Abstract
In the 1990s an unexpected gene-silencing phenomena in plants, the later called RNA interference (RNAi), perplexed scientists. Following the proof of activity in mammalian cells, small interfering RNAs (siRNAs) have quickly crept into biomedical research as a new powerful tool for the potential treatment of different human diseases based on altered gene expression. In the past decades, several promising data from ongoing clinical trials have been reported. However, despite surprising successes in many pre-clinical studies, concrete obstacles still need to be overcome to translate therapeutic siRNAs into clinical reality. Here, we provide an update on the recent advances of RNAi-based therapeutics and highlight novel synthetic platforms for the intracellular delivery of siRNAs.
Introduction
The goal of RNA interference-based therapy is to silence the expression of ‘uncontrollable’ genes involved in human diseases that are un-targetable by conventional drugs. siRNAs mediate gene silencing by inducing sequence-specific cleavage of complementary messenger RNA (mRNA). Gene silencing can be induced by chemical synthesized short double-stranded RNA, including siRNA and microRNA (miRNA), and double-stranded hairpin RNA (shRNA) transcribed in vitro or from viral vectors and plasmids. In the endogenous pathway, siRNA is incorporated into the RNA-induced silencing complex (RISC), a ubiquitous process in mammalian cells, where it seeks and binds perfect or nearly perfect complementary regions of target mRNA and causes translational repression and suppression of its expression (1, 2). Then, when the cleaved mRNA is released, the siRNA-RISC complex binds to another mRNA and starts a new round of cleavage (3, 4). The potency of siRNAs in knocking down the expression of specific targets has been widely demonstrated in vivo for the treatment of different diseases, such as hepatitis B virus (HBV) (5), human papilloma virus (6), ovarian cancer (7) and hypercholesterolemia (8).
However, in order to have a concrete impact on the health of patients, promising strategies should be proposed to translate therapeutics siRNAs into the clinical setting. Naked siRNAs are unstable in human plasma and too large and negatively charged to cross the cellular membranes. Chemically modified siRNAs have been used extensively to enhance the stability of siRNA in biological fluids. Chemical modifications on the backbone, sugar moiety and nucleobase of the siRNA, are usually introduced to the 5′ or 3′ end of the terminal regions of the sense-strand (non-guide strand) (9). This approach definitely increases the stability of siRNA in human blood often without affecting the silencing activity.
Nevertheless, the silencing effect of chemically modified siRNA has been shown only in certain body tissues, such as brain and lung (10, 11). Wider systemic delivery of siRNA to various organs and tissues possesses its unique advantages as well as challenges that deserve special focus. Using the rational siRNA design, nuclease resistant siRNA molecules could be synthesized (12, 13), however, then the longevity in the circulation becomes the new challenge. One of the main siRNA elimination pathways is through the kidneys and the pore size of the glomerular filtration limit is around 8 nm. Free siRNA with the average diameter less than 10 nm can accumulate in the kidney and pass through the filtration to urine within 5-to-10 min of administration (14, 15). This obstacle can be overcome by using a nanosized siRNA carrier system, which is designed to be larger than 20-nm size to avoid the renal clearance. At the same time, to exploit the leaky vasculature of the tumors and benefit from the enhanced permeability and retention (EPR) effect, the size of the carriers should be limited to 200–400 nm, depending on the various cut-off sizes of different tumors (16, 17). However, when a carrier has been used, the prevention of phagocytosis becomes crucial by using materials to form a steric barrier around the siRNA carrier, such as polyethylene glycol (PEG) (18). Following extravasation, siRNA and its carrier must overcome multiple diffusion barriers through the extracellular matrix to reach the target tissue or cells and activate the RNAi. Cellular uptake of siRNA or its carrier by the target cells is the next roadblock along the journey of successful siRNA delivery. It should also be noted that PEG, that provides vital benefits until this step, could also hinder the cellular interaction by a phenomena called ‘PEG dilemma’. Various strategies that could be implemented to enhance the cellular uptake of the carrier systems do not guarantee the effective protein silencing because the siRNA still needs to escape endosomal encapsulation and subsequent lysosomal degradation. Endosomal escape has become the new point of focus for successful in vivo siRNA delivery since the nanocarrier based approaches helped to avoid most of the obstacles mentioned above in the last decade.
Realization of the challenges of in vivo siRNA applications makes clear that without siRNA delivery systems, the true potential of RNAi as a therapeutic approach cannot be fulfilled. Delivery strategies include physical methods, conjugation methods, and viral or non viral drug delivery systems. The physical approaches increase the siRNA uptake in certain target tissues using the gene gun, electroporation, pressure, sonoporation, but are generally difficult to apply in humans or unrealistic for a commercial translation. Modified viruses such as retrovirus, lentivirus, adeno-virus and adeno-associated viruses (AAV) are widely use to harness the endogenous pathway of RNAi and so far almost 70% of advanced clinical trials use viral vectors for gene therapy. The AAV-based gene therapeutic Glybera (alipogene tiparvovec) which contains the human lipoprotein lipase gene variant LPLS447X in a vector, has been approved for use in Europe to treat adult patients diagnosed with familial lipoprotein lipase deficiency.
Although there have been many advances in this field, several limitations are associated with the use of viral vectors including carcinogenesis (19), immunogenicity (20), no-selectivity (21), and difficulty of scale-up (22). The design of an effective and non-toxic delivery system that is able to selectively accumulate siRNA in the tissue of interest remains the greatest hurdle to widespread the therapeutic application of RNAi of all the diseases. The systemic delivery of siRNA has been extensively investigated in the recent past (23–26). The number of publications on siRNA delivery strategies has steadily increased in the last decade. Different siRNA delivery approaches have been proposed, including conjugated or modified siRNAs and several delivery vehicles such as lipid nanoparticles (27–29), polymer-conjugates (30, 31), inorganic nanoparticles (32, 33), aptamer-based approaches (34–36), peptide-mediated delivery (37–39), and other non-viral systems (40).
To date several clinically tested siRNA therapeutics are administrated intravenously by synthetic carriers. Among them, six use cationic liposomes, including stable nucleic-acid lipid particles (SNALPs), carriers developed by Tekmira (Barnaby, Canada), and siRNA-lipoplex known as AtuPLEX™ developed by Silence Therapeutics (London, UK), currently in phase IIa trial for the treatment of advanced solid tumors. In contrast to cationic liposomal carriers, Calando Pharmaceuticals (Pasadena, CA, USA) developed cyclodextrin-based polymer nanoparticles as a drug delivery system for siRNA, CALAA-01. Moreover, another siRNA drug that uses polymeric carriers includes a biodegradable polymeric matrix known as LODER.
Here, we review a selection of non-viral siRNA delivery systems. In this review, we highlight and provide recent advances on non-viral siRNA delivery systems as well as underline the established strategies used for successful siRNA applications.
siRNA conjugation technology
Covalent conjugation of siRNAs with other ligands and molecules can be used to increase the internalization of siRNA in specific cells as well as to increase its stability in vivo (41). The ligands can be placed at the 3′ or 5′ end of the sense or passenger strand without affecting the silencing activity of the siRNA. To date, the most advanced therapeutic program engaging siRNA conjugation strategy is Alnylam’s (Cambridge, MA, USA) Phase II ALN-TTRsc (USA clinical trial NCT01981837; http://clinicaltrials.gov/show/NCT01981837). The clinical trial targets a siRNA against transthyretin (TTR) to the liver hepatocytes by conjugating the siRNA with a N-acetylgalactosamine (GalNAc) ligand, which mediates the cell uptake by the asialoglycoprotein receptor, for the treatment of TTR amyloidosis, an inherited and fatal disease. TTR is a primarily liver-expressed tetrameric protein. Following mutations of the TTR gene, the TTR protein destabilizes in tetramer with amyloid formation and it accumulates abnormally in tissues, including heart, causing amyloidosis. The results from the Phase I study showed a robust silencing of the TTR levels in the serum (≥90%) in an encouraging non-toxic profile (http://investors.alnylam.com/releasedetail.cfm?ReleaseID=814002). In a recent in vivo study, a similar concept has been used to covalently conjugate a VEGFR2 siRNA to a cyclic arginine-glycine-aspartate (RGD) peptide (cRGD), known to bind αvβ3 integrin receptors. αvβ3 integrin is significantly up-regulated in tumor blood vessels, as well as in invasive tumor cells of many cancer types (42). To evaluate the potential of the developed cRGD-siRNAs conjugate as an anticancer agent, its effect on the tumor development and angiogenesis was investigated in a mouse model of tumor. Interestingly, in mice xenografted with A549 luciferase-expressing cells, the systemic delivery of the tumor-targeted conjugate resulted in a significant down-regulation of the mRNA and protein levels of the targeted gene into the tumors together with an overall reduction of tumor volume (43).
Another approach is to conjugate siRNA to natural ligands, such as cholesterol, lipids and long fatty acids in order to improve the stability of the siRNA against nuclease as well as to increase the cell uptake. These lipophilic siRNAs usually interact with lipoprotein particles and harness the RNAi machinery without needing a delivery system (13). Kubo et al. (44) developed a simple method to covalently conjugate fatty acids, such as palmitic and lauric acids, at the 5′-end of the sense strand of siRNAs via amide linkage. By using a very simple conjugation method, the gene-silencing activity, membrane permeability, and nuclease resistance were significantly enhanced in vitro in HeLa cells. Moreover, the lipophilic siRNA-conjugates exhibited strong RNAi potency in absence of any transfection reagents, such as Lipofectamine 2000.
Aptamer-based delivery systems
In this review it is also worth mentioning the very recent advances in aptamer-based siRNA delivery systems. Aptamers are single stranded, short, synthetic DNA or RNA molecules with high affinity and specificity to various targets (45). The target can be small molecules, proteins, cell surface receptors or even bacteria and viruses. Aptamers are selected from a pool by a process called Systematic Evolution of Ligands by Exponential Enrichment (SELEX) (46, 47), an in vitro step-by-step selection process with increasingly stringent conditions that ensures the resulting molecules have the highest selectivity against their target. Easy modifications of the in vitro synthesis process, very high selectivity against the target structure and dissociation constant (Kd) values in the nanomolar range (48, 49) make them a promising technology for siRNA delivery area. They could be used as a drug on their own, as in the example of first FDA approved aptamer-drug Pegaptanib (Macugen) by Eyetech (Palm Beach Gardens, FL, USA) that targets VEGF 165 for ocular vascular disease (50) or AS1411, an anti-nucleolin aptamer drug in clinical trials which prevents nucleolin from binding to and stabilizing mRNA of the anti-apoptotic Bcl-2 (51). There are two main strategies for aptamer-based siRNA delivery; (i) using aptamers as the targeting ligands on the surface of nanocarriers, (ii) attaching siRNAs directly to aptamers in which aptamers directly deliver the siRNA both as a carrier and as a ligand. In recent years, aptamer-siRNA conjugates (chimeras) have gained a lot of attention and evaluated for drug hypersensitivity (52), HIV treatment (53, 54) and cancer therapy (35, 55–57).
Giangrande and colleagues were the first to describe a first generation aptamer-siRNA chimera (36). In this study, an aptamer that specifically bound the prostate-specific membrane antigen (PSMA), a receptor over-expressed in prostate cancer, has been covalently linked to the passenger strand of siRNAs. The conjugate was able to significantly inhibit the mRNA expression of targeted pro-survival genes, PLK1 and Bcl2 (of 80% and 90%, respectively) in vitro and when injected intratumorally, the tumor volume of xenografted mouse model of prostate cancer was significantly decreased. After few years, they improved both physiochemical properties and the in vivo stability in order to use the chimera for systemic therapy (35). In particular, to facilitate the synthesis of the chimera in a large-scale production, the length of the aptamer was significantly decreased (from 71 nt to 39 nt) without affecting the binding and targeting affinity. In addition, the introduction of a PEG moiety on the antisense strand of the siRNA increased significantly the circulation time (from 30 min to 30 h) as well as the immunogenicity was significantly reduced, making the second-generation chimera suitable for systemic therapy. This approach seems to possess minimal immunogenicity together with the possibility to easily synthesize the platform in large quantities at a relatively low cost. More recently, Lai et al. used the aptamer-siRNA chimera approach to simultaneously deliver two different siRNAs to lung cancer in vitro and in vivo (58). They reported that the combined treatment of nucleolin aptamer conjugated snail family zinc finger 2 (SLUG) siRNA (aptNCL-SLUGsiR) and neuropilin 1 (NRP1) siRNA (aptNCL-NRP1siR) synergistically suppress lung cancer cell invasion, tumor growth and angiogenesis.
While the exciting studies proved the potential of successful in vivo siRNA delivery by aptamer-siRNA chimeras (summarized in Table 1), the challenge of endosomal escape and intracellular fate remains unclear. In very recent years, using aptamers as highly specific targeting ligands for siRNA delivery began to attract attention. Li et al. (59) combined the advantages of PEGylated long-cationic liposomes as effective siRNA carriers and an aptamer shows the specific binding to nucleolin, a phosphoprotein overexpressed in various cancer types. Liposome formulations, consist of 3β-[N-(N′,N′-dimethylaminoethane) carbamoyl] cholesterol (DC-Chol), cationic lipid dioleoylphosphatidylethanolamine (DOPE) and maleimide-PEG3400-DOPE at a mole ratio of 1:1:0.1 molar ratio, was surface modified with aptamer AS1411 via disulfide linkage. Aptamer-targeted liposomes containing anti-BRAF siRNA fully protected siRNA at N/P ratio of 20 and selectively uptaken only by cancer cells. This resulted in up to ~34% BRAF mRNA downregulation in A375 melanoma cells in vitro, while the liposomes modified with scrambled aptamer caused only 4% mRNA downregulation. The in vitro downstream protein downregulation and proliferation tests confirmed the in vitro results. More importantly, the aptamer targeted liposomes accumulated in tumors within 3–6 h after injection in a xenograft model of A375. In vivo gene silencing was investigated by real time PCR and BRAF mRNA levels decreased ca. 45% in aptamer-targeted liposomal siRNA delivery group. The study supported that siRNA delivery with aptamer modified liposomes for melanoma treatment is a very promising option with strong specificity to cancer cells and reduced overall toxicity.
Recent aptamer-siRNA conjugate systems (chimeras) for successful in vivo siRNA delivery.
| Aptamer | siRNA | Model | Outcome | References |
|---|---|---|---|---|
| CTLA4, targeting tumor-associated T cells | STAT3 | Athymic nu/nu mice engrafted with Karpas299 human lymphoma cells | Promoted tumor cell apoptosis and tumor growth inhibition, activation of tumor antigen-specific T cells, reduced tumor associated Tregs | (61) |
| gp120, targeting HIV-1 glycoprotein gp120 | Three different Dicer substrate siRNAs (as a cocktail) against; Anti-tat/rev, anti-CD4, Anti-TNPO3 | Humanized BALB/ c-Rag2-/-γc-/- infected with HIV-1 | Knockdown of all three target mRNAs, potent inhibition of HIV-1 replication, protection against viral induced CD4+ T-cell depletion, long-term suppression of HIV-1 viral loads | (62) |
| 4-1BB, targeting CD8+ T cells following TCR stimulation | mTOR complex 1 (mTORC1) | Transgenic OT-I, C57BL/6 mice | Enhanced memory CD8+ T cell development, superior cytotoxic effector functions compared to pharmacological agents, enhanced vaccine induced protective immunity and rejection of subsequent tumor challenge | (63) |
| EpCAM, targeting EpCAM+ epithelial cancers and their tumor-initiating cells | PLK1 | MDA-MB-468 (Basal A triple-negative breast cancer/TNBC) nude mice xenografts | Selective uptake by only EpCAM+ tumor cells but not by normal tissues, inhibition of tumor initiation by EpCAM+ luminal and basal A TNBC cell lines, selective accumulation in EpCAM+ Her2+ and TNBC tumors and suppression of tumor growth | (64) |
Another recent study that underlines the advantages of successful in vivo siRNA delivery by using aptamers as the targeting ligands was very recently published by Liang et al. (60). Following the screening by cell-SELEX, the authors have chosen CH6 aptamer that targets both rat and human osteoblasts for developing CH6 aptamer-functionalized lipid nanoparticles (LNPs). The researchers encapsulated osteogenic pleckstrin homology domain-containing family O member 1 (plekho1) siRNA in LNPs that have ~84 nm size with ca. 80% siRNA encapsulation efficiency and ~83% aptamer loading efficiency. Shorter sequences and satisfactory secondary structure of CH6 aptamer compared to other osteoblast specific aptamers investigated made it easier to conjugate to LNPs. CH6-modified LNPs were internalized selectively by the osteoblast cells, as confirmed by siRNA co-localization with osteoblast markers, via macropinocytosis and clathrin-mediated endocytosis and were able to escape from lysosomes. The aptamer LNPs (CH6-LNPs-siRNA) silenced the target mRNA in vitro in a siRNA dose dependent manner with up to 90% at 80 nm siRNA without any cytotoxicity to the osteoblast cells. Successful in vitro results are translated into in vivo effectively using Sprague-Dawley rat model. It has been shown that the CH6-LNPs-siRNA accumulated in the bone significantly higher even after 12 h following injection while the liver and kidney accumulations were significantly lower compared to non-aptamer targeted LNPs. Gene silencing indicated more than 60% in vivo plekho1 downregulation. This osteoblast-specific silencing promoted bone formation, improved bone microarchitecture, increased bone mass and proved that the aptamers are indeed highly effective targeting ligands for cell type specific in vivo siRNA delivery.
Lipid-based nanocarrier delivery systems
In the late 1987 Felgner and colleagues introduced the term ‘lipofection’for a very efficient method to deliver both DNA and RNA into eukaryotic cells by using cationic lipids (65, 66). Cationic liposomes for gene delivery, the so-called lipoplexes, are simply complexes between cationic lipids and negatively charged nucleic acids. Lipoplexes are routinely used for gene transfection in vitro. Although the success of cationic liposomes in efficiently take up nucleic acids, the low silencing activity in vivo and the high systemic toxicity strongly limited their clinical applicability. The introduction of additional compounds to the lipoplexes, such as PEG-lipids, helper lipids as well as targeting moieties (transferrin, anisamide ect), has been proposed for systemic translation of the siRNA-based complex and it has been shown to improve the pharmacokinetic profile and supposedly to target the siRNA to specific tissues. Silence Therapeutics developed a novel liposomal siRNA formulation, AtuPLEX®, based on cationic lipids containing neutral fusogenic and PEG-modified lipid components to target the endothelial cells of the vascular system. In previous pre-clinical studies, they have shown a selective inhibition of both mRNA and protein levels of the targeted gene in vivo together with therapeutic efficacy, with not significant toxic effects, in xenograft mouse tumor models (67). Then, a siRNA specifically targeting the kinase N3 protein (PKN3) was formulated in the same liposomes, the so-called Atu027, for the treatment of advanced cancer (68). Atu027 uses AtuPLEX® technology to target the expression of the protein PKN3 which is involved in cancer progression and metastasis. From in vivo studies, Atu027 was able to restrict tumor growth, local invasion and both, lymph node as well as pulmonary metastasis in mouse xenograft models. A significant down-regulation of the expression of PKN3 has been shown in mice, rats and non-human primates. To date, Atu027 is being tested in a Phase IIa trial in patients with advanced solid tumors, with the aim to evaluate its safety. In patients with advanced solid tumors, the treatment with Atu027 was well tolerated and, in a significant percentage of patients (of about 50%) Atu027 caused stabilization of the disease. Interestingly, the treatment with Atu027 elicited decreases of sFLT1 (sVEGF-R1), suggesting a potential of Atu027 as a new vascular stabilizing agent (69).
Shen et al. (70) have recently proposed a multistage vector based on the 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) neutral liposomes loaded in micro-porous silicon particles to create a nanoparticle-in-microparticle delivery system for siRNA. They described the potential of silicon particles in modulating the circulation and the interaction with the tumor vascular area without using the well known enhanced permeability and retention (EPR) effect. In particular, once in circulation, the round silicon microparticles with large surface area interact with the tumor vasculature effectively where the porous silicon degrades and the incorporated nanotherapeutics get released. The sustained release of siRNA into the tumor tissues achieved a significant reduction of the tumor growth in animal model of ovarian cancer. In addition, the treatment with the multistage vector strongly sensitized the tumors to chemotherapeutic agents, such as paclitaxel (PTX), resulting in a complete regression of the tumor growth. Same group reported various studies involving successful application of EphA2 siRNA-lipid conjugates in vitro and in vivo (71–73). Their approach granted them a Phase I clinical study (NCT01591356) for advanced cancers in 2014.
In 1998 Huang and co-workers have engineered new self-assembled nanoparticles, the so-called LPD, a cationic liposomes-polycation-DNA complex. Briefly, oligonucleotide or siRNA are first mixed with a calf thymus DNA to increase the negative charge of the nucleic acids, and then complexed with protamine, a highly positively charged peptide (74). Then, the resulting complex is coated with cationic liposomes to obtain LPD. Over the years, the LPD has been upgraded by introducing PEG-lipid moiety for sterical stabilization. For tumor target therapy, LPD have been functionalized with a specific ligand, anisamide, which targets the sigma receptor overexpressed in many human cancer cells, including lung cancer cells. A significant improvement of the in vivo silencing activity of the targeted LPD has been shown in a tumor model of lung cancer (75). Moreover, from biodistribution studies, tumor targeted-LPDs were preferentially accumulated in the tumor (76). Interestingly, the same lab has recently proposed an alternative platform, LCP (lipid-calcium-phosphate NPs), a mixture of lipids and calcium phosphate NPs (77). The presence of a pH sensitive CaP core allows to incorporate and to deliver more efficiently the nucleic acids in a biocompatible, biodegradable and low cost profile (78). In addition, compared to the LPD formulation, the LCP NP releases more siRNA into the cytoplasm, leading to a significant (~40-fold in vitro and ~4-fold in vivo) improvement of the siRNA delivery. Another strongly promising platform for intravenous injection of siRNA are the stable nucleic acid lipid particles (SNALP). SNALP are, right now, one of the most potent delivery technologies for gene knockdown in the liver. Many examples of siRNA efficiently delivered by SNALPs are reported and many of them are in different stages of clinical trials. Zimmermann et al. reported that a single injection of ApoB-specific siRNAs encapsulated in SNALP dramatically knocked-down the mRNA levels of ApoB in the livers of both mice and non-human primates. Moreover, the silencing effect lasted at least 11 days (79). Significant silencing activity has been shown by using the same formulation for the delivery of siRNA targeting hepatitis B virus (HBV) (5). Tekmira (Barnaby, Canada) started in 2015 a Phase II clinical trial to evaluate the efficacy of siRNA containing SNALP for the treatment of Ebola virus (TKM-100201). In previous monkey studies, a 0.5 mg/kg dose of siRNA SNALP achieved complete protection. However, this dose was the one that in the previous clinical trial (TKM-HBV) caused idiosyncratic immune stimulations and other side effects and the last results from the Phase II trial showed a not significant therapeutic benefit of TKM-100201 in Ebola virus infected patients (TKM-Ebola-Guinea). If successful, this study will strengthen the position of SNALP as the most potent nucleic acid based therapeutics technology for gene knockdown in the liver, for Ebola treatment, and supposedly for cancer treatment.
Polymer-based nanocarriers
Although many of the polymeric carriers were initially designed for plasmid DNA delivery, their usage extended to cover siRNA delivery in the recent years. Different nanocarriers composed of mainly polymeric materials and that do not fall into lipid-based nanoparticles (such as liposomes or solid lipid nanoparticles) are included in this section. Polymeric systems for effective siRNA delivery must comply with several important factors such as having cationic charge to bind and condense the negatively charged siRNA but at the same time having low toxicity of the cationic polymer, steric stabilization for a longer time in the circulation in cases of systemic delivery and successful endocytosis followed by endosomal escape. Among the variety of polymer-based nanoparticles, recent advances with organic and inorganic synthetic delivery systems based on polyethyleneimine, cyclodextrin, dendrimer and mesoporous silica nanoparticles were summarized in here.
Polyethyleneimine
Polyethyleneimine (PEI), in both its linear and branched form, is one of the most commonly used polymers in siRNA delivery systems (80, 81). Due to its proton-accepting amino groups, cationic PEI and its derivatives have a high positive charge density that enables effective condensation of siRNA by electrostatic interactions into a more compact state, referred to as a ‘complex’ or ‘polyplex’. High molecular weight PEI is a popular gene transfection agent, both in vitro and in vivo due to its relatively high efficiency. Moreover, transfection ability is also associated with its ability to avoid lysosomal encapsulation and thus degradation of its siRNA cargo. Upon PEI mediated entry into the cell, the endosomes polymer acts as a sponge that adsorbs protons due to its high buffering capacity and primary, secondary and tertiary amine groups. This protonation causes an influx of H+ and Cl- ions and water into the endosome, and eventually leads to swelling and bursting of the endosomes because of the osmotic pressure (82–84). The escape ability of PEI from the endo/lysosomal pathway is hypothesized by the ‘proton-sponge effect’. Due to the given advantages, in recent years PEI-based siRNA delivery systems were used alone or linked to a variety of targeting moieties such as cell penetrating peptides (CPPs), folate or galactose (85–87). However, additional studies in the last years investigated further the possible intracellular trafficking mechanisms of PEI polyplexes and pointed out the possibility that the proton-sponge effect is not the dominant factor of endosomal escape (88–90). Moreover, they found for PEI polyplexes that, only a very small fraction of complexes were able to escape from endosomes (90). Recent findings indicate that endosomal escape, even for PEI polyplexes, is still a very important barrier for PEI-based siRNA delivery and optimization for more effective systems needs to be continued.
In general, high molecular weight PEIs provide high transfection efficiency but they also cause interaction with blood components and opsonization, leading to rapid clearance from the blood circulation. As a result, the PEI complexes are cleared from circulation in a few minutes and accumulate mainly in RES organs such as liver and spleen (91). Moreover, the positive charge of the high molecular weight PEI is usually linked to high non-specific toxicity (92, 93). Low molecular weight PEIs are more biocompatible but much less efficient.
To improve the balance between efficacy and toxicity of PEI, recently a series of phospholipid-modified PEI-based nanopreparations for siRNA-mediated gene silencing was introduced. Navarro et al. covalently conjugated phosphatidyl choline (PC), dipalmitoyl phosphoethanolamine (DPPE) and dioleoyl phosphoethanolamine (DOPE) to two different molecular weight PEI backbones (1.8 kDa and 25 kDa) and investigated the structure/activity relationships for the optimization of these phospholipid-PEI amphiphiles (94). They found that although the physicochemical properties and siRNA complexation capacity of conjugates were the same, their cellular interaction and silencing potency varied dramatically depending on the choice of lipidation. Modification of PEI with DOPE and DPPE produced di-block amphiphiles able to self-assemble into micellar aggregates with critical micelle concentrations of 97 μg/ml and 72 μg/ml, respectively, whereas PC-PEI showed no micellization. The micelle forming ability of DPPE-PEI and DOPE-PEI completely changed PEI’s interaction with the cell membrane and increased the cellular internalization up to 80% in the first hour of incubation with GFP-overexpressing cells. Among the conjugates, DOPE-PEI displayed a more effective GFP silencing (60%) compared to DPPE-PEI (30%) and PC-PEI (5%). Use of DOPE as a helper lipid for fusogenic functionality increased the overall efficacy of the lipid-conjugates. Moreover, the cytotoxicity of low-molecular weight PEI conjugates was 10 times less than that of 25 kDa PEIs. Using this structure/activity relationship information, Navarro et al. (95) formulated a novel siRNA carrier, micelle-like nanoparticle (MNP), based on the combination with DOPE-PEI and PEG-lipid/lipid mixtures for improved bioavailability (Figure 1). While DOPE-PEI polyplexes had positive surface charge (31±2 mV) the developed MNPs consisted of DOPE-PEI/POPC/cholesterol/PEG-PE (4:3:3:0.3 mol/mol) had neutral zeta potential due to the shielding effect of PEG and lipids. They showed that MNPs effectively delivered FAM-labeled siRNA to B16F10 cells with more than 70% siRNA-positive cells. The potential of MNPs were demonstrated by 20% GFP downregulation on C166-GFP cells without any cytotoxicity (95).
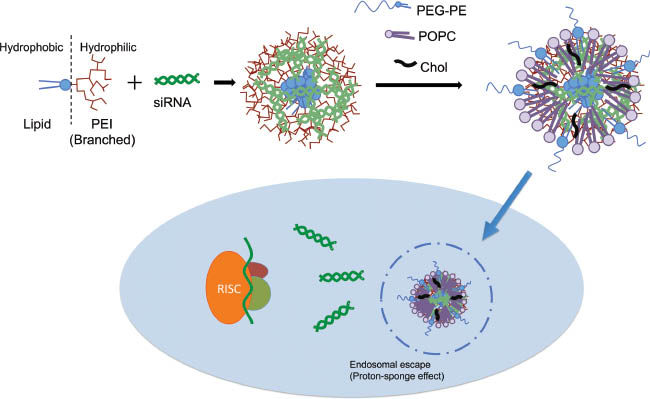
Schematic representation of RNA interference by self-assembled micelle-like nanoparticles (MNPs).
More recently, MNPs were used for therapeutic protein downregulation targeting p-glycoprotein (P-gp) (96). The presence of P-gp on the surface of resistant cells decreased after treating cells with DOPE-PEI or MNPs loaded siMDR-1. This P-gp downregulation was translated into an effective inhibition of doxorubicin (DOX) efflux activity and P-gp downregulation mediated by DOPE-PEI and MNPs significantly improved DOX toxicity in multidrug resistant cells.
Another very recent approach for modification of low molecular weight PEI with different carbon length lipids was introduced by Akinc et al. (97). They have used a synthesis method based on conjugate addition alkly-acrylates or alkyl-acrylamides to primary or secondary amines. They synthesized a total of 1200 different lipid-like siRNA delivery nanoparticles (LNP) and evaluated their efficacy using in vitro and in vivo models, including cynomolgus monkeys and created a library of these particles. Chen et al. (98) combined this combinatorial library approach with a microfluidic formulation method to produce siRNA-LNPs in a microliter scale and a high-throughput production need. They were able to create LNPs with 70 nm size, named as C12-200, which consisted of a cationic lipid from the library, cholesterol, distearoyl phosphatidylcholine and a methoxyPEG-modified lipid. The latest successful example of this approach was reported by Dhalman et al. (99) to demonstrate in vivo endothelial siRNA delivery using the 7C1 formulation. The lipid-like structure of 7C1 was synthesized by reacting C15 epoxide-terminated lipids with low molecular weight PEI (PEI600) at 14:1 molar ratio. While the conjugate itself was effective alone, like the C12-200 example, investigators preferred the MNP approach and formulated the polymer with C14PEG2000 to increase the colloidal and in vivo stability. They showed 90% and 50% of in vivo target gene expression silencing in lung endothelial cells at doses of 0.1 mg/kg and 0.02 mg/kg. The 7C1 was able to transfect endothelial cells selectively at low doses, but more importantly it did not cause gene knockdown in hepatocytes, peritoneal immune cells, pulmonary epithelial cells or pulmonary immune cells.
Cyclodextrins
Cyclodextrins (CD) are natural polymers, which can form water-soluble inclusion complexes with small and large molecules (100). CDs are short polycationic polymers (typically n=5) with amide functional groups that assemble with siRNA via electrostatic interactions (101, 102). The most important aspect of using CDs in siRNA delivery is that they are the first systemically administered siRNA in clinical trials and with direct evidence of RNAi in phase I study (103). CALAA-01 by Calando Pharmaceuticals (Pasadena, CA, USA) was developed using a linear CD-based polymer with adamantane (AD)-PEG chains for in vivo stabilization and efficacy. It incorporates a duplex of synthetic, non-chemically-modified siRNA directed against the M2 subunit of ribonucleotide reductases (RRM2), a critical component in the proliferation of cancer cells. To increase the cellular uptake that has been reduced by PEG shielding, transferrin receptor (CD71) targeting introduced to the system using AD-PEG-Tf conjugate allowing multivalent binding to the cancer cells (104). Patients with solid tumors refractory to conventional therapies received these nanoparticles systematically in two 21-day cycles. The phase I trial (NCT00689065) showed successful delivery of CD nanoparticles by biopsies and knockdown of target protein by qRT-PCR. 5′-RLM-RACE analyses were also confirmed that the delivered siRNA engages the RNAi machinery (103). This first reported clinical trial of siRNA is additionally important due to the explanation of the factors needed to be evaluated for successful delivery strategies such as low cytotoxicity of the main cationic polymer, steric stabilization for improved circulation times and protection, using a ligand for multivalent binding and effective internalization. Moreover, using imidazole end groups in the main polymer for buffering of endocytic vesicles proved the necessity of effective endosomal escape mechanisms. The trial that started in 2008 was completed in 2013, however, currently there are no active phase II clinical trials related to CALAA-01 or other cyclodextrin-based siRNA delivery systems.
In recent years, using CD in the non-viral siRNA delivery systems as combinatory component started to become more and more popular and common. Some examples of these combination structures are with PEI (105–107), poly-L-lysine (108) and dendrimers (109–111). In the last years, PEG is one the most used modification/combination option for CD nanoparticles. Direct modification of CD with PEG chains has proved to be a challenging area with most of the research focused on PEG conjugation on the primary side of CDs. Secondary side PEG modification of CDs could be performed using copper(I)-catalyzed ‘click’ chemistry to form the polar secondary side (112). O’Driscoll’s group has adopted the ABCD approach (112–114) introduced by Kostarelos and Miller (115) to prepare PEG conjugated CD polymers for developing co-formulations blended with CD:siRNA complexes. In these systems ‘A’ donates the siRNA and ‘B’ the amphiphilic CDs. ‘C’ is the stability enhancer of the formulation, which is PEG conjugated to modified 2′-hydroxyl position of β-CD by click chemistry. PEGylation significantly improved the stability of CD:siRNA formulations in high salt concentration and lowered the surface charge and reduced the cytotoxicity significantly. However, compared to non-PEGylated CD:siRNA complexes, gene silencing dramatically reduced due to the steric and charge hindrance of cationic CDs in different cell lines in vitro (112, 113) including the neuronal mHypoE N41 and non-neuronal Caco-2, but stayed significantly higher than naked siRNA. The translation of this approach to in vivo was investigated to reduce the Huntingtin (HTT) gene in Huntington’s Disease (HD) using the R6/2 mouse model. The silencing of HTT was achieved after single direct injections and after repeated brain injections, alleviation of motor deficits was observed without toxicity (116). To enhance the efficacy of these ‘ABC’ type systems, a targeting moiety was introduced by O’Mahony et al. and insertion of a cell penetrating peptide (CPP) octaarginine (R8) conjugated to PEG-DSPE resulted in a complete ‘ABCD’ delivery system (114) with 20% more cellular internalization of FAM-labeled siRNA over the non-R8 modified CD delivery system. They reported the recovered silencing effect of luciferase reporter gene by 80% knockdown and endogenous GAPDH by 40% knockdown at mass-ratio of 20 (MR20) in mHypoE N41 mouse embryonic hypothalamic cell line as a model for neuronal cells (114).
Dendrimers
Dendrimers are highly branched synthetic macromolecules that represent another promising siRNA delivery platform (117). Dendrimers depict a structure that consists of a central core molecule that acts as a root, from which a number of highly branched, tree-like arms originate in a symmetrical manner. The repetition of these symmetrical tree-like units during their synthesis is defined by the term ‘generation’. Their unique structural properties such as precisely controlled radial symmetry, tunable size, easily accessible functional groups and high cargo encapsulation in a nanometer size surely increase this potential. For better complexation with negatively charged siRNA, cationic dendrimers have found applications as successful non-viral siRNA delivery vectors. Among them poly(amidoamine) (PAMAM) and poly(propyleneimine) (PPI) dendrimers were of interest. The elucidation of the complexation mechanism of the PAMAM-siRNA dendriplex self assembly revealed that generation four (G4) and G7 dendrimers displayed equal efficiency for dendriplex formation, while G1 with much less positive charge density, lacked the siRNA condensation (118). However, PAMAM dendrimers demonstrated immunogenicity and cytotoxicity associated with surface charge (119, 120). To avoid these disadvantages, Minko et al. introduced internally cationic dendrimers, where the system has a neutral surface for low cytotoxicity while the internal cationic charge of the dendrimer allows siRNA complexation (120, 121). Established strategies such as PEGylation to extend circulation time and attachment of targeting peptides at the distal ends of PEG to increase the specificity could also be performed effectively (122, 123). PEG is also served as a di-functional or mono-functional core for dendrimers (124, 125) and very recently cationic dendrimers with 4-arm PEG-core were synthesized using an accelerated AB2/CD2 dendritic growth approach by Albertazzi et al. (126) for DNA transfection with promising results and approach for translation into siRNA delivery.
In recent years, PAMAM dendrimers gained attention for siRNA delivery to the lung; an application that mostly faced important challenges based on oral inhalation device compatibility. Conti et al. (127) developed 4th-generation PAMAM dendrimer-siRNA dendriplexes and incorporated them in chitosan-g-lactic acid or mannitol microparticles for the preparation of hydrofluoroalkane (HFA)-based pressurized metered-dose inhalers (pMDI). They reported that siRNA formulated as dendriplexes in pMDI was stable and intact during the particle formation process and even after long exposure times to HFA. Respirable fractions up to 77% and 85% recovery of total siRNA with chitosan-based formulation indicates that dendrimers could be used for developing pMDIs to deliver siRNA to deep parts of the lungs.
The versatility of the dendrimers can be increased by surface modification using ligands such as CPPs, for better intracellular siRNA delivery by enhancing the cellular uptake. Liu et al. developed arginine-terminated generation-four PAMAM dendrimers for enhanced cellular uptake of siRNA to silence heat shock protein 27 (Hsp27) in various cell lines including prostate cancer PC-3 and C4-2 cells as well as breast cancer MDA-MB-231 cells (128, 129). More interestingly, Zeng et al. designed a biodegradable and multifunctional dendronized polypeptide (denpol) platform that combines the multivalency of dendrimers and the conformational flexibility of linear polymers. While the aromatic resides in the polymer enhanced the cellular uptake, the buffering capacity of histidine facilitated the endosomal escape for successful siRNA delivery (130).
Recently Biswas et al. developed a triphenylphophonium (TPP)-conjugated, acetylated, fluorescently-labeled generation-five PAMAM dendrimer (131). Neutralization of the surface charge of the dendrimer by acetylation prevented the non-specific interaction and binding of the molecule with cell organelles and membrane, and thus reduced the cytotoxicity while the fluorescent labeling allowed researchers to follow the dendrimers intracellularly. The cation TPP has lipophilicity due to the presence of three phenyl groups and a stable delocalized positive charge, properties that give it the targeting ability to mitochondria (132). The association of two TPP molecules on the surface of a single dendrimer helped them to reach the mitochondria of HeLa cells compared to parent and acetylated dendrimer, confirmed by confocal laser scanning microscopy.
The unique micellar-dendrimer system, where a newly synthesized construct, a PEG-DOPE modified generation-four PAMAM dendrimer (G(4)-PAMAM-PEG2k-DOPE) (MD) was utilized to prepare a 1:1 mixed micellar formulation with PEG5k-PE (MDM) (133). It was shown that MD and MDM delivered significantly higher amounts of Cy3-labeled GAPDH siRNA than the parent G(4) dendrimers (G(4)-D), approx. 3 and 2-fold, respectively. The GFP silencing was approx. 10% with the G(4)-D, 22% for MD and 18% for MDM. Lipid modification of the dendrimer surface resulted in higher cellular association and facilitated the cell membrane penetration of the system. Moreover, MDM proved superior properties for MDR1 siRNA and anticancer drug loading confirmed by the higher co-delivery of DOX and siRNA into the cancer cells simultaneously, which makes the system a true multifunctional nanocarrier for siRNAs.
Successful in vitro results obtained with dendrimers were put to the challenge for in vivo siRNA delivery by many groups. One of the recent examples published by Zheng et al. (134) described the G5 PAMAM dendrimer-modified selenium nanoparticles (G5Se) for P-gp downregulation in cisplatin (DDP) resistant A549/DDP tumors. Nude mice bearing A549/DDP tumors were injected with G5Se loaded with mdr1 siRNA and cisplatin (G5Se-DDP-siRNA) daily for 2 weeks. The authors showed that the G5Se dendrimers increased the endosomal escape efficiency compared to positively charged G5-NH2 dendrimers without selenium at the cellular level and yielded significantly higher downregulation of cyclin D1, c-myc and P-Akt. The dendrimers containing both DDP and siRNA suppressed the tumor volume effectively in vivo compared to only DDP containing dendrimers, without any apparent toxicity.
Finlay et al. targeted the in vivo breast cancer cell metastasis by downregulating TWIST1, a transcription factor activates the epithelial-mesenchymal transition (EMT), using third generation amphiphilic PAMAM dendrimer YTZ3-15 complexed with TWIST1 siRNA (135). The formulation was able to accumulate and remain in the nude mice xenograft orthotopic TNBC tumors, however, in vivo silencing of the target proteins was not investigated. Rajasekaran et al. (136) developed PEGylated G5 PAMAM dendrimers complexed with astrocyte elevated gene-1 (AEG-1) siRNA and loaded with all-trans retinoic acid (ATRA) to counteract hepatocellular carcinoma (HCC). Further modification of distant PEG chains on the dendrimers with lactobionic acid (Gal) allowed the formulation to interact with asialoglycoprotien receptors that are overexpressed in liver hepatocytes. Athymic nude mice implanted with QGY-7703 cells treated with ATRA resulted in low efficacy due to high AEG-1 levels. Dendrimers delivering AEG-1 siRNA caused significant decrease in AEG-1 levels in vivo. Mice treated with Gal-PEG-PAMAM dendrimers delivering siRNA and ATRA simultaneously showed increased necrosis, inhibition of proliferation and increased apoptosis. The study marks the importance of successful in vivo siRNA delivery by dendrimers by increasing the efficacy of an approved anticancer agent.
Mesoporous silica nanoparticles
Among various inorganic nanoparticles that have been used for siRNA delivery such as carbon nanotubes (137–142), iron oxide (143–146), quantum dots (147–150) and gold (32, 151–155), mesoporous silica nanoparticles (MSN) emerged as a very promising choice in very recent years. Even they have and still do draw attention as efficient drug carriers in the early development years (156–160), the advantages like large surface area, ordered pore structures, tunable pore size and volume, easy surface modification and ability to encapsulate both small and large molecules make them an excellent candidate for effective siRNA delivery platform (161). In the scope of this review, recent advances related to MSN-based siRNA delivery is summarized in this section.
The initial strategy for negatively charged siRNA loading/complexation to MSNs involves coating or modification of the MSN surface with polycationic polymers such as poly-L-lysine and PEI (162) followed by a charge driven adsorption of siRNA on the particle surface. Hom et al. developed MSNs with surface conjugated PEI at a nanoparticle:siRNA ratio of 1:25 by mass and reported better GFP silencing compared to Lipofectamine 2000 and significant down-regulation of Akt by Western blotting (163). Bhattarai et al. (164) used the well-established PEGylation and surface modification with polycation followed by siRNA complexation on the surface of the particles. They reported low cytotoxicity of the particles and high RNA interference but also the loss of mesoporous structure after incubation in cell culture media. The adsorption/complexation of siRNA on the surface of MSNs indeed limit MSNs advantages over non-porous nanoparticle-based systems that uses the same loading strategy by depleting the surface amino groups and preventing further modifications. With this in mind, in the last years several groups developed MSNs, which encapsulates siRNA in the mesopores. If the MSNs pore size is bigger than the siRNA diameter (~2.6 nm), siRNA molecules could enter the pores if they adopt the necessary conformation (165). The shape of both the pores and siRNA is cylindrical so if the inner surface of the mesopores could be modified with positive charge, the interaction area would be much more higher than the surface adsorption. Moreover, after siRNA encapsulation the pores could be capped with PEI, which facilitates the cellular internalization and endosomal escape of the system. It was shown that if the ultra-large pore sizes are used, siRNA encapsulation increases significantly without any cytotoxicity of the system. Na et al. exploited this hypothesis and created MSNs with 23 nm pore sizes and encapsulated 5800 siRNA molecule per particle (166) to obtain significant VEGF downregulation in vivo. However, without any steric stabilization and tendency to aggregate, the system was injected intratumorally instead of systematically. Successful in vivo attempts have been reported very recently by different groups using modifications that allow the systemic administration such as magnetic MSNs that have PEI coating and fusogenic peptide (KALA) on the surface of MSNs (167) to silence GFP in vivo. In vivo therapeutic siRNA (PKM2) silencing with cyclodextrin and PEI functionalized MSNs were also reported the year previous to this review with very promising results (105).
Co-delivery of siRNA and anticancer drugs
The combination therapies act generally in two ways; one agent may increase the action of another drug or two drugs may combine to exert effects that are distinct from either individual compound (168). In general, single drug therapy is typically not effective in cancer treatment and thus, combination therapy has found a wide usage in this area. Even though combination chemotherapy regimens were developed during the mid-19th century, after the development of RNA interference technology, new windows are opened in this area. Combination of siRNA with anticancer small molecules benefit both individual active substances. Tumors are highly prone to genetic mutations, which may hinder the effectiveness of siRNA as a single agent in the treatment of malignancies. Moreover, conventional anticancer agents also suffer from limitations like multidrug resistance (MDR), which hampers cancer therapy significantly. There are number of studies reporting that the pre-treatment of cancer cells with siRNAs followed by conventional anticancer small molecules could sensitize/re-sensitize the cells towards the drugs and enhance the efficacy of treatment (96, 169–171). However, they can be considered as early attempts and strategies for siRNA and small molecule combination therapy. To gain the maximum effect from both siRNA and drug in vivo, they must be delivered simultaneously to the same cancer cell following systemic administration for maximal cooperation. Here, we summarize the recent developments and approaches related to siRNA and small molecule combination therapy for cancer treatment involving co-incorporated nanocarrier systems.
Several studies were reported for P-gp specific siRNA and anticancer drug combinations in the past few years using different nanocarriers. Meng et al. (172) used MSNs to incorporate DOX and P-gp siRNA simultaneously to overcome drug resistance in squamous carcinoma cell line KB-V1, which has >1000 times higher expression of P-gp compared to its drug sensitive counterpart KB-31. They encapsulated positively charged DOX into the negatively charged 2–2.5 nm diameter pores’ inner surface and modified the outer surface of MSNs (100–120 nm size) with different molecular weight PEI for siRNA complexation. They reported full complexation of siRNA at N/P ratios of 80, 80 and 10 for PEI 1.8 kDa, PEI 10 kDa and PEI 25 kDa, respectively. While the MSN formulation with 1.8 kDa PEI was not effective for P-gp siRNA delivery and knockdown, the 25 kDa PEI formulation was considerably cytotoxic due to proton sequestration by unsaturated PEI amines in the lysosomal compartment. The DOX loading into the inner pores did not alter the siRNA loading and PEI coating did not affect the DOX release either. They confirmed by confocal microscopy that while the cellular internalization of DOX is increased significantly by PEI-coated MSNs over non-coated MSNs, its nuclear localization stayed the same. Only siRNA-DOX co-loaded MSN formulation resulted in significantly higher nuclear DOX localization and thus, lower IC50 values (~10 μg/ml). However, the authors could not overcome resistance and restore the sensitivity to the level in KB-31 cells (~0.2 μg/ml).
More recently, the same group adopted a systematic approach and silenced different targets by siRNA including P-gp, MRP1, ABCG2, Bcl-2, cMyc and PXR (173) to define the optimal siRNA to be delivered together with DOX. They found that P-gp knockdown resulted in highest DOX cytotoxicity at siRNA dose range of 0.002–1 μg/ml and DOX dose of 0.0066–3.3 μg/ml, with siRNA:MSN mass ratio of 1:100. To demonstrate the in vivo efficacy of the MSN formulations, they modified the surface of MSNs with the PEI-PEG copolymer with 1.8 kDa PEI and 5 kDa PEG. At a dose of 4 mg/kg DOX and 1.2 mg/kg siRNA every 3–6 days for 30 days, P-gp siRNA-DOX co-loaded MSN showed significantly higher tumor growth inhibition (up to 80%) over free DOX (62%) or MSN loaded with DOX only (59%), due to the synergistic effect. Moreover, PEI-PEG modified MSN showed decreased MPS uptake. Li et al. (174) reported L-Arg or L-His coupled β-CD modified CdSe/ZnSe quantum dots to simultaneously delivery DOX and MDR-1 siRNA for multidrug resistance reversal of HeLa cells. Co-delivery of siRNA and DOX was confirmed by confocal microscopy as well as TEM imaging. Seventy-two hours after treatment of the MDR HeLa cells P-gp levels were significantly reduced due to successful siRNA delivery and enhanced accumulation of DOX resulted in enhanced apoptosis levels.
Another important target for RNA interference is Bcl-2, an anti-apoptotic protein that is over-expressed in MDR cancer cells (175, 176). Cheng et al. studied Bcl-2 and DOX co-delivery in a rat model with an in situ C6 glioma implant (177). The nanocarrier system consisted of electrostatic folic acid (FA) conjugated poly(ethylene glycol)-block-poly(glutamic acid) (FA-PEG-PGA) coating on the surface of cationic poly(etyhleneimine)-block-poly(ε-caprolactone) (PEI-PCL) that is preloaded with DOX and siRNA. This targeted co-delivery system induced significant apoptosis in vitro. In the animal studies folate-targeted co-delivery caused significant downregulation of Bcl-2 and also up-regulated the pro-apoptotic Bax gene. The synergistic effect of DOX resulted in effective tumor growth inhibition and prolonged survival time over treatment with non-targeted or single agent loaded system. This system was further optimized by Zou et al. using PEG-PEI-PCL which resulted in better stability properties due to covalent binding of PEG on the surface rather than electrostatic interaction (178).
In recent years, star-shaped co-polymers consisting of a CD core and cationic arms have attracted attention due to their ability to co-load small molecules and siRNA and rose as an alternative system to micellar nanocarriers. They include a CD core (α, β or γ) and poly(amidoamine) dendron arms (179, 180), poly(glycidyl methacrylate) derivative arms (181) and non-targeted (182) or folic acid targeted (183) oligoethylenimine arms. In 2014, Lie et al. developed a copolymer with a β-CD core and poly(L-lysine) dendron arms for co-delivery of docetaxel and MMP-9 siRNA plasmid (108). The system (with a size around 125 nm) could encapsulate hydrophobic docetaxel in the CD core and positively charged arms complexed siRNA at N/P ratios 10 and higher. The system silenced the MMP-9 mRNA and protein in HNE-1 cells as confirmed by RT-PCR and Western blot, with the efficacy of 50% compared to control. At doses of 2.5 μg/ml siRNA and 0.33 μg/ml docetaxel, co-delivery of compounds resulted in significantly higher apoptosis increase and polymer itself was shown to have better blood compatibility and lower cytotoxicity compared to PEI-25 kDa.
A phase II study was recently initiated by Silenseed Ltd to evaluate the efficacy of a siRNA targeted against KRAS mutations, a driving oncogene in most of human pancreatic cancer cases, in combination with Gemcitabine or Folfirinox in patients with unresectable locally advanced pancreatic cancer (NCT01188785). The siRNA is formulated in LODER, a miniature biodegradable polymeric matrix, designed to continuously release the siRNA for 4 months within the pancreatic tumor. From previous pre-clinical studies, the so-called siG12D LODER was able to effectively induce cell death, to decrease the KRAS levels as well as halting the tumor growth of human pancreatic tumor cells and prolonged mouse survival (184). Encouraging preliminary results were recently noted from a phase I/IIa study, where a single dose of siG12D LODER together with Gemcitabine or Folfirinox, were administrated in patients with non-operable, locally advanced pancreatic cancer. A high safety profile and an inhibition of the tumor progression were observed in all the treated patients, suggesting that the combination of siG12D-LODER and chemotherapy was well tolerated.
Stimuli-responsive siRNA delivery
Stimuli-sensitive or responsive carriers can release siRNAs in response to a given stimulus that is characteristic of the area of interest. For all siRNA delivery platforms, one of the major focus is on preventing siRNA degradation from the time it is introduced in the systemic circulation until it reaches the RNAi machinery in the cytoplasm (185). This main goal can be realized by using multifunctional and stimuli-sensitive siRNA delivery systems and longevity in the circulation is one of the necessities. Although PEGylation have clear advantages on that manner, paradoxically PEGylation also causes a steric hindrance for the vectors that are used for endosomal escape and prevents them to freely interact with the endosomal/lysosomal membranes. Thus reduced interaction causes insufficient endosomal escape and eventually can decrease the intracellular delivery of the system and siRNA. This effect of PEG is known as the ‘PEG dilemma’. Most ideal peptide and protein carrier system should have the PEG coating layer for long circulation times and decreased opsonization in vivo, but this PEG layer should also dissociate from the carrier surface at the right place and time based on different abnormalities in the tumor microenvironment such as acidic pH, altered redox potential, upregulated proteins and hypoxia as stimulus (Figure 2).

Schematic representation of stimuli-sensitive approaches used for the preparation of NPs for siRNA delivery.
Matrix metalloproteinases (MMPs), especially MMP2, are known to be involved in cancer invasion, progression and metastasis (186). In a recent study, Zhu et al. (187) reported the development of MMP2 sensitive polymeric micelles for siRNA and and paclitaxel (PTX) co-delivery. They used an MMP2-sensitive self-assembling copolymer, PEG-pp-PEI-PE, consisted of branched PEI (1800 Da), DOPE and a synthetic peptide (GPLGIAGQ) for PEG (2000 Da) linkage. In the presence of MMP2 enzyme, the peptide linker between the PEG and PEI is being cleaved and this cleavage caused ‘de-shielding’ due to PEG chain removal from the rest of the polymer. Following the PEG chain removal, the remaining PEI-DOPE micelles could be internalized by the cells effectively due to exposure of the high positive charge of PEI. Moreover, after the internalization of the PEI-DOPE-siRNA complexes, both of the components of the system help endosomal escape, PEI by proton-sponge effect and DOPE by endosomal membrane destabilization. The same peptide have been used by researchers previously with both liposomes (188) and micelles (189) for MMP2-sensitive tumor targeting. PTX was loaded into the hydrophobic PE core of the polymer with 2.3 wt% incorporation efficiency and polymeric micelles were capable of forming complexes with different siRNAs and protected them from RNAse degradation. The MMP2-sensitive micelles significantly silenced the GFP in copGFP A549 cells by around 55% only after one administration. Three consecutive administrations resulted in more pronounced GFP silencing up to 65%. The survivin silencing was significantly higher in the MMP2 sensitive group in multidrug resistant A549 T24 cells. PEG-pp-PEI-PE/PTX micelles significantly increased the cytotoxicity of PTX in both drug sensitive and resistant A549 cells compared to free PTX or non-sensitive micelles prepared with an uncleavable peptide. Simultaneous co-delivery of anti-survivin siRNA and PTX was confirmed with FACS and confocal studies and resulted in a synergistic effect. The IC50 of PTX in PEG-pp-PEI-PE micelles significantly decreased to 15 nm from 96 nm for free PTX and 28 nm for only PTX loaded PEG-pp-PEI-PE micelles. Moreover, MMP2-sensitive micelles showed a 2.4-fold increase of PTX and siRNA co-internalization than that of non-sensitive micelles and about 14.4% of total cells in tumor internalized both compounds simultaneously (187).
pH-sensitive siRNA delivery nanocarriers are another class of stimuli-sensitive systems designed to enhance the effectiveness of siRNA delivery. However, there have been different approaches and thus strategies involved in pH-sensitive delivery of siRNA. The first approach involves pH-sensitive systems to exploit the low endosomal pH for enhanced endosomal escape of internalized siRNA delivery systems. For lipid-based systems it can be achieved by an amine-rich group in the cationic structure. Following their encapsulation in the endosomes-lysosomes, these cationic lipoplexes fuse with the membranes and disrupt them. In recent years, various studies adopted this approach to develop new lipids and siRNA carrier systems for enhanced pH-dependent endosomal escape. Malamas et al. synthesized new amphiphilic cationic siRNA carriers consisted of protonable amine-based ethylenediamine head group, a hydrophobic group containing two mono-unsaturated oleic acid tails and a histidine-cysteine amino acid based linker (190). They reported higher luciferase silencing in HT29 cells than lipofectamine RNAiMax with members of this new lipids from their library. Their findings suggest that the increased number of amines in the protonable head group and removal of histidine along with the increasing degree of unsaturation on the lipid tails resulted in improved siRNA delivery into the cytoplasm. The Harashima group introduced a novel pH-sensitive cationic lipid, YSK05, and incorporated this lipid in the multifunctional envelope-type nano device (MEND) (191). They found that YSK05-MEND has higher ability for endosomal escape than other MENDs containing conventional cationic lipids such as DOTAP and DODAP in the endosomal pH range, while showing no membrane disruption in the physiological pH. The PEGylated version of this carrier further demonstrated higher in vivo polo-like kinase 1 (PLK1) downregulation compared to DOTAP modified MENDs, however, the injections were intratumoral. Combining stearylated-octaarginine as cationic polycation, stearylated-octahistidine (STR-H8) as a pH responsive polycation in the MEND system, Toriyabe et al. achieved controlled siRNA to the cytoplasm (192). They used R8 and GALA, a pH-sensitive fusogenic peptide, backbone structure to prepare MENDs that shows enhanced cellular uptake and loaded STR-H8/siRNA complexes into these MENDs for decondensation of siRNA in the cytoplasm. While the above-mentioned systems focused on endosomal escape via pH-dependence, they rely first on accumulation and second on internalization of the siRNA carriers as a first step.
The second approach in the pH-sensitive siRNA delivery systems involves low pH in the tumor microenvironment as the stimulus. While these kind of multifunctional stimuli systems were successfully used earlier for mainly small molecule delivery (193, 194), recently Sawant et al. used PEI-lipid conjugate-based pH sensitive micellar nanocarriers for gene delivery (195). They incorporated PEI-DOPE in low-pH-degradable PEG-hydrazone-PE micelles. These PEGylated systems have better stability characteristics due to PEG shielding but also stimuli-sensitive PEG detachment in the relatively acidic tumor microenvironment, which shows promise as a site-specific siRNA delivery applications.
Another stimuli used for siRNA delivery systems is the higher intracellular concentration of glutathione (GSH) (~2–10 mm) compared to extracellular (~2–10 μm) (196). Moreover, tumor microenvironment was found to be more reductive compared to healthy tissues (197). Musacchio et al. (198) previousy reported bio-reductive polymeric micelles for siRNA delivery based on siRNA conjugated to phosphothioethanol (PE) via disulfide linkage. Very recently, using this reversibly phospholipid modified siRNA approach; Salzano et al. (199) synthesized anti-survivin siRNA-S-S-PE conjugate by using SPDP-activated siRNA and PE-SH. After purification by a desalting column, resulted siRNA-S-S-PE conjugate incorporated in PEG2000-PE micelles encapsulating PTX. While in the non-reductive environment, the polymeric micelles and siRNA conjugate reported to be highly stable. Polymeric micelles consisted of 1:750 weight ratio of siRNA-S-S-PE/ PEG2000-PE resulted in approx. Twenty-five nanometer sized micelles with 50% siRNA and 70% PTX loading efficiency. However, in the presence of GSH, such as intracellular compartments, the disulfide linkage between the siRNA and lipid is cleaved and siRNA is released into the cytosol. The cell growth inhibition effect of survivin siRNA-S-S-PE incorporated micelles on the growth of different human cancer cell lines, including MDA-MB-231, A2780, SKOV-3 and PTX-resistant SKOV-3TR was investigated after treatment of cells with 200 nm survivin siRNA for 48 h. While formulations caused approx. 30% survivin silencing in all cell lines as confirmed by ELISA, cell growth inhibition was only achieved in drug sensitive cells and not in the drug resistant SKOV-3TR cell line. However, simultaneous delivery of PTX and survivin siRNA-S-S-PE at different ratios in polymeric micelles to SKOV-3TR cells led to a significant inhibition of cell growth compared to free PTX and survivin siRNA-only micelles. This synergistic effect was able to reverse drug resistance in an aggressive cell line in a stimuli-sensitive manner. Another report on the GSH dependent siRNA delivery system published by Zhao et al. (200) for herceptin targeted docetaxel and PLK1 co-delivery. The authors conjugated siPLK1 to vitamin E TPGS (TPGS) using the disulfide bond and further modified the system with herceptin conjugated TPGS for targeting which resulted in effective internalization and cytotoxicity against cancer cells.
Perche et al. (201) developed hypoxia-responsive siRNA nanocarrier using PEG2000, azobenzene, PEI (1.8 kDa) and DOPE where azobenzene imparts hypoxia sensitivity and specificity. The resulting polymer (PEG-azobenzene-PEI-DOPE), which is able to complex siGFP and form micelle-like structures in the aqueous environment, showed hypoxia-selective PEG detachment in hypoxic conditions. This detachment exposed the highly cationic PEI-DOPE/siRNA complexes to the cells and resulted in higher cellular internalization. Significantly higher GFP downregulation was achieved in various GFP overexpressing cancer cells under hypoxic conditions compared to normoxic conditions. Hypoxia insensitive polymer did not cause any GFP downregulation due to PEG shielding. The authors also reported enhanced spheroid penetration and siRNA delivery under hypoxic conditions using 3-D cell culture models. Moreover, in vivo efficacy and selectivity of the system was investigated using mice bearing GFP expressing B16F10 and A2780 tumors. A two-fold increase in tumor-cell-associated fluorescence intensity was observed only with rhodamine-labeled hypoxia-sensitive polymer while the hypoxia-insensitive polymer was not found to accumulate in the tumors. Substantial GFP downregulation was detected after intravenous injection hypoxia-sensitive formulation by ex vivo imaging and by flow cytometry. This study is the first of its kind with a hypoxia-activated siRNA nanocarrier achieving silencing in vivo.
Expert opinion
Here we reviewed recent siRNA delivery strategies that have proven to be successful and effective. The summarized systems exhibit a wide diversity from simple conjugates to organic and inorganic nanoparticle carriers with different size, surface charge, chemistry and preparation methods. But regardless of this overwhelming variety, some of the basic guidelines became available in recent years thanks to the increasing number of in vivo studies and clinical trials. We know that the nanoparticle-based systems should have a size range between 20 nm and 200–400 nm, PEGylation or similar surface modification is necessary for longevity in circulation and shielding of positive charge (in case of complexation-based systems) to prevent non-specific interaction and toxicity at the off-site target is required for successful systemic application. However, after delivering the siRNA to the target site, following internalization by the target cells, an additional mechanism, which grants the endo/lysosomal escape, is another must-have. The use of targeting ligands, despite the cost and regulatory hurdles associated with the targeting strategy, still is a big motivation and the benefits are undeniable (202). While some think that combining all these characteristics in one-for-all system seems utopic, the introduction and fast-paced development of multifunctional and stimuli-sensitive siRNA delivery systems hold great possibilities in an area that is so young and needs specific requirements every day (185, 203).
References
1. Zamore PD, Tuschl T, Sharp PA, Bartel DP. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell 2000; 101: 25–33.10.1016/S0092-8674(00)80620-0Search in Google Scholar
2. Martinez J, Patkaniowska A, Urlaub H, Luhrmann R, Tuschl T. Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell 2002; 110: 563–74.10.1016/S0092-8674(02)00908-XSearch in Google Scholar
3. Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001; 411: 494–8.10.1038/35078107Search in Google Scholar PubMed
4. Robb GB, Brown KM, Khurana J, Rana TM. Specific and potent RNAi in the nucleus of human cells. Nat Struct Mol Biol 2005; 12: 133–7.10.1038/nsmb886Search in Google Scholar PubMed
5. Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, Hartsough K, Machemer L, Radka S, Jadhav V, Vaish N, Zinnen S, Vargeese C, Bowman K, Shaffer CS, Jeffs LB, Judge A, MacLachlan I, Polisky B. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol 2005; 23: 1002–7.10.1038/nbt1122Search in Google Scholar PubMed
6. Niu XY, Peng ZL, Duan WQ, Wang H, Wang P. Inhibition of HPV 16 E6 oncogene expression by RNA interference in vitro and in vivo. Int J Gynecol Cancer 2006; 16: 743–51.10.1111/j.1525-1438.2006.00384.xSearch in Google Scholar PubMed
7. Halder J, Kamat AA, Landen CN, Han LY, Lutgendorf SK, Lin YG, Merritt WM, Jennings NB, Chavez-Reyes A, Coleman RL, Gershenson DM, Schmandt R, Cole SW, Lopez-Berestein G, Sood AK. Focal adhesion kinase targeting using in vivo short interfering RNA delivery in neutral liposomes for ovarian carcinoma therapy. Clin Cancer Res 2006; 12: 4916–24.10.1158/1078-0432.CCR-06-0021Search in Google Scholar PubMed PubMed Central
8. Frank-Kamenetsky M, Grefhorst A, Anderson NN, Racie TS, Bramlage B, Akinc A, Butler D, Charisse K, Dorkin R, Fan Y, Gamba-Vitalo C, Hadwiger P, Jayaraman M, John M, Jayaprakash KN, Maier M, Nechev L, Rajeev KG, Read T, Rohl I, Soutschek J, Tan P, Wong J, Wang G, Zimmermann T, de Fougerolles A, Vornlocher HP, Langer R, Anderson DG, Manoharan M, Koteliansky V, Horton JD, Fitzgerald K. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc Natl Acad Sci USA 2008; 105: 11915–20.10.1073/pnas.0805434105Search in Google Scholar PubMed PubMed Central
9. Corey DR. Chemical modification: the key to clinical application of RNA interference? J Clin Invest 2007; 117: 3615–22.10.1172/JCI33483Search in Google Scholar PubMed PubMed Central
10. DiFiglia M, Sena-Esteves M, Chase K, Sapp E, Pfister E, Sass M, Yoder J, Reeves P, Pandey RK, Rajeev KG, Manoharan M, Sah DW, Zamore PD, Aronin N. Therapeutic silencing of mutant huntingtin with siRNA attenuates striatal and cortical neuropathology and behavioral deficits. Proc Natl Acad Sci USA 2007; 104: 17204–9.10.1073/pnas.0708285104Search in Google Scholar PubMed PubMed Central
11. de Fougerolles A, Novobrantseva T. siRNA and the lung: research tool or therapeutic drug? Curr Opin Pharmacol 2008; 8: 280–5.10.1016/j.coph.2008.04.005Search in Google Scholar
12. Guo P, Coban O, Snead NM, Trebley J, Hoeprich S, Guo S, Shu Y. Engineering RNA for targeted siRNA delivery and medical application. Adv Drug Deliv Rev 2010; 62: 650–66.10.1016/j.addr.2010.03.008Search in Google Scholar
13. Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J, John M, Kesavan V, Lavine G, Pandey RK, Racie T, Rajeev KG, Röhl I, Toudjarska I, Wang G, Wuschko S, Bumcrot D, Koteliansky V, Limmer S, Manoharan M, Vornlocher HP. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature 2004; 432: 173–8.10.1038/nature03121Search in Google Scholar
14. Gao S, Dagnaes-Hansen F, Nielsen EJ, Wengel J, Besenbacher F, Howard KA, Kjems J. The effect of chemical modification and nanoparticle formulation on stability and biodistribution of siRNA in mice. Mol Ther 2009; 17: 1225–33.10.1038/mt.2009.91Search in Google Scholar
15. Iversen F, Yang C, Dagnaes-Hansen F, Schaffert DH, Kjems J, Gao S. Optimized siRNA-PEG conjugates for extended blood circulation and reduced urine excretion in mice. Theranostics 2013; 3: 201–9.10.7150/thno.5743Search in Google Scholar
16. Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci USA 1998; 95: 4607–12.10.1073/pnas.95.8.4607Search in Google Scholar
17. Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, Jain RK. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res 1995; 55: 3752–6.Search in Google Scholar
18. Torchilin VP, Trubetskoy VS. Which polymers can make nanoparticulate drug carriers long-circulating? Adv Drug Deliv Rev 1995; 16: 141–55.10.1016/0169-409X(95)00022-YSearch in Google Scholar
19. Baum C, Kustikova O, Modlich U, Li Z, Fehse B. Mutagenesis and oncogenesis by chromosomal insertion of gene transfer vectors. Hum Gene Ther 2006; 17: 253–63.10.1089/hum.2006.17.253Search in Google Scholar PubMed
20. Bessis N, GarciaCozar FJ, Boissier MC. Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Ther 2004; 11(Suppl 1): S10–7.10.1038/sj.gt.3302364Search in Google Scholar PubMed
21. Waehler R, Russell SJ, Curiel DT. Engineering targeted viral vectors for gene therapy. Nat Rev Genet 2007; 8: 573–87.10.1038/nrg2141Search in Google Scholar PubMed PubMed Central
22. Bouard D, Alazard-Dany D, Cosset FL. Viral vectors: from virology to transgene expression. Br J Pharmacol 2009; 157: 153–65.10.1038/bjp.2008.349Search in Google Scholar PubMed PubMed Central
23. Kawakami S. Development and application of glycosylated particulate carriers for delivery of nucleic acid medicine. Yakugaku Zasshi 2008; 128: 1743–9.10.1248/yakushi.128.1743Search in Google Scholar PubMed
24. White PJ. Barriers to successful delivery of short interfering RNA after systemic administration. Clin Exp Pharmacol Physiol 2008; 35: 1371–6.10.1111/j.1440-1681.2008.04992.xSearch in Google Scholar PubMed
25. Tseng YC, Huang L. Self-assembled lipid nanomedicines for siRNA tumor targeting. J Biomed Nanotechnol 2009; 5: 351–63.10.1166/jbn.2009.1044Search in Google Scholar PubMed PubMed Central
26. Peer D, Shimaoka M. Systemic siRNA delivery to leukocyte-implicated diseases. Cell cycle 2009; 8: 853–9.10.4161/cc.8.6.7936Search in Google Scholar PubMed
27. Judge AD, Robbins M, Tavakoli I, Levi J, Hu L, Fronda A, Ambegia E, McClintock K, MacLachlan I. Confirming the RNAi-mediated mechanism of action of siRNA-based cancer therapeutics in mice. J Clin Invest 2009; 119: 661–73.10.1172/JCI37515Search in Google Scholar PubMed PubMed Central
28. Tao W, Davide JP, Cai M, Zhang GJ, South VJ, Matter A, Ng B, Zhang Y, Sepp-Lorenzino L. Noninvasive imaging of lipid nanoparticle-mediated systemic delivery of small-interfering RNA to the liver. Mol Ther 2010; 18: 1657–66.10.1038/mt.2010.147Search in Google Scholar PubMed PubMed Central
29. Basha G, Novobrantseva TI, Rosin N, Tam YY, Hafez IM, Wong MK, Sugo T, Ruda VM, Qin J, Klebanov B, Ciufolini M, Akinc A, Tam YK, Hope MJ, Cullis PR. Influence of cationic lipid composition on gene silencing properties of lipid nanoparticle formulations of siRNA in antigen-presenting cells. Mol Ther 2011; 19: 2186–200.10.1038/mt.2011.190Search in Google Scholar PubMed PubMed Central
30. Rozema DB, Lewis DL, Wakefield DH, Wong SC, Klein JJ, Roesch PL, Bertin SL, Reppen TW, Chu Q, Blokhin AV, Hagstrom JE, Wolff JA. Dynamic PolyConjugates for targeted in vivo delivery of siRNA to hepatocytes. Proc Natl Acad Sci USA 2007; 104: 12982–7.10.1073/pnas.0703778104Search in Google Scholar PubMed PubMed Central
31. Kim SH, Jeong JH, Kim TI, Kim SW, Bull DA. VEGF siRNA delivery system using arginine-grafted bioreducible poly(disulfide amine). Mol Pharm 2009; 6: 718–26.10.1021/mp800161eSearch in Google Scholar PubMed PubMed Central
32. Bishop CJ, Tzeng SY, Green JJ. Degradable polymer-coated gold nanoparticles for co-delivery of DNA and siRNA. Acta Biomater 2015; 11: 393–403.10.1016/j.actbio.2014.09.020Search in Google Scholar PubMed PubMed Central
33. Krais A, Wortmann L, Hermanns L, Feliu N, Vahter M, Stucky S, Mathur S, Fadeel B. Targeted uptake of folic acid-functionalized iron oxide nanoparticles by ovarian cancer cells in the presence but not in the absence of serum. Nanomedicine 2014; 10: 1421–31.10.1016/j.nano.2014.01.006Search in Google Scholar PubMed
34. Thiel KW, Giangrande PH. Therapeutic applications of DNA and RNA aptamers. Oligonucleotides 2009; 19: 209–22.10.1089/oli.2009.0199Search in Google Scholar PubMed PubMed Central
35. Dassie JP, Liu XY, Thomas GS, Whitaker RM, Thiel KW, Stockdale KR, Meyerholz DK, McCaffrey AP, McNamara JO, Giangrande PH. Systemic administration of optimized aptamer-siRNA chimeras promotes regression of PSMA-expressing tumors. Nat Biotechnol 2009; 27: 839–49.10.1038/nbt.1560Search in Google Scholar PubMed PubMed Central
36. McNamara JO, Andrechek ER, Wang Y, Viles KD, Rempel RE, Gilboa E, Sullenger BA, Giangrande PH. Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat Biotechnol 2006; 24: 1005–15.10.1038/nbt1223Search in Google Scholar PubMed
37. Jafari M, Chen P. Peptide mediated siRNA delivery. Curr Top Med Chem 2009; 9: 1088–97.10.2174/156802609789630839Search in Google Scholar PubMed
38. Hoyer J, Neundorf I. Knockdown of a G protein-coupled receptor through efficient peptide-mediated siRNA delivery. J Control Release 2012; 161: 826–34.10.1016/j.jconrel.2012.05.017Search in Google Scholar PubMed
39. Hayashi Y, Yamauchi J, Khalil IA, Kajimoto K, Akita H, Harashima H. Cell penetrating peptide-mediated systemic siRNA delivery to the liver. Int J Pharm 2011; 419: 308–13.10.1016/j.ijpharm.2011.07.038Search in Google Scholar PubMed
40. Chen Y, Huang L. Tumor-targeted delivery of siRNA by non-viral vector: safe and effective cancer therapy. Expert Opin Drug Deliv 2008; 5: 1301–11.10.1517/17425240802568505Search in Google Scholar PubMed PubMed Central
41. Jeong JH, Mok H, Oh YK, Park TG. siRNA conjugate delivery systems. Bioconjug Chem 2009; 20: 5–14.10.1021/bc800278eSearch in Google Scholar PubMed
42. Pasqualini R, Koivunen E, Ruoslahti E. α v Integrins as receptors for tumor targeting by circulating ligands. Nat Biotechnol 1997; 15: 542–6.10.1038/nbt0697-542Search in Google Scholar PubMed
43. Liu X, Wang W, Samarsky D, Liu L, Xu Q, Zhang W, Zhu G, Wu P, Zuo X, Deng H, Zhang J, Wu Z, Chen X, Zhao L, Qiu Z, Zhang Z, Zeng Q, Yang W, Zhang B, Ji A. Tumor-targeted in vivo gene silencing via systemic delivery of cRGD-conjugated siRNA. Nucleic Acids Res 2014; 42: 11805–17.10.1093/nar/gku831Search in Google Scholar PubMed PubMed Central
44. Kubo T, Yanagihara K, Sato Y, Nishimura Y, Kondo S, Seyama T. Gene-silencing potency of symmetric and asymmetric lipid-conjugated siRNAs and its correlation with dicer recognition. Bioconjug Chem 2013; 24: 2045–57.10.1021/bc400391nSearch in Google Scholar PubMed
45. Nimjee SM, Rusconi CP, Sullenger BA. Aptamers: an emerging class of therapeutics. Annu Rev Med 2005; 56: 555–83.10.1146/annurev.med.56.062904.144915Search in Google Scholar PubMed
46. Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990; 249: 505–10.10.1126/science.2200121Search in Google Scholar PubMed
47. Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature 1990; 346: 818–22.10.1038/346818a0Search in Google Scholar PubMed
48. Jenison RD, Gill SC, Pardi A, Polisky B. High-resolution molecular discrimination by RNA. Science 1994; 263: 1425–9.10.1126/science.7510417Search in Google Scholar PubMed
49. Balamurugan S, Obubuafo A, Soper SA, Spivak DA. Surface immobilization methods for aptamer diagnostic applications. Anal Bioanal Chem 2008; 390: 1009–21.10.1007/s00216-007-1587-2Search in Google Scholar PubMed
50. Ng EW, Shima DT, Calias P, Cunningham ET Jr, Guyer DR, Adamis AP. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov 2006; 5: 123–32.10.1038/nrd1955Search in Google Scholar PubMed
51. Bates PJ, Laber DA, Miller DM, Thomas SD, Trent JO. Discovery and development of the G-rich oligonucleotide AS1411 as a novel treatment for cancer. Exp Mol Pathol 2009; 86: 151–64.10.1016/j.yexmp.2009.01.004Search in Google Scholar PubMed PubMed Central
52. Wang CW, Chung WH, Cheng YF, Ying NW, Peck K, Chen YT, Hung SI. A new nucleic acid-based agent inhibits cytotoxic T lymphocyte-mediated immune disorders. The J Allergy Clin Immunol 2013; 132: 713–22 e11.10.1016/j.jaci.2013.04.036Search in Google Scholar PubMed
53. Wheeler LA, Vrbanac V, Trifonova R, Brehm MA, Gilboa-Geffen A, Tanno S, Greiner DL, Luster AD, Tager AM, Lieberman J. Durable knockdown and protection from HIV transmission in humanized mice treated with gel-formulated CD4 aptamer-siRNA chimeras. Mol Ther 2013; 21: 1378–89.10.1038/mt.2013.77Search in Google Scholar PubMed PubMed Central
54. Wheeler LA, Trifonova R, Vrbanac V, Basar E, McKernan S, Xu Z, Seung E, Deruaz M, Dudek T, Einarsson JI, Yang L, Allen TM, Luster AD, Tager AM, Dykxhoorn DM, Lieberman J. Inhibition of HIV transmission in human cervicovaginal explants and humanized mice using CD4 aptamer-siRNA chimeras. J Clin Invest 2011; 121: 2401–12.10.1172/JCI45876Search in Google Scholar PubMed PubMed Central
55. Hussain AF, Tur MK, Barth S. An aptamer-siRNA chimera silences the eukaryotic elongation factor 2 gene and induces apoptosis in cancers expressing αvβ3 integrin. Nucleic Acid Ther 2013; 23: 203–12.10.1089/nat.2012.0408Search in Google Scholar PubMed
56. Zhou J, Tiemann K, Chomchan P, Alluin J, Swiderski P, Burnett J, Zhang X, Forman S, Chen R, Rossi J. Dual functional BAFF receptor aptamers inhibit ligand-induced proliferation and deliver siRNAs to NHL cells. Nucleic Acids Res 2013; 41: 4266–83.10.1093/nar/gkt125Search in Google Scholar PubMed PubMed Central
57. Pastor F, Kolonias D, Giangrande PH, Gilboa E. Induction of tumour immunity by targeted inhibition of nonsense-mediated mRNA decay. Nature 2010; 465: 227–30.10.1038/nature08999Search in Google Scholar PubMed PubMed Central
58. Lai WY, Wang WY, Chang YC, Chang CJ, Yang PC, Peck K. Synergistic inhibition of lung cancer cell invasion, tumor growth and angiogenesis using aptamer-siRNA chimeras. Biomaterials 2014; 35: 2905–14.10.1016/j.biomaterials.2013.12.054Search in Google Scholar PubMed
59. Li L, Hou J, Liu X, Guo Y, Wu Y, Zhang L, Yang Z. Nucleolin-targeting liposomes guided by aptamer AS1411 for the delivery of siRNA for the treatment of malignant melanomas. Biomaterials 2014; 35: 3840–50.10.1016/j.biomaterials.2014.01.019Search in Google Scholar PubMed
60. Liang C, Guo B, Wu H, Shao N, Li D, Liu J, Dang L, Wang C, Li H, Li S, Lau WK, Cao Y, Yang Z, Lu C, He X, Au DW, Pan X, Zhang BT, Lu C, Zhang H, Yue K, Qian A, Shang P, Xu J, Xiao L, Bian Z, Tan W, Liang Z, He F, Zhang L, Lu A, Zhang G. Aptamer-functionalized lipid nanoparticles targeting osteoblasts as a novel RNA interference-based bone anabolic strategy. Nat Med 2015; 21: 288–94.10.1038/nm.3791Search in Google Scholar PubMed PubMed Central
61. Herrmann A, Priceman SJ, Swiderski P, Kujawski M, Xin H, Cherryholmes GA, Zhang W, Zhang C, Lahtz C, Kowolik C, Forman SJ, Kortylewski M, Yu H. CTLA4 aptamer delivers STAT3 siRNA to tumor-associated and malignant T cells. J Clin Invest 2015; 125: 2547.10.1172/JCI82555Search in Google Scholar PubMed PubMed Central
62. Zhou J, Neff CP, Swiderski P, Li H, Smith DD, Aboellail T, Remling-Mulder L, Akkina R, Rossi JJ. Functional in vivo delivery of multiplexed anti-HIV-1 siRNAs via a chemically synthesized aptamer with a sticky bridge. Mol Ther 2013; 21: 192–200.10.1038/mt.2012.226Search in Google Scholar PubMed PubMed Central
63. Berezhnoy A, Castro I, Levay A, Malek TR, Gilboa E. Aptamer-targeted inhibition of mTOR in T cells enhances antitumor immunity. J Clin Invest 2014; 124: 188–97.10.1172/JCI69856Search in Google Scholar PubMed PubMed Central
64. Gilboa-Geffen A, Hamar P, Le MT, Wheeler LA, Trifonova R, Petrocca F, Wittrup A, Lieberman J. Gene knockdown by EpCAM aptamer-siRNA chimeras suppresses epithelial breast cancers and their tumor-initiating cells. Mol Cancer Ther 2015; 14: 2279–91.10.1158/1535-7163.MCT-15-0201-TSearch in Google Scholar PubMed
65. Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, Northrop JP, Ringold GM, Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci USA 1987; 84: 7413–7.10.1073/pnas.84.21.7413Search in Google Scholar PubMed PubMed Central
66. Malone RW, Felgner PL, Verma IM. Cationic liposome-mediated RNA transfection. Proc Natl Acad Sci USA 1989; 86: 6077–81.10.1073/pnas.86.16.6077Search in Google Scholar PubMed PubMed Central
67. Aleku M, Schulz P, Keil O, Santel A, Schaeper U, Dieckhoff B, Janke O, Endruschat J, Durieux B, Röder N, Löffler K, Lange C, Fechtner M, Möpert K, Fisch G, Dames S, Arnold W, Jochims K, Giese K, Wiedenmann B, Scholz A, Kaufmann J. Atu027, a liposomal small interfering RNA formulation targeting protein kinase N3, inhibits cancer progression. Cancer Res 2008; 68: 9788–98.10.1158/0008-5472.CAN-08-2428Search in Google Scholar PubMed
68. Santel A, Aleku M, Keil O, Endruschat J, Esche V, Fisch G, Dames S, Löffler K, Fechtner M, Arnold W, Giese K, Klippel A, Kaufmann J. A novel siRNA-lipoplex technology for RNA interference in the mouse vascular endothelium. Gene Ther 2006; 13: 1222–34.10.1038/sj.gt.3302777Search in Google Scholar PubMed
69. Schultheis B, Strumberg D, Santel A, Vank C, Gebhardt F, Keil O, Lange C, Giese K, Kaufmann J, Khan M, Drevs J. First-in-human phase I study of the liposomal RNA interference therapeutic Atu027 in patients with advanced solid tumors. J Clin Oncol 2014; 32: 4141–8.10.1200/JCO.2013.55.0376Search in Google Scholar PubMed
70. Shen H, Rodriguez-Aguayo C, Xu R, Gonzalez-Villasana V, Mai J, Huang Y, Zhang G, Guo X, Bai L, Qin G, Deng X, Li Q, Erm DR, Aslan B, Liu X, Sakamoto J, Chavez-Reyes A, Han HD, Sood AK, Ferrari M, Lopez-Berestein G. Enhancing chemotherapy response with sustained EphA2 silencing using multistage vector delivery. Clin Cancer Res 2013; 19: 1806–15.10.1158/1078-0432.CCR-12-2764Search in Google Scholar PubMed PubMed Central
71. Tanaka T, Mangala LS, Vivas-Mejia PE, Nieves-Alicea R, Mann AP, Mora E, Han HD, Shahzad MM, Liu X, Bhavane R, Gu J, Fakhoury JR, Chiappini C, Lu C, Matsuo K, Godin B, Stone RL, Nick AM, Lopez-Berestein G, Sood AK, Ferrari M. Sustained small interfering RNA delivery by mesoporous silicon particles. Cancer Res 2010; 70: 3687–96.10.1158/0008-5472.CAN-09-3931Search in Google Scholar PubMed PubMed Central
72. Shahzad MM, Lu C, Lee JW, Stone RL, Mitra R, Mangala LS, Lu Y, Baggerly KA, Danes CG, Nick AM, Halder J, Kim HS, Vivas-Mejia P, Landen CN, Lopez-Berestein G, Coleman RL, Sood AK. Dual targeting of EphA2 and FAK in ovarian carcinoma. Cancer Biol Ther 2009; 8: 1027–34.10.4161/cbt.8.11.8523Search in Google Scholar PubMed PubMed Central
73. Landen CN Jr, Chavez-Reyes A, Bucana C, Schmandt R, Deavers MT, Lopez-Berestein G, Sood AK. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res 2005; 65: 6910–8.10.1158/0008-5472.CAN-05-0530Search in Google Scholar PubMed
74. Tan Y, Li S, Pitt BR, Huang L. The inhibitory role of CpG immunostimulatory motifs in cationic lipid vector-mediated transgene expression in vivo. Hum Gene Ther 1999; 10: 2153–61.10.1089/10430349950017149Search in Google Scholar PubMed
75. Chen Y, Sen J, Bathula SR, Yang Q, Fittipaldi R, Huang L. Novel cationic lipid that delivers siRNA and enhances therapeutic effect in lung cancer cells. Mol Pharm 2009; 6: 696–705.10.1021/mp800136vSearch in Google Scholar PubMed PubMed Central
76. Li SD, Huang L. Nanoparticles evading the reticuloendothelial system: role of the supported bilayer. Biochim Biophys Acta 2009; 1788: 2259–66.10.1016/j.bbamem.2009.06.022Search in Google Scholar PubMed PubMed Central
77. Yang Y, Li J, Liu F, Huang L. Systemic delivery of siRNA via LCP nanoparticle efficiently inhibits lung metastasis. Mol Ther 2012; 20: 609–15.10.1038/mt.2011.270Search in Google Scholar PubMed PubMed Central
78. Cheng X, Kuhn L. Chemotherapy drug delivery from calcium phosphate nanoparticles. Int J Nanomedicine 2007; 2: 667–74.Search in Google Scholar
79. Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, Harborth J, Heyes JA, Jeffs LB, John M, Judge AD, Lam K, McClintock K, Nechev LV, Palmer LR, Racie T, Röhl I, Seiffert S, Shanmugam S, Sood V, Soutschek J, Toudjarska I, Wheat AJ, Yaworski Ed, Zedalis W, Koteliansky V, Manoharan M, Vornlocher HP, MacLachlan I. RNAi-mediated gene silencing in non-human primates. Nature 2006; 441: 111–4.10.1038/nature04688Search in Google Scholar PubMed
80. Yuan X, Naguib S, Wu Z. Recent advances of siRNA delivery by nanoparticles. Expert Opin Drug Deliv 2011; 8: 521–36.10.1517/17425247.2011.559223Search in Google Scholar PubMed
81. Wu ZW, Chien CT, Liu CY, Yan JY, Lin SY. Recent progress in copolymer-mediated siRNA delivery. J Drug Target 2012; 20: 551–60.10.3109/1061186X.2012.699057Search in Google Scholar PubMed
82. Akinc A, Thomas M, Klibanov AM, Langer R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J Gene Med 2005; 7: 657–63.10.1002/jgm.696Search in Google Scholar PubMed
83. Cho YW, Kim JD, Park K. Polycation gene delivery systems: escape from endosomes to cytosol. J Pharm Pharmacol 2003; 55: 721–34.10.1211/002235703765951311Search in Google Scholar PubMed
84. Behr J-P. The proton sponge: a trick to enter cells the viruses did not exploit. CHIMIA International Journal for Chemistry 1997; 51: 34–6.10.2533/chimia.1997.34Search in Google Scholar
85. Hobel S, Koburger I, John M, Czubayko F, Hadwiger P, Vornlocher HP, Aigner A. Polyethylenimine/small interfering RNA-mediated knockdown of vascular endothelial growth factor in vivo exerts anti-tumor effects synergistically with Bevacizumab. J Gene Med 2010; 12: 287–300.Search in Google Scholar
86. Biswal BK, Debata NB, Verma RS. Development of a targeted siRNA delivery system using FOL-PEG-PEI conjugate. Mol Biol Rep 2010; 37: 2919–26.10.1007/s11033-009-9853-3Search in Google Scholar PubMed
87. Nie C, Liu C, Chen G, Dai J, Li H, Shuai X. Hepatocyte-targeted psiRNA delivery mediated by galactosylated poly(ethylene glycol)-graft-polyethylenimine in vitro. J Biomater Appl 2011; 26: 255–75.10.1177/0885328210364678Search in Google Scholar PubMed
88. Benjaminsen RV, Mattebjerg MA, Henriksen JR, Moghimi SM, Andresen TL. The possible “proton sponge” effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol Ther 2013; 21: 149–57.10.1038/mt.2012.185Search in Google Scholar PubMed PubMed Central
89. Yue Y, Jin F, Deng R, Cai J, Dai Z, Lin MC, Kung HF, Mattebjerg MA, Andresen TL, Wu C. Revisit complexation between DNA and polyethylenimine–effect of length of free polycationic chains on gene transfection. J Control Release 2011; 152: 143–51.10.1016/j.jconrel.2011.03.020Search in Google Scholar PubMed
90. ur Rehman Z, Hoekstra D, Zuhorn IS. Mechanism of polyplex- and lipoplex-mediated delivery of nucleic acids: real-time visualization of transient membrane destabilization without endosomal lysis. ACS Nano 2013; 7: 3767–77.10.1021/nn3049494Search in Google Scholar PubMed
91. Neu M, Fischer D, Kissel T. Recent advances in rational gene transfer vector design based on poly(ethylene imine) and its derivatives. J Gene Med 2005; 7: 992–1009.10.1002/jgm.773Search in Google Scholar PubMed
92. Thomas M, Klibanov AM. Enhancing polyethylenimine’s delivery of plasmid DNA into mammalian cells. Proc Natl Acad Sci USA 2002; 99: 14640–5.10.1073/pnas.192581499Search in Google Scholar PubMed PubMed Central
93. Thomas M, Ge Q, Lu JJ, Chen JZ, Klibanov AM. Cross-linked small polyethylenimines: While still nontoxic, deliver DNA efficiently to mammalian cells in vitro and in vivo. Pharmaceut Res 2005; 22: 373–80.10.1007/s11095-004-1874-ySearch in Google Scholar PubMed PubMed Central
94. Navarro G, Essex S, Sawant RR, Biswas S, Nagesha D, Sridhar S, de ILarduya CT5, Torchilin VP. Phospholipid-modified polyethylenimine-based nanopreparations for siRNA-mediated gene silencing: implications for transfection and the role of lipid components. Nanomedicine 2014; 10: 411–9.10.1016/j.nano.2013.07.016Search in Google Scholar PubMed PubMed Central
95. Navarro G, Sawant RR, Essex S, Tros de Ilarduya C, Torchilin VP. Phospholipid-polyethylenimine conjugate-based micelle-like nanoparticles for siRNA delivery. Drug Deliv Transl Res 2011; 1: 25–33.10.1007/s13346-010-0004-0Search in Google Scholar PubMed PubMed Central
96. Navarro G, Sawant RR, Biswas S, Essex S, Tros de Ilarduya C, Torchilin VP. P-glycoprotein silencing with siRNA delivered by DOPE-modified PEI overcomes doxorubicin resistance in breast cancer cells. Nanomedicine (Lond) 2012; 7: 65–78.10.2217/nnm.11.93Search in Google Scholar PubMed PubMed Central
97. Akinc A, Zumbuehl A, Goldberg M, Leshchiner ES, Busini V, Hossain N, Bacallado SA, Nguyen DN, Fuller J, Alvarez R, Borodovsky A, Borland t, Constien r, Fougerolles ad, Dorkin JR, Jayaprakash KN, Jayaraman M, John M, Koteliansky V. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol 2008; 26: 561–9.10.1038/nbt1402Search in Google Scholar PubMed PubMed Central
98. Chen D, Love KT, Chen Y, Eltoukhy AA, Kastrup C, Sahay G, Jeon A, Dong Y, Whitehead KA, Anderson DG. Rapid discovery of potent siRNA-containing lipid nanoparticles enabled by controlled microfluidic formulation. J Am Chem Soc 2012; 134: 6948–51.10.1021/ja301621zSearch in Google Scholar PubMed
99. Dahlman JE, Barnes C, Khan OF, Thiriot A, Jhunjunwala S, Shaw TE, Xing Y, Sager HB, Sahay G, Speciner L, Bader A, Bogorad RL, Yin H, Racie T, Dong Y, Jiang S, Seedorf D, Dave A, Singh Sandhu K, Webber MJ, Novobrantseva T, Ruda VM, Lytton-Jean AK, Levins CG, Kalish B, Mudge DK, Perez M, Abezgauz L. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat Nanotechnol 2014; 9: 648–55.10.1038/nnano.2014.84Search in Google Scholar PubMed PubMed Central
100. Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov 2004; 3: 1023–35.10.1038/nrd1576Search in Google Scholar PubMed
101. Gonzalez H, Hwang SJ, Davis ME. New class of polymers for the delivery of macromolecular therapeutics. Bioconjug Chem 1999; 10: 1068–74.10.1021/bc990072jSearch in Google Scholar PubMed
102. Davis ME. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol Pharm 2009; 6: 659–68.10.1021/mp900015ySearch in Google Scholar PubMed
103. Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 2010; 464: 1067–70.10.1038/nature08956Search in Google Scholar PubMed PubMed Central
104. Hu-Lieskovan S, Heidel JD, Bartlett DW, Davis ME, Triche TJ. Sequence-specific knockdown of EWS-FLI1 by targeted, nonviral delivery of small interfering RNA inhibits tumor growth in a murine model of metastatic Ewing’s sarcoma. Cancer Res 2005; 65: 8984–92.10.1158/0008-5472.CAN-05-0565Search in Google Scholar PubMed
105. Shen J, Kim HC, Su H, Wang F, Wolfram J, Kirui D, Mai J, Mu C, Ji LN, Mao ZW, Shen H. Cyclodextrin and polyethylenimine functionalized mesoporous silica nanoparticles for delivery of siRNA cancer therapeutics. Theranostics 2014; 4: 487–97.10.7150/thno.8263Search in Google Scholar PubMed PubMed Central
106. Liu X, Wang G, You Z, Qian P, Chen H, Dou Y, Wei Z, Chen Y, Mao C, Zhang J. Inhibition of hypoxia-induced proliferation of pulmonary arterial smooth muscle cells by a mTOR siRNA-loaded cyclodextrin nanovector. Biomaterials 2014; 35: 4401–16.10.1016/j.biomaterials.2014.02.009Search in Google Scholar PubMed
107. Li JM, Wang YY, Zhang W, Su H, Ji LN, Mao ZW. Low-weight polyethylenimine cross-linked 2-hydroxypopyl-β-cyclodextrin and folic acid as an efficient and nontoxic siRNA carrier for gene silencing and tumor inhibition by VEGF siRNA. Int J Nanomedicine 2013; 8: 2101–17.Search in Google Scholar
108. Liu T, Xue W, Ke B, Xie MQ, Ma D. Star-shaped cyclodextrin-poly(l-lysine) derivative co-delivering docetaxel and MMP-9 siRNA plasmid in cancer therapy. Biomaterials 2014; 35: 3865–72.10.1016/j.biomaterials.2014.01.040Search in Google Scholar PubMed
109. Motoyama K, Mitsuyasu R, Akao C, Tanaka T, Ohyama A, Sato N, Higashi T, Arima H. Design and evaluation of thioalkylated mannose-modified dendrimer (G3)/α-cyclodextrin conjugates as antigen-presenting cell-selective siRNA carriers. The AAPS J 2014; 16: 1298–308.10.1208/s12248-014-9665-9Search in Google Scholar PubMed PubMed Central
110. Anno T, Higashi T, Hayashi Y, Motoyama K, Jono H, Ando Y, Arima H. Potential use of glucuronylglucosyl-β-cyclodextrin/dendrimer conjugate (G2) as a siRNA carrier for the treatment of familial amyloidotic polyneuropathy. J Drug Target 2014; 22: 883–90.10.3109/1061186X.2014.939984Search in Google Scholar PubMed
111. Abdelwahab AF, Ohyama A, Higashi T, Motoyama K, Khaled KA, Sarhan HA, Hussein AK, Arima H. Preparation and evaluation of polyamidoamine dendrimer conjugate with glucuronylglucosyl-β-cyclodextrin (G3) as a novel carrier for siRNA. J Drug Target 2014; 22: 927–34.10.3109/1061186X.2014.950663Search in Google Scholar PubMed
112. O’Mahony AM, Ogier J, Desgranges S, Cryan JF, Darcy R, O’Driscoll CM. A click chemistry route to 2-functionalised PEGylated and cationic β-cyclodextrins: co-formulation opportunities for siRNA delivery. Org Biomol Chem 2012; 10: 4954–60.10.1039/c2ob25490eSearch in Google Scholar PubMed
113. O’Mahony AM, Ogier J, Darcy R, Cryan JF, O’Driscoll CM. Cationic and PEGylated Amphiphilic Cyclodextrins: Co-Formulation Opportunities for Neuronal Sirna Delivery. PloS one 2013; 8: e66413.10.1371/journal.pone.0066413Search in Google Scholar PubMed PubMed Central
114. O’Mahony AM, Desgranges S, Ogier J, Quinlan A, Devocelle M, Darcy R, Cryan JF, O’Driscoll CM. In vitro investigations of the efficacy of cyclodextrin-siRNA complexes modified with lipid-PEG-Octaarginine: towards a formulation strategy for non-viral neuronal siRNA delivery. Pharm Res 2013; 30: 1086–98.10.1007/s11095-012-0945-8Search in Google Scholar PubMed
115. Kostarelos K, Miller AD. Synthetic, self-assembly ABCD nanoparticles; a structural paradigm for viable synthetic non-viral vectors. Chem Soc Rev 2005; 34: 970–94.10.1039/b307062jSearch in Google Scholar PubMed
116. Godinho BM, Ogier JR, Darcy R, O’Driscoll CM, Cryan JF. Self-assembling modified β-cyclodextrin nanoparticles as neuronal siRNA delivery vectors: focus on Huntington’s disease. Mol Pharm 2013; 10: 640–9.10.1021/mp3003946Search in Google Scholar PubMed
117. Biswas S, Torchilin VP. Dendrimers for siRNA Delivery. Pharmaceuticals 2013; 6: 161–83.10.3390/ph6020161Search in Google Scholar PubMed PubMed Central
118. Jensen LB, Pavan GM, Kasimova MR, Rutherford S, Danani A, Nielsen HM, Foged C. Elucidating the molecular mechanism of PAMAM-siRNA dendriplex self-assembly: effect of dendrimer charge density. Int J Pharm 2011; 416: 410–8.10.1016/j.ijpharm.2011.03.015Search in Google Scholar PubMed
119. Singha K, Namgung R, Kim WJ. Polymers in small-interfering RNA delivery. Nucleic Acid Ther 2011; 21: 133–47.10.1089/nat.2011.0293Search in Google Scholar PubMed PubMed Central
120. Patil ML, Zhang M, Betigeri S, Taratula O, He H, Minko T. Surface-modified and internally cationic polyamidoamine dendrimers for efficient siRNA delivery. Bioconjug Chem 2008; 19: 1396–403.10.1021/bc8000722Search in Google Scholar PubMed
121. Patil ML, Zhang M, Taratula O, Garbuzenko OB, He H, Minko T. Internally cationic polyamidoamine PAMAM-OH dendrimers for siRNA delivery: effect of the degree of quaternization and cancer targeting. Biomacromolecules 2009; 10: 258–66.10.1021/bm8009973Search in Google Scholar PubMed PubMed Central
122. Waite CL, Roth CM. PAMAM-RGD conjugates enhance siRNA delivery through a multicellular spheroid model of malignant glioma. Bioconjug Chem 2009; 20: 1908–16.10.1021/bc900228mSearch in Google Scholar PubMed PubMed Central
123. Tang Y, Li YB, Wang B, Lin RY, van Dongen M, Zurcher DM, Gu XY, Banaszak Holl MM, Liu G, Qi R. Efficient in vitro siRNA delivery and intramuscular gene silencing using PEG-modified PAMAM dendrimers. Mol Pharm 2012; 9: 1812–21.10.1021/mp3001364Search in Google Scholar PubMed PubMed Central
124. Fernandez-Megia E, Correa J, Riguera R. “Clickable” PEG-dendritic block copolymers. Biomacromolecules 2006; 7: 3104–11.10.1021/bm060580dSearch in Google Scholar PubMed
125. Clementi C, Miller K, Mero A, Satchi-Fainaro R, Pasut G. Dendritic poly(ethylene glycol) bearing paclitaxel and alendronate for targeting bone neoplasms. Mol Pharm 2011; 8: 1063–72.10.1021/mp2001445Search in Google Scholar PubMed
126. Albertazzi L, Mickler FM, Pavan GM, Salomone F, Bardi G, Panniello M, Amir E, Kang T, Killops KL, Bräuchle C, Amir RJ, Hawker CJ. Enhanced bioactivity of internally functionalized cationic dendrimers with PEG cores. Biomacromolecules 2012; 13: 4089–97.10.1021/bm301384ySearch in Google Scholar PubMed PubMed Central
127. Conti DS, Brewer D, Grashik J, Avasarala S, da Rocha SR. Poly(amidoamine) dendrimer nanocarriers and their aerosol formulations for siRNA delivery to the lung epithelium. Mol Pharm 2014; 11: 1808–22.10.1021/mp4006358Search in Google Scholar PubMed PubMed Central
128. Liu X, Liu C, Zhou J, Chen C, Qu F, Rossi JJ, Rocchi P, Peng L. Promoting siRNA delivery via enhanced cellular uptake using an arginine-decorated amphiphilic dendrimer. Nanoscale 2015; 7: 3867–75.10.1039/C4NR04759ASearch in Google Scholar
129. Liu C, Liu X, Rocchi P, Qu F, Iovanna JL, Peng L. Arginine-terminated generation 4 PAMAM dendrimer as an effective nanovector for functional siRNA delivery in vitro and in vivo. Bioconjug Chem 2014; 25: 521–32.10.1021/bc4005156Search in Google Scholar PubMed
130. Zeng H, Little HC, Tiambeng TN, Williams GA, Guan Z. Multifunctional dendronized peptide polymer platform for safe and effective siRNA delivery. J Am Chem Soc 2013; 135: 4962–5.10.1021/ja400986uSearch in Google Scholar PubMed
131. Biswas S, Dodwadkar NS, Piroyan A, Torchilin VP. Surface conjugation of triphenylphosphonium to target poly(amidoamine) dendrimers to mitochondria. Biomaterials 2012; 33: 4773–82.10.1016/j.biomaterials.2012.03.032Search in Google Scholar PubMed PubMed Central
132. Smith RA, Porteous CM, Gane AM, Murphy MP. Delivery of bioactive molecules to mitochondria in vivo. Proc Natl Acad Sci USA 2003; 100: 5407–12.10.1073/pnas.0931245100Search in Google Scholar PubMed PubMed Central
133. Biswas S, Deshpande PP, Navarro G, Dodwadkar NS, Torchilin VP. Lipid modified triblock PAMAM-based nanocarriers for siRNA drug co-delivery. Biomaterials 2013; 34: 1289–301.10.1016/j.biomaterials.2012.10.024Search in Google Scholar PubMed PubMed Central
134. Zheng W, Cao C, Liu Y, Yu Q, Zheng C, Sun D, Ren X, Liu J. Multifunctional polyamidoamine-modified selenium nanoparticles dual-delivering siRNA and cisplatin to A549/DDP cells for reversal multidrug resistance. Acta Biomater 2015; 11: 368–80.10.1016/j.actbio.2014.08.035Search in Google Scholar PubMed
135. Finlay J, Roberts CM, Lowe G, Loeza J, Rossi JJ, Glackin CA. RNA-based TWIST1 inhibition via dendrimer complex to reduce breast cancer cell metastasis. Biomed Res Int 2015; 2015: 382745.10.1155/2015/382745Search in Google Scholar PubMed PubMed Central
136. Rajasekaran D, Srivastava J, Ebeid K, Gredler R, Akiel M, Jariwala N, Robertson CL, Shen XN, Siddiq A, Fisher PB, Salem AK, Sarkar D. Combination of nanoparticle-delivered siRNA for astrocyte elevated gene-1 (AEG-1) and all-trans retinoic acid (ATRA): an effective therapeutic strategy for hepatocellular carcinoma (HCC). Bioconjug Chem 2015; 26: 1651–61.10.1021/acs.bioconjchem.5b00254Search in Google Scholar PubMed PubMed Central
137. Liu Z, Winters M, Holodniy M, Dai HJ. siRNA delivery into human T cells and primary cells with carbon-nanotube transporters. Angew Chem Int Edit 2007; 46: 2023–7.10.1002/anie.200604295Search in Google Scholar PubMed
138. Podesta JE, Al-Jamal KT, Herrero MA, Tian B, Ali-Boucetta H, Hegde V, Bianco A, Prato M, Kostarelos K. Antitumor activity and prolonged survival by carbon-nanotube-mediated therapeutic siRNA silencing in a human lung xenograft model. Small 2009; 5: 1176–85.10.1002/smll.200801572Search in Google Scholar PubMed
139. Yang R, Yang X, Zhang Z, Zhang Y, Wang S, Cai Z, Jia Y, Ma Y, Zheng C, Lu Y, Roden R, Chen Y. Single-walled carbon nanotubes-mediated in vivo and in vitro delivery of siRNA into antigen-presenting cells. Gene Ther 2006; 13: 1714–23.10.1038/sj.gt.3302808Search in Google Scholar PubMed
140. Varkouhi AK, Foillard S, Lammers T, Schiffelers RM, Doris E, Hennink WE, Storm G. SiRNA delivery with functionalized carbon nanotubes. Int J Pharm 2011; 416: 419–25.10.1016/j.ijpharm.2011.02.009Search in Google Scholar PubMed
141. Siu KS, Zheng X, Liu Y, Zhang Y, Zhang X, Chen D, Yuan K, Gillies ER, Koropatnick J, Min WP. Single-walled carbon nanotubes noncovalently functionalized with lipid modified polyethylenimine for siRNA delivery in vitro and in vivo. Bioconjug Chem 2014; 25: 1744–51.10.1021/bc500280qSearch in Google Scholar PubMed
142. Battigelli A, Wang JT, Russier J, Da Ros T, Kostarelos K, Al-Jamal KT, Prato M, Bianco A. Ammonium and guanidinium dendron-carbon nanotubes by amidation and click chemistry and their use for siRNA delivery. Small 2013; 9: 3610–9.10.1002/smll.201300264Search in Google Scholar PubMed
143. Lee JH, Lee K, Moon SH, Lee Y, Park TG, Cheon J. All-in-one target-cell-specific magnetic nanoparticles for simultaneous molecular imaging and siRNA delivery. Angew Chem Int Ed Engl 2009; 48: 4174–9.10.1002/anie.200805998Search in Google Scholar PubMed
144. Taratula O, Garbuzenko O, Savla R, Wang YA, He H, Minko T. Multifunctional nanomedicine platform for cancer specific delivery of siRNA by superparamagnetic iron oxide nanoparticles-dendrimer complexes. Curr Drug Deliv 2011; 8: 59–69.10.2174/156720111793663642Search in Google Scholar PubMed
145. Duan J, Dong J, Zhang T, Su Z, Ding J, Zhang Y, Mao X. Polyethyleneimine-functionalized iron oxide nanoparticles for systemic siRNA delivery in experimental arthritis. Nanomedicine (Lond) 2014; 9: 789–801.10.2217/nnm.13.217Search in Google Scholar PubMed
146. Chen J, Zhu S, Tong L, Li J, Chen F, Han Y, Zhao M, Xiong W. Superparamagnetic iron oxide nanoparticles mediated (131)I-hVEGF siRNA inhibits hepatocellular carcinoma tumor growth in nude mice. Bmc Cancer 2014; 14: 114.10.1186/1471-2407-14-114Search in Google Scholar PubMed PubMed Central
147. Derfus AM, Chen AA, Min DH, Ruoslahti E, Bhatia SN. Targeted quantum dot conjugates for siRNA delivery. Bioconjug Chem 2007; 18: 1391–6.10.1021/bc060367eSearch in Google Scholar PubMed
148. Tan WB, Jiang S, Zhang Y. Quantum-dot based nanoparticles for targeted silencing of HER2/neu gene via RNA interference. Biomaterials 2007; 28: 1565–71.10.1016/j.biomaterials.2006.11.018Search in Google Scholar PubMed
149. Zhao MX, Li JM, Du L, Tan CP, Xia Q, Mao ZW, Ji LN. Targeted cellular uptake and siRNA silencing by quantum-dot nanoparticles coated with β-cyclodextrin coupled to amino acids. Chemistry 2011; 17: 5171–9.10.1002/chem.201003523Search in Google Scholar PubMed
150. Li S, Liu Z, Ji F, Xiao Z, Wang M, Peng Y, Zhang Y, Liu L, Liang Z, Lia F. Delivery of Quantum Dot-siRNA Nanoplexes in SK-N-SH Cells for BACE1 Gene Silencing and Intracellular Imaging. Mol Ther Nucleic Acids 2012; 1: e20.10.1038/mtna.2012.11Search in Google Scholar PubMed PubMed Central
151. Kong WH, Bae KH, Jo SD, Kim JS, Park TG. Cationic lipid-coated gold nanoparticles as efficient and non-cytotoxic intracellular siRNA delivery vehicles. Pharm Res 2012; 29: 362–74.10.1007/s11095-011-0554-ySearch in Google Scholar PubMed
152. Bonoiu AC, Mahajan SD, Ding H, Roy I, Yong KT, Kumar R, Hu R, Bergey EJ, Schwartz SA, Prasad PN. Nanotechnology approach for drug addiction therapy: gene silencing using delivery of gold nanorod-siRNA nanoplex in dopaminergic neurons. Proc Natl Acad Sci USA 2009; 106: 5546–50.10.1073/pnas.0901715106Search in Google Scholar PubMed PubMed Central
153. Niikura K, Kobayashi K, Takeuchi C, Fujitani N, Takahara S, Ninomiya T, Hagiwara K, Mitomo H, Ito Y, Osada Y, Ijiro K. Amphiphilic gold nanoparticles displaying flexible bifurcated ligands as a carrier for siRNA delivery into the cell cytosol. ACS Appl Mater Interfaces 2014; 6: 22146–54.10.1021/am505577jSearch in Google Scholar PubMed
154. Lytton-Jean AK, Langer R, Anderson DG. Five years of siRNA delivery: spotlight on gold nanoparticles. Small 2011; 7: 1932–7.10.1002/smll.201100761Search in Google Scholar PubMed
155. Lee Y, Lee SH, Kim JS, Maruyama A, Chen X, Park TG. Controlled synthesis of PEI-coated gold nanoparticles using reductive catechol chemistry for siRNA delivery. J Control Release 2011; 155: 3–10.10.1016/j.jconrel.2010.09.009Search in Google Scholar PubMed
156. Yang P, Gai S, Lin J. Functionalized mesoporous silica materials for controlled drug delivery. Chem Soc Rev 2012; 41: 3679–98.10.1039/c2cs15308dSearch in Google Scholar PubMed
157. Yanes RE, Tamanoi F. Development of mesoporous silica nanomaterials as a vehicle for anticancer drug delivery. Ther Deliv 2012; 3: 389–404.10.4155/tde.12.9Search in Google Scholar PubMed PubMed Central
158. Vivero-Escoto JL, Slowing II, Trewyn BG, Lin VSY. Mesoporous silica nanoparticles for intracellular controlled drug delivery. Small 2010; 6: 1952–67.10.1002/smll.200901789Search in Google Scholar PubMed
159. Lu J, Liong M, Zink JI, Tamanoi F. Mesoporous silica nanoparticles as a delivery system for hydrophobic anticancer drugs. Small 2007; 3: 1341–6.10.1002/smll.200700005Search in Google Scholar PubMed
160. He Q, Shi J, Chen F, Zhu M, Zhang L. An anticancer drug delivery system based on surfactant-templated mesoporous silica nanoparticles. Biomaterials 2010; 31: 3335–46.10.1016/j.biomaterials.2010.01.015Search in Google Scholar PubMed
161. Slowing II, Vivero-Escoto JL, Wu CW, Lin VSY. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv Drug Deliv Rev 2008; 60: 1278–88.10.1016/j.addr.2008.03.012Search in Google Scholar PubMed
162. Xia T, Kovochich M, Liong M, Meng H, Kabehie S, George S, Zink JI, Nel AE. Polyethyleneimine coating enhances the cellular uptake of mesoporous silica nanoparticles and allows safe delivery of siRNA and DNA constructs. ACS Nano 2009; 3: 3273–86.10.1021/nn900918wSearch in Google Scholar PubMed PubMed Central
163. Hom C, Lu J, Liong M, Luo H, Li Z, Zink JI, Tamanoi F. Mesoporous silica nanoparticles facilitate delivery of siRNA to shutdown signaling pathways in mammalian cells. Small 2010; 6: 1185–90.10.1002/smll.200901966Search in Google Scholar PubMed PubMed Central
164. Bhattarai SR, Muthuswamy E, Wani A, Brichacek M, Castaneda AL, Brock SL, Oupicky D. Enhanced gene and siRNA delivery by polycation-modified mesoporous silica nanoparticles loaded with chloroquine. Pharm Res 2010; 27: 2556–68.10.1007/s11095-010-0245-0Search in Google Scholar PubMed PubMed Central
165. Li X, Xie QR, Zhang J, Xia W, Gu H. The packaging of siRNA within the mesoporous structure of silica nanoparticles. Biomaterials 2011; 32: 9546–56.10.1016/j.biomaterials.2011.08.068Search in Google Scholar PubMed
166. Na HK, Kim MH, Park K, Ryoo SR, Lee KE, Jeon H, Jeon H, Ryoo R, Hyeon C, Min DH. Efficient functional delivery of siRNA using mesoporous silica nanoparticles with ultralarge pores. Small 2012; 8: 1752–61.10.1002/smll.201200028Search in Google Scholar PubMed
167. Li X, Chen Y, Wang M, Ma Y, Xia W, Gu H. A mesoporous silica nanoparticle–PEI–fusogenic peptide system for siRNA delivery in cancer therapy. Biomaterials 2013; 34: 1391–401.10.1016/j.biomaterials.2012.10.072Search in Google Scholar PubMed
168. Pritchard JR, Bruno PM, Gilbert LA, Capron KL, Lauffenburger DA, Hemann MT. Defining principles of combination drug mechanisms of action. Proc Natl Acad Sci USA 2013; 110: E170–9.10.1073/pnas.1210419110Search in Google Scholar PubMed PubMed Central
169. Spankuch B, Kurunci-Csacsko E, Kaufmann M, Strebhardt K. Rational combinations of siRNAs targeting Plk1 with breast cancer drugs. Oncogene 2007; 26: 5793–807.10.1038/sj.onc.1210355Search in Google Scholar PubMed
170. MacDiarmid JA, Amaro-Mugridge NB, Madrid-Weiss J, Sedliarou I, Wetzel S, Kochar K, Brahmbhatt VN, Phillips L, Pattison ST, Petti C, Stillman B, Graham RM, Brahmbhatt H. Sequential treatment of drug-resistant tumors with targeted minicells containing siRNA or a cytotoxic drug. Nat Biotechnol 2009; 27: 643–51.10.1038/nbt.1547Search in Google Scholar PubMed
171. Zhang L, Lu Z, Zhao Q, Huang J, Shen H, Zhang Z. Enhanced chemotherapy efficacy by sequential delivery of siRNA and anticancer drugs using PEI-grafted graphene oxide. Small 2011; 7: 460–4.10.1002/smll.201001522Search in Google Scholar PubMed
172. Meng H, Liong M, Xia T, Li Z, Ji Z, Zink JI, Nel AE. Engineered design of mesoporous silica nanoparticles to deliver doxorubicin and P-glycoprotein siRNA to overcome drug resistance in a cancer cell line. ACS Nano 2010; 4: 4539–50.10.1021/nn100690mSearch in Google Scholar PubMed PubMed Central
173. Meng H, Mai WX, Zhang H, Xue M, Xia T, Lin S, Wang X, Zhao Y, Ji Z, Zink JI, Nel AE. Codelivery of an optimal drug/siRNA combination using mesoporous silica nanoparticles to overcome drug resistance in breast cancer in vitro and in vivo. ACS Nano 2013; 7: 994–1005.10.1021/nn3044066Search in Google Scholar PubMed PubMed Central
174. Li JM, Wang YY, Zhao MX, Tan CP, Li YQ, Le XY, Ji LN, Mao ZW. Multifunctional QD-based co-delivery of siRNA and doxorubicin to HeLa cells for reversal of multidrug resistance and real-time tracking. Biomaterials 2012; 33: 2780–90.10.1016/j.biomaterials.2011.12.035Search in Google Scholar PubMed
175. Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nature Med 1997; 3: 614–20.10.1038/nm0697-614Search in Google Scholar PubMed
176. Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 1997; 275: 1129–32.10.1126/science.275.5303.1129Search in Google Scholar PubMed
177. Cheng D, Cao N, Chen J, Yu X, Shuai X. Multifunctional nanocarrier mediated co-delivery of doxorubicin and siRNA for synergistic enhancement of glioma apoptosis in rat. Biomaterials 2012; 33: 1170–9.10.1016/j.biomaterials.2011.10.057Search in Google Scholar PubMed
178. Zou S, Cao N, Cheng D, Zheng R, Wang J, Zhu K, Shuai X. Enhanced apoptosis of ovarian cancer cells via nanocarrier-mediated codelivery of siRNA and doxorubicin. Int J Nanomedicine 2012; 7: 3823–35.Search in Google Scholar
179. Liang B, Deng JJ, Yuan F, Yang N, Li W, Yin JR, Pu SX, Xie LC, Gao C, Zhang LM. Efficient gene transfection in the neurotypic cells by star-shaped polymer consisting of β-cyclodextrin core and poly(amidoamine) dendron arms. Carbohyd Polym 2013; 94: 185–92.10.1016/j.carbpol.2012.12.070Search in Google Scholar PubMed
180. Deng J, Li N, Mai K, Yang C, Yan L, Zhang L-M. Star-shaped polymers consisting of a [small β]-cyclodextrin core and poly(amidoamine) dendron arms: binding and release studies with methotrexate and siRNA. J Mater Chem 2011; 21: 5273–81.10.1039/c0jm03030aSearch in Google Scholar
181. Hu Y, Zhu Y, Yang WT, Xu FJ. New star-shaped carriers composed of β-cyclodextrin cores and disulfide-linked poly(glycidyl methacrylate) derivative arms with plentiful flanking secondary amine and hydroxyl groups for highly efficient gene delivery. ACS Appl Mater Interfaces 2013; 5: 703–12.10.1021/am302249xSearch in Google Scholar PubMed
182. Yang C, Li H, Goh SH, Li J. Cationic star polymers consisting of α-cyclodextrin core and oligoethylenimine arms as nonviral gene delivery vectors. Biomaterials 2007; 28: 3245–54.10.1016/j.biomaterials.2007.03.033Search in Google Scholar PubMed
183. Zhao F, Yin H, Zhang Z, Li J. Folic acid modified cationic gamma-cyclodextrin-oligoethylenimine star polymer with bioreducible disulfide linker for efficient targeted gene delivery. Biomacromolecules 2013; 14: 476–84.10.1021/bm301718fSearch in Google Scholar PubMed
184. Zorde Khvalevsky E, Gabai R, Rachmut IH, Horwitz E, Brunschwig Z, Orbach A, et al. Mutant KRAS is a druggable target for pancreatic cancer. Proc Natl Acad Sci USA 2013; 110: 20723–8.10.1073/pnas.1314307110Search in Google Scholar PubMed PubMed Central
185. Jhaveri AM, Torchilin VP. Multifunctional polymeric micelles for delivery of drugs and siRNA. Front Pharmacol 2014; 5: 77.Search in Google Scholar
186. Mansour AM, Drevs J, Esser N, Hamada FM, Badary OA, Unger C, Nat Rev Drug Discov. A new approach for the treatment of malignant melanoma: enhanced antitumor efficacy of an albumin-binding doxorubicin prodrug that is cleaved by matrix metalloproteinase 2. Cancer Res 2003; 63: 4062–6.Search in Google Scholar
187. Zhu L, Perche F, Wang T, Torchilin VP. Matrix metalloproteinase 2-sensitive multifunctional polymeric micelles for tumor-specific co-delivery of siRNA and hydrophobic drugs. Biomaterials 2014; 35: 4213–22.10.1016/j.biomaterials.2014.01.060Search in Google Scholar PubMed PubMed Central
188. Zhu L, Kate P, Torchilin VP. Matrix metalloprotease 2-responsive multifunctional liposomal nanocarrier for enhanced tumor targeting. ACS Nano 2012; 6: 3491–8.10.1021/nn300524fSearch in Google Scholar PubMed PubMed Central
189. Zhu L, Wang T, Perche F, Taigind A, Torchilin VP. Enhanced anticancer activity of nanopreparation containing an MMP2-sensitive PEG-drug conjugate and cell-penetrating moiety. Proc Natl Acad Sci USA 2013; 110: 17047–52.10.1073/pnas.1304987110Search in Google Scholar PubMed PubMed Central
190. Malamas AS, Gujrati M, Kummitha CM, Xu R, Lu ZR. Design and evaluation of new pH-sensitive amphiphilic cationic lipids for siRNA delivery. J Control Release 2013; 171: 296–307.10.1016/j.jconrel.2013.06.019Search in Google Scholar PubMed PubMed Central
191. Sato Y, Hatakeyama H, Sakurai Y, Hyodo M, Akita H, Harashima H. A pH-sensitive cationic lipid facilitates the delivery of liposomal siRNA and gene silencing activity in vitro and in vivo. J Control Release 2012; 163: 267–76.10.1016/j.jconrel.2012.09.009Search in Google Scholar PubMed
192. Toriyabe N, Hayashi Y, Harashima H. The transfection activity of R8-modified nanoparticles and siRNA condensation using pH sensitive stearylated-octahistidine. Biomaterials 2013; 34: 1337–43.10.1016/j.biomaterials.2012.10.043Search in Google Scholar PubMed
193. Sawant RM, Hurley JP, Salmaso S, Kale A, Tolcheva E, Levchenko TS, Torchilin VP. “SMART” drug delivery systems: double-targeted pH-responsive pharmaceutical nanocarriers. Bioconjug Chem 2006; 17: 943–9.10.1021/bc060080hSearch in Google Scholar PubMed PubMed Central
194. Koren E, Apte A, Jani A, Torchilin VP. Multifunctional PEGylated 2C5-immunoliposomes containing pH-sensitive bonds and TAT peptide for enhanced tumor cell internalization and cytotoxicity. J Control Release 2012; 160: 264–73.10.1016/j.jconrel.2011.12.002Search in Google Scholar PubMed PubMed Central
195. Sawant RR, Sriraman SK, Navarro G, Biswas S, Dalvi RA, Torchilin VP. Polyethyleneimine-lipid conjugate-based pH-sensitive micellar carrier for gene delivery. Biomaterials 2012; 33: 3942–51.10.1016/j.biomaterials.2011.11.088Search in Google Scholar PubMed PubMed Central
196. Estrela JM, Ortega A, Obrador E. Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci 2006; 43: 143–81.10.1080/10408360500523878Search in Google Scholar PubMed
197. Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater 2013; 12: 991–1003.10.1038/nmat3776Search in Google Scholar PubMed
198. Musacchio T, Vaze O, D’Souza G, Torchilin VP. Effective stabilization and delivery of siRNA: reversible siRNA-phospholipid conjugate in nanosized mixed polymeric micelles. Bioconjug Chem 2010; 21: 1530–6.10.1021/bc100199cSearch in Google Scholar PubMed
199. Salzano G, Riehle R, Navarro G, Perche F, De Rosa G, Torchilin VP. Polymeric micelles containing reversibly phospholipid-modified anti-survivin siRNA: a promising strategy to overcome drug resistance in cancer. Cancer Lett 2014; 343: 224–31.10.1016/j.canlet.2013.09.037Search in Google Scholar PubMed PubMed Central
200. Zhao J, Mi Y, Feng SS. Targeted co-delivery of docetaxel and siPlk1 by herceptin-conjugated vitamin E TPGS based immunomicelles. Biomaterials 2013; 34: 3411–21.10.1016/j.biomaterials.2013.01.009Search in Google Scholar PubMed
201. Perche F, Biswas S, Wang T, Zhu L, Torchilin VP. Hypoxia-targeted siRNA delivery. Angew Chem Int Ed Engl 2014; 53: 3362–6.10.1002/anie.201308368Search in Google Scholar PubMed PubMed Central
202. Cheng Z, Al Zaki A, Hui JZ, Muzykantov VR, Tsourkas A. Multifunctional nanoparticles: cost versus benefit of adding targeting and imaging capabilities. Science 2012; 338: 903–10.10.1126/science.1226338Search in Google Scholar PubMed PubMed Central
203. Torchilin VP. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discov 2014; 13: 813–27.10.1038/nrd4333Search in Google Scholar PubMed PubMed Central
©2015 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Reviews
- Recent advances in siRNA delivery
- Trefoil factor family peptides – friends or foes?
- Developments and new vistas in the field of melanocortins
- Cell-cell and cell-matrix adhesion in survival and metastasis: Stat3 versus Akt
- Glyoxalase biochemistry
- Short Conceptual Overviews
- Recent data concerning heparanase: focus on fibrosis, inflammation and cancer
- Adaptive regulation of glucose transport, glycolysis and respiration for cell proliferation
Articles in the same Issue
- Frontmatter
- Reviews
- Recent advances in siRNA delivery
- Trefoil factor family peptides – friends or foes?
- Developments and new vistas in the field of melanocortins
- Cell-cell and cell-matrix adhesion in survival and metastasis: Stat3 versus Akt
- Glyoxalase biochemistry
- Short Conceptual Overviews
- Recent data concerning heparanase: focus on fibrosis, inflammation and cancer
- Adaptive regulation of glucose transport, glycolysis and respiration for cell proliferation

