Abstract
Objectives
This study aimed to evaluate clinicians’ awareness, perspectives, and expectations regarding critical value reporting in laboratories through both a survey and retrospective data analysis.
Methods
Critical value data were retrospectively collected from the hospital information system between September 2021 and February 2022. A 17-item survey, comprising six demographic questions and 11 items related to critical values (eight closed-ended, two semi-open-ended, and one open-ended), was administered to clinicians at two tertiary hospitals using Google Forms. The survey was piloted on 10 clinicians for validation. Data were analyzed using Microsoft Excel 2013 (Microsoft Corp., WA, USA) and SPSS version 25.0 (IBM Corp., NY, USA).
Results
Over the six-month period, a total of 4,899 critical values were reported, with platelets, hemoglobin, and glucose being the most frequently flagged parameters. The median critical value notification time was found to be 7 min. A total of 321 clinicians participated in the survey. Among them, 97.8 % considered critical value reporting essential; 44.6 % preferred notification via text message, while 10.8 % favored phone calls. Most respondents found the current critical value test list adequate; however, 8.4 % suggested that troponin should be added.
Conclusions
Our findings indicate a high level of clinician awareness and acceptance of the importance of critical value reporting. Timely notification and the selection of appropriate communication methods were identified as key factors. In addition, recommendations to add tests such as troponin to the critical value list highlight the importance of regularly updating the list based on clinical needs.
Introduction
A critical laboratory result, also known as a ‘panic value’ or ‘critical value,’ refers to a significantly abnormal test result that may indicate a life-threatening condition requiring immediate medical intervention. This concept was first introduced by Lundberg to emphasize the need for urgent clinician attention to prevent adverse patient outcomes [1]. Since laboratories serve a wide range of patients (inpatients, primary care, emergency outpatients, etc.), laboratory results that may be critical in some patients may not indicate an acute risk for others. For instance, a creatinine level that would be alarming in a healthy outpatient might be expected in a patient undergoing dialysis [2]. As an important post-analytical quality indicator of the total testing process in clinical laboratories, reporting critical values and communicating them to clinicians in a timely and secure manner is crucial, and much effort is required to improve harmonization throughout the process [3]. Many international healthcare regulatory bodies have developed requirements for the reporting of critical values. Since 2003, the Joint Commission on Accreditation of Healthcare Organizations (JACHO) began implementing the National Patient Safety Goals, where reporting of critical values is one of the most focused goals [4]. In addition, the Clinical and Laboratory Improvement Amendments of 1988 (CLIA ‘88), the Laboratory Accreditation Program proposed by the College of American Pathologists (CAP) and the International Organization for Standardization (ISO) 15,189 have also stated the necessity of reporting critical values [5], 6].
Although the reporting of critical results has been in practice for more than 40 years, considerable variation still exists among clinical laboratories in both the selection of analytes and the methods of communication with clinicians. In 2018, Türkiye took an important step toward standardization by initiating the Rational Laboratory Use Project. This national initiative defined decision limits (threshold values), critical values (panic values), and procedures for the harmonization of measurement units [7]. As a result of this project, standardization has been achieved in terms of which tests are considered critical and at what values critical alerts should be triggered.
Although standardization has been achieved in terms of the tests and threshold values for critical results, variations still exist in how these values are communicated and reported to clinicians. Evaluating critical values solely from a laboratory perspective presents an incomplete approach; clinicians’ opinions are also essential in managing this process effectively. In our study, we aimed to evaluate clinicians’ perspectives on critical values and their expectations of laboratories through a dedicated survey. Additionally, we sought to assess whether the current critical value notification process aligns with clinicians’ expectations. To do so, we retrospectively analyzed tests reported with critical values over a 6-month period to identify areas for improvement.
Materials and methods
The study was carried out in accordance with the principles of the Declaration of Helsinki. Ethics approval was obtained from the Ethics Committee of the University of Health Sciences, Tepecik Training and Research Hospital, with the decision dated December 15th, 2021 (2021/12-09). Informed consent was obtained from all clinicians who participated in the survey.
Critical laboratory tests and predefined critical value limits used in Tepecik Training and Research Hospitalare shown in Table 1. The critical value list used in our laboratory was established based on the harmonisation procedure for decision limits (threshold values), critical values (panic values), and measurement units published by the Turkish Ministry of Health in 2018. The final list was determined in consultation with clinicians. Our laboratory follows this procedure for handling critical values: When a test result exceeds the predefined critical value limits in the Laboratory Information Management System (LIMS), the test result appears in purple. The requesting physician is contacted by phone, and informed of the critical value. Based on mutual agreement, the test can be repeated or analyzed using a new sample. If the result is deemed clinically consistent, the laboratory physician approves the result. When the test result is approved via LIMS, the critical values are automatically sent via message to the relevant clinician’s mobile phone. In case of ongoing critical values, especially in hospitalized patients, there is no need to make repeated notifications.
Critical laboratory tests and predefined critical value limits used in Tepecik Training and Research Hospital.
| Test name | Age | Lower critical value | Upper critical value |
|---|---|---|---|
| APTT, seconds | General | – | ≥ 150 |
| Fibrinogen (mg/dL (g/L)) | General | ≤ 60 (≤ 0.6) | – |
| PT (INR) | General | – | ≥ 5.0 |
| Leukocyte (x109/L) | General | ≤ 2 | ≥ 100.0 |
| Absolute neutrophil count (x109/L) | General | ≤ 0.5 | – |
| Hemoglobin (g/dL (g/L)) | General | ≤ 6.0 (≤ 60) | ≥ 20.0 (≥ 200) |
| Platelet (x109/L) | General | ≤ 40 | ≥ 1,000 |
| Ammonia, μmol/L | ≥ 1 year | – | ≥ 200 |
| Ammonia, μmol/L | < 1 year | – | ≥ 100 |
| Bilirubin, total, mg/dL | < 1 year | – | ≥ 5.0 |
| Calcium, total, mg/dL | General | ≤ 6.5 | ≥ 13.0 |
| Creatinine, mg/dL | 1 day-4 weeks | – | ≥ 1.5 |
| Creatinine, mg/dL | 5 weeks-23 months | – | ≥ 2.0 |
| Creatinine, mg/dL | 2–11 years | – | ≥ 2.5 |
| Creatinine, mg/dL | 12–15 years | – | ≥ 3.0 |
| Creatinine, mg/dL | ≥ 16 years | – | ≥ 10.0 |
| Creatine kinase, total, U/L | General | – | ≥ 10,000 |
| Glucose, mg/dL | < 4 weeks | ≤ 40 | ≥ 400 |
| Glucose, mg/dL | ≥ 4 weeks | ≤ 50 | ≥ 400 |
| Magnesium, mg/dL | General | ≤ 1.0 | ≥ 9.0 |
| pH | General | ≤ 7.200 | ≥ 7.600 |
| Phosphorus, mg/dL | General | ≤ 1.0 | – |
| Potassium, mmol/L | General | ≤ 2.5 | ≥ 6.0 |
| Sodium, mmol/L | General | ≤ 120 | ≥ 160 |
| Chloride, mmol/L | General | ≤ 75 | ≥ 130 |
| Paracetamol, 4 h after last dose, μg/mL | General | – | > 150 |
| Digoxin, ng/mL | General | – | ≥ 4.0 |
| Ethanol, mg/dL | General | – | ≥ 400 |
| Salicylate, mg/dL | General | – | ≥ 50.0 |
| Phenytoin, mg/L | General | – | ≥ 30 |
| Phenobarbital, mg/L | General | – | ≥ 60 |
| Carbamazepine, mg/L | General | – | ≥ 15 |
| Valproic acid, mg/L | General | – | ≥ 150 |
-
PT, prothrombin time; INR, international normalized ratio; APTT, activated partial thromboplastin time.
Critical value data of Tepecik Training and Research Hospital between September 2021 and February 2022 were obtained retrospectively from the hospital information management system.
The survey consisted of two main sections. The first section included six questions to determine the demographic characteristics of the participants. The second section included 11 questions (8 closed-ended, 2 semi-open-ended, and 1 open-ended) to assess the awareness, opinions, and expectations of clinicians regarding critical values. The survey was prepared based on the Ministry of Health’s critical value notification procedure and was piloted on 10 clinicians to test its comprehensibility before the study commenced. The survey was administered to clinicians using Google Forms at the Health Sciences University, Izmir Tepecik Training and Research Hospital, and Bakırçay University, Cigli Training and Research Hospital. Participation was conducted on an institutional basis and based on voluntariness.
A priori power analysis was conducted using G*Power 3.1 program to determine the minimum sample size. With an expected effect size of 0.3, a significance level of 0.05, and a power of 0.80, the required minimum number of participants was calculated as 175.
Survey responses were obtained using Google Forms. Microsoft Excel 2013 (Microsoft Corp., WA, USA) and SPSS 25.0 (IBM Corp., NY, USA) were used for statistical analysis. Categorical variables were presented as frequencies and percentages. Associations between demographic variables and responses to survey questions were assessed using Pearson’s Chi-square test. A p-value of less than 0.05 was considered statistically significant. The distribution of the critical value notification time was assessed using the Kolmogorov–Smirnov test. As the data were not normally distributed, the results were expressed as median and interquartile range (IQR).
Results
A total of 4,955 critical values were identified in our laboratory over a six-month period. Critical values were analyzed, and the samples of 48 patients with high potassium levels were rejected due to hemolysis. In addition, samples from 4 patients were rejected because they simultaneously showed extremely high potassium and extremely low calcium levels. These errors were attributed to tube-to-tube transfer from K2EDTA tubes to gel serum tubes. Following rejected tests due to preanalytical errors, 4,899 critical values were reported.
The most frequently reported critical tests were platelets (27.03 %), hemoglobin (14.86 %), and glucose (12.82 %) (Table 2). In platelet tests, 90 % of the critical values were low, while in glucose tests, 70.5 % of the critical values were high (Figure 1). A repeat test with a new sample was requested on the same day in 20 % of the notified critical value cases. An additional 17 % were repeated on the following day. In 30 % of the repeated tests, the new result was determined as a critical value. The median notification time for critical values was 7 min (IQR: 6–15 min).
Analysis of critical values (6 months).
| Test | Total test volume | Critical test values, n | Percentage of all critical test values | Percentage of total test volume with a critical value |
|---|---|---|---|---|
| Platelet | 269,936 | 1,324 | 27.03 | 0.490 |
| Hemoglobin | 269,936 | 728 | 14.86 | 0.270 |
| Glucose | 229,636 | 628 | 12.82 | 0.273 |
| Leukocyte | 269,936 | 594 | 12.12 | 0.220 |
| pH | 69,585 | 500 | 10.21 | 0.719 |
| Absolute neutrophil count | 269,936 | 334 | 6.82 | 0.124 |
| Potassium | 195,254 | 257 | 5.25 | 0.132 |
| PT (INR) | 90,835 | 127 | 2.59 | 0.140 |
| Bilirubin, total | 132,138 | 113 | 2.31 | 0.086 |
| Calcium, total | 179,781 | 93 | 1.90 | 0.052 |
| Creatinine | 245,260 | 82 | 1.67 | 0.033 |
| Sodium | 196,191 | 42 | 0.86 | 0.021 |
| Creatine kinase, total | 49,673 | 32 | 0.65 | 0.064 |
| Magnesium | 101,750 | 15 | 0.31 | 0.015 |
| Phosphorus | 101,500 | 8 | 0.16 | 0.008 |
| Carbamazepine | 742 | 7 | 0.14 | 0.943 |
| Ammonia | 582 | 5 | 0.10 | 0.859 |
| Chloride | 156,159 | 2 | 0.04 | 0.001 |
| Fibrinogen | 31,241 | 2 | 0.04 | 0.006 |
| Ethanol | 1,495 | 2 | 0.04 | 0.134 |
| Digoxin | 206 | 2 | 0.04 | 0.971 |
| APTT | 87,039 | 1 | 0.02 | 0.001 |
| Phenobarbital | 80 | 1 | 0.02 | 1.250 |
| Salicylate | 21 | 0 | 0 | 0 |
| Phenytoin | 108 | 0 | 0 | 0 |
| Valproic acid | 1857 | 0 | 0 | 0 |
-
PT, prothrombin time; INR, international normalized ratio; APTT, activated partial thromboplastin time.
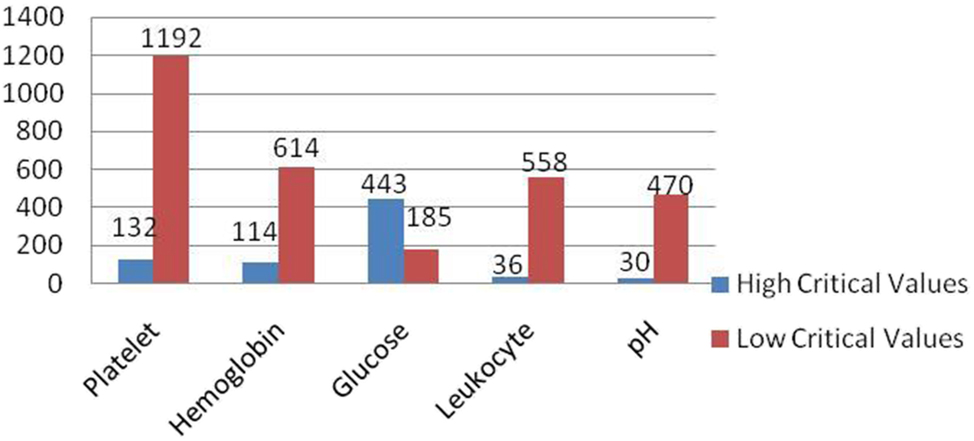
Distribution of critical values. Distribution of high and low critical laboratory values across five commonly monitored parameters: platelet, hemoglobin, glucose, leukocyte, and pH. Blue bars indicate high critical values, while red bars represent low critical values. The most frequent low critical value was observed in platelet count (n=1,192), followed by hemoglobin (n=614) and leukocyte count (n=558). In contrast, glucose had the highest number of high critical values (n=443).
A total of 321 clinicians participated in the survey. Based on the results of the power analysis, this number exceeded the minimum required sample size, indicating that the study had sufficient statistical power. The majority of participants (60.4 %) were between the ages of 24–35, and 59.2 % were female physicians. Most participants (70.1 %) work at the Health Sciences University, Izmir Tepecik Training and Research Hospital (Table 3).
Evaluations of the questions related to the first part of the questionnaire.
| Questionnaire questions | Categorization | n (%) |
|---|---|---|
| Age | 24–29 | 127 (39.6) |
| 30–34 | 60 (18.7) | |
| 35–39 | 49 (15.3) | |
| 40–44 | 33 (10.3) | |
| 45–49 | 27 (8.4) | |
| >50 | 25 (7.7) | |
| Gender | Female | 190 (59.2) |
| Male | 131 (40.8) | |
| Department | Gynecology and obstetrics | 52 (16.2) |
| Internal medicine | 41 (12.8) | |
| Anesthesia and reanimation | 40 (12.5) | |
| Child health and diseases | 39 (12.1) | |
| Emergency medicine | 29 (9) | |
| Family medicine | 25 (7.8) | |
| Other | 95 (29.6) | |
| Title | General practitioner | 5 (1.6) |
| Assistant doctor | 168 (52.3) | |
| Specialist doctor | 117 (36.4) | |
| Associate professor | 22 (6.9) | |
| Professor doctor | 9 (2.8) | |
| How many years are you in the profession? | 1–4 years | 130 (40.5) |
| 5–9 years | 60 (18.7) | |
| 10–14 years | 52 (16.2) | |
| 15–19 years | 25 (7.8) | |
| >20 years | 54 (16.8) | |
| Institution | Bakırcay University Cigli Training and Research Hospital | 96 (29.9) |
| Health Sciences University Tepecik Training and Research Hospital | 225 (70.1) |
A total of 97.8 % of clinicians considered critical value notification necessary, 44.6 % preferred to be informed via phone message, and 40.5 % preferred both phone message and phone call (Figure 2). In contrast, 2.2 % of participants stated that critical value reporting was not necessary, as examination results are already closely monitored in emergency departments. However, they acknowledged its potential usefulness in outpatient settings and emphasized that critical value limits may vary depending on the patient. After the critical value notification, 19.6 % of the physicians requested a new sample, 12.2 performed direct clinical intervention, and 49.8 % requested a new sample after the intervention. It was stated that factors such as the patient’s general condition, accompanying diseases, and the type of test were effective in determining the clinical decision. While 77.9 % of the participants found the Medical Biochemistry critical value test list sufficient, 8.4 % stated that the troponin test should be added to the critical value list (Table 4).

Clinician preferences for critical value notification. Distribution of clinician preferences regarding the method of notification for critical values. The most preferred method was phone message (45 %), followed closely by a combination of phone message and direct call (40 %). A smaller proportion preferred phone calls alone (11 %) or notification through auxiliary healthcare personnel (4 %).
Evaluations of the questions related to the second part of the questionnaire.
| Questionnaire questions | Categorization | n (%) |
|---|---|---|
| 1.What do you think is critical value? | A. The laboratory test result is outside the reference range. | 8 (2.5) |
| B. The limit values of a laboratory test result that indicate that the patient’s life may be in danger without immediate intervention. | 308 (95.9) | |
| C. I have no idea. | 5 (1.6) | |
| 2.Do you know that the notification of critical value is mandatory according to health quality standards? | A. I know | 243 (75.7) |
| B. I don’t know | 78 (24.3) | |
| 3.Have you ever received a critical value notification about your patient from the medical biochemistry laboratory? | A. Yes | 265 (82.5) |
| B. No | 56 (17.5) | |
| 4. If your answer to question 3 is YES, how many critical value notifications do you receive on average in a month? | ||
| 5.Do you think critical value notification is necessary? | Yes | 314 (97.8) |
| No (if your answer is NO, please state the reason) | 7 (2.2) | |
| 6. If your answer to question 5 is YES, how would you like the critical values to be notified to you by the medical biochemistry laboratory? | A. Phone message | 140 (44.6) |
| B. Phone call | 34 (10.8) | |
| C. Phone message and call | 127 (40.5) | |
| D. Through auxiliary health personnel (nurse, emergency medical technician, midwife, etc.) | 13 (4.1) | |
| 7.Would you like to be notified if the patient’s previous test result, which also had a critical value, shows a critical value again? | A. Yes | 277 (86.3) |
| B. No | 44 (13.7) | |
| 8.Do you think that the critical values were reported to you late by the medical biochemistry laboratory? | A. Yes | 51 (15.9) |
| B. No | 270 (84.1) | |
| 9.What would be your attitude when a critical value is reported about your patient? | A. I request a repeat test with a new sample. | 63 (19.6) |
| B. Based on this result, I provide the patient with the necessary medical intervention. | 39 (12.2) | |
| C. I request a repeat test after the necessary medical intervention is given to the patient. | 160 (49.8) | |
| D. If other, please specify… | 59 (18.4) | |
| 10.For which one or which ones of the tests listed below in the critical value list in the ministry of health guide for medical biochemistry laboratories do you think critical value notification is not required? Please tick. | Glucose | 29 (9) |
| Total bilirubin (<1 year) | 35 (10.9) | |
| Calcium | 30 (9.3) | |
| Phosphorus | 72 (22.4) | |
| Magnesium | 61 (19) | |
| Sodium | 20 (6.2) | |
| Potassium | 14 (4.4) | |
| Creatinine | 32 (10) | |
| Creatine kinase | 56 (17.4) | |
| Ammonia | 35 (10.9) | |
| PT (INR) | 18 (5.6) | |
| aPTT | 22 (6.9) | |
| pH, arterial | 32 (10) | |
| pC02, arterial | 38 (11.8) | |
| pO2, arterial | 50 (15.6) | |
| Paracetamol | 21 (6.5) | |
| Digoxin | 19 (5.9) | |
| Ethanol | 21 (6.5) | |
| Salicylate | 21 (6.5) | |
| Platelet | 19(5.9) | |
| Hemoglobin | 33(10.3) | |
| Leukocyte | 13(4.1) | |
| Absolute neutrophil count | 9(2.8) | |
| All notification required | 179 (55.8) | |
| No notification required for any | 3 (0.9) | |
| 11.Do you think there is any other test or tests studied in the medical biochemistry laboratory that should be added to the critical value test list mentioned above? | A. No | 278 (86.6) |
| B. If yes, please specify | 43 (13.4) |
-
PT, prothrombin time; INR, international normalized ratio; APTT, activated partial thromboplastin time; pC02, partial pressure of carbon dioxide; p02, partial pressure of oxygen; n, number of individuals.
When the relationships between the demographic data collected in the study and the responses given to the survey questions were evaluated, a significant relationships were found between the status of knowing that critical value notification is mandatory according to the Quality Standards in Healthcare and the variables of years in the profession (χ2=55.89, df=33, p=0.008) and branch (χ2=48.90, df=29, p=0.012). Whether the clinician had received a critical value notification before showed a significant relationship with the title (χ2=23.81, df=4, p<0.001). Opinions on whether critical value reporting is necessary were found to be significantly related to age (χ2=52.77, df=34, p=0.021), gender (χ2=5.97, df=1, p=0.015), and title (χ2=13.44, df=4, p=0.009). Statistically significant relationships were also found between participants’ perceptions of the reporting time of critical value results and title (χ2=28.60, df=16, p=0.027) and age (χ2=61.63, df=34, p=0.003) variables.
Discussion
Differences between laboratories in terms of the terminology used for critical values, parameters, limit values, notification methods, and responsible contact persons affect the overall management of critical values. The lack of standardization and quality indicators in these areas leads to various problems [8]. The Clinical and Laboratory Standards Institute (CLSI) GP47 guideline recommends that each laboratory tailor its critical value strategy based on the patient population it serves [9]. Our findings support this recommendation and highlight the importance of assessing current critical value practices and identifying opportunities for improvement. Sonjic et al. investigated doctors’ attitudes towards critical value lists and emphasized the need for tailored approaches across hospital departments [10]. Salinas et al. reported that efficiency could be improved by customizing the critical value reporting procedure based on the patient’s clinical condition, rather than relying on a rigid list of predefined laboratory values. Portuguese laboratories report critical values solely based on the patient’s age, regardless of their location, disease type, or ethnicity [8]. Similar results were previously reported in a study conducted by the College of American Pathologists (CAP) [2]. Laboratories should collaborate with clinicians to customize critical value lists for patient groups in different departments according to their clinical needs.
The reference range represents the statistical limits derived from healthy individuals, whereas the critical value defines specific thresholds that may pose a threat to the patient’s life and require urgent clinical intervention. Therefore, a test result may fall outside the reference range, yet it does not necessarily warrant immediate notification or intervention. In our study, clinicians appeared to be generally aware of the definition of critical values and the importance of reporting them. We believe that regular reporting is effective in raising clinicians’ awareness of critical values. Overall, the majority of clinicians consider notification to be necessary. However, some clinicians argue that critical values should be patient-specific and, since results are already closely monitored in emergency settings, notification may be unnecessary and may lead to inefficient use of clinical personnel. On the other hand, others emphasized that such notifications could be beneficial in outpatient settings.
Published guidelines and accreditation standards require laboratories to establish a communication strategy and reporting protocol for critical values [9], [11], [12], [13]. In our study, the method most preferred by clinicians for reporting critical values was phone messaging, primarily due to time constraints and the need for documentation. Additionally, the high frequency of both phone messages and verbal call notifications highlights the need for two-way communication systems to address potential communication gaps with clinicians. Plebani et al. reported that one of the major barriers to the reporting of critical values is ineffective communication. In outpatient settings, it has been reported that it is difficult not only to reach the responsible healthcare personnel but also to ensure timely notification of critical values [14]. Digh et al. reported that critical values for outpatients are often communicated late. The primary reasons include the absence of a fixed patient location, as in inpatient settings, and missing or illegible information regarding the clinician or relevant unit on request forms [15]. Phone calls, text messages, or automated electronic systems can be employed as communication tools between laboratories and clinicians for the reporting of critical values. However, when automated electronic systems are used, a feedback mechanism must be in place to enable laboratories to monitor whether the alerts sent to clinical teams have been received, read, and acted upon [16].
Half of the laboratories in Portugal reanalyze critical values before reporting, and a study conducted in Spain also recommended repeat testing as part of the reporting protocol. However, some studies have indicated that this practice may result in unnecessary delays in the reporting of critical values [17], [18], [19]. The majority of clinicians in our study indicated that they would intervene directly upon receiving a critical value result, reflecting their confidence in the laboratory findings. Clinicians also reported that their decisions are guided by the patient’s overall condition and clinical context. When a faster confirmatory test is available (e.g., blood gas for hyperkalemia or glucometer for hypo/hyperglycemia), they prefer to rely on it.
When the retrospective data were compared with the survey results, a strong alignment was observed between clinician behaviour and survey responses. 19.6 % of the survey respondents stated that they requested a repeat test with a new specimen following a critical value, while 49.8 % stated that they requested a repeat test after applying the necessary medical intervention. Similarly, the retrospective analysis found that approximately 37 % of the critical values reported required retesting with a new specimen on the same day or the following day. These findings show that clinicians’ approach to critical values is consistent with the survey responses and that the reported attitudes are reflected in clinical practice.
Although there was broad consensus among clinicians on the necessity and adequacy of the critical value tests listed in the Ministry of Health guidelines, opinions on test selection varied by clinical specialty. Emergency department clinicians frequently stated that troponin should be added to the critical value test list.
Knowledge and attitudes regarding critical value reporting show significant differences depending on demographic and professional factors such as age, title, professional experience and field of expertise. Clinical experience and field-specific practices play an important role in increasing awareness on this issue. Therefore, these variables should be taken into account when planning critical value reporting processes and in-house practices should be organized in accordance with these differences.
Agarwal et al. evaluated 1,279 critical values in clinical chemistry over a 25-month period, and the most frequently reported analytes were sodium and potassium. It has been suggested that critical value reporting is high in the intensive care unit and emergency department, with 64.61 % of critical values reported between 30 and 120 min after samples are taken [20]. In our laboratory, the total number of tests in the critical value list for 6 months is 2,950,877, and there are 4,899 critical value notifications. The parameters for which critical values are most frequently reported are platelet, hemoglobin, and glucose. In our study, the average reporting time of critical values was determined as 9 min. This finding reveals that our laboratory is successful in terms of time management in critical value reporting.
This study has some limitations. First, retrospective data on critical value reporting were obtained only from Tepecik Training and Research Hospital. Therefore, the generalizability of the results is limited. There is a risk of response bias because opinions of clinicians were collected via a survey. In addition, the clinical impact of critical value reporting on treatment efficacy was not evaluated.
Conclusions
Improvement of the critical value notification process is necessary to improve patient safety and the effectiveness of clinical care, as well as reduce the delay in identifying patients at risk. The urgent and necessary first step to be taken for this is to ensure standardization. Because there is a lot of variability between laboratories regarding critical value policies, critical value practices and critical value lists. For this purpose, a critical value notification procedure should be created, which includes information such as the list of critical values to be notified, the person to notify, to whom the notification will be made, the targeted time for notification, and the method of notification.
Since critical values vary between patient groups, independent critical value lists need to be created to reduce the workload in the laboratory and prevent unnecessary interruption of clinicians. For this purpose, the critical value list should be created in a short and concise manner in consultation with clinicians and updated periodically.
The findings obtained in our study led to an important step towards adding the troponin test to the critical value test list in our hospital in order to better meet the needs and expectations of clinicians regarding critical value notifications. It is thought that this regulation will contribute to clinicians’ early detection of critical situations and increase patient safety and rapid intervention opportunities.
Harmonization of institutional practices with national standards will ensure consistency across healthcare systems. In the future, it may be possible to develop patient-based, dynamic critical value alerts with the integration of decision support systems supported by artificial intelligence.
Acknowledgments
The authors thank all clinicians who voluntarily participated in this study.
-
Research ethics: The study was carried out in accordance with the principles of the Declaration of Helsinki and the ethics committee approval was obtained with the decision of the Ethics Committee of University of Health Sciences, Tepecik Training and Research Hospital, dated 15.12.2021 and numbered 2021/12–09.
-
Informed consent: Informed consent for participation was obtained from all patients included in the study.
-
Author contributions: MA, MZA, AC and BIB: conceptualization of the study. MA, MZA, AC, BIB, IK and FDA methodology. MA, MZA, AC and BIB: investigation.MA, MZA, AC, BIB, IK, EK, FDA and ZC: data curation and analysis. MA, MZA: writing of the manuscript. MA, MZA, AC, BIB, IK and FDA: reviewing and editing of the manuscript. MA, MZA, AC and BIB: supervision of the study. All authors have read and agreed to the version of the manuscript submitted for publication.
-
Use of Large Language Models, AI and Machine Learning Tools: Artificial Intelligence (AI) and/or Machine Learning Tools were not used in the writing of this article.
-
Conflict of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
-
Research funding: The authors received no financial support for the research, authorship, and/or publication of this article.
-
Data availability: The raw data can be obtained on request from the corresponding author.
References
1. Lundberg, GD. When to panic over abnormal values. MLO Med Lab Obs 1972;4:47–54.Search in Google Scholar
2. Salinas, M, López-Garrigós, M, Gutiérrez, M, Lugo, J, Flors, L, Leiva-Salinas, C. Should we customise critical value procedure according to patient origin and laboratory turnaround time? J Clin Pathol 2013;66:269–72. https://doi.org/10.1136/jclinpath-2012-201030.Search in Google Scholar PubMed
3. Piva, E, Plebani, M. From “panic” to “critical” values: which path toward harmonization? Clin Chem Lab Med 2013;51:2069–71. https://doi.org/10.1515/cclm-2013-0459.Search in Google Scholar PubMed
4. Zeng, R, Wang, W, Wang, Z. National survey on critical values notification of 599 institutions in China. Clin Chem Lab Med 2013;51:2099–107. https://doi.org/10.1515/cclm-2013-0183.Search in Google Scholar PubMed
5. Centers for Medicare and Medicaid Services. Department of health and human services. Part 493. Laboratory requirements: Clinical laboratory improvement amendments of 1988. Code of federal regulations, title 42. Parts 430 to end. Washington (DC): US Government Printing Office. Published annually.Search in Google Scholar
6. ISO. Medical laboratories: particular requirements for quality and competence. ISO 15189. Geneva: International Organization for Standardization; 2007.Search in Google Scholar
7. Health Services General Directorate. Procedure for harmonization of decision limit (threshold value), critical value (panic value) and measurement units within the framework of the rational laboratory use project. Ankara: Ministry of Health; 2018. Available from: https://shgmtetkikdb.saglik.gov.tr/Eklenti/15143/0/karar-siniri-esik-deger-kriek42009846pdf.pdf.Search in Google Scholar
8. Vuljanić, D, Pereira, M, Santos, S, Nikler, A, Biljak, VR, Cachapuz, I. Critical results reporting in Portuguese hospital laboratories: state-of-the-art. EJIFCC 2020;31:145–56.Search in Google Scholar
9. Clinical and Laboratory Standards Institute (CLSI). Management of critical and significant risk results, 1st ed. CLSI guideline GP47. Wayne, PA: CLSI; 2015.Search in Google Scholar
10. Šonjić, P, Nikler, A, Vuljanić, D, Dukić, L, Šimundić, AM. Clinician’s opinion about critical risk results proposed by the croatian chamber of medical biochemists: a survey in one croatian tertiary hospital. Biochem Med (Zagreb). 2019;29:030711. https://doi.org/10.11613/BM.2019.030711.Search in Google Scholar PubMed PubMed Central
11. Lam, Q, Ajzner, E, Campbell, CA, Young, A. Critical risk results – an update on international initiatives. EJIFCC 2016;27:66–76.10.1002/pu.30145Search in Google Scholar
12. Campbell, CA, Horvath, AR. Harmonization of critical result management in laboratory medicine. Clin Chim Acta 2014;432:135–47. https://doi.org/10.1016/j.cca.2013.11.004.Search in Google Scholar PubMed
13. International Organization for Standardization. ISO 15189:2012. Medical laboratories – requirements for quality and competence (requirement 5.8). Geneva: International Organization for Standardization; 2012.Search in Google Scholar
14. Plebani, M, Zaninotto, M, Sciacovelli, L, Piva, E. Critical laboratory results: communication is just one of the problems. Am J Clin Pathol 2012;137:164. Author reply 165. https://doi.org/10.1309/AJCPTCJQAO1SV8IJ.Search in Google Scholar PubMed
15. Dighe, AS, Rao, A, Coakley, AB, Lewandrowski, KB. Analysis of laboratory critical value reporting at a large academic medical centre. Am J Clin Pathol 2006;125:758–64. https://doi.org/10.1309/r53x-vc2u-5ch6-tng8.Search in Google Scholar
16. Croal, B. The communication of critical and unexpected pathology results. The Royal College of Pathologists. https://www.rcpath.org/resourceLibrary/the-communication-of-critical-and-unexpected-pathology-results-pdf.html [Accessed 20 Mar 2024].Search in Google Scholar
17. Kopcinovic, LM, Trifunović, J, Pavosevic, T, Nikolac, N. Croatian survey on critical results reporting. Biochem Med (Zagreb) 2015;25:193–202. https://doi.org/10.11613/bm.2015.019.Search in Google Scholar PubMed PubMed Central
18. Keng, TB, De, LSB, Bourner, G, Merino, A, Han, JY, Kawai, Y. Standardization of haematology critical results management in adults: an international council for standardization in haematology (ICSH) survey and recommendations. Int J Lab Hematol 2016;38:457–71.10.1111/ijlh.12526Search in Google Scholar PubMed
19. Delgado Rodríguez, JA, Pastor García, MI, Gómez Cobo, C, Pons Más, AR, Llompart Alabern, I, Bauça, JM. Assessment of a laboratory critical risk result notification protocol in a tertiary care hospital and their use in clinical decision making. Biochem Med (Zagreb). 2019;29:030703. https://doi.org/10.11613/BM.2019.030703.Search in Google Scholar PubMed PubMed Central
20. Agarwal, R, Chhillar, N, Tripathi, CB. Study of variables affecting critical value notification in a laboratory catering to tertiary care hospital. Indian J Clin Biochem 2015;30:89–93. https://doi.org/10.1007/s12291-013-0409-x.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

