Inconclusive evidence of sexual reproduction of invasive Halophila stipulacea: a new field guide to encourage investigation of flower and fruit production throughout its invasive range
-
Fee O.H. Smulders
, Kelcie L. Chiquillo
Abstract
The dioecious seagrass species Halophila stipulacea reproduces mainly through fast clonal growth, underlying its invasive behavior. Here, we provide morphological evidence to show that the first findings of fruits in the Caribbean were misidentified. Consequently, H. stipulacea reproduction is likely still only asexual in the Caribbean. Therefore, we introduce an identification key of H. stipulacea reproductive structures to encourage careful identification and quantification throughout its invasive range. Until large-scale seed production in invaded habitats is reported, the apparent low rate of sexual reproduction needs to be considered in current studies investigating the invasion capacity of this species.
Native to the Red Sea and Western Indo-Pacific, the seagrass Halophila stipulacea (Forsk.) Ascherson, was reported to have invaded the Mediterranean Sea in 1894 (Lipkin 1975) and the Caribbean in 2002 (Ruiz and Ballantine 2004), after which it spread successfully in both regions (Winters et al. 2020). Similar to many invasive macrophytes, H. stipulacea expands mainly through asexual clonal growth and fragmentation (Lipkin 1975; Smulders et al. 2017), a feature believed to characterize the colonization of multiple Caribbean islands (Willette et al. 2014).
Sexual reproduction increases the genetic variability and dispersal potential of seagrasses, which is important for long-term stability of populations under dynamic change (Ackerman 2006). In its native range, Halophila stipulacea flowers predictably with both female and male flower production, followed by seed formation within 1–2 months after fertilization (Dural 2020; Malm 2006; Nguyen et al. 2018 see Figure 1A and B). Male flowering has commonly been reported throughout its invasive range; both in the Mediterranean as well as in the Caribbean (Chiquillo et al. 2019; Dural et al. 2020; Gambi et al. 2018; Procaccini et al. 1999; Vera et al. 2014). In contrast, documentation of female flowers and fruits is rare, and was limited to four studies in the Mediterranean (Dural et al. 2020; Gerakaris and Tsiamis 2015; Lipkin 1975; Nguyen et al. 2018), until a recent report of fruits in the US Virgin Islands (Chiquillo et al. 2019).
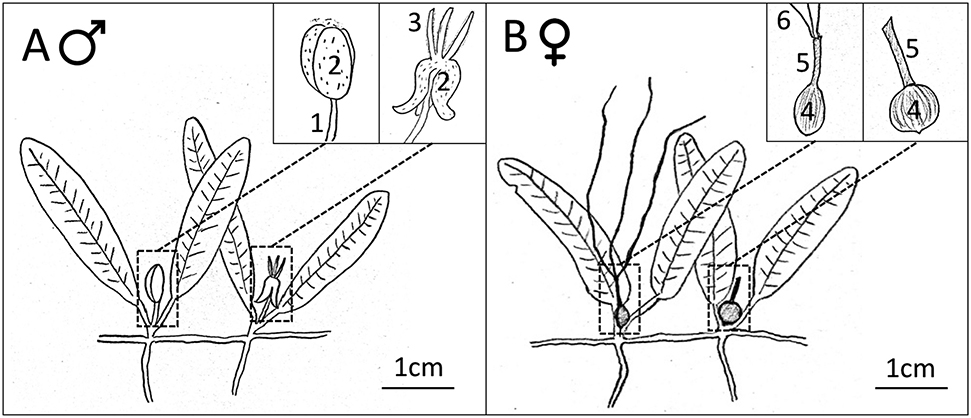
Halophila stipulacea reproductive structures of (A) male and (B) female plants. Structures of the male flower bud and male flower are 1: pedicel, 2: tepals, 3: stamens. Structures of the female flower and developing seed are 4: ovary, 5: hypanthium, 6: styles.
In 2017, male flowers and fruits of Halophila stipulacea were reported in the US Virgin Islands, implying the existence of sexual reproduction of this species in the Caribbean (Chiquillo et al. 2019). Here we reevaluate the morphological features of the proposed male fruits presented in Chiquillo et al. (2019) and compare them with reproductive structures from Caribbean H. stipulacea that flowered in a controlled laboratory environment (Figure 2). The images of the proposed fruit (Chiquillo et al. 2019, Figure 1B) did not match the description of previous published work on H. stipulacea fruit morphology. Gerakaris and Tsiamis (2015) and Nguyen et al. (2018) published images that show that the fruit of H. stipulacea is spherical, attached to the base of the shoot, and that the hypanthium (enlargement of the flower receptacle) remains attached to the fruit (see Figure 1B). Images by Vohník et al. (2017) show that female flowers and fruits mature progressively at each node along the rhizome and they discuss the possibility of confusion with a fungal phytomixid infection.

Halophila stipulacea male flower buds in different stages of development. The arrows in (A) and (B) depict flower buds attached with a pedicel to the base of the shoot. The arrows in (C) and (D) depict male flowers emerging from flower buds, with three stamens that release pollen grains. Halophila stipulacea male flower buds and flowers shown were harvested on Bonaire, Dutch Caribbean and grown in the laboratory on coral sand, at 25 °C and a salinity of 35. The plants were observed flowering in January 2020 in three separate mesocosms (length × width × height: 0.6 × 0.3 × 0.25 m).
Contrary to these accounts, the photograph of Chiquillo et al. (2019, Figure 1B) shows an oblong shaped flower, attached to the base of the shoot by a stem. Additionally, the distinctive female hypanthium, attached to mature fruits (Figure 1B), is missing and no female flowers at various stages of development are documented. The proposed fruits in Chiquillo et al. (2019) are more likely to be male flower buds, which have similar dimensions to fruits, but are more oval in shape (see Figures 1A, 2A, 2B and dichotomous key in Appendix 1). The male flower bud is attached to a stem (pedicel) of varying length, similar to the image in Chiquillo et al. (2019). The flower buds presented in Figure 2 have all been observed to develop in male flowers (Figures 2C and D).
Based on these observations, the existence of female flowers and fruits of Halophila stipulacea in the Caribbean Sea is open to question. Consequently, the rapid spread of this species is currently likely to be limited to asexual reproduction: clonal growth and fragmentation. Why male plants of this species seem to be more successful in colonizing new areas outside its native range compared to female plants, and why female plants do not flower regularly outside the native range, remain important open questions.
Collecting improved pictures of sampled or in situ fruits and female flowers of Halophila stipulacea would increase our understanding of sexual reproduction in the Caribbean as would more frequent monitoring of plants for reproductive structures and, once they are located in situ, monitoring of flowers approximately every 2 weeks (Dural et al. 2020). In-depth studies of male and female flowering rates and ratios, seed production per area and seed viability within the invasive range would increase our knowledge on the invasion capacity of H. stipulacea. To facilitate such studies, we provide a field guide with a dichotomous key for identifying the reproductive structures of H. stipulacea (Appendix 1).
Understanding reproduction of Halophila stipulacea in its invasive range is critical to managing this species. For example, sexual reproduction may increase physical dispersal capacity (Ackerman 2006). Increased genetic diversity associated with sexual reproduction could also amplify the adaptive capacity of this tropical species, for instance, to colder water conditions in subtropical regions. Furthermore, the development of seed banks could increase the resilience of H. stipulacea to physical disturbance such as storms, or seasonal differences (Unsworth et al. 2015). Sexual reproduction therefore adds an understudied dimension to seagrass invasive potential. The only other transoceanic invasive marine angiosperm, Zostera japonica, is believed to be transported by seeds in shipments of Japanese oysters to the northeast Pacific, where it has spread along the west coast of the USA (Harrison and Bigley 1982; Williams 2007). High reproductive output is suggested to be one of the main reasons for the invasion success of Z. japonica, and increased output following disturbance events was found to provide a temporal and/or spatial escape, facilitating its invasion (Bando 2006; Henderson and Hacker 2015; Ruesink et al. 2010). Therefore, monitoring the occurrence and rate of sexual reproduction is vital in understanding invasive seagrass dynamics and this knowledge needs to be taken into account in further studies on dispersal, genetic diversity and resilience of these species in invasive habitats.
We strongly encourage new monitoring efforts that target Halophila stipulacea flowers and fruits across its invasive range, as shifts in temperature and disturbance regimes may be accelerating the plant’s growth and spread (Nguyen et al. 2020; Willette et al. 2020). Until future studies quantify the production of invasive H. stipulacea fruits, we should take into consideration that sexual reproduction is either absent or largely insignificant throughout its Caribbean invasive range.
Funding source: Nederlandse Organisatie voor Wetenschappelijk Onderzoek
Award Identifier / Grant number: NWO016.VENI.181.002
About the authors

Fee O. H. Smulders is a PhD candidate of the Aquatic Ecology and Water Quality Management group at Wageningen University and Research in Wageningen, the Netherlands. In her project she investigates the impacts of seagrass invasion and herbivore grazing on the productivity and ecosystem services of Caribbean seagrass meadows in order to improve conservation of these valuable coastal ecosystems.

Kelcie L. Chiquillo is a PhD candidate of the Ecology and Evolutionary Biology Department at the University of California, Los Angeles (UCLA). Her research uses multidisciplinary approaches to advance marine conservation and biodiversity. Her project begins to understand the origins and ecological dynamics of an invasive seagrass. Kelcie hopes to become a professor in academia and continue mentoring women of color (WOC) to increase participation in marine biology.

Demian A. Willette is an assistant professor of the Biology Department at Loyola Marymount University, USA. His research interests include applied ecology, biological invasions, conservation biology, and sustainable fisheries. He is a Fulbright Global Scholar and conducts research in Los Angeles and across the Pacific and Caribbean regions.

Paul H. Barber is a professor of the Ecology and Evolutionary Biology Department at the University of California, Los Angeles (UCLA). Paul’s research program integrates genetics, genomics, ecology, and oceanography to understand the evolution of marine biodiversity, and uses this information to promote marine conservation. He was recently named HHMI Professor, and continues to integrate this research in the context of educational programs, like The Diversity Project, to promote and increase minority participation in marine science.

Marjolijn J.A. Christianen is a marine ecologist and an assistant professor of the Aquatic Ecology and Water Quality Management Group at Wageningen University and Research in the Netherlands. She is an NWO VENI Scholar and investigates the cascading effects of large consumers, plant invasion and habitat degradation to ecosystem services of coastal ecosystems, both in the tropics and in temperate regions.
Acknowledgments
This study was carried out as part of the project ‘Global defaunation and plant invasion: cascading effects on seagrass ecosystem services’ appointed to MJAC (NWO 016.Veni.181.002). The authors thank Gidon Winters for providing pictures of Halophila stipulacea female flowers and fruits.
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
Ackerman, J.D. (2006). Sexual reproduction of seagrasses: pollination in the marine context. In: Seagrasses: biology, ecology and conservation: Springer, Netherlands, pp. 89–109.10.1007/978-1-4020-2983-7_4Search in Google Scholar
Bando, K.J. (2006). The roles of competition and disturbance in a marine invasion. Biol. Invasions 8: 755–763, https://doi.org/10.1007/s10530-005-3543-4.Search in Google Scholar
Chiquillo, K.L., Barber, P.H., and Willette, D.A. (2019). Fruits and flowers of the invasive seagrass Halophila stipulacea in the Caribbean Sea. Bot. Mar. 62: 109–112, https://doi.org/10.1515/bot-2018-0052.Search in Google Scholar
Dural, B., Şükran Okudan, E., Demir, N., Şenkardeşler, A., Erduğan, H., and Aysel, V. (2020). Observations on the flowering and fruit developments in Halophila stipulacea (Hydrocharitaceae) in the Aegean. J. Black Sea/Mediterranean Environ. 26: 1–16.Search in Google Scholar
Gambi, M.C., Gaglioti, M., and Barbieri, F. (2018). Sometimes they come back: the re-colonization of the alien seagrass Halophila stipulacea (forsskål) ascherson, 1867 (hydrocharitaceae) in the palinuro harbor (tyrrhenian sea, Italy). BioInvasions Rec 7: 215–221, https://doi.org/10.3391/bir.2018.7.3.01.Search in Google Scholar
Gerakaris, V. and Tsiamis, K. (2015). Sexual reproduction of the lessepsian seagrass Halophila stipulacea in the Mediterranean Sea. Bot. Mar. 58: 51–53, https://doi.org/10.1515/bot-2014-0091.Search in Google Scholar
Harrison, P.G. and Bigley, R.E. (1982). The recent introduction of the seagrass Zostera japonica Aschers, and Graebn. to the Pacific coast of North America. Can. J. Fish. Aquat. Sci. 39: 1642–1648, https://doi.org/10.1139/f82-221.Search in Google Scholar
Henderson, J. and Hacker S, S. (2015). Buried alive: an invasive seagrass (Zostera japonica) changes its reproductive allocation in response to sediment disturbance. Mar. Ecol. Prog. Ser. 532: 123–136, https://doi.org/10.3354/meps11335.Search in Google Scholar
Lipkin, Y. (1975). Halophila stipulacea, a review of a successful immigration. Aquat. Bot. 1: 203–215, https://doi.org/10.1016/0304-3770(75)90023-6.Search in Google Scholar
Malm, T. (2006). Reproduction and recruitment of the seagrass Halophila stipulacea. Aquat. Bot. 85: 345–349, https://doi.org/10.1016/j.aquabot.2006.05.008.Search in Google Scholar
Nguyen, H.M., Kleitou, P., Kletou, D., Sapir, Y., and Winters, G. (2018). Differences in flowering sex ratios between native and invasive populations of the seagrass Halophila stipulacea. Bot. Mar. 61: 337–342, https://doi.org/10.1515/bot-2018-0015.Search in Google Scholar
Nguyen, H.M., Yadav, N.S., Barak, S., Lima, F.P., Sapir, Y., and Winters, G. (2020). Responses of invasive and native populations of the seagrass Halophila stipulacea to simulated climate change. Front. Mar. Sci. 6: 812, https://doi.org/10.3389/fmars.2019.00812.Search in Google Scholar
Procaccini, G., Acunto, S., Famà, P., and Maltagliati, F. (1999). Structural, morphological and genetic variability in Halophila stipulacea (Hydrocharitaceae) populations in the western Mediterranean. Mar. Biol. 135: 181–189, https://doi.org/10.1007/s002270050615.Search in Google Scholar
Ruesink, J.L., Hong, J.S., Wisehart, L., Hacker, S.D., Dumbauld, B.R., Hessing-Lewis, M., and Trimble, A.C. (2010). Congener comparison of native (Zostera marina) and introduced (Z. japonica) eelgrass at multiple scales within a Pacific Northwest estuary. Biol. Invasions 12: 1773–1789, https://doi.org/10.1007/s10530-009-9588-z.Search in Google Scholar
Ruiz, H. and Ballantine, D.L. (2004). Occurrence of the seagrass Halophila stipulacea in the tropical west Atlantic. Bull. Mar. Sci. 75: 131–135.Search in Google Scholar
Smulders, F.O.H., Vonk, J.A., Engel, M.S., and Christianen, M.J.A. (2017). Expansion and fragment settlement of the non-native seagrass Halophila stipulacea in a Caribbean bay. Mar. Biol. Res. 13: 967–974, https://doi.org/10.1080/17451000.2017.1333620.Search in Google Scholar
Unsworth, R.K.F., Collier, C.J., Waycott, M., Mckenzie, L.J., and Cullen-Unsworth, L. (2015). A framework for the resilience of seagrass ecosystems. Mar. Pollut. Bull. 100: 34–46, https://doi.org/10.1016/j.marpolbul.2015.08.016.Search in Google Scholar
Vera, B., Collado-Vides, L., Moreno, C., and van Tussenbroek, B.I. (2014). Halophila stipulacea (Hydrocharitaceae): a recent introduction to the continental waters of Venezuela. Caribb. J. Sci. 48: 66–70, https://doi.org/10.18475/cjos.v48i1.a11.Search in Google Scholar
Vohník, M., Borovec, O., and Özbek, E.Ö. (2017). Rare phytomyxid infection on the alien seagrass Halophila stipulacea in the southeast Aegean Sea. Mediterr. Mar. Sci. 18: 433–442, https://doi.org/10.12681/mms.14053.Search in Google Scholar
Willette, D.A., Chalifour, J., Debrot, A.O.O.D., Engel, M.S., Miller, J., Oxenford, H.A., Short, F.T., Steiner, S.C.C., and Védie, F. (2014). Continued expansion of the trans-Atlantic invasive marine angiosperm Halophila stipulacea in the eastern Caribbean. Aquat. Bot. 112: 98–102, https://doi.org/10.1016/j.aquabot.2013.10.001.Search in Google Scholar
Willette, D. A., Chiquillo, K.L., Cross, C., Fong, P., Kelley, T., Toline, C.A., Zweng, R., and Muthukrishnan, R. (2020). Growth and recovery after small-scale disturbance of a rapidly-expanding invasive seagrass in St. John, U.S. Virgin Islands. J. Exp. Mar. Biol. Ecol. 523: 151265, https://doi.org/10.1016/j.jembe.2019.151265.Search in Google Scholar
Williams, S.L. (2007). Introduced species in seagrass ecosystems: status and concerns. J. Exp. Mar. Biol. Ecol. 350: 89–110, https://doi.org/10.1016/j.jembe.2007.05.032.Search in Google Scholar
Winters, G., Beer, S., Willette, D.A., Viana, I.G., Chiquillo, K.L., Beca-Carretero, P., Villamayor, B., Azcárate-García, T., Shem-Tov, R., Mwabvu, B., et al.. (2020). The tropical seagrass Halophila stipulacea: reviewing what we know from its native and invasive habitats, Alongside identifying knowledge gaps. Front. Mar. Sci. 7: 300, https://doi.org/10.3389/fmars.2020.00300.Search in Google Scholar
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/bot-2020-0046).
© 2020 Fee O.H. Smulders et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- In this issue
- Physiology and ecology
- Physiological and structural responses of the seagrass Cymodocea nodosa to titanium dioxide nanoparticle exposure
- Iodine and fluorine concentrations in seaweeds of the Arabian Gulf identified by morphology and DNA barcodes
- The influence of migratory birds on the distribution of the seagrass Zostera japonica
- Taxonomy/phylogeny and biogeography
- Molecular analysis confirms Laurenciella marilzae (Rhodophyta, Rhodomelaceae) in the Mediterranean Sea, a species often misidentified as Laurencia dendroidea
- Inconclusive evidence of sexual reproduction of invasive Halophila stipulacea: a new field guide to encourage investigation of flower and fruit production throughout its invasive range
- Genomics
- Superoxide dismutase and ascorbate peroxidase genes in Antarctic endemic brown alga Ascoseira mirabilis (Ascoseirales, Phaeophyceae): data mining of a de novo transcriptome
- Chemistry and applications
- Monitoring environmental risk of the exotic species Kappaphycus alvarezii (Rhodophyta), after two decades of introduction in southeastern Brazil
- Photo-bleached agar extracts from Gracilariopsis heteroclada
Articles in the same Issue
- Frontmatter
- In this issue
- Physiology and ecology
- Physiological and structural responses of the seagrass Cymodocea nodosa to titanium dioxide nanoparticle exposure
- Iodine and fluorine concentrations in seaweeds of the Arabian Gulf identified by morphology and DNA barcodes
- The influence of migratory birds on the distribution of the seagrass Zostera japonica
- Taxonomy/phylogeny and biogeography
- Molecular analysis confirms Laurenciella marilzae (Rhodophyta, Rhodomelaceae) in the Mediterranean Sea, a species often misidentified as Laurencia dendroidea
- Inconclusive evidence of sexual reproduction of invasive Halophila stipulacea: a new field guide to encourage investigation of flower and fruit production throughout its invasive range
- Genomics
- Superoxide dismutase and ascorbate peroxidase genes in Antarctic endemic brown alga Ascoseira mirabilis (Ascoseirales, Phaeophyceae): data mining of a de novo transcriptome
- Chemistry and applications
- Monitoring environmental risk of the exotic species Kappaphycus alvarezii (Rhodophyta), after two decades of introduction in southeastern Brazil
- Photo-bleached agar extracts from Gracilariopsis heteroclada

