Abstract
In this study, surface modifications for the biodegradable polymers poly(ε-caprolactone) (PCL) and poly(3-hydroxybutyrate) [P(3HB)] were developed in order to improve their suitability as scaffold material for bioartificial vessel prostheses. The challenge of wet-chemical surface modifications is to avoid bulk adjustments resulting in undesired changes in mechanical properties of these polymers. Nevertheless subsequent immobilization and controlled release of potent angiogenic biomolecules like vascular endothelial growth factor (VEGF) from the polymer surface is required. In order to improve the biocompatibility of PCL and P(3HB), terminal hydroxyl groups on the surface of these polymers were generated via oxygen (O2) and carbon dioxide (CO2) plasma. Then, the immobilization of VEGF was achieved through hydrolysable ester bonds using the crosslinker N,N′-disuccinimidyl carbonate (DSC). Our studies demonstrated that the plasma surface activations have no negative influence on the viability of L929 mouse fibroblasts and hemocompatibility. The immobilization of VEGF on the modified polymers via DSC is improved compared to the untreated reference. For the CO2 plasma-chemical activated surfaces we observed the highest VEGF surface immobilization. Our release studies also reveal the highest cumulative release using CO2 plasma-chemical activated samples.
Introduction
The application of tissue engineered scaffolds and implants in the cardiovascular field require vascularity sufficient for nutrient transport of cells over a distance of roughly 100 μm [1]. In this context, biodegradable polymers such as poly(ε-caprolactone) (PCL) and poly(3-hydroxybutyrate) [P(3HB)] are approved and promising candidates as implant materials regarding the immobilization and controlled release of biomolecules. For instance, synthetic degradable PCL and P(3HB) are widely used in the biomedical field, such as material for sutures, wound dressings, drug delivery devices and scaffolds for tissue engineering, especially in the development of scaffolds for tissue-engineered vessel grafts or prostheses [1], [2], [3], [4].
Successful integration of implants in the cardiovascular field is vital to an early vascularization after implantation. The same applies for tissue engineered scaffolds. Both need to attract and stimulate adherence and growth of endothelial cells or their progenitors especially for neovascularization [1]. The incorporation of growth factors to support and stimulate cell growth is a widely used method [5], [6]. Several of them are used to regulate migration and proliferation of cells and are vital for angiogenesis. Among them, vascular endothelial growth factor (VEGF) is one of the most used biomolecules. Administered soluble VEGF is rapidly diluted and degrades fast in the human body [7]. However, possible side effects, such as provoking potential malignant growth, are caused by the systemic administration of too much VEGF [1]. Therefore, the immobilization of VEGF at the scaffold site seems a more promising mode of therapy because of its increased stability, its presence at the implantation site and the need of lower local dosages [8]. The release of immobilized VEGF will allow the creation of a growth factor gradient to attract and trap progenitor endothelial cells at the implantation site.
The coupling of growth factors to the mostly inert polymeric implant surface requires a generation of functional groups, such as hydroxyl groups which are mostly coupled to the C or the N terminus of proteins by a crosslinker, such as NHS-homobifunctional crosslinker N,N′-Disuccinimidyl carbonate (DSC).
Plasma-chemical processes are widely used for functional group generation on polymeric surfaces. Minor morphological surface changes can only be observed although bulk characteristics of materials remain unchanged [1], [4]. Although, for PCL and P(3HB) studies were performed using O2 or CO2 plasma [9], [10].
Our study focuses on the development of a surface modification for the degradable polymers PCL and P(3HB) without changing bulk mechanics including the immobilization and controlled release of biomolecules leading to improved suitability as scaffold materials for bioartificial vessel prostheses. With respect to the structural and chemical composition of these polymers, we adjusted CO2 and O2 plasma-parameters in order to induce the desirable chemical surface changes by generating hydroxyl groups while maintaining surface morphology. Furthermore, we analyzed the impact of the modified polymers on the viability of L929 fibroblasts as well as their hemocompatibility. To overcome the problematic of burst release by mainly diffusion controlled drug delivery system we developed a chemical controlled hydrolysis release system. Therefore, modified polymer surfaces were immobilized with VEGF via DSC generating hydrolysable covalent carbamate and carbonate ester bonds [11], [12]. Hydrolyzation of these bonds resulted in the release of CO2 and unmodified VEGF.
Results
Characterization of modified PCL and P(3HB) surfaces
The surface reactions leading to the formation of hydroxyl or carboxy groups were analyzed by means of contact angle measurements. To achieve the highest degree of surface activation and functionalization, the conditions for the plasma-chemical surface reactions were optimized by determination of contact angles (data not shown).
As shown in Table 1all contact angles are significantly decreased by the plasma-chemical modification of the polymer surfaces. The decrease of the contact angles on the modified surfaces of PCL and P(3HB) were in a similar range between 20 and 25°, indicating high hydrophilicity and prolific generation of oxygen containing functional groups (Figure 1).
Mean water contact angle ΘW±standard deviation (SD) on untreated and modified PCL and P(3HB) surfaces for sessile drop method.
| Modification | PCL contact angle Θw±SD (°) | P(3HB) contact angle Θw±SD (°) |
|---|---|---|
| Untreated | 71±5 | 69±7 |
| CO2 plasma | 50±4a | 44±3a |
| O2 plasma | 51±4a | 44±3a |
n=24, ap<0.001, significant after plasma-chemical treatment.
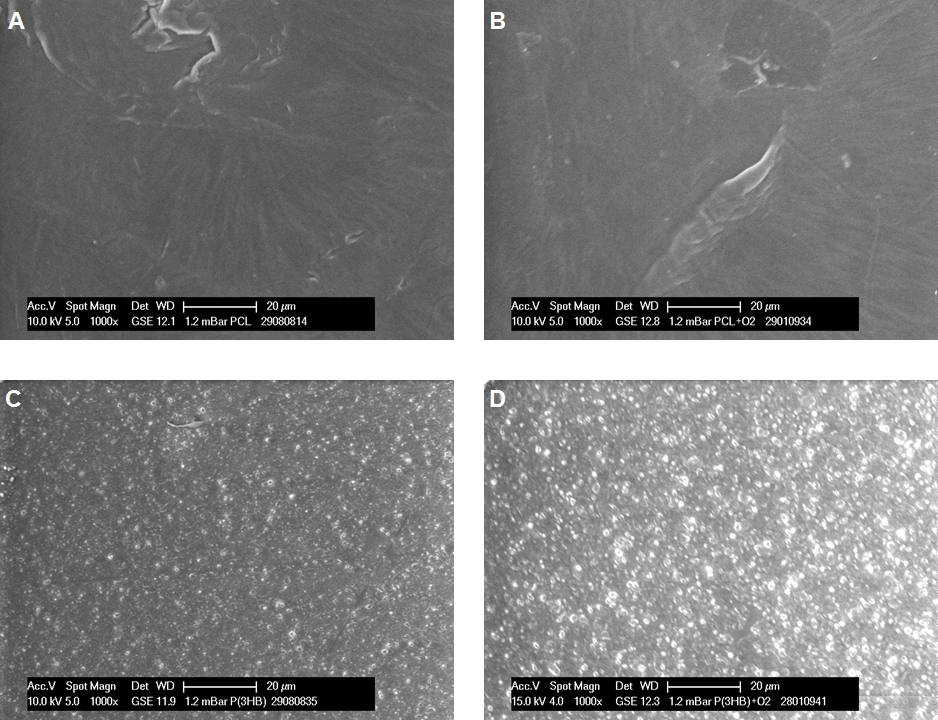
ESEM images of PCL and P(3HB) films at 1000× magnification. PCL surface before plasma-chemical activation (untreated) (A), after O2 plasma (B) and P(3HB) surface before plasma-chemical activation (untreated) (C), after O2 plasma (D).
Surface morphology for both PCL and P(3HB), reveals no relevant changes after the applied plasma-chemical treatments, as exemplarily shown for the O2 plasma-chemical modification in Figure 1.
Biocompatibility of modified PCL and P(3HB) surfaces
In order to investigate the cell viability on the modified PCL and P(3HB) surfaces, L929 mouse fibroblasts were cultivated onto the untreated and plasma-chemical modified surfaces for 48 h. As shown in Figure 2, relative cell viability of L929 fibroblasts on plasma-chemical modified PCL and P3(HB) surfaces ranged between 76 and 114%. In particular, relative viability of L929 cells on PCL was similar on both plasma-chemical modified surfaces compared to untreated PCL, with nearly similar cell viabilities of 98% and 107% for CO2 and O2 plasma-chemical modified surfaces, respectively. In contrast, on P(3HB), cell viability of L929 cells was slightly enhanced on the CO2 and significantly decreased on the O2 plasma-chemical modified surfaces with 114% and 76% in comparison to the untreated P(3HB) surface. Positive control Zinc diethyldithiocarbamate (ZDEC) was at 3% and negative control tissue-culture polystyrene was calculated as 100%.
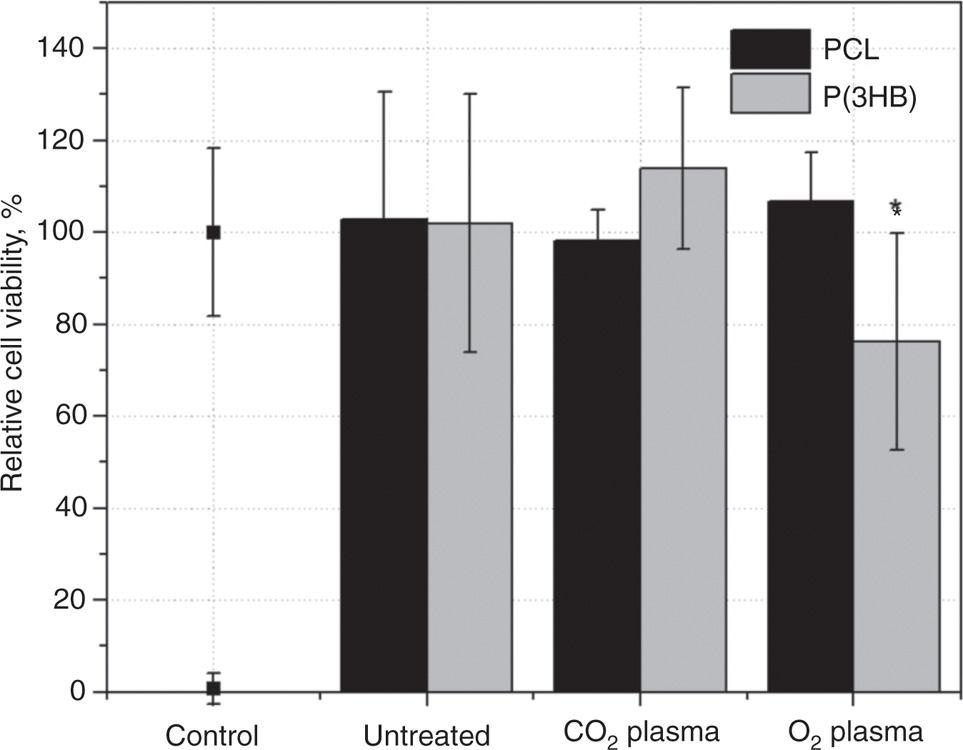
Relative cell viability of L929 fibroblast after 48 h cultivation on plasma-chemical modified PCL and P(3HB) surfaces in comparison to untreated PCL and P(3HB) surfaces (mean+SD, n=6, p<0.05).
Beside cell viability also hemocompatibility of plasma-chemical activated polymer surfaces was analyzed. Primary hemostasis was determined via β-thromboglobulin activity (Figure 3). The specific activity for the positive control thrombin was around 120 IU/mL. Untreated PCL samples presented an activity nearly as high as the positive control around 118 IU/mL. Plasma-chemical modified surfaces reduce the activity clearly by around 45% (CO2 plasma: 66 IU/mL) and 55% (O2 plasma: 51 IU/mL). Untreated P(3HB) (61 IU/mL) induced β-thromboglobulin activity about half of the negative control. Furthermore, the CO2 and O2 plasma-chemical modified P(3HB) reduced the activity by around 20% (CO2 plasma: 47 IU/mL) or 10% (O2 plasma: 58 IU/mL) than untreated P(3HB), respectively.
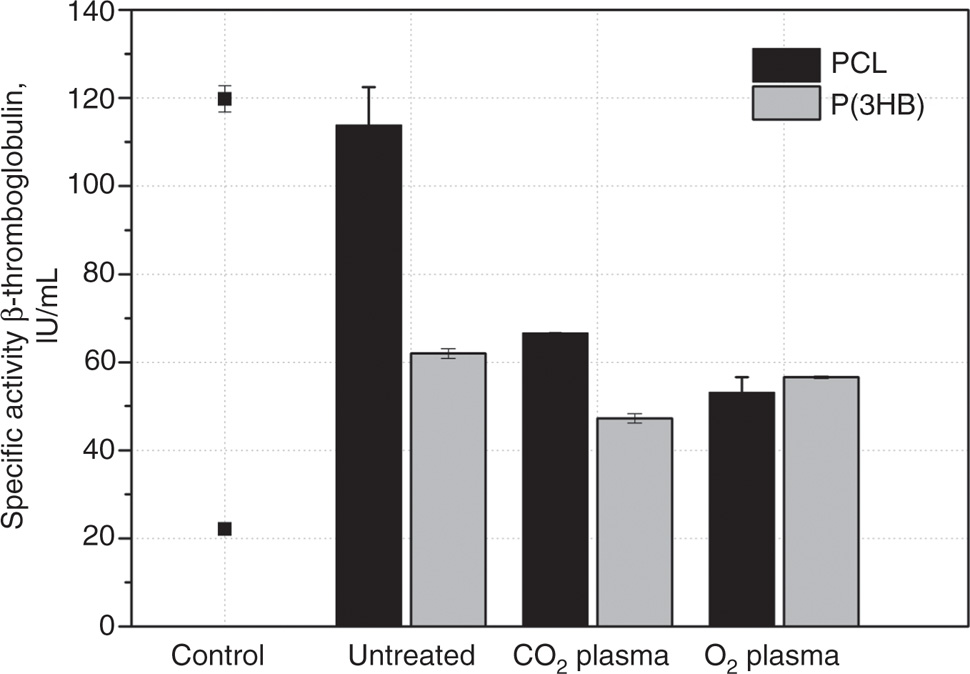
Specific activity of β-thromboglobulin after 30 min incubation of untreated and modified P(3HB) and PCL (each with n=2) with human blood plasma in comparison to the control: PC, thrombin (120 IU/mL), NC, polystyrene (22 IU/mL).
Secondary hemostasis was determined via prothrombin fragment F1+2 concentration. The P(3HB) surfaces demonstrated lower values than the correlating PCL surfaces (Figure 4). Furthermore, we observed highest F1+2 concentration for untreated PCL surfaces and lowest for O2 plasma-chemical activated surfaces. Therefore, it seems that all examined plasma-chemical modifications of P(3HB) and PCL samples have no negative influence on the secondary hemostasis.
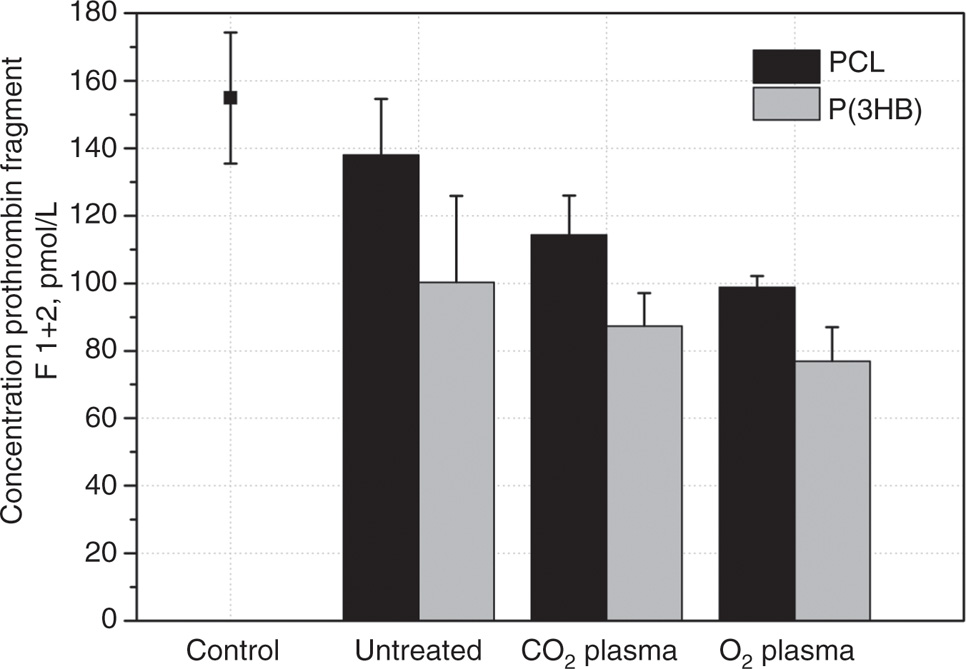
Concentration of prothrombin fragment F 1+2 after 30 min incubation of untreated and modified P(3HB) and PCL (each with n=2) with human plasma in comparison to control: NC, polystyrene and PC, kaolinite (data not shown; 1188±46 pmol/L).
Immobilization of VEGF
VEGF was immobilized on the PCL and P(3HB) surfaces via coupling with DSC. Either terminal hydroxyl groups of VEGF react with DSC forming of a hydrolyzable covalent carbonate ester bond or terminal amino groups of VEGF react with DSC creating a hydrolyzable covalent carbamate ester bond (Figure 5(II)).
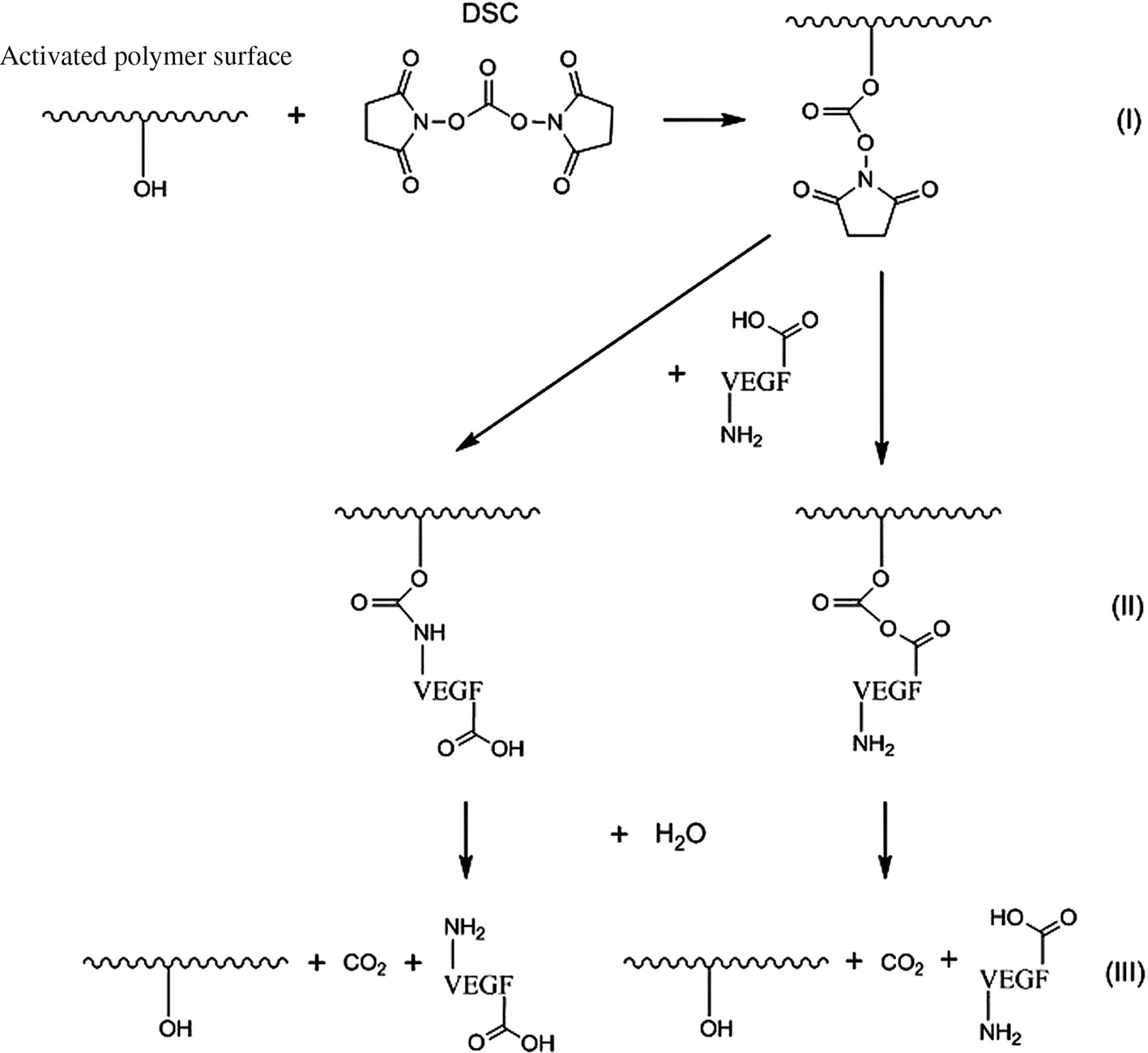
VEGF coupling scheme on activated P(3HB)/PCL surface via DSC: coupling of DSC (I), covalent bonding of VEGF via a functional amino or carboxy group (II) and VEGF release by hydrolysis resulting in VEGF and CO2 (III).
The immobilized VEGF on the P(3HB) surfaces was quantified by a VEGF peroxidase-antiperoxidase (PAP)-enzyme-linked immunosorbent assay (ELISA) to enhance the measurement signal. The immobilized VEGF was calculated by a sandwich-avidin-biotin complex (ABC)-ELISA to convert the PAP-ELISA signal. VEGF loading was determined via ELISA using PAP for signal amplification according to Wulf et al. [2]. In order to eliminate positive immobilization results by unspecific VEGF adhesion and to measure only covalently bounded VEGF, we optimized the washing procedures for PCL and P(3HB) surfaces.
It was found that the VEGF content on modified polymer surfaces is higher than on untreated surfaces. The DSC crosslinked surfaces strongly varied in VEGF amount with plasma-chemical modification (Table 2). The VEGF amount detected on both CO2 plasma-chemical modified polymer surfaces is significantly higher compared to O2 plasma-chemical modified systems. Similar results were observed for O2 plasma-chemical modified PCL or P(3HB) surfaces. Crosslinked VEGF amount was four times higher for the CO2 plasma-chemical modified P(3HB) surface compared to PCL.
Mean VEGF amount±standard deviation (SD) on PCL and P(3HB) surfaces after VEGF coupling with DSC.
| Modification | Absolute VEGF amount on PCL (pg/sample±SD) | Absolute VEGF amount on P(3HB) (pg/sample±SD) |
|---|---|---|
| Untreated | Not determinable | Not determinable |
| CO2 plasma | 367±106 | 1589±254 |
| O2 plasma | 172±55b | 175±58a |
n=5, ap<0.05; bp<0.01 significant difference between CO2 and O2 plasma-chemical treated samples.
In addition, in vitro VEGF release studies, employing a Sandwich-ABC-ELISA, were performed (Figure 6).
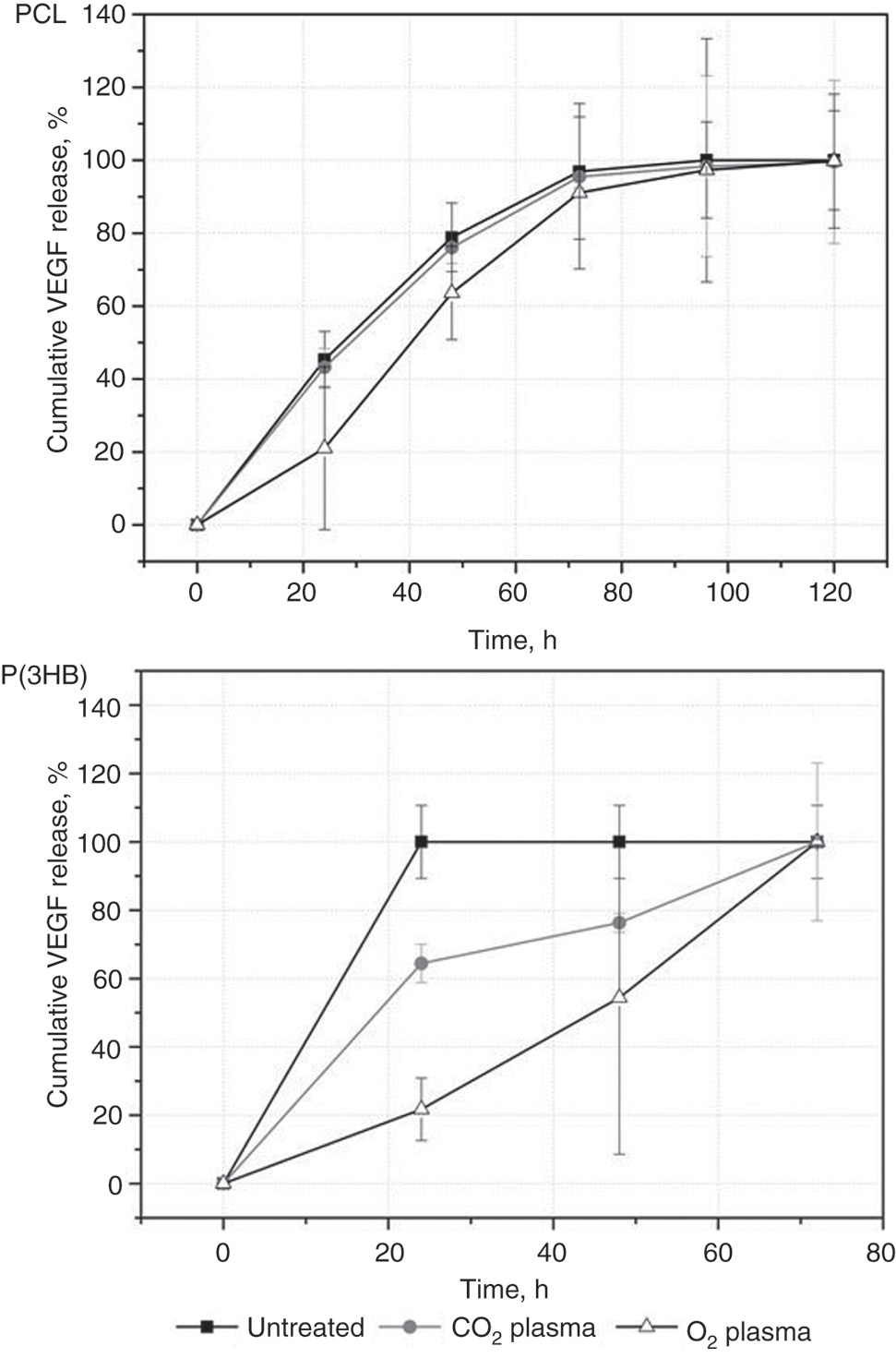
Time-dependent cumulative release of VEGF coupled with DSC as crosslinker on untreated and plasma-chemical modified PCL and P(3HB) (each with n=3).
The in vitro VEGF release rate is higher for all P(3HB) samples compared to the investigated PCL samples. The VEGF release profile for DSC functionalized, untreated PCL and CO2 plasma-chemical modified PCL is similar. For O2 plasma-chemical modified PCL, a slower release rate and an increase of release between 24 and 48 h was detected. The immobilized VEGF was completely released within 24 h from untreated P(3HB) and within 72 h from plasma-chemical modified P(3HB). Also, both plasma-chemical modified P(3HB) samples showed partially increased VEGF release between 24 and 48 h.
Discussion
Surface modifications of polymer scaffolds by biomolecule immobilization are a promising method to improve cell-implant interactions. In this context, the biodegradable polymers PCL and P(3HB) play an important role for drug delivery systems. In former investigations we already described promising results of amino group modified PCL surfaces [2].
Plasma-chemical treated surface characterization
Within this investigation we established plasma-chemical methods to generate oxygen containing groups on PCL and P(3HB) surfaces. For both investigated PCL and P(3HB) samples, the contact angle significantly decreased after the plasma-chemical modification. However, the contact angles for each polymer after plasma-chemical modification by either O2 or CO2 are rather similar with 50° or 51° for PCL and 44° for P(3HB), indicating higher hydrophilicity and prolific generation of oxygen containing functional groups. Obvious morphological changes due to the chemical plasma surface activation were not observed. This is in line with the findings in the literature [1], [4]. The CO2 or O2 plasma-chemical treatments are promising methods for the increase of hydrophilicity of the used polymer surfaces. Same results are also reported by Pappa et al. [13] and Syromotina et al. [3]. Furthermore, these results correlate with our earlier study of the ammonia plasma-chemical activation [2].
Biocompatibility
To improve cell adhesion and proliferation we analyzed the influence of plasma-chemical surface activation on biocompatibility.
In direct contact with fibroblasts no significant changes between the plasma-modified and untreated surfaces were observed, except for P(3HB) after O2 plasma-chemical modification. Nevertheless, no cytotoxic effects before and after plasma-chemical modification were apparent according to DIN EN ISO 10993-5:2009-10 (cell viability higher than 70%), as previously described in the literature [3], [4], [13].
Possible applications of implants or tissue engineered scaffolds in the cardiovascular system also require hemocompatibility analyses. Therefore, we analyzed primary hemostasis via β-thromboglobulin activity. According to the low number of samples we disclaim statistical analyses according to low reliability. For PCL, we observed a strong improvement in primary hemostasis after plasma-chemical modifications. In contrast to P(3HB), only minor improvements after plasma-chemical modifications were observed. Perhaps these findings are due to the fact that the results of the untreated P(3HB) are already within the range of the plasma-chemical modified PCL, which was observed in the literature [2], [14]. Furthermore, several interactions can occur between blood and foreign materials like the intrinsic coagulation pathway or the complement system [15]. Both pathways can also influence platelet activation and perhaps cause the higher β-thromboglobulin activity of untreated PCL compared to untreated P(3HB).
Influence of secondary hemostasis was analyzed via prothrombin fragment F1+2 formation. We also observed better results after plasma-chemical modification PCL than for untreated PCL. Same was observed for P(3HB) which has a better hemocompatibility than PCL. We also detected slightly better results for the O2 plasma modified surfaces than for the CO2 plasma modified surfaces. In this context, Mao et al. described that positively charged surfaces seem to induce activation of platelets. On the other hand negatively charged surfaces, like intact intima, have a good blood compatibility [16]. Similar influence of plasma-chemical modifications was also described by Wulf et al. for positively charged surfaces [2].
Thus, regarding the hemocompatibility and cell viability, plasma-chemical modifications do not have negative effects in vitro. However, the different tendencies for both modifications indicate the generation of different oxygen containing groups and also their amount which is important for the biocompatibility and further biofunctionalization. The use of DSC as crosslinking agent for the immobilization of VEGF enables the covalent binding of the bioactive substances.
VEGF loading and drug release
Plasma-chemical modification drastically increases the amount of VEGF loading via DSC coupling on PCL and P(3HB) surfaces. This is also reported in literature and is caused by the generation of functional chemical groups on the surface and possibly also by nanoscale surface structuring by the sandblasting effect [1], [2]. Furthermore, for CO2 plasma-modified surfaces we detected significantly higher VEGF amounts than for O2 plasma-modification. This could be due to the higher amount of generated functional groups which can react with DSC after CO2 plasma than after O2 plasma activation.
For the plasma-modified P(3HB) compared to the untreated, slower release rates were detected suggesting a stronger interaction between modified surfaces and the more hydrophilic surface of the plasma-chemical modified P(3HB) (lower contact angle than for PCL) which generally causes a decreased unspecific absorption of proteins on surfaces [17].
A slower VEGF release was observe only for O2 plasma-modified PCL surfaces over the whole timeframe of the in vitro release studies. For O2 plasma-modified PCL and for CO2 and O2 plasma-chemical modified P(3HB), an increased VEGF release rate were observed between 24 and 48 h and within 24 and 72 h suggesting a cleavage of the hydrolyzable ester bonds [Figure 5(III)], respectively. Possible reasons for the time difference in the hydrolyzation between the PCL and P(3HB) could be the different type of the ester bonds and the chemical environment which influences the hydrolyzation process.
Generally we observed higher amounts of released VEGF compared to the immobilized VEGF detected on the polymer surface. Thus, VEGF is possibly immobilized within the polymer bulk and perhaps could not be detected with the used PAP-ELISA.
The potential use of VEGF for implants in the cardiovascular field such as stents or small vascular grafts was already demonstrated in the literature [18], [19], [20], [21]. In particular, Storm et al. described a beneficial increase of the viability of human umbilical vein endothelial cells (HUVECs) on untreated PCL in vitro, which is furthermore increased for O2 plasma-chemical modified PCL after stimulation with 25 ng/mL VEGF. Besides, there are still functional differences in the biological effect among soluble VEGF and immobilized VEGF on polymer surfaces. Scaffold/implant immobilized VEGF still retains its mitogenic effect on endothelial cells as described in literature [1], [22], [23], while released VEGF does no longer affect the implantation site. Furthermore, immobilized VEGF still promotes endothelial cell growth in scaffolds [21]. In comparison to Wulf et al. [2], we achieved similar VEGF amounts for our PCL CO2 and O2 plasma-chemical modified surfaces as well as for ammonia-plasma modified surfaces. P(3HB) CO2 plasma-chemical modified surfaces have four times more immobilized VEGF than the PCL CO2 plasma-chemical modified surfaces.
Conclusion
The results confirm that the efficiency of the immobilization of VEGF depends on the chosen surface modification. The highest drug loading and cumulative release was obtained by using CO2 plasma-chemical activated PCL and P(3HB). Plasma conditions for surface activation were properly chosen with respect to morphology changes. The conditions of the plasma-chemical modification were suitable for scaffolds material of PCL and P(3HB) without changing surface morphology. Plasma-chemical modifications did not diminish hemocompatibility. For viability of L929 fibroblasts only P(3HB) treated with O2 plasma has shown a slight decrease, meanwhile all other samples showed an excellent biocompatibility. VEGF coupling via DSC results in hydrolysable ester bonds with retarding effect on drug release. The biofunctionalization presented here seems to be a suitable method to modify scaffold materials for bioartificial vessel prostheses.
Materials and methods
Materials
All chemicals were purchased from Sigma-Aldrich (Taufkirchen, Germany), Mallinckrodt Baker (Griesheim, Germany), SERVA Feinbiochemica (Heidelberg, Germany), Thermo Scientific (Karlsruhe, Germany) or Merck (Darmstadt, Germany) in p. a. quality or higher. L929 mouse fibroblasts (ATCC number CCL1) were purchased from Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany).
Film fabrication
The poly(3-hydroxybutyrate) (P(3HB)) foils were prepared via dip-coating. Therefore, 4 g P(3HB) (Mw=850,000 g/mol, Helmholtz-Zentrum für Umweltforschung, Leipzig, Germany) was dissolved in 100 mL chloroform. An immersed body (master steel pattern) was then dipped into the bubble-free P(3HB) solution for 10 times. After each dipping, the master steel pattern was turned by 180° to assure a homogeneous surface and foil thickness. The remaining chloroform was allowed to evaporate for 1 day until a 115 μm thick film had formed, which was cut from the master steel pattern.
In 25 mL chloroform 1 g poly(ε-caprolactone) (PCL, Mn=80,000 g/mol, Cappa 6800, Perstorp UK Limited, Warrington, UK) was dissolved and poured into a glass Petri dish (Ø=9 cm). The remaining chloroform was allowed to evaporate until a 130 μm thick film had formed, which was cut from the Petri dish [2].
The resulting P(3HB) and PCL foils were washed in methanol for 2 days and afterwards 2 days in water. Foils were finally dried in a vacuum cabinet drier at 40 °C and 40 mbar. The film thickness was analyzed by means of a thickness gage (2109 Mitutoyo, Mitutoyo Europe GmbH, Neuss, Germany).
Plasma-chemical activation
The plasma-chemical activation process for P(3HB) and PCL was performed via plasma-etching (PE) electrode in a carbon dioxide (CO2) or Oxygen (O2) plasma using a radio frequency (RF) plasma generator (frequency 13.56 MHz, power 100 W, Diener electronic GmbH & Co. KG, Ebhausen, Germany) at a low pressure of 0.3 mbar (Figure 7). Parameters for plasma-chemical activation (generator power and time) were optimized by using contact angle measurements (screening data not shown). A summary of the used parameters are given in Table 3.
Surface plasma-chemical activation scheme for P(3HB) using O2 or CO2 plasma.
Summary of the used plasma-chemical conditions for surface activation of PCL and P(3HB).
| Polymer | 1. O2 plasma | 2. CO2 plasma |
|---|---|---|
| P(3HB) | ||
| Time: | 3 min | 5 min |
| Power: | 45 W | 45 W |
| PCL | ||
| Time: | 1 min | 1 min |
| Power: | 30 W | 30 W |
Generator: f=13.56 MHz; temperature: <40 °C; pressure: 0.3 mbar.
Biofunctionalization of VEGF via DSC
The coupling procedure was performed according to [2]. P(3HB) or PCL samples were immersed in 5 mM DSC in dry DMSO for 4 h at room temperature. Afterwards, samples were washed with DMSO and reacted with 1 μg/mL VEGF in dry DMSO for 1 h at room temperature. Finally, samples were washed with DMSO, methanol and tris(hydroxymethyl)aminomethane buffer (pH 7.6, TRIS buffer, 4.4 g NaCl and 3 g TRIS filled to 500 mL with pure water). Finished samples were used for VEGF eluting and loading analysis.
Contact angle measurements
Sessile drop method (Contact Angle System, OCA 20, Dataphysics Instruments GmbH, Filderstadt, Germany) with n=24 (one topside and one backside) was performed.
Statistical analysis
Statistical analyses of contact angle measurements, cell viability and VEGF surface immobilization results were performed using the software SPSS 15.0. To evaluate statistical significance in contact angle differences a Wilcoxon-test at ***p<0.001 was used. Cell viability on activated surfaces was compared to the untreated surface via Mann-Whitney U-test at *p<0.05. For the VEGF immobilization the Wilcoxon-test was also used at *p<0.05 and **p<0.01. Mean values and standard deviations were calculated and presented in the results.
Morphology analysis
The morphology of the PCL and P(3HB) specimens were examined with a Philips XL 30 ESEM (Philips ElectronOptics, Eindhoven, The Netherlands) in the ESEM mode. The samples were fixed on aluminum trays with conductive tape. The images were taken at a 1000× magnification and representative micrographs are shown.
In vitro VEGF release
The in vitro VEGF release from polymer samples (Ø=6 mm) was carried out in 0.35 mL cell culture medium MCDB131 (PAN Biotech, Aidenbach, Germany), with 2.5% fetal calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin and 2 mM L-glutamine, at pH 7.4, at 37 °C and was analyzed by a Sandwich-ABC-ELISA (human VEGF ELISA Kit RayBio®, Hölzel Diagnostika, Köln, Germany). The ELISA was carried out according to manufacturer’s instructions of the ELISA VEGF Kit. Tests were performed with three individual specimens.
VEGF surface loading
VEGF loading was determined via ELISA using PAP for signal amplification according to Wulf et al. [2]. To preferably analyze only covalently bound VEGF we developed blocking and washing procedures for PCL and P(3HB) surfaces to eliminate unspecific adhesion of VEGF. Therefore, the untreated and modified polymer samples (Ø=6 mm) were added for 1 h to 2 mL of a block solution, containing 1% bovine serum albumine (BSA) in PBS (pH=7.6), and washed with PBS/0.05%Tween 20 (wash buffer). To the polymer samples the primary antibody (1 μL/mL, anti-VEGF, host: goat), diluted in the BSA block solution, was added, to the polymer stirred for 1 h at room temperature and washed with wash buffer. Diluted in block solution the secondary antibody (2 μL/mL, IgG peroxidase conjugate, host: mouse anti-goat) was added to the polymer sample, stirred for 1 h at room temperature and samples were washed. Two millilitres of a solution of peroxidase anti-peroxidase complex labeled with horseradish peroxidase (HRP) (1 μg/μL, PAP-complex) in BSA block solution was added to the polymer sample, stirred for 1 h at room temperature and samples were washed. The VEGF amount was determined by blue color of oxidized 3,3′,5,5′ -tetramethylbenzidine (TMB). The reaction was stopped via 2 M H2SO4 in distilled water. The resulting absorption (450 nm) was measured by means of the plate reader FLUOstar Optima (FLUOstar Optima, BMG LABTECH, Ortenberg, Germany). A Sandwich-ABC-ELISA (human VEGF ELISA Kit RayBio®, Hölzel Diagnostika, Köln, Germany) according to manufacturer’s instructions was used to convert PAP-ELISA signal into VEGF amount. Tests were performed with five individual specimens. The method is described in detail in Teske [24].
Cell viability assay
For the determination of the biocompatibility of the PCL and P(3HB) samples, cell viability of L929 mouse fibroblasts (DSZM, Braunschweig, Germany) was determined with the CellQuanti-Blue assay (BioAssay Systems, Hayward, CA, USA). The tests were performed according to Wulf et al. [2]. Zinc diethyldithiocarbamate (ZDEC) and tissue-culture polystyrene served as positive and negative controls (PC, NC), respectively. In total, six independent biological replications for each surface were performed. Data are calculated from n=6 independent biological experiments.
Hemocompatibility assay (β-thromboglobulin)
The activation of primary hemostasis was analyzed in vitro by reading out the formation of β-thromboglobulin, which is released by the activation of platelets. Human blood was collected, sodium citrate (0.11 M) added and centrifuged. The platelet-rich plasma supernatant (PRP) was collected and used for analysis. One millilitre PRP was added to the sample in a 24-well plate and incubated for 30 min at 37 °C. As references a sample without test specimens (NC = polystyrene) and a sample with thrombin (3 IU/mL PRP, PC) were analyzed. After incubation platelets were arrested using citrate-theophylline-adenosine-dipyridamole. The arrested PRP was centrifuged and the supernatant diluted and analyzed via a Sandwich-ELISA (Asserachrom b-TG, Diagnostica Stago, Asniere, France) according to the description of the manufacturer. All samples were run in duplicate. A detailed description is published in Wulf et al. [2].
Hemocompatibility assay (prothrombin fragment F1+2)
The formation of prothrombin fragment F1+2, formed by the generation of thrombin, was analyzed in order to determine the activation of secondary hemostasis. The analyses were performed by means of a Sandwich-ELISA (Sandwich-ELISA Kit EnzygnostVR F1+2 of Siemens Healthcare Diagnostics Products GmbH, Marburg, Germany). Human blood was collected, lithium heparin added, centrifuged and the plasma supernatant removed. 1 mL of the heparinized blood plasma was added to the sample in a 24-well plate and incubated for 30 min at 37 °C. As references a sample without test specimens (NC = polystyrene) and a sample with kaolinite (1190±47 pmol/L, PC) were analyzed. One hundred microliters aliquots were subjected to the prothrombin ELISA according to manufacturer’s instructions. Each sample was run in duplicate. A detailed description is published in Wulf et al. [2].
Acknowledgements
The authors would like to thank Jaqueline Bohm, Babette Hummel, Martina Nerger, Martina Schröder and especially Marian Löbler and Katharina Wulf for their technical assistance. The partial financial support by the DFG within SFB Transregio 37 and the state government Mecklenburg-Vorpommern (AZ: V230-630-08-TFMV-S-005) is gratefully acknowledged. The presented data are results of the doctoral thesis of Michael Teske under the supervision of Katrin Sternberg.
Author’s statement
Conflict of interest: Authors state no conflict of interest.
Materials and methods
Informed consent: Informed consent has been obtained from all individuals included in this study.
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee.
1. Guex AG, Hegemann D, Giraud MN, Tevaearai HT, Popa AM, Rossi R, et al. Covalent immobilisation of VEGF on plasma-coated electrospun scaffolds for tissue engineering applications. Colloids Surf B Biointerfaces. 2014;123:724–33.10.1016/j.colsurfb.2014.10.016Search in Google Scholar PubMed
2. Wulf K, Teske M, Löbler M, Luderer F, Schmitz K-P, Sternberg K. Surface functionalization of poly(ε-caprolactone) improves its biocompatibility as scaffold material for bioartificial vessel prostheses. J Biomed Mater Res. 2011;98B:89–100.10.1002/jbm.b.31836Search in Google Scholar PubMed
3. Syromotina DS, Surmenev RA, Surmeneva MA, Boyandin AN, Nikolaeva ED, Prymak O, et al. Surface wettability and energy effects on the biological performance of poly-3-hydroxybutyrate films treated with RF plasma. Mater Sci Eng C Mater Biol Appl. 2016;62:450–7.10.1016/j.msec.2016.01.075Search in Google Scholar PubMed
4. Syromotina DS, Surmenev RA, Surmeneva MA, Boyandin AN, Epple M, Ulbricht M, et al. Oxygen and ammonia plasma treatment of poly(3-hydroxybutyrate) films for controlled surface zeta potential and improved cell compatibility. Mater Lett. 2016a;163:277–80.10.1016/j.matlet.2015.10.080Search in Google Scholar
5. Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface. 2011;8:153–70.10.1098/rsif.2010.0223Search in Google Scholar PubMed PubMed Central
6. Chen FM, Zhang M, Wu ZF. Toward delivery of multiple growth factors in tissue engineering. Biomaterials. 2010;31:6279–308.10.1016/j.biomaterials.2010.04.053Search in Google Scholar PubMed
7. Simon-Yarza T, Formiga FR, Tamayo E, Pelacho B, Prosper F, Blanco-Prieto MJ. Vascular endothelial growth factor-delivery systems for cardiac repair: an overview. Theranostics. 2012;2:541–52.10.7150/thno.3682Search in Google Scholar PubMed PubMed Central
8. Masters KS. Covalent growth factor immobilization strategies for tissue repair and regeneration. Macromol Biosci. 2011;11:1149–63.10.1002/mabi.201000505Search in Google Scholar PubMed
9. Bak T-Y, Kook M-S, Jung S-C, Kim B-H. Biological effect of gas plasma treatment on CO2 gas foaming/salt leaching fabricated porous polycaprolactone scaffolds in bone tissue engineering. J Nanomater. 2014;20141–6.10.1155/2014/657542Search in Google Scholar
10. Patra S, Anjum S, Ray AR, Gupta B. Effect of CO2 plasma exposure on physico-chemical properties of porous polycaprolactone scaffold. Polym Bull. 2016;73:1875–90.10.1007/s00289-015-1582-2Search in Google Scholar
11. Mercado AE, He X, Xu W, Jabbari E. The release characteristics of a model protein from self-assembled succinimide-terminated poly(lactide-co-glycolide ethylene oxide fumarate) nanoparticles. Nanotechnology. 2008;19:325609.10.1088/0957-4484/19/32/325609Search in Google Scholar
12. Morpurgo M, Bayer EA, Wilchek M. N-hydroxysuccinimide carbonates and carbamates are useful reactive reagents for coupling ligands to lysines on proteins. J Biochem Biophys Methods. 1999;38:17–28.10.1016/S0165-022X(98)00027-XSearch in Google Scholar
13. Pappa AM, Karagkiozaki V, Krol S, Kassavetis S, Konstantinou D, Pitsalidis C, et al. Oxygen-plasma-modified biomimetic nanofibrous scaffolds for enhanced compatibility of cardiovascular implants. Beilstein J Nanotechnol. 2015;6:254–62.10.3762/bjnano.6.24Search in Google Scholar
14. Rudolph A, Teske M, Illner S, Kiefel V, Sternberg K, Grabow N, et al. Surface modification of biodegradable polymers towards better biocompatibility and lower thrombogenicity. PLoS One. 2015;10:e0142075.10.1371/journal.pone.0142075Search in Google Scholar
15. Bernacca GM, Gulbransen MJ, Wilkinson R, Wheatley DJ. In vitro blood compatibility of surface-modified polyurethanes. Biomaterials. 1998;19:1151–65.10.1016/S0142-9612(98)00016-7Search in Google Scholar
16. Mao C, Qiu Y, Sang H, Mei H, Zhu A, Shen J, et al. Various approaches to modify biomaterial surfaces for improving hemocompatibility. Adv Colloid Interface Sci. 2004;110:5–17.10.1016/j.cis.2004.02.001Search in Google Scholar
17. Rabinow BE, Ding YS, Qin C, McHalsky ML, Schneider JH, Ashline KA, et al. Biomaterials with permanent hydrophilic surfaces and low protein adsorption properties. J Biomater Sci Polym Ed. 1995; 6(1):91–109. DOI:10.1163/156856295X00788.Search in Google Scholar
18. van Belle E, Tio FO, Couffinhal T, Maillard L, Passeri J, Isner JM. Stent endothelialization. Time course, impact of local catheter delivery, feasibility of recombinant protein administration, and response to cytokine expedition. Circulation. 1997;95:438–8.10.1161/01.CIR.95.2.438Search in Google Scholar
19. Swanson N, Hogrefe K, Javed Q, Gershlick AH. In vitro evaluation of vascular endothelial growth factor (VEGF)-eluting stents. Int J Cardiol. 2003;92:247–51.10.1016/S0167-5273(03)00102-5Search in Google Scholar
20. Storm T, Wulf K, Teske M, Löbler M, Kundt G, Luderer F, et al. (2014): Chemical activation and changes in surface morphology of poly(epsilon-caprolactone) modulate VEGF responsiveness of human endothelial cells. J Mater Sci Mater Med. 2014;25:2003–15.10.1007/s10856-014-5226-0Search in Google Scholar PubMed
21. Antonova LV, Sevostyanova VV, Kutikhin AG, Mironov AV, Krivkina EO, Shabaev AR, et al. Vascular endothelial growth factor improves physico-mechanical properties and enhances endothelialization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/poly(epsilon-caprolactone) small-diameter vascular grafts in vivo. Front Pharmacol. 2016;7:230.10.3389/fphar.2016.00230Search in Google Scholar PubMed PubMed Central
22. Stone D, Phaneuf M, Sivamurthy N, LoGerfo FW, Quist WC. A biologically active VEGF construct in vitro: implications for bioengineering-improved prosthetic vascular grafts. J Biomed Mater Res. 2002;59:160–5.10.1002/jbm.1229Search in Google Scholar PubMed
23. Shen YH, Shoichet MS, Radisic M. Vascular endothelial growth factor immobilized in collagen scaffold promotes penetration and proliferation of endothelial cells. Acta Biomater. 2008;4:477–89.10.1016/j.actbio.2007.12.011Search in Google Scholar PubMed
24. Teske M. Nass- und plasmachemische Oberflächenmodifizierung biodegradierbarer, polymerer Implantatwerkstoffe unter Immobilisierung von Wirkstoffen zur Optimierung der Zelle-Implantat-Interaktion 2014.Search in Google Scholar
©2017, Michael Teske et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Biofunctionalization
- Biofunctionalization of surfaces using polyelectrolyte multilayers
- Focal adhesion stabilization by enhanced integrin-cRGD binding affinity
- Surface functionalization of poly(ε-caprolactone) and poly(3-hydroxybutyrate) with VEGF
- Aptamer-modified nanomaterials: principles and applications
- Bioconjugation of SERS nanotags and increasing the reproducibility of results
- Functionalization of polyethetherketone for application in dentistry and orthopedics
- Influence of surface roughness of dental zirconia implants on their mechanical stability, cell behavior and osseointegration
Articles in the same Issue
- Biofunctionalization
- Biofunctionalization of surfaces using polyelectrolyte multilayers
- Focal adhesion stabilization by enhanced integrin-cRGD binding affinity
- Surface functionalization of poly(ε-caprolactone) and poly(3-hydroxybutyrate) with VEGF
- Aptamer-modified nanomaterials: principles and applications
- Bioconjugation of SERS nanotags and increasing the reproducibility of results
- Functionalization of polyethetherketone for application in dentistry and orthopedics
- Influence of surface roughness of dental zirconia implants on their mechanical stability, cell behavior and osseointegration

