Abstract
MicroRNAs are short non-coding RNA molecules encoded by distinct genes involved in the posttranscriptional regulation of gene expression. Forming part of the epigenetic machinery, microRNAs are involved in several aspects of tumorigenesis. Deregulation of microRNA expression is a common feature of tumors. Overexpressed oncogenic and underexpressed tumor suppressor microRNAs have been described in many different tumors. MicroRNAs are released from tumors that might affect other cells within and outside the tumor. Circulating microRNAs might also be involved in a tumor surveillance mechanism. In this short overview, some important aspects of microRNA in tumors are discussed.
Introduction
MicroRNAs (miR, miRNA) are short non-coding ribonucleic acid molecules primarily involved in the postransciptional regulation of gene expression (1). MicroRNAs were discovered as endogenous mediators of RNA interference: mature single-stranded microRNA bind to the 3′ untranslated region (3′ UTR) of messenger RNA (mRNA) molecules and thereby inhibit their translation or induce their degradation (1). MicroRNA are encoded by distinct genes that are mostly located in non-coding parts in the genome (the former ‘dark matter’), and also many are found in introns of protein coding genes (2). MicroRNA genes are often found in clusters that often comprise microRNAs of similar sequence or function (microRNA families) (3). Because microRNAs affect gene expression without altering the nucleotide sequence, they can be considered parts of the epigenetic machinery. The number of microRNAs is steadily increasing, and their number exceeds 2500 in humans at present (2588 mature microRNAs in the miRBase registry: www.mirbase.org, release June 21, 2014).
A sophisticated maturation process gives rise to mature microRNAs, whereby primary hairpin structure transcripts of microRNA genes synthesized by RNA polymerase II, called pri-microRNA are first cleaved by intranuclear RNAse III Drosha in complex with DGCR8, and then the resulting pre-microRNAs are transported into the cytoplasm via the nuclear membrane by Exportin 5/Ran GTPase (XPO5) proteins. Mature 19–25 nucleotide long microRNAs are produced from pre-microRNAs by cytoplasmic Dicer. Mature single stranded microRNAs are stabilized in the RISC (RNA-induced silencing complex) containing transactivation-responsive RNA-binding protein (TRBP) and Argonaute 2 (Ago2) as major components and several other factors associated to Ago proteins such as helicases (4, 5) (Figure 1).

Biogenesis and maturation of microRNA.
MicroRNAs are very well-conserved molecules that can be found in a wide range of species ranging from viruses and plants to mammals. MicroRNAs mostly negatively regulate gene expression by inhibiting mRNA translation or inducing their degradation; however, there are data that in some cases show that microRNA might even promote translation (6). A single microRNA can target several hundreds of different mRNAs, thus the action of microRNA is pleiotropic (4). Moreover, a single mRNA can be targeted by several different microRNAs, whose action is often synergistic (1). This complex interaction between microRNAs and target mRNAs constitutes very complicated interaction networks that appear to be tissue specific. MicroRNAs are predicted to regulate 30%–60% of human protein coding genes (7–9).
Apart from this classic mRNA-targeting action of microRNA taking place in the cytoplasm, novel data show that microRNA have other basic functions as well. MicroRNAs have been shown to affect gene transcription directly in the nucleus (10) and thereby participate in forming the genomic landscape of transcription (7). MicroRNAs also influence other epigenetic pathways including chromatin remodeling, methylation, etc. (7). Conversely, the expression of microRNAs is also affected by epigenetic pathways (7).
MicroRNAs affect several basic biological phenomena via targeting crucial mRNAs involved in the regulation of cell proliferation, differentiation, apoptosis, ontogenesis, immune regulation, etc. (11). Given their basic biological functions, it was not surprising to uncover their relevance in several diseases. The differential expression of microRNA has been described in a wide variety of diseases including cancer (3, 12). MicroRNA deregulation is considered to be a major phenomenon of tumor formation (3, 4). Significant differences of microRNA expression have been described between benign and malignant tumors; moreover, subclassification of cancer might be also feasible by microRNA profiles (13). MicroRNA profiling in tumor metastases might be helpful for identifying the primary tumor (14); moreover, microRNA expression changes were identified in the stroma surrounding the tumor that might also be exploited for diagnostic purposes (15). Differential expression of tissue microRNAs can be used for the diagnosis of malignancy or as prognostic markers. MicroRNA markers of malignancy can be especially useful for the diagnosis of tumors whose histological analysis is difficult [e.g., differentiated thyroid tumors (16, 17) and adrenal tumors (12)]. The diagnostic utility of microRNAs is further extended due to their stability, because formalin-fixed, paraffin embedded tissue blocks might also be efficiently used for microRNA analysis (18). In this brief overview, the most important aspects of microRNA in tumors are discussed.
MicroRNA as oncogenes and tumor suppressors
MicroRNA deregulation is considered to be an early event in tumorigenesis (19). Altered expression of microRNAs was described even in the histologically normal tissue surrounding the tumor, e.g., in papillary thyroid carcinoma (16). Several microRNAs in tumors have been found to be over- or underexpressed relative to normal tissues. Following the classical tumor suppressor-oncogene dichotomy, underexpressed microRNAs are termed tumor suppressors, whereas overexpressed are considered to be oncogenic (oncomiR) (20). However, as most cytoplasmic actions of microRNAs are exerted via targeting various messenger RNAs, overexpressed oncogenic microRNAs actually mostly target tumor suppressor mRNAs, whereas the underexpression of tumor suppressor microRNAs results in the overexpression of oncogenic mRNAs (20) (Figure 2).
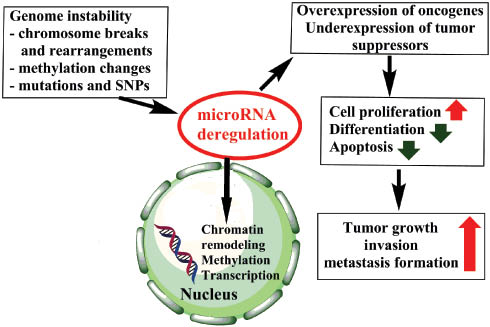
Schematic representation of the relevance of microRNA dysregulation in tumors.
Green arrows represent inhibition, red arrows stimulation.
About 50% of microRNA genes are located in fragile chromosomal regions that are often damaged in tumor tissues (21). However, the association with copy number variations and microRNA expression alterations is not clear cut (22).
In the first study describing the loss of a tumor suppressor microRNA gene involved in disease pathogenesis, miR-15 and miR-16 expression was found to be down-regulated in chronic lymphocytic leukemia (23). Both miR-15 and miR-16 were revealed to target the anti-apoptotic gene BCL-2 (B-cell leukemia 2), and therefore their underexpression resulted in BCL-2 overexpression leading to increased cell proliferation (24, 25). Later, several other microRNA genes have been found with predominant tumor suppressor or oncogenic activity such as the class of let-7 microRNAs that are mainly tumor suppressors (26) or miR-21 that is mainly oncogenic (27).
The oncogene-tumor-suppressor dichotomy is, however, further complicated by the tissue specific action of microRNAs. The same microRNA can be tumor suppressor in one tissue but oncogenic in another. For example, miR-503 is overexpressed in adrenocortical cancer (28) but underexpressed in pituitary tumors (29). There are numerous examples of such discrepancies among various tumors (30). This tissue specific behavior of microRNAs may represent a major hurdle in the development of microRNA-based treatment for cancer.
The expression of microRNA is influenced by transcription factors that, in turn, can be targeted by microRNAs. Interesting regulatory loops may thus arise, such as the regulatory network including the c-MYC protooncogene, miR-17-5p and miR-20a and the transcription factor E2F1 (31). In this network, c-MYC induces the transcription of both E2F1 and the miR17-92 cluster including these microRNAs, but miR-17-5p and miR-20a target the E2F1 mRNA, whereby a fine control of E2F1 activity might be achieved (31). The TP53 (tumor protein 53) is another major transcription factor that is often lost in cancer tissues. Because TP53 induces the transcription of miR-34a that is mostly a tumor suppressor microRNA inducing growth arrest in several cell lines and tissues, loss of TP53 might be associated with decreased miR-34a and thus increased cell proliferation (32). Moreover, TP53 appears to modulate microRNA processing and this phenomenon was suggested to take part in its tumor suppressive activity (33). Conversely, the expression of miR-34a was shown to be affected by a TP53-independent pathway too, as the ETS family transcription factor, ELK1, was found to induce miR-34a expression during oncogene induced senescence (34); c-MYC is known to be among the targets for miR-34a (34). These examples highlight the complex interactions among different players of the gene expression regulation machinery including both transcription factors and microRNAs as posttranscriptional mediators. Fine-tuned regulation of cell cycle and other basic cellular phenomena is based on these sophisticated interactions. Derailment of these balanced regulatory networks can be observed in malignant transformation.
MicroRNA genes and microRNA processing enzymes affected by mutations and single nucleotide polymorphisms (SNP)
Because the classic mRNA-targeting action of microRNAs involves base pairing between the microRNA and the target mRNA, genetic variation in either the microRNA or its recognition sequence might affect the activity of microRNAs (35). The first example of this phenomenon was again delivered by chronic lymphocytic leukemia, where a mutation in the pri-microRNA sequence of miR-16 was found in a familial case of the disease (36). This was an interesting example of an inherited germ-line mutation affecting a non-coding RNA involved in gene expression regulation. In contrast with mutations, single nucleotide polymorphisms (SNP) are much more frequent (found at approximately every 1000 nucleotides of the genome). Variation in both microRNA and target sequences appears to be involved in a wide variety of human cancers (35) and might affect drug sensitivity and treatment response as well. Moreover, there are data that the biogenesis of microRNA might be affected by SNPs in microRNA processing enzymes, and these could be associated with some tumors (35). Thus, the SNP profile of an individual might affect the microRNA expression profile (miRNome). There are several data on the potential association of certain microRNA sequence or target sequence SNPs and tumor risk (35).
Considering mutations of microRNA processing enzymes, frequent hemizygous deletions of Dicer1 resulting in haploinsufficiency has been described in human cancers (37, 38), and Dicer1 can thus be regarded as a tumor suppressor (38). Somatic mutations including a recurrent mutation of Drosha and other microRNA processing enzyme genes have been noted in Wilms tumor samples (39).
It can be hypothesized that due to the genomic instability characteristic for malignant tumors (40), the accumulation of genetic variants and mutations might affect the biogenesis and action of microRNAs that could be relevant in tumor progression. Moreover, the microRNA target mRNAs are also more prone to alternative splicing in proliferating cells that might result in shorter 3′ UTR sequences harboring fewer microRNA binding sites (41).
MicroRNA released from tumor cells
Apart from tissue microRNAs, novel data show that microRNAs are released from cells and enter body fluids and excrements (blood, urine, feces, saliva, semen, milk, etc.) (42). MicroRNAs are released in three major ways: (i) passive release through damage (necrosis, inflammation), (ii) active release packed in extracellular membrane vesicles (microvesicles, exosomes, and apoptotic bodies), or (iii) associated with protein complexes such as Argonaute 2 and high density lipoprotein (HDL) (43). The microRNAs released in the blood are surprisingly stable and the circulating microRNAs can be exploited as minimally invasive biomarkers of tumors even in their very early stages (44). Blood-borne circulating microRNA might be regarded as hormones conveying epigenetic information to distant tissues (19) (Figure 3). Circulating microRNA markers are very promising biomarkers that could be used as minimally invasive biomarkers of tumor malignancy or prognosis (44).

Relevance for tumor-secreted microRNAs in tumor pathogenesis and progression.
MicroRNA in extracellular vesicles might enter other tumor and normal cells. MicroRNA in macromolecular complexes might also be relevant, but these warrant experimental validation, as well.
A growing body of experimental evidence shows that tumor cells release microRNAs in extracellular membrane vesicles, and the activity of this release process seems to be correlated with tumor stage, i.e., advanced tumors release more extracellular vesicles. These extracellular vesicles are very important in the communication among tumor cells but also with the surrounding normal tissue and immune cells (45). In a rat model, exosome-derived microRNAs were shown to affect non-transformed cells by modulating the expression of proteases, adhesion molecules, etc., and so might be involved in tumor invasion and metastasis formation (46); miR-1227 contained in large extracellular vesicles (oncosomes) released from a prostate cancer cell line enhanced the migration of cancer associated fibroblasts (47). Beside microRNAs, extracellular vesicles contain mRNAs and proteins as well. The vesicles might fuse with cell membranes of other cells, whereby their contents might enter these cells. The entry of microRNA into recipient cells could be involved in the epigenetic reprogramming of these cells (45, 48, 49).
Circulating microRNA constituting a tumor surveillance mechanism?
By looking through the relative abundance of circulating microRNAs in the blood of healthy individuals, we have noted a relative abundance of microRNAs with predominant tumor suppressor activity, e.g., miR-451, miR-223, let-7, miR-16 (50). Based on this observation, we have raised a novel hypothesis claiming that the relative abundance of tumor suppressor microRNAs in the circulation might represent a tumor surveillance mechanism. There are experimental data that tumor-cell-derived extracellular vesicles might enter other cells (both tumor and normal) (45), but the activity of microRNAs in recipient cells warrants experimental validation. We hypothesize that circulating tumor suppressor microRNAs packed in membrane vesicles (or perhaps in macromolecular complexes, but this awaits experimental validation) entering transforming cells might halt their transformation in their early phase or induce their apoptosis. Such a circulating microRNA-mediated tumor surveillance might complement the well-known immune tumor surveillance or cancer immunoediting (51). There are, however, microRNAs with predominant oncogenic activity in the circulation, such as miR-21, and the tissue specific activity of microRNAs can be raised also as a counter arguments against this hypothesis. It is not clear at present how efficient these circulating microRNAs might be as tumor repressors, and intensive experimental work-up is needed to confirm this hypothesis (50).
The relative abundance of predominantly tumor suppressive circulating microRNAs has been observed in advanced cancer patients, too. In a hypothesis put forward by Chen et al., the abundance of tumor suppressor microRNAs was suggested to represent an anti-cancer activity defense mechanism (52). As most deregulated microRNAs were shown to be implicated in the regulation of the immune response, this anti-cancer activity might affect the immune system.
MicroRNA in anti-tumor therapy
Given their major cell biological roles and relevance in tumorigenesis, intensive efforts are underway to target microRNAs for treatment purposes. Based on the oncogene-tumor suppressor dichotomy, two major directions can be taken: (i) to decrease the activity of oncogenic microRNAs, and (ii) to enhance or replace the missing activity of tumor suppressor microRNAs. Given the often synergistic action of microRNAs, it could be useful to target several microRNAs simultaneously.
There are some promising in vitro and animal data on the anti-tumor application of microRNAs, e.g., overexpressed miR-21, miR-221, and miR-214 (53), but introduction to human clinical treatment is still far away. In adrenocortical tumors, we have proposed that miR-483-5p/miR-483-3p, miR-195, and miR-210 would be the most suitable candidates for treatment targets (12). Modulation of microRNAs might facilitate other anti-tumor treatments, e.g., the up-regulation of miR-195 in breast cancer cells enhanced their sensitivity to adriamycin administration (54), or down-regulation of the main hypoxia-associated miR-210 improved the outcome of radiation therapy in a human hepatoma xenograft model (55).
There are several available molecular techniques that could make these goals feasible in the clinical setting, e.g., microRNA mimics for replacing tumor suppressors or antagomiRs to counteract overexpressed oncogenic microRNAs (56). There are, however, major problems to overcome. Technical issues include the efficient administration of microRNA (liposomes, plasmids, viral vectors, nanoparticles) and the dose to be used. A major biological difficulty is related to the tissue specific action of microRNA, whereby off-target actions may arise in other organs or tissues with undesirable side effects. If the dose of the administered pre-microRNA is too high, it might saturate the endogenous microRNA processing machinery that might lead to unforeseen complications (12, 56).
Conclusions
Since their discovery two decades ago, microRNAs have experienced an unprecedented career in molecular medicine including oncology. Altered expression of microRNA appears to be a cornerstone of tumor formation and can be exploited in tumor diagnosis and even in therapy. It must be emphasized, however, that microRNAs act together with other molecular players, and the different layers of molecular alterations including the genome, miRNome, transcription factors, proteome, methylome, and metabolome interact with each other. Major developments are to be expected in the field of microRNAs in tumor biology, including their roles in pathogenesis, diagnosis, and as treatment targets. The advent of next generation sequencing and novel bioinformatics approaches might help to unravel this fascinating field of biology.
Acknowledgments
This study has been supported by a grant from the Hungarian Scientific Research Fund (OTKA K100295) to Dr. Peter Igaz.
References
1. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009; 136: 215–33.10.1016/j.cell.2009.01.002Search in Google Scholar PubMed PubMed Central
2. Igaz I, Igaz P. Possible role for microRNAs as inter-species mediators of epigenetic information in disease pathogenesis: Is the non-coding dark matter of the genome responsible for epigenetic interindividual or interspecies communication? Med Hypotheses 2015; 84: 150–4.10.1016/j.mehy.2014.11.021Search in Google Scholar PubMed
3. Iorio MV, Croce CM. microRNA involvement in human cancer. Carcinogenesis 2012; 33: 1126–33.10.1093/carcin/bgs140Search in Google Scholar PubMed PubMed Central
4. Acunzo M, Romano G, Wernicke D, Croce CM. MicroRNA and cancer – a brief overview. Adv Biol Regul 2015; 57: 1–9.10.1016/j.jbior.2014.09.013Search in Google Scholar PubMed
5. Kawamata T, Tomari Y. Making RISC. Trends Biochem Sci 2010; 35: 368–76.10.1016/j.tibs.2010.03.009Search in Google Scholar PubMed
6. Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science 2007; 318: 1931–4.10.1126/science.1149460Search in Google Scholar PubMed
7. Malumbres M. miRNAs and cancer: an epigenetics view. Mol Aspects Med 2013; 34: 863–74.10.1016/j.mam.2012.06.005Search in Google Scholar PubMed PubMed Central
8. Berezikov E, Guryev V, van de Belt J, Wienholds E, Plasterk RH, Cuppen E. Phylogenetic shadowing and computational identification of human microRNA genes. Cell 2005; 120: 21–4.10.1016/j.cell.2004.12.031Search in Google Scholar PubMed
9. Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res 2008; 36: D154–8.10.1093/nar/gkm952Search in Google Scholar PubMed PubMed Central
10. Salmanidis M, Pillman K, Goodall G, Bracken C. Direct transcriptional regulation by nuclear microRNAs. Int J Biochem Cell Biol 2014; 54: 304–11.10.1016/j.biocel.2014.03.010Search in Google Scholar PubMed
11. Alvarez-Garcia I, Miska EA. MicroRNA functions in animal development and human disease. Development 2005; 132: 4653–62.10.1242/dev.02073Search in Google Scholar PubMed
12. Igaz P, Igaz I, Nagy Z, Nyiro G, Szabo PM, Falus A, Patocs A, Racz K. MicroRNAs in adrenal tumors: relevance for pathogenesis, diagnosis, and therapy. Cell Mol Life Sci 2015; 72: 417–28.10.1007/s00018-014-1752-7Search in Google Scholar PubMed
13. Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature 2005; 435: 834–8.10.1038/nature03702Search in Google Scholar PubMed
14. Rosenfeld N, Aharonov R, Meiri E, Rosenwald S, Spector Y, Zepeniuk M, Benjamin H, Shabes N, Tabak S, Levy A, Lebanony D, Goren Y, Silberschein E, Targan N, Ben-Ari A, Gilad S, Sion-Vardy N, Tobar A, Feinmesser M, Kharenko O, Nativ O, Nass D, Perelman M, Yosepovich A, Shalmon B, Polak-Charcon S, Fridman E, Avniel A, Bentwich I, Bentwich Z, Cohen D, Chajut A, Barshack I. MicroRNAs accurately identify cancer tissue origin. Nat Biotechnol 2008; 26: 462–9.10.1038/nbt1392Search in Google Scholar PubMed
15. Nielsen BS, Jorgensen S, Fog JU, Sokilde R, Christensen IJ, Hansen U, Brunner N, Baker A, Moller S, Nielsen HJ. High levels of microRNA-21 in the stroma of colorectal cancers predict short disease-free survival in stage II colon cancer patients. Clin Exp Met 2011; 28: 27–38.10.1007/s10585-010-9355-7Search in Google Scholar PubMed PubMed Central
16. He H, Jazdzewski K, Li W, Liyanarachchi S, Nagy R, Volinia S, Calin GA, Liu CG, Franssila K, Suster S, Kloos RT, Croce CM, de la Chapelle A. The role of microRNA genes in papillary thyroid carcinoma. Proc Natl Acad Sci USA 2005; 102: 19075–80.10.1073/pnas.0509603102Search in Google Scholar PubMed PubMed Central
17. Weber F, Teresi RE, Broelsch CE, Frilling A, Eng C. A limited set of human MicroRNA is deregulated in follicular thyroid carcinoma. J Clin Endocrinol Metab 2006; 91: 3584–91.10.1210/jc.2006-0693Search in Google Scholar PubMed
18. Klopfleisch R, Weiss AT, Gruber AD. Excavation of a buried treasure – DNA, mRNA, miRNA and protein analysis in formalin fixed, paraffin embedded tissues. Histol Histopathol 2011; 26: 797–810.Search in Google Scholar
19. Cortez MA, Bueso-Ramos C, Ferdin J, Lopez-Berestein G, Sood AK, Calin GA. MicroRNAs in body fluids – the mix of hormones and biomarkers. Nat Rev Clin Oncol 2011; 8: 467–77.10.1038/nrclinonc.2011.76Search in Google Scholar PubMed PubMed Central
20. Chen CZ. MicroRNAs as oncogenes and tumor suppressors. New Engl J Med 2005; 353: 1768–71.10.1056/NEJMp058190Search in Google Scholar PubMed
21. Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA 2004; 101: 2999–3004.10.1073/pnas.0307323101Search in Google Scholar PubMed PubMed Central
22. Volinia S, Galasso M, Costinean S, Tagliavini L, Gamberoni G, Drusco A, Marchesini J, Mascellani N, Sana ME, Abu Jarour R, Desponts C, Teitell M, Baffa R, Aqeilan R, Iorio MV, Taccioli C, Garzon R, Di Leva G, Fabbri M, Catozzi M, Previati M, Ambs S, Palumbo T, Garofalo M, Veronese A, Bottoni A, Gasparini P, Harris CC, Visone R, Pekarsky Y, de la Chapelle A, Bloomston M, Dillhoff M, Rassenti LZ, Kipps TJ, Huebner K, Pichiorri F, Lenze D, Cairo S, Buendia MA, Pineau P, Dejean A, Zanesi N, Rossi S, Calin GA, Liu CG, Palatini J, Negrini M, Vecchione A, Rosenberg A, Croce CM. Reprogramming of miRNA networks in cancer and leukemia. Genome Res 2010; 20: 589–99.10.1101/gr.098046.109Search in Google Scholar PubMed PubMed Central
23. Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, Bullrich F, Croce CM. Frequent deletions and down-regulation of microRNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci USA 2002; 99: 15524–9.10.1073/pnas.242606799Search in Google Scholar PubMed PubMed Central
24. Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA 2005; 102: 13944–9.10.1073/pnas.0506654102Search in Google Scholar PubMed PubMed Central
25. Aqeilan RI, Calin GA, Croce CM. miR-15a and miR-16-1 in cancer: discovery, function and future perspectives. Cell Death Differ 2010; 17: 215–20.10.1038/cdd.2009.69Search in Google Scholar PubMed
26. Boyerinas B, Park SM, Hau A, Murmann AE, Peter ME. The role of let-7 in cell differentiation and cancer. Endocr-Relat Cancer 2010; 17: F19–36.10.1677/ERC-09-0184Search in Google Scholar PubMed
27. Hong L, Han Y, Zhang Y, Zhang H, Zhao Q, Wu K, Fan D. MicroRNA-21: a therapeutic target for reversing drug resistance in cancer. Expert Opin Ther Targets 2013; 17: 1073–80.10.1517/14728222.2013.819853Search in Google Scholar PubMed
28. Tombol Z, Szabo PM, Molnar V, Wiener Z, Tolgyesi G, Horanyi J, Riesz P, Reismann P, Patocs A, Liko I, Gaillard RC, Falus A, Racz K, Igaz P. Integrative molecular bioinformatics study of human adrenocortical tumors: microRNA, tissue-specific target prediction, and pathway analysis. Endocr Rrelat Cancer 2009; 16: 895–906.10.1677/ERC-09-0096Search in Google Scholar PubMed
29. Butz H, Liko I, Czirjak S, Igaz P, Korbonits M, Balint K, Racz K, Patocs A. Downregulation of Wee1 kinase by a specific subset of microRNAs in human sporadic pituitary adenomas. J Clin Endocrinol Metab 2010; 95: E181–91.10.1210/jc.2010-0581Search in Google Scholar PubMed
30. Palanichamy JK, Rao DS. miRNA dysregulation in cancer: towards a mechanistic understanding. Front Genet 2014; 5: Article number: 54.10.3389/fgene.2014.00054Search in Google Scholar PubMed PubMed Central
31. O’Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature 2005; 435: 839–43.10.1038/nature03677Search in Google Scholar PubMed
32. He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, Jackson AL, Linsley PS, Chen C, Lowe SW, Cleary MA, Hannon GJ. A microRNA component of the p53 tumour suppressor network. Nature 2007; 447: 1130–4.10.1038/nature05939Search in Google Scholar PubMed PubMed Central
33. Suzuki HI, Yamagata K, Sugimoto K, Iwamoto T, Kato S, Miyazono K. Modulation of microRNA processing by p53. Nature 2009; 460: 529–33.10.1038/nature08199Search in Google Scholar PubMed
34. Christoffersen NR, Shalgi R, Frankel LB, Leucci E, Lees M, Klausen M, Pilpel Y, Nielsen FC, Oren M, Lund AH. p53-independent upregulation of miR-34a during oncogene-induced senescence represses MYC. Cell Death Differ 2010; 17: 236–45.10.1038/cdd.2009.109Search in Google Scholar PubMed
35. Ryan BM, Robles AI, Harris CC. Genetic variation in microRNA networks: the implications for cancer research. Nat Rev Cancer 2010; 10: 389–402.10.1038/nrc2867Search in Google Scholar PubMed PubMed Central
36. Calin GA, Ferracin M, Cimmino A, Di Leva G, Shimizu M, Wojcik SE, Iorio MV, Visone R, Sever NI, Fabbri M, Iuliano R, Palumbo T, Pichiorri F, Roldo C, Garzon R, Sevignani C, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. New Engl J Med 2005; 353: 1793–801.10.1056/NEJMoa050995Search in Google Scholar PubMed
37. Lambertz I, Nittner D, Mestdagh P, Denecker G, Vandesompele J, Dyer MA, Marine JC. Monoallelic but not biallelic loss of Dicer1 promotes tumorigenesis in vivo. Cell Death Differ 2010; 17: 633–41.10.1038/cdd.2009.202Search in Google Scholar PubMed PubMed Central
38. Kumar MS, Pester RE, Chen CY, Lane K, Chin C, Lu J, Kirsch DG, Golub TR, Jacks T. Dicer1 functions as a haploinsufficient tumor suppressor. Genes Dev 2009; 23: 2700–4.10.1101/gad.1848209Search in Google Scholar PubMed PubMed Central
39. Torrezan GT, Ferreira EN, Nakahata AM, Barros BD, Castro MT, Correa BR, Krepischi AC, Olivieri EH, Cunha IW, Tabori U, Grundy PE, Costa CM, de Camargo B, Galante PA, Carraro DM. Recurrent somatic mutation in DROSHA induces microRNA profile changes in Wilms tumour. Nat Commun 2014; 5: 4039.10.1038/ncomms5039Search in Google Scholar PubMed PubMed Central
40. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–74.10.1016/j.cell.2011.02.013Search in Google Scholar PubMed
41. Sandberg R, Neilson JR, Sarma A, Sharp PA, Burge CB. Proliferating cells express mRNAs with shortened 3′ untranslated regions and fewer microRNA target sites. Science 2008; 20: 1643–7.10.1126/science.1155390Search in Google Scholar PubMed PubMed Central
42. Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, Galas DJ, Wang K. The microRNA spectrum in 12 body fluids. Clin Chem 2010; 56: 1733–41.10.1373/clinchem.2010.147405Search in Google Scholar PubMed PubMed Central
43. Redis RS, Calin S, Yang Y, You MJ, Calin GA. Cell-to-cell miRNA transfer: from body homeostasis to therapy. Pharmacol Ther 2012; 136: 169–74.10.1016/j.pharmthera.2012.08.003Search in Google Scholar PubMed PubMed Central
44. Reid G, Kirschner MB, van Zandwijk N. Circulating microRNAs: Association with disease and potential use as biomarkers. Crit Rev Oncol/Hematol 2011; 80: 193–208.10.1016/j.critrevonc.2010.11.004Search in Google Scholar PubMed
45. Katsuda T, Kosaka N, Ochiya T. The roles of extracellular vesicles in cancer biology: toward the development of novel cancer biomarkers. Proteomics 2014; 14: 412–25.10.1002/pmic.201300389Search in Google Scholar PubMed
46. Rana S, Malinowska K, Zoller M. Exosomal tumor microRNA modulates premetastatic organ cells. Neoplasia 2013; 15: 281–95.10.1593/neo.122010Search in Google Scholar PubMed PubMed Central
47. Morello M, Minciacchi VR, de Candia P, Yang J, Posadas E, Kim H, Griffiths D, Bhowmick N, Chung LW, Gandellini P, Freeman MR, Demichelis F, Di Vizio D. Large oncosomes mediate intercellular transfer of functional microRNA. Cell Cycle 2013; 12: 3526–36.10.4161/cc.26539Search in Google Scholar PubMed PubMed Central
48. Muralidharan-Chari V, Clancy JW, Sedgwick A, D’Souza-Schorey C. Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci 2010; 123:1603–11.10.1242/jcs.064386Search in Google Scholar PubMed PubMed Central
49. Camussi G, Deregibus MC, Bruno S, Grange C, Fonsato V, Tetta C. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am J Cancer Res 2011; 1: 98–110.Search in Google Scholar
50. Igaz I, Igaz P. Tumor surveillance by circulating microRNAs: a hypothesis. Cell Mol Life Sci 2014; 71: 4081–7.10.1007/s00018-014-1682-4Search in Google Scholar PubMed PubMed Central
51. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002; 3: 991–8.10.1038/ni1102-991Search in Google Scholar PubMed
52. Chen G, Wang J, Cui Q. Could circulating miRNAs contribute to cancer therapy? Trends Mol Med 2013; 19: 71–3.10.1016/j.molmed.2012.10.006Search in Google Scholar PubMed
53. Farooqi AA, Rehman ZU, Muntane J. Antisense therapeutics in oncology: current status. OncoTargets Ther 2014; 7: 2035–42.10.2147/OTT.S49652Search in Google Scholar PubMed PubMed Central
54. Yang G, Wu D, Zhu J, Jiang O, Shi Q, Tian J, Weng Y. Upregulation of miR-195 increases the sensitivity of breast cancer cells to Adriamycin treatment through inhibition of Raf-1. Oncol Rep 2013; 30: 877–89.10.3892/or.2013.2532Search in Google Scholar PubMed
55. Yang W, Wei J, Sun T, Liu F. Effects of knockdown of miR-210 in combination with ionizing radiation on human hepatoma xenograft in nude mice. Radiat Oncol 2013; 8: 102.10.1186/1748-717X-8-102Search in Google Scholar PubMed PubMed Central
56. McDermott AM, Heneghan HM, Miller N, Kerin MJ. The therapeutic potential of microRNAs: disease modulators and drug targets. Pharm Res 2011; 28: 3016–29.10.1007/s11095-011-0550-2Search in Google Scholar PubMed
©2015 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Reviews
- Transgenerational epigenetic inheritance: resolving uncertainty and evolving biology
- Longevity: epigenetic and biomolecular aspects
- Stepping inside the realm of epigenetic modifiers
- Numb family proteins: novel players in cardiac morphogenesis and cardiac progenitor cell differentiation
- Short Conceptual Overviews
- Suggested roles for microRNA in tumors
- The TATA-box motif and its impact on transcriptional gene regulation by miRNAs
Articles in the same Issue
- Frontmatter
- Reviews
- Transgenerational epigenetic inheritance: resolving uncertainty and evolving biology
- Longevity: epigenetic and biomolecular aspects
- Stepping inside the realm of epigenetic modifiers
- Numb family proteins: novel players in cardiac morphogenesis and cardiac progenitor cell differentiation
- Short Conceptual Overviews
- Suggested roles for microRNA in tumors
- The TATA-box motif and its impact on transcriptional gene regulation by miRNAs

