Abstract
Objectives
Threatened abortion (TA) is a common condition in pregnant women. The role of CARMN in the onset and progression of TA is increasingly recognized as significant. However, the precise mechanisms of its influence are not yet fully understood. This study was to investigate the clinical value of CARMN in TA patients and the mechanism of action that may influence TA.
Methods
This study included 105 patients aged 22–38 years old, 6–8 weeks pregnant and with early TA of single pregnancy. In addition, 63 healthy women of the same age group who were 6–8 weeks pregnant were included as controls. qRT-PCR was employed to assess the expression levels of CARMN and miR-515-5p. The clinical diagnostic utility of CARMN in patients with TA and the predictive value of CARMN in pregnancy outcome of TA patients were analyzed using ROC curve methodology. Cell viability in HTR-8/SVneo cells was measured utilizing a cell counting kit-8 (CCK-8) kit. Apoptosis rates were quantified through flow cytometry. Cell migration was investigated using a transwell assay.
Results
The level of serum CARMN decreased and miR-515-5p increased in patients with TA. CARMN could predict pregnancy outcomes in patients with TA. Functionally, CARMN enhanced the viability and migration of HTR-8/SVneo cells by regulating the expression of miR-515-5p, while inhibiting apoptosis.
Conclusions
LncRNA CARMN may serve as a diagnostic marker for TA and participate in the progression of TA by regulating the expression of miR-515-5p.
Introduction
Threatened abortion (TA) is a common pathological symptom in women during pregnancy. The main clinical symptoms are a small amount of vaginal bleeding, paroxysmal back pain or lower abdominal pain, etc., which can lead to abortion in severe cases [1]. The incidence of TA is 20–25 %, which seriously affects women’s physical and mental health [2]. At present, the cause of TA is not clear, and the research believes that its occurrence is related to chromosomes, maternal disease, endocrine, intake of substances and mental stress [3]. At present, the clinical diagnosis of TA needs to be combined with Gynecological examination, B-ultrasound and blood markers and other tests can be determined [4]. The complexity of its examination has significantly reduced the early diagnostic rate of TA, which is also one of the main reasons for the rising incidence of TA over the years [5]. Consequently, the search for an effective, rapid, and accurate diagnostic method for TA is currently a hot topic and a challenge in clinical research [6].
Research has indicated that certain long non-coding RNA (lncRNAs) play significant regulatory roles in the behavior of trophoblast cells and abortion. Such as the upregulation of DANCR enhances the migratory and invasive capabilities of chorionic trophoblast cells [7]. Investigation of lncRNA expression in villous tissues from patients experiencing recurrent abortion via microarray analysis revealed that 4,421 lncRNAs exhibited altered expression, with 1,537 upregulated and 2,884 downregulated [8].
The recent studies identified and characterized CARMN as a smooth muscle cell-specific lnc RNA that is highly abundant and conserved [9]. CARMN is involved in the pathogenesis of various diseases, such as CARMN serves as a potential biomarker for psoriasis [10], as well as for endometrial cancer [11]. One study showed that CARMN expression was downregulated in people with unexplained recurrent pregnancy loss [12]. Nevertheless, the function of CARMN in cases of TA remains unexplored.
MicroRNAs (miRNAs) typically exert their function post-transcriptionally by engaging in base-pairing interactions with the 3′ untranslated region of mRNA molecules [13]. We found that miR-515-5p is a regulatory target of CARMN through the starBase database. It was found that miR-515-5p mediated the proliferation and migration of human chorionic cancer cells [14] Research showed that maternal serum miR-515-5p level is noticeably increased in individuals suffering from preeclampsia [15]. In the starBase database, it is predicted that there are binding sites in the sequence of CARMN and miR-515-5p, however, whether CARMN regulates the expression of miR-515-5p and participates in the progression of TA has not been confirmed.
In this study, we investigated the clinical value of CARMN in TA patients and the mechanism of action that may influence TA for the first time.
Materials and methods
Study subjects
A total of 105 patients aged 22–38 years old, 6–8 weeks pregnant and with early TA of single pregnancy treated in The 2nd Affiliated Hospital of Chengdu Medical College (Nuclear Industry 416 Hospital) were included as the TA group. In addition, 63 normal pregnant women who underwent normal labor examination in The 2nd Affiliated Hospital of Chengdu Medical College (Nuclear Industry 416 Hospital) were included as control group. All participants provided written consent for participation. This study was approved by the Ethics Committee of The 2nd Affiliated Hospital of Chengdu Medical College (Nuclear Industry 416 Hospital).
Inclusion and exclusion criteria
Inclusion criteria: 1) The patients in the TA group were accompanied by small amounts of vaginal bleeding and slight abdominal pain to varying degrees. The cervical opening was found to be closed by gynecological examination, and a small amount of bleeding was caused by vaginal bleeding in the uterine cavity. 2) Women with intrauterine single pregnancy. 3) Participants have complete medical records.
Exclusion criteria: 1) Patients with severe abnormalities of liver, kidney, heart, and lung function. 2) Patients with other serious endocrine system diseases. 3) People with immune system diseases. 4) Patients with abnormal coagulation function. 5) Patients with adverse pregnancy history. 6) Patients with gestational comorbidities, such as gestational diabetes, gestational hypertension. 7) People with mental illness, who are unable to cooperate with the completion of this study.
Collection of general information
General information was collected for all researchers, including age, gestational age, smoking history, and miscarriage history. In the morning, two tubes of 5 mL venous blood from the two groups of pregnant women were taken under a fasting state, and the serum was obtained after standing at room temperature for 30 min at 3,000 r/min and centrifuged for 10 min. The contents of serum β-human chorionic gonadotropin (β-hCG), progesterone and estradiol (E2) in one tube were detected in the laboratory of The 2nd Affiliated Hospital of Chengdu Medical College (Nuclear Inoustry 416 Hospital), and the other tube was stored at −80 °C for reserve.
RNA extraction and qRT-PCR
Total RNA was isolated from the serum and transfected cells following the manufacturer’s guidelines provided with the TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). The extracted RNA samples showed an acceptable quality, as indicated by their A260/A280 ratios, which fell within the range of 1.8–2.1. The reverse transcription and quantitative analysis were conducted with the PrimerScript RT Reagent Kit (TaKaRa, Otsu, Shiga, Japan). qRT-PCR was performed on the StepOne Plus Real-Time PCR System (Applied Biosystems). The expression level was determined using the 2−ΔΔCt method. The primers used for qRT-PCR included:
CARMN forward: 5′-TGTGCTTGGCTGTCCTTTCT-3′.
CARMN reverse: 5′-AGGTGAATAGGGGGCAGACT-3′.
miR-515-5p forward:5′-TCGGCAGGUUCUCCAAAAGAAA-3′.
miR-515-5p reverse5′-CTCGCTTCGGCAGCACA-3′.
GAPDH forward: 5′-GTTAGGAAAGCCTGCCGGTG-3′.
GAPDH reverse: 5′-AGCATCGCCCCACTTGATTT-3′.
Cell culture and transfection
HTR-8/SVneo cells (Shaun Biotechnology, Wuhan, China) were cultured in DMEM medium containing 10 % FBS and incubated in a cell incubator with a CO2 saturation of 5 % at 37 °C. The shRNA- CARMN (sh-CARMN) and negative control (sh-NC) were sourced from Transheep Bio (Shanghai, China). Furthermore, the pcDNA3.1-CARMN (pcDNA-CARMN), an empty pcDNA3.1 vector used for overexpression, as well as the miR-515-5p mimics/mimics NC and miR-515-5p inhibitor/inhibitor NC, were sourced from BeNa Bio (Beijing, China). HTR-8/SVneo cells were infected with the aforementioned agents via Lipofectamine 3,000 (Thermo Fisher Scientifc, Waltham, MA, USA).
CCK-8 assay
HTR-8/SVneo cells were inoculated into 96-well plates and allowed to proliferate for a 48-h period. 10 μL of CCK-8 solution (Procell Life Science & Technology) were added to each well. After a 2-h incubation at a temperature of 37 °C, the absorbance at 450 nm was determined using a microplate spectrophotometer.
Cell apoptosis assay
HTR-8/SVneo cells were inoculated into six-well plates. To assess apoptosis, the Annexin V-Fluos and Propidium Iodide (PI) Apoptosis Detection Kit (Sigma, Germany) was employed to detect the apoptosis rate. The examination was executed in line with the producer’s instructions, and the procedure was replicated three times independently.
Transwell assay
The cellular populations were re-dispersed within a medium devoid of serum, subsequent to which they were introduced into the upper compartment of a transwell plate. A DMEM medium with a high sugar content, which also included 20 % FBS, was poured into the lower chamber and allowed to incubate for a 24-h period. Following this, the medium was removed, the cells were immobilized through a 30-min immersion in methanol. Subsequently, the cells were colored using a 0.1 % crystal violet solution for an additional 20 min. Thereafter, the upper layer of cells that had not migrated was carefully removed using cotton swabs. These cells were then observed, and images were captured using an optical microscope for documentation purposes.
Dual-luciferase reporter assay
The CARMN sequence was inserted into the pmirGLO vector (Promega, Shanghai, China), leading to the production of two distinct luciferase reporter constructs: CARMN-WT (for wild-type) and CARMN-MUT (for mutant). Subsequently, these vectors were coinjected into HTR-8/SVneo cells employing Lipofectamine 2000. After a 48-h incubation period, the Promega Dual-Luciferase Reporter Assay Kit was utilized in compliance with the producer’s instructions.
Statistical analysis
Data statistical analysis was conducted using SPSS version 26.0 and GraphPad Prism version 9. The data was displayed as mean ± standard deviation (SD) and examined using an independent sample t-test or one-way ANOVA. The diagnostic utility of CARMN in TA was evaluated using a ROC curve. Additionally, logistic regression was utilized to determine the risk factors associated with the onset of TA. p<0.05 were deemed as being statistically significant differences.
Results
Comparison of general data from different study groups
Individuals experiencing TA did not show a variation from those with uncomplicated pregnancies in terms of age, gestational age, BMI, or smoking history. However, significant disparities were noted between the two groups in terms of a prior history of abortion and serum levels of β-hCG, progesterone, and E2 (Table 1).
Comparison of basic data between normal pregnancy and threatened abortion population.
| Parameters | Normal (n=63) | TA (n=105) | p-Value |
|---|---|---|---|
| Age, year | 29.89 ± 4.78 | 29.57 ± 4.59 | 0.669 |
| Gestational weeks | 7.16 ± 0.63 | 6.95 ± 0.55 | 0.109 |
| BMI, kg/m2 | 24.31 ± 4.01 | 23.23 ± 1.83 | 0.157 |
| Smoking history (n, %) | 7 (11.11) | 10 (9.52) | 0.741 |
| Abortive history (n, %) | 6 (9.52) | 25 (23.81) | 0.021a |
| β-hCG, mIU/mL | 10,397.51 ± 1732.57 | 8,619.97 ± 997.87 | <0.001b |
| Progesterone, nmol/mL | 55.55 ± 13.22 | 43.23 ± 9.07 | <0.001b |
| E2, pmol/L | 800.36 ± 78.19 | 722.77 ± 86.70 | <0.001b |
-
BMI, body mass index; β-hCG, β-human chorionic gonadotropin; E2, estradiol; SD, standard deviation. Data are expressed as mean ± SD or n, %. aP<0.05, bP<0.001.
Differential expression of CARMN in serum of TA patients and predicted value of TA
Reduced expression of CARMN was observed in the serum of individuals experiencing TA (Figure 1A). In these patients with TA, CARMN expression was lower in women who terminated pregnancy than in women who continued pregnancy (Figure 1B). The ROC curve analysis revealed that the CARMN biomarker yielded an AUC of 0.891 for predicting TA, exhibiting a sensitivity of 85.71 % and a specificity of 82.54 % (Figure 1C). In addition, CARMN predicted the pregnancy outcome of TA patients with an AUC of 0.814, a sensitivity of 82.93 %, and a specificity of 78.13 % (Figure 1D).

The level of CARMN in patients with TA and has diagnostic value for TA. (A) The expression level of CARMN in serum of TA patients was detected by qRT-PCR. (B) The level of CARMN in patients with continuation pregnancy and those with termination pregnancy was detected by qRT-PCR. (C) ROC curve of serum CARMN for predicting TA. ∗∗∗P<0.001. (D) ROC curve of serum CARMN for predicting continuation and termination in TA patients.
Logistic regression analysis of risk factors for TA
With the occurrence of TA as the dependent variable and age, gestatory week, BMI, smoking history, abortion history, serum β-hCG, progesterone, E2 levels, and CARMN expression as the independent variables, binary logistic regression analysis showed that abortion history, serum β-hCG, progesterone, E2 levels, and the expression of CARMN were risk factors for TA (Table 2).
Logistic regression analysis of risk factors for threatened abortion.
| Parameters | Standard error | p-Value | OR | 95 %CI |
|---|---|---|---|---|
| Age | 0.486 | 0.362 | 0.642 | 0.248–1.665 |
| Gestational weeks | 0.479 | 0.466 | 0.705 | 0.276–1.802 |
| BMI | 0.493 | 0.181 | 0.518 | 0.197–1.360 |
| Smoking history | 0.750 | 0.707 | 1.325 | 0.305–5.764 |
| Abortive history | 0.740 | 0.046a | 4.385 | 1.028–18.707 |
| β-hCG | 0.492 | <0.001c | 0.148 | 0.056–0.387 |
| Progesterone | 0.495 | 0.003b | 0.231 | 0.088–0.610 |
| E2 | 0.485 | 0.039a | 0.367 | 0.142–0.950 |
| CARMN | 0.497 | <0.001c | 0.094 | 0.036–0.249 |
-
BMI, body mass index; β-hCG, β-human chorionic gonadotropin; E2, estradiol. aP<0.05, bP<0.01, cP<0.001.
Relevance of CARMN to clinical features of TA patients
According to the average level of serum CARMN expression in patients with TA, the participants were categorized into groups with low and high expression levels, and the Chi-square test was performed. Patients with a history of miscarriage and low serum levels of β-hCG, progesterone, and E2 had lower serum CARMN expression (Table 3).
Relevance of CARMN to clinical data of patients with threatened abortion.
| Parameters | Patients (n=105) | Low CARMN expression (n=57) | High CARMN expression (n=48) | p-Value | |
|---|---|---|---|---|---|
| Age, year | <30 | 59 | 28 | 31 | 0.112 |
| ≥30 | 46 | 29 | 17 | ||
| Gestational weeks | <7 | 59 | 35 | 24 | 0.241 |
| ≥7 | 46 | 22 | 24 | ||
| BMI, kg/m2 | <23.6 | 60 | 31 | 29 | 0.534 |
| ≥23.6 | 45 | 26 | 19 | ||
| Smoking history | No | 95 | 53 | 42 | 0.340 |
| Yes | 10 | 4 | 6 | ||
| Abortive history | No | 80 | 39 | 41 | 0.042a |
| Yes | 25 | 18 | 7 | ||
| β-hCG, mIU/ml | <8,620.0 | 55 | 37 | 18 | 0.005b |
| ≥8,620.0 | 50 | 20 | 30 | ||
| Progesterone, nmol/ml | <43.2 | 63 | 41 | 22 | 0.007b |
| ≥43.2 | 42 | 16 | 26 | ||
| E2, pmol/l | <722.8 | 57 | 37 | 20 | 0.017a |
| ≥722.8 | 48 | 20 | 28 | ||
-
BMI, body mass index; β-hCG, β-human chorionic gonadotropin; E2, estradiol. aP<0.05, bP<0.01.
Effect of CARMN on HTR-8/SVneo cells
In order to assess the impact of CARMN on HTR-8/SVneo cells, we introduced pcDNA-CARMN and sh-CARMN into these cells and determined the functional role of CARMN under vitro conditions. Transfection of pcDNA-CARMN markedly increased the levels of CARMN expression, while the transfection with sh-CARMN noticeably decreased CARMN expression (Figure 2A). Subsequently, the influence of CARMN overexpression and knockdown on the cell viability, apoptosis, and migration was investigated. Figure 2B–D demonstrated that pcDNA-CARMN transfection significantly enhanced the cell viability, and migration, and conversely, suppressed the apoptosis rate. sh-CARMN markedly suppressed the cell viability, and migration, while enhancing the apoptotic ratio.

Effects of overexpression or knockdown of CARMN on the function of HTR-8/SVneo cells. (A) The transfection efficiency of overexpression or knockdown of CARMN was detected by qRT-PCR. (B) The changes in cell viability after CARMN overexpression or knockdown were detected by CCK-8. (C) Annexin V/PI assay was used to detect apoptosis after CARMN overexpression or knockdown. (D) The transwell assay was employed to assess the capacity of cell migration following CARMN overexpression or knockdown. **p<0.01. ***p<0.001.
miR-515–5p is a target of CARMN
Figure 3A illustrated that the miR-515-5p level was elevated in TA compared to normal pregnant women, and this upregulation was inversely associated with the levels of CARMN expression (Figure 3B). Figure 3C showed the binding sites of CARMN and miR-515-5p. The relative luciferase activity in cells was substantially augmented following the co-transfection of CARMN-WT with miR-515-5p inhibitors. Conversely, when CARMN-WT and miR-515-5p mimics were co-inoculated, a marked decrease in the relative luciferase activity was observed.

Expression of miR-515-5p in TA patients and miR-515-5p is a target of CARMN. (A) The expression level of miR-515-5p in serum of TA was detected by qRT-PCR. (B) Pearson correlation analysis revealed a negative association between CARMN and miR-515–5p. (C) The sequence of the binding site between CARMN and miR-515-5p was forecasted utilizing the starbase database and the specific interaction between CARMN and miR-515-5p was investigated using a dual luciferase reporter gene assay. **p<0.01. ***p<0.001.
CARMN regulates HTR-8/SVneo cell viability, migration, and apoptosis by regulating miR-515-5p
CARMN overexpression significantly decreased the level of miR-515-5p, this trend was reversed after transfection with miR-515-5p mimic (Figure 4A). miR-515-5p overexpression reversed the promoting effect of CARMN overexpression on the cell viability (Figure 4B). miR-515-5p mimics partially offset the inhibitory effect of CARMN overexpression on cell apoptosis (Figure 4C). Transwell experiments showed that miR-515-5p mimics partially reversed the promotional impacts of elevated CARMN expression on the migration of HTR-8/SVneo cells (Figure 4D).
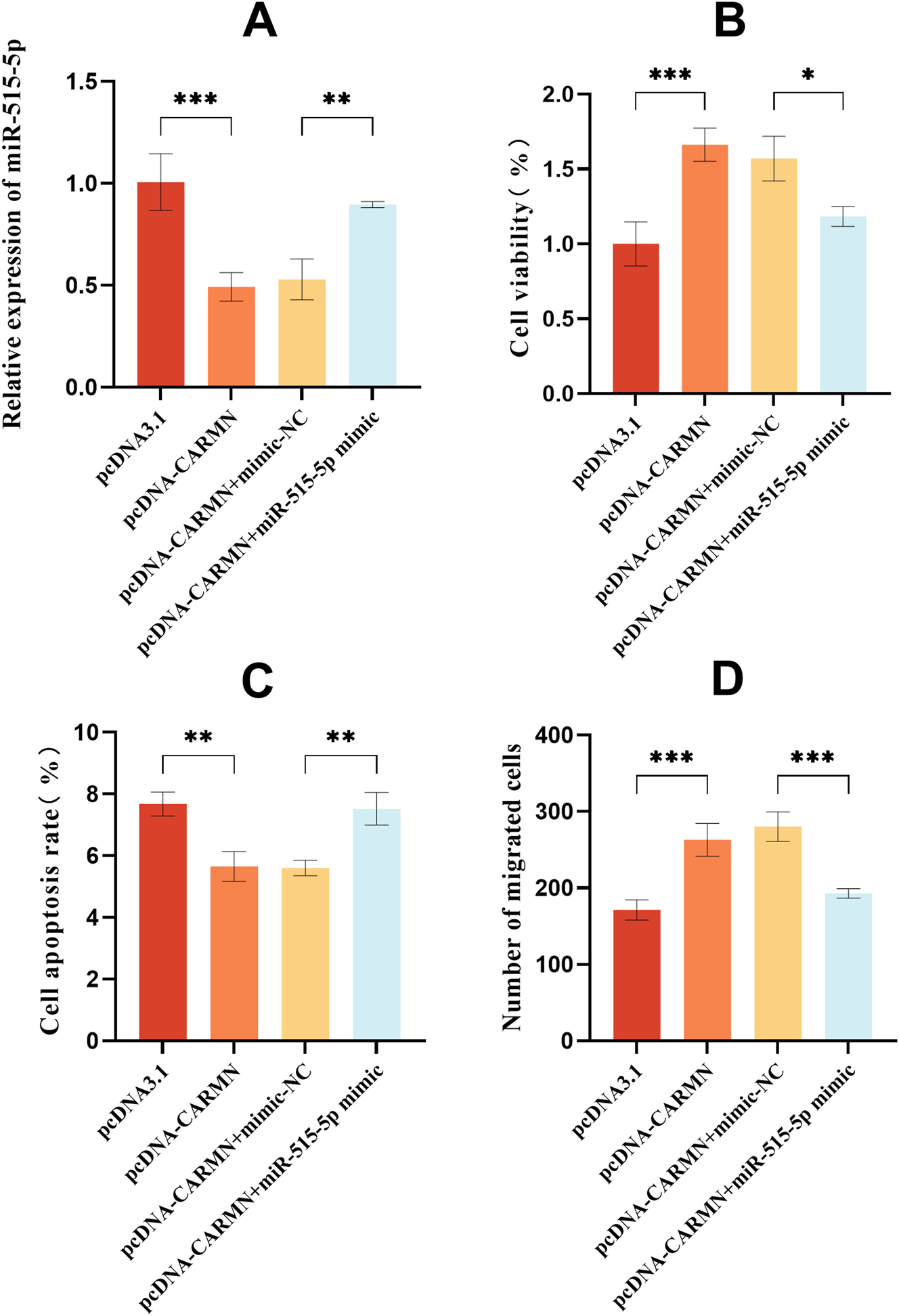
miR-515-5p partially counteracts the effects of sh-CARMN on cellular function. (A) miR-515-5p level was assessed by qRT-PCR after sh-CARMN and miR-515-5p inhibitor treatment. (B) Cell viability was assessed using the CCK-8 assay. (C) Cell apoptosis was quantified by the annexin V/PI assay. (D) The migration capability of cells was assessed by the transwell assay. *p<0.05, **p<0.01, ***p<0.001.
Discussion
According to reports, approximately one-fifth of pregnancies experience TA, among which 3–16 % of patients with TA subsequently miscarry [16]. Women experiencing a risk of pregnancy loss frequently experience profound distress. Such individuals often require serial prenatal assessments, which can heighten their apprehension. The utilization of dependable predictive biomarkers may alleviate the psychological burden on these patients.
Despite the clinical suggestion of multiple pregnancy hormones as viable diagnostic tools for the early stages of pregnancy, the earliest identifiable marker, β-hCG, remains the primary standard in contemporary pregnancy detection [17]. Progesterone is a steroid hormone that plays an important role in reproduction. It has been proposed and widely used to treat different gynecological diseases as well as assisted reproductive technology and maintenance of pregnancy [18]. While blood β-hCG and progesterone levels are the main indicators used to diagnose and identify women with threatened miscarriage, E2 levels are also used in some cases [19]. In this study, by comparing the serum levels of β-hCG, progesterone and E2 in normal pregnancy and TA patients, it was found that the serum levels of β-hCG, progesterone and E2 in TA patients were reduced compared with the normal pregnancy population. More importantly, in this study, the expression level of CARMN was significantly correlated with the levels of β-hCG, progesterone and E2 of patients, which may suggest that the level of CARMN is closely related to the pathology and disease of patients.
Many previous reports have confirmed that lncRNAs are differentially expressed in many pregnancy complications. An extensive examination of lncRNA expression patterns in individuals experiencing unexplained recurrent miscarriage showed that 683 lncRNAs were differentially expressed in unexplained recurrent abortion [20]. Compared with normal pregnancy, lncRNA TCL6 is highly expressed in placental tissue of preeclampsia pregnancy, promoting the progression of preeclampsia [21]. Dysregulation of imprinted lncRNA MEG8 leads to trophoblast malfunction and pregnancy loss [22]. In the current investigation, we observed a reduced expression of lncRNA CARMN in the serum of individuals experiencing TA, corroborating findings from prior research [12]. It was observed that the levels of CARMN was a significant risk factor for TA in pregnant women. The data indicated that lncRNA CARMN may serve as a significant biomarker for the prediction of TA and predict pregnancy outcomes in patients with TA, suggesting a strong association with the onset of this condition. The results of our study provide evidence for the clinical application of CARMN, but more experiments are needed to prove the conclusion of this experiment.
To delve deeper into the underlying mechanism of CARMN’s impact on TA, we searched for the target genes of CARMN through the database and found that miR-515-5p could specifically bind to CARMN. CARMN participates in the advancement of cancer by modulating the expression levels of miRNAs [23], 24]. In this study, we confirmed that CARMN can bind miR-515-5p and regulate the expression of miR-515-5p. Furthermore, miR-515-5p is overexpressed in individuals with TA, and it suppresses the growth of HTR-8/SVneo cells while facilitating cellular apoptosis. Moreover, we delved into the interaction between lncRNA CARMN and miR-515-5p, noting that the upregulation of miR-515-5p partially counteracts the effects of CARMN overexpression on cellular function. Previous studies have reported that lncRNAs affect the function of HTR-8/SVneo cells through multiple mechanisms. For instance, lncRNA ZEB2-AS1 regulates proliferation and invasion potential of HTR-8/SVneo in preeclampsia [25]. HOTAIR significantly enhanced the viability, migration and invasion of HTR-8/SVneo cells in recurrent spontaneous abortion [26]. In this study, we demonstrated that CARMN may affect the proliferation, apoptosis, and migration of HTR-8/SVneo cells by regulating the expression of miR-515-5p, thus affecting the progression of TA. Cell experiments provide more evidence for the role of CARMN in TA.
In summary, this study explored the clinical value of lncRNA CARMN expression in the early diagnosis of TA and its predictive value of pregnancy outcomes in patients with TA and explored the effect of CARMN on the function of HTR-8/SVneo cells. In clinical practice, overexpression of CARMN may inhibit the progression of TA, but this needs to be confirmed by more experiments. Nonetheless, this research is subject to certain constraints. The sample size included in this study was not large enough and more patients may be needed to validate the findings of this study. CARMN may affect the occurrence of TA through a variety of mechanisms, and there may be multiple miRNAs that be used as target for CARMN, which are worthy of further exploration in future studies.
Conclusions
The level of serum CARMN decreased and miR-515-5p increased in patients with TA. CARMN could predict pregnancy outcomes in patients with TA. Functionally, CARMN enhanced the viability and migration of HTR-8/SVneo cells by regulating the expression of miR-515-5p, while inhibiting apoptosis. CARMN may serve as a diagnostic marker for TA and participate in the progression of TA by regulating the expression of miR-515-5p.
-
Research ethics: The study protocol was approved by The Ethics Committee of the 2nd Affiliated Hospital of Chengdu Medical College (Nuclear Industry 416 Hospital) and followed the principles outlined in the Declaration of Helsinki.
-
Informed consent: Informed consent has been obtained from the participants involved.
-
Author contributions: W. L and R. Z designed the research study. X.Y. W and X.H. W performed the research and analyzed the data. W. L and R. Z wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors declare that they have no competing interests.
-
Research funding: The authors did not receive support from any organization for the submitted work.
-
Data availability: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
1. Pillai, RN, Konje, JC, Tincello, DG, Potdar, N. Role of serum biomarkers in the prediction of outcome in women with threatened miscarriage: a systematic review and diagnostic accuracy meta-analysis. Hum Reprod Update 2016;22:228–39. https://doi.org/10.1093/humupd/dmv054.Search in Google Scholar PubMed
2. Greene, MF. Progesterone for threatened abortion. N Engl J Med 2019;380:1867–8. https://doi.org/10.1056/nejme1903069.Search in Google Scholar PubMed
3. Zhou, J, Huang, Z, Pan, X, Leung, WT, Li, C, Chen, L, et al.. New thoughts in exploring the pathogenesis, diagnosis, and treatment of threatened abortion. Biosci Trends 2019;13:284–5. https://doi.org/10.5582/bst.2019.01155.Search in Google Scholar PubMed
4. Traianov, I, Dimitrakova, E. Left and right uterine artery Doppler as early screening test of threatened abortion outcome. Akusherstvo i ginekologiia 2016;55:34–8.Search in Google Scholar
5. Soltan, MH. Buphenine and threatened abortion. Eur J Obstet Gynecol Reprod Biol 1986;22:319–24. https://doi.org/10.1016/0028-2243(86)90120-6.Search in Google Scholar PubMed
6. Póvoa, A, Xavier, P, Matias, A, Blickstein, I. First trimester β-hCG and estradiol levels in singleton and twin pregnancies after assisted reproduction. J Perinat Med 2018;46:853–6. https://doi.org/10.1515/jpm-2017-0132.Search in Google Scholar PubMed
7. Zhang, Q, Wang, Z, Cheng, X, Wu, H. lncRNA DANCR promotes the migration an invasion and of trophoblast cells through microRNA-214-5p in preeclampsia. Bioengineered 2021;12:9424–34. https://doi.org/10.1080/21655979.2021.1988373.Search in Google Scholar PubMed PubMed Central
8. Wang, H, Cao, Q, Ge, J, Liu, C, Ma, Y, Meng, Y, et al.. LncRNA-regulated infection and inflammation pathways associated with pregnancy loss: genome wide differential expression of lncRNAs in early spontaneous abortion. Am J Reprod Immunol 2014;72:359–75. https://doi.org/10.1111/aji.12275.Search in Google Scholar PubMed
9. Billing, AM, Dib, SS, Bhagwat, AM, da Silva, IT, Drummond, RD, Hayat, S, et al.. A systems-level characterization of the differentiation of human embryonic stem cells into mesenchymal stem cells. Mol Cell Proteomics: MCP 2019;18:1950–66. https://doi.org/10.1074/mcp.ra119.001356.Search in Google Scholar PubMed PubMed Central
10. Fan, F, Huang, Z, Chen, Y. Integrated analysis of immune-related long noncoding RNAs as diagnostic biomarkers in psoriasis. PeerJ 2021;9:e11018. https://doi.org/10.7717/peerj.11018.Search in Google Scholar PubMed PubMed Central
11. Hao, C, Lin, S, Liu, P, Liang, W, Li, Z, Li, Y. Potential serum metabolites and long-chain noncoding RNA biomarkers for endometrial cancer tissue. J Obstet Gynaecol Res 2023;49:725–43. https://doi.org/10.1111/jog.15494.Search in Google Scholar PubMed
12. Wang, Y, Cheng, Q, Xia, Z, Zhou, R, Li, Y, Meng, L, et al.. Whole-transcriptome sequencing identifies key mRNAs, miRNAs, lncRNAs, and circRNAs associated with unexplained recurrent pregnancy loss. Cell Tissue Res 2022;389:129–43. https://doi.org/10.1007/s00441-022-03632-x.Search in Google Scholar PubMed
13. Fabian, MR, Sonenberg, N, Filipowicz, W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem 2010;79:351–79. https://doi.org/10.1146/annurev-biochem-060308-103103.Search in Google Scholar PubMed
14. Zhang, L, Wan, Q, Zhou, H. Targeted-regulating of miR-515-5p by LncRNA LOXL1-AS1 on the proliferation and migration of trophoblast cells. Exp Mol Pathol 2021;118:104588. https://doi.org/10.1016/j.yexmp.2020.104588.Search in Google Scholar PubMed
15. Nunode, M, Hayashi, M, Nagayasu, Y, Sawada, M, Nakamura, M, Sano, T, et al.. miR-515-5p suppresses trophoblast cell invasion and proliferation through XIAP regulation in preeclampsia. Mol Cell Endocrinol 2023;559:111779. https://doi.org/10.1016/j.mce.2022.111779.Search in Google Scholar PubMed
16. Makrydimas, G, Sebire, NJ, Lolis, D, Vlassis, N, Nicolaides, KH. Fetal loss following ultrasound diagnosis of a live fetus at 6-10 weeks of gestation. Ultrasound Obstet Gynecol: Official J Int Soc Ultrasound Obstet Gynecol 2003;22:368–72. https://doi.org/10.1002/uog.204.Search in Google Scholar PubMed
17. Duan, L, Yan, D, Zeng, W, Yang, X, Wei, Q. Predictive power progesterone combined with beta human chorionic gonadotropin measurements in the outcome of threatened miscarriage. Arch Gynecol Obstet 2011;283:431–5. https://doi.org/10.1007/s00404-010-1367-7.Search in Google Scholar PubMed
18. Di Renzo, GC, Giardina, I, Clerici, G, Brillo, E, Gerli, S. Progesterone in normal and pathological pregnancy. Horm Mol Biol Clin Invest 2016;27:35–48. https://doi.org/10.1515/hmbci-2016-0038.Search in Google Scholar PubMed
19. Günzel-Apel, A, Urhausen, C, Wolf, K, Einspanier, A, Oei, C, Piechotta, M. Serum progesterone in pregnant bitches supplemented with progestin because of expected or suspected luteal insufficiency. Reprod Domest Anim = Zuchthygiene 2012;47:55–60. https://doi.org/10.1111/rda.12029.Search in Google Scholar PubMed
20. Zhu, X, Du, M, Gu, H, Wu, R, Gao, M, Xu, H, et al.. Integrated analysis of lncRNA and mRNA expression profiles in patients with unexplained recurrent spontaneous abortion. Am J Reprod Immunol 2023;89:e13691. https://doi.org/10.1111/aji.13691.Search in Google Scholar PubMed
21. Wu, JL, Wang, YG, Gao, GM, Feng, L, Guo, N, Zhang, CX. Overexpression of lncRNA TCL6 promotes preeclampsia progression by regulating PTEN. Eur Rev Med Pharmacol Sci 2019;23:4066–72. https://doi.org/10.26355/eurrev_201905_17907.Search in Google Scholar PubMed
22. Sheng, F, Sun, N, Ji, Y, Ma, Y, Ding, H, Zhang, Q, et al.. Aberrant expression of imprinted lncRNA MEG8 causes trophoblast dysfunction and abortion. J Cell Biochem 2019;120:17378–90. https://doi.org/10.1002/jcb.29002.Search in Google Scholar PubMed
23. Wang, L, Zhao, H, Fang, Y, Yuan, B, Guo, Y, Wang, W. LncRNA CARMN inhibits cervical cancer cell growth via the miR-92a-3p/BTG2/Wnt/β-catenin axis. Physiol Genom 2023;55:1–15. https://doi.org/10.1152/physiolgenomics.00088.2022.Search in Google Scholar PubMed
24. Wang, X, Wu, S, Yang, Y, Zhao, J. LncRNA CARMN affects hepatocellular carcinoma prognosis by regulating the miR-192-5p/LOXL2 Axis. Oxid Med Cell Longev 2022;2022:9277360. https://doi.org/10.1155/2022/9277360.Search in Google Scholar PubMed PubMed Central
25. Gao, Y, Guo, X, Li, Y, Sha, W, She, R. The decreased lncRNA ZEB2-AS1 in pre-eclampsia controls the trophoblastic cell line HTR-8/SVneo’s invasive and migratory abilities via the miR-149/PGF axis. J Cell Biochem 2019;120:17677–86. https://doi.org/10.1002/jcb.29034.Search in Google Scholar PubMed
26. Long, N, Sun, RL, Lai, QH, Lu, M, Li, X, Chen, Y, et al.. HOTAIR/miR-1277-5p/FBN2 signaling axis is involved in recurrent spontaneous abortion by regulating the growth, migration, and invasion of HTR-8/SVneo cells. Biol Reprod 2024;111:135–47. https://doi.org/10.1093/biolre/ioae030.Search in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

