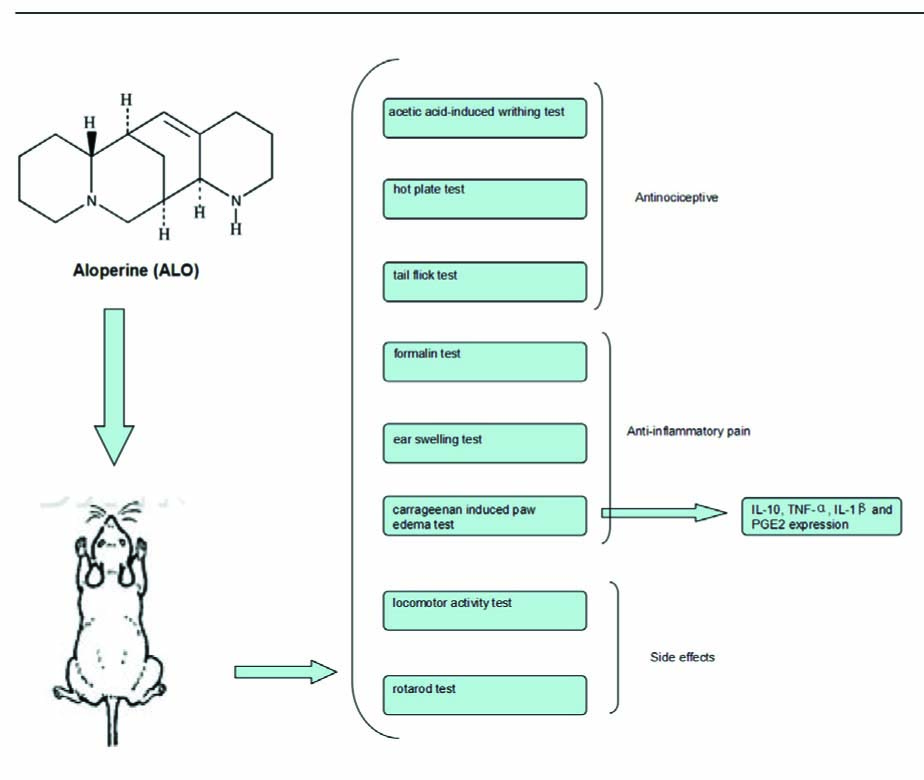
Graphical Abstract
Abstract
Background and objectives
Aloperine (ALO) is an alkaloid compound and presents in several medicinal plants. This study was undertaken to investigate the anti-nociceptive and anti-inflammatory activities of ALO on various chemical- and thermal-induced hypersensitivity models in mice.
Methods
The anti-nociceptive effect of ALO was evaluated using acetic acid-induced writhing test, hot plate test, tail flick test, formalin test, ear swelling test, locomotor activity test, rota-rod test and carrageenan-induced paw edema test in mice. Inflammatory cytokines including interleukin-10 (IL-10), tumor necrosis factor α (TNF-α), interleukin-1 beta (IL-1β) and prostaglandin E2 (PGE2) expression were examined in ALO- and vehicle-treated mice.
Results
The results showed that ALO significantly attenuated acetic acid-induced writhing numbers in mice in a dose-dependent manner. ALO showed no effect on prolonging latency in the hot plate test and the tail-flick test. ALO showed analgesic activity in the inflammatory phase of formalin-induced pain. Its anti-inflammatory effect was also confirmed in the ear-swelling test. In the carrageenan-induced paw edema model, ALO significantly and dose-dependently reduced the carrageenan-induced paw edema, decreased the contents of TNF-α IL-1β and PGE2, but increased the IL-10 production. On the other hand, ALO showed no influence on the rota-rod performance time or on spontaneous locomotor activity.
Conclusion
It is concluded that ALO has both anti-inflammatory and analgesic effects, especially in the field of inflammation pain.
Implications
Our findings support the hypothesis that ALO ameliorates inflammatory pain induced by chemical and thermal stimuli and provides a scientific basis for the resource development and clinical use of aloperine.
1 Introduction
The plant, Sophora alopecuroides Linn, grows richly in the Ningxia Autonomic Hui Region located in the northwest of the People’s Republic of China. It has been used as an important medicinal plant to treat sore throat, diarrhea and eczema [1]. In addition, it has been commonly used as a pain-relief drug in traditional Chinese medicine in the northwest of China for several thousand years. Aloperine (ALO) is a monomer alkaloid purified from Sophora alopecuroides Linn and there is evidence that it possesses free radical-scavenging, anti-inflammatory and anti-allergic properties, and that it regulates immune function and attenuates neuropathic pain. However, there still has been no comprehensive evaluation of its analgesic activity against acute and inflammatory pain [2,3,4,5]. In this study, we examined the effects of ALO on hypersensitivity using mouse nociception models induced by chemical and thermal stimuli [6]. Carrageenan-induced inflammatory pain is believed to be mediated by prostaglandin. Recent studies have revealed that inflammation evokes prostaglandin E2 (PGE2) and the latter sensitizes the peripheral primary nociceptive neurons [7,8]. It has been previously reported that during inflammation, the pro-inflammatory cytokines, such as tumor necrosis factor α (TNF-α) and interleukin-1 beta (IL-1 beta or IL-1β) are produced, which leads to an increased production of PGE2. Anti-inflammatory cytokine interleukin-10 (IL-10) inhibits the production of TNF-α and IL-1 β [9]. Therefore, modulating cytokine production is an important anti-inflammatory and anti-hyperalgesic approach. Although aspirin reduces hypersensitivity by inhibiting inflammatory cytokines [10,11], it produces serious side effects such as stomach upset, gastric bleeding and peptic ulcer. Inflammatory pain is generally treated with opioids and non-steroid anti-inflammatory drugs (NSAIDs), but both are limited by side effects. Therefore, a search for potential drugs that could be used for anti-inflammatory pain has been a challenge. The objective of this study was to elucidate the analgesic effects of ALO on inflammatory pain induced by chemical and thermal stimuli and provide a scientific basis for the resource development and clinical use of aloperine.
2 Materials and methods
2.1 Animals and housing conditions
Two tothree month old ICRmice with equal distribution of male and female, weighing between 18 and 23 g, were provided by the Experimental Animal Center of Ningxia Medical University (Certificate number was SYXK Ningxia 2014-0001). The experimental protocol was duly approved by the Institutional Animal Care and Usage Committee of Ningxia Medical University, Yinchuan city, Ningxia.
2.2 Drugs and chemicals
Aloperine (C15H24N2) was purchased from Yan Chi pharmaceutical (Yanchi, Ningxia) with purity >98.5%. Its chemical structure is given in Fig. 1. Mouse ELISA kits (IL-10, TNF-α, IL-1β) were purchased from Neobioscience Ltd (Shenzhen, China). Aspirin (Asp) was purchased from Sigma-Aldrich (Steinheim, Germany). Acetic acid was obtained from Tianjin Bei Lian Ltd (Tianjin, China). Xylene was obtained from Beijing Chemical Plant (Beijing, China). Carrageenan was purchased from Shanghai Kai Yang Biotechnology Co., Ltd (Shanghai, China). Morphine hydrochloride was obtained from Northeast Pharmaceutical Group Shenyang No.1 Pharmaceutical Co., Ltd (Shenyang, China). Formalin was purchased from Yantai San He Chemical Reagent Co. (Shandong, China).
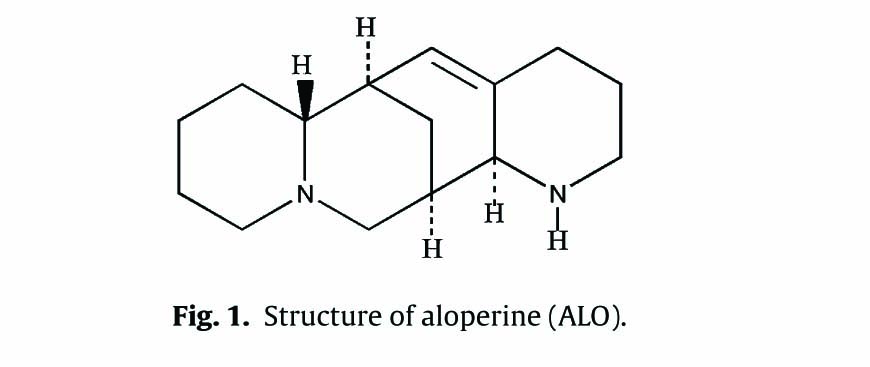
Structure of aloperine (ALO).
2.3 Sample preparation
ALO was dissolved in normal saline (NS, 0.9% NaCl diluted in distilled water). Morphine was diluted in NS. Aspirin was dissolved in 0.05% sodium carboxymethyl cellulose solution. Carrageenan was dissolved in distilled water. NS, morphine (10mg/kg) and ALO (20, 40, 80 mg/kg) were administered by intraperitoneal injection (i.p.) in a volume of 0.1 ml/10g. Aspirin (400mg/kg) was administered by intragastric gavage (i.g.) in a volume of 0.1 ml/10g.
2.4 Acetic acid-induced writhing test
Acetic acid-induced writhing test has been widely used to test the analgesic effect [6]. Briefly, each mouse was placed in a large glass beaker and the total number of writhing was recorded for 15 min after 0.6% (v/v, diluted in saline) acetic acid intraperitoneal administration in a volume of 0.1 ml/10g. The writhing response consisted of a contraction of the abdominal muscle together with a stretching of the hind limbs. Mice were pre-treated with the drugs 60 min before administration of acetic acid. The mouse was put on a large beaker and the intensity of the writhing response was expressed as the number of writhing.
2.5 Formalin test
The formalin test was performed by injecting 20 μl of 2% formalin into the intraplantar of the right hind paw and then recording the length of time the animal spends licking that right hind paw. One hour after administration of drugs, the animals were injected with 20 μl of 2% formalin, transferred to a large beaker, and the licking right hind pawtime then recorded. Results were recorded for both the first (0-10 min) and second (10-60 min) phases [6].
2.6 Hot plate latency test
The hot-plate test evaluates the thermal pain reflex of paw licking due to footpad contact with a heated surface. The plate temperature was maintained at 55.0 ± 0.5 °C. Mice (female) were placed on the heated surface one at a time and the response latency until each animal began to lick its paw was recorded [6]. Mice with a normal latency (reaction time, 5–30 s approximately) were selected. Mice were pre-treated with the drugs, and placed on the heated surface. A maximum latency (cut off) was set at 60 s to avoid paw tissue damage.
2.7 Tail flick test
The effect of ALO on physiological pain was determined by the tail flick test [12]. Animal tails were immersed in a water bath (50.0 ± 0.1 °C) about 3 cm under the water and the latency time or time before they flicked their tail was recorded. Mice with normal latency of 2–5 s were selected. The tail-flick latencies were measured after administration of drugs. Cut-off times set at 15 s to avoid tail tissue damage.
2.8 Spontaneous locomotor (exploratory) activity test
The locomotor activity test was performed to detect any drug side effects on the exploratory behavior after drug administration [13]. In this test, locomotor activity in an open field was determined by YLS-1A locomotor activity recorder (Shandong Academy of Medical Science Device Station, Jinan, China). Mice with a locomotor activity of 200 ± 20 times per 5 min prior to the initiation of drug treatment were included in the study.
2.9 Motor coordination test
A rota-rod treadmill device (YLS-4C, Shandong Academy of Medical Science Device Station, Jinan, China) was used for evaluation of motor coordination [13]. Initially, 3 days prior to the formal test, the mice were acclimatized to the revolving drum by a training run of 5 min each day and those that could stay on the rota-rod apparatus longer than 300 s were selected for the experiment. The rod was set to accelerate from 5 to 40rpm over a 90s period and then revolve at 40rpm for a total time of 300 s. One hour after drug administration, each animal was tested on the rota-rod apparatus. The elapsed times before the mice fell from the drum onto the plate were recorded. The maximum time measured was 300 s. The mice were able to remain on the bar was recorded for 300 s.
2.10 Mouse ear swelling test (MEST)
The mouse ear-swelling test (MEST) was developed in the early 1980s to provide a lower cost, shorter and objectively graded test for the assessment of anti-inflammatory activity of the drug [14]. Mice were pre-treated with the drugs. One hour later, 50 μl of xylene was smeared onto the anterior and posterior surfaces of the right ear of each mouse. Forty min after xylene application, mice were sacrificed and both right and left ears were taken circularly and weighed. The weight difference between the right and left ears is the measured mouse ear swelling.
2.11 Carrageenan-induced paw edema test
The anti-inflammatory effect of plant extract against paw edema was assessed in mice [15]. Paw edema was induced by injection of 30 μl of 1% carrageenan (prepared in distilled water) into plantar surface of the right hind paw. Mice were treated with drug 30 min before the administration of carrageenan. Paw edema was assessed by toe volumetric measuring instrument (YLS-7A, Shandong Academy of Medical Science Device Station, Jinan, China) both before carrageenan injection and 1,2,3,4 and 5 h after carrageenan injection.
2.12 Content of inflammatory cytokines in paw edema tissue
Mice were euthanized after 5 h of treatment and the skin tissue was removed from the injected right hind paws. The samples were homogenized in 800 μl of 0.86% saline and centrifuged at 800 g for 10 min. The protein concentration of the supernatants was measured. For PGE2 measurement, the supernatant (150 μl) was added into 2 ml of KOH methanol solution (0.5 mol/l) and incubated at 50 °C for 20 min. Then 2.5 ml of methanol was added and OD value was measured at 278 nm using an UV-vis Spectrophotometer (UV-2550, Shimadzu, Japan) [15,16]. TNF-α, IL-1β, and IL-10 levels were measured using ELISA kits (Neobioscience Ltd, Shenzhen, China) according to the manufacturer’s instruction. The results were expressed as picograms (pg) of cytokine per gram protein [16,17].
2.13 Statistical analysis
Results are presented as the mean±S.E.M. Statistical analysis were performed using GraphPad Prism Version-5.0 software. Oneway analysis of variance (ANOVA) followed by Dunnet’s test was used for multiple comparisons. p < 0.05 was considered as statistically significant.
3 Results
3.1 Acetic acid-induced writhing test
As shown in Fig. 2A, average number of writhes was about 40 times in 0.6% acetic acid i.p. injected mice. ALO at the dosages of 80, and 40mg/kg significantly reduced the number of writhes induced by acetic acid (p < 0.01), but not at the 20mg/kg. Asp (400 mg/kg) had a similar effect as ALO in suppressing the writhing (p < 0.01), while morphine (10 mg/kg) almost completely inhibited the writhing response (p < 0.01).
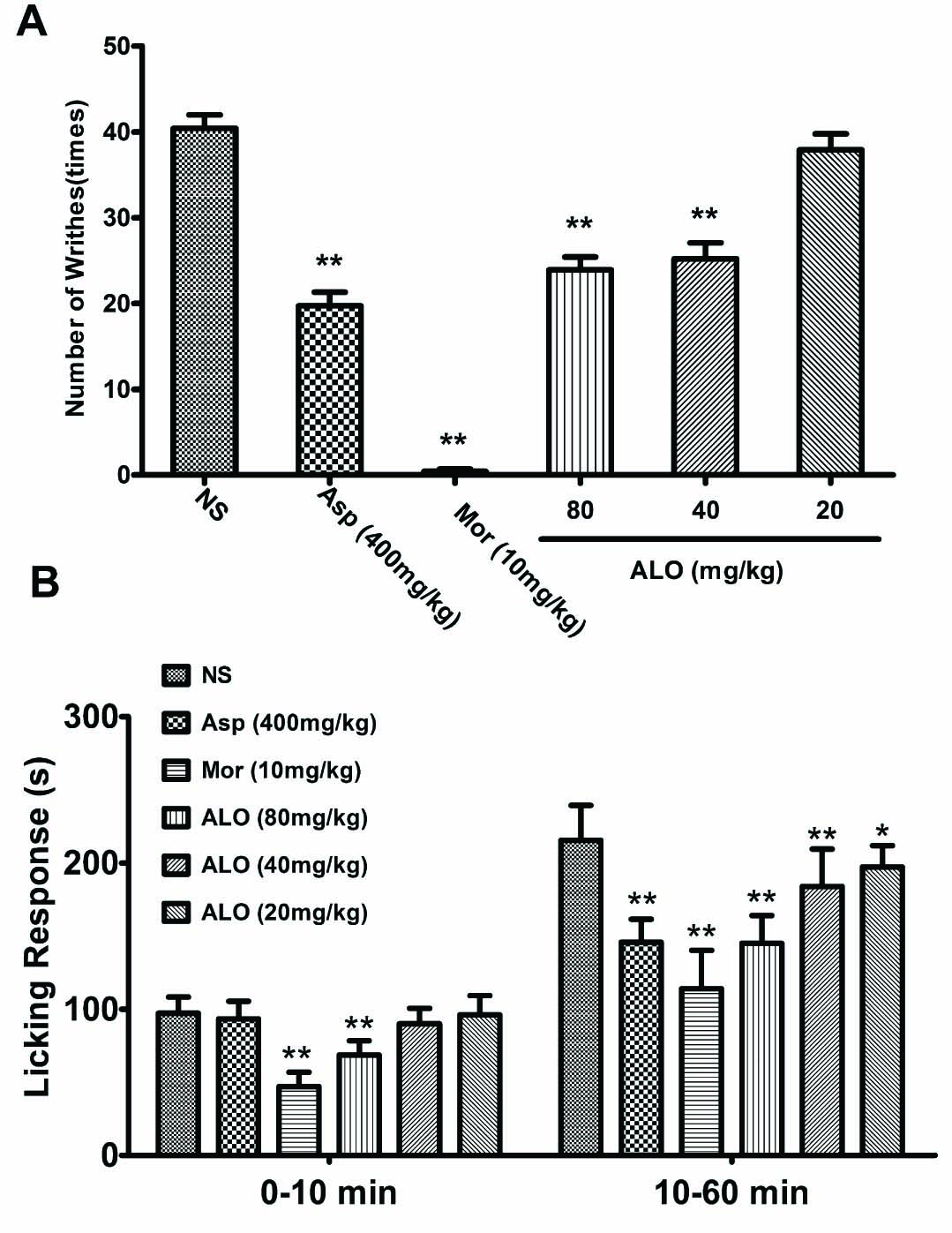
(A) Effects of ALO on acetic acid-induced writhing test. (B) Effects of ALO on formalin-induced pain test. Mice were treated with saline, aspirin (400 mg/kg), morphine (10mg/kg) or ALO (20, 40 and 80mg/kg). N = 10 in each group. * p < 0.05, ** p < 0.01 vs. control group (NS). Data were presented as the mean±S.E.M.
3.2 Formalin test
Injection of formalin into the right hind paw induces irritation and tissue swelling. The reaction can be divided into two phases. The first phase is the neurogenic phase that occurs between 0 and 10 min following the injection. The second phase is inflammatory phase that occurs after 10 min. Both neurogenic and inflammatory processes induced a licking response in the animals. In saline-pretreated mice, the average licking period was 100 s in the neurogenic phase and 220 s in the inflammatory phase (10-60 min). ALO at dose of 80 mg/kg inhibited the licking response in the neurogenic phase (p < 0.01). However, at lower dosages (40 and 20 mg/kg), ALO failed to suppress the licking response. ALO also suppressed the licking response in the inflammatory phase in a dose-dependent manner. In comparison, Asp (400mg/kg) reduced the licking time only in the inflammatory phase, but not in the neurogenic phase. Morphine offered the strongest inhibition in both neurogenic and inflammatory phases (p < 0.01) (Fig. 2B).
3.3 Hot-plate latency test
The normal reactionofananimal standingonahot plateistolick its paws to prevent tissue injury. The average (response) latency to licking was about 18s in saline pretreated mice (Fig. 3A). Pretreatment with ALO (20, 40, 80mg/kg) or Asp (400mg/kg) did not affect the latency as observed up to 120min following drug administration (p > 0.05). In contrast, morphine (10 mg/kg), which served as a reference pain suppressant in this study, significantly delayed latency (Fig. 3A).
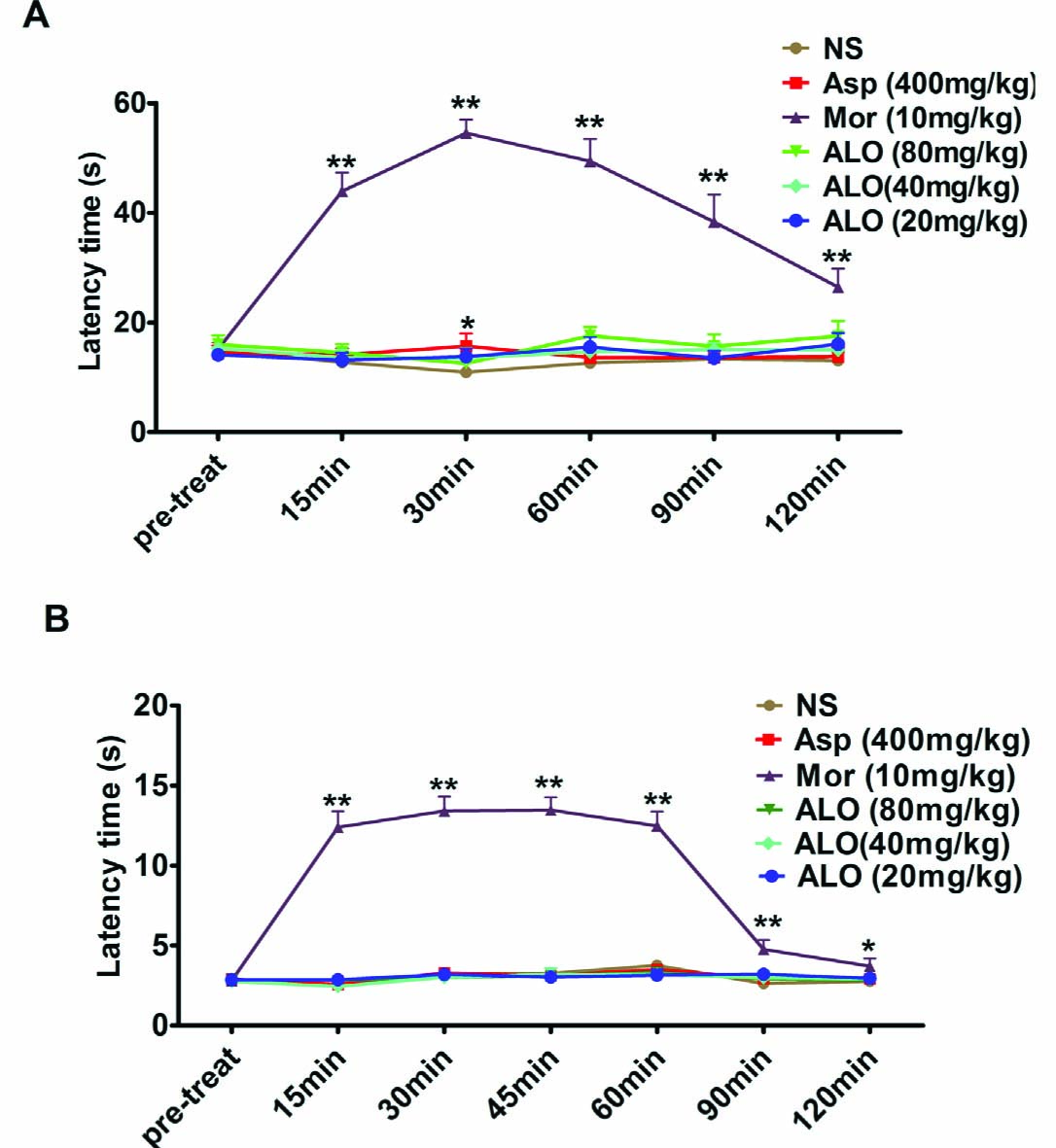
(A) Effects of ALO (ALO) on latencyto paw-lickingin the hot plate testin mice. (B) Influence of ALO on warm water tail-flick test in mice. Animals were treated intraperitoneally with saline, morphine (10 mg/kg) or ALO (20, 40 and 80mg/kg), or intragastric with aspirin (400 mg/kg). N = 10 in each group. * p < 0.05, ** p < 0.01 vs. control group (NS). Data were presented as the mean±S.E.M.
3.4 Tail flick test
Tail flick responseis often observed after submersion of amouse tail in hot water for 2–5s. As shown in Fig. 3B, saline pretreated mice had a 2–3s latency before a tail flick. Pretreatment with ALO (20, 40, 80mg/kg) or Asp (400mg/kg) had no effects on the latency to tail flick as observed for 0–120 min after drug administration (p > 0.05). In contrast, morphine (10 mg/kg) significantly increased the response latency (p < 0.01).
3.5 Spontaneous locomotor (exploratory) activity test and motor coordinate test
To exclude the possibility that ALO inhibits the pain-like behaviors responses through suppression of motor activity, we performed spontaneous locomotor activity and motor coordinate tests. The saline pretreated mice had an average spontaneous exploratory movement of 200 times per 5min. Pretreatment with ALO (80, 40, 20 mg/kg) did not influence the motor activity (Fig. 4A). Similarly, the average time that amouse could stay on a rotating rod was 290 s in saline pretreated group. Pretreatment with ALO had no effect on the time mice could stay on the rod. (Fig. 4B). These results suggest that ALO has no motor inhibitory effect.
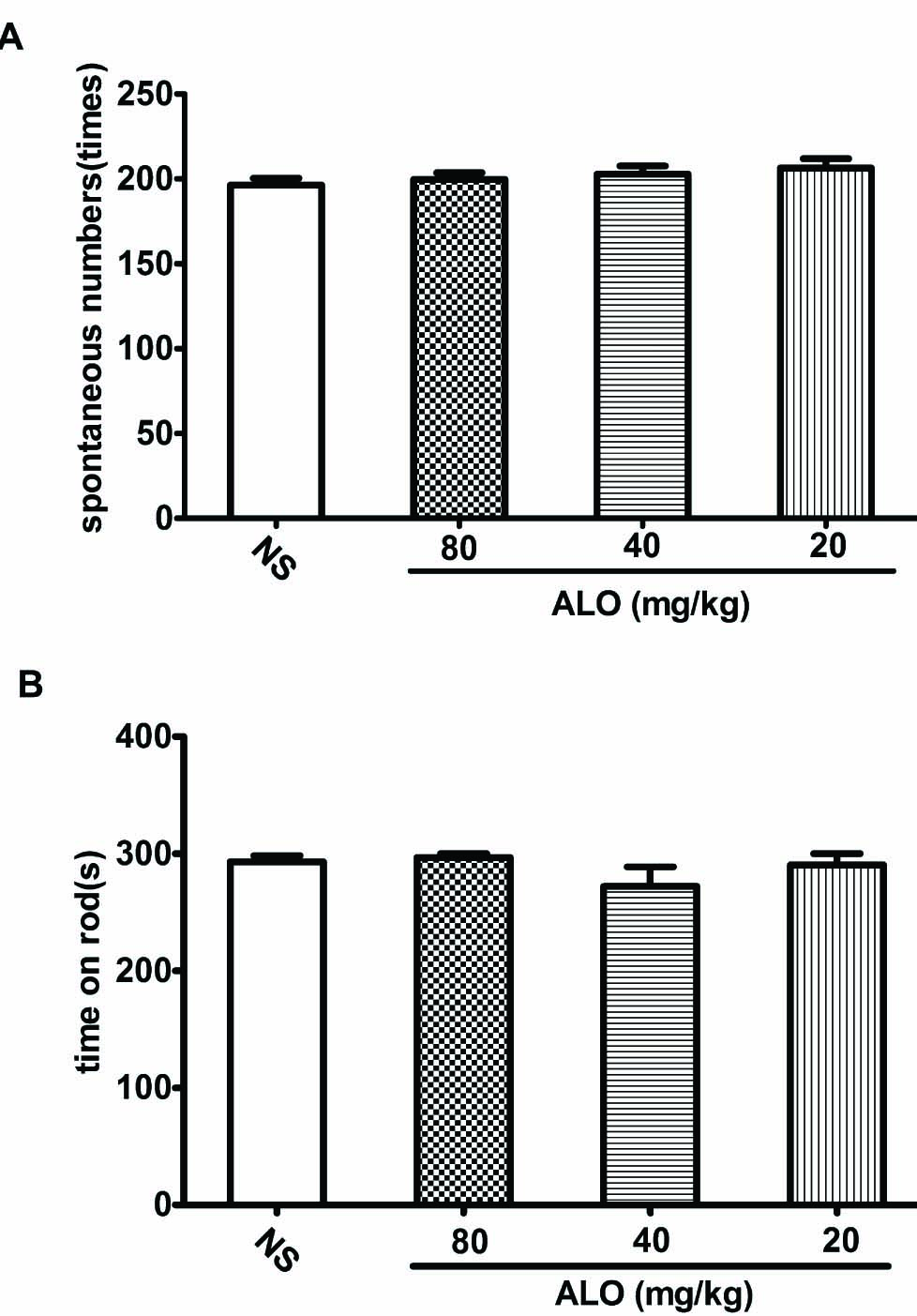
(A) Effects of ALO on locomotor activity test. (B) Effects of ALO on motor coordination test. Mice were treated with saline or ALO (20, 40 and80mg/kg). N = 10 in each group. * p < 0.05, ** p < 0.01 vs. control group. Data were presented as the mean±S.E.M.
3.6 Mouse ear swelling test (MEST)
The MEST was performed on the right ear and left ear served as reference. Pretreatment with ALO at dosages of 40 and 80mg/kg significantly reduced ear swelling comparing to that of saline-treated control (p < 0.01). Low dose of ALO (20mg/kg) did not reduce the ear swelling (p > 0.05). The Asp (400 mg/kg) effect was equivalent to that of 80mg/kg ALO (p < 0.01) in reducing ear swelling (Fig. 5).
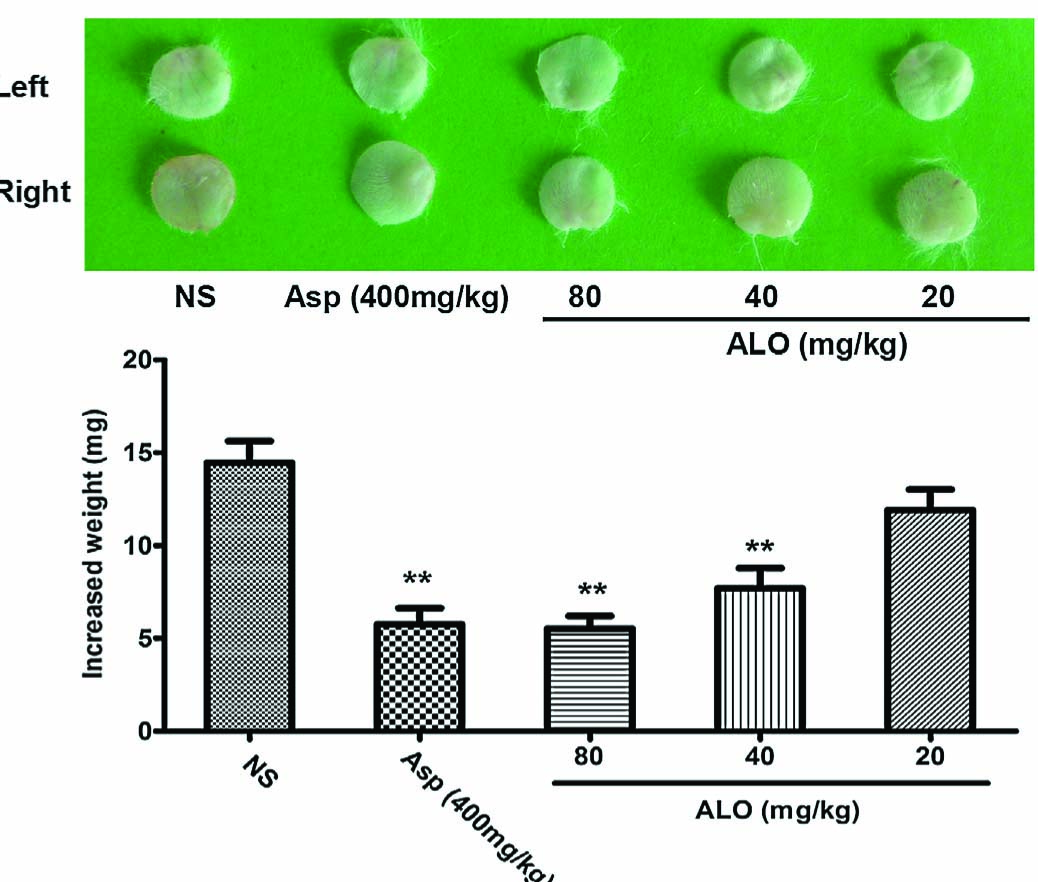
Effects of ALO on ear swelling test. Mice were treated with saline, aspirin (400 mg/kg), or ALO (20, 40 and 80mg/kg). N = 10 in each group. * p <0.05, ** p <0.01 vs. control group (xylene topical ear application). Data were presented as the mean±S.E.M.
3.7 Carrageenan induced paw edema test
Carrageenan was injected into the plantar of the right foot to induce edema. The left foot served as a reference. As shown in Fig. 6, carrageenan injection induced significant paw swelling as reflected by increased right foot volume. The edema increased with time after carrageenan injection. ALO (80 mg/kg) significantly reduced paw edema observed between 2h and 5h after carrageenan injection (p < 0.01). The reduction was lessened when ALO dose was reduced to 40 and 20 mg/kg. Treatment with Asp (400 mg/kg) produced similar effects as ALO (80 mg/kg) (p < 0.01).
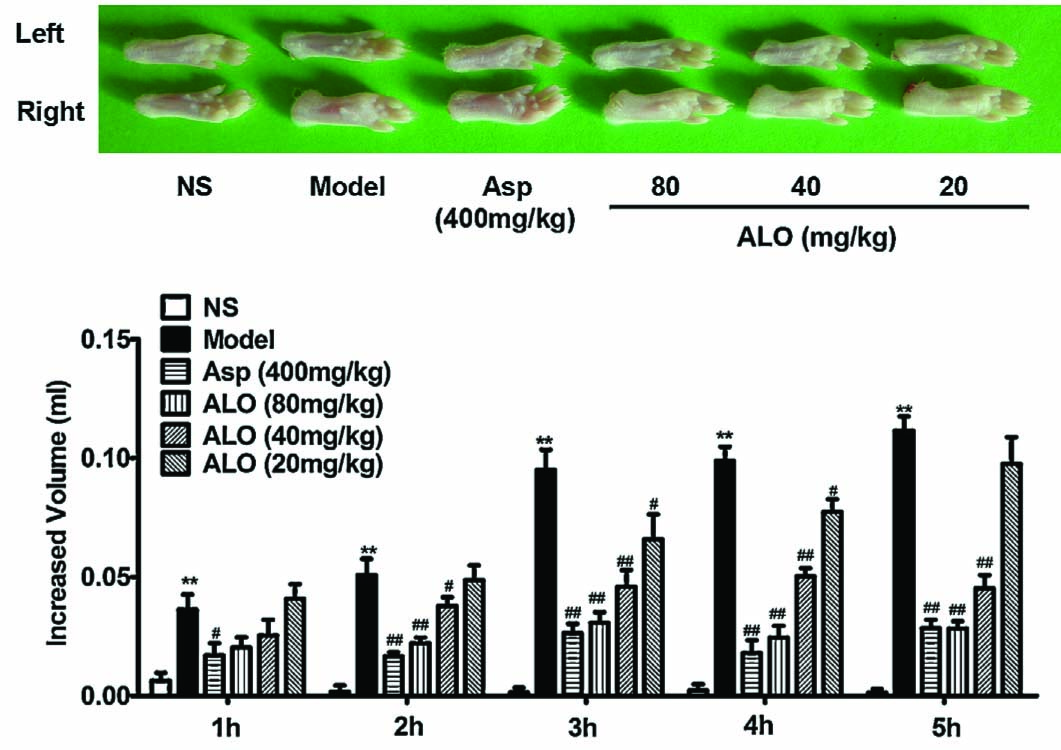
Effects of ALO on carrageenan-induced paw edema test. Mice were treated with saline, aspirin (400 mg/kg), or ALO (20,40 and 80 mg/kg). N = 10 in each group. # p < 0.05, ## p < 0.01 vs. control group; * p < 0.05, ** p < 0.01 vs. model group (xylene topical ear application). Data were presented as the mean ± S.E.M.
3.8 Contents of inflammatory cytokines in carrageenan-induced paw edema tissue
As shown in Fig. 7, carrageenan caused marked increases of PGE2, Il-1β, TNF-α and IL-10. ALO pretreatment at dosages of 40 and 80 mg/kg markedly reduced the levels of PGE2, Il-1 β and TNF-α, and also increased levels of IL-10. A lower dose of ALO (20 mg/kg) reduced Il-1 β, but failed to reduce PGE2 or TNF-α levels nor did it enhance IL-10 content. Asp (400 mg/kg) significantly reduces the contents of PGE2, IL-1 β and TNF-α and increased IL-10 (p < 0.01). The effect of ALO 80 mg/kg on inflammatory cytokines was equivalent to that of Asp 400 mg/kg.
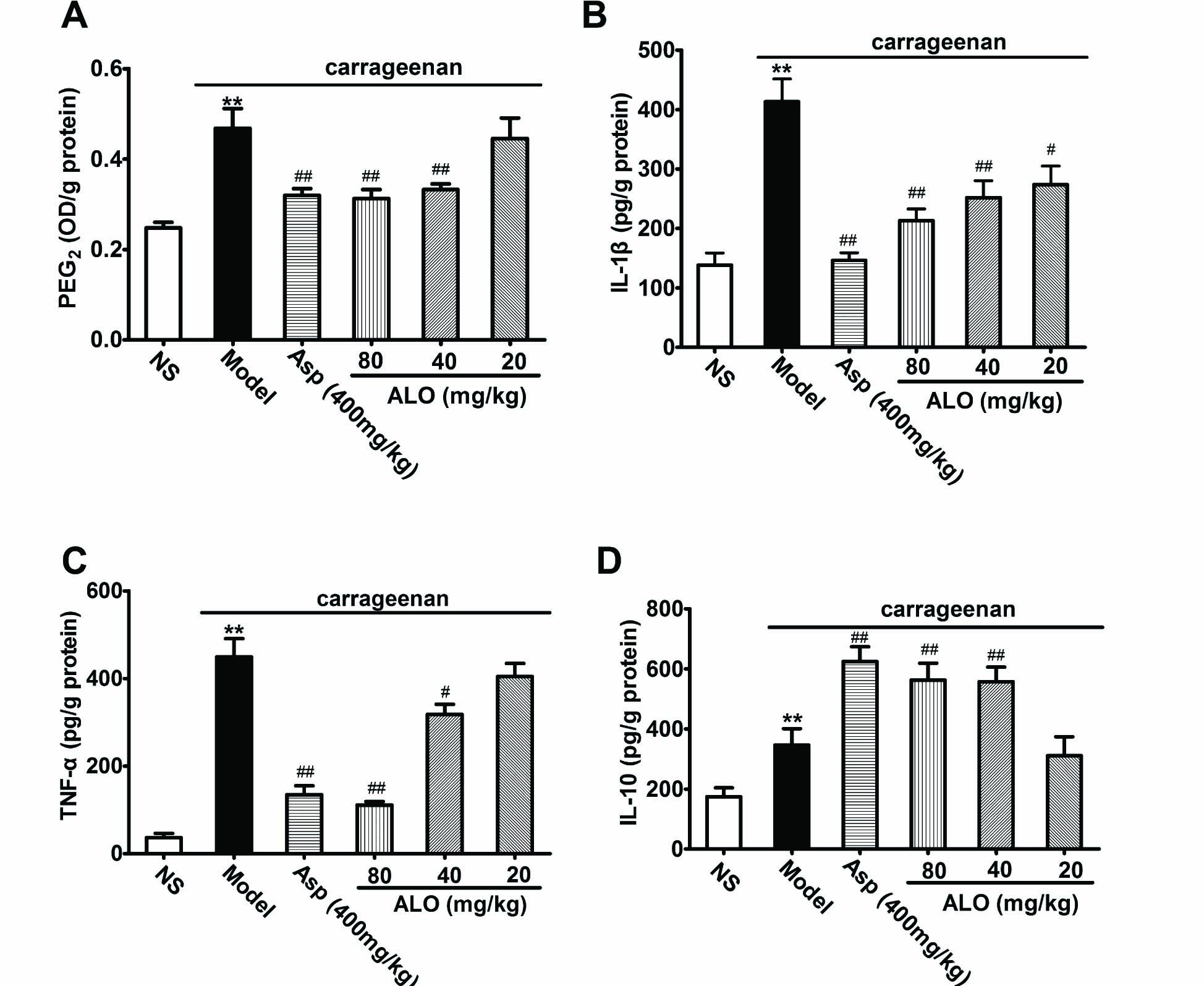
(A) Effects of ALO on content of PGE2; (B) effects of ALO on content of IL-1β; (C) effects of ALO on content of TNF-α; (D) effects of ALO on content of IL-10 in paw edema tissue induced by carrageenan. Mice were treated with saline, aspirin (400mg/kg), or ALO (20, 40 and 80mg/kg). N = 10 in each group. # p < 0.05, ## p < 0.01 vs. the control group; * p < 0.05, ** p < 0.01 vs. the model group, content of PGE2 was expressed as OD value/g of protein. Data were presented as the mean ± S.E.M.
4 Discussion
Pain is classified as acute pain, inflammatory pain and neuropathic pain. It functions as a protective response and warning of contact with potentially damaging stimuli [18]. In the present study, we evaluated the anti-nociceptive effects of ALO on mouse nociception models induced by the thermal and chemical stimuli. The results showed that ALO suppressed inflammatory pain and decreased protein levels of pro-inflammatory cytokines.
The acetic acid-induced writhing test has the advantage of detecting effects produced by weak analgesics and is widely employed as a method to screen novel analgesic agents. However, it is not a specific method for evaluating hypersensitivity [19]. It is generally believed that acetic acid injection induces secretions of endogenous mediators, such as bradykinin and serotonin, which stimulate the peripheral nociceptive receptors. Acetic acid also increases PGE2 level that is known to mediate inflammatory responses [20]. In this study, we observed that ALO reduced the number of writhes induced by acetic acid i.p. injection, suggesting that ALO has an analgesic effect.
The formalin-induced pain can be divided into two phases: the first phase (0-10 min) representing direct stimulating effects of formalin on nociceptors and the second phase (10-60 min) reflecting effects of formalin on inflammatory nociceptive responses [21,22]. High concentration of ALO (80 mg/kg) increased the latency to licking response during both the first and second phases. However, at low concentrations (40 and 20 mg/kg), ALO moderately decreased the licking response in the inflammatory phase but not in the neurogenic phase. These data suggest that ALO primarily inhibited inflammatory pain.
In order to exclude the possibility that reduced response to hypersensitivity in the ALO group might be caused by an unknown motor inhibitory side effect of the ALO, we performed the spontaneous locomotor activity test and rota-rod motor coordinate test [13]. ALO did not affect the motor activity of the animals in either test. This suggests that the observed inhibitory effects of ALO on pain-like behaviors cannot be ascribed to motor inhibition. This confirms that ALO has no sedative or hypnotic side effects, which is a desirable feature for analgesic drugs.
The hot plate test and tail-flick test examined the response to acute pain [23]. The tail-flick response is thought to be a reflex mediated by the spinal cord, whereas the hind paw licking response on a hot plate is a more complex. Normally, Aδ nerve fibers, serve as nociceptors, and conduct stimuli such as heat and pressures to the central nervous system where the signals are interpreted as fast/first pain. In response, the CNS initiates a protective reaction manifested as a withdrawal reflex to avoid the noxious stimuli [24]. We used the tail-flick and hot plate tests to examine if ALO could suppress nociception [25]. The results showed that neither ALO nor aspirin were able to delay the response latency to heat in the hot plate test, whereas morphine significantly prolonged the latency. Further, neither ALO, nor aspirin affected the latency to tail flick, whereas morphine did again. These results suggested that ALO had no effect on acute pain.
We then focused on the effects of ALO on inflammatory pain using the mouse ear swelling test and paw edema test. Topical application of xylene produces neurogenic acute inflammatory responses, such as vasodilatation, plasma leakage, and erythema, which result in mouse ear swelling. Our results demonstrated that ALO (80 and 40mg/kg) profoundly reduced the ear swelling, suggesting that ALO possesses anti-inflammatory effect. This was further supported by the results obtained from the carrageenan-induced paw edema test showing that ALO significantly reduced the animal foot swelling caused by carrageenan injection that has been commonly used to assess the anti-inflammatory effect of natural products. Carrageenan is a mucopolysaccharide derived from Irish Sea moss Chondrus. It produces localized acute inflammatory responses without inducing systemic reactions. The carrageenan-induced acute inflammation can be divided into two phases. In early phase (1–2h), histamine and serotonin mediate the formation of edema. In late phase (2–5 h), the high vascular permeability is mediated by capillary endothelial secretions of bradykinin and PGE2. These mediators participate in the inflammatory response, stimulate the nociceptors, induce and exacerbate hypersensitivity [26]. Our results demonstrated that ALO reduced the levels of pro-inflammatory cytokines and increased the levels of anti-inflammatory cytokines. It has previously been reported that during inflammation processes, prostaglandins released from damaged tissue directly excite sensory nerve endings and cause hypersensitivity [26,27]. Pro-inflammatory cytokines, such as TNF-aand IL-1 β play vital roles in mediating inflammatory pain. These cytokines increase the expression of COX-2, leading to an increase of PGE2. Further, PGE2 reduces the noxious stimulation threshold and enhances the sensitivity of peripheral nerve endings in inflammation pain. Meanwhile, anti-inflammatory cytokines, such as IL-10, inhibit the production and action of TNF-α and IL-1 β and suppress the expression of COX-2, causing further decrease of the PGE2 content. Our results showed that ALO markedly suppressed the expression of TNF-α, IL-1 β and PGE2, but increased the IL-10 levels. These data suggest that the anti-inflammatory effect of ALO is associated with suppression of pro-inflammatory cytokines and enhancement of anti-inflammatory cytokines.
It can be concluded that ALO is effective as an analgesic agent in various inflammatory pain models. These effects are most likely mediated via regulation of cytokine and prostaglandin synthesis. Our results support hypothesis that ALO has therapeutic potential for the management of inflammatory pain.
Highlights
Aloperine suppresses hypersensitivity in mouse.
Aloperine reduces inflammatory pain.
Aloperine exerts analgesic effect by suppressing pro-inflammatory cytokines.
DOI of refers to article: http://dx.doi.org/10.1016/j.sjpain.2015.04.025
-
Conflict of interest
Conflict of interest statement: The authors declare that there are no conflicts of interest.
Acknowledgments
This study received support from Ningxia Medical University General Program (NXM2013001) and Ningxia Science and Technology Research Projects (NGY2013073), China. The authors greatly appreciate Mr. David Lamson for proof-read and editing the manuscript.
References
[1] Brosius AD, Overman LE. Aloperine stereocontrolled synthesis of two stereoisomers and determination of absolute configuration. J OrgChem 1997;62:440–1.Search in Google Scholar
[2] Lin WC, Lin JY. Five bitter compounds display different anti-inflammatory effects through modulating cytokine secretion using mouse primary splenocytes in vitro. J Agric Food Chem 2011;59:184–92.Search in Google Scholar
[3] Xu YQJin SJ, Liu N. Aloperine attenuated neuropathic pain induced by chronic constriction injury via anti-oxidation activity and suppression of the nuclear factor kappa B pathway. Biochem Biophys Res Commun 2014;451:568–73.Search in Google Scholar
[4] Yuan XY, Liu W, Zhang P, Wang RY, Guo JY. Effects and mechanisms of Aloperine on 2, 4-dinitrofluorobenzene-induced allergic contact dermatitis in BALB/c mice. EurJ Pharmacol 2010;629:147–52.Search in Google Scholar
[5] Zhou Y, Wang H, Liang L, Zhao WC, Chen Y, Deng HZ. Total alkaloids of Sophora alopecuroides increase the expression of CD4+ CD25+ Tregs and IL10 in rats with experimental colitis. Am J Chin Med 2010;38:265–77.Search in Google Scholar
[6] Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev 2001;53:597–652.Search in Google Scholar
[7] Ulmann L, Hirbec H, Rassendren F. P2X4 receptors mediate PGE2 release by tissue-resident macrophages and initiate inflammatory pain. EMBO J 2010;29:2290–300.Search in Google Scholar
[8] Mahgoub AA. Grapefruit juice potentiates the anti-inflammatory effects of diclofenac on the carrageenan induced rat’s paw oedema. Pharmacol Res 2002;45:1–4.Search in Google Scholar
[9] Chang TN, Deng JS, Chang YC, Lee CY, Jung-Chun L, Lee MM, Peng WH, Huang SS, Huang GJ. Ameliorative effects of scopoletin from Crossostephium chinensis against inflammation pain and its mechanisms in mice. Evid Based Complement Alternat Med 2012;2012:595–603.Search in Google Scholar
[10] Vervoordeldonk MJ, Pineda Torra IM, Aarsman AJ, van den Bosch H. Aspirin inhibits expression of the interleukin-1beta-inducible group II phospholipase A2. FEBS Lett 1996;397:108–12.Search in Google Scholar
[11] Shackelford RE, Alford PB, Xue Y, Thai SF, Adams DO, Pizzo S. Aspirin inhibits tumor necrosis factoralphagene expressioninmurine tissue macrophages. Mol Pharmacol 1997;52:421–9.Search in Google Scholar
[12] Keyhanfar F, Shamsi Meymandi M, Sepehri G, Rastegaryanzadeh R, Heravi G. Evaluation of antinociceptive effect of pregabalin in mice and its combination with tramadol using tail flick test. Iran J Pharm Res 2013;12:483–93.Search in Google Scholar
[13] Haiyan W, Yuxiang L, Linglu D, Tingting X, Yinju H, Hongyan L, Lin M, Yuanxu J, Yanrong W, Jianqiang Y. Antinociceptive effects of matrine on neuropathic pain induced by chronic constriction injury. Pharm Biol 2013;51:844–50.Search in Google Scholar
[14] Chao J, Liao JW, Peng WH, Lee MS, Pao LH, Cheng HY. Antioxidant, analgesicanti-inflammatory, and hepatoprotective effects of the ethanol extract of Mahonia oiwakensis stem. Int J Mol Sci 2013;14:2928–45.Search in Google Scholar
[15] Mahdi MF, Mohammed MH, Jassim AA. Design, synthesis and preliminary pharmacological evaluation of new non-steroidal anti-inflammatory agents having a 4-(methylsulfonyl) aniline pharmacophore. Molecules 2012;17:1751–63.Search in Google Scholar
[16] Borghi SM, Carvalho TT, Staurengo-Ferrari L, Hohmann MS, Pinge-Filho P, Casagrande R, Verri Jr WA. Vitexin inhibits inflammatory pain in mice by targeting TRPV1 oxidative stress, and cytokines. J Nat Prod 2013;76:1141–9.Search in Google Scholar
[17] Panthong A, Kanjanapothi D, Taesotikul T, Wongcome T, Reutrakul V. Anti-inflammatory and antipyretic properties of Clerodendrum petasites S Moore. J Ethnopharmacol 2003;85:151–6.Search in Google Scholar
[18] Bocheva A, Mikhova B, Taskova R, Mitova M, Duddeck H. Anti-inflammatory and analgesic effects of Carthamus lanatus aerial parts. Fitoterapia 2003;74:559–63.Search in Google Scholar
[19] Ji W, Cui C, Zhang Z, Liang J. Paradoxic effects of propofol on visceral pain induced by various TRPV1 agonists. Exp Ther Med 2013;5:1259–63.Search in Google Scholar
[20] Bouffi C, Bony C, Courties G, Jorgensen C, Noël D. IL-6-dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PLoS ONE 2010;5:e142–7.Search in Google Scholar
[21] Devi BP, Boominathan R, Mandal SC. Anti-inflammatory, analgesic and antipyretic properties of Clitoria ternatea root. Fitoterapia 2003;74:345–9.Search in Google Scholar
[22] Sahranavard S, Kamalinejad M, Faizi M. Evaluation of anti-inflammatory and anti-nociceptive effects of defatted fruit extract of Olea europaea. Iran J Pharm Res 2014;13:119–23.Search in Google Scholar
[23] Lin YR, Chen HH, Ko CH, Chan MH. Effects of honokiol and magnolol on acute and inflammatory pain models in mice. Life Sci 2007;81:1071–8.Search in Google Scholar
[24] Radulović NS, Miltojević AB, McDermott M, Waldren S, Parnell JA, Pinheiro MM, Fernandes PD, de Sousa Menezes F. Identification of a new antinociceptive alkaloid isopropyl N-methylanthranilate from the essential oil of Choisya ternata Kunth. J Ethnopharmacol 2011;135:610–9.Search in Google Scholar
[25] Nwafor PA, Okwuasaba FK. Anti-nociceptive and anti-inflammatory effects of methanolic extract of Asparagus pubescens root in rodents. J Ethnopharmacol 2003;84:125–9.Search in Google Scholar
[26] Kim HS, Park JW, Kwon OK, Kim JH, Oh SR, Lee HK, Bach TT, Quang BH, Ahn KS. Anti-inflammatory activity of a methanol extract from Ardisia tinctoria on mouse macrophages and paw edema. Mol Med Rep 2014;9:1388–94.Search in Google Scholar
[27] Lee JH, Choi YH, Choi BT. The anti-inflammatory effectsof2Hz electroacupuncture with different intensities on acute carrageenan-induced inflammation in the rat paw. Int J Mol Med 2005;16:99–102.Search in Google Scholar
© 2015 Scandinavian Association for the Study of Pain
Articles in the same Issue
- Editorial comment
- An overlooked cause of head- and neck-pain: Chronic canalithiasis, or Benign Paroxysmal Positional Vertigo – BPPV
- Observational study
- Treatment of chronic canalithiasis can be beneficial for patients with vertigo/dizziness and chronic musculoskeletal pain, including whiplash related pain
- Editorial comment
- Exploring the impact of comorbid primary headaches and neck pain
- Clinical pain research
- Migraine co-existing tension-type headache and neck pain: Validation of questionnaires
- Editorial comment
- Pain outside of the hospital: What is the situation in pre-hospital care, and how could it be improved?
- Observational study
- Prehospital personnel’s attitudes to pain management
- Editorial comment
- The anti-inflammatory alkaloid aloperine in Chinese herbal medicine is potentially useful for management of pain and itch
- Original experimental
- Effects of aloperine on acute and inflammatory pain models in mice
- Editorial comment
- The sodium-channel blocker lidocaine in subanesthetic concentrations reduces spontaneous and evoked pain in human painful neuroma
- Original experimental
- Differential analgesic effects of subanesthetic concentrations of lidocaine on spontaneous and evoked pain in human painful neuroma: A randomized, double blind study
- Editorial comment
- Advances in pain management and research presented at the 2015 Scientific Meeting of the Scandinavian Association for the Study of Pain (SASP)
- Abstracts
- Spinal disulfide HMGB1, but not all-thiol HMGB1, induces mechanical hypersensitivity in a TLR4-dependent manner
- Abstracts
- Practitioners’ perspective on pain disabilities in Ghanaian women. A qualitative study
- Abstracts
- Association between chronic pain and the sperm motion characteristics
- Abstracts
- Spontaneous burrowing behavior as an outcome measure of the global impact of chronic pain in preclinical models of arthritis
- Abstracts
- Effects of habituation to the testing facility on mechanical nociceptive thresholds of pigs
- Abstracts
- Chronic nonmalignant pain at a mono-disciplinary pain clinic. Identifying the key patient groups for targeted health promotion – A cohort study
- Abstracts
- Characterization of complex chronic pain patients
- Abstracts
- Increased C-fiber response induced by experimental disc herniation is associated with upregulation of fractalkine and its receptor in nucleus pulposus and dorsal root ganglion
- Abstracts
- Chronic pain-related patient-provider communication: The significance of health related quality of life and satisfaction
- Abstracts
- Gender differences in chronic pain related health care utilization
- Abstracts
- Cerebrospinal fluid levels of substance P (SP) N-terminal fragment SP1–7 in patients with neuropathic pain
- Abstracts
- Characterization of small nerve fibers in painful distal symmetric polyneuropathy and healthy controls
- Abstracts
- High-throughput screening reveals enzyme and GPCR targets as putative binding sites for D-deprenyl
- Abstracts
- New players in the mechanism of spinal cord stimulation for neuropathic pain
- Abstracts
- Hyperalgesia after experimental and work-related sleep restriction
- Abstracts
- Local up-regulation of interferon-γ (IFN-γ following disc herniation is involved in the inflammatory response underlying acute lumbar radicular pain
- Abstracts
- The effect of tail docking in neonatal pigs on the central expression of genes involved in modulating anxiety-like behaviour
- Abstracts
- Blockage of lysophosphatidic acid reverses arthritis-induced hypersensitivity and Cavα2δ1 and P2X3 expression in dorsal root ganglia
- Abstracts
- Pain intensity and duration in a cohort of Norwegian construction workers
- Abstracts
- The effectiveness of a nursing staff development Intervention to improve pain management – A randomized controlled trial
Articles in the same Issue
- Editorial comment
- An overlooked cause of head- and neck-pain: Chronic canalithiasis, or Benign Paroxysmal Positional Vertigo – BPPV
- Observational study
- Treatment of chronic canalithiasis can be beneficial for patients with vertigo/dizziness and chronic musculoskeletal pain, including whiplash related pain
- Editorial comment
- Exploring the impact of comorbid primary headaches and neck pain
- Clinical pain research
- Migraine co-existing tension-type headache and neck pain: Validation of questionnaires
- Editorial comment
- Pain outside of the hospital: What is the situation in pre-hospital care, and how could it be improved?
- Observational study
- Prehospital personnel’s attitudes to pain management
- Editorial comment
- The anti-inflammatory alkaloid aloperine in Chinese herbal medicine is potentially useful for management of pain and itch
- Original experimental
- Effects of aloperine on acute and inflammatory pain models in mice
- Editorial comment
- The sodium-channel blocker lidocaine in subanesthetic concentrations reduces spontaneous and evoked pain in human painful neuroma
- Original experimental
- Differential analgesic effects of subanesthetic concentrations of lidocaine on spontaneous and evoked pain in human painful neuroma: A randomized, double blind study
- Editorial comment
- Advances in pain management and research presented at the 2015 Scientific Meeting of the Scandinavian Association for the Study of Pain (SASP)
- Abstracts
- Spinal disulfide HMGB1, but not all-thiol HMGB1, induces mechanical hypersensitivity in a TLR4-dependent manner
- Abstracts
- Practitioners’ perspective on pain disabilities in Ghanaian women. A qualitative study
- Abstracts
- Association between chronic pain and the sperm motion characteristics
- Abstracts
- Spontaneous burrowing behavior as an outcome measure of the global impact of chronic pain in preclinical models of arthritis
- Abstracts
- Effects of habituation to the testing facility on mechanical nociceptive thresholds of pigs
- Abstracts
- Chronic nonmalignant pain at a mono-disciplinary pain clinic. Identifying the key patient groups for targeted health promotion – A cohort study
- Abstracts
- Characterization of complex chronic pain patients
- Abstracts
- Increased C-fiber response induced by experimental disc herniation is associated with upregulation of fractalkine and its receptor in nucleus pulposus and dorsal root ganglion
- Abstracts
- Chronic pain-related patient-provider communication: The significance of health related quality of life and satisfaction
- Abstracts
- Gender differences in chronic pain related health care utilization
- Abstracts
- Cerebrospinal fluid levels of substance P (SP) N-terminal fragment SP1–7 in patients with neuropathic pain
- Abstracts
- Characterization of small nerve fibers in painful distal symmetric polyneuropathy and healthy controls
- Abstracts
- High-throughput screening reveals enzyme and GPCR targets as putative binding sites for D-deprenyl
- Abstracts
- New players in the mechanism of spinal cord stimulation for neuropathic pain
- Abstracts
- Hyperalgesia after experimental and work-related sleep restriction
- Abstracts
- Local up-regulation of interferon-γ (IFN-γ following disc herniation is involved in the inflammatory response underlying acute lumbar radicular pain
- Abstracts
- The effect of tail docking in neonatal pigs on the central expression of genes involved in modulating anxiety-like behaviour
- Abstracts
- Blockage of lysophosphatidic acid reverses arthritis-induced hypersensitivity and Cavα2δ1 and P2X3 expression in dorsal root ganglia
- Abstracts
- Pain intensity and duration in a cohort of Norwegian construction workers
- Abstracts
- The effectiveness of a nursing staff development Intervention to improve pain management – A randomized controlled trial

