Monoclonal whole IgG impairs both fibrin and thrombin formation: hemostasis and surface plasmon resonance studies
-
Lénárd Gonda
Abstract
Objectives
Monoclonal gammopathies frequently associate with hemostatic alterations. Thrombotic events occur with high incidence particularly upon treatment, while in rarer cases hemorrhagic diathesis can be observed. The pathology of these tendencies could be caused by thrombocytopenia or hyperviscosity burden of circulating monoclonal antibodies. Studies also suggest interference of monoclonal antibodies with primary hemostasis. We isolated monoclonal whole IgG paraproteins from two myeloma patients to observe their effect on thrombin formation and fibrin polymerization.
Methods
Monoclonal whole IgG was prepared from sera of two newly diagnosed untreated multiple myeloma patients and control normal plasma samples. Fibrin formation was measured using thrombin time and dilute prothrombin time tests and thrombin formation was detected with a fluorimetric thrombin generation assay. In addition, molecular interactions were investigated by surface plasmon resonance (SPR).
Results
Thrombin time was prolonged upon addition of monoclonal IgG even at 30 g/L by 12 %, increasing up to 36 % at 60 g/L concentration. Dilute prothrombin time was prolonged by 20 % even at 30 g/L. Thrombin generation assay indicated an impairment in thrombin formation at the presence of monoclonal IgG compared to polyclonal at equivalent concentration. By an SPR assay we determined that both clonality IgG preparations interacted with fibrinogen, however interaction with human thrombin was only detected with monoclonal immunoglobulins (KD=1.03 × 10-7 M).
Conclusions
Here we provide evidence that isolated monoclonal whole IgG from myeloma patients can impair both fibrin and thrombin formation and we demonstrate by SPR assay that it interacts with components of the final phase of the coagulation system.
Introduction
Patients diagnosed with monoclonal gammopathies are prone to develop hemostatic alterations.
Although most of these can only be observed in a developed full-blown disease like myeloma, some pathological hemostatic alterations have also been described in its precursor state the monoclonal gammopathy of undetermined significance (MGUS). Hemostatic changes are best exemplified by the higher incidence of thrombotic events in untreated myeloma patients [1], [2], [3], [4], [5], that further increases upon treatment with immunomodulatory drugs. Even though, 15 % of myeloma patients may also exhibit bleeding symptoms. One trivial cause for this could be the anemia and thrombocytopenia observed in these patients, as red blood cells and platelets can provide the physiological surface for the assembly of coagulation factor complexes. Another potential cause for the bleeding symptoms are the defects in primary hemostasis [6]. Regarding the coagulation phase impairments in myeloma, one study investigating a large number of patients observed a clear prognostic role of prolongations in prothrombin time (PT) and activated partial thromboplastin time (APTT) in newly diagnosed patients [7]. In addition to these generally used screening tests there are studies that demonstrate that the presence of paraproteins interfere with the thrombin time assay that sometimes results in an unmeasurably long thrombin time [8, 9]. Most authors agreed upon that these prolongation effects are mere measurement interferences as the patients did not have any bleeding tendency even with very long thrombin times [10]. There is no consensus however on what may cause these prolongations in clotting times. Some authors concluded that it is unrelated to the amount of paraproteins but rather related to the presence of free light chains [6].
In our experiments we hypothesized that the monoclonal whole IgG paraprotein is capable of interacting with the coagulation system and thus can exert an inhibitory effect. To investigate these effects, we isolated monoclonal IgG from two myeloma patients and used these preparations to spike normal pool plasma samples to investigate fibrin and thrombin formation, and the results were compared to data obtained after adding equivalent amounts of isolated polyclonal pooled IgG. To identify interactions of the coagulation proteins and quantify their binding affinities, surface plasmon resonance studies were carried out to observe that monoclonal IgG molecules, unlike polyclonal IgG, interacts with human thrombin and this impairs both fibrin and thrombin formation in human plasma.
Materials and methods
Immunoglobulin isolation
Immunoglobulin purification was carried out by affinity chromatography method described previously [11]. Citrated blood samples were first centrifuged (1,500 g, 15 min, room temperature) and the supernatants were collected and were frozen and stored at −20 °C until the purification process. Plasma samples were diluted by a fivefold volume binding buffer 20 mM sodium phosphate, pH 7.0 before the application to the chromatography column (HiTrap™ Protein G HP [5 mL], GE Healthcare Uppsala, Sweden) with a peristaltic pump. After washing the column to clear residual proteins, elution buffer (0.1 M glycine pH 2.7) was used to create aliquots preloaded with neutralizing buffer (1 M Tris pH 9.0) to create a neutral solution. Collected sample was dialyzed three times against 1× PBS, pH 7.0. Ultrafiltration was done by (Amicon® Ultra 100K) centrifugal filter device (4,000 g, 15 min, room temperature). The final IgG concentration of our stock was 400 g/L. Our method resulted in a yield of 95 % of IgG purified from sample plasma. The purity from residual proteins was confirmed by electrophoresis.
Thrombin time
Thrombin time was measured by ST4 hemostasis analyzer (Stago International S.C.A., Asnières sur Seine Cedex, France). Previously described isolated immunoglobulins were diluted in normal pool plasma in a ratio of 1:4, at the final concentration of 30 and 60 g/L. According to manufacturer’s instruction 50 µL of sample was diluted with 50 µL of Owren’s buffer and incubated for 30 min. Human thrombin reagent was used at a concentration of 3 NIH units/mL (Sigma Aldrich, Thrombin from Human plasma, T7009). Measurements were done in quadruplicates to ensure consistency.
Dilute prothrombin time
Dilute prothrombin time was measured using BCS® XP System hemostasis analyzer (Siemens Healthineers AG, Forchheim, Germany). Previously described isolated immunoglobulins were diluted in normal pool plasma in a ratio of 1:4, at a final concentration of 30 and 60 g/L. Preincubation was performed at 37 °C for 30 min. Fifty µL of samples were added to 50 µL of 50× and 500× prediluted human recombinant tissue factor (Dade™ Innovin™ PT Reagent, Siemens Healthineers). Initiation of clotting was achieved by adding 50 µL 20 mmol/L CaCl2. Measurements were repeated four times to ensure precision.
Thrombin generation assay
Thrombin generation was measured by the calibrated automated thrombogram (CAT) method (Fluoroskan Ascent FL fluorimeter, Thrombinoscope BV, Maastricht, The Netherlands). Twenty µL of the reagent (1 pM recombinant tissue factor, 4 µM phospholipid) and thrombin calibrator (Thrombinoscope BV, Maastricht, Netherlands) were pipetted into the wells of a 96-well plate, to which was added 80 µL of normal platelet poor plasma containing 30 g/L of isolated immunglobulins. The control consisted of normal platelet poor plasma diluted with PBS with corresponding volume to match the composition of samples with isolated IgG. Incubation was performed at 37 °C for 30 min. Thrombin generation was started by dispensation of 20 μL of FluCa-Kit including 100 mmol/L CaCl2 and Z-Gly-Gly-Arg AMC (Thrombinoscope BV, Maastricht, Netherlands) into each well. Measurement time was 1 h. Thrombin generation measurements have been repeated to demonstrate reproducibility (n=3). Thrombinoscope software (Thrombinoscope BV, Maastricht, Netherlands) was used to calculate thrombin activity over time. The analyzed parameters were lagtime, endogenous thrombin potential (ETP), thrombin peak, time to peak and velocity index.
Surface plasmon resonance (SPR)
SPR assay was performed on a Biacore X instrument (GE Healthcare, Uppsala, Sweden). Ligands of the experiment was obtained as described earlier by affinity chromatography. The SPR sensor chip (CMD 500, XanTec bioanalytics GmbH, Duesseldorf, Germany) covered by a 500 nm normal matrix carboxymethyl dextran hydrogel coating was used and immobilization process was performed according to manufacturer’s instruction. Activation of the chip was done with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS) with the ratio of 1:1. Separated immunoglobulins were diluted in sodium-acetate (10 nM, pH 8.0) before immobilization. Excess NHS groups were deactivated with ethanolamine. Human thrombin (Sigma Aldrich, Thrombin from Human plasma, T7009) analyte with different concentrations (100, 200, 300, 500, 1,000 nM) was injected into the microflow chamber with running buffer HBS (10 mM HEPES, pH: 7.4, 150 mM NaCl, 50 % (v/v) glycerol) with a flow rate of 10 μL/min at 37 °C during measurements. Regeneration between measurements was achieved with running 70 % (v/v) glycerol. Evaluation process was performed by BIAevaluation software (GE Healthcare, Uppsala, Sweden).
Results
Hemostasis parameters of patients
We investigated 10 de novo and untreated patients all with IgG type multiple myeloma, who all had elevated total protein and monoclonal IgG with variable kappa or lambda light chains (data not shown). Patients have not received anticoagulant, antiplatelet or antimyeloma treatment at the time of sampling. Although the mean values of the coagulation screening tests were all within the reference range, in case of three patients the thrombin time value reached or exceeded the upper reference limit. It is important to emphasize that prolonged thrombin time tests were not normalized in the presence of polybrene so they were not caused by an endogenous heparin-like activity. Regarding activated partial thrombin time and prothrombin time our samples were within reference range (Table 1).
Clinical laboratory parameters of patient samples.
| Patient A | Patient B | Reference range | |
|---|---|---|---|
| Total protein, g/L | 114 | 139 | 60–80 |
| IgG, g/L | 85.7 | 100.1 | 7.0–16.0 |
| IgA, g/L | <0.5 | <0.5 | 0.7–4.0 |
| IgM, g/L | <0.25 | <0.25 | 0.4–2.3 |
| Monoclonal component, g/L | 61.9 | 83.3 | |
| β₂ microglobulin, mg/L | 7.1 | 9.21 | 1.09–2.53 |
| Light chain kappa, mg/L | 16.8 | 2,000 | 3.3–19.4 |
| Light chain lambda, mg/L | 240 | 7.67 | 5.71–26.3 |
| Serum light chain ratio | 0.07 | 260.8 | 0.26–1.65 |
| Prothrombin time, s | 10.3 | 11.9 | 8–12 |
| Activated partial thromboplastin time, s | 32.1 | 29.2 | 28–37 |
| Thrombin time, s | 24 | 31.4 | 16–24 |
| Fibrinogen, g/L | 2.06 | 3.77 | 1.5–4.0 |
| Factor II activity, % | 74 | 94 | 70–120 |
| Factor V activity, % | 109 | 62 | 70–120 |
| Factor VII activity, % | 72 | 60 | 70–130 |
| Factor VIII activity, % | 378 | 438 | 60–150 |
| Factor X activity, % | 81 | 84 | 70–120 |
Effect of immunoglobulins on thrombin time
Out of the three patients with prolonged thrombin time in two cases the IgG was isolated and its effects was investigated in functional assays. Thrombin time of a control plasma in the presence of isolated monoclonal immunoglobulin were prolonged and dependent on the concentration of paraprotein. Control polyclonal immunoglobulins at 30 g/L had thrombin time of 20.7 s while paraproteins at 30 g/L had thrombin time of 23.3 s and 23.1 respectively. Furthermore, immunoglobulin concentration of 60 g/L demonstrated even longer thrombin times. In the presence of control polyclonal immunoglobulin the thrombin time was 21.6 s contrasting the thrombin time of 24.2 and 29.4 s in the presence of isolated myeloma proteins. This meant that thrombin times were prolonged by 9.6 and 12.3 % even at the concentration of 30 g/L, whereas at 60 g/L concentration of monoclonal IgG caused 11.9 and 36.2 % prolongation in thrombin time compared to plasmas containing equivalent amount of polyclonal immunoglobulins (Figure 1A).
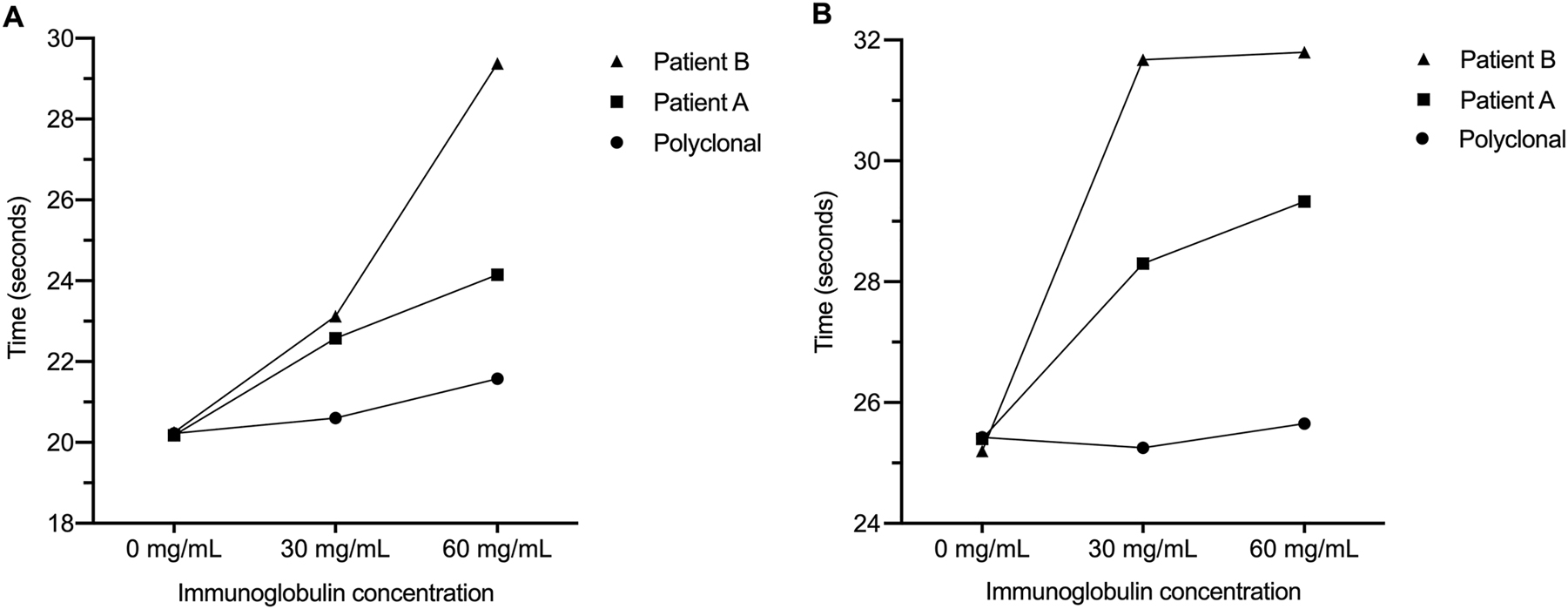
The effect of immunoglobulins on thrombin time and dilute prothrombin time results thrombin time after the addition of isolated monoclonal (Patient A and B) and polyclonal immunoglobulins (Control) in control pooled plasma (A). Prothrombin time results obtained with 50× diluted recombinant tissue factor reagent (B) after the addition of isolated monoclonal (Patient A and B) and polyclonal (Control) immunoglobulins.
Effect of immunoglobulins on dilute prothrombin time
Our control whole IgG with 50× diluted prothrombin time was 25.3 s at 30 g/L and 25.7 s at 60 g/L. Myeloma proteins caused an increase to 28.3 and 31.7 s even at 30 g/L. A similar tendency was observed with monoclonal IgG present at 60 g/L as 29.3 and 31.8 s were measured in the two spiked samples. Measurements were also carried out with 500× diluted thromboplastin reagent, which suggested corresponding prolongation upon introduction of monoclonal paraproteins. In summary dilute prothrombin time was prolonged by monoclonal protein 10.6–20.2 % compared to control healthy protein depending on investigated concentrations (Figure 1B).
Effect of isolated IgG on plasma thrombin generation assay
Introduction of IgG to thrombin generation assay at the concentration of 30 g/L despite the clonality, have resulted in an increased thrombotic state (Figure 2). Presence of IgG decreased lagtime by 18–22 % and time to peak by 28–35 %. However, the presence of a monoclonal immunoglobulin mitigated thrombin generation compared to polyclonal immunoglobulins. Endogenous thrombin potential was decreased by 15–21 % and peak thrombin was reduced from 285 nM by 24 and 22 % in the presence of monoclonal proteins compared to plasmas containing equivalent amount of polyclonal IgG. The velocity index – that integrates quantity and time parameters – showed considerable decrease as it was by 19 and 33 % lower when monoclonal IgG was added (Table 2). Notably monoclonal IgG at this pathological plasma concentration of 30 g/L is still in a prothrombotic state compared to control plasma, but the impairment of thrombin generation in contrast to polyclonal IgG is evident.
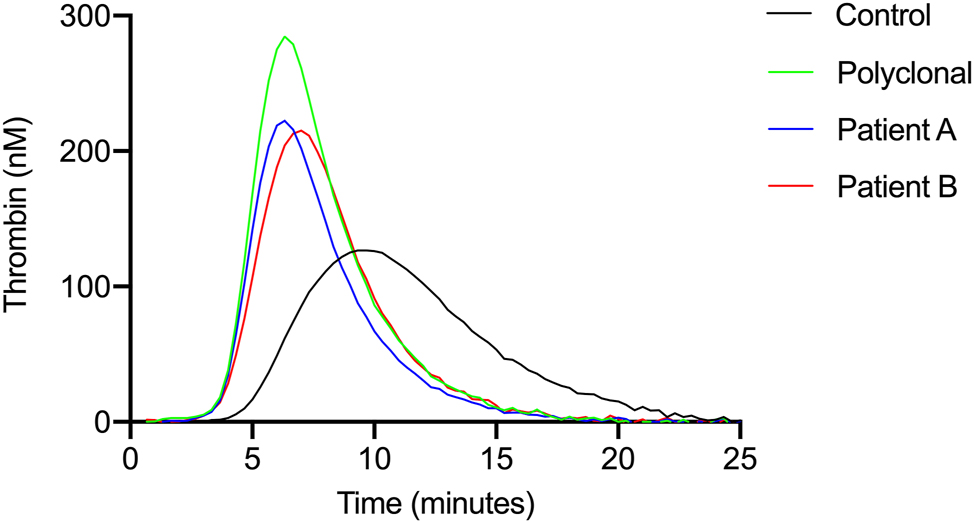
Thrombin generation curves upon addition of isolated immunoglobulins the thrombin generation curves show the attenuation of thrombin generation after the isolated paraproteins were added to control platelet-poor plasma at a final concentration of 30 g/L.
Thrombin generation assay results Effects of monoclonal and control whole IgG at concentration of 30 g/L on velocity and quantity of thrombin generation. Results of three replicate measurements.
| Average ± SD | Control | Polyclonal | Patient A | Patient B |
|---|---|---|---|---|
| Lagtime, min | 5.0 ± 0 | 3.9 ± 0.2 | 3.9 ± 1.8 | 4.1 ± 0.2 |
| ETP, nM × min | 1,102 ± 147 | 1,338 ± 46 | 1,051 ± 49 | 1,136 ± 174 |
| Peak, nM | 128 ± 26 | 285 ± 14 | 222 ± 7 | 216 ± 24 |
| ttPeak, min | 9.7 ± 0.3 | 6.4 ± 0.2 | 6.3 ± 0 | 7.0 ± 0.3 |
| Vel index, nM/min | 27 ± 7 | 112 ± 13 | 91 ± 7 | 75 ± 13 |
Binding characteristics of paraproteins to human thrombin and fibrinogens
We hypothesize that these interfering effects with fibrin and thrombin formation can be demonstrated by a binding technique using non-labeled technology as this would not alter the binding characteristic of the investigated ligands. Polyclonal immunoglobulins isolated from healthy controls showed no interaction with human thrombin at the concentration of 100, 200 and 1,000 nM (Figure 3A), contrary to immunoglobulins separated from monoclonal gammopathies. Paraprotein interaction with human thrombin at concentrations of 300–1,000 nM resulted in equilibrium dissociation constants, KD=1.03 × 10−7 M and KD=1.67 × 10−7 M respectively (Figure 3B, Table 3).
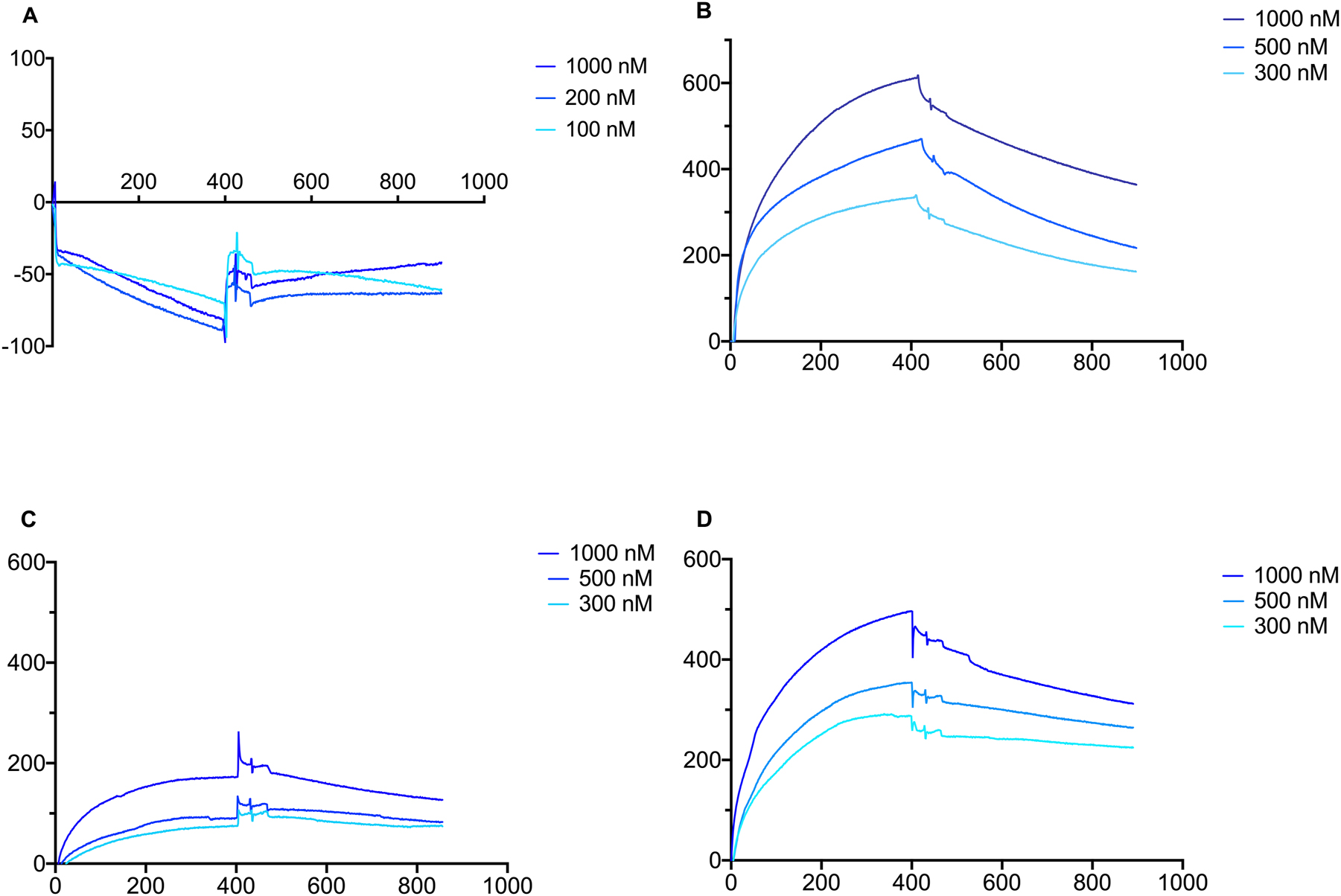
Surface plasmon resonance results of isolated immunoglobulins effect on thrombin with polyclonal IgG, monoclonal IgG, fibrinogen with polyclonal IgG and monoclonal IgG on the vertical axis response unit and on horizontal axis time in seconds are displayed. No interaction of isolated polyclonal immunoglobulin with human thrombin was recorded at various thrombin concentrations (A). Evident interaction was observed of isolated monoclonal immunoglobulin with human thrombin at different concentrations for patient A (B). Interaction of isolated immunoglobulin with fibrinogen at various concentrations. Binding of polyclonal IgG (C), and that of monoclonal IgG from patient A (D) were observed.
Surface plasmon resonance calculated constants Thrombin interferes only with monoclonal IgG. Fibrinogen displays binding to both monoclonal and polyclonal IgG with similar affinity.
| KD (M) | ka (1/Ms) | kd (1/s) | |
|---|---|---|---|
| “Patient A” IgG to thrombin | 1.03 × 10−7 | 1.59 × 104 | 1.22 × 10−3 |
| “Patient B” IgG to thrombin | 1.67 × 10−7 | 8.19 × 103 | 1.18 × 10−3 |
| “Patient A” IgG to fibrinogen | 3.08 × 10−8 | 2.06 × 104 | 4.09 × 10−4 |
| Control IgG to fibrinogen | 5.05 × 10−8 | 1.71 × 104 | 7.45 × 10−4 |
We also studied the binding characteristics of monoclonal and polyclonal IgG to the different forms of human fibrinogen. Human plasma fibrinogen composes mostly of the fraction that contains gamma A/gamma A subunits and this encompasses 85 % of the plasma fibrinogen (Peak 1) while another fraction contains gamma A/gamma‘ subunits and this is the fraction that is bound to the circulating coagulation factor XIII (Peak 2) [12]. A neglectable amount (0.5 %) contains gamma’/gamma’ chains only. We studied the binding of polyclonal and monoclonal IgG to Peak 1 and Peak 2 of the fibrinogen molecule (Figure 3C, D) and the following binding characteristics were measured (Table 3). As can be seen in case of Peak 1 and Peak 2 fibrinogen bound to mono- and polyclonal IgG with similarly high affinity (KD 10−8).
Discussion
In a recent study authors investigated thrombin generation in whole blood in multiple myeloma patients [13]. Similarly, to our study done on plasma samples of myeloma patients they observed an enhanced thrombin formation as measured by the ETP values. However, they also observed that thrombin formation was delayed, that resulted in the prolongation of lag time and time to peak parameters. These results reflect a disbalanced hemostasis that is indicative for both hypo- and hypercoagulable state [13]. Similarly in a more recent study we have also observed enhanced thrombin generation in myeloma cases and we also described that multiple myeloma cases are resistant to the effect of activated protein C unlike MGUS cases or healthy controls [1]. In addition to the literature on enhanced thrombin generation there were also observations on prolongation of clotting times in multiple myeloma cases [8, 9]. Some authors previously described an association with elevated monoclonal IgG and prothrombin time [14]. Our 10 patients had a significant correlation of monoclonal component and prothrombin time (p=0.027), suggesting an impairment in fibrin formation. In order to clarify the cause of these prolongations we isolated IgG from two myeloma cases and studied their effect in coagulation tests and on thrombin formation. We have observed that fibrin formation – as detected in different types of clotting assays – were prolonged 9.6–36.2 % upon addition of monoclonal IgG to control plasma. The dilute PT test proved to be more sensitive at both applied dilutions as here considerable prolongation was observed already at 30 g/L IgG concentration that is the upper reference limit that is also observed in MGUS. Previous studies have indicated that the reduction in the concentration of agonist to 1 pM tissue factor and 4 µM phospholipid in thrombin generation improved sensitivity for modulators [1, 15]. The thrombin generation assay using this method was also quite sensitive in the detection of this impairment in thrombin formation as at 30 g/L final concentration of the monoclonal IgG the speed of thrombin formation (velocity index) values were impaired by 19 and 33 %. These values correlated with the thrombin times measured in the patients’ plasma as the larger effect on thrombin generation assay also resulted in a longer thrombin time in the patient. Although the two isolated IgG preparations elicited considerably different impairment in thrombin formation, their binding affinity to thrombin was in a similar range. Thrombin generation also highlighted the prothrombotic state of the pathological plasma IgG concentration of 30 g/L. This effect could correlate with publications describing thromboembolic events upon administering intravenous IgG [16].
In case of thrombin binding to polyclonal IgG resulted in no SPR binding signal. Nevertheless, we found that fibrinogen established a strong interaction with the normal IgG. These results are in accordance with previously published data when Boehm and coworkers have observed that fibrinogen specifically binds to IgG and IgA but not to IgM antibodies and this interaction is substantiated via the Fab and Fc portions of the heavy chain, although the strength of the binding via the heavy chain Fc portion is considerably stronger. It was also demonstrated that this binding alters the binding ability of the IgG as at high fibrinogen concentration these antibodies may bind roughly twice as much fibrinogen and this can alter the composition of the fibrinogen aggregates [17, 18].
Then binding affinities of fibrinogen with polyclonal and monoclonal IgG were compared and we found that normal polyclonal IgG and monoclonal IgG strongly binds in similar magnitude to fibrinogen (KD 10−8). As most myeloma patients display an elevated fibrinogen level, these observations may have implications in the formation of thrombotic and bleeding complications as observed in myeloma patients [19]. Furthermore, we have investigated separately the portion of the fibrinogen that binds coagulation factor XIII and designated as Peak 2. We have observed that the binding affinities with monoclonal and polyclonal IgG are in similar range (KD=10−8). Multiple myeloma and its precursor state MGUS exemplifies clinical entities that interact with the hemostatic system via several potential mechanisms. The inhibitory effect of isolated monoclonal whole IgG on fibrin and thrombin formation does not contradict the observations of the enhanced thrombin formation in multiple myeloma. The cause of the enhanced procoagulant activity is mostly related to a pronounced cellular hypercoagulability elicited by the phosphatidylserine exposing cells [20] with simultaneous endothelial cell activation [21] and the important role of procoagulant phospholipid and tissue factor bearing microvesicles has also been demonstrated [22]. Our study has some limitations. It is an observational experimental study on only two isolated monoclonal IgGs that does not allow statistical evaluation of the results. Nevertheless, the observed effects in both fibrin and thrombin formation are substantial and the three different functional studies provided concordant results on the interaction of monoclonal IgG with thrombin and fibrin formation.
Venous thromboembolism remains a dreadful complication in multiple myeloma, with an increased rate during antimyeloma treatment [23]. Moreover appropriate prophylaxis for this complication still remains a matter of debate [24] and the mechanisms behind this complication is yet to be fully understood. A comprehensive understanding of the pathomechanism is essential for management and prevention of hemostasis complications in monoclonal gammopathies [25]. In this report we describe a phenomenon in paraproteinemias, that so far have not been published. It is also important to emphasize that these effects were observed at the 30 g/L IgG concentration that represents the borderline IgG limit between MGUS and multiple myeloma, thus the described changes may also occur in the precursor state of this disease.
-
Research ethics: The study was conducted in accordance with the Declaration of Helsinki and the protocol was approved by the Scientific and Research Ethics Committee of the University of Debrecen (protocol number: RKEB/IKEB 5906-2021).
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: The authors state no conflict of interest.
-
Research funding: János Kappelmayer was supported by a Research Group Grant provided by the Faculty of Medicine, University of Debrecen in 2023.
-
Data availability: The raw data can be obtained on request from the corresponding author.
References
1. Ghansah, H, Debreceni, IB, Váróczy, L, Rejtő, L, Lóczi, L, Bagoly, Z, et al.. Patients with multiple myeloma and monoclonal gammopathy of undetermined significance have variably increased thrombin generation and different sensitivity to the anticoagulant effect of activated protein C. Thromb Res 2023;223:44–52. https://doi.org/10.1016/j.thromres.2023.01.010.Search in Google Scholar PubMed
2. Ghansah, H, Orbán-Kálmándi, R, Debreceni, IB, Katona, É, Rejtő, L, Váróczy, L, et al.. Low factor XIII levels and altered fibrinolysis in patients with multiple myeloma. Thromb Res 2023;234:12–20. https://doi.org/10.1016/j.thromres.2023.12.004.Search in Google Scholar PubMed
3. Li, P, Xu, B, Xu, J, Xu, Y, Wang, Y, Chen, C, et al.. Lenalidomide promotes thrombosis formation, but does not affect platelet activation in multiple myeloma. Int J Mol Sci 2023;24:14097. https://doi.org/10.3390/ijms241814097.Search in Google Scholar PubMed PubMed Central
4. Nielsen, T, Kristensen, SR, Gregersen, H, Teodorescu, EM, Pedersen, S. Prothrombotic abnormalities in patients with multiple myeloma and monoclonal gammopathy of undetermined significance. Thromb Res 2021;202:108–18. https://doi.org/10.1016/j.thromres.2021.03.015.Search in Google Scholar PubMed
5. Yokoyama, K. Thrombosis in lymphoma patients and in myeloma patients. Keio J Med 2015;64:37–43. https://doi.org/10.2302/kjm.2014-0017-re.Search in Google Scholar
6. Hinterleitner, C, Pecher, AC, Kreiselmeier, KP, Budde, U, Kanz, L, Kopp, HG, et al.. Disease progression and defects in primary hemostasis as major cause of bleeding in multiple myeloma. Eur J Haematol 2020;104:26–35. https://doi.org/10.1111/ejh.13331.Search in Google Scholar PubMed
7. Geng, C, Yang, G, Wang, H, Zhang, Z, Zhou, H, Chen, W. The prognostic role of prothrombin time and activated partial thromboplastin time in patients with newly diagnosed multiple myeloma. BioMed Res Int 2021;2021:6689457. https://doi.org/10.1155/2021/6689457.Search in Google Scholar PubMed PubMed Central
8. Njegovan, M, Margetic, S, Kuna, AT, Derek, L, Celap, I, Pavicic, T, et al.. Interference of M-protein on thrombin time test a: a case report. Lab Med 2020;51:545–9. https://doi.org/10.1093/labmed/lmz103.Search in Google Scholar PubMed
9. Huang, H, Li, H, Li, D. Effect of serum monoclonal protein concentration on haemostasis in patients with multiple myeloma. Blood Coagul Fibrinolysis 2015;26:556–9. https://doi.org/10.1097/mbc.0000000000000296.Search in Google Scholar PubMed
10. Gogia, A, Sikka, M, Sharma, S, Rusia, U. Hemostatic abnormalities in multiple myeloma patients. Asian Pac J Cancer Prev 2018;19:127–30. https://doi.org/10.22034/APJCP.2018.19.1.127.Search in Google Scholar PubMed PubMed Central
11. Grodzki, AC, Berenstein, E. Antibody purification: affinity chromatography – protein A and protein G Sepharose. Methods Mol Biol 2010;588:33–41. https://doi.org/10.1007/978-1-59745-324-0_5.Search in Google Scholar PubMed
12. Siebenlist, KR, Meh, DA, Mosesson, MW. Plasma factor XIII binds specifically to fibrinogen molecules containing gamma chains. Biochemistry 1996;35:10448–53. https://doi.org/10.1021/bi9606206.Search in Google Scholar PubMed
13. Li, L, Roest, M, Remijn, J, Fijnheer, R, Smit, K, Huskens, D, et al.. Patients with multiple myeloma have disbalanced whole blood thrombin generation profile. Front Cardiovasc Med 2022;9:919495. https://doi.org/10.3389/fcvm.2022.919495.Search in Google Scholar PubMed PubMed Central
14. Pandey, S, Post, SR, Alapat, DV, Smock, KJ, Post, GR. Prolonged prothrombin time correlates with serum monoclonal protein concentration in patients with plasma cell dyscrasia. Int J Lab Hematol 2013;35:421–7. https://doi.org/10.1111/ijlh.12036.Search in Google Scholar PubMed
15. Szabó, G, Debreceni, IB, Tarr, T, Soltész, P, Østerud, B, Kappelmayer, J. Anti-β2-glycoprotein I autoantibodies influence thrombin generation parameters via various mechanisms. Thromb Res 2021;197:124–31. https://doi.org/10.1016/j.thromres.2020.10.032.Search in Google Scholar PubMed
16. Ramírez, E, Romero-Garrido, JA, López-Granados, E, Borobia, AM, Pérez, T, Medrano, N, et al.. Symptomatic thromboembolic events in patients treated with intravenous-immunoglobulins: results from a retrospective cohort study. Thromb Res 2014;133:1045–51. https://doi.org/10.1016/j.thromres.2014.03.046.Search in Google Scholar PubMed
17. Boehm, TK, DeNardin, E. Fibrinogen binds IgG antibody and enhances IgG-mediated phagocytosis. Hum Antibodies 2008;17:45–56. https://doi.org/10.3233/hab-2008-173-401.Search in Google Scholar
18. Boehm, TK, Sojar, H, Denardin, E. Concentration-dependent effect of fibrinogen on IgG-specific antigen binding and phagocytosis. Cell Immunol 2010;263:41–8. https://doi.org/10.1016/j.cellimm.2010.02.014.Search in Google Scholar PubMed PubMed Central
19. O’Sullivan, LR, Meade-Murphy, G, Gilligan, OM, Mykytiv, V, Young, PW, Cahill, MR. Platelet hyperactivation in multiple myeloma is also evident in patients with premalignant monoclonal gammopathy of undetermined significance. Br J Haematol 2021;192:322–32. https://doi.org/10.1111/bjh.16774.Search in Google Scholar PubMed
20. Guo, L, Tong, D, Yu, M, Zhang, Y, Li, T, Wang, C, et al.. Phosphatidylserine-exposing cells contribute to the hypercoagulable state in patients with multiple myeloma. Int J Oncol 2018;52:1981–90. https://doi.org/10.3892/ijo.2018.4354.Search in Google Scholar PubMed
21. Fotiou, D, Sergentanis, TN, Papageorgiou, L, Stamatelopoulos, K, Gavriatopoulou, M, Kastritis, E, et al.. Longer procoagulant phospholipid-dependent clotting time, lower endogenous thrombin potential and higher tissue factor pathway inhibitor concentrations are associated with increased VTE occurrence in patients with newly diagnosed multiple myeloma: results of the prospective ROADMAP-MM-CAT study. Blood Cancer J 2018;8:102. https://doi.org/10.1038/s41408-018-0135-y.Search in Google Scholar PubMed PubMed Central
22. Nielsen, T, Kristensen, SR, Gregersen, H, Teodorescu, EM, Christiansen, G, Pedersen, S. Extracellular vesicle-associated procoagulant phospholipid and tissue factor activity in multiple myeloma. PLoS One 2019;14:e0210835. https://doi.org/10.1371/journal.pone.0210835.Search in Google Scholar PubMed PubMed Central
23. Carrier, M, Le Gal, G, Tay, J, Wu, C, Lee, AY. Rates of venous thromboembolism in multiple myeloma patients undergoing immunomodulatory therapy with thalidomide or lenalidomide: a systematic review and meta-analysis. J Thromb Haemostasis 2011;9:653–63. https://doi.org/10.1111/j.1538-7836.2011.04215.x.Search in Google Scholar PubMed
24. Frenzel, L, Decaux, O, Macro, M, Belhadj-Merzoug, K, Manier, S, Touzeau, C, et al.. Venous thromboembolism prophylaxis and multiple myeloma patients in real-life: results of a large survey and clinical guidance recommendations from the IFM group. Thromb Res 2024;233:153–64. https://doi.org/10.1016/j.thromres.2023.11.021.Search in Google Scholar PubMed
25. Chalayer, E, Talbot, A, Frenzel, L, Karlin, L, Collet, P, Guyotat, D, et al.. Prediction of venous thromboembolism in patients with multiple myeloma treated with lenalidomide, bortezomib, dexamethasone, and transplantation: lessons from the substudy of IFM/DFCI 2009 cohort. J Thromb Haemostasis 2022;20:1859–67. https://doi.org/10.1111/jth.15758.Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorials
- EFLM European Urinalysis Guideline
- Clinical Chemistry Laboratory Medicine in the post-acute COVID-19 era
- EFLM Guideline
- The EFLM European Urinalysis Guideline 2023
- Review
- Approaching sustainability in Laboratory Medicine
- Opinion Papers
- New reimbursement models to promote better patient outcomes and overall value in laboratory medicine and healthcare
- Screening for sickle cell disease: focus on newborn investigations
- Genetics and Molecular Diagnostics
- The role of Killer immunoglobulin-like receptors (KIRs) in the genetic susceptibility to non-celiac wheat sensitivity (NCWS)
- General Clinical Chemistry and Laboratory Medicine
- Understanding the limitations of your assay using EQA data with serum creatinine as an example
- Add-on testing: stability assessment of 63 biochemical analytes in centrifuged and capped samples stored at 16 °C
- Smartphone swabs as an emerging tool for toxicology testing: a proof-of-concept study in a nightclub
- Allergy: Evaluation of 16 years (2007–2022) results of the shared external quality assessment program in Belgium, Finland, Portugal and The Netherlands
- Cancer Diagnostics
- Monoclonal whole IgG impairs both fibrin and thrombin formation: hemostasis and surface plasmon resonance studies
- Cardiovascular Diseases
- Patients with antiphospholipid syndrome and a first venous or arterial thrombotic event: clinical characteristics, antibody profiles and estimate of the risk of recurrence
- Letters to the Editor
- Familial dysalbuminemic hyperthyroxinemia coexisting with a Grave’s disease: a Belgian case report
- Diagnostic challenge between a frequent polygenic hypocholesterolemia and an unusual Smith Lemli Opitz syndrome related to bi-allelic DHCR7 mutations
- First reported co-occurrence of Philadelphia chromosome-positive B-cell acute lymphoblastic leukemia with pseudo Chediak-Higashi anomaly and complex karyotype
- Facing the new IVD Regulation 2017/746: Contract Research Organizations (CROs), key partners of IVDs manufacturers for compliance
- Comprehensive analysis and clinical case studies on pseudoeosinophilia: insights and implications – unraveling the complexity: analytical approaches and clinical significance
- Misdiagnosis of type 2B von Willebrand disease as immune thrombocytopenia in a thrombocytopenic patient
- Biological matrices, reagents and turnaround-time: the full-circle of artificial intelligence in the pre-analytical Phase. Comment on Turcic A, et al., Machine learning to optimize cerebrospinal fluid dilution for analysis of MRZH reaction. CCLM 2024;62:436–41
Articles in the same Issue
- Frontmatter
- Editorials
- EFLM European Urinalysis Guideline
- Clinical Chemistry Laboratory Medicine in the post-acute COVID-19 era
- EFLM Guideline
- The EFLM European Urinalysis Guideline 2023
- Review
- Approaching sustainability in Laboratory Medicine
- Opinion Papers
- New reimbursement models to promote better patient outcomes and overall value in laboratory medicine and healthcare
- Screening for sickle cell disease: focus on newborn investigations
- Genetics and Molecular Diagnostics
- The role of Killer immunoglobulin-like receptors (KIRs) in the genetic susceptibility to non-celiac wheat sensitivity (NCWS)
- General Clinical Chemistry and Laboratory Medicine
- Understanding the limitations of your assay using EQA data with serum creatinine as an example
- Add-on testing: stability assessment of 63 biochemical analytes in centrifuged and capped samples stored at 16 °C
- Smartphone swabs as an emerging tool for toxicology testing: a proof-of-concept study in a nightclub
- Allergy: Evaluation of 16 years (2007–2022) results of the shared external quality assessment program in Belgium, Finland, Portugal and The Netherlands
- Cancer Diagnostics
- Monoclonal whole IgG impairs both fibrin and thrombin formation: hemostasis and surface plasmon resonance studies
- Cardiovascular Diseases
- Patients with antiphospholipid syndrome and a first venous or arterial thrombotic event: clinical characteristics, antibody profiles and estimate of the risk of recurrence
- Letters to the Editor
- Familial dysalbuminemic hyperthyroxinemia coexisting with a Grave’s disease: a Belgian case report
- Diagnostic challenge between a frequent polygenic hypocholesterolemia and an unusual Smith Lemli Opitz syndrome related to bi-allelic DHCR7 mutations
- First reported co-occurrence of Philadelphia chromosome-positive B-cell acute lymphoblastic leukemia with pseudo Chediak-Higashi anomaly and complex karyotype
- Facing the new IVD Regulation 2017/746: Contract Research Organizations (CROs), key partners of IVDs manufacturers for compliance
- Comprehensive analysis and clinical case studies on pseudoeosinophilia: insights and implications – unraveling the complexity: analytical approaches and clinical significance
- Misdiagnosis of type 2B von Willebrand disease as immune thrombocytopenia in a thrombocytopenic patient
- Biological matrices, reagents and turnaround-time: the full-circle of artificial intelligence in the pre-analytical Phase. Comment on Turcic A, et al., Machine learning to optimize cerebrospinal fluid dilution for analysis of MRZH reaction. CCLM 2024;62:436–41

