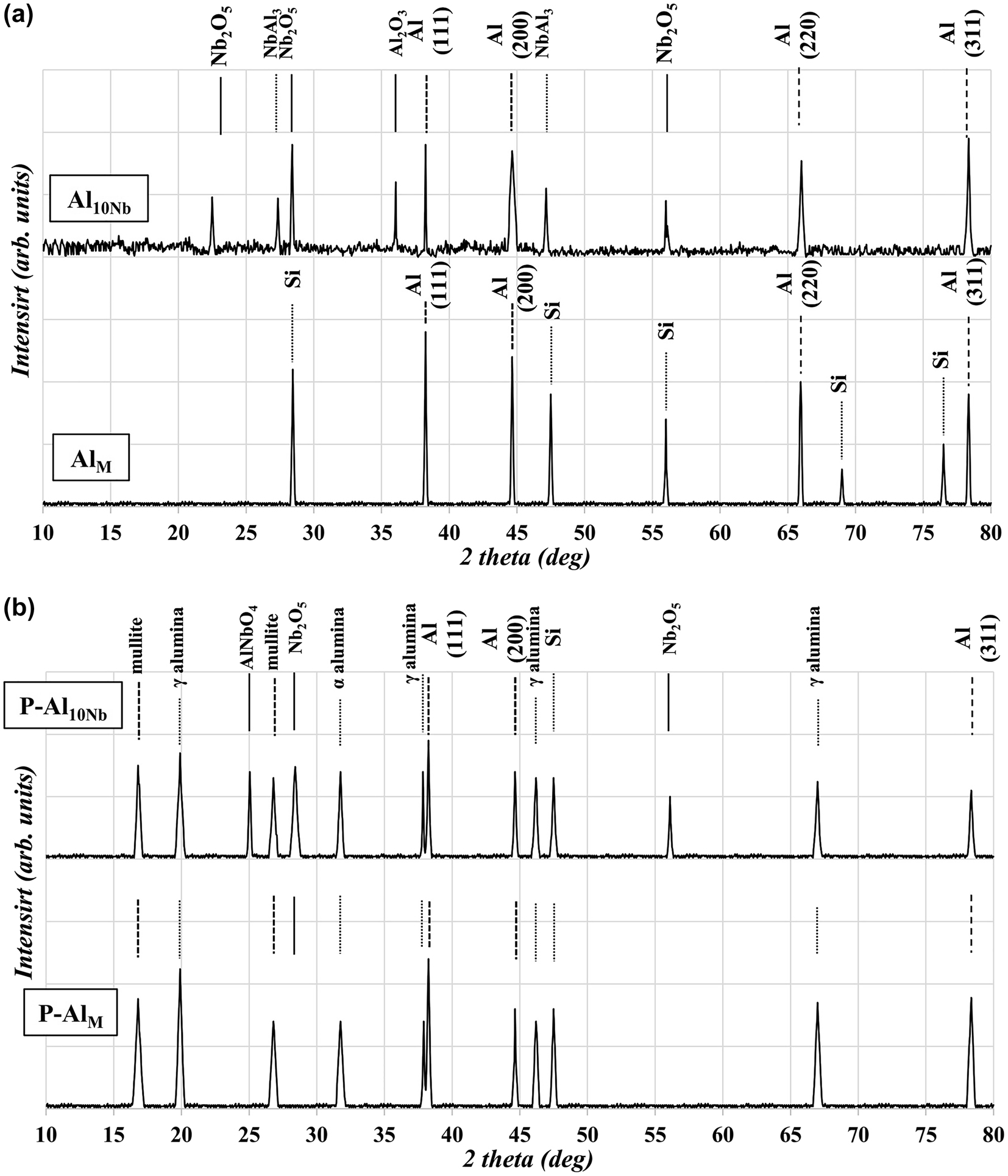
XRD (a) (AlM, Al10Nb) and GI-XRD (b) (P−AlM, P−Al10Nb) patterns of the as-prepared monolithic, composite, and coated samples gathered at ambient temperature.
The PEO modification of samples’ surface area creates irregular volcanic cones (white arrow), smooth and flat zones (yellow arrow), adjacent crater structures, and scattered micropores and microcracks (red arrow) along the surface (Figure 6a and b). The PEO treatment inclines samples to heavy oxidation and mixed-oxides formation, which are evident in the XRD patterns (Figure 5b). This oxidation provides oxide-based microstructures at the outer surface of samples containing α, γ alumina in monolithic alloy and AlNbO4, Nb2O5, and alumina phases in composite samples. Even the residual Si from aluminum alloy and electrolyte forms into mullite phase (Al6Si2O13). According to these patterns, the microstructure consists of mixed oxide and composite high-oxygen interoxide systems of Al2O3–Nb2O5–SiO2.
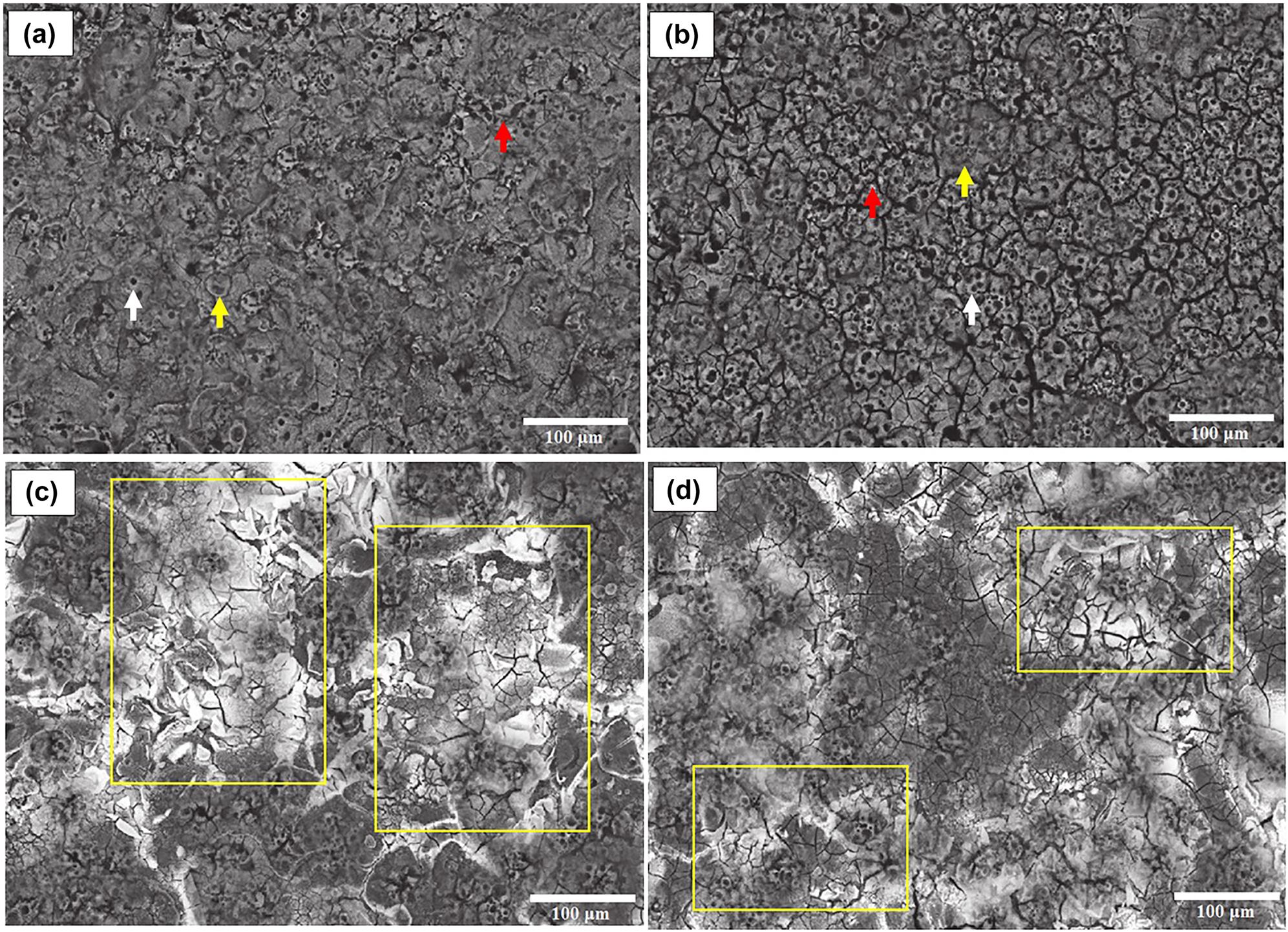
FE-SEM micrographs of PEO as-coated monolithic and composite samples: (a) P−AlM, (b) P−Al10Nb and after immersion coated monolithic (c) and coated composite (d) samples.
The smooth surface of AlM, containing alloy hypoeutectic phases with a few casting defects (white arrows) prior to B100 3000 h immersion (Figure 7a) with uniform elemental distribution (inlet EDS pattern is included), changes into spot-covered corrosion deposits (white arrows) of mixed aluminum and alloying elements oxide, as shown in (Figure 7b). The SEM micrographs of Al10Nb show the needlelike intermetallic Si2Fe2Al9 (Bahador et al. 2020) with signs of niobium present with the formation of Al−Nb oxide-dispersed semicircular deposits after 3000 h immersion (Figure 7c and d). The formation of deposits and possible corrosion by-products was even detectable as white spots and flakes on the P−AlM and P−Al10Nb samples with extra surface cracks due to expansions via the comparison of micrographs before (Figure 6a and b) and after immersion (Figure 6c and d). The semicontinuous coverage of deposits in P−AlM (yellow boxes) and isolated scattered by-products in P−Al10Nb show the scarce corrosion susceptibility of the PEO-coated composite sample (the coating morphology remains intact). It should be mentioned that deposit accumulation zones were detected after careful examination of many SEM sample, which indicates how scarcely corrosion by-products were formed on the PEO coating protected samples. The XRD patterns of after-immersion samples (not included) were not conclusive, due to phase similarity of inner and outer oxide compounds to corrosion by-products. The elemental analysis of EDS results (Table 2) indicates the main oxides and extra mixed-oxide formation in surface-modified samples. The significant increase in oxygen content (quantified by stoichiometry) alongside depreciation of magnesium (from 0.42 to 0.13), copper/iron (from 0.2 to near nil), and silicon (from 7.23 to 5.78) in both AlM and Al10Nb are indications of possible dissolution due to corrosion reactions.

FE-SEM micrographs of the as-cast monolithic (a) and composite (c) prior to immersion and after immersion monolithic (b) and composite (d).
The EDS elemental results of samples based on the EC (B100).
| Sample code | Immersion time (h) | Elements (wt% ± 0.02) | ||||||
|---|---|---|---|---|---|---|---|---|
| Al | Nb | Mg | O | Fe | Cu | Si | ||
| AlM | 0 | 91.55 | 0 | 0.42 | 0.32 | 0.2 | 0.2 | 5.78 |
| Al10Nb | 0 | 82.32 | 9.14 | 0.39 | 0.39 | 0.2 | 0.19 | 6.5 |
| AlM | 3000 | 90.49 | 0 | 0.1 | 1.99 | 0.02 | 0.03 | 5.78 |
| Al10Nb | 3000 | 81 | 8.85 | 0.18 | 1.27 | 0.01 | 0.06 | 6.14 |
| P−AlM | 0 | 40.18 | 0 | 0.11 | 50.18 | 0.19 | 0.15 | 8.99 |
| P−Al10Nb | 0 | 31.17 | 10.21 | 0.09 | 50.17 | 0.13 | 0.11 | 8.11 |
| P−AlM | 3000 | 38.18 | 0 | 0.11 | 52.13 | 0.16 | 0.18 | 9.18 |
| P−Al10Nb | 3000 | 30.89 | 11.03 | 0.18 | 51.94 | 0.15 | 0.17 | 5.52 |
Similar high polarization resistance (Rp) can be expected due to these oxides, which is evident by a significant decrease in CRs. The presence of microcracks and micropores in these coatings are caused by thermal stresses and are generated during the rapid solidification of the molten oxide products in the strong discharge channels. This structure can create channels of contact with immersion medium and possible under coating corrosion. This penetration has resulted in a small amount of corrosion in PEO-coated samples (P−AlM and P−Al10Nb) at less than 0.4 range CRs, which drops into less than half after first 1500 h of immersion in all mediums.
The improved corrosion resistivity via (Oxide)P reinforcement and surface modification can also be detected via EIS studies (Nyquist plots and anodic potentiodynamic polarization curves), and the results prior and after immersion in fuel blends are compared. The recorded data were used to study the corrosion resistance of the samples by qualitatively comparison and based on the larger semicircles and higher impedance modulus at lower frequencies as common indicators for higher corrosion resistance. The Nyquist plot of the AlM before composite and PEO coating was used as the baseline to indicate the cumulative effect of process and immersions. The Nyquist plots contained only one capacitive loop for AlM and Al10Nb before and after immersion for 3000 h in all samples (Figure 8a and c), which is attributed to gradual changes in the conductivity of mediums. The comparison between nonimmersed AlM and Al10Nb samples with semicircular shape shows the significant increase in impedance values that indicates the decrease in corrosion susceptibility at the presence of (Nb2O5)P reinforcement. The presence of inductive loop showed that the medium aggressive agents (fatty acids and water) have corroded the AlMsubstrate, which specifies the processes of the dissolution of aluminum to ions, start of the formation of corrosion products, and the adsorption of electrolyte-active compounds such as Al(OH)3−AlOOH at the localized defective sites.

EIS (Nyquist impedance plots and anodic potentiodynamic polarization curves) of the as-cast monolithic (a) and (b) and composite (c) and (d) samples before and after immersion in fuel blends gathered at ambient temperature.
This impedance response of electrochemical system is similar to a circuit with parallel resistor and capacitor. The effect of fuel immersion on the increase in corrosion resistance of samples, while keeping one capacitive loop for all immersed samples, can be seen in the plot of B100, D100, and B50 for both samples. A close study of anodic potentiodynamic polarization response (Figure 8b and d) also indicates higher corrosion susceptibility of AlM when compared to that of Al10Nb with higher values of current density, which tend to decrease the following fuel immersions.
The significant changes in Nyquist plots of P−AlM and P−Al10Nb samples, with increased radius of circles and Z impedance values (mean 150 times) show the positive effect of PEO modification, and the similar recorded trend confirms the effect of medium immersion on corrosion control (Figure 9a and b). The lack of inductive loops specifies that localized degradation has not occurred in these samples, which alongside Z values indicate the significant level of protection at the substrate-coating interface. The obvious changes in plots with excess loop are related to the additional effect of PEO-coating inner and outer layers (causing further block of medium penetration and prevention of direct contact with base metal), which translates to two RC parallels linked in series in the equivalent electrical circuit for the impedance response of samples. In the EC (Figure 9c) for (I) Al10Nb and (II) P−Al10Nb systems, R1 is the resistance of the electrolyte, and R2 and Q1 are the resistance and capacitance of oxide layer in P−AlM sample, respectively. The presence of extra (oxide)P particles in P−Al10Nb sample results in parallel particle resistance (R3) and constant phase element (CPE) internal barrier layer of Q2 in the EC. The EIS BODE plots (Figure 9d) also indicate the corrosion resistance effect of oxide reinforcement and also, clearly, the PEO coatings. The difference in corrosion resistance between P−AlM and P−Al10Nb is related to a much denser nature of the outer oxide layer and the mixed-oxide compound formation in P−Al10Nb, which considerably decreases the chances for medium penetration. The Bode phase angle plot (Figure 9e) consists of time constant at intermediate frequencies (102 Hz) with a metal surface corrosion part at low frequencies of (10−2 Hz) that indicates the deterioration of metal surface oxides by the electrolyte in base AlM and Al10Nb samples. The time constant of the compact layer of oxide coatings in P−AlM and P−Al10Nb samples appears at high frequencies, whereas the diffusion tail appears at low frequencies in the Bode phase angle plots. The detailed information on EIS plots for samples after 3000 h immersion in B100 are reported in Table 3, including CPE, n, and R values for base and PEO-coated substrates.
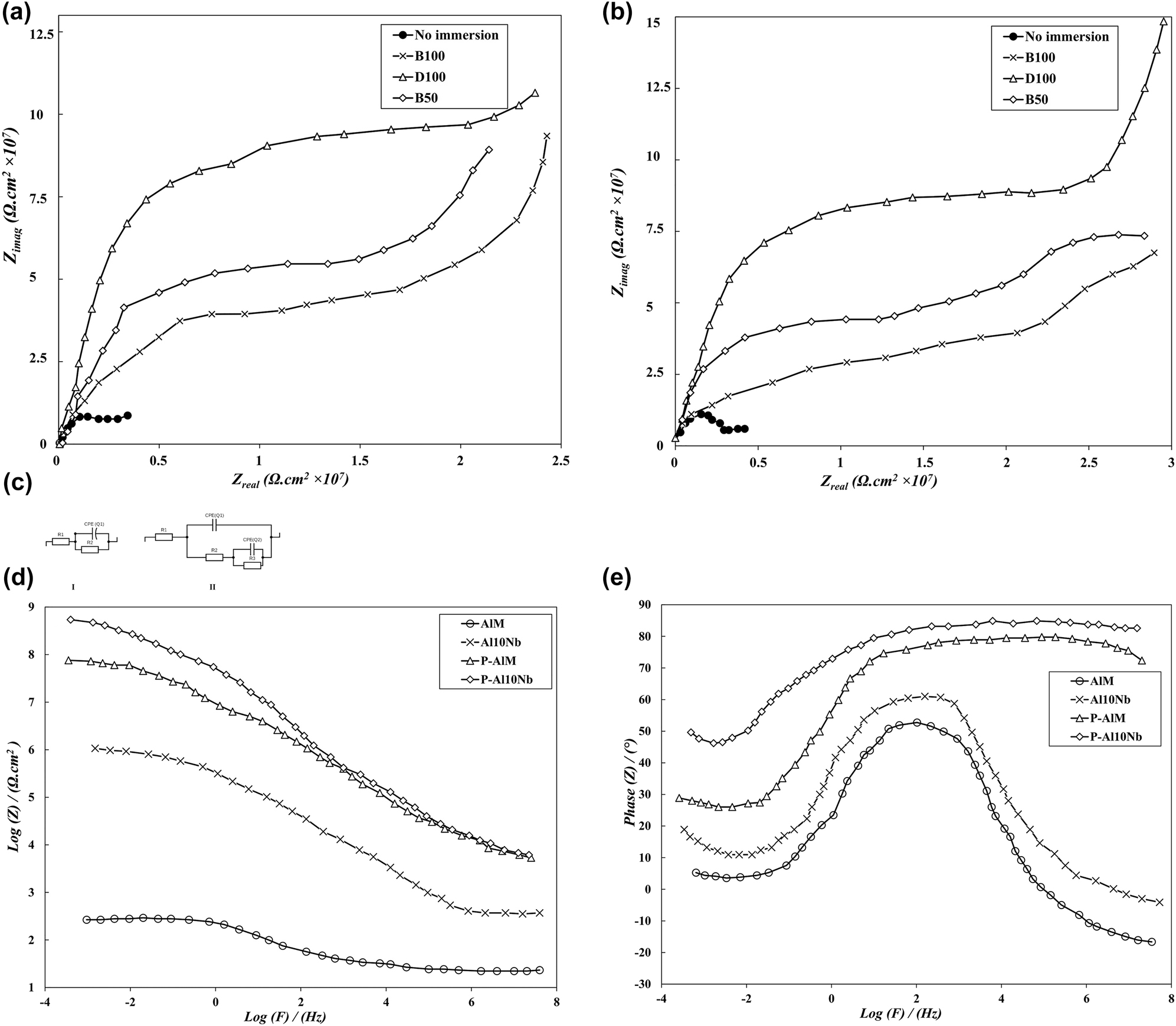
EIS (Nyquist impedance plots) of the as-coated monolithic (a) and composite (b) samples. The ECs for samples (c) with (d) Bode modulus plots and (e) Bode phase plots for samples gathered at ambient temperature.
Electrical elements result of EIS plots of samples based on the EC (B100).
| Sample code | Immersion time (h) | Inner composite layer | Outer layer | ||||
|---|---|---|---|---|---|---|---|
| CPE (μF cm−2 Sn−1) | n | R (kΩ cm2) | CPE (μF cm−2 Sn−1) | n | R (kΩ cm2) | ||
| AlM | 3000 | 0.911 | 0.84 | 0.068 | 7.002 | 0.77 | 162 |
| Al10Nb | 3000 | 3.902 | 0.88 | 0.079 | 37.056 | 0.83 | 322 |
| P−AlM | 3000 | 56.015 | 0.93 | 0.152 | 50.002 | 0.86 | 533 |
| P−Al10Nb | 3000 | 58.273 | 0.96 | 0.168 | 66.413 | 0.87 | 608 |
The chemical reaction between samples and mediums at different immersion periods has the mutual effect of changing elemental profile of surface area and also the composition of the blends. These dissolved ions can cause biodiesel oxidization, and the metal species can react as a catalyst in that reaction. A closer study of the physical properties of blends showed changes in the visual profiles of blends in contact with metal, composite, and oxide coating surfaces, which is an indication for the presence of dissolved metal ions and bond changes in the biodiesel/petrodiesel. The color difference (ΔE) measurements and its comparison with the original color profile prior to immersion (as the reference) facilitates the precise and quantitative study of changes in blends and deterioration due to corrosion phenomena. The ΔE parameter was recorded to indicate the changes proportionally with an actual change in the observed colors, and was calculated using equation (2). The recorded results (Figure 10a–d) show perceptible at a glance (ΔE ≤ 2) and perceptible through close observation changes (2 < ΔE ≤ 10) in color profiles which makes the experiments viable for interpretation and explanation.

Color changes L * ab (expressed as ΔE) for (a) AlM, (b) Al10Nb, (c) P−AlM, and (d) P−Al10Nb compared to original color profiles of fuel blends. The images of the residual blends compared to original: (e) B100 medium before and after AlM immersion, (f) B50 medium before and after AlM immersion, (g) D100 medium before and after AlM immersion, (h) after immersion of P−AlM in B100-D100-B50 (from left to right), and (i) after immersion of P−Al10Nb in B100–B90–B50–B10–D100 (from left to right).
The general examination of plots indicates less susceptibility of petrodiesel (ΔE: 1.5–2) and significantly higher sensitivity of biodiesel blends (ΔE: 1.5–8) to chemically induced changes due to ion solutions and formation of corrosion products. The highest ΔE values were recorded for high biodiesel content blends (B100) in contact with AlM and Al10Nb metallic surface samples (ΔE: 7–8), which notably decrease in P−AlM and P−Al10Nb samples to ΔE: 1.5–1.7 range. Similar trends are detected in measured data for B90 or even B50 blends, whereas B10 blends show properties similar to D100 medium. The diffusion barrier effect of surface oxide and mixed-oxide coating layer and the isolation of samples from medium direct contact improved the preservation of biofuel blends chemical integrity.
The total acid number (TAN) of exposed fuel blends to samples was also chosen as an applicable parameter for fuel stability or vulnerability toward metal surface corrosion reactions, especially in comparison with the specification of ASTM D6751/EN 14214 (max 0.5 mgKOH/g). The measured TAN value for B100, B50, and D100 blends after 3000 h immersion of samples are shown in Figure 11, with original before immersion TAN of 0.35 for biodiesel (red dashed line) and 0.22 for petrodiesel (orange solid line). The increase in acidity of the high biodiesel content blends in contact with AlM and Al10Nb samples (about 1.7 times) is more severe than that of petrodiesel (near 1.5 times), whereas it changes to moderate and near standard values in contact with P−AlM and P−Al10Nb samples to about 0.4 mgKOH/g.

TAN changes due to contact with different samples compared to original values gathered at ambient temperature.
4 Conclusions
The mutual effect of different biodiesel/petrodiesel blends on the corrosion susceptibility of Si-rich aluminum matrix (Nb2O5)P reinforced composites was evaluated by the corrosion study of samples upon exposure. The noncoated monolithic and composite materials were slightly affected by petrodiesel and 10 wt% biodiesel blends. The higher water content in pure biodiesel than 90 wt% blends increased the weight changes and CRs upon exposure up to 0.11 g (1.6 mm y−1) which increased to 0.14 g (1.9 mm y−1) in B10 blend. The oxide reinforcement decreased the corrosion susceptibility due to the formation of internal high electrical resistivity content interoxide compounds between naturally formed alumina and added niobia. The PEO coating increased the corrosion resistivity of both materials significantly to 0.0.4 g (0.18 mm y−1). The oxide surfaces also kept the chemical integrity of fuel blends.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: None declared.
-
Conflicts of interest: The authors declare that they have no conflicts of interest regarding this article.
References
Adegboye, M.F., Ristivojevic, P., and Gegechkori, V. (2021). Bioprospecting of microbial strains for biofuel production: metabolic engineering, applications, and challenges. Biotechnol. Biofuels 14: 5, https://doi.org/10.1186/s13068-020-01853-2.Search in Google Scholar PubMed PubMed Central
Agatonovic-Kustrin, S., Ristivojevic, P., and Gegechkori, V. (2020). Essential oil quality and purity evaluation via FT-IR spectroscopy and pattern recognition techniques. Appl. Sci. 10: 7294, https://doi.org/10.3390/app10207294.Search in Google Scholar
Akaehomen, O., Ibhadode, A., and Ebhojiaye, R.S. (2018). A new lightweight material for possible engine parts manufacture. In: Carlucci, A.P. (Ed.), The future of internal combustion engines. IntechOpen, London, Uk.Search in Google Scholar
Akubude, V.C., Nwaigwe, K.N., and Dintwa, D. (2019). Production of biodiesel from microalgae via nanocatalyzed transesterification process: a review. Mater. Sci. Technol. 2: 216–225, https://doi.org/10.1016/j.mset.2018.12.006.Search in Google Scholar
Ananthi, V., Raja, R., and Carvalho, I.S. (2020). A realistic scenario on microalgae-based biodiesel production: third generation biofuel. Fuel 284: 118965, https://doi.org/10.1016/j.fuel.2020.118965.Search in Google Scholar
Aquino, I.P. and Hernandez, R.P.B. (2012). Influence of light, temperature and metallic ions on biodiesel degradation and corrosiveness to copper and brass. Fuel 102: 795–807, https://doi.org/10.1016/j.fuel.2012.06.011.Search in Google Scholar
Asrat Mengesha, G., Chu, J.P., and Lou, B.S. (2020). Corrosion performance of plasma electrolytic oxidation grown oxide coating on pure aluminum: effect of borax concentration. J. Mater. Res. Technol. 9: 8766–8779, https://doi.org/10.1016/j.jmrt.2020.06.020.Search in Google Scholar
Asselin, E., Ahmed, T.M., and Alfantazi, A. (2007). Corrosion of niobium in sulphuric and hydrochloric acid solutions at 75 and 95 °C. Corrosion Sci. 49: 694–710, https://doi.org/10.1016/j.corsci.2006.05.028.Search in Google Scholar
Bahador, R., Hosseinabadi, N., and Yaghtin, A.H. (2020). Microstructural and mechanical characterizations of stir cast aluminum 356–Nb2O5 composite. Adv. Compos. Hybrid. Mater. 3: 594–608, https://doi.org/10.1007/s42114-020-00173-1.Search in Google Scholar
Bahador, R., Hosseinabadi, N., and Yaghtin, A.H. (2021). Effect of power duty cycle on plasma electrolytic oxidation of A356-Nb2O5 metal matrix composites. J. Mater. Eng. Perform. 30: 2586–2604, https://doi.org/10.1007/s11665-021-05597-4.Search in Google Scholar
Bihari, B. (2018). Corrosion behaviour of Al 7075/Al2O3/graphite hybrid composite in 3.5% sodium chloride solution. Int. J. Eng. Res. Technol. 7: 1.Search in Google Scholar
Cursaru, D. and Nassreddine, S. (2018). Impact of moisture on the corrosion behavior of copper and mild carbon steel in corn biodiesel. Corrosion Rev. 36: 559–574, https://doi.org/10.1515/corrrev-2018-0015.Search in Google Scholar
Díaz-Ballote, L. and López-Sansores, J.F. (2009). Corrosion behavior of aluminum exposed to a biodiesel. Electrochem. Commun. 11: 41–44, https://doi.org/10.1016/j.elecom.2008.10.027.Search in Google Scholar
Fazal, M. (2014). Effect of copper and mild steel on the stability of palm biodiesel properties: a comparative study. Ind. Crop. Prod. 58: 8–14, https://doi.org/10.1016/j.indcrop.2014.03.019.Search in Google Scholar
Geller, T.D. (2008). Fuel, and undefined, storage stability of poultry fat and diesel fuel mixtures: specific gravity and viscosity. Elsevier, London, Uk, pp. 118–135.10.1016/j.fuel.2007.03.043Search in Google Scholar
Hussain, F., Shah, S.Z., and Ahmed, H. (2021). Microalgae an ecofriendly and sustainable wastewater treatment option: biomass application in biofuel and bio-fertilizer production. a review. Renew. Sustain. Energy Rev. 137: 110603, https://doi.org/10.1016/j.rser.2020.110603.Search in Google Scholar
Jankowski, A. (2017). Design of a new alloy for internal combustion engines pistons. In: Silva Gomes, J.F., and Meguid, S.A. (Eds.). Proceedings of the 7th international conference on mechanics and materials. Publ. INEGI/FEUP.Search in Google Scholar
Kramer, G., Mendez, C.M., and Ares, A.E. (2018). Evaluation of corrosion resistance of commercial aluminum alloys in ethanol solutions. Mater. Res. 21: 20170272, https://doi.org/10.1590/1980-5373-MR-2017-0272.Search in Google Scholar
Kugelmeier, C.L., Monteiro, M.R., da Silva, R., Kuri, S.E., Sordi, V.L., and Della Rovere, C.A. (2021). Corrosion behavior of carbon steel, stainless steel, aluminum and copper upon exposure to biodiesel blended with petrodiesel. Energy 226: 120344, https://doi.org/10.1016/j.energy.2021.120344.Search in Google Scholar
Kumar, S. and Kumar, A. (2019). Corrosion behaviour of Al 7075/TiC composites processed through friction stir processing. Mater. Today Proc. 15: 21–29, https://doi.org/10.1016/j.matpr.2019.05.019.Search in Google Scholar
Kumar Kurre, S. (2017). A review of biofuel generated contamination, engine oil degradation and engine wear. Biofuels 8: 273–280, https://doi.org/10.1080/17597269.2016.1224291.Search in Google Scholar
Li, F. and Liu, Z. (2019). Effect of biodiesel components on its lubrication performance. J. Mater. Res. Technol. 8: 3681–3687, https://doi.org/10.1016/j.jmrt.2019.06.011.Search in Google Scholar
Liu, W. and Pu, Y. (2020). Corrosion and wear behavior of PEO coatings on D16T aluminum alloy with different concentrations of graphene. Coatings 10: 249, https://doi.org/10.3390/coatings10030249.Search in Google Scholar
Luís Pereira Elias, A., Kuzumi, M.S., and Ortiz, E.L. (2020). Corrosion behavior of an Al–Si casting and a sintered Al/Si composite immersed into biodiesel and blends. Fuel Process. Technol. 202: 106360, https://doi.org/10.1016/j.fuproc.2020.106360.Search in Google Scholar
Magara-Gomez, K.T. and Olson, M.R. (2012). Sensitivity of diesel particulate material emissions and composition to blends of petroleum diesel and biodiesel fuel. Aerosol Sci. Technol. 46: 1109–1118, https://doi.org/10.1080/02786826.2012.696315.Search in Google Scholar
Mahmoud, T. (2012). Corrosion behavior of Al/SiC and Al/Al2O3 nanocomposites. Mater. Res. 15: 903–910, https://doi.org/10.1590/S1516-14392012005000113.Search in Google Scholar
Maleque, M.A. (2012). Aluminium–copper–SiCp composite materials corrosion in biodiesel. Adv. Mater. Res. 576: 425–428, https://doi.org/10.4028/www.scientific.net/AMR.576.425.Search in Google Scholar
Meenakshi, H.N. (2021). Corrosion behavior of brass in methanol-gasoline fuel blends. In: Kumaresan, G., Shanmugam, N.S., and Dhinakaran, V. (Eds.), Advances in materials research. Springer Ser. Mater. Sci. 5, Singapore.10.1007/978-981-15-8319-3_40Search in Google Scholar
Mroczkowska, K.M. and Antończak, A.J. (2019). The corrosion resistance of aluminum alloy modified by laser radiation. Coatings 9: 672–685, https://doi.org/10.3390/coatings9100672.Search in Google Scholar
Pereira Franco dos Santos, A., Kropf da Silva, K., Alves Borges, G., and d’Avila, L.A. (2019). Fuel quality monitoring by color detection. In: Zeng, L.-W. and Cao, S.-L. (Eds.), Color detection. IntechOpen, London.10.5772/intechopen.86531Search in Google Scholar
Posmyk, A. and Filipczyk, J. (2013). Aspects of the applications of composite materials in combustion engines. J. Kones Powertrain Transp. 20: 357–362, https://doi.org/10.5604/12314005.1137847.Search in Google Scholar
Ravikumar, M. and Reddapa, H.N. (2018). Electrochemical studies of aluminium 7075 reinforced with Al2O3/SiCp hybrid composites in acid chloride medium. AIP Conf. Proc. 1943: 020096.10.1063/1.5029672Search in Google Scholar
Schloss, K.B. and Lessard, L. (2018). Modeling color preference using color space metrics. Vision. Res. 151: 99–116, https://doi.org/10.1016/j.visres.2017.07.001.Search in Google Scholar PubMed
Silva, J.B., Almeida, J.S., and Barbosa, R.V. (2021). Thermal oxidative stability of biodiesel/petrodiesel blends by pressurized differential scanning calorimetry and its calculated cetane index. Processes 9: 174, https://doi.org/10.3390/pr9010174.Search in Google Scholar
Shi, Y. and Wang, Y. (2021). Properties and structure of PEO treated aluminum alloy. J. Wuhan Univ. Technol. Mater. Sci. Ed. 36: 424–432, https://doi.org/10.1007/s11595-021-2426-6.Search in Google Scholar
Siddharth, J. and Sharma, M.P. (2014). Effect of metal contents on oxidation stability of biodiesel/diesel blends. Fuel 116: 14–18, https://doi.org/10.1016/j.fuel.2013.07.104.Search in Google Scholar
Soares, M., Berber, L.O., and Vieira, C. (2020). Study of corrosion of AA 3003 aluminum in biodiesel, diesel, ethanol and gasoline media. Mater. Sci. Technol. 1012: 407–411, https://doi.org/10.4028/www.scientific.net/MSF.1012.407.Search in Google Scholar
Sorate, A. and Bhaleh, P. (2018). Corrosion behavior of automotive materials with biodiesel: a different approach. SAE Int. J. Fuels Lub. 11: 147–162, https://doi.org/10.4271/04-11-02-0007.Search in Google Scholar
Trost, D., Polcar, A., Boldor, D., and Nde, D. (2021). Temperature dependence of density and viscosity of biobutanol-gasoline blends. Appl. Sci. 11: 3172, https://doi.org/10.3390/app11073172.Search in Google Scholar
Wood, D.A. (2021). Microalgae to biodiesel – review of recent progress. Bioresour. Technol. Rep. 14: 100665, https://doi.org/10.1016/j.biteb.2021.100665.Search in Google Scholar
Xia, C., Huang, J., Tao, J., and Wang, S. (2020). The preparation and properties of the brown film by micro-arc oxidized on in-situ TiB2/7050Al matrix composites. Coatings 10: 615, https://doi.org/10.3390/coatings10070615.Search in Google Scholar
Zhang, F.D., Liu, H., Suebka, S., and Liu, Z. (2018). Corrosion behaviour of laser-cleaned AA7024 aluminium alloy. Appl. Surf. Sci. 435: 452–461, https://doi.org/10.1016/j.apsusc.2017.11.141.Search in Google Scholar
Zhang Nie, P.X. and Han, L. (2010). Wear protection of Al383/SiO2 metal matrix composites by plasma electrolytic oxidation (PEO) process. SAE Int. J. Mater. Manuf. 3: 55–62.10.4271/2010-01-0024Search in Google Scholar
Zhen, Z., Yue, Y., and Li, D. (2020). Determination of water content of crude oil by azeotropic distillation Karl Fischer coulometric titration. Anal. Bioanal. Chem. 412: 4639–4645, https://doi.org/10.1007/s00216-020-02714-5.Search in Google Scholar PubMed
Zhu, L., Huo, S., and Qin, L. (2015). A microalgae-based biodiesel refinery: sustainability concerns and challenges. Int. J. Green Energy 12: 595–602, https://doi.org/10.1080/15435075.2013.867406.Search in Google Scholar
© 2022 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- In this issue
- Reviews
- An overview of anti-corrosion properties of ionic liquids for corrosion of carbon steel in acidic media
- Corrosion monitoring techniques for concrete in corrosive environments
- Graphene-based coatings for magnesium alloys: exploring the correlation between coating architecture, deposition methods, corrosion resistance and materials selection
- Original Articles
- Decision support system to evaluate a vandalized and deteriorated oil pipeline transportation system using artificial intelligence techniques. Part 1: modeling
- The third-generation biodiesel blends corrosion susceptibility of oxide particle-reinforced Si-rich aluminum alloy matrix composites
- Electrodeposition and corrosion characterization of epoxy/polyaniline coated AZ61 magnesium alloy
- Novel anticorrosive coating of silicone acrylic resin modified by graphene oxide and polyaniline
Articles in the same Issue
- Frontmatter
- In this issue
- Reviews
- An overview of anti-corrosion properties of ionic liquids for corrosion of carbon steel in acidic media
- Corrosion monitoring techniques for concrete in corrosive environments
- Graphene-based coatings for magnesium alloys: exploring the correlation between coating architecture, deposition methods, corrosion resistance and materials selection
- Original Articles
- Decision support system to evaluate a vandalized and deteriorated oil pipeline transportation system using artificial intelligence techniques. Part 1: modeling
- The third-generation biodiesel blends corrosion susceptibility of oxide particle-reinforced Si-rich aluminum alloy matrix composites
- Electrodeposition and corrosion characterization of epoxy/polyaniline coated AZ61 magnesium alloy
- Novel anticorrosive coating of silicone acrylic resin modified by graphene oxide and polyaniline

