External quality assessment practices in medical laboratories: an IFCC global survey of member societies
-
Ivan M. Blasutig
, Renze Bais
and Qing H. Meng
Abstract
Objectives
Clinical laboratory results are required for critical medical decisions, underscoring the importance of quality results. As part of total quality management, external quality assessment (EQA) is a vital component to ensure laboratory accuracy. The goal of this survey was to evaluate the current status of global laboratory quality systems and assess the need for implementation, expansion, or harmonization of EQA programs (EQAP) for Clinical Chemistry and Laboratory Medicine.
Methods
The International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) Task Force on Global Laboratory Quality (TF-GLQ) conducted a survey of IFCC full and affiliate members (n=110) on laboratory quality practice. A total of 41 (37.3%) countries representing all IFCC regions except North America provided responses about EQA availability and practices.
Results
All 41 countries perform EQA, 38 reported that their laboratories had EQA policies and procedures, and 39 further act/evaluate unacceptable EQA results. 39 countries indicated they have international and/or national EQAP and 30 use alternative performance assessments. EQA frequency varied among countries. Generally, an EQAP provided the EQA materials (40/41) with four countries indicating that they did not have an EQAP in their country.
Conclusions
Globally, most laboratories participate in an EQAP and have defined quality procedures for EQA. There remain gaps in EQA material availability and implementation of EQA as a part of a total laboratory quality system. This survey highlights the need for education, training, and harmonization and will guide efforts of the IFCC TF-GLQ in identifying areas for enhancing global laboratory quality practices.
Introduction
Clinical laboratory test results are involved in guiding patient management including diagnosis, treatment, and monitoring, underscoring the critical importance of ensuring quality results. The quality of the lab results can be affected by any number of factors spanning the entire testing process. As a result, an all-encompassing total quality management system (TQM) is imperative to ensure the reporting of reliable patient results.
To assess analytical reliability, internal quality control (IQC) is the primary method used by clinical laboratories. While IQC is critical for daily operation to evaluate changes in instrumentation and reagent lots, provide a regular check on the continued precision of the test system, and identify other possible analytical errors, it is of limited utility in evaluating result accuracy and it often does not encompass the entire testing process. External quality assessment programs (EQAP) are a tool that can aid in bridging these limitations of IQC.
EQA is a vital component of a laboratory’s TQM system, and is required by many national regulations, for International Organization for Standardization (ISO) 15189 accreditation [1], and by the Clinical and Laboratory Standards Institute (CLSI) QMS24 document [2, 3]. When EQA schemes are not available for a specific analyte or methodology, alternative assessment procedures are necessary, and these vary significantly between laboratories and regions [3, 4]. EQA provides an external assessment of laboratory performance over time. Depending on the design, EQA can provide an assessment of trueness, intra and interlaboratory variation, linearity, identify differences between methods, and monitor continued efforts at harmonization [5], [6], [7], [8]. While there continue to be challenges with EQA programs and materials [2], currently available programs provide necessary external verification for clinical laboratories. Unfortunately, EQA programs can be costly, especially for remote laboratories; and its appropriate implementation to improve diagnostic accuracy and total quality of the laboratory can be complex.
Global practices in EQA vary considerably as national regulations are not harmonized across nations, and in rare cases may be lacking completely [8]. To clarify the current state of EQA practices globally, the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) Task Force on Global Laboratory Quality (TF-GLQ), consisting of global experts in clinical laboratory quality, was convened in 2021. The TF-GLQ conducted a survey of IFCC full and affiliate members on laboratory quality practices. The goal of the survey was to evaluate the current state of global quality systems and to assess the need for standardizing or improving quality programs for clinical laboratories. In this article we outline our findings as it relates to EQA practices.
Materials and methods
Survey design
A two-part survey was drafted by a IFCC TF-GLQ subcommittee of members addressing global IQC and EQA programs in clinical chemistry (including immunoassays), hematology, and serology. This draft was circulated for feedback by all TF-GLQ members and corporate representatives, and the feedback was then incorporated. The final survey was in English and included 10 questions regarding EQA with an emphasis on providing simple, multiple-choice responses to limit language barriers and increase participation.
Survey deployment and participation
The final survey was sent to IFCC National Member Societies through National Representatives for response via SurveyMonkey on February 10, 2021 and closed on March 15, 2021 with the express statement that “This survey’s intention is to determine your country’s current quality laboratory system and assess the need for implementation or expansion of IQC and EQA programs for Clinical Chemistry and Laboratory Medicine.” Reminder emails were sent to promote participation. The survey was distributed to 93 full members and 17 affiliate members for a total of 110 recipients. A total of 66 responses were received with some duplication within countries. Excluding duplicates 52 countries (47.3%) responded to the survey, with 41 (37.3%) providing responses to questions regarding EQA (Table 1). Only countries that provided responses to EQA questions were included in survey result assessment, incomplete responses were not evaluated. One country provided duplicate responses while another submitted multiple responses. These were combined to create a single response per country.
Number of countries in each IFCC federation that provided a response.
| Provided contact information | Completed at least one survey question | |
|---|---|---|
| African Federation of Clinical Chemistry (AFCC) | 3 | 3 |
| Arab Federation of Clinical Biology (AFCB) | 2 | 1 |
| Asia-Pacific Federation for Clinical Biochemistry and Laboratory Medicine (APFCB) | 9 | 9 |
| European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) | 30 | 26 |
| Latin-American Confederation of Clinical Biochemistry (COLABIOCU) | 8 | 6 |
| North American Federation of Clinical Chemistry and Laboratory Medicine (NAFCC) | 0 | 0 |
| Total countries | 52 | 45 |
Survey analysis
Survey results were exported to a comma separated file and compiled in Excel (Microsoft, Redmond, WA, USA). Tables, figures, and calculations were performed in Excel and Prism (version 9; GraphPad, San Diego, CA). To understand geographic variability, where pertinent, responses were stratified by IFCC region: African Federation of Clinical Chemistry (AFCC), Arab Federation of Clinical Biology (AFCB), Asia-Pacific Federation for Clinical Biochemistry and Laboratory Medicine (APFCB), European Federation of Clinical Chemistry and Laboratory Medicine (EFLM), Latin American Confederation of Clinical Biochemistry (COLABIOCLI). No responses were received from members of the North America Federation of Clinical Chemistry (NAFCC).
Results
EQA availability and type
We first assessed if countries had EQA policies within laboratories and access to EQA within the country. Most countries (38 of 41) reported that their laboratories had EQA policies and procedures, though three countries reported that there were none (Figure 1A). Similarly, 36 of 40 of responding countries indicated that they have EQA programs available in their country, while 4 countries reported they did not (1 from the AFCC and 3 from the EFLM) (Figure 1B). In response to this question one country from COLABIOCLI had responded both Yes and No and was excluded from this question. Alternative performance assessments are often necessary for uncommon and unstable analytes and 30 countries responded to a query about the type of alternative performance assessments used in their country. This was a multiple-choice question with the possibility to choose multiple options and 24 countries indicated that they use between laboratory alternative performance assessments. Among laboratory and within laboratory assessments each had 6 countries respond affirmatively (Figure 1C).
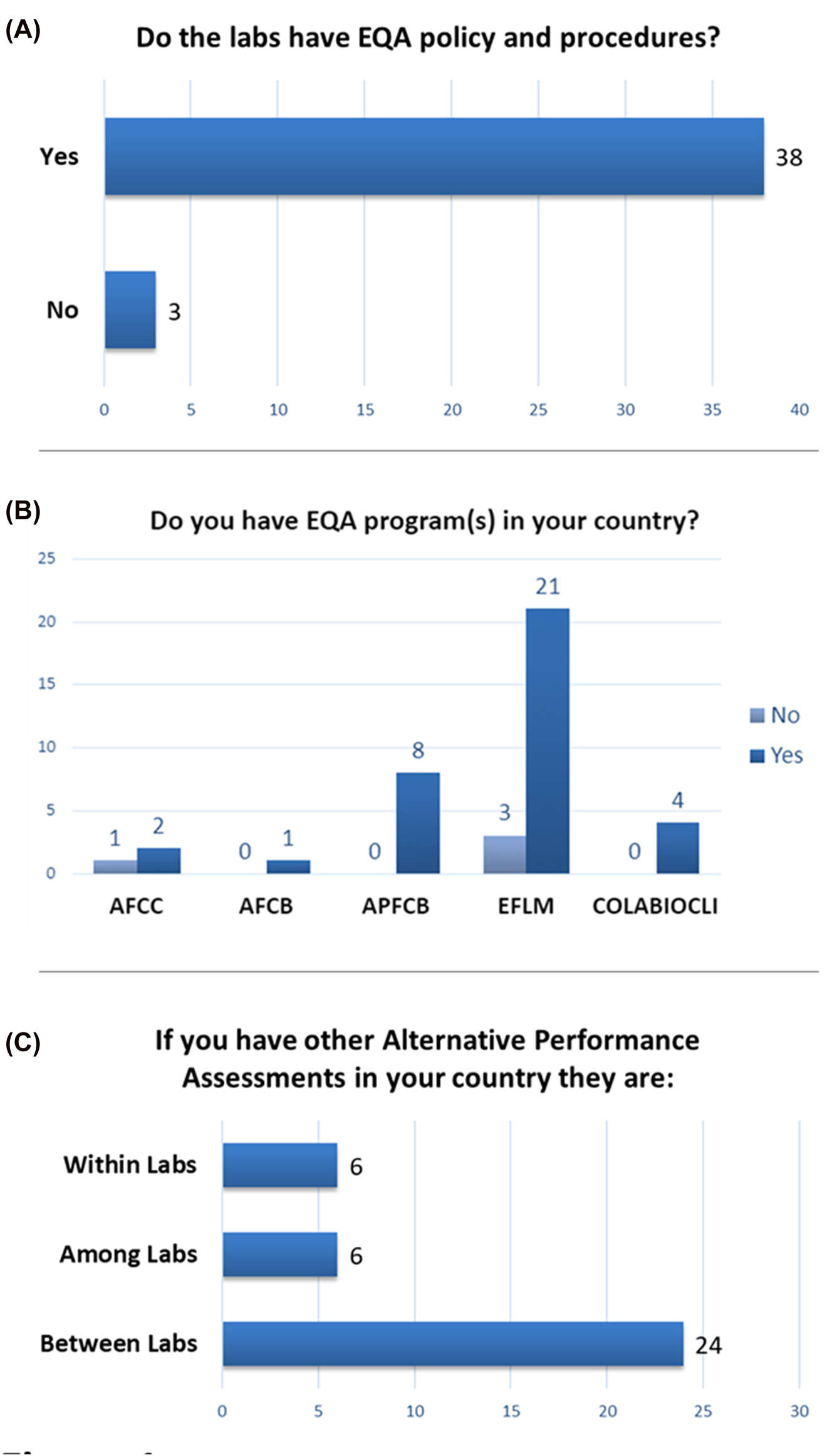
External quality assessment programs and policies. (A) Response indicates if laboratories in a country have EQA policies and procedures (n=41). (B) Response indicates if a formal EQA program is present in the respondent’s country. Responses are stratified by IFCC region. AFCC, African Federation of Clinical Chemistry; AFCB, Arab Federation of Clinical Biology; APFCB, Asia-Pacific Federation for Clinical Biochemistry and Laboratory Medicine; EFLM, European Federation of Clinical Chemistry and Laboratory Medicine; COLABIOCLI, Latin American Confederation of Clinical Biochemistry (n=40). (C) Response indicates the type of EQA alternative performance assessments utilized by countries (n=30).
We found that with only one exception, countries carried out their EQA programs using materials and resources provided by an available EQA program (40 of 41 countries; Figure 2A). The one country that reported ‘other’ for EQA program materials provision indicated that split patient samples were created by the laboratory. All 41 countries responded to the question regarding the type of their EQA program, 2 indicated they have a regional or local program (1 each from the AFCC and EFLM), 22 have a national program (6 from the APFCB, 14 in the EFLM, and 2 from COLABIOCLI), and 17 countries, spanning all regions, have international programs available (Figure 2B).

Information about external quality assessment programs. (A) Response indicates if EQA materials are provided by the EQA program, or other source (n=41). (B) Response indicates if the formal EQA programs are regional/local, national, or international in origin. Responses are stratified by IFCC region. AFCC, African Federation of Clinical Chemistry; AFCB, Arab Federation of Clinical Biology; APFCB, Asia-Pacific Federation for Clinical Biochemistry and Laboratory Medicine; EFLM, European Federation of Clinical Chemistry and Laboratory Medicine; COLABIOCLI, Latin American Confederation of Clinical Biochemistry (n=41).
Methods of EQA performance
The survey asked two questions regarding how EQA was performed. Frequency for EQA performance varied greatly with almost half of respondents choosing ‘other’ (19 of 41; Figure 3A). Under ‘other’ the majority of respondents indicated that the frequency varied depending on the analyte or program. Two respondents did not provide any further clarification, while the rest indicated frequencies ranging from monthly (16 respondents) up to yearly (1 respondent). Next most commonly, countries run EQA quarterly (11 countries), every 6 months (7 countries), and few run EQA every 4 months (4 countries). The activity menu for EQA also varied considerably. All 41 countries listed chemistry in the EQA activity menu and 40 of 41 countries listed hematology in the activity menu, while immunoassays, blood gas, infectious diseases, urinalysis, TDM/toxicology, and serology were not uniformly listed as available in all countries (Figure 3B). Most countries (95.0%) indicated that they evaluate/act on unacceptable EQA results, however, two countries responded that they do not (Figure 4A). While most countries have EQA available and participate in EQA programs, 59% (23 of 39 respondents) were interested in being selected for a pilot IFCC training program in EQA (Figure 4B).

How external quality assessment is run. (A) Responses for how frequently EQA is performed (n=41). (B) Responses to the EQA test/activity menu that were listed by the respondents (n=41).

Evaluation of external quality assessments. (A) Response indicates if there are actions or evaluations of unacceptable EQA results (n=41). (B) Respondent interest in a pilot training program organized by the IFCC. Responses are stratified by IFCC region. AFCC, African Federation of Clinical Chemistry; AFCB, Arab Federation of Clinical Biology; APFCB, Asia-Pacific Federation for Clinical Biochemistry and Laboratory Medicine; EFLM, European Federation of Clinical Chemistry and Laboratory Medicine; COLABIOCLI, Latin American Confederation of Clinical Biochemistry (n=39).
Discussion
EQA is a critical pillar in TQM to ensure continuing laboratory accuracy and reliability. Prior work has found that while participation in an EQA program may not directly improve analytical quality, it is a critical external measure for identifying methodologic and process issues to improve, and for spurring improved technical performance [1, 6, 8, 9]. Local, national, and international programs and organizations continue to require and recommend EQA as a necessary means of benchmarking laboratory performance to ensure ongoing quality [1, 2, 9, 10]. This survey provided a robust sampling of national EQA practices from global laboratories with 37.3% participation from the 110 IFCC full and affiliate members. There was notably an over-representation of members from the EFLM and no representation from the NAFCC. However, there was a diversity in the responses within the EFLM which demonstrates the current lack of global standardization in EQA practices. The lack of response from the NAFCC is not highly relevant as the two members of this federation, Canada and the United States of America (USA), have stringent national requirements for EQA in the clinical laboratory that are thoroughly enforced. The participation of COLABIOCLI, AFCC, AFCB, and APFCB encourages interpretation of this survey as reasonably representative of the current state of EQA globally.
While most countries reported having EQA policies and procedures, it reflects the ongoing need for quality training and education that approximately 7% of respondents do not. Policies and procedures for EQA are critical to ensure uniform assessment and follow-up of EQA failures and continued process improvements [2, 8], [9], [10]. This is an area where virtual or in-person training can be of immense value to aid in establishing the metrics and workflows to appropriately implement the quality aspect of EQA [11].
We found that though not all countries reported having EQA programs in their country, all countries indicated having access to some form of EQAP. The frequency of EQA varied considerably among countries, with some countries reporting that there were analytes for which EQA was assessed only annually. There continues to be debate around monthly assessment of a single sample compared to quarterly or semi-annual assessment of multiple levels of EQA, making application of a best practice for frequency difficult [12]. Thus, the variability in responses is not unexpected. The test menus for EQA likewise varied, likely reflecting the common testing within a country (every country reported clinical chemistry), and highlighting possible current limitations due to costs, regulations, or material stability (e.g. toxicology and blood gas EQA). The EQA activity menu is a question that is likely highly limited by the possibility that a response may not accurately reflect the practice of laboratories across the entire country. However, the prominent availability of the most common menu items is as expected.
We found that most countries participated in some sort of alternative performance assessment. Alternative performance assessments, which do not use a formal test material, require thorough quality systems to ensure proper utilization [2, 3]. The high utilization of these assessments highlights an important area for further guidance and education. The variability in the survey responses and complexity of appropriate implementation of EQA is reflected in the interest of 59% of respondents in an IFCC sponsored pilot training program.
This survey was created to gain a better understanding of global EQA practices among clinical laboratories. As this survey was sent to 110 National Representatives across the globe, the questions were designed to explore fundamental EQA practices. Additionally, questions were not open-ended, limiting the amount of information obtained. Despite these facts, we observed some discrepancies in responses across questions from National Representatives. This may reflect issues with interpreting the survey questions. Lastly, responses were sought at the national level rather than the level of individual laboratories. It must be assumed that there are variations in laboratory practice within a country which may lead to difficulty in determining the best response for some of the survey questions.
While this survey has the noted limitations discussed above, it represents the current state of global EQA practices and provides evidence on areas in need of further improvement. Expansion of availability of EQA materials and additional training on EQA best practices, procedures, and appropriate troubleshooting are vital to ensure the continuous improvement of global laboratory quality necessary for optimal patient care. Based on this survey, we note that laboratory quality practices vary among countries and quality policies, procedures, and practices are not harmonized. There is a need for the improvement of laboratory quality through education, training, and harmonization, which fits well with the mission and mandates of IFCC TF-GLQ.
Acknowledgments
The IFCC TF-GLQ would like to thank Silvia Cardinale for her help and expertise in survey distribution and collation.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflicts of interest: JL is an employee of Abbott Labs, RB owns rbaisconsulting, KC is an employee of Bio-Rad Laboratories, JMG is an employee of Technical Direction Biogroup and Labac, EA owns Amann Consulting.
-
Informed consent: Not applicable.
-
Ethical approval: Not applicable.
References
1. Schneider, F, Maurer, C, Friedberg, RC. International organization for standardization (ISO) 15189. Ann Lab Med 2017;37:365–70. https://doi.org/10.3343/alm.2017.37.5.365.Search in Google Scholar PubMed PubMed Central
2. Sciacovelli, L, Secchiero, S, Padoan, A, Plebani, M. External quality assessment programs in the context of ISO 15189 accreditation. Clin Chem Lab Med 2018;56:1644–54. https://doi.org/10.1515/cclm-2017-1179.Search in Google Scholar PubMed
3. Darcy, T. CLSI. QMS24 using proficiency testing and alternative assessment to improve medical laboratory quality, Darcy, T, editor, 3rd ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2016.Search in Google Scholar
4. Payne, DA, Russomando, G, Linder, MW, Baluchova, K, Ashavaid, T, Steimer, W, et al.. External quality assessment (EQA) and alternative assessment procedures (AAPs) in molecular diagnostics: findings of an international survey. Clin Chem Lab Med 2021;59:301–6. https://doi.org/10.1515/cclm-2020-0101.Search in Google Scholar PubMed
5. Plebani, M. Harmonization in laboratory medicine: the complete picture. Clin Chem Lab Med 2013;51:741–51. https://doi.org/10.1515/cclm-2013-0075.Search in Google Scholar PubMed
6. Ceriotti, F. The role of external quality assessment schemes in monitoring and improving the standardization process. Clin Chim Acta 2014;432:77–81. https://doi.org/10.1016/j.cca.2013.12.032.Search in Google Scholar PubMed
7. Ceriotti, F, Cobbaert, C. Harmonization of external quality assessment schemes and their role – clinical chemistry and beyond. Clin Chem Lab Med 2018;56:1587–90. https://doi.org/10.1515/cclm-2018-0265.Search in Google Scholar PubMed
8. James, D, Ames, D, Lopez, B, Still, R, Simpson, W, Twomey, P. External quality assessment: best practice. J Clin Pathol 2014;67:651. https://doi.org/10.1136/jclinpath-2013-201621.Search in Google Scholar PubMed
9. Peterson, JC, Hill, RH, Black, RS, Winkelman, J, Tholen, D. Review of the proficiency testing services for clinical laboratories in the United States. Final report of a technical working group; 2008. Available from: https://www.cdc.gov/labbestpractices/pdfs/proficiency-testing-report-2007.pdf.Search in Google Scholar
10. Miller, WG, Jones, GR, Horowitz, GL, Weykamp, C. Proficiency testing/external quality assessment: current challenges and future directions. Clin Chem 2011;57:1670–80. https://doi.org/10.1373/clinchem.2011.168641.Search in Google Scholar PubMed
11. Luzzi, V, Algeciras-Schimnich, A, Calderón, B, Colón-Franco, JM, Garcia, JD, Goldsmith, BM, et al.. Impact of the AACC global laboratory quality initiative in partnership with professional societies and universities in Latin America and the caribbean. J Appl Lab Med 2022;7:596–606. https://doi.org/10.1093/jalm/jfab111.Search in Google Scholar PubMed
12. Thomas, A. External quality assessment in laboratory medicine: is there a rationale to determine frequency of surveys? Accred Qual Assur 2009;14:439–44. https://doi.org/10.1007/s00769-009-0563-2.Search in Google Scholar
© 2023 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial
- Addressing standardized definitions of post-COVID and long-COVID
- Reviews
- The chitinases as biomarkers in immune-mediate diseases
- Pitfalls in the diagnosis of hematuria
- Opinion Papers
- Remote decentralized clinical trials: a new opportunity for laboratory medicine
- Striving for a pragmatic contribution of biomarkers results to lifelong health care
- IFCC Paper
- External quality assessment practices in medical laboratories: an IFCC global survey of member societies
- Guidelines and Recommendations
- Antibody-mediated interferences affecting cardiac troponin assays: recommendations from the IFCC Committee on Clinical Applications of Cardiac Biomarkers
- General Clinical Chemistry and Laboratory Medicine
- Evaluation of four automated clinical analyzers for the determination of total 25(OH)D in comparison to a certified LC-MS/MS
- Standard −20 °C freezer storage protocols may cause substantial plasma renin cryoactivation
- Lower accuracy of testosterone, cortisol, and free T4 measurements using automated immunoassays in people undergoing hemodialysis
- Multicenter study to compare the diagnostic performance of CLIA vs. FEIA transglutaminase IgA assays for the diagnosis of celiac disease
- Imprecision remains to be improved in the measurement of serum cystatin C with heterogeneous systems
- Analytical validation of the modified Westergren method on the automated erythrocyte sedimentation rate analyzer CUBE 30 touch
- Reference Values and Biological Variations
- Systematic review and meta-analysis of within-subject and between-subject biological variation data of coagulation and fibrinolytic measurands
- Biological variation estimates for spot urine analytes and analyte/creatinine ratios in 33 healthy subjects
- Short-term biological variation of plasma uracil in a Caucasian healthy population
- Cardiovascular Diseases
- Elevated Hemolysis Index is associated with higher risk of cardiovascular diseases
- Infectious Diseases
- Clinical assessment of SNIBE Maglumi SARS-CoV-2 antigen fully-automated chemiluminescent immunoassay
- Pre-analytical considerations in the development of a prototype SARS-CoV-2 antigen ARCHITECT automated immunoassay
- SARS CoV-2 spike protein-guided exosome isolation facilitates detection of potential miRNA biomarkers in COVID-19 infections
- Monocyte distribution width alterations and cytokine storm are modulated by circulating histones
- Letters to the Editor
- Letter to the Editor regarding the article by Wayne J. Dimech et al. Time to address quality control processes applied to antibody testing for infectious diseases. Clin Chem Lab Med 2023; 61(2):205–212
- Response to Tony Badrick regarding “Letter to the Editor regarding the article by Wayne J. Dimech et al. Time to address quality control processes applied to antibody testing for infectious diseases. Clin Chem Lab Med 2023; 61(2):205–212 by”
- Monocyte distribution width (MDW) as a reliable biomarker for urosepsis
- A consistency analysis of common biochemical tests in arterial blood and venous blood of critically ill patients
- Test results comparison: is the S-Monovette® Lithium-Heparin Gel+ a suitable replacement for the S-Monovette® Lithium-Heparin Gel on Alinity Abbott®?
- Analytical performance of Abbott’s ARCHITECT and Alinity TSH-receptor antibody (TRAb) assays
- Cis-AB showing discrepant results across different automated and manual methods: a case report and review of the literature
- A graphical tool to investigate method validation
- Live lab-monitor; a customizable HTML-based and systems independent, real-time laboratory overview screen
- Congress Abstracts
- 61st National Congress of the Hungarian Society of Laboratory Medicine
- 9th Annual Meeting of the Austrian Society for Laboratory Medicine and Clinical Chemistry (ÖGLMKC)
Articles in the same Issue
- Frontmatter
- Editorial
- Addressing standardized definitions of post-COVID and long-COVID
- Reviews
- The chitinases as biomarkers in immune-mediate diseases
- Pitfalls in the diagnosis of hematuria
- Opinion Papers
- Remote decentralized clinical trials: a new opportunity for laboratory medicine
- Striving for a pragmatic contribution of biomarkers results to lifelong health care
- IFCC Paper
- External quality assessment practices in medical laboratories: an IFCC global survey of member societies
- Guidelines and Recommendations
- Antibody-mediated interferences affecting cardiac troponin assays: recommendations from the IFCC Committee on Clinical Applications of Cardiac Biomarkers
- General Clinical Chemistry and Laboratory Medicine
- Evaluation of four automated clinical analyzers for the determination of total 25(OH)D in comparison to a certified LC-MS/MS
- Standard −20 °C freezer storage protocols may cause substantial plasma renin cryoactivation
- Lower accuracy of testosterone, cortisol, and free T4 measurements using automated immunoassays in people undergoing hemodialysis
- Multicenter study to compare the diagnostic performance of CLIA vs. FEIA transglutaminase IgA assays for the diagnosis of celiac disease
- Imprecision remains to be improved in the measurement of serum cystatin C with heterogeneous systems
- Analytical validation of the modified Westergren method on the automated erythrocyte sedimentation rate analyzer CUBE 30 touch
- Reference Values and Biological Variations
- Systematic review and meta-analysis of within-subject and between-subject biological variation data of coagulation and fibrinolytic measurands
- Biological variation estimates for spot urine analytes and analyte/creatinine ratios in 33 healthy subjects
- Short-term biological variation of plasma uracil in a Caucasian healthy population
- Cardiovascular Diseases
- Elevated Hemolysis Index is associated with higher risk of cardiovascular diseases
- Infectious Diseases
- Clinical assessment of SNIBE Maglumi SARS-CoV-2 antigen fully-automated chemiluminescent immunoassay
- Pre-analytical considerations in the development of a prototype SARS-CoV-2 antigen ARCHITECT automated immunoassay
- SARS CoV-2 spike protein-guided exosome isolation facilitates detection of potential miRNA biomarkers in COVID-19 infections
- Monocyte distribution width alterations and cytokine storm are modulated by circulating histones
- Letters to the Editor
- Letter to the Editor regarding the article by Wayne J. Dimech et al. Time to address quality control processes applied to antibody testing for infectious diseases. Clin Chem Lab Med 2023; 61(2):205–212
- Response to Tony Badrick regarding “Letter to the Editor regarding the article by Wayne J. Dimech et al. Time to address quality control processes applied to antibody testing for infectious diseases. Clin Chem Lab Med 2023; 61(2):205–212 by”
- Monocyte distribution width (MDW) as a reliable biomarker for urosepsis
- A consistency analysis of common biochemical tests in arterial blood and venous blood of critically ill patients
- Test results comparison: is the S-Monovette® Lithium-Heparin Gel+ a suitable replacement for the S-Monovette® Lithium-Heparin Gel on Alinity Abbott®?
- Analytical performance of Abbott’s ARCHITECT and Alinity TSH-receptor antibody (TRAb) assays
- Cis-AB showing discrepant results across different automated and manual methods: a case report and review of the literature
- A graphical tool to investigate method validation
- Live lab-monitor; a customizable HTML-based and systems independent, real-time laboratory overview screen
- Congress Abstracts
- 61st National Congress of the Hungarian Society of Laboratory Medicine
- 9th Annual Meeting of the Austrian Society for Laboratory Medicine and Clinical Chemistry (ÖGLMKC)

