Abstract
Objectives
Dental caries is a multifactorial infectious disease caused by the colonization and proliferation of bacteria in the mouth. Recently, it has been reported that local antioxidant and oxidant status may play an important role in the pathogenesis of dental caries. Visfatin is an adipocytokine that enhances leukocyte activation and release of proinflammatory cytokines. In this study, our aim was to investigate the salivary visfatin levels, total antioxidant capacity (TAC), and total oxidant status (TOS) in patients with and without dental caries.
Methods
Saliva samples were collected from 50 caries-free individuals and 115 patients with dental caries who were admitted to Selcuk University Restorative Dentistry Clinics. Saliva samples were collected based on the stimulated saliva collection procedure. Visfatin levels were measured using enzyme-linked immunosorbent assay. Spectrophotometric methods were used to determine salivary TAC and TOS.
Results
Salivary TAC, TOS, and visfatin levels were statistically higher in patients with dental caries compared to caries-free group (p=0.035; p=0.003; p<0.001 respectively). There was a positive correlation between caries number and salivary TOS and visfatin levels.
Conclusions
Findings of this prospective study demonstrated that oxidative stress may be involved in the pathogenesis of dental caries. Salivary visfatin, TAC, and TOS may be novel markers to evaluate dental caries.
Introduction
Dental caries is one of the most common chronic diseases in the world. It is an infectious disease [1] with multifactorial etiology resulting from the disruption of the dynamic balance between pathological factors and protective factors [2]. This disease is a high burden on individuals, and it also causes serious expenses in health systems [3].
Saliva is the exocrine secretion of the major and minor salivary glands. Saliva, recently used in the diagnosis of many diseases, can be an alternative to time-consuming and invasive diagnostic procedures, as it is always readily available, easily and cost-free collected without the need for an invasive procedure [4]. Although 99% of its content is water, it consists of various organic and inorganic substances such as proteins, immunoglobulins, electrolytes, minerals, enzymes, and cytokines. The proportions of saliva components are important for the function of protection and maintaining the health of the oral cavity [5]. It is known that many components of saliva have been associated with caries risk [6].
Visfatin is a 52 kDa adipokine, discovered by Fukuhara in 2005, that has been shown to lower plasma glucose levels by binding insulin receptors in mice [7]. However, the same molecule was previously discovered and named as Pre-B Colony Enhancing Factor, involved in the early development of B-cell growth factor and cytokine-like effects. Visfatin is also known as nicotinamide phosphoribosyl transferase (NAMPT) which is the rate-limiting step in the biosynthesis of nicotinamide adenine dinucleotide (NAD). Visfatin is synthesized from lymphocytes, activated macrophages, neutrophils, hepatocytes, and pneumocytes [8]. It is found in many biological fluids including blood, saliva, and gingival crevicular fluid and is considered as an inflammatory cytokine.
Oxidative stress is a pathological condition that occurs as a result of impaired balance of the oxidative system in favor of oxidants through excessive free radical production or decreased antioxidant system function. In recent years, it has been revealed that many diseases are associated with increased free radical activity, and the active role of oxidative stress in the pathogenesis of diseases has been pointed out. Since measuring different antioxidant or oxidant molecules separately is impractical, requires complicated techniques and their effects would probably be additive, the total antioxidant capacity (TAC) and oxidant capacity of a sample is measured and defined as TAC and total oxidant status (TOS), respectively. Reactive oxygen species (ROS) are released in metabolic and physiological processes in the human body, and they are removed through enzymatic and non-enzymatic antioxidative mechanisms.
Dental plaque consists of a large number of bacteria that adhere to the tooth surface. These bacteria metabolize carbohydrates to provide energy and produce organic acids as a byproduct, which induces demineralization of tooth enamel and ultimately causes tooth decay. Cariogenic bacteria also enhance the release of many virulence factors, which is the main determinant of cariogenic plaque [9]. NADH oxidase in Streptococcus mutans, a major pathogen of dental caries, is an essential enzyme for the regeneration of NAD+ during glycolysis as well as the reduction of diatomic oxygen in order to prevent ROS formation [10]. Moreover, in response to the colonization of microorganisms, the host immune system can induce the secretion of salivary antioxidants and many cytokines as a result of tissue defense mechanism [11]. Free radicals and ROS in the mouth also originate from polymorphonuclear neutrophils, primarily to inhibit bacterial growth [12].
The oxidant and antioxidant systems have been confirmed as one of the important contributory etiologic factors in many oral conditions including dental caries and it has been argued that saliva may be the first line of defense against free radical-mediated oxidative stress [13, 14]. Saliva plays an important role in caries development and its composition can be changed by any physiological and pathological conditions including dental caries. There are many studies investigating the relationship between salivary visfatin levels and periodontitis and gingivitis in recent years, and most of these studies showed increased visfatin levels [15, 16]. No study has been conducted yet with regard to salivary visfatin evaluation in patients with dental caries. Visfatin has also been shown to increase ROS derivatives [17]. Although several studies have shown that salivary TAC is elevated in early childhood caries [12, 13], the role of antioxidants and antioxidant-related mechanisms is not fully understood. There is a lacuna of studies examining the relationship between salivary TAC and TOS levels with dental caries, particularly in adults. Therefore, in this present study, our aim was to evaluate the levels and role of salivary visfatin, TAC, and TOS in dental caries to determine the role of oxidative stress in the pathogenesis of dental caries and tissue destruction.
Materials and methods
Study population
A total of 165 individuals aged 18–54 years, who were admitted to Selcuk University Department of Restorative Dentistry, were included in the study. Saliva samples were collected from 50 healthy individuals and 133 patients with dental caries, 18 patients were excluded from the study due to incomplete clinical data (the remaining 115 patients with dental caries, 50 caries-free controls). General exclusion criteria included chronic systemic disease, active systemic inflammatory disease, use of the drug, BMI ≥ 30 and pregnant women. Local exclusion criteria included oral tumor, aggressive periodontitis, oral infection, and salivary gland dysfunction. The participants met the following inclusion criteria: they should be over 18 years old, have good general health status, have caries-free teeth for the control group, and have at least one dental caries for the experimental group.
Clinical examination
Clinical assessment procedures included teeth examination, oral-periodontal evaluation of mucosal status, and collection of saliva samples. Clinical and radiographic examinations were carried out to determine the number of carious teeth of the patients. Before the examination phase, the occlusal surfaces of the teeth were cleaned carefully with soft brushes and then dried. Intra-oral examinations were performed using a dental mirror and ball-ended dental probe by a single examiner. Clinically visible large carious surfaces are detected, and diagnoses are supported with panoramic and bitewing radiographs. Decayed, Missing, and Filled Teeth (DMFT) score was calculated for each patient.
Saliva collection
A standard stimulated saliva collection method is used. Saliva samples from the subjects were collected between 09:00 and 11:00 a.m. in order to prevent diurnal variation. All participants were asked to abstain from eating and drinking for at least 2 h. Individuals were seated comfortably in an upright position, rinsed their mouths with water before collecting saliva, and then waited 10 min prior to collection. Participants chewed 0.5 g of paraffin for 30 s, the accumulated saliva was spat into a disposable sterile laboratory container with a wide opening and saliva collection was performed for 90 more seconds with continuous spitting. After, whole stimulated saliva samples were transferred into Eppendorf microtubes, and then samples were centrifuged at 10,000×g for 5 min to remove tissue debris. Saliva supernatants were stored at −80 °C until the analysis time. The informed consent form was signed by all patients prior to saliva collection. The study was approved by Selcuk University Defense Research Ethics Committee (2017/172).
Visfatin analysis
Salivary visfatin levels were determined using the enzyme-linked immunosorbent assay (ELISA) method by Human Visfatin C – terminal ELISA kit (Phoenix Pharmaceuticals Inc., CA, USA) (Cat. No: EK-003-80). The analytic sensitivity for visfatin was 2.3 ng/mL, intra-assay coefficient variation (CV) was <10%, and inter-assay CV was <15%.
TAC–TOS analysis
Salivary TAC and TOS were determined using an automated measurement method (Rel Assay Diagnostics kit, Mega Tıp, Gaziantep, Turkey), by Erel’s colorimetric method [18, 19]. Briefly, TOS was measured by oxidizing ferrous ions (Fe+2) to ferric ions (Fe+3) in the presence of varied oxidative species in an acidic environment. Xylenol orange was used as an indicator and results were expressed in μmol/L H2O2 equivalent (μmol/L H2O2). TAC measurement was relied on the change in the absorbance at 660 nm due to the reduction of ABTS+ to ABTS [2,2-azino-bis-(3-ethyl-benzothiazoline-6-sulphonate)] and results were expressed in mmol/L Trolox. Oxidative stress index (OSI) was counted according to TOS/TAC formula. For TAC measurement, inter-assay CV was 2.8% and the intra-assay CV was 3.3%. For TOS measurement, inter-assay CV was 3.2% and the intra-assay CV was 3.9%.
Statistical analysis
Data analysis was performed using SPSS version 15 (SPSS Inc, Chicago, IL, USA). Kolmogorov–Smirnov analysis was used to test for Gaussian or non-Gaussian distribution. For Gaussian-distributed data, an independent samples t-test was used to assess the significance of differences between two groups. If the data does not have a normal distribution, the Mann–Whitney U test was performed for comparisons between groups. The correlations were assessed using Spearman’s rank test for non-Gaussian-distributed variables. Based on power analysis for the sample size, the power was calculated as 99.8%. A p-value ≤ 0.05 was considered statistically significant.
Results
A total of 50 caries-free individuals (23 males, 27 females) with a mean age of 28.1 ± 6.2 years, 115 patients (52 males, 63 females) with dental caries had a mean age of 32.8 ± 8.3 years were included in this study. No statistical differences were found between groups in regard to age and sex (p > 0.05).
Visfatin, TAC, and TOS were detectable in all samples. The median salivary TAC, TOS, and visfatin values were significantly higher in the experimental group than in the caries-free group (p=0.035, p=0.003, p<0.001 respectively). The descriptive statistics (median and percentage values) and their comparisons are provided in Table 1.
Comparison of laboratory parameters in caries active group and caries-free group.
| Parameters | Healthy | Patients with dental caries | p-Value |
|---|---|---|---|
| Median (25–75%) | Median (25–75%) | ||
| n=50 | n=115 | ||
| Visfatin, ng/mL | 66.58 (40.4–88.9) | 136.12 (80.4–204.3) | <0.001 |
| TAC, mmol Trolox Eq/L | 0.42 (0.32–0.6) | 0.51 (0.43–0.67) | 0.035 |
| TOS, µmol H2O2/L | 0.74 (0.2–5.28) | 2.6 (0.98–11.79) | 0.003 |
| OSI, arbitrary units | 1.69 (0.58–9.35) | 5.42 (1.37–20.96) | 0.008 |
-
TAC, total antioxidant capacity; TOS, total oxidant status; OSI, oxidative stress index.
Spearmen’s correlation analysis showed a positive correlation between salivary visfatin levels with TOS and OSI. Similarly, TOS had a positive correlation with visfatin, OSI, and TAC. There was no significant correlation between salivary TAC and visfatin levels. Correlations of laboratory parameters with each other are given in Table 2.
The correlations of the laboratory parameters (visfatin, TAC, TOS, and OSI) with each other.
| Spearman’s Rho | TAC | TOS | OSI | Visfatin | |
|---|---|---|---|---|---|
| TAC | r | 1 | 0.412 | 0.202 | 0.092 |
| p | – | <0.001 | 0.009 | 0.238 | |
| TOS | r | 1 | 0.968 | 0.244 | |
| p | – | <0.001 | 0.002 | ||
| OSI | r | 1 | 0.256 | ||
| p | – | 0.001 | |||
| Visfatin | r | 1 | |||
| p | – |
-
TAC, total antioxidant capacity; TOS, total oxidant status; OSI, oxidative stress index.
In terms of clinical parameters, there was a positive correlation between DMFT index, caries number, and salivary visfatin levels. Salivary TOS and OSI were also positively correlated with DMFT index and caries number. On the other hand, TAC had no correlation with the DMFT index and caries number. Correlation analysis results between laboratory tests and clinical parameters are given in Table 3.
Correlation analysis of laboratory parameters (visfatin, TAC, TOS, and OSI) and clinical parameters (DMFT index and caries number).
| Spearman’s Rho | TAC | TOS | OSI | Visfatin | |
|---|---|---|---|---|---|
| Caries number | r | 0.052 | 0.283 | 0.314 | 0.502 |
| p | 0.582 | 0.002 | 0.001 | <0.001 | |
| DMFT index | r | 0.120 | 0.245 | 0.240 | 0.326 |
| p | 0.153 | 0.003 | 0.004 | <0.001 |
-
DMFT, Decayed, Missing, and Filled Teeth; TAC, total antioxidant capacity; TOS, total oxidant status; OSI, oxidative stress index.
Based on the Receiver operating characteristic curve (ROC) analysis, which was carried out to determine the diagnostic value of visfatin, TAC and TOS in detecting dental caries, the area under the curve (AUC) values were 0.771, 0.604, and 0.643, respectively. ROC analysis and AUC data are shown in Table 4 and Figure 1.
The diagnostic value of laboratory parameters and ROC analysis.
| AUC | SE | 95% CI | |
|---|---|---|---|
| Visfatin | 0.771 | 0.037 | 0.699–0.844 |
| TAC | 0.604 | 0.049 | 0.508–0.700 |
| TOS | 0.643 | 0.048 | 0.550–0.737 |
-
TAC, total antioxidant capacity; TOS, total oxidant status; AUC, area under the curve; SE, standart error; CI, confidence interval.
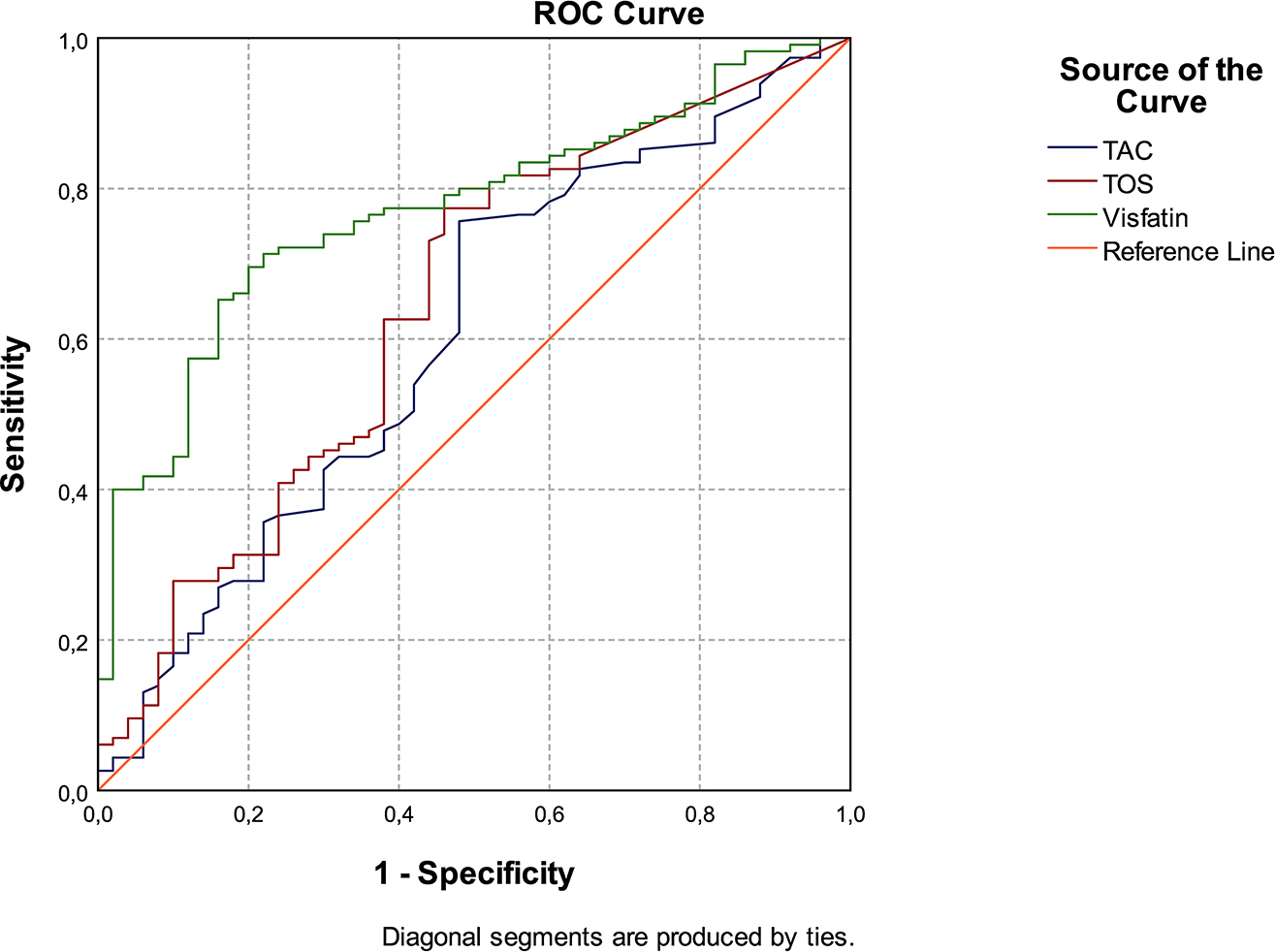
The ROC curve for TAC, TOS, and visfatin in patients with dental caries and caries-free individuals.
Discussion
The analysis of saliva biomarkers is accepted as an alternative to serum and plasma by many authorities [20]. Since saliva is easy and non-invasive to collect, contains many biomarkers in its composition, and is affected by both intraoral and systemic events, it is a valuable diagnostic and research tool. In case of dental caries, some alterations in its composition may occur, increasing its diagnostic value.
Oxidative stress can be defined as the impaired balance between the production of free radicals and the antioxidant defense mechanism of the organism, which directly or indirectly results in cell damage. Oxidative stress can be responsible, with varying degrees of importance, for the pathogenesis of many diseases such as diabetes, atherosclerosis, hypertension, obesity, cancer, and inflammation [21]. Recently, attention has focused on the role of oxidative stress in the onset and/or progression of dental diseases including dental caries, which is a common and preventable chronic disease. According to the results of this present study, salivary TAC and TOS were found to be higher in the dental caries group compared to healthy subjects, thus also confirming the hypothesis established by this study. TAC is the amount of free radical scavenged by a test solution used to determine the antioxidant capacity of biological samples [22]. The general belief is that salivary TAC levels are increased in dental caries, although there are few studies to suggest otherwise [23, 24]. Ahmadi-Motamayel et al. indicated that salivary TAC was higher in individuals with dental caries compared to the control group among high school students with the age range of 15–17 years [25]. Several further studies in the pediatric age group have shown that salivary TAC is higher in patients with active caries [13, 26], [27], [28]. Contrary to these papers, studies investigating the relationship between periodontitis and salivary TAC indicated decreased salivary TAC in patients with periodontitis [29, 30] and this finding can reveal that salivary TAC plays a role in the pathogenesis of dental caries independently of other dental conditions. Studies conducted in the adult age group are very limited. For this reason, our study was planned to be conducted on adult individuals, where there are fewer studies on this subject in the literature. Hegde et al. showed that saliva and serum TAC levels were higher in patients with dental caries compared to caries-free group, and both serum and saliva TAC values increased with the severity of caries [31]. If the antioxidant capacity of the tissues is insufficient, free radicals interact with cellular structures and cause disruption of their structure by affecting components of the cells such as lipid, protein, DNA, and carbohydrates. The increase in TAC could be explained by the fact that higher TAC levels in experimental group with dental caries may be a compensatory mechanism against oxidative stress due to an altered response to infection. The other mechanism that may be effective is diet. Excessive fructose consumption leads to an elevation in uric acid concentrations, which is the main antioxidant of the saliva [29]. Therefore, consumption of sugars not only increases the risk of developing dental caries, but also contributes to raised salivary TAC levels.
In the literature review, very few studies were identified that evaluated the link between dental caries and TOS levels. Guler et al. found that TOS levels were higher in the experimental group with dental caries (n:20) compared to caries free group (n:20) in individuals aged 8–12 years [27]. Similarly, Guzelcicek et al. reported elevated salivary OSI and TOS levels in the group of children with dental caries than in the caries-free group [32]. Since dental caries is an infectious condition with multifactorial etiology, it can cause an increase in oxidative stress and an increase in antioxidant levels in order to balance this increase. According to the results of our study, it can be taught that both oxidative and antioxidative pathways are closely related to caries pathogenesis.
Saliva may contribute a first line of defense against free radical-mediated oxidative stress that may take part in the pathogenesis of dental conditions. Current study findings that there may be a link between salivary TAC and TOS levels and dental caries suggest that salivary tests can be used to take preventive measures against caries development. The relationship between enzymatic endogenous antioxidants, non-enzymatic endogenous antioxidants, exogenous antioxidants, and dental caries, which are evaluated separately, may contribute to the understanding of the dental caries mechanism. At rest, the submandibular salivary gland secretes 65% of all saliva. However, parotid salivary gland secretion is more dominant in stimulated saliva and the parotid salivary gland is the main source of antioxidants and proteins [25]. Since total protein, nitrite, TAC and TOS concentrations increased in stimulated saliva [4, 33], stimulated saliva was preferred in our study.
Visfatin acts as an inflammatory cytokine by increasing leukocytes activation, adhesion molecules’ synthesis, proangiogenic activity and production of proinflammatory and inflammatory cytokines [34]. Furthermore, it provides macrophages to survive longer and inhibit neutrophil apoptosis, prolonging the duration of neutrophils and leading to tissue destruction [35]. Recent studies demonstrated a complex and close interaction between visfatin and oxidative stress and concluded that visfatin can induce oxidative stress, as well as in the modulation of some miRNA and target genes [17, 36]. Büyükaydın et al. showed a positive correlation between circulating visfatin levels and TOS [37]. In this present study, salivary visfatin levels were evaluated and salivary visfatin levels were found to be higher in patients with dental caries than those of healthy subjects. Visfatin concentrations were also positively correlated with caries number. According to our hypothesis, increased visfatin levels may mediate tissue damage in the formation or pathogenesis of dental caries as well as disruption in the integrity of dental tissue. Mamali et al. reported that contrary to resistin and adiponectin, there was no correlation between salivary visfatin levels and serum visfatin levels [20]. The reason of these differences may be that, in response to intraoral changes, salivary glands also secrete many proteins including visfatin which can take part in the pathogenesis of caries by locally affecting dental tissues that may result in the destruction of dental structures. There are several that demonstrated a significant increase in salivary visfatin levels in periodontitis and gingivitis [15, 16]. Ozcan et al. reported that salivary visfatin levels are higher particularly in the gingivitis group (n:24) and in the periodontitis group (n:25) than in the healthy group (n:23) [16]. However, salivary visfatin measurement was inadequate in the differentiation of gingivitis and periodontitis. Tabari et al. reported that salivary visfatin levels were higher in subjects with periodontitis (n:20) than in healthy subjects (n:20) [15]. On the contrary to these researches, Kadkhodazadeh et al. found no association between periodontitis and salivary visfatin levels [38]. Therefore, gingivitis and periodontitis may also have an effect on salivary visfatin levels.
To the author’s knowledge, this present study is the first study to investigate the relationship between salivary visfatin concentrations and dental caries. The number of samples in our study is considerably higher than in previous studies. In addition, our results show a positive correlation between visfatin concentrations and caries number. Therefore, salivary visfatin concentration can be used as a marker to assess the potential caries risk of individuals. In the future, both visfatin and oxidative stress in saliva could be used for screening purposes by evaluating the risk of dental caries in large populations. Moreover, a better understanding of the role of oxidative stress in dental caries can provide new treatment strategies using host modulation techniques.
The limitations of this study are the difficulties of constructing a control group without periodontal tissue diseases and dental caries, and an experimental group with only dental caries without periodontal tissue diseases. In view of the above studies, more comprehensive studies with larger groups with both dental caries and periodontal diseases are needed.
Funding source: Selcuk University
Award Identifier / Grant number: 17701269
-
Research funding: This study was supported by the Scientific Research Projects Unit of Selcuk University (grand number: 17701269).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: No conflict of interest was declared by the authors.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Ethical approval: The study was approved by Selcuk University Defense Research Ethics Committee (2017/172).
References
1. Caufield, PW, Li, Y, Dasanayake, A. Dental caries: an infectious and transmissible disease. Comp Cont Educ Dent 2005;26:10–6.Search in Google Scholar
2. Featherstone, JD. The caries balance: the basis for caries management by risk assessment. Oral Health Prev Dent 2004;2:259–64.Search in Google Scholar
3. Listl, S, Galloway, J, Mossey, PA, Marcenes, W. Global economic impact of dental diseases. J Dent Res 2015;94:1355–61. https://doi.org/10.1177/0022034515602879.Search in Google Scholar PubMed
4. Justino, AB, Teixeira, RR, Peixoto, LG, Jaramillo, OLB, Espindola, FS. Effect of saliva collection methods and oral hygiene on salivary biomarkers. Scand J Clin Lab Invest 2017;77:415–22. https://doi.org/10.1080/00365513.2017.1334261.Search in Google Scholar PubMed
5. de Almeida Pdel, V, Grégio, AM, Machado, MA, de Lima, AA, Azevedo, LR. Saliva composition and functions: a comprehensive review. J Contemp Dent Pract 2008;9:72–80.10.5005/jcdp-9-3-72Search in Google Scholar
6. Guo, L, Shi, W. Salivary biomarkers for caries risk assessment. J Calif Dent Assoc 2013;41:107–18.10.1080/19424396.2013.12222284Search in Google Scholar
7. Fukuhara, A, Matsuda, M, Nishizawa, M, Segawa, K, Tanaka, M, Kishimoto, K, et al.. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science 2005;307:426–30. https://doi.org/10.1126/science.1097243.Search in Google Scholar PubMed
8. Hivert, M-F, Sullivan, LM, Fox, CS, Nathan, DM, D’Agostino RB, Sr., Wilson, PWF, et al.. Associations of adiponectin, resistin, and tumor necrosis factor-alpha with insulin resistance. J Clin Endocrinol Metab 2008;93:3165–72. https://doi.org/10.1210/jc.2008-0425.Search in Google Scholar PubMed PubMed Central
9. Kuramitsu, HK, Wang, B-Y. Virulence properties of cariogenic bacteria. BMC Oral Health 2006;6:S11. https://doi.org/10.1186/1472-6831-6-s1-s11.Search in Google Scholar PubMed PubMed Central
10. Baker, JL, Derr, AM, Faustoferri, RC, Quivey, RGJr. Loss of NADH oxidase activity in Streptococcus mutans leads to Rex-mediated overcompensation in NAD+ regeneration by lactate dehydrogenase. J Bacteriol 2015;197:3645–57. https://doi.org/10.1128/jb.00383-15.Search in Google Scholar PubMed PubMed Central
11. Jurczak, A, Kościelniak, D, Skalniak, A, Papież, M, Vyhouskaya, P, Krzyściak, W. The role of the saliva antioxidant barrier to reactive oxygen species with regard to caries development. Redox Rep 2017;22:524–33. https://doi.org/10.1080/13510002.2017.1301625.Search in Google Scholar PubMed PubMed Central
12. Mahjoub, S, Ghasempour, M, Gharage, A, Bijani, A, Masrourroudsari, J. Comparison of total antioxidant capacity in saliva of children with severe early childhood caries and caries-free children. Caries Res 2014;48:271–5. https://doi.org/10.1159/000355581.Search in Google Scholar PubMed
13. Kumar, D, Pandey, RK, Agrawal, D, Agrawal, D. An estimation and evaluation of total antioxidant capacity of saliva in children with severe early childhood caries. Int J Paediatr Dent 2011;21:459–64. https://doi.org/10.1111/j.1365-263x.2011.01154.x.Search in Google Scholar PubMed
14. Battino, M, Ferreiro, MS, Gallardo, I, Newman, HN, Bullon, P. The antioxidant capacity of saliva. J Clin Periodontol 2002;29:189–94. https://doi.org/10.1034/j.1600-051x.2002.290301x.x.Search in Google Scholar PubMed
15. Tabari, ZA, Azadmehr, A, Nohekhan, A, Naddafpour, N, Ghaedi, FB. Salivary visfatin concentrations in patients with chronic periodontitis. J Periodontol 2014;85:1081–5. https://doi.org/10.1902/jop.2013.130388.Search in Google Scholar PubMed
16. Özcan, E, Saygun, NI, Serdar, MA, Kurt, N. Evaluation of the salivary levels of visfatin, chemerin, and progranulin in periodontal inflammation. Clin Oral Invest 2015;19:921–8. https://doi.org/10.1007/s00784-014-1308-0.Search in Google Scholar PubMed
17. Oita, RC, Ferdinando, D, Wilson, S, Bunce, C, Mazzatti, DJ. Visfatin induces oxidative stress in differentiated C2C12 myotubes in an Akt- and MAPK-independent, NFkB-dependent manner. Pflügers Archiv 2010;459:619–30. https://doi.org/10.1007/s00424-009-0752-1.Search in Google Scholar PubMed
18. Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem 2005;38:1103–11. https://doi.org/10.1016/j.clinbiochem.2005.08.008.Search in Google Scholar PubMed
19. Erel, O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem 2004;37:112–9. https://doi.org/10.1016/j.clinbiochem.2003.10.014.Search in Google Scholar PubMed
20. Mamali, I, Roupas, ND, Armeni, AK, Theodoropoulou, A, Markou, KB, Georgopoulos, NA. Measurement of salivary resistin, visfatin and adiponectin levels. Peptides 2012;33:120–4. https://doi.org/10.1016/j.peptides.2011.11.007.Search in Google Scholar PubMed
21. Pizzino, G, Irrera, N, Cucinotta, M, Pallio, G, Mannino, F, Arcoraci, V, et al.. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev 2017;2017: 8416763. https://doi.org/10.1155/2017/8416763.Search in Google Scholar PubMed PubMed Central
22. Rubio, CP, Hernández-Ruiz, J, Martinez-Subiela, S, Tvarijonaviciute, A, Ceron, JJ. Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: an update. BMC Vet Res 2016;12:166. https://doi.org/10.1186/s12917-016-0792-7.Search in Google Scholar PubMed PubMed Central
23. Rahmani, M, Ghorchi, V, Rezaei, F, Vaisi-Raygani, A. Evaluation of total antioxidant capacity of saliva in high school students. Global J Health Sci 2015;8:89–94. https://doi.org/10.5539/gjhs.v8n4p89.Search in Google Scholar PubMed PubMed Central
24. Krawczyk, D, Błaszczak, J, Borowicz, J, Mielnik-Błaszczak, M. Life style and risk of development of dental caries in a population of adolescents. Ann Agric Environ Med 2014;21:576–80. https://doi.org/10.5604/12321966.1120605.Search in Google Scholar PubMed
25. Ahmadi-Motamayel, F, Goodarzi, MT, Hendi, SS, Kasraei, S, Moghimbeigi, A. Total antioxidant capacity of saliva and dental caries. Med Oral Patol Oral Cir Bucal 2013;18:e553–56. https://doi.org/10.4317/medoral.18762.Search in Google Scholar PubMed PubMed Central
26. Dodwad, R, Betigeri, AV, Preeti, BP. Estimation of total antioxidant capacity levels in saliva of caries-free and caries-active children. Contemp Clin Dent 2011;2:17–20. https://doi.org/10.4103/0976-237x.79296.Search in Google Scholar PubMed PubMed Central
27. Guler, C, Karabulut, A, Görgen, V, Güneş, D. Evaluation of changes in salivary oxidative stress and antioxidant levels after restored with a color compomer of caries teeth in children. Yeditepe Dent J 2021;17:31–6. https://doi.org/10.5505/yeditepe.2021.19870.Search in Google Scholar
28. Araujo, HC, Nakamune, ACMS, Garcia, WG, Pessan, JP, Antoniali, C. Carious lesion severity induces higher antioxidant system activity and consequently reduces oxidative damage in children’s saliva. Oxid Med Cell Longev 2020;2020: 3695683. https://doi.org/10.1155/2020/3695683.Search in Google Scholar PubMed PubMed Central
29. Toczewska, J, Maciejczyk, M, Konopka, T, Zalewska, A. Total oxidant and antioxidant capacity of gingival crevicular fluid and saliva in patients with periodontitis: review and clinical study. Antioxidants 2020;9: 450. https://doi.org/10.3390/antiox9050450.Search in Google Scholar PubMed PubMed Central
30. Baltacıoğlu, E, Yuva, P, Aydın, G, Alver, A, Kahraman, C, Karabulut, E, et al.. Lipid peroxidation levels and total oxidant/antioxidant status in serum and saliva from patients with chronic and aggressive periodontitis. Oxidative stress index: a new biomarker for periodontal disease? J Periodontol 2014;85:1432–41. https://doi.org/10.1902/jop.2014.130654.Search in Google Scholar PubMed
31. Hegde, M, Hegde, N, Ashok, A, Shetty, S. Evaluation of total antioxidant capacity of saliva and serum in caries-free and caries-active adults: an in-vivo study. Indian J Dent Res 2013;24:164–7. https://doi.org/10.4103/0970-9290.116670.Search in Google Scholar PubMed
32. Guzelcicek, A, Demir, M, Kirmit, A, Dogan, M. Evaluation of the relationshıp between dental caries and oxidatıve stress and antioxidant capacity. Authorea; March 31, 2021.10.22541/au.161717895.53907771/v1Search in Google Scholar
33. Maciejczyk, M, Zalewska, A, Ładny, JR. Salivary antioxidant barrier, redox status, and oxidative damage to proteins and lipids in healthy children, adults, and the elderly. Oxid Med Cell Longev 2019;2019: 4393460. https://doi.org/10.1155/2019/4393460.Search in Google Scholar PubMed PubMed Central
34. Kukla, M, Mazur, W, Bułdak, RJ, Zwirska-Korczala, K. Potential role of leptin, adiponectin and three novel adipokines—visfatin, chemerin and vaspin—in chronic hepatitis. Mol Med 2011;17:1397–410. https://doi.org/10.2119/molmed.2010.00105.Search in Google Scholar PubMed PubMed Central
35. Tabari, ZA, Ghaedi, FB, Azadmehr, A, Nohekhan, A, Tabrizi, MAA, Ardakani, MRT, et al.. Salivary visfatin concentration in response to non-surgical periodontal therapy. J Clin Diagn Res 2015;9:ZC05–08. https://doi.org/10.7860/JCDR/2015/11537.5773.Search in Google Scholar PubMed PubMed Central
36. El-Taweel, HMA, Salah, NA, Selem, AK, El-Refaeey, AA, Abdel-Aziz, AF. Visfatin gene expression and oxidative stress in pregnancy induced hypertension. Egypt J Basic Appl Sci 2018;5:69–74. https://doi.org/10.1016/j.ejbas.2017.12.002.Search in Google Scholar
37. Buyukaydin, B, Guler, EM, Karaaslan, T, Olgac, A, Zorlu, M, Kiskac, M, et al.. Relationship between diabetic polyneuropathy, serum visfatin, and oxidative stress biomarkers. World J Diabetes 2020;11:309–21. https://doi.org/10.4239/wjd.v11.i7.309.Search in Google Scholar PubMed PubMed Central
38. Kadkhodazadeh, M, Amid, R, Torshabi, M, Yahyazadeh, N, Youssefi, N, Ekhlasmand, M. Comparison of salivary visfatin in patients with periodontitis and peri-implantitis in an Iranian population. J Periodontol Implant Dent 2016;8:37–42.10.15171/jpid.2016.007Search in Google Scholar
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review Article
- The implication of molecular markers in the early stage diagnosis of colorectal cancers and precancerous lesions
- Research Articles
- What are the predominant parameters for Down syndrome risk estimation in first-trimester screening: a data mining study
- Comparison between liquid chromatography-tandem mass spectrometry and immunoassay methods for measurement of plasma 25 (OH) vitamin D
- Comparison of Barricor tube and serum separator tube in outpatients
- Effect of hemoglobin, triglyceride, and urea in different concentrations on compatibility between methods used in HbA1c measurement
- Evaluation of hemolysis interference and possible protective effect of N-phenyl maleimide on the measurement of small peptides
- Neuroprotective and metabotropic effect of aerobic exercise training in female patients with type 2 diabetes mellitus
- Serum asprosin levels in patients with retinopathy of prematurity
- Neutrophil/lymphocyte and platelet/lymphocyte ratios as a biomarker in postoperative wound infections
- Relationship between dental caries and saliva’s visfatin levels, total antioxidant capacity (TAC) and total oxidant status (TOS)
- Might periostin serve as a marker of bone marrow involvement in patients with diffuse large B-cell lymphoma?
- A new approach for the pleiotropic effect of metformin use in type 2 diabetes mellitus
- IGFBP7 is a predictor of diuretic-induced acute kidney injury in the patients with acute decompensated heart failure
- Serum vitamin D receptor and fibroblast growth factor-23 levels in postmenopausal primary knee osteoarthritis patients
- Antioxidant activity of ethanol extract and fractions of Piper crocatum with Rancimat and cuprac methods
- Lycorine impedes 7,12-dimethylbenz(a)anthracene exposed hamster oral carcinogenesis through P13K/Akt and NF-κB inhibition
- Sublethal effects of acrylamide on thyroid hormones, complete blood count and micronucleus frequency of vertebrate model organism (Cyprinus carpio)
- Acknowledgment
- Acknowledgment
Articles in the same Issue
- Frontmatter
- Review Article
- The implication of molecular markers in the early stage diagnosis of colorectal cancers and precancerous lesions
- Research Articles
- What are the predominant parameters for Down syndrome risk estimation in first-trimester screening: a data mining study
- Comparison between liquid chromatography-tandem mass spectrometry and immunoassay methods for measurement of plasma 25 (OH) vitamin D
- Comparison of Barricor tube and serum separator tube in outpatients
- Effect of hemoglobin, triglyceride, and urea in different concentrations on compatibility between methods used in HbA1c measurement
- Evaluation of hemolysis interference and possible protective effect of N-phenyl maleimide on the measurement of small peptides
- Neuroprotective and metabotropic effect of aerobic exercise training in female patients with type 2 diabetes mellitus
- Serum asprosin levels in patients with retinopathy of prematurity
- Neutrophil/lymphocyte and platelet/lymphocyte ratios as a biomarker in postoperative wound infections
- Relationship between dental caries and saliva’s visfatin levels, total antioxidant capacity (TAC) and total oxidant status (TOS)
- Might periostin serve as a marker of bone marrow involvement in patients with diffuse large B-cell lymphoma?
- A new approach for the pleiotropic effect of metformin use in type 2 diabetes mellitus
- IGFBP7 is a predictor of diuretic-induced acute kidney injury in the patients with acute decompensated heart failure
- Serum vitamin D receptor and fibroblast growth factor-23 levels in postmenopausal primary knee osteoarthritis patients
- Antioxidant activity of ethanol extract and fractions of Piper crocatum with Rancimat and cuprac methods
- Lycorine impedes 7,12-dimethylbenz(a)anthracene exposed hamster oral carcinogenesis through P13K/Akt and NF-κB inhibition
- Sublethal effects of acrylamide on thyroid hormones, complete blood count and micronucleus frequency of vertebrate model organism (Cyprinus carpio)
- Acknowledgment
- Acknowledgment

