Abstract
In the second half of the Twentieth Century, the chemical and molecular sciences experienced a deep transformation with regard to the types of research instruments used, and the associated methods involved. Historians have coined this development the Instrumental Revolution, and even described it as the Second Chemical Revolution [1]. With the latter notion, they referred to the First Chemical Revolution of the late eighteenth century, when Antoine Laurent Lavoisier and his allies transformed chemistry’s theoretical framework along with its nomenclature, creating modern chemistry. The “second” chemical revolution of the twentieth century had an equally deep impact on chemistry’s theoretical base, linking chemistry to quantum physics, and expanding its range into the life sciences and technologies, the material sciences, and engineering. However, the related changes in terminology and nomenclature have largely escaped the historian’s attention, and this might explain why IUPAC’s role in the Instrumental Revolution has not been investigated in any detail. In the following, I will first briefly describe the Instrumental Revolution, and its main impact on chemistry and related fields, before sketching IUPAC’s role in facilitating and enhancing it [2].
Physical Methods Reach the Core of Chemistry
The key development of the Instrumental Revolution has been the introduction of techniques and methods that had mostly been developed in physics and were based on advanced electrical engineering as well as concepts of theoretical physics. Examples include Infrared and Ultraviolet Spectroscopy, Raman Spectroscopy, Mass Spectrometry, Nuclear Magnetic Resonance (NMR), Electron Spin Resonance (ESR), and many others. These spectroscopic methods were used for the identification of molecules and elements, and they often came hand in hand with chromatographic techniques applied for separating single compounds, or groups thereof, from mixtures, in this sense isolating them.
It is of course true that physical methods were applied in chemistry from very early on—Lavoisier’s first chemical revolution being a famous case in point—but arguably the usage of such old physical methods was rather limited, both in scale and scope. Even in the late nineteenth century, when modern physical chemistry came into being with the “push” of concepts and techniques of physics, the “pull” of most chemical subdisciplines was rather slow in coming, and weak. Notably organic chemistry, chemistry’s most dynamic and powerful subdiscipline in the late nineteenth and early twentieth centuries, only reluctantly applied methods that were not relying on wet chemistry. Still, infrared spectroscopy had its impact on organic chemistry even then, and ultraviolet was soon joining, but the bulk of the organic and inorganic chemists’ benchwork was still done with test tubes and consisted mainly in carrying out chemical reactions. Analytical and synthetic chemistry still worked hand in glove sharing the same basics in methodology. The cornerstone of the feat of the elucidation of an unknown molecule’s structure was its synthesis.
Much of this changed in the mid twentieth century, and it changed very rapidly so. The above-mentioned techniques, and others, took chemistry by storm. In the year of Sputnik (1957), elite US scientists assembled in the newly founded National Science Foundation (NSF), were drumming the news that “much can still be done with test tubes, slide rule, paper and pencil, but there is now convincing evidence that great scientific discoveries are to be expected through the development and use of the expensive new tools of scientific research [3]”. In 1965, a detailed survey by the US National Academy of Science, aptly entitled Chemistry: Opportunities and Needs, brought up empirical evidence that the experimental practice of all chemists had deeply changed. A graph showed the seven most widely used new instruments in chemistry, based on their citation rate in articles of academic journals. Most of these techniques (Infrared, Ultraviolet, NMR, VPC (vapor-phase or gaz chromatography), Computer, Mass Spectrometry, and ESR) came into existence only after 1940. Their citation rate (which supplied a measure for actual use) in the early 1950s equaled only ca. 43 per 100 articles, thus less than a half of all relevant publications used one of these techniques. In 1964, about 120 citations per 100 articles made clear that the authors of the articles used on average 1.2 of these techniques in the work leading to a single publication. What was once unusual became routine, and a necessity, in roughly a decade and a half. See Figure 1.
What were the reasons for this rapid and wide-ranging transformation of chemical practice? How did it unfold? To answer the first question, historians have pointed to the impact of World War II. Armament research and development led to major advances in electrical engineering, and many appliances, for example those related to radar, found their way out of military establishments into the scientific laboratories. In addition, physical theory since the 1920s has brought about major shifts in chemistry as well. The theoretical understanding of chemical bonds and molecular structure made such clear advances that all chemists had to take notice—mentioning the names of Linus Pauling (1901-1994) and Robert S. Mulliken (1896-1986) might suffice here. All of this was connected to the use of physical methods, and soon, all chemists had to apply them, because colleagues that had a tendency to “ride the waves” of new technologies used the generous funding available after the start of the Korean War to speed up their efforts. Thus, technology push and demand pull came together with the felicitous rise of governmental spending on science.

Walmer Studios. “Beckman Instruments International Touring Laboratory,” 1964. Beckman Historical Collection, Box 57, Folder 24. Science History Institute. Philadelphia. https://digital.sciencehistory.org/works/ws859f652.
However, methods development is still seen today by many as a marginal affair, relegated to the methods sections in articles, and finding refuge only in the technical bulletins of industrial suppliers. In contrast to this neglect, the role of methods development loomed large in science: method makers developed and applied all kinds of research instrumentation, from big science to table-top instruments. They often worked in an interdisciplinary fashion, and played a major role in the tremendous expansion of the chemical sciences in the second half of the twentieth century. Arguably, without the inroads of physical methods into chemistry, many of the recent advancements in science and technology—from material science and nanotechnology through molecular biology and the life sciences to medicine—would not have been possible. Crucially, many activities of the method makers involve standardization, terminology and nomenclature. Thus, it does not come as a surprise that IUPAC and its commissions had a major role in the rise of physical methods in chemistry, and in the expansion of chemistry’s reach into neighboring fields beyond chemistry’s original turf. In the following, I will sketch briefly some of the major hubs of IUPAC’s activities in this regard, with special attention to the strategies deployed, and the goals envisaged.
Facing new methods in chemistry
In 1960, early in the Instrumental Revolution, William Albert Noyes (1898-1980), then president of IUPAC, together with Harold Warris Thompson (1908-1983) gave methods center stage in a description of IUPAC’s major functions. Referring to the longstanding goals of IUPAC to facilitate international agreement, and to bring about “uniform practice,” they included “certain methods of analysis and assay.” [4] In IUPAC’s characteristic structure, commissions took over this task, notably those in the Analytical Chemistry Division (ACD), and especially the Commission on Molecular Structure and Spectroscopy (CMSS) of the Physical Chemistry Division.
Plans for including spectroscopic methods in IUPAC’s portfolio reach back to the year 1951, when IUPAC’s division structure was firmly in place. After some delays, the CMSS was founded in 1955 at the meeting in Zurich, with Thompson as president. This was done with the awareness that existing sub-commissions on absorption and emission spectroscopy of the Division of Analytical Chemistry were only focusing on procedures directly relevant for analysis. Moreover, it was felt that European and US activities needed more coordination. In taking up this task, the CMSS in the late 1950s focused on standardization and presentation issues, mainly of Infrared and NMR Spectroscopy, adding Optical Rotatory Dispersion, the latter a technique developed by Carl Djerassi (1923-2015) in the United States. In addition, activities unfolded to coordinate between IUPAC, the International Union of Pure and Applied Physics (IUPAP), and the International Astronomical Union (IAU), which later led to the creation of the Triple Commission on Spectroscopy—since 1966 under the aegis of ICSU. Inside IUPAC, in the Analytical Chemistry Division, a commission of optical data was formed in 1955, which in 1959 changed its name into Commission on Spectrochemical and Other Optical Procedures for Analysis [5].
Chemists in IUPAC were swift in their actions; most of them were crucially impacted by the Instrumental Revolution, and they were eager to develop guidelines and signposts. For example, a task force in the ACD focusing on gas chromatography formed in 1959, less than ten years after this technique had been invented. Among their aims was “to recognize and encourage existing, well established conventions, to the extent that they are basically sound (consistent with accepted theory) and of general utility.” To “suppress confusion” their only weapon was the argument, making “these Recommendations as clear and concise that they will find universal acceptance.” [6]
During this rather early period, most recommendations of members of the Analytical Chemistry Division were concerned with traditional techniques, such as titrimetry, trace analysis and microchemistry. For example, in the late 1960s and early 1970s the pace picked up and included General Atomic Emission Spectroscopy, Mass Spectrometry, and Analytical Flame Spectroscopy, among others [7]. At the same time, however, many of the other relevant, and booming techniques, such as Infrared, Raman, and Mössbauer Spectroscopy, NMR, and Optical Rotary Dispersion, were covered by the Physical Chemistry Division’s Commission on Molecular Structure and Spectroscopy [8]. In 1963 they were also aware of the new challenges of computing and creating a sub-commission on storage and retrieval of spectroscopic data [9]. In the mid 1970s, the Tables of Wavenumbers for the Calibration of Infrared Spectrometers was IUPAC’s best-selling book [10]. This followed an international trend, focusing many spectroscopies in the new subdiscipline of chemical physics, thus not in the general practitioners’ domain of analytical chemistry. However, this was subject to change, beginning with the plans starting in 1971 to assemble the recommendations in a compendium. Following the models of compendia on nomenclature in organic and inorganic chemistry, and the Manual of Symbols and Terminology for Physical Quantities and Units, the new compendium was named the “Orange Book,” adopting the tradition of color nicknames for IUPAC titles. Published in 1978, the Orange Book contained the “definitive” (thus, approved by IUPAC) guidelines published before in Pure and Applied Chemistry. Today, the Orange Book is in the 3rd edition, first published in 1998, and available online.
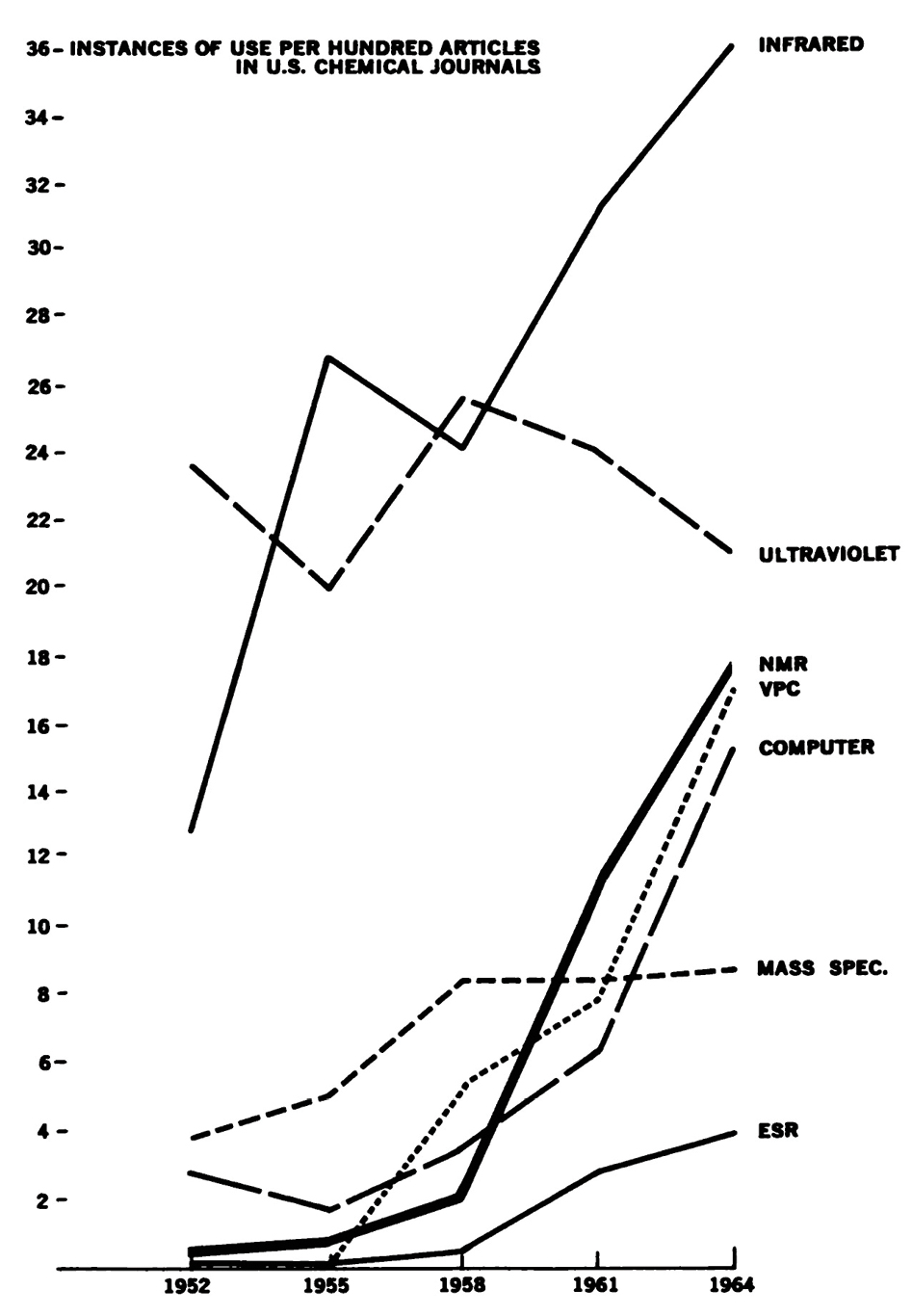
The use rate in chemical research in the United States for seven of the most commonly used types of instruments. The "use rate" is defined as the number of instances of use cited per 100 papers in selected representative journals. (reproduced from Chemistry: opportunities and needs; a report on basic research in U.S. chemistry. National Academy of Science, National Research Council, Committee for the Survey of Chemistry, 1965, fig 16)
In the 1970s, the goals of the IUPAC commissions consisted mainly in solving nomenclature and terminology issues, and there were many. In addition, they focused on calibration and standardization, thus covering the expected range of IUPAC. In the 1980s and 1990s, a new task gained prominence: performance evaluation, and related quality management both with regards to intra- and inter-laboratory evaluations. Increasingly, analysis was seen as a Chemical Measurement Process, a term for an analytical method with defined structure and statistical control. In this regard, Lloyd Currie in 1995 differentiated between sampling; preparation, measurement and evaluation; and presentation, and highlighting the middle pair as the central part of the measurement process. Equally important were the checkpoints of Standard (Certified) Reference Materials, and Standard Reference Data. Here, the IUPAC Analytical Chemistry Division was involved with publications and databases. Clarification of terminology continued, starting with general notions such as Precision, Accuracy, Technique, Methods, Procedure, and Protocol. IUPAC commission members were careful to apply precise definitions to practice, not just terminology. In 1995 the Commission of Analytical Nomenclature of the ACD distinguished between definitive methods (as stand alone, establishing accuracy), reference methods (related to a definitive method, or to certified materials), and standardized chemical measurement processes (used in the general practitioners’s laboratories), thus establishing a hierarchy of status between the methods developed and used. The first, definitive, rank was accorded to methods “of exceptional status…sufficiently accurate to stand alone in the determination of a given property for the Certification of a Reference Material” [11].
On this basis, in the early 2000s, David Moore, then chairman of the Analytical Chemistry Division of IUPAC, very aptly pointed to the Division’s impact during the last 50 years in an article describing the reform of its structure [12]. He emphasized the intermediary role of the IUPAC commissions between communities of academic scientists on the one hand and associations and organizations engaged in standardization on the other hand. Thus, facilitating the instrumental revolution led to an important role of IUPAC in the service of society, a role that continues to the present day. 
Über den Autor / die Autorin
Carsten Reinhardt, <carsten.reinhardt@uni-bielefeld.de> is Professor for Historical Studies of Science, University of Bielefeld.
References
1. Peter J. T. Morris, ed., From Classical to Modern Chemistry. The Instrumental Revolution, Cambridge: Royal Society of Chemistry 2002; Carsten Reinhardt, Shifting and Rearranging. Physical Methods and the Transformation of Modern Chemistry, Sagamore Beach: Science History Publications 2006.Search in Google Scholar
2. The first part of this is based on C. Reinhardt, “Forschungstechnologien im 20. Jahrhundert. Transfer und Transformationen,” in Klaus Hentschel, ed., Zur Geschichte von Forschungstechnologien. Generizität – Interstitialität – Transfer, Diepholz: GNT-Verlag 2012, pp. 277-307. (The author wishes to thank Danielle Fauque for crucial help in preparing this article.)Search in Google Scholar
3. National Science Foundation (NSF), MPE Divisional Committee, Chairman Thomas K. Sherwood to Bronk and Waterman, 21 January 1957. National Archives Record Administration (NARA), RG 307, Office of the Director, General Records, 1949-63, 1960-61, Box 48, folder Division of M, P, and ES.Search in Google Scholar
4. W. A. Noyes, H.W. Thompson, “The organization and functions of the International Union of Pure and Applied Chemistry (I.U.P.A.C.),” PAC 1 (1960), 5-10, https://doi.org/10.1351/pac19600101000510.1351/pac196001010005Search in Google Scholar
5. Based on IUPAC Comptes Rendus 1955-1961.Search in Google Scholar
6. Anon., “Preliminary Recommendations on nomenclature and presentation of data in gas chromatography,” PAC 1 (1960), 177-186, https://doi.org/10.1351/pac19600101017710.1351/pac196001010177Search in Google Scholar
7. See the list in H.M.N.H. Irving, H. Freiser, T. S. West, Compendium of Analytical Nomenclature. Definitive Rules 1977, Oxford: Pergamon Press 1978, pp. 5-6.Search in Google Scholar
8. “Commission on Molecular Structure and Spectroscopy (I.5)”, CMSS report, in IUPAC Comptes Rendus XXVI Conference, Washington DC, 15-24 July 1971, pp. 126-128Search in Google Scholar
9. R. C. Lord, “CMSS (I.5)”, in IUPAC Comptes Rendus XXII Conference, London, 5-9 July 1963, pp. 185-186Search in Google Scholar
10. “CMSS (I.5)”, report, in IUPAC Comptes Rendus, 28th Conference, Madrid, 2-11 Sept 1975, part B, pp. 193-195. Search in Google Scholar
11. Lloyd Currie, “Nomenclature in evaluation of analytical methods including detection and quantification capabilities (IUPAC Recommendations 1995),” PAC 67 (1995), 1699-1723; https://doi.org/10.1351/pac19956710169910.1351/pac199567101699Search in Google Scholar
12. David Moore, “IUPAC News, The Analytical Chemistry Division,” Chem Int 24(4) (2002), 16-19, https://doi.org/10.1515/ci.2002.24.4.16b10.1515/ci.2002.24.4.16bSearch in Google Scholar
©2019 IUPAC & De Gruyter. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. For more information, please visit: http://creativecommons.org/licenses/by-nc-nd/4.0/
Articles in the same Issue
- Masthead - Full issue pdf
- Special IUPAC100
- The Union in the Interwar period
- 1919-1939: The First Life of the Union
- The International Research Council and Its Unions: 1919-1931
- Ernst Cohen and the Challenge of a Truly International Union
- IUPAC in Brussels in 1921: A Historical Photo
- Pioneers of Japanese Participation in IUPAC
- After WWII: a fresh start
- London 1947: A Caricature
- Rebuilding IUPAC after WWII
- Ellen Gleditsch: Woman Chemist in IUPAC’s Early History
- IUPAC Expansion from 1957 to 1975
- The First Russian President of IUPAC: Victor Kondratiev
- IUPAC Engagement in the Instrumental Revolution
- IUPAC’s achievements and actions
- A History of CNIC
- IUPAC and the Naming of Elements
- A Century of Nomenclature for Chemists and Machines
- The mole and IUPAC: a brief history
- Stepping in the new century
- Women’s Increasing Responsibilities in IUPAC since 1975
- The Historical Archives of IUPAC at the Science History Institute
- Not an Epilogue, but a Commencement!
- Mark Your Calendar
Articles in the same Issue
- Masthead - Full issue pdf
- Special IUPAC100
- The Union in the Interwar period
- 1919-1939: The First Life of the Union
- The International Research Council and Its Unions: 1919-1931
- Ernst Cohen and the Challenge of a Truly International Union
- IUPAC in Brussels in 1921: A Historical Photo
- Pioneers of Japanese Participation in IUPAC
- After WWII: a fresh start
- London 1947: A Caricature
- Rebuilding IUPAC after WWII
- Ellen Gleditsch: Woman Chemist in IUPAC’s Early History
- IUPAC Expansion from 1957 to 1975
- The First Russian President of IUPAC: Victor Kondratiev
- IUPAC Engagement in the Instrumental Revolution
- IUPAC’s achievements and actions
- A History of CNIC
- IUPAC and the Naming of Elements
- A Century of Nomenclature for Chemists and Machines
- The mole and IUPAC: a brief history
- Stepping in the new century
- Women’s Increasing Responsibilities in IUPAC since 1975
- The Historical Archives of IUPAC at the Science History Institute
- Not an Epilogue, but a Commencement!
- Mark Your Calendar

