Abstract
Hexagonal α-Al2O3 platelets with about 1-μm diameter and 0.2-μm thickness are prepared by the calcination of a milled Al(OH)3 precursor with NH4F and NH4Cl additives. The influences of the ball milling treatment and the composite additive on the microstructure of α-Al2O3 micro-powders are studied. The results indicate that the particle size distribution of α-Al2O3 is highly dependent on the aggregate size of the Al(OH)3 precursor and that the morphology of α-Al2O3 can be significantly favored by the addition of 5 wt.% NH4F and 5 wt.% NH4Cl as additives. The growth velocity of the (0001) plane is significantly reduced because of the accelerated growth rate of other crystal faces caused by the increase of gas phase mass transfer. The combined effect of composite additives and precursor ball milling treatment leads to the generation of well-developed α-Al2O3 platelets with uniform size distribution.
1 Introduction
Plate-like α-Al2O3 powders have been widely applied in the fields of high-strength ceramics, abrasives and other refractory materials owing to its excellent properties including good chemical stability, high elastic modulus as well as high tensile and compression strength [1], [2], [3], [4], [5], [6], [7].
Until now, many methods have been developed to synthesize α-Al2O3 platelets [8], [9], [10], such as the melt salt method [11], hydrothermal synthesis [12], laser scanning [13], electrostatic spray-assisted chemical vapor deposition [14] and high-temperature calcination [15]. As the crystal growth of α-Al2O3 is mainly determined by the relative growth rates of various crystal faces, not only the internal structural factors but also the external conditions [16], [17], [18], such as the type of precursor, the reaction temperature and the addition of crystal seeds (including α-Al2O3, α-Fe2O3, α-Cr2O3, etc.), have been considered to control the morphology of α-Al2O3 powders.
In particular, it has been demonstrated that the type and microstructure (morphology, aggregate size and shape) of the precursor are crucial to control the morphology of α-Al2O3 powders [19], [20]. As the larger-sized precursor aggregates could be broken into highly active fragments under high-energy ball milling, the neck growth of α-Al2O3 during calcination could be efficiently suppressed, and almost-spherical α-Al2O3 powders of ~1.0-μm size were synthesized by the calcination of the ground precursor at 1450°C [21]. Moreover, although many kinds of additives [15], [22], [23], [24], such as AlF3, ZnF2, NH4F, etc., have been introduced to improve the α-phase transformation and the morphology of α-Al2O3, the effect of fluoride additives on the morphology modification of α-Al2O3 particles is different, depending on the precursor [20].
Therefore, considering the above aspects, in current technology, commercial Al(OH)3 with spherical agglomerates and about 100-μm size is ball milled as the precursor for the preparation of α-Al2O3. Composite additives (NH4F and NH4Cl) are introduced to modify the α-Al2O3 microstructure. In the present work, hexagonal α-Al2O3 micro-platelets with a uniform distribution are prepared by high-temperature calcination, and the growth process is discussed.
2 Experimental procedure
The preparation of α-Al2O3 powders from a commercial Al(OH)3 precursor (Shandong Branch of CHACLO, China) was carried out in the following way. First, ball milling treatment of commercial Al(OH)3 precursor was carried out for 9 h in a batch-type planetary mill (ND7-2L, Nanjing LAB Industrial Co., Ltd., China) with zirconia balls using ethanol as the dispersion medium. The ratio of grinding balls to material was 15:1 in weight, and a rotational speed of 240 r min−1 was employed in the experiments. The milled Al(OH)3 samples were oven dried at T=80°C for 24 h. Then, 5 wt.% NH4F (Sinopharm Chemical Reagent Beijing Co., Ltd., China) and 5 wt.% NH4Cl (Sinopharm Chemical Reagent Beijing Co., Ltd., China) additives were introduced into the milled Al(OH)3 precursor, and the mixture was ground in an agate mortar for 1 h. Finally, the commercial Al(OH)3 precursor, the Al(OH)3 precursor milled for 9 h and the milled Al(OH)3 precursor with 5 wt.% NH4F and 5 wt.% NH4Cl as additives were calcined separately at T=1300°C for 3 h for comparison.
The phase compositions and the crystallinity of the samples after milling and calcination were identified by powder X-ray diffraction (XRD; X′ Pert Pro MPD, Philips, Eindhoven, Netherlands) with CuKα radiation (λ =0.154 nm, 40 kV, 40 mA) in the 2θ range 20–80° and with a scan rate of 5° min−1. Fourier transform infrared (FTIR; Tensor II, Bruker) spectra of the α-Al2O3 samples were collected between 4000 and 400 cm−1 on a Tensor 37 infrared spectrometer with a wavenumber resolution of 4 cm−1. The microstructure of the milled and unmilled Al(OH)3 samples was analyzed by cold field emission scanning electron microscopy (SEM; JSM-7500F, JEOL, Tokyo, Japan) at an accelerating voltage of 15 kV, and the morphology evolution of the α-Al2O3 samples upon calcination at T=1300°C was observed by field emission SEM (Sigma HD, Zeiss, Oberkochen, Germany) at an accelerating voltage of 10 kV.
3 Results and discussion
The effect of the ball milling treatment on the phase composition and crystallinity of the precursor is shown in Fig. 1. The XRD patterns of the commercial samples and those milled for 9 h indicate that the crystallinity of the precursor could be significantly decreased by the ball milling treatment, because the diffraction peaks (shown in the XRD pattern of the 9-h milled precursor) related to gibbsite (JCPDS File No: 74-1775) are weakened and broadened after 9 h of ball milling. In particular, the peak centered at 68.88º corresponding to the 026 reflection of gibbsite even disappears. These results demonstrate that the 9-h ball milling treatment results in a grain refinement and poor crystallinity of the Al(OH)3 precursor [25], although the milled and unmilled Al(OH)3 precursors are identical in phase composition.
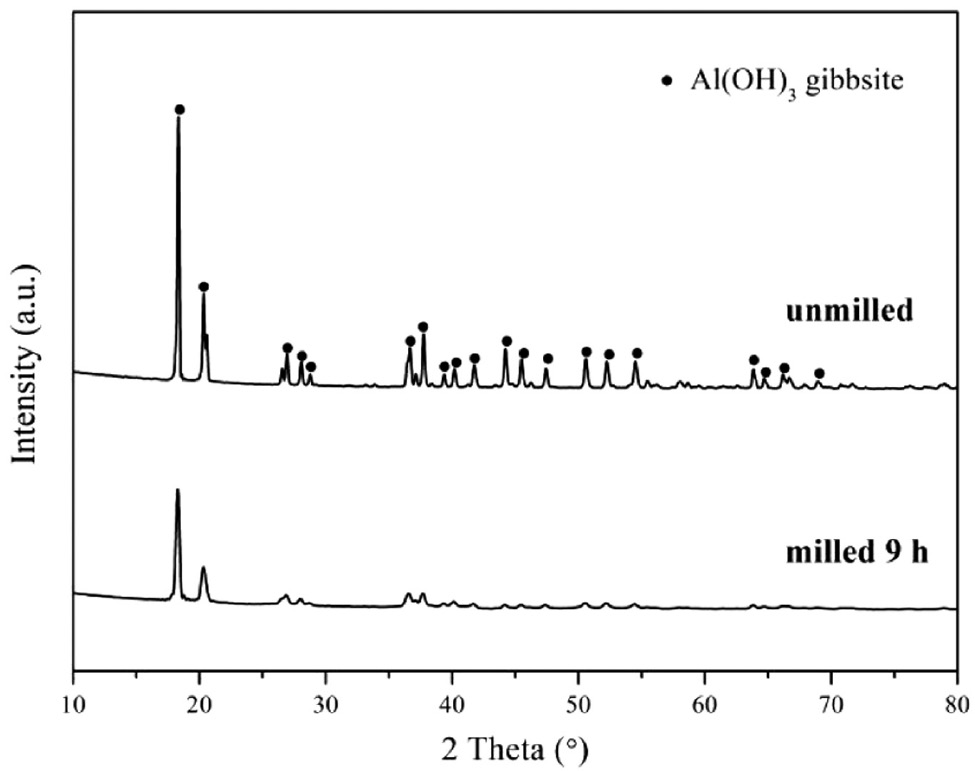
XRD patterns of the Al(OH)3 precursors before and after ball milling.
Figure 2 presents the microstructure of the commercial Al(OH)3 precursor. It is seen that the Al(OH)3 precursor without ball milling consists mainly of spherical agglomerates in the size range of 100–200 μm (as shown in Fig. 2a), which consist of many smaller-sized grains (as shown in Fig. 2b). In comparison, the morphology of the ball-milled Al(OH)3 precursor shown in Fig. 3 indicates that these large agglomerates are mostly broken into irregularly shaped particles in the size range of ≤5 μm by 9-h ball milling, accompanied by a formation of a small amount of irregular pieces in the size range of 10–30 μm. The above results reveal that the ball milling treatment leads to a significant refinement in morphology and particle size distribution of the Al(OH)3 precursor.
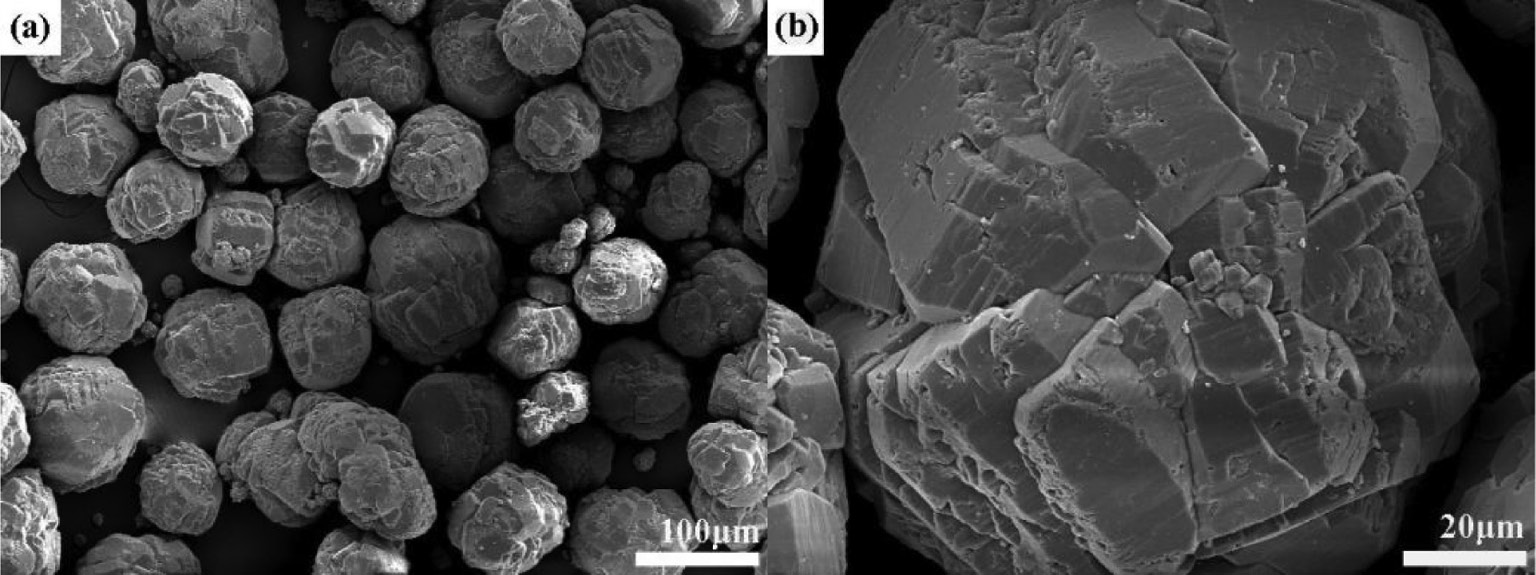
SEM photographs of the commercial Al(OH)3 precursor. Picture (b) is the magnification of the selected area in picture (a).
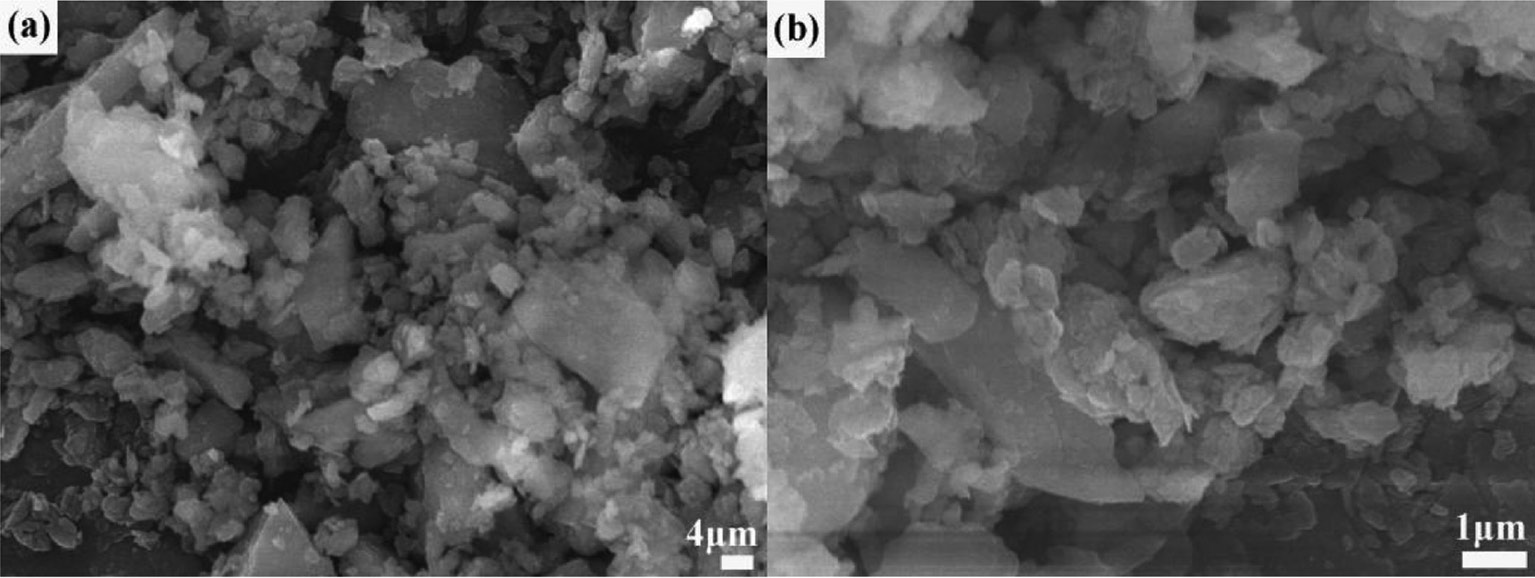
SEM photographs of the Al(OH)3 precursor after 9 h of ball milling. Picture (b) is the magnification of the selected area in picture (a).
Figure 4 shows the XRD patterns of the samples obtained by calcination of the commercial Al(OH)3 precursor, the 9-h ball-milled Al(OH)3 precursor and the milled Al(OH)3 precursor with 5 wt.% NH4F and 5 wt.% NH4Cl additives at 1300°C for 3 h. The characteristic peaks at 25.590º, 35.155º, 37.781º, 43.353º, 52.561º, 57.504º, 61.310º, 66.530º, 68.215º and 76.883º corresponding to the 012, 104, 110, 113, 024, 116, 018, 214, 300 and 1010 reflections of α-Al2O3, respectively [15], confirm that single-phase α-Al2O3 could be synthesized by the calcination at T=1300°C.
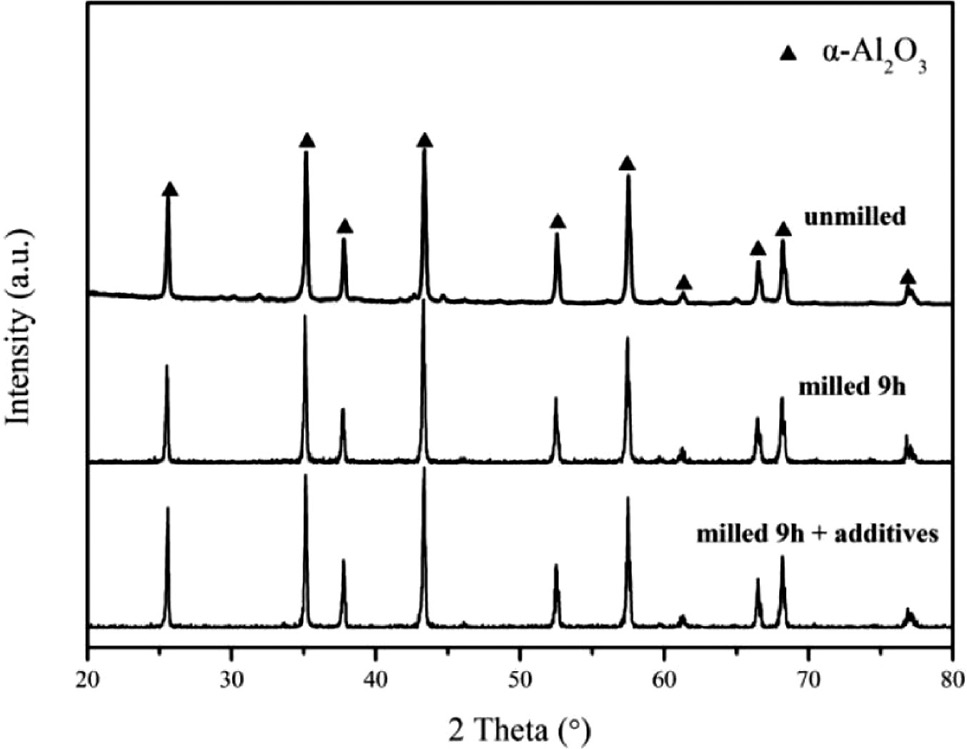
XRD patterns of samples obtained by calcination at T=1300°C.
To further prove the above result, these calcined samples were analyzed by FTIR. As shown in Fig. 5, intense bands between 1000 and 400 cm−1 assigned to the Al–O–Al stretching emerge in the spectra [26], while the peaks at 688, 641, 595, 493, 469 and 453 cm−1 result from the vibrations of Al–O bonds of the AlO6 units [21]. The FTIR results confirm that the samples calcined at T=1300°C are composed of α-Al2O3.
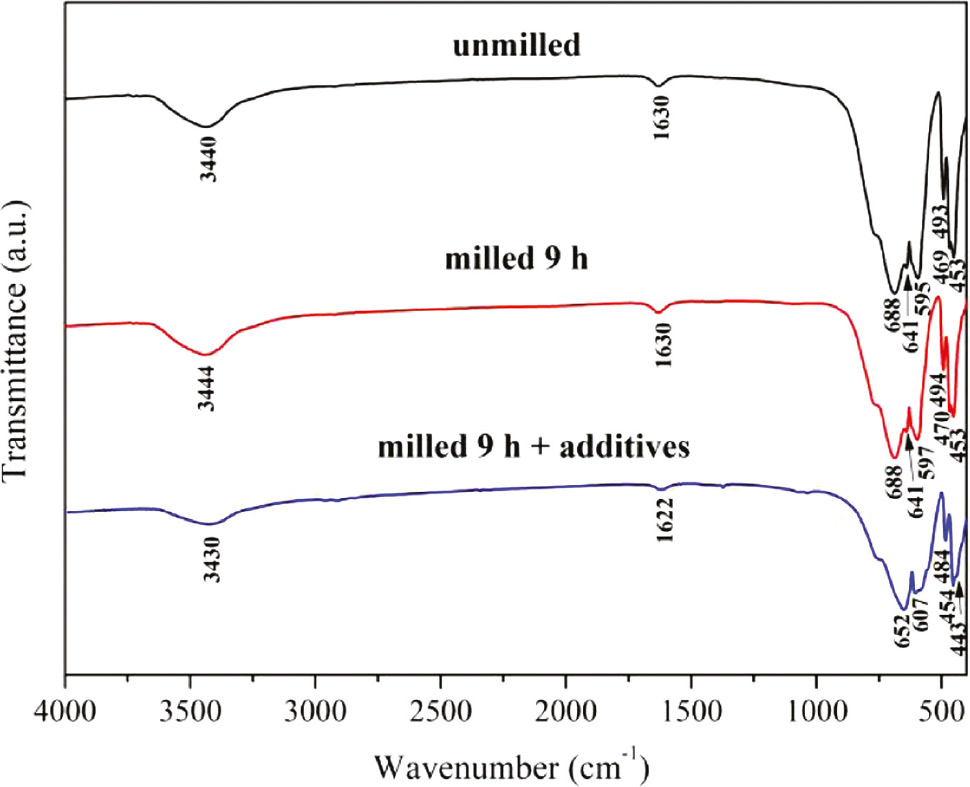
FTIR spectra of samples obtained by calcination at T=1300°C.
The morphological evolution of the α-Al2O3 samples can be followed in Figs. 6–8 , which present SEM photographs of the α-Al2O3 prepared by the calcination of commercial Al(OH)3, 9-h ball-milled Al(OH)3 and 9-h ball-milled Al(OH)3 with NH4F and NH4Cl additives at 1300°C, respectively. As shown in Fig. 6a, the α-Al2O3 particles obtained from the commercial Al(OH)3 precursor fired at 1300°C maintain the initial aggregate shape with a worm-like structure formed by the neck growth of α-Al2O3 grains. By comparing Fig. 7 with Fig. 6, it is seen that the aggregate size has been significantly decreased due to the morphology refinement of the milled Al(OH)3 (see Fig. 2). The above results indicate that the α-Al2O3 powders obtained by the calcination of the unmilled and milled Al(OH)3 precursors at 1300°C have similar worm-like microstructures because the formation of the as-synthesized α-Al2O3 mainly follows the solid phase mass transfer mechanism [27].
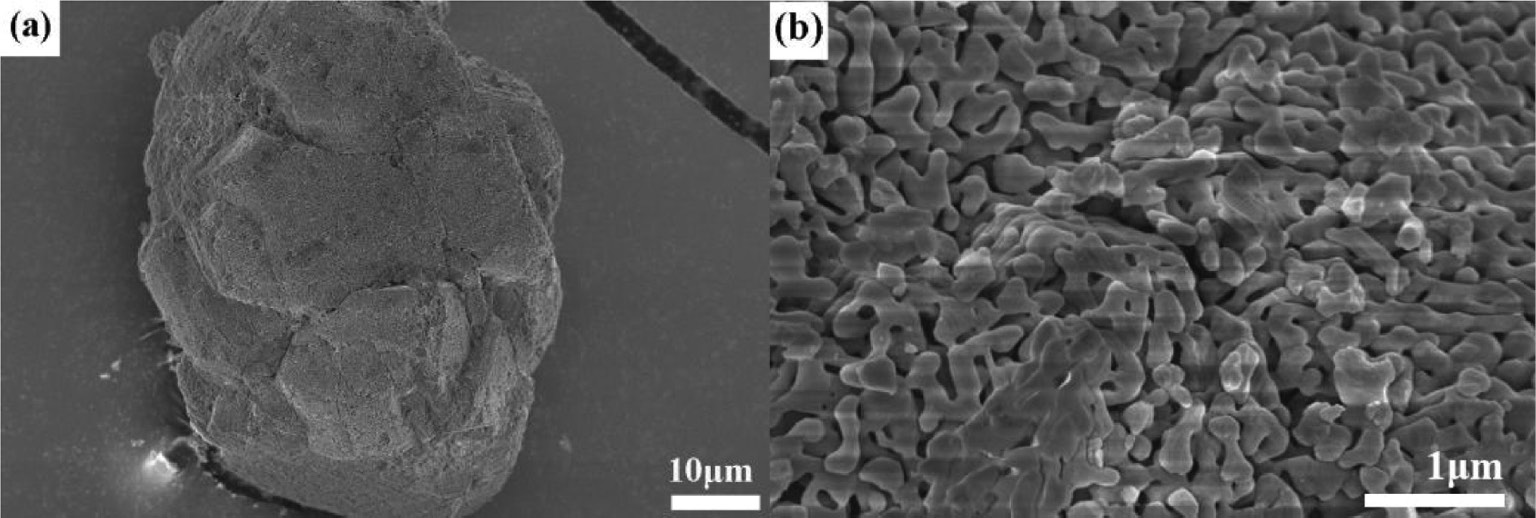
SEM photographs of the α-Al2O3 sample obtained by the calcination of commercial Al(OH)3 at T=1300°C for 3 h. Picture (b) is the magnification of the selected area in picture (a).
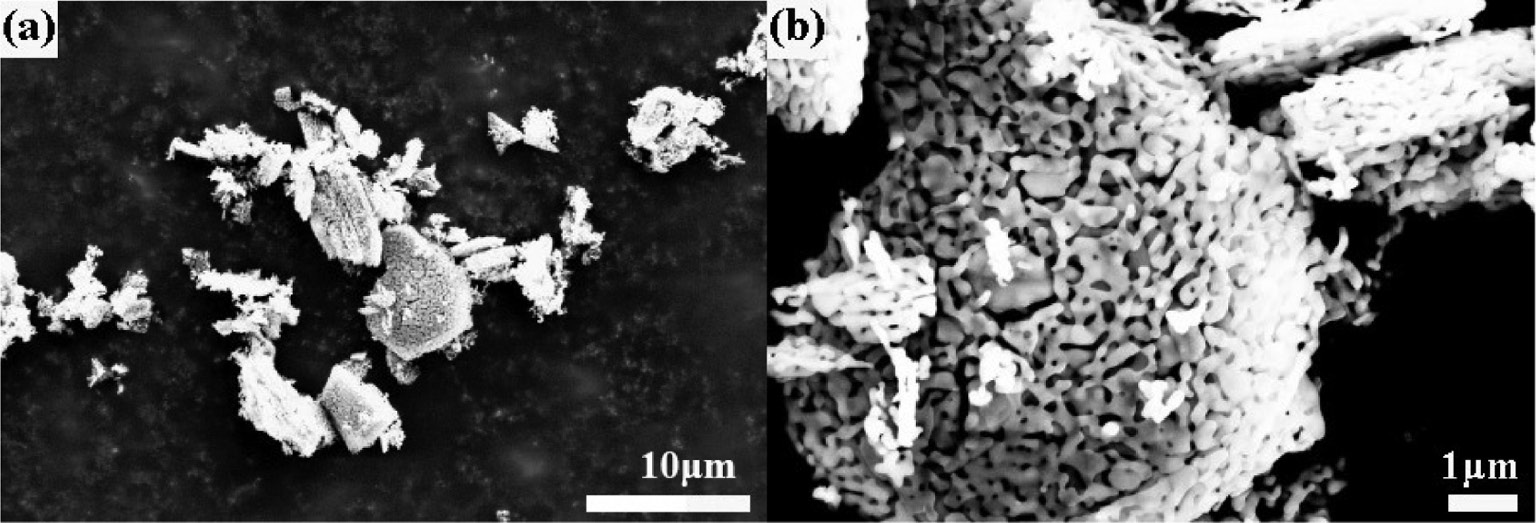
SEM photographs of the α-Al2O3 sample obtained by the calcination of 9-h ball-milled Al(OH)3 at T=1300°C for 3 h. Picture (b) is the magnification of the selected area in picture (a).
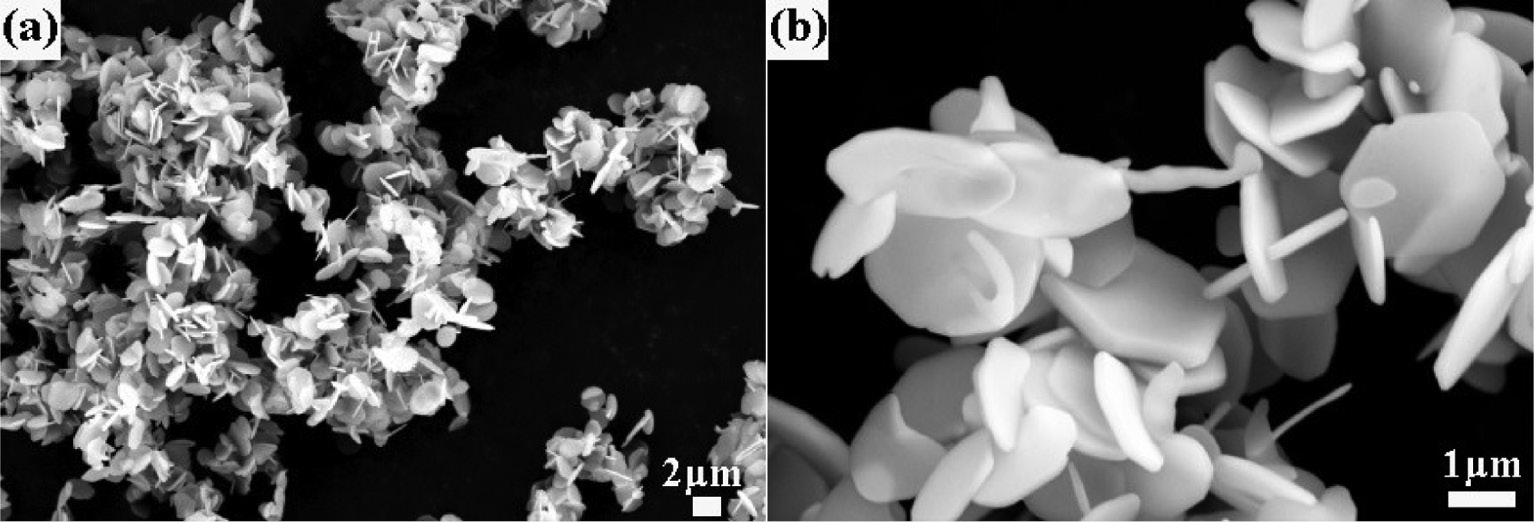
SEM photographs of the α-Al2O3 sample obtained by the calcination of 9-h ball-milled Al(OH)3 containing NH4F and NH4Cl additives. Picture (b) is the magnification of the selected area in picture (a).
In contrast, α-Al2O3 powders obtained from the milled Al(OH)3 precursor with the 5 wt.% NH4Cl and 5 wt.% NH4F additives exhibit a well-defined hexagonal platelet morphology as shown in Fig. 8. The diameters of the α-Al2O3 platelets are about 1 μm, and the thicknesses are about 200 nm. It can be seen that the introduction of the NH4Cl and NH4F additives results in a significant morphological modification of the α-Al2O3 powders from the milled Al(OH)3 precursor calcined at T=1300°C.
The phenomenon suggests that the formation of hexagonal α-Al2O3 platelets is highly related to the addition of NH4Cl and NH4F, because the additives cause a different growth rate along the respective crystal axis and consequently influence the microstructure of α-Al2O3. Previous studies have reported [9], [28], [29] that the (0001) surface is the most stable surface of α-Al2O3 and appears predominantly, thereby promoting the formation of hexagonal platelets. In the present study, the addition of NH4Cl and NH4F accelerates gas phase mass transport with formation of gaseous intermediates including mainly AlOF and AlOCl, etc. [23], [30], and hence promotes the growth rate of α-Al2O3 along the crystal orientation (101̅0). As a result, the growth velocity of the (101̅0) crystal faces is significantly increased, and the growth velocity of the (0001) plane is greatly reduced in the presence of NH4Cl and NH4F. Thus, the α-Al2O3 powders prepared by calcination of the milled Al(OH)3 precursor with NH4Cl and NH4F additives appear as well-developed hexagonal platelets.
4 Summary
Well-shaped hexagonal α-Al2O3 platelets of ~1.0-μm diameter and ~0.2-μm thickness have been synthesized from a ball-milled Al(OH)3 precursor with NH4Cl and NH4F additives calcined at 1300°C. As spherical Al(OH)3 agglomerates in the size range of 100–200 μm are mostly broken into irregularly shaped particles in the ≤5 μm size range, the size distribution of α-Al2O3 powders is significantly improved through the incorporation of the morphology refinement of the precursor caused by the ball milling treatment. Moreover, the growth velocity of the (0001) plane could be reduced by the introduction of NH4Cl and NH4F additives, because the growth of α-Al2O3 crystals along (101̅0) is greatly promoted by gaseous AlOF and AlOCl or other intermediates. Therefore, the addition of NH4Cl and NH4F appears to be helpful in the generation of α-Al2O3 platelets.
Acknowledgments
The authors acknowledge the National Natural Science Foundation of China (contract no. U1504526), the Key Scientific Research Project for Universities and Colleges in Henan Province (contract no. 19A430028) and the Open Foundation of the State Key Laboratory of Refractories and Metallurgy (G201909) for financial support.
References
[1] Y. H. Huang, Y. Xia, S. Liao, Z. F. Tong, G. Liu, Y. Li, Z. P. Chen, Ceram. Int.2014, 40, 8071.10.1016/j.ceramint.2013.12.161Suche in Google Scholar
[2] Y. Q. Wu, Y. F. Zhang, X. X. Huang, J. K. Guo, Ceram. Int.2001, 27, 265.10.1016/S0272-8842(00)00074-2Suche in Google Scholar
[3] W. J. Gu, L. L. Zhu, X. J. Shang, D. F. Ding, L. Q. Liu, G. L. Chen, G. T. Ye, J. Alloys Compd.2019, 772, 637.10.1016/j.jallcom.2018.09.128Suche in Google Scholar
[4] J. Ding, C. Yu, J. P. Liu, C. J. Deng, H. X. Zhu, Mater. Chem. Phys.2018, 206, 193.10.1016/j.matchemphys.2017.11.011Suche in Google Scholar
[5] S. S. Li, J. H. Liu, J. K. Wang, L. Han, H. J. Zhang, S. W. Zhang, Ceram. Int.2018, 44, 12940.10.1016/j.ceramint.2018.04.108Suche in Google Scholar
[6] A. Huang, Y. J. Wang, Y. S. Zou, H. Z. Gu, L. P. Fu, Ceram. Int.2018, 44, 14617.10.1016/j.ceramint.2018.05.085Suche in Google Scholar
[7] Y. Li, L. L. Zhu, K. Liu, D. F. Ding, J, Zhang, G. T. Ye, Ceram. Int.2019, 45, 12066.10.1016/j.ceramint.2019.03.103Suche in Google Scholar
[8] X. H. Jin, L. Gao, J. Am. Ceram. Soc.2004, 87, 533.10.1111/j.1551-2916.2004.00533.xSuche in Google Scholar
[9] W. L. Wang, J. Q. Bi, Y. Q. Qi, Z. Zhang, Z. Xing, H. L. Zhu, Y. J. Bai, Powder Technol.2010, 201, 273.10.1016/j.powtec.2010.04.010Suche in Google Scholar
[10] Y. F. Chang, J. Wu, M. M. Zhang, E. Kupp, G. L. Messing, Ceram. Int.2017, 43, 12684.10.1016/j.ceramint.2017.06.150Suche in Google Scholar
[11] L. H. Zhu, R. R. Tu, Q. W. Huang, Ceram. Int.2012, 38, 901.10.1016/j.ceramint.2011.08.008Suche in Google Scholar
[12] W. L. Suchanek, J. M. Garcés, P. F. Fulvio, M. Jaroniec, Chem. Mater.2010, 22, 6564.10.1021/cm102158wSuche in Google Scholar
[13] Y. Q. Wu, K. L. Choy, L. L. Hench, J. Am. Ceram. Soc.2004, 87, 3.Suche in Google Scholar
[14] M. Wei, D. Zhi, K. L. Choy, Nanotechnology2006, 17, 181.10.1088/0957-4484/17/1/029Suche in Google Scholar
[15] L. L. Zhu, C. H. Sun, L. G. Chen, X. F. Lu, S. Li, G. T. Ye, L. Q. Liu, Z. Naturforsch.2017, 72b, 665.10.1515/znb-2017-0079Suche in Google Scholar
[16] H. I. Hsiang, C. C. Chuang, T. H. Chen, F. S. Yen, Ceram. Int.2010, 36, 1467.10.1016/j.ceramint.2010.01.004Suche in Google Scholar
[17] M. M. Zhang, Y. F. Chang, N. Pulati, S. T. Zhang, Y. Sun, J. Wu, Y. C. Liu, B. Yang, W. W. Cao, Mater. Lett.2018, 210, 182.10.1016/j.matlet.2017.09.025Suche in Google Scholar
[18] K. L. Gavrilov, S. J. Bennison, K. R. Mikeska, R. Levi-Setti, J. Mater. Sci.2003, 38, 3965.10.1023/A:1026206414377Suche in Google Scholar
[19] H. J. Kim, T. G. Kim, J. J. Kim, S. S. Park, S. S. Hong, G. D. Lee, J. Phys. Chem. Solids2008, 69, 1521.10.1016/j.jpcs.2007.10.024Suche in Google Scholar
[20] H. Chen, Q. D. Wu, T. X. Yang, M. Q. Li, H. C. Xie, C. Lin, Ceram. Int.2015, 41, 12288.10.1016/j.ceramint.2015.06.054Suche in Google Scholar
[21] S. Li, L. L. Zhu, L. Q. Liu, L. G. Chen, H. X. Li, C. H. Sun, Z. Naturforsch.2018, 73b, 589.10.1515/znb-2018-0080Suche in Google Scholar
[22] J. Li, Y. S. Wu, Y. B. Pan, W. B. Liu, J. K. Guo, Ceram. Int.2007, 33, 919.10.1016/j.ceramint.2006.02.002Suche in Google Scholar
[23] H. S. Kim, N. K. Park, T. J. Lee, M. Kang, Ceram. Int.2014, 40, 3813.10.1016/j.ceramint.2013.08.033Suche in Google Scholar
[24] F. Mirjalili, Bull. Mater. Sci.2014, 37, 1709.10.1007/s12034-014-0734-6Suche in Google Scholar
[25] Z. J. Liu, W. C. Wang, D. Z. Yang, S. Wang, L. Y. Dai, Z. L. Yang, Ceram. Int.2015, 42, 3411.10.1016/j.ceramint.2015.10.136Suche in Google Scholar
[26] X. L. Du, Y. Q. Wang, X. H. Su, J. G. Li, Powder Technol.2009, 192, 40.10.1016/j.powtec.2008.11.008Suche in Google Scholar
[27] D. Riello, C. Zetterström, C. Parr, M. A. L. Braulio, M. Moreira, J. B. Gallo, V. C. Pandolfelli, Ceram. Int.2016, 42, 9804.10.1016/j.ceramint.2016.03.074Suche in Google Scholar
[28] B. Hinnemann, E. A. Carter, J. Phys. Chem. C.2007, 111, 7105.10.1021/jp068869cSuche in Google Scholar
[29] N. G. Petrik, P. L. Huestis, J. A. LaVerne, A. B. Aleksandrov, T. M. Orlando, G. A. Kimmel, J. Phys. Chem. C2018, 122, 9540.10.1021/acs.jpcc.8b01969Suche in Google Scholar
[30] P. F. Tan, J. Müller, D. Neuschütz, J. Electrochem. Soc.2005, 152, C288.10.1149/1.1883236Suche in Google Scholar
© 2019 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- In this Issue
- Research Articles
- RbKLi2[Li3SiO4]4:Eu2+ an ultra narrow-band phosphor
- 2D-Coordination polymer containing lead(II) in a hemidirected PbO4S3 environment formed by molecular breaking of the 1,3-oxathiolane ligand
- Photoluminescence and photocatalytic activity of a 3D framework based on 4,4′-bis(1,2,4-triazol-1-ylmethyl)biphenyl as a ligand for cadmium
- TiO2–SiO2 nanocomposite as a highly efficient catalyst for the solvent-free cyclocondensation reaction of isatins, cyclohexanones, and urea
- Crystal structure of a new silver(I) coordination polymer assembled from imidazolidine-2-thione (Imt), {[Ag2(Imt)3](NO3)2}n
- Volume increments of some pseudohalide anions
- Synthesis, crystal structure and photoluminescence of [NHEt3][Re(I)(quinaldate)(CO)3Cl]
- Preparation of hexagonal micro-sized α-Al2O3 platelets from a milled Al(OH)3 precursor with NH4F and NH4Cl additives
- Iminium-functionalized 1,2,3-triazoles by [3+2] cycloaddition reactions of internal acetylenic iminium triflates with organoazides
- Synthesis of 4-arylaminoquinazoline-2-carboxylic acid derivatives by the reaction of (Z)-2-amino-N′-aryl-benzimidamides with some selected anhydrides
- Surface phenomena related to applications regarding optimum dosages of casein superplasticizer in self-leveling underlayment cements
- Phase equilibrium in the Gd-Ni-In system at 870 K
- A new Co(II) coordination polymer with the 2-(4-pyridyl)-terephthalate ligand: synthesis, crystal structure and magnetic properties
Artikel in diesem Heft
- Frontmatter
- In this Issue
- Research Articles
- RbKLi2[Li3SiO4]4:Eu2+ an ultra narrow-band phosphor
- 2D-Coordination polymer containing lead(II) in a hemidirected PbO4S3 environment formed by molecular breaking of the 1,3-oxathiolane ligand
- Photoluminescence and photocatalytic activity of a 3D framework based on 4,4′-bis(1,2,4-triazol-1-ylmethyl)biphenyl as a ligand for cadmium
- TiO2–SiO2 nanocomposite as a highly efficient catalyst for the solvent-free cyclocondensation reaction of isatins, cyclohexanones, and urea
- Crystal structure of a new silver(I) coordination polymer assembled from imidazolidine-2-thione (Imt), {[Ag2(Imt)3](NO3)2}n
- Volume increments of some pseudohalide anions
- Synthesis, crystal structure and photoluminescence of [NHEt3][Re(I)(quinaldate)(CO)3Cl]
- Preparation of hexagonal micro-sized α-Al2O3 platelets from a milled Al(OH)3 precursor with NH4F and NH4Cl additives
- Iminium-functionalized 1,2,3-triazoles by [3+2] cycloaddition reactions of internal acetylenic iminium triflates with organoazides
- Synthesis of 4-arylaminoquinazoline-2-carboxylic acid derivatives by the reaction of (Z)-2-amino-N′-aryl-benzimidamides with some selected anhydrides
- Surface phenomena related to applications regarding optimum dosages of casein superplasticizer in self-leveling underlayment cements
- Phase equilibrium in the Gd-Ni-In system at 870 K
- A new Co(II) coordination polymer with the 2-(4-pyridyl)-terephthalate ligand: synthesis, crystal structure and magnetic properties

