Abstract
This paper presents a research on the corrosion behavior of Ti-15-3 alloy overlapped with aluminized PVC film in salt spray. It was found that severe corrosion occurred on aluminized PVC film in the coupled regions because of crevice corrosion and/or galvanic corrosion whereas Ti-15-3 alloy in the coupled regions experienced minor corrosion. Scanning electron microscope and Energy-dispersive X-ray spectroscopy analyses demonstrated the corrosion products adhered to the surface of Ti-15-3 alloy within the crevice. To evaluate the effect of aluminized PVC film on the crevice corrosion of Ti-15-3 alloy in salt spray condition, it is necessary to compare with the corrosion resistance of Ti-15-3 overlapped with polytetrafluoroethylene (PTFE) in different neutral salt spray. Further, the tests were performed by electrochemical impedance spectroscopy and potentiodynamic polarization. Combining the graphical model, an in-depth understanding of the crevice and galvanic corrosion mechanism of Ti-15-3 alloy overlapped with aluminized PVC film has been revealed.
1 Introduction
Metastable beta (β) titanium alloys have been concerned for years about practical fields of the aerospace industry, marine climate conditions, and severe chemical circumstances. These alloys are becoming increasingly popular because of their high mechanical strength, tractility, and good anti-corrosion (Lin et al. 2018), especially Ti-15V-3Cr-3Al-3Sn (commonly referred to as Ti-15-3) alloy was developed as an alternate for Ti-6Al-4V to settle the requirement of thin sheet metal for aerospace applications (Santhosh et al. 2014). However, Ti-15-3 alloy used in the aerospace launch tower is very prone to sensitive electrochemical corrosion attack in chloride-containing media of the tropical ocean. This pitting corrosion, an extremely localized form of corrosion, the tiny pits or holes invisible to the naked eye come into being as a result of corrosion environment, which accordingly grow inside the alloy and destroy the passive film (Wang and Han 2013). For instance, Li et al. 2011 reported that pitting is the most common and damaging form of corrosion in offshore structures. Sidharth and Plato (2009) showed that pitting corrosion is extremely risky, universal, and unnoticed, which has been a cause for concern to marine and offshore industries in recent years. Hence, the pitting corrosion leads to loss of mechanical performances of the offshore structure such as strength, ductility, and impact strength, sometimes even, to the equipment fracture and failure (Popoola et al. 2013).
There are many reasons for the corrosion of titanium alloys. In general, titanium alloys have good corrosion resistance. However, in the salt spray environment, corrosion occurs because chlorine ions destroy or pass through the passivation film of titanium alloy. In engineering applications, for salt spray environments, a measure of titanium alloys covered with aluminized PVC film has been used to prevent chloride ions from entering the surface of titanium alloy. This is since aluminized PVC film is a typical general-purpose composite with lightweight, strong interface adhesion, and low-cost production (Li et al. 2014). Currently, the protective measures on metal surfaces have the protective coating, paint, and cathodic protection method (Bhandari et al. 2015). Hence, two experimental ways of Ti-15-3 alloy overlapped with aluminized PVC film and polytetrafluoroethylene (PTFE) plate are adopted to evaluate the corrosion effect of aluminized PVC film on Ti-15-3 alloy. Similarly, in corrosion science and protection engineering, galvanic corrosion is an important topic (Song et al. 2004). By this time, galvanic corrosion occurs in lap joints of dissimilar materials in contact with each other. Meanwhile, crevice corrosion can also occur in crevices where the two materials overlap each other with an environment prone to developing acidity (Makhlouf and Botello 2018). Crevice corrosion is also a kind of localized corrosion, pitting corrosion and crevice corrosion are originally identical phenomena in the view of electrochemical corrosion, though there are geometrical diversities between them (Galvele 1976; Zadorozne et al. 2012). In terms of specific crevice corrosion, a chemistry in the occluded area is more aggressive than the bulk solution, which is largely based on a separation of dominant anodic and cathodic reaction sites and a large increase in the concentration of chloride in crevice (Kelly and Lee 2018). At the moment, Liang et al. 2015 reported that the crevice corrosion process of titanium alloy is affected by the temperature of media and dissolution of crevice surface chlorides salt membrane.
Many efforts were made to focus on the galvanic corrosion behavior of titanium alloy coupled to other materials in seawater (Barik et al. 2007; Du et al. 2014). Moreover, there is an extensive amount of analytical work reported in the literature to investigate the crevice corrosion and galvanic corrosion behavior of titanium alloys coupled to aluminum alloy or magnesium alloy (Coelho et al. 2019; Snihirova et al. 2019; Zhu et al. 2015). In these couples, the less inert material usually experiences severe corrosion and the more inactive material is protected, and corrosion degradation of electrodes inside the crevice tends to increase. However, it was found in this work that the galvanic couple between Ti-15-3 alloy and aluminized PVC shows differently from traditional understandings when a crevice forms between the dissimilar materials, the present work aims to study crevice corrosion of cathode material (Ti-15-3) for Ti-15-3 alloy overlapped with aluminized PVC film used in tropical oceans facilities that were exposed in a salt spray environment. We happened to find that the galvanic effect of Ti-15-3 alloy/aluminized PVC film coupling could decrease the corrosion rate of Ti-15-3 alloy inside the crevice and that Ti-15-3 alloy surface outside the crevice also suffers from some corrosion damages, by comparing with crevice corrosion of Ti-15-3 alloy overlapped with PTFE plate, which has guiding significance for the service life and protection measures of the aerospace launch tower in the tropical ocean.
In this paper, the present work aims to perform a detailed study of the effect of different salt spray time and aluminized PVC film on crevice corrosion of Ti-15-3 alloy. Initially, according to electrochemical impedance spectroscopy (EIS) and polarization curves, corrosion resistance of Ti-15-3 alloy inside the crevice for Ti-15-3 alloy overlapped with aluminized PVC film was estimated through a comparative test of Ti-15-3 alloy overlapped with PTFE in neutral salt spray (NSS). In addition, the following research can determine how the galvanic effect of Ti-15-3 alloy overlapped with aluminized PVC film affect crevice corrosion of Ti-15-3 alloy.
2 Materials and methods
2.1 Specimen preparation and salt spray test
The material used in this work was Ti-15-3 alloy in the form of rolled plates of 2 mm thickness, PTFE plate of 2 mm thickness, and aluminized PVC film of 1 Å (1 × 10−7 mm) thickness, these specimens were cut to a required size of 50 × 25 mm. Aluminized PVC film is a kind of composite flexible packaging material which is formed by coating the plastic film surface with a very thin metal aluminum (aluminum wire with a purity of 99.99% or more) in the vacuum aluminized machine (Li et al. 2014). The chemical compositions of Ti-15-3 alloy were listed in Table 1. Two experimental ways of Ti-15-3 alloy overlapped with aluminized PVC film-PTFE (aluminized PVC film was pasted on the PTFE plate) or only PTFE plate to evaluate the crevice corrosion behavior for Ti-15-3 alloy in NSS, as shown in Figure 1. These overlap regions had a dimension of approximately 25 × 25 mm. The coupon surfaces of Ti-15-3 alloy were ground by emery paper (no. 240, 320, 400, 600) gradually. Then, the substrates were ultrasonically cleaned in acetone for 35 min, followed by rinsing with deionized water, and then cleaned with alcohol, finally dried with cold air. The Surface morphologies of the unexposed Ti-15-3 alloys and aluminized PVC films were showed in Figure 2. According to the ASTM B117 (NSS test), the NSS test was carried out with the YQW-250 type salt spray test chamber. The overlapped coupons were exposed to the NSS environment for 2, 6, 24, 72, and 120 h respectively. After the salt spray test, the specimens were taken out and rinsed with deionized water to remove extra salts. Then, the specimens were dried and placed in a desiccator for 0.5 h at a temperature of 35 °C to ensure that no further surface reactions occurred. Three parallel specimens were exposed to the salt spray test chamber at each salt spray time.
Chemical composition (wt%) of Ti-15-3 alloy.
| V | Cr | Sn | Al | Fe | Si | C | N | H | O | Ti |
|---|---|---|---|---|---|---|---|---|---|---|
| 13.493 | 4.181 | 3.232 | 5.763 | 0.073 | 0.178 | 0.05 | 0.05 | 0.015 | 0.13 | Balance |

Surface morphologies of (a) Ti-15-3 alloys, (b) aluminized PVC film, (c) cross-section of aluminized PVC film-PTFE, (d) PTFE, and (e) configuration diagram of the crevice that is unexposed to NSS.
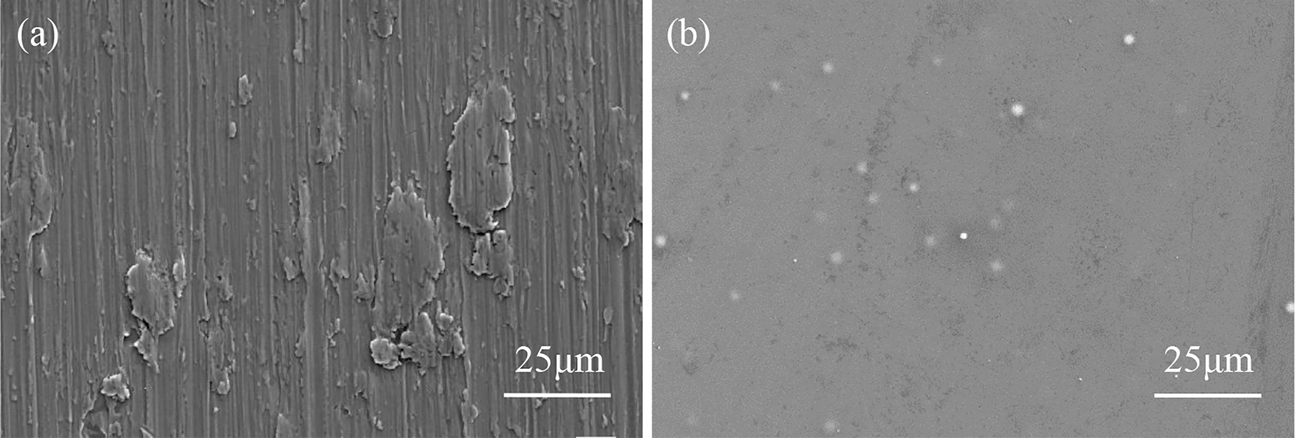
Surface morphologies of (a) Ti-15-3 alloys and (b) aluminized PVC film that are unexposed to NSS.
2.2 Microstructural characterization
The morphologies of the corrosion surface after different NSS tests were examined with the SUPRA55 type scanning electron microscope (SEM) and stereo microscope. Energy-dispersive X-ray Spectroscopy (EDS) was used to carry out surface analysis of Ti-15-3 alloy.
2.3 Electrochemical test
Before the electrochemical tests, the samples after exposure to NSS were immersed in the 3.5 wt% NaCl solution for 0.5 h, The electrochemical tests used a three-electrode system, reference electrode of the saturated calomel electrode (SCE), auxiliary electrode of a platinum sheet, and Ti-15-3 alloy with a surface area of about 1 cm2 as the working electrode. Primarily, the specimen as a working electrode was immersed in the 3.5 wt% NaCl solution to reach a steady-state open circuit potential (OCP) before the EIS and polarization measurements. Secondly, the EIS of specimens were obtained at the OCP, with a frequency domain of 100 kHz–10 mHz and an amplitude of 10 mV. Then, polarization curves were traced at a scan rate of 5 mV/s starting from −0.9 to 0.9 V vs. SCE. In the end, cyclic polarization (CP) curves were performed with a scanning rate of 1 mV/s starting from 200 mV below the corrosion potential. The scan direction was reversed when a potential of 5 VSCE was reached. Three parallel samples of each salt spray time were prepared for electrochemical measurements to ensure the reliability of the experiments.
3 Results
3.1 Microstructure and surface characterization
Figure 3 shows the appearance of Ti-15-3 alloy overlapped with aluminized PVC film at 2, 6, 24, 72, and 120 h from the beginning of the salt spray. An obvious interface appears inside and outside the crevice, indicating that the corrosion sensitivity of the two regions is different. The aluminized PVC films in the coupled regions were severely corroded, and most of the aluminum film was peeled off. With the salt spray corrosion time extends, the spalling of the aluminum film aggravates continually, and the uncoupled area also appears the aluminum film spalling phenomenon after 72 h of NSS. All surfaces of Ti-15-3 alloy in the coupled regions have obvious corrosion rust spots (the corrosion rust spots are derived from the anodic dissolution of the aluminized PVC film), which were found to be oxychloride or aluminum oxide by EDS analyses below whereas there is no change on all surfaces of Ti-15-3 alloy outside the crevice.
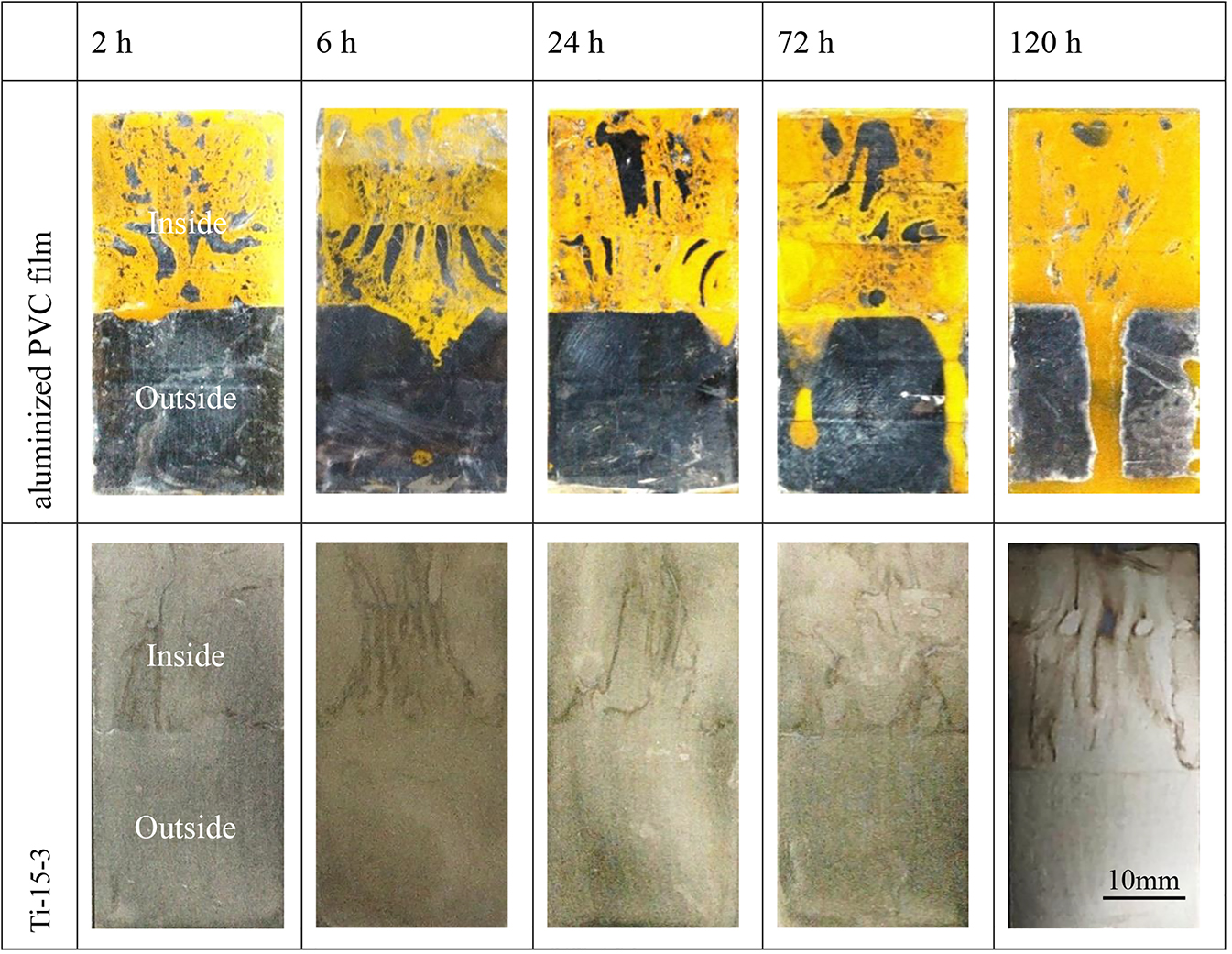
The photos of Ti-15-3 overlapped with aluminized PVC film during the NSS test.
The EDS analyses of Ti-15-3 alloys inside the crevice after 2 and 6 h of the NSS were showed in Figure 4. It is seen that light colors indicate the presence of the elements whereas the dark colors imply the absence of elements in these regions. For 2 h in Figure 4a, oxygen, aluminum, and chlorine are concentrated in both the same shape whereas titanium and vanadium are identical in shape. And oxygen and chloride are not elements of the Ti-15-3 matrix. It can be determined that the lamellar corrosion products may be oxychloride. This indicates that the lamellar corrosion products produced by the galvanic corrosion of Ti-15-3 alloy overlapped with aluminized PVC film are attached to the surface of Ti-15-3 alloy. For 6 h in Figure 4b, oxygen and aluminum are centralized in both the block corrosion products whereas titanium and vanadium are the elements in the Ti-15-3 matrix. This indicates that the block corrosion products can be aluminum hydroxide or aluminum oxide.

SEM-EDS mapping of Ti-15-3 alloys inside the crevice after 2 h (a) and 6 h (b) of NSS.
Figure 5 shows the surface SEM images of Ti-15-3 alloys within the crevice for Ti-15-3 overlapped with aluminized PVC film that are exposed to different NSS times, respectively. As can be seen in Figure 5a, pitting corrosions are observed on the surface of Ti-15-3 alloy within the crevice after 2 h of NSS. In addition, some lamellar corrosion products (oxychloride) adhere to the surface of Ti-15-3 alloys whereas lamellar corrosion product was not observed on the surface of Ti-15-3 alloy outside the crevice, and only pitting corrosions exist (see Figure 5b). Many researchers have pointed out that the pits are connected with the rises in the localized current density, proving quickly localized metal corrosion (Burstein et al. 2005). It remains to be further proved by electrochemical techniques whether stable pits or metastable pits are formed on the surface of Ti-15-3 alloys. Similarly, as shown in Figure 5c and d (for 24 h), there exist some corrosion pits and cracked corrosion products after 24 h of NSS, but only pitting corrosions exist on the surface of Ti-15-3 alloy outside the crevice. Figure 5e shows that cracked corrosion products on the surface of Ti-15-3 alloy increase and there exist some corrosion pits after 120 h of NSS. Figure 5f shows that pitting corrosions on the surface of Ti-15-3 alloy outside the crevice increases obviously (for 120 h).

Surface morphologies of Ti-15-3 alloys within the crevice after 2 h (a), 24 h (c), and 120 h (e) of NSS; surface morphologies of Ti-15-3 alloys outside the crevice after 2 h (b), 24 h (d), and 120 h (f) of NSS.
Figure 6 shows the surface SEM images of Ti-15-3 alloys within the crevice for Ti-15-3 overlapped with PTFE after different NSS times. As can be seen in for Figure 6, the cracked corrosion products do not appear, and there only exist some corrosion pits on the surface of Ti-15-3 alloy, this suggests that the cracked corrosion products are derived from the anodic dissolution of the aluminized PVC film rather than from the Ti-15-3 alloy itself. And pitting corrosion on the surface of Ti-15-3 alloy inside the crevice increases obviously with increasing salt spray time. According to observations, the number of these corrosion pits is more than that on the Ti-15-3 alloy surface within the crevice for Ti-15-3 alloy overlapped with aluminized PVC film.

Surface morphologies of Ti-15-3 alloys within the crevice after 2 h (a), 24 h (b), 72 h (c), and 120 h (d) of NSS.
3.2 Electrochemical behavior of the Ti-15-3 alloy
The OCP of the electrode is a thermodynamic criterion for the possibility of metal corrosion. The more negative the potential is, the greater the tendency to convert metals into metal ions and enter into solutions will be. The OCPs measured of the Ti-15-3 alloy and aluminized PVC film (they are unexposed to NSS) in 3.5 wt% NaCl solution are summarized in Table 2.
The open-circuit potentials measured of Ti-15-3 alloy and aluminized PVC film in 3.5 wt% NaCl solution.
| Materials | Ti-15-3 | Aluminized PVC film |
|---|---|---|
| OCP (VSCE) | −0.131 | −0.750 |
From Table 2, it can be seen that the OCP of aluminized PVC film is relatively negative, which implies that aluminized PVC film serves as the anode. Hence, severe corrosion occurred on aluminized PVC film in the coupled regions because of galvanic corrosion. The galvanic corrosion reaction of Ti-15-3 alloy and aluminized PVC film is as follows:
Figure 7 shows the time dependence of the OCP measured of Ti-15-3 alloy electrodes inside the crevice for Ti-15-3 alloy overlapped with PTFE plate or aluminized PVC film after different NSS time (2, 24 72, and 120 h) in 3.5 wt% NaCl solution. As can be seen in Figure 7, it is observed that the potential of Ti-15-3 alloy inside the crevice for Ti-15-3 alloy overlapped with aluminized PVC film is more positive than that inside the crevice for Ti-15-3 alloy overlapped with PTFE plate after different NSS time, which implies that the aluminized PVC film inside the crevice serves as the anode and its corrosion will be accelerated whereas the Ti-15-3 alloy electrode inside the crevice serves as the cathode and its corrosion will be restrained for Ti-15-3 alloy/aluminized PVC film coupling.

Time dependence of the OCP measured of Ti-15-3 alloy electrode inside the crevice for Ti-15-3 alloy overlapped with aluminized PVC film or PTFE after 2 h (a), 24 h (b), 72 h (c), and 120 h (d) of NSS time in 3.5 wt% NaCl solution.
Figure 8 shows the polarization curves measured of Ti-15-3 alloy electrodes inside and outside the crevice for Ti-15-3 alloy overlapped with PTFE plate or aluminized PVC film after different NSS times (2, 24, 72, and 120 h) in 3.5 wt% NaCl solution. It serves to show that the self-corrosion current density inside the crevice of Ti-15-3 alloy overlapped with aluminized PVC film is smaller than that inside the crevice of Ti-15-3 alloy overlapped with PTFE plate after different NSS time, which implies that the galvanic effect of Ti-15-3 alloy/aluminized PVC film coupling could decrease the corrosion rate of Ti-15-3 alloy inside the crevice.

Polarization curves examined of Ti-15-3 alloy electrode inside the crevice of Ti-15-3 alloy overlapped with PTFE plate or aluminized PVC film after 2 h (a), 24 h (b), 72 h (c), and 120 h (d) of NSS in 3.5 wt% NaCl solution (scan rate: 5 mV/s).
Figure 9a depicts the CP curves measured of Ti-15-3 alloy electrode inside the crevice for Ti-15-3 alloy overlapped with aluminized PVC film after different NSS times (2, 24, 72, and 120 h) in 3.5 wt% NaCl solution. Three curves show negative hysteresis features exhibiting the self-repair of a damaged passive film and no initiation of pits in the test environment (Tait 2018). Whereas, although they did not continue evolving into stable pits, they may grow into metastable growing pits (Burstein et al. 2005). Three curves depict two current density plateaus. This characteristic was formerly observed for titanium alloy (Alkhateeb and Virtanen 2005). The first passivation current density plateau corresponds to the formation of titanium suboxides, TiO, and Ti2O3 (Kovačević et al. 2012). Then, the current density starts to increase suddenly for all of NSS, which could be related to a partial protective oxide layer. All of the second passivation characteristics is not obvious and corresponds to the formation of the partial titanium oxides, TiO2 (Fernández-Domene et al. 2011). These oxides act as barrier layers to restrain further corrosion of matrix alloy and give rise to the start of passivation (Liu et al. 2015). The corrosion repassivation reaction during polarization is given below:

CP curves measured of Ti-15-3 alloy inside the crevice in 3.5 wt% NaCl solution (scan rate: 1 mV/s): (a) For Ti-15-3 alloy overlapped with aluminized PVC film after different NSS time; for Ti-15-3 alloy overlapped with PTFE plate or aluminized PVC film after 72 h (b), and 120 h (c) of NSS.
It is obvious that the Ip of Ti-15-3 decreased gradually when salt spray time increased from 2 to 72 h. This could imply that the corrosion resistance of the Ti-15-3 alloy electrode inside the crevice increased gradually. However, the Ip of Ti-15-3 increased after 120 h of NSS. This could imply that the corrosion resistance of the Ti-15-3 alloy electrode inside the crevice decreased again. As can be seen in Figure 9b and c, it is apparent that Ip of Ti-15-3 alloy electrode inside the crevice for Ti-15-3 alloy overlapped with aluminized PVC film after 72 and 120 h of NSS are more lesser than that inside the crevice for Ti-15-3 alloy overlapped with PTFE, which implies that the galvanic effect of Ti-15-3 alloy/aluminized PVC film coupling can retard the corrosion of the Ti-15-3 alloy inside the crevice.
For Figure 10a, for all NSS time, the capacitive reactance arcs display that the arc radii inside the crevice for Ti-15-3 alloy overlapped with aluminized PVC film are larger than that inside the crevice for Ti-15-3 alloy overlapped with PTFE, indicating that the galvanic effect of Ti-15-3 alloy/aluminized PVC film coupling inhibits the corrosion of the Ti-15-3 alloy electrode inside the crevice. This result is in accordance with that of polarization curves in Figure 8. As can be seen in Figures 10b and d, it is observed that the arc radii of Ti-15-3 alloy outside the crevice are larger than that inside the crevice for Ti-15-3 alloy overlapped with aluminized PVC film after 2 and 120 h of NSS. For 72 h in Figure 10c, it shows that the arc radii of Ti-15-3 alloy outside the crevice are smaller than that inside the crevice, which implies that the galvanic effect caused by the Ti-15-3 alloy surface outside and inside the crevice weaken after 72 h of NSS time.

(a) Nyquist plots examined of Ti-15-3 alloy electrode inside the crevice for Ti-15-3 alloy overlapped with PTFE plate or aluminized PVC film after different NSS time; Nyquist plot examined of Ti-15-3 alloy electrode inside and outside the crevice for Ti-15-3 alloy overlapped with aluminized PVC film after 2 h (b), 72 h (c), and 120 h (d) of NSS.
4 Discussion
The crevice and galvanic corrosion mechanisms for the Ti-15-3/aluminized PVC film couple exposed to NSS environment are proposed by the corrosion morphology characterization and EDS analyses of corrosion product, combined with the potentiodynamic polarization and EIS results measured in 3.5 wt% NaCl solution. The initial stage of the salt spray, aluminized PVC film serves as the anode and it was corroded seriously because of galvanic corrosion of Ti-15-3 alloy/aluminized PVC film coupling. Meanwhile, the hydrolysis reaction (Eq. (4)) and electro-migration of chloride ions into the crevice led to the establishment of a critical crevice solution and depassivation of Al inside the crevice (Li et al. 2018).
With the increase of corrosion time, some aluminized PVC films inside the crevice are corroded. The electrochemical reaction occurred on the surface of the Ti-15-3 alloy inside and outside the crevice, as follows:
However, O2 inside the crevice is consumed by Eq. (6), O2 of the Ti-15-3 alloy surface within the crevice cannot be compensated because of the long narrow diffusion path formed by the crevice. Thus, an oxygen concentration difference cell is formed between the Ti-15-3 alloy surface outside and inside the crevice. This oxygen concentration difference effect accelerated the corrosion of the Ti-15-3 alloy surface within the crevice.
Then, the anodic reaction is restrained because of the replenishment of oxygen and the accumulative effect of galvanic corrosion for Ti-15-3 alloy/aluminized PVC film coupling inside the crevice. However, under conditions of high cathodic current density where the Ti-15-3 alloy surface outside the crevice may become strongly alkaline, this amphoteric metal such as titanium may suffer a severe attack. Meanwhile, the passivation of the anodic pitting on the surface of the Ti-15-3 alloy within the crevice can occur. Wherein possible reactions of dissolution and simultaneous passivation at the Ti-15-3 alloy surface inside and outside the crevice are given as follows:
From Figure 11, the anodic reaction within the crevice after 72 h of NSS is restrained, that is, passive film of the anodic pitting inside the crevice is in passivation state because of the galvanic effect of Ti-15-3 alloy/aluminized PVC film coupling whereas passive film of the anodic pitting on the surface of the Ti-15-3 alloy outside the crevice is dissolved. At this time, OH− and Cl− double erosion promoted the destruction of the Ti-15-3 alloy outside the crevice (Hu et al. 2017). The dissolution reaction of the bare metal/passive film interface outside the crevice can occur (Fernández-Domene et al. 2014):

The combination schematic illustrations of the proposed physic patterns for Ti-15-3 alloy overlapped with aluminized PVC film after 72 h of NSS.
5 Conclusions
The corrosion process of Ti-15-3 alloy overlapped with aluminized PVC film includes metastable pitting, crevice, and galvanic corrosion.
The corrosion resistance of the Ti-15-3 alloy electrodes inside the crevice for Ti-15-3 alloy overlapped with aluminized PVC film increase gradually when salt spray time increases from 2 to 72 h. However, the corrosion resistance of the Ti-15-3 alloy electrode inside the crevice decreased again after 120 h of NSS.
The galvanic effect of Ti-15-3 alloy overlapped with aluminized PVC film restrains the corrosion of Ti-15-3 alloy electrode inside the crevice.
The galvanic effect caused by the Ti-15-3 alloy surface outside and inside the crevice weaken because of the strong alkalinity of the Ti-15-3 alloy surface outside the crevice in certain salt spray time.
In summary, the corrosion mechanism diagrams of Ti-15-3 alloy overlapped with aluminized PVC film were revealed by microstructure observation of SEM, morphology analyses of EDS, and the discussions of EIS and potentiodynamic polarization.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 51975084
Award Identifier / Grant number: 51405059
Funding source: Fundamental Research Funds for the Central Universities
Award Identifier / Grant number: DUT19LAB16
Acknowledgments
The authors wish to thank the Analytical and Testing Center of the Dalian University of Technology for the support.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the National Natural Science Foundation of China (grant no.: 51975084, 51405059), and the Fundamental Research Funds for the Central Universities (DUT19LAB16).
-
Conflicts of interest: The authors declare no conflicts of interest regarding this article.
References
Alkhateeb, E., and Virtanen, S. (2005). Influence of surface self-modification in Ringer’s solution on the passive behavior of titanium. J. Biomed. Mater. Res. 75: 934–940, https://doi.org/10.1002/jbm.a.30508.Search in Google Scholar PubMed
Barik, R.C., Wharton, J.A., Wood, R.J.K., and Stokes, K.R. (2007). 8: galvanic corrosion of nickel–aluminium bronze coupled to titanium or Cu-15Ni alloy in brackish seawater. In: Féron, D. (Ed.), Corrosion behaviour and protection of copper and aluminium alloys in seawater. Woodhead Publishing, Cambridge, pp. 128–141, https://doi.org/10.1533/9781845693084.3.128.10.1533/9781845693084.3.128Search in Google Scholar
Bhandari, J., Khan, F., Abbassi, R., Garaniya, V., and Ojeda, R. (2015). Modelling of pitting corrosion in marine and offshore steel structures. A technical review. J. Loss Prev. Process. Ind. 37: 39–62, https://doi.org/10.1016/j.jlp.2015.06.008.Search in Google Scholar
Burstein, G.T., Liu, C., and Souto, R.M. (2005). The effect of temperature on the nucleation of corrosion pits on titanium in Ringer’s physiological solution. Biomaterials 26: 245–256, https://doi.org/10.1016/j.biomaterials.2004.02.023.Search in Google Scholar PubMed
Coelho, L.B., Hacha, M., Paint, Y., and Olivier, M.-G. (2019). Highlighting the effect of the aluminium alloy self-corrosion on the AA2024-T3/Ti6Al4V galvanic coupling in NaCl media. Surf. Interface 16: 15–21, https://doi.org/10.1016/j.surfin.2019.04.004.Search in Google Scholar
Du, X.-Q., Yang, Q.-S., Chen, Y., Yang, Y., and Zhang, Z. (2014). Galvanic corrosion behavior of copper/titanium galvanic couple in artificial seawater. Trans. Nonferrous Metals Soc. China 24: 570–581, https://doi.org/10.1016/S1003-6326(14)63097-1.Search in Google Scholar
Fernández-Domene, R.M., Blasco-Tamarit, E., García-García, D.M., and García-Antón, J. (2011). Cavitation corrosion and repassivation kinetics of titanium in a heavy brine LiBr solution evaluated by using electrochemical techniques and confocal laser scanning microscopy. Electrochim. Acta 58: 264–275, https://doi.org/10.1016/j.electacta.2011.09.034.Search in Google Scholar
Fernández-Domene, R.M., Blasco-Tamarit, E., García-García, D.M., and García Antón, J. (2014). Passivity breakdown of titanium in LiBr solutions. J. Electrochem. Soc. 161: C25–C35, https://doi.org/10.1149/2.035401jes.Search in Google Scholar
Galvele, J.R. (1976). Transport processes and the mechanism of pitting of metals. J. Electrochem. Soc. 123: 464–474, https://doi.org/10.1149/1.2132857.Search in Google Scholar
Hu, P., Song, R., Wang, K., Yang, F., Hu, B., Chen, Z., Li, Q., Cao, W., Liu, D., and Guo, L., et al. (2017). Electrochemical corrosion behavior of titanium-zirconium-molybdenum alloy. Rare Met. Mater. Eng. 46: 1225–1230, https://doi.org/10.1016/s1875-5372(17)30141-8.Search in Google Scholar
Kelly, R.G., and Lee, J.S. (2018). Localized corrosion: crevice corrosion. In: Wandelt, K. (Ed.), Encyclopedia of interfacial chemistry. Elsevier, Oxford, pp. 291–301, https://doi.org/10.1016/B978-0-12-409547-2.13420-1.Search in Google Scholar
Kovačević, N., Pihlar, B., Selih, V.S., and Milošev, I. (2012). The effect of pH value of a simulated physiological solution on the corrosion resistance of orthopaedic alloys. Acta Chim. Slov. 59: 144–155.Search in Google Scholar
Li, H., Brown, B., and Nešic, S. (2011). Predicting localized CO2 corrosion in carbon steel pipelines. In: Corrosion 2011. NACE International, Houston, TX, USA, pp. 13–17.10.5006/C2011-11253Search in Google Scholar
Li, D., Tai, Q., Feng, Q., Li, Q., Xu, X., Li, H., Huang, J., Dong, L., Xie, H., and Xiong, C., et al. (2014). Highly reflective and adhesive surface of aluminized polyvinyl chloride film by vacuum evaporation. Appl. Surf. Sci. 311: 541–548, https://doi.org/10.1016/j.apsusc.2014.05.106.Search in Google Scholar
Li, S., Khan, H.A., Hihara, L.H., Cong, H., and Li, J. (2018). Corrosion behavior of friction stir blind riveted Al/CFRP and Mg/CFRP joints exposed to a marine environment. Corrosion Sci. 132: 300–309, https://doi.org/10.1016/j.corsci.2018.01.005.Search in Google Scholar
Liang, C., Jia, L.’n., Yuan, C., and Huang, N. (2015). Crevice corrosion behavior of CP Ti, Ti-6Al-4V alloy and Ti-Ni shape memory alloy in artificial body fluids. Rare Met. Mater. Eng. 44: 781–785, https://doi.org/10.1016/S1875-5372(15)30046-1.Search in Google Scholar
Lin, F., Marteleur, M., Jacques, P.J., Jacques, and Delannay, L. (2018). Transmission of {332}⟨113⟩ twins across grain boundaries in a metastable β-titanium alloy. Int. J. Plast. 105: 195–210, https://doi.org/10.1016/j.ijplas.2018.02.012.Search in Google Scholar
Liu, J.-C., Park, S., Nagao, S., Nogi, M., Koga, H., Ma, J.-S., Zhang, G., and Suganuma, K. (2015). The role of Zn precipitates and Cl− anions in pitting corrosion of Sn–Zn solder alloys. Corrosion Sci. 92: 263–271, https://doi.org/10.1016/j.corsci.2014.12.014.Search in Google Scholar
Makhlouf, A.S.H. and Botello, M.A. (2018). Chapter 1: failure of the metallic structures due to microbiologically induced corrosion and the techniques for protection. In: Makhlouf, A.S.H. and Aliofkhazraei, M. (Eds.), Handbook of materials failure analysis. Butterworth-Heinemann, pp. 1–18.10.1016/B978-0-08-101928-3.00001-XSearch in Google Scholar
Popoola, L.T., Grema, A.S., Latinwo, G.K., Gutti, B., and Balogun, A.S. (2013). Corrosion problems during oil and gas production and its mitigation. Int. J. Ind. Chem. 4: 35.10.1186/2228-5547-4-35Search in Google Scholar
Santhosh, R., Geetha, M., Saxena, V.K., and Nageswararao, M. (2014). Studies on single and duplex aging of metastable beta titanium alloy Ti–15V–3Cr–3Al–3Sn. J. Alloys Compd. 605: 222–229, https://doi.org/10.1016/j.jallcom.2014.03.183.Search in Google Scholar
Sidharth and Plato, A.A. (2009). Effect of pitting corrosion on ultimate strength and buckling strength of plate-a review. Dig. J. Nanomater. Biostruct. 4: 783–788.Search in Google Scholar
Snihirova, D., Höche, D., Lamaka, S., Mir, Z., Hack, T., and Zheludkevich, M.L. (2019). Galvanic corrosion of Ti6Al4V-AA2024 joints in aircraft environment: modelling and experimental validation. Corrosion Sci. 157: 70–78, https://doi.org/10.1016/j.corsci.2019.04.036.Search in Google Scholar
Song, G., Johannesson, B., Hapugoda, S., and StJohn, D. (2004). Galvanic corrosion of magnesium alloy AZ91D in contact with an aluminium alloy, steel and zinc. Corrosion Sci. 46: 955–977, https://doi.org/10.1016/S0010-938X(03)00190-2.Search in Google Scholar
Tait, W.S. (2018). Chapter 5: electrochemical corrosion basics. In: Kutz, M. (Ed.), Handbook of environmental degradation of materials, 3rd ed. William Andrew Publishing, New York, pp. 97–115, https://doi.org/10.1016/B978-0-323-52472-8.00005-8.10.1016/B978-0-323-52472-8.00005-8Search in Google Scholar
Wang, H., and Han, E.-H. (2013). Simulation of metastable corrosion pit development under mechanical stress. Electrochim. Acta 90: 128–134, https://doi.org/10.1016/j.electacta.2012.11.056.Search in Google Scholar
Zadorozne, N.S., Giordano, C.M., Rodríguez, M.A., Carranza, R.M., and Rebak, R.B. (2012). Crevice corrosion kinetics of nickel alloys bearing chromium and molybdenum. Electrochim. Acta 76: 94–101, https://doi.org/10.1016/j.electacta.2012.04.157.Search in Google Scholar
Zhu, R., Zhang, J., and Gao, W. (2015). Effect of silane on galvanic corrosion between EW75 magnesium alloy and TC4 alloy. Rare Metal Mat. Eng. 44: 1838–1844, https://doi.org/10.1016/S1875-5372(15)30110-7.Search in Google Scholar
© 2021 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- In this issue
- Reviews
- Adoption of strategies for clean combustion of biomass in boilers
- Evolution of TSA/TSZ coatings: a review on recent advances on cold gas spraying for steel corrosion protection
- Original articles
- Pitting corrosion and crevice corrosion behaviors of titanium alloy overlapped with aluminized PVC film in neutral salt spray
- Prediction of corrosion rates of a ship under the flow accelerated corrosion mechanism
- Anodic protection of 316L stainless steel piping in sulfuric acid service: failure causes and remedial actions
- Structure related corrosion behavior of DLC films in high Cl− environment
- Comparison of corrosion behavior of 2205 and 2507 duplex stainless steel in simulated flue gas condensate of a waste incineration power plant
Articles in the same Issue
- Frontmatter
- In this issue
- Reviews
- Adoption of strategies for clean combustion of biomass in boilers
- Evolution of TSA/TSZ coatings: a review on recent advances on cold gas spraying for steel corrosion protection
- Original articles
- Pitting corrosion and crevice corrosion behaviors of titanium alloy overlapped with aluminized PVC film in neutral salt spray
- Prediction of corrosion rates of a ship under the flow accelerated corrosion mechanism
- Anodic protection of 316L stainless steel piping in sulfuric acid service: failure causes and remedial actions
- Structure related corrosion behavior of DLC films in high Cl− environment
- Comparison of corrosion behavior of 2205 and 2507 duplex stainless steel in simulated flue gas condensate of a waste incineration power plant

