Abstract
Background and purpose
Spinal cord injury (SCI) has detrimental consequences that include chronic neuropathic pain, which is seen in 40-50% of patients, and symptoms of anxiety and depression, which affect 13-45% of SCI patients. The coexistence of pain, anxiety, and depression is known from other neuropathic pain conditions, but the relationship between these symptoms is not clear and has not been investigated in a preclinical model of SCI so far.
The aim of this study was to investigate anxiety-like behavior and at-level mechanical hypersensitivity following experimental spinal cord contusion (SCC) in female Sprague-Dawley rats, and the effects of analgesic and anxiolytic drugs.
Methods
Mechanical sensitivity and elevated plus maze (EPM) behavior were measured pre- and postinjury in SCC and sham animals. Pregabalin 30 mg/kg, morphine 3 mg/kg, midazolam 0.5 mg/kg, and 0.9% NaCl were evaluated in a randomly allocated, blinded balanced design.
Results
SCC animals developed persistent at-level mechanical hypersensitivity and decreased open arm activity in the EPM, which indicates an anxiety-like state. Pregabalin, a dual-acting analgesic and anxiolytic drug reduced both hypersensitivity and anxiety-like behavior, while the analgesic drug morphine only reduced hypersensitivity. The anxiolytic drug midazolam in the dose used had no effect on either parameter.
Conclusions
SCC animals developed long lasting coexisting at-level mechanical hypersensitivity and anxiety-like behavior, but there was no evidence to support a causal relationship between pain and anxiety following SCI.
Implications
The findings that at-level mechanical hypersensitivity and anxiety-like behavior develops concomitantly in the spinal cord contusion models and that both symptoms is persistent provide basis for further investigation of the mechanisms and connection behind these two clinically relevant symptoms after injury to the central nervous system.
1 Introduction
A spinal cord injury (SCI) may have many detrimental consequences including chronic neuropathic pain [1], which is seen in 40-50% of patients [2,3,4], and symptoms like anxiety and depression, which affect 13-45% of SCI patients [5,6,7,8,9]. Other serious problems include pareses, spasticity and spasms, decreased bladder and bowel function, and autonomic dysfunction. The coexistence of pain, anxiety and depression is known from other neuropathic pain conditions [10,11,12,13,14,15], but the underlying mechanisms are not clear, although a common pathophysiological mechanism seems unlikely [16,17,18].
The intensity of pain in SCI has been associated with increased levels of anxiety [19], and Nicholson et al. found a significant difference in pain severity when comparing those with and without possible clinical levels of anxiety and depression [8]. However, the relation between pain and anxiety is complex, and anxiety levels in SCI have also been shown to relate to, e.g., gastrointestinal symptoms [20], autonomic dysfunction [21], severe complications, and level of autonomy [6], and to be more affected by other consequences of SCI than pain [22].
Given the unclear relationship between symptoms of anxiety and pain in SCI, there is a need for additional research, and preclinical studies may represent a novel approach. Preclinical pain research has frequently utilized simple measurements such as paw withdrawal or other reflexive behaviour as the only outcomemeasure, representing, at best, only the sensory component of the pain experience [23,24]. In contrast, multiple different endpoints are recommended in clinical trials on pain conditions, including measurements of anxiety, depression, sleep disturbances, and quality of life [25]. Recently, focus on the importance of these comorbidities as well as on the need to include measurements of the affective-motivational component of pain has increased in preclinical research. Methodology has been updated with novel pain assays developed to measure the affective component of pain (e.g., the place escape/avoidance paradigm and operant escape) [26,27,28,29], and endpoints that measure symptoms such as anxiety are included (e.g., the elevated plus maze or the open field) [30,31], thereby attempting to bridge the gap between preclinical and clinical research.
Distance travelled in the open field and time spent in the EPM by animals with a 12.5mm SCC at bone segment T10. Data is presented as average distance (cm)±S.E.M.
| Total distance (cm) open field | Open arms (cm) elevated plus maze | |||
|---|---|---|---|---|
|
|
|
|||
| SCC | Sham | SCC | Sham | |
| Preinjury | 4009 ± 91 | 3942 ± 151 | 76.58 ± 9.74 | 74.73 ± 8.03 |
| 4 weeks | 2277 ± 177[*] | 2478 ± 213[*] | 35.72 ± 11.83[*] | 34.58 ± 8.10[*] |
| 6 weeks | - | - | 13.58 ± 5.16* | 58.95 ± 8.49 |
| 12 weeks | 854 ± 58[*] | 969 ± 93 | 10.01 ± 3.66[*] | 39.73 ± 8.28[*] |
| NaCl | 2187 ± 179 | 2249 ± 261 | 8.35 ± 4.11 | 74.41 ± 16.93 |
| Pregabalin | 2139 ± 164 | 2597 ± 269 | 17.20 ± 8.42[#] | 59.27 ± 16.64 |
| Morphine | 2249 ± 249 | 2765 ± 182 | 2.63 ± 1.87 | 56.46 ± 20.11 |
| Midazolam | 1895 ± 212 | 1712 ± 272[##] | 10.10 ± 3.97 | 68.45 ± 11.28 |
-
The total distances for both SCC and sham animals were not different at any time point (Injury group: p = 0.564; mixed model) or between treatments (Injury group: p = 0.290, mixed model). There was a significant effect of time (p < 0.001, mixed model) and treatment (p = 0.008, mixed model).
Previous studies that have combined measurements of mechanical hypersensitivity and anxiety-like behaviour in models of neuropathic pain, e.g., partial nerve ligation [32,33], sciatic nerve ligation in mice [34] and rats [35], varicella zoster-associated pain [35], and HIV-associated neuropathic pain [36], have found significantly increased anxiety-like behaviour 0-4 weeks after injury. Moreover, analgesics reversed both mechanical hypersensitivity and anxiety, which supports the hypothesis that anxiety may be a consequence of persistent pain in these models. Other studies using the spinal nerve ligation model [37] and a mouse model of sciatic nerve ligation [38] did, however, not find significant signs of anxiety. No preclinical studies have so far investigated the relation between hypersensitivity and anxiety in a model of SCI or in an extended period including the later more chronic stage.
We have previously characterized the central pain syndrome following spinal cord contusion (SCC) in rodents. This clinically relevant model [39,40] resulted in persistent and robust evoked at-level mechanical hypersensitivity [23,29,41].
The aim of the study was to investigate whether anxiety-like behaviour could be observed following SCC, and if the anxiety-like behaviour would be present concomitantly with evoked at-level mechanical hypersensitivity. Furthermore, we compared the antihypersensitivity and analgesic and anxiolytic properties of a classical anxiolytic drug (midazolam), an analgesic drug (morphine), and a mixed anxiolytic and analgesic drug (pregabalin).
2 Results
2.1 General observations
A total of 42 animals were included in the study. The average time to a locomotor score ≥4 was 12 days for SCC animals and 1 day for sham animals. Sporadic mild spontaneous spasms were initially observed in SCC animals, but the mobility, evaluated by the total distance travelled in the open field, was similar for both SCC and sham animals at all times tested (injury group: p = 0.564, time: p < 0.001; mixed model) (Table 1). There were no significant differences in general health (e.g., weight and fur coat) or behaviour between the injury groups, nor were any signs of distress observed (e.g., self-inflicted abrasions, spontaneous or handlingevoked vocalization).
2.2 Development of mechanical hypersensitivity
The animals were tested for mechanical sensitivity preinjury and 4, 6, and 12 weeks postinjury. Preinjury, no difference between the injury groups was observed (p = 0.131, Student's t-test) (Fig. 1a). Following injury, SCC animals (n = 20) had significantly lower at-level mechanical sensitivity thresholds than sham animals (n = 21) (p < 0.001, mixed model excluding preinjury). At-level mechanical sensitivity thresholds of SCC animals were, in contrast to the sham animals (p = 0.156, mixed model), significantly decreased postinjury compared with preinjury (p < 0.001, mixed model) remaining low throughout the test period. The thresholds were on average decreased by 71% (8.51-2.46 g) in the SCC animals as compared with preinjury, and at 6 and 12 weeks postinjury, SCC animals had a 65% lower threshold than sham animals. One SCC animal did not develop any mechanical hypersensitivity and was thus excluded from the drug study.
2.3 Development of anxiety
Anxiety levels were determined by the time spent in the open arms of the EPM. Preinjury, there was no difference between the injury groups (p = 0.884, Student's t-test) (Fig. 1b). An overall effect of injury group was observed (p = 0.036, mixed model excluding preinjury), and both SCC and sham animals experienced an initial decrease in time spent in open arms 2 weeks postinjury (p < 0.001, SCC and p = 0.003, sham; mixed model contrast), with a further decrease in SCC animals at 6weeks (p = 0.017, mixed model contrast) lasting throughout the test period. The decrease amounted on average to 85% at 6 and 12 weeks compared with preinjury. In sham animals, in contrast, there was no difference at 6weeks compared with preinjury (p = 0.101, mixed model contrast), nor were the levels at 6 and 12 weeks significantly different (p = 0.067, mixed model contrast). SCC animals spent less time (76% on average) in the open arms compared with sham animals at both 6 and 12 weeks postinjury (p < 0.001 and p = 0.003 respectively, Student's t-test). Repeating the mixed model analysis including the total distance travelled in the EPM as a covariate did not affect the above conclusions.
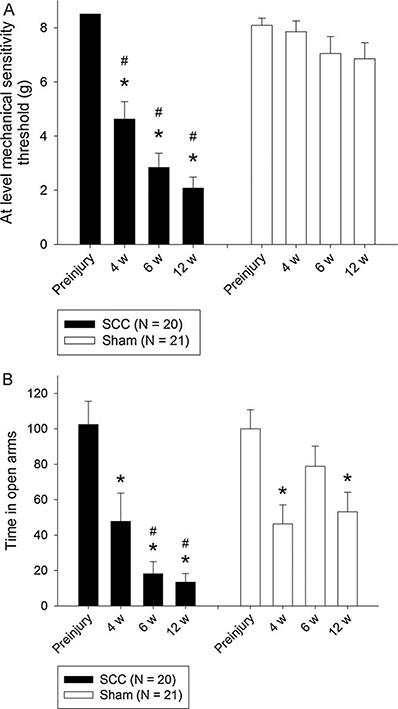
Development of mechanical hypersensitivity (a) and anxiety (b) in animals with a 12.5mm SCC at bone segment T10. Data are presented as average at-level mechanical sensitivity thresholds (g)±S.E.M. *p < 0.05 compared with preinjury. #p < 0.05 compared with sham group. (A) SCC animals had a lower threshold 4, 6, and 12 weeks postinjury as compared with preinjury (p < 0.001, mixed model). There is no significant difference between 6 and 12 weeks postinjury (pre- and postpharmacological testing) (p = 0.191, mixed model contrast). (B) SCC animals spent less time in the open arms of the EPM 4, 6, and 12 weeks postinjury compared with preinjury (p = 0.036, mixed model excluding preinjury). There is no significant difference between 6 and 12 weeks postinjury (pre- and postpharmacological treatment). Data have been normalized to the mean value of the preinjury measurement.
2.4 Correlation between mechanical sensitivity and anxiety
Linear correlation between mechanical sensitivity and anxiety was evaluated based on the time spent in the open arms as a function of at-level mechanical sensitivity thresholds at 6 weeks postinjury. There was no apparent relationship (R2 = 0.378, p = 0.091, Spearman ranked correlation) between the two parameters.
2.5 Effect of pregabalin, morphine, and midazolam on mechanical sensitivity
The effect on mechanical hypersensitivity of a single dose of an anxiolytic and two analgesics was examined in a subgroup of animals (n = 23). In SCC animals, a statistically significant effect of pregabalin (p < 0.001, mixed model contrast) and morphine treatment (p = 0.001, mixed model contrast) compared with saline treatment was observed, whereas treatment with midazolam resulted in no significant effect (p = 0.323, mixed model contrast) (Fig. 2a). Pregabalin and morphine both increased the at-level mechanical sensitivity thresholds to approximately the same level as seen in the saline-treated sham animals, but not to preinjury level. The at-level mechanical sensitivity thresholds for sham animals were not affected by either treatment (p = 0.178, mixed model).
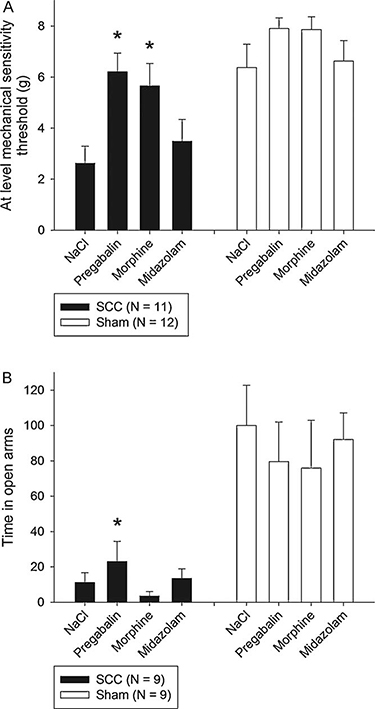
Treatment effect on mechanical hypersensitivity (a) and anxiety (b) in animals with a 12.5mm SCC at T10. Data are presented as average at-level mechanical sensitivity thresholds (g)±S.E.M. *p < 0.05 compared with NaCl 0.9%. (A) SCC animals had an increase in at-level mechanical sensitivity threshold after treatment with 30 mg/kg pregabalin IP (p < 0.001, mixed model contrast) and 3 mg/kg morphine IP (p = 0.001, mixed model contrast) but no significant effect of midazolam 0.5 mg/kg IP (p = 0.323, mixed model contrast). Sham animals had no treatment effect of either drug (p = 0.178, mixed model). (B) SCC animals had a twofold increase in open arm activity after treatment with 30 mg/kg pregabalin IP (p < 0.001, mixed model contrast), whereas 3mg/kg morphine IP (p = 0.425, mixed model contrast) and 0.5 mg/kg midazolam IP (p = 0.675, mixed model contrast) had no significant effect. Sham animals had no treatment effect of either drug (p = 0.710, mixed model). Data have been normalized to the mean value of the saline measurement.
2.6 Effect of pregabalin, morphine, and midazolam on anxiety
The effect on anxiety of a single dose of one anxiolytic and two analgesic drugs was estimated in another subgroup of animals (n = 18). Pregabalin increased the open arm occupation of SCC animals by twofold (p < 0.001, mixed model contrast), though the absolute level was still substantially lower than the saline-treated sham animals (increase from 11% to 23%) and preinjury levels (Fig. 2b). The second analgesic drug morphine did not have any treatment effect (p = 0.425, mixed model contrast), and it appeared to decrease the open arm activity rather than increase it. The anxiolytic drug midazolam had no apparent effect compared with saline (p = 0.675, mixed model contrast). None of the pharmacological drugs were effective in sham animals (p = 0.710, mixed model). Repeating the mixed model analysis including the total distance travelled in the EPM as a covariate did not affect the above conclusions.
2.7 Effect of pregabalin, morphine, and midazolam on mobility
Side effects of the drugs, e.g., sedation and somnolence, were evaluated by the total distance travelled in the open field following administration (Table 1). An overall treatment effect was observed (p = 0.006, mixed model), but no group difference (p = 0.290, mixed model). Midazolam treatment of sham animals resulted in borderline significantly lower distances travelled than saline-treated sham animals (p = 0.054, mixed model contrast), but this effect was not observed for SCC animals. None of the other treatments had a significant effect on either group.
3 Discussion
The current study demonstrated that amild to moderate SCC in rats resulted in the development of persistent at-level mechanical hypersensitivity with a 71% decrease in thresholds throughout the 12-week study period compared with sham animals. This is consistent with other studies [41] and previous results from our group using a more severe contusion with a 25mm weight drop [23]. Furthermore, the mechanical hypersensitivity of SCC animals had decreased with a single dose of the analgesic drugs pregabalin and morphine to levels comparable to those in saline-treated sham animals, but not with the anxiolytic drug midazolam, which supports that the behaviour may be comparable to at-level SCI pain.
The inflicted injury furthermore resulted in significant and persistently decreased exploratory behaviour of SCC animals in the open arms of the EPM compared with sham animals, which may indicate the development of a chronic anxiety-like state. This result is in concurrence with other similar studies using different injury models and of shorter duration [32,34-36], supporting that injury-related anxiety is a general phenomenon in animal models of hypersensitivity. Treatment of SCC animals with a single injection of pregabalin significantly reduced the level of anxiety (by nearly 100%); an effect that could not be seen after treatment with either morphine or midazolam. No effect of either treatment was observed in the sham group.
We saw no differences in the total distance travelled between the groups or between the drug treatments in the open field, suggesting no bias from sedation, somnolence, or motor disability.
The results of the current study also provide evidence of the validity of the two applied methods. Two important questions can be raised regarding the method specificity: Is the mechanical sensitivity method only measuring modulation of sensitivity or will a modulation of anxiety also attenuate at-level mechanical sensitivity thresholds? And is the EPM measuring anxiety, or can the observed effect be a result of increased mobility due to pain relief?
Inhouse observations suggest that measurements of mechanical sensitivity were biased by the current emotional state of the animal. Thus, externally induced vigilance and state anxiety (due to noise, handling, etc.) may be, at least partly, responsible for the observed temporal interindividual differences. The small nonsignificant positive effect of midazolam on mechanical hypersensitivity in SCC, but not sham animals, indicates that the method may be confounded by more persistent emotional states such as anxiety induced by the SCI rather than short-term state vigilance and anxiety. However, the lack of effect of midazolam on anxiety prevents conclusions to be made based on midazolam treatment of hypersensitivity. Further studies are needed to clarify this. On the other hand, the ability of morphine to almost completely reverse at-level mechanical sensitivity thresholds in both SCC and sham animals (not significant) combined with no effect on the anxiety component in the EPM suggests that the effect in the sensitivity method may not be driven by anxiety modulation.
There was no observation of behaviour to suggest that the SCC animals preferred the closed arms of the EPM because the walls offered them physical support to compensate for motor disability and symptoms of the spastic syndrome. The effect of pregabalin, but not morphine, on anxiety-like behaviour suggests that the explorative behaviour in the open arms was not reduced because of pain or motor disability-induced immobility. The total distance travelled in the open field was not different between the groups, suggesting no reluctance in general exploratory behaviour. Furthermore, none of the applied drugs has any known effect on motor disability and the spastic syndrome.
A limitation of the study is the lack of effect by midazolam in the EPM. Midazolam has a strong immediate anxiolytical effect after the first dose both in man and animals, and a single dose should hence be sufficient to elicit an anxiolytic response [42,43,44]. Even though the same dosage and treatment time were used in the Roeska study (different animal strain: Wistar) [32], the most likely explanation remains that the applied dosage was outside the therapeutic index. The lack of effect in the sham animals supports this argument. Another possibility is that the midazolam dose caused sedation and hence a decrease in the overall exploratory behaviour of the animals, supported by a slight decrease (not significant) in the distance travelled in the open field by the midazolam- versus saline-treated sham animals. Further studies with both higher and lower doses of midazolam need to be conducted in order to determine the actual cause.
The open arm activity of the sham animals in the EPM 4-12 weeks postinjury was significantly higher than that of the SCC animals, but the activity was not as stable as preferable, which would have strengthened the method validity. One possible explanation may be test habituation or sensitization. The effect of period was significant in both groups in the EPM, indicating that some habituation, sensitization, or one- trial tolerance occurs [31,45,46], even with repetitions of the EMP separated by 1 week, and thus more frequent measurements or treatments cannot be recommended. The conclusions are not sensitive to this, since a balanced treatment design was used.
3.1 Pain and anxiety relationship
The relationship between pain and anxiety is still hypothetical. Several explanations have been suggested [5].
The mediational model, i.e., development of anxiety leads to increased pain perception or presence of anxiety may prevent the ability to experience pain relief. Only one factor changes, but affects the experience of the other.
Linear causality model, i.e., pain and anxiety are directly correlated; thus, either pain causes anxiety or anxiety causes pain. Changing one will equally change the other.
Coexistence model, i.e., pain and depression are related to SCI but remain independent. Changing one, does not necessarily affect the other.
In most of the previous studies it is hypothesized that anxiety is a consequence of persistent hypersensitivity (linear causality), supported by the fact that both morphine and gabapentin reversed pain and anxiety-like behaviour without having an effect on anxiety in the sham animals [35]. Furthermore, midazolam reduced anxiety in both groups without affecting mechanical hypersensitivity [32,36].
In this study, however, pregabalin and morphine reduced mechanical hypersensitivity, while pregabalin, but not morphine, reduced anxiety-like behaviour in SCC animals. Since anxiety could not be attenuated by pain relief (morphine), but only by the dual-acting pregabalin, this study supports the coexistence model: that following SCC, at least partly, anxiety-like behaviour develops independently of SCI pain. Since we did not observe the expected effect of midazolam on anxiety, the lack of effect of midazolam on hypersensitivity may be due to inadequate dosage.
The coexistence model is further supported by the fact that we saw no significant correlation between postinjury time spent in open arms and at-level mechanical sensitivity thresholds, although this may be due to an insufficient number of animals. The development and level of pain have previously been reported to not be related to preinjury anxiety levels, but no reports on the relation to postinjury anxiety levels were included [47].
To the best of our knowledge all preclinical studies on pain and anxiety correlation use evoked sensitivity as outcome for pain-like behaviour. Evidence from clinical research with SCI patients suggests that anxiety may be correlated to spontaneous rather than evoked pain. Hence, patients with continuous pain had a higher score on the Hospital Anxiety and Depression Scale than those with intermittent or without pain, and thus 33% of SCI patients with continuous pain had probable clinically relevant anxiety compared with 21% of patients with intermittent and 6% without pain [48].
Finally, anxiety has also been related to a number of other consequences of SCI in humans [22], which highlights that anxiety may be generated and maintained by multiple factors and mechanisms.
Our results are in concordance with findings in clinical trials of SCI pain, showing effect of pregabalin on pain and anxiety [49,50] and of the opioid tramadol on pain [51].
Even though pregabalin reduced the anxiety behaviour in the SCC animals, it was not reversed to the level of the saline-treated sham animals (only to approx. 23%), whereas mechanical hypersensitivity was decreased to the level of the saline-treated sham animals. This suggests that pregabalin is more effective in reducing the sensory pain experience than the anxiety component, at least with the applied dosage. The observation is not likely caused by dosage issues, since the recommended dose of pregabalin for treatment of neuropathic pain and generalized anxiety is the same, although we cannot exclude that a longer treatment period may be required to modulate anxiety behaviour.
4 Conclusion
In conclusion, our study found a significant concomitant development of both at-level mechanical hypersensitivity and anxiety-like behaviour following experimental SCI. The dual-acting analgesic and anxiolytic drug pregabalin reduced both behaviours, while the analgesic drug morphine only reduced mechanical hypersensitivity, and the anxiolytic drug midazolam, in the dose used, had no effect on either anxiety or mechanical sensitivity. The results did thus not support a causal relationship between pain and anxiety following SCI.
5 Materials and methods
5.1 Animals
All experiments were performed according to the Ethical Guidelines of the International Association for the Study of Pain [52]. The study protocol and experimental design, animal housing, husbandry, and handing were in compliance with European and Danish legislation and approved by the Danish Animal Experiments Inspectorate (2006/561-1211).
In the experiment, 42 female Sprague-Dawley rats (200 g) (Taconic Farms Inc., Borup, Denmark) were used. All animals were housed in pairs in a temperature-controlled environment with a 12-h light/dark cycle. Food and water was available ad libitum. Special soft bedding was used after surgery to minimize contact pressure on the rat hind quarters. Animals were acclimatized to the housing facility for one week upon arrival from the vendor.
5.2 Model of central neuropathic pain: spinal cord contusion (SCC)
Animals were randomly assigned using random allocation software [53] and allocated to one of two groups: SCC (SCI by contusion, n = 21) and sham (laminectomy, but no contusion, n = 21).
The surgical procedure has been described in detail elsewhere [23]. In short, surgery was performed in an isolated facility intended for the purpose. Animals were anesthetized initially with 1ml/kg SC 1:1:2 Hypnorm® (0.315 mg/ml fentanyl/10 mg/ml fluanisone)/Dormicum® (5 mg/ml midazolam)/NaCl 0.9% (w/w). After laminectomy of bone segment T10, a 10 g rod was dropped 12.5mm onto spinal cord segments T12-13 using the MASCIS impactor (Rutgers, New Jersey)[39], generating a 1.0-2.0mmvertical compression of the cord (mild to moderate injury). The surgical site was closed in two layers with 4.0 re-absorbable sutures. Fluid replacement of 5ml 0.9% NaCl was administered IP, and the animals were allowed to recover under a heating lamp. Average time for the procedure was 1 h.
Postoperatively, animals required daily manual expression of the bladder until self-voiding (4 days on average). Responses to handling, fur condition, weight, porphyrin production (red tears), and signs of bladder infections (urine pH, colour, and clarity) were collected on daily inspections. A combination of sulfamethoxazole and trimethoprim (200 + 40mg/kg) (Tribissen®, Boxmeer, the Netherlands) was administered prophylactically SC for 5 days postoperatively to prevent bladder infections.
Locomotor recovery and presence of spastic syndrome were evaluated regularly as described in Baastrup et al. [23]. A locomotor score of 4 (walks with deficit (balance and foot positioning)) out of 6 (normal walking) was required for performance of the behavioural testing.
Excessive grooming behaviour/autophagia was monitored [23] and any score >0 (normal) prompted topical treatment with Clindamycin gel 1% and grade >3 (penetration through subcutaneous layers) or refractory grade 2 (penetration through the dermis) served as human endpoint.
5.3 Measurements of at-level mechanical sensitivity
Animals in groups of four were placed in individual 25cm × 10cm × 15cm Plexiglas boxes with black opaque walls (excluding the wall facing the investigator). A 30-min acclimatization period to the testing facility was allowed. Von Frey filaments (Semmes Weinstein nylon monofilaments, Stoelting, IL, USA) (0.07, 0.17, 0.69, 1.20, 2.04, 3.63, 5.50 g) were applied approx. 2 cm lateral to the incision site on the thorax until bending of the filament according to the up-down method [54]. Any brainstem reflex response (licking, guarding, struggling, vocalizing, jumping, and biting) [55] indicated a positive response, and results were converted to an at-level mechanical sensitivity threshold. All testing sessions were performed by the same trained investigator (CB). In sessions including pharmacological treatment, a pretreatment baseline threshold was measured.
5.4 Measurement of motility: open field
The effect of locomotor dysfunction on normal spontaneous exploratory behaviour, signs of spontaneous pain, and sedative side effects were evaluated in a black square 100cm × 100cm × 30cm open field. All animals were in turn placed in the same corner of the open field close to the wall and allowed to freely explore the environment for a test period of 5min. All trials were tracked and recorded using the Ethovision XT 4.0 software (Noldus Information Technology b.v., Wageningen, The Netherlands). The total distance travelled was calculated and used as outcome measure. The open field was thoroughly cleaned with 70% ethanol between the testing of each animal.
5.5 Measurement of anxiety: elevated plus maze (EPM)
The elevated plus maze (EPM) (Stoelting, IL, USA) consists of two opposing open and two closed arms (50cm × 10 cm) extending from a central platform (10cm × 0 cm), all made of odourless opaque gray plastic. The maze was elevated 40cm above floor level and equally illuminated. The animal was placed in the middle of the EPM facing an open arm and allowed to freely explore the environment for a test period of 10 min. All trials were tracked and recorded using the Ethovision XT 4.0 software (Noldus Information Technology b.v., Wageningen, The Netherlands). The time spent in the open arms was calculated by the software (outcome measure). After each test, the maze was carefully cleaned with 70% ethanol.
5.6 Experimental design
All 42 SCC and sham animals performed the anxiety, mechanical sensitivity, and motility tests prior to injury, 4 weeks postinjury, and 1 week after completion of the treatment study (12 weeks postinjury) to monitor temporal development. Mechanical sensitivity and anxiety tests were furthermore performed at 6weeks postinjury.
SCC and sham animals were randomly allocated to two separate test groups: one was tested for anxiety behaviour (n = 18), and one for mechanical sensitivity and motility testing (n = 24). All pharmacological treatments were performed 7-11 weeks postinjury. Each animal was treated with single doses of all four drugs in a balanced design, with each test session separated by 1 week to avoid carry-over effects and test habituation.
Testing was performed in an isolated testing facility to avoid inauspicious and unfavourable visual, olfactory, and auditory cues, minimize anticipatory hypervigilance and variations in stress levels induced by external factors [56,57]. Animals were tested in randomized order to avoid group bias. Drug solutions were prepared and drawn from identical vials, and the investigator was blinded both to injury groups and pharmacological treatments.
5.7 Drugs
Drug dosages were pregabalin 30 mg/kg, morphine 3mg/kg, midazolam 0.5 mg/kg, and 0.9% NaCl, based on previous studies with peripheral pain models. Pregabalin (pure substance) was supplied by Pfizer Inc., CT, USA; morphine (Morfin “SAD” 1mg/ml) was obtained from SAD, Copenhagen, Denmark; and midazolam (Midazolam “Hameln” 5mg/ml) was obtained from Hameln Pharmaceuticals, Hameln, Germany. All compounds were dissolved or diluted in saline (0.9% NaCl) and administered intraperitoneally 1ml/kg 45 min prior to testing.
5.8 Statistics
Data are presented as means ± SEM. Data for each test and each injury group were analyzed for temporal development and treatment effect with a linear mixed model including time/treatment and test sequence as a fixed effect and animals as a random effect, and for at-level mechanical sensitivity, the pretest threshold was included as a covariate. Appropriate post hoc contrast testing was predefined and performed. A p-value less than 0.05 indicates a statistical significance.
Results from the EPM test are graphically presented with data normalized to the mean value of the sham group (vehicle or preinjury).
DOI of refers to article: 10.1016/j.sjpain.2011.05.001
Acknowledgements
This study was supported by grants from the Danish Agency for Science, Technology and Innovation; the Faculty of Health Sciences, Aarhus University; Velux Fonden; the Ludvig and Sara Elsass Foundation; the Department of Clinical Medicine, Aarhus University; and the Aarhus University Research Foundation. None of the authors have any financial conflicts of interest. The authors would like to thank medical student Elise Klaestrup for experimental assistance and research secretary Helle Obenhausen Andersen for language revision. This study is a part of the Europain project, funded by the Innovative Medicines Initiative Joint Undertaking (IMI JU), Grant no 115007.
Abbreviations
- SCI
-
spinal cord injury
- SCC
-
spinal cord contusion
- EPM
-
elevated plus maze
References
[1] Finnerup NB, Baastrup C, Jensen TS. Neuropathic apin following spinal cord injury: mechanisms and treatment. Scandinavian Journal of Pain 2009;1:S3-11.Search in Google Scholar
[2] Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJ. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain 2003;103:249-57.Search in Google Scholar
[3] Werhagen L, Budh CN, Hultling C, Molander C. Neuropathic pain after traumatic spinal cord injury-relations to gender, spinal level, completeness, and age at the time of injury. Spinal Cord 2004;42:665-73.Search in Google Scholar
[4] Norrbrink BC, Lund I, Ertzgaard P, Holtz A, Hultling C, Levi R, Werhagen L, Lundeberg T. Pain in a Swedish spinal cord injury population. Clin Rehabil 2003;17:685-90.Search in Google Scholar
[5] Cairns DM, Adkins RH, Scott MD. Pain and depression in acute traumatic spinal cord injury: origins of chronic problematic pain? Arch Phys Med Rehabil 1996;77:329-35.Search in Google Scholar
[6] Scivoletto G, Petrelli A, Di LL, Castellano V. Psychological investigation of spinal cord injury patients. Spinal Cord 1997;35:516-20.Search in Google Scholar
[7] Woolrich RA, Kennedy P, Tasiemski T. A preliminary psychometric evaluation of the Hospital Anxiety and Depression Scale (HADS) in 963 people living with a spinal cord injury. Psychol Health Med 2006;11:80-90.Search in Google Scholar
[8] Nicholson PK, Nicholas MK,Middleton J. Spinal cord injury-related pain in rehabilitation: a cross-sectional study of relationships with cognitions, mood and physical function. Eur J Pain 2009;13:511-7.Search in Google Scholar
[9] Hancock KM, Craig AR, Dickson HG, Chang E, Martin J. Anxiety and depression over the first year of spinal cord injury: a longitudinal study. Paraplegia 1993;31:349-57.Search in Google Scholar
[10] Nicholson PK, Nicholas MK,Middleton J, Siddall P. Psychological characteristics of people with spinal cord injury-related persisting pain referred to a tertiary pain management center. J Rehabil Res Dev 2009;46:57-67.Search in Google Scholar
[11] Gormsen L, Rosenberg R, Bach FW, Jensen TS. Depression, anxiety, healthrelated quality of life and pain in patients with chronic fibromyalgia and neuropathic pain. Eur J Pain 2010;14:127-8.Search in Google Scholar
[12] Haythornthwaite JA, Sieber WJ, Kerns RD. Depression and the chronic pain experience. Pain 1991;46:177-84.Search in Google Scholar
[13] Haythornthwaite JA, Benrud-Larson LM. Psychological aspects of neuropathic pain. Clin J Pain 2000;16:S101-5.Search in Google Scholar
[14] McCarberg B. Managing the comorbidities of postherpetic neuralgia. J AmAcad Nurse Pract 2003;15:16-21.Search in Google Scholar
[15] Nicholson B, Verma S. Comorbidities in chronic neuropathic pain. Pain Med 2004;5(Suppl. 1):S9-27.Search in Google Scholar
[16] Leo RJ. Chronic pain and comorbid depression. Curr Treat Options Neurol 2005;7:403-12.Search in Google Scholar
[17] Romano JM, Turner JA. Chronic pain and depression: does the evidence support a relationship? Psychol Bull 1985;97:18-34.Search in Google Scholar
[18] Ulrich-Lai YM, Xie W, Meij JT, Dolgas CM, Yu L, Herman JP. Limbic and HPA axis function in an animal model of chronic neuropathic pain. Physiol Behav 2006;88:67-76.Search in Google Scholar
[19] Richards JS, Meredith RL, Nepomuceno C, Fine PR, Bennett G. Psycho-social aspects of chronic pain in spinal cord injury. Pain 1980;8:355-66.Search in Google Scholar
[20] Hocevar B, Gray M. Intestinal diversion (colostomy or ileostomy) in patients with severe bowel dysfunction following spinal cord injury. J Wound Ostomy Continence Nurs 2008;35:159-66.Search in Google Scholar
[21] Widerstrom-Noga EG, Turk DC. Exacerbation of chronic pain following spinal cord injury. J Neurotrauma 2004;21:1384-95.Search in Google Scholar
[22] Widerstrom-Noga EG. Evaluation of clinical charateristics of pain and psycosocial factors after spinal cord injury. In: Yezierski RP, Burchiel KJ, editors. Spinal Cord Injury Pain: Assessment. Mechanisms, Management. Seattle: IASP Press; 2002. p. 53-82.Search in Google Scholar
[23] Baastrup C, Maersk-Moller CC, Nyengaard JR, Jensen TS, Finnerup NB. Spinal-, brainstem- and cerebrally mediated responses at- and below-level of a spinal cord contusion in rats: evaluation of pain-like behavior. Pain 2010;151:670-9.Search in Google Scholar
[24] Vierck CJ, Hansson PT, Yezierski RP. Clinical and pre-clinical pain assessment: are we measuring the same thing? Pain 2008;135:7-10.Search in Google Scholar
[25] Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113:9-19.Search in Google Scholar
[26] Pedersen LH, Blackburn-Munro G. Pharmacological characterisation of place escape/avoidance behaviour in the rat chronic constriction injury model of neuropathic pain. Psychopharmacology (Berl) 2006;185:208-17.Search in Google Scholar
[27] LaBuda CJ, Fuchs PN.Abehavioral test paradigm to measure the aversive quality of inflammatory and neuropathic pain in rats. Exp Neurol 2000;163:490-4.Search in Google Scholar
[28] Mauderli AP, costa-Rua A, Vierck CJ. An operant assay of thermal pain in conscious, unrestrained rats. J Neurosci Methods 2000;97:19-29.Search in Google Scholar
[29] Baastrup C, Jensen TS, Finnerup NB. Pregabalin attenuates place escape/avoidance behavior in a rat model of spinal cord injury. Brain Res 2011;1370:129-35. Epub;%2010 Nov 9.:129-35.Search in Google Scholar
[30] Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 1985;14:149-67.Search in Google Scholar
[31] Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav 1996;54:21-30.Search in Google Scholar
[32] Roeska K, Doods H, Arndt K, Treede RD, Ceci A. Anxiety-like behaviour in rats with mononeuropathy is reduced by the analgesic drugs morphine and gabapentin. Pain 2008;139:349-57.Search in Google Scholar
[33] Roeska K, Ceci A, Treede RD, Doods H. Effect of high trait anxiety on mechanical hypersensitivity in male rats. Neurosci Lett 2009;464:160-4.Search in Google Scholar
[34] Narita M, Kaneko C, Miyoshi K, Nagumo Y, Kuzumaki N, Nakajima M, Nanjo K, Matsuzawa K, Yamazaki M, Suzuki T. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology 2006;31:739-50.Search in Google Scholar
[35] Hasnie FS, Breuer J, Parker S, Wallace V, Blackbeard J, Lever I, Kinchington PR, Dickenson AH, Pheby T, Rice AS. Further characterization of a rat model of varicella zoster virus-associated pain: relationship between mechanical hypersensitivity and anxiety-related behavior, and the influence of analgesic drugs. Neuroscience 2007;144:1495-508.Search in Google Scholar
[36] Wallace VC, Segerdahl AR, Blackbeard J, Pheby T, Rice AS. Anxiety-like behaviour is attenuated by gabapentin, morphine and diazepam in a rodent model of HIV anti-retroviral-associated neuropathic pain. Neurosci Lett 2008;19(448):153-6.Search in Google Scholar
[37] Kontinen VK, Kauppila T, Paananen S, Pertovaara A, Kalso E. Behavioural measures of depression and anxiety in rats with spinal nerve ligation-induced neuropathy. Pain 1999;80:341-6.Search in Google Scholar
[38] Hasnie FS, Wallace VC, Hefner K, Holmes A, Rice AS. Mechanical and cold hypersensitivity in nerve-injured C57BL/6 J mice is not associated with fear-avoidance- and depression-related behaviour. Br J Anaesth 2007;98:816-22.Search in Google Scholar
[39] Young W. Spinal cord contusion models. Prog Brain Res 2002;137:231-55.Search in Google Scholar
[40] Robins SL, Fehlings MG. Models of experimental spinal cord injury: translational relevance and impact. Drug Discovery Today: Disease Models 2008;5:5-11.Search in Google Scholar
[41] Siddall P, Xu CL, Cousins M. Allodynia following traumatic spinal cord injury in the rat. Neuroreport 1995;19:1241-4.Search in Google Scholar
[42] Haefely W. Psychopharmacology of anxiety. Eur Neuropsychopharmacol 1991;1:89-95.Search in Google Scholar
[43] Albrechet-Souza L, Cristina de CM, Rodrigues FC, Brandao ML. Increases in plasma corticosterone and stretched-attend postures in rats naive and previously exposed to the elevated plus-maze are sensitive to the anxiolytic-like effects of midazolam. Horm Behav 2007;52:267-73.Search in Google Scholar
[44] Bertoglio LJ, Carobrez AP. Prior maze experience required to alter midazolam effects in rats submitted to the elevated plus-maze. Pharmacol Biochem Behav 2002;72:449-55.Search in Google Scholar
[45] Holmes A, Rodgers RJ. Prior exposure to the elevated plus-maze sensitizes mice to the acute behavioral effects of fluoxetine and phenelzine. Eur J Pharmacol 2003;459:221-30.Search in Google Scholar
[46] Stern CA, Carobrez AP, Bertoglio LJ. Aversive learning as a mechanism for lack of repeated anxiolytic-like effect in the elevated plus-maze. Pharmacol Biochem Behav 2008;90:545-50.Search in Google Scholar
[47] Wilson HD, Boyette-Davis J, Fuchs PN. The relationship between basal level of anxiety and the affective response to inflammation. Physiol Behav 2007;90:506-11.Search in Google Scholar
[48] Norrbrink BC, Hultling C, Lundeberg T. Quality of sleep in individuals with spinal cord injury: a comparison between patients with and without pain. Spinal Cord 2005;43:85-95.Search in Google Scholar
[49] Baastrup C, Finnerup NB. Pharmacological management of neuropathic pain following spinal cord injury. CNS Drugs 2008;22:455-75.Search in Google Scholar
[50] Siddall PJ, Cousins MJ, Otte A, Griesing T, Chambers R, Murphy TK. Pregabalin in central neuropathic pain associated with spinal cord injury: a placebo-controlled trial. Neurology 2006;67:1792-800.Search in Google Scholar
[51] Norrbrink C, Lundeberg T. Tramadol in neuropathic pain after spinal cord injury: a randomized, double-blind, placebo-controlled trial. Clin J Pain 2009;25:177-84.Search in Google Scholar
[52] Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983;16:109-10.Search in Google Scholar
[53] Saghaei M. Random allocation software for parallel group randomized trials. BMC Med Res Methodol 2004;4(26):26.Search in Google Scholar
[54] Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53:55-63.Search in Google Scholar
[55] Vierck CJ. Animal models of pain. In: McMahon S, Koltzenburg M, editors. Wall and Melzack's Textbook of Pain. Philadelphia: Elsevier Churchill Livingstone; 2006. p. 175-86.Search in Google Scholar
[56] Langford DJ, Crager SE, Shehzad Z, Smith SB, Sotocinal SG, Levenstadt JS, Chanda ML, Levitin DJ, Mogil JS. Social modulation of pain as evidence for empathy in mice. Science 2006;312:1967-70.Search in Google Scholar
[57] Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL, Mogil JS. Identification and ranking of genetic and laboratory environment factors influencing a behavioral trait, thermal nociception, via computational analysis of a large data archive. Neurosci Biobehav Rev 2002;26:907-23.Search in Google Scholar
© 2011 Scandinavian Association for the Study of Pain
Articles in the same Issue
- Editorial comment
- Looking at visceral pain: New vistas
- Review
- Neuroimaging of the human visceral pain system–A methodological review
- Editorial comment
- The pain modulatory cocktail
- Review
- Review of neuroimaging studies related to pain modulation
- Editorial comment
- Combination of physiotherapy and cognitive therapy in chronic pain
- Review
- Somatocognitive therapy in the management of chronic gynaecological pain. A review of the historical background and results of a current approach
- Editorial comment
- Effects of the excitatory amino acid transporter subtype 2 (EAAT-2) transporter inducer ceftriaxone (an antibiotic) on different pain modalities in rat
- Original experimental
- Effects of the excitatory amino acid transporter subtype 2 (EAAT-2) inducerceftriaxone on different pain modalities in rat
- Editorial comment
- Is finding the common biological link(s) between pain and affect an infinity quest?
- Original experimental
- Coexisting mechanical hypersensitivity and anxiety in a rat model of spinal cord injury and the effect of pregabalin, morphine, and midazolam treatment
- Editorial comment
- The burden of headache, also for the adolecents?
- Observational studies
- The Nord-Trøndelag Health Study shows increased prevalence of primary recurrent headaches among adolescents over a four-year period
Articles in the same Issue
- Editorial comment
- Looking at visceral pain: New vistas
- Review
- Neuroimaging of the human visceral pain system–A methodological review
- Editorial comment
- The pain modulatory cocktail
- Review
- Review of neuroimaging studies related to pain modulation
- Editorial comment
- Combination of physiotherapy and cognitive therapy in chronic pain
- Review
- Somatocognitive therapy in the management of chronic gynaecological pain. A review of the historical background and results of a current approach
- Editorial comment
- Effects of the excitatory amino acid transporter subtype 2 (EAAT-2) transporter inducer ceftriaxone (an antibiotic) on different pain modalities in rat
- Original experimental
- Effects of the excitatory amino acid transporter subtype 2 (EAAT-2) inducerceftriaxone on different pain modalities in rat
- Editorial comment
- Is finding the common biological link(s) between pain and affect an infinity quest?
- Original experimental
- Coexisting mechanical hypersensitivity and anxiety in a rat model of spinal cord injury and the effect of pregabalin, morphine, and midazolam treatment
- Editorial comment
- The burden of headache, also for the adolecents?
- Observational studies
- The Nord-Trøndelag Health Study shows increased prevalence of primary recurrent headaches among adolescents over a four-year period

