Abstract
Objectives
Calcium/calmodulin-dependent protein kinase-2 (CaMKII) is a serine/threonine kinase prevalent in neuronal cells, playing a key role in memory, learning, and synaptic plasticity. Nonspecific CaMKII inhibition can prevent apoptosis in neuronal cells and reduce glutamate-induced cell death. Additionally, variations in CaMKK enzyme levels affect hemopoietic stem cell proliferation, although the effects of CaMKII on stem cell responses during stress remain unclear. This study aims to explore CaMKII’s impact on the survival and proliferation of mesenchymal stem cells under stress and analyze the expression of its isoforms (alpha, beta, gamma, and delta) in these conditions.
Methods
The study included the characterization of MSCs, followed by an evaluation of the effects of KN-93, a CaMKII inhibitor, on the viability and proliferation of stem cells both in the presence and absence of H2O2 treatment. The toxicity caused by the application of 1 mM H2O2 further increased by inhibitor treatment. Additionally changes in the gene expression levels of CaMKII isoforms were analyzed.
Results
The application of H2O2 significantly decreased the total expression levels of CaMKII, with a significant reduction in the delta isoform. Furthermore, CaMKII inhibition by KN-93 increased the toxicity induced by H2O2. Viability and proliferation of stem cells were negatively impacted by the combined treatment of KN-93 and H2O2 compared to H2O2 alone.
Conclusions
Our findings provide a strong foundation to understand the response mechanisms of MSCs under stress conditions and could inform strategies for targeted stem cell therapies in oxidative stress conditions.
Introduction
The term “kinase” encompasses a broad category of enzymes that facilitate a phosphate group transfer from ATP to Serine, Threonine, or Tyrosine hydroxyl groups in protein substrates [1]. The calcium/calmodulin-dependent protein kinase (CaM-kinase) family, including CaMKI, CaMKII, CaMKIII, CaMKIV, and CaMKK, consists of Serine/Threonine kinases activated primarily by the calcium (Ca2+)/calmodulin (CaM) complex. Some members can also undergo Ca2+/CaM-independent activation through additional modifications, enabling precise regulation of diverse cellular functions [2]. The pursuit of a “memory molecule” led to significant findings, suggesting that these molecules could be kinases or phosphatases that act as switches for memory and cognitive processes [3]. Specifically, calcium/calmodulin-dependent protein kinase II (CaMKII) has garnered attention due to its pivotal role in learning and memory [4]. Beyond neuronal function, CaMKII is involved in regulating tumor cell survival and proliferation. Recent research has elucidated its integral role in autophagy and cellular responses to various stimuli [5], 6].
CaMKII is found in many tissues, but in higher concentrations in some parts of the brain, constituting as much as 2 % of the total protein especially in neuron cells [7]. CaMKII is abundantly present in brain tissues and throughout the body, playing a key role in coordinating Ca2+ signal transduction. Its phosphorylated substrates maintain cellular homeostasis and support activity-dependent neuronal modifications essential for learning and memory [8]. CaMKII holoenzymes have a unique structure, consisting of 12 functional domains arranged in a cogwheel-like shape with two clusters of six domains. Each subunit contains catalytic, autoregulatory, and helper domains. Binding of Ca2+/CaM to the holoenzyme releases the autoregulatory inhibition, triggering autophosphorylation and resulting in changes such as Ca2+/CaM-independent activity, CaM capture, and altered sensitivity. These mechanisms grant CaMKII a molecular memory capacity for its activity and autoregulation [9]. In mammals, CaMKII is encoded by four genes: α, β, γ, and δ isoenzymes. The α and β isoenzymes are more abundant in the brain. Each of these isoenzymes has many linkage variations. In invertebrates, a single alternatively folded gene encodes CaMKII [10]. It has been proposed that nonspecific inhibition of CaMKII can avert apoptosis in neuronal cells. Additionally, inhibiting both induced and autonomic CaMKII activity reduces glutamate-induced neuronal cell death in primary cultures [11].
The mechanism of CaMKII inhibition by KN-93 and its structural analog KN-92 involves the disruption of CaMKII’s interaction with calmodulin (CaM), a process that is essential for its activation. KN-93 is a well-known inhibitor that specifically targets the CaMKII pathway, affecting various physiological processes. The inhibition of CaMKII by KN-93 has been shown to have significant effects on cellular functions, such as delaying ovarian follicle development [12] and increasing neuronal excitability [13], which are mediated through distinct molecular pathways. In contrast, KN-92 does not inhibit CaMKII, as it cannot interfere with the CaM-CaMKII interaction, thus lacking the physiological effects of KN-93. This highlights the specificity of KN-93 as a CaMKII inhibitor and the significant physiological changes resulting from its targeted inhibition [12].
Hydrogen peroxide (H2O2) is a crucial metabolite involved in various cellular processes and redox metabolism. It functions as a key secondary messenger, alongside hydrogen sulfide (H2S) and nitric oxide (NO), triggering downstream protein cascades through specific oxidations. This response determines whether a cell proliferates, survives, or undergoes death, depending on the activated pathways (pathological, homeostatic, or protective) [14]. It is shown that H2O2 potently upregulates endothelial NO synthase (eNOS) gene expression in endothelial cells via activation of CaMKII and Janus kinase 2 (JAK2) [15]. Conversely, oxidative stress injury results in significant localized and widespread tissue damage, leading to functional failure in distant organs, such as cardiovascular system, the gastrointestinal tract, liver, and brain [16]. A study has demonstrated the function of 17b-estradiol (17b-E2) against oxidative stress on mice bone marrow mesenchymal stem cells (BMSCs) death triggered by hydrogen peroxide (H2O2) [17].
In the 1960s, stem cells first described in adult mouse bone marrow and they are categorized as embryonic, fetal or adult stem cells, according to the period of origin during ontogenesis [18]. Stem cells have the capacity to renew themselves over time, as they have a non-limited number of mitosis divisions and can be obtained both in the embryonic and in the adult period, and either prenatally or postnatally [19]. It has been shown that CaMKII is crucial in the bone marrow-derived mesenchymal stem cells chondrogenesis [20]. Another study has demonstrated a direct involvement of CaMKII in TGF-β and bone morphogenetic protein-mediated responses in chondrocytes derived from primary and pluripotent stem cells [21]. Although the specific interaction between WJ-MSCs and CaMKII is not directly addressed, CaMKII is known to regulate cellular signaling pathways involved in differentiation and proliferation, indicating its potential role in the neural differentiation of WJ-MSCs. The regulatory mechanisms of WJ-MSCs, including transcriptional and epigenetic modifications, may involve CaMKII, which is essential for maintaining pluripotency and guiding differentiation [22].
Additionally, recent research suggests that variations in the levels of Calcium/calmodulin-dependent protein kinase (CaMKK) enzyme, which belongs to the same family as CaMKII, regulate the proliferation of hematopoietic stem cells [23]. During embryogenesis and postnatal development, generating differentiated cells for tissue growth must balance stem cell continuity for future growth. This balance is maintained by initiating a proliferative phase that increases progenitor cell numbers before their differentiation into hypertrophic chondrocytes. The study found that increased CaMKII activity prevented hypertrophy, while loss of CaMKII function disrupted the transition from proliferation to hypertrophy [24]. It is known that the multifunctional CaMKII enzyme is involved in the phenotype change and maintenance of smooth muscle cells. In a study, it was determined that CaMKIIγ isoform plays a role in human adipose tissue-derived mesenchymal stem cell differentiation into contractile smooth muscle cells. Throughout the differentiation process, the level of CaMKIIγ gradually increased [25]. However, there remains a lack of clarity over the effects of CaMKII and its isoenzymes’ on cellular response in stem cell viability and proliferation in stressful conditions. In this study, we aimed to investigate the effect of CAMKII enzyme on the survival and proliferation of mesenchymal stem cells both with and without stress conditions, and also, to determine the alterations in gene expression and protein levels of CAMKII isoforms (alpha, beta, gamma, delta) under stress conditions. This study posits that inhibition of CaMKII will lead to a decrease in the survival and proliferation rates of mesenchymal stem cells, particularly under oxidative stress.
Materials and methods
Obtaining of umbilical cords, Wharton’s jelly-derived MSCs isolation & culture and mesenchymal stem cell characterization
This study involved seven completely healthy pregnant women (aged 18–40) at term 37–40 weeks undergoing elective cesarean sections at Tepecik Obstetrics and Research Hospital, Izmir (For the study, ethical approval and informed written consent were obtained). Exclusion criteria included pregnancies from assisted reproductive techniques, fetal abnormalities, intrauterine growth retardation (fetal weight<2500 g), maternal disorders like diabetes or any other maternal risk factors. A 10–20 cm segment of the umbilical cord was collected, cleaned of excess blood, and preserved in +4 °C sterile phosphate buffer (PBS) containing 100 μg/ml streptomycin and 100 U/ml penicillin. The samples were then transferred to Dokuz Eylül University’s Medical Biochemistry Department for further analysis.
The cleaned cord was dissected longitudinally using a sterile scalpel, removing the vessels, and the section containing the mucous layer (Wharton’s jelly) was separated into 0.4–0.5 cm pieces (explant). These pieces were placed into 6-well plates. DMEM/F-12 medium supplemented with 10 % fetal bovine serum (FBS), 1 % penicillin/streptomycin and 1 % L-glutamine was used to culture the cells and the plates were cultured in a 37 °C adjusted 5 % CO2 incubator. Cell output from explants was observed after an average of 9–11 days. The medium of the cells was changed every three days for more efficient feeding and proliferation. Isolated cells were grown in plates for experiments or they were frozen at −80 °C until usage.
Isolated WJ-MSC’s were characterized with Human Mesenchymal Stem Cell Functional Identification Kit (R&D Systems, Cat:SC006) like in our previous study [26]. In this study, the minimal criteria for characterizing Mesenchymal Stem Cells (MSCs), according to the International Society for Cellular Therapy (ISCT), were the ability to differentiate into adipogenic, osteogenic and chondrogenic cells. These criteria were demonstrated histochemically by the use of Wharton Jelly derived stem cells. The characterization of MSCs were isolated using explant method from Wharton jelly. During this process, multi lineage differentiation capacity and stemness-related cell surface antigen expressions were revealed by immunofluorescence microscopy, flow cytometry and q-PCR The reedited data from our previous study was shown in Supplementary Material [26].
Hydrogen peroxide (H2O2) toxicity, cell viability and proliferation assays
In this study, optimal doses of inhibitors and hydrogen peroxide (H2O2) were determined through preliminary experiments, focusing on viability, effect dose, and IC50 values. The study involved six experimental conditions: control, H2O2(Sigma, Cat:H1009) group, KN-93(BioVision, Cat:1909) inhibitor+H2O2, KN-93 inhibitor, KN-92(BioVision, Cat:B1643) negative inhibitor+H2O2, and KN-92 negative inhibitor. Cells (8×103) were cultured in 96-well plates and 200×103 cells in 6-well plates with complete DMEM/F-12 medium. After incubation at 37 °C in 5 % CO2 for 16–18 h, cells were pre-treated with 1 µM KN-93 or KN-92 inhibitors for 24 h. Subsequently, 1 mM H2O2 was added, and plates were incubated for 30 min. All experiments were conducted a minimum of three times.
Thiazolyl blue tetrazolium bromide (MTT) Kit (Applichem, Cat:A2231) was used to determine cell viability and CellTiter96® AQueous One Solution Cell Proliferation (MTS) Kit (Promega, Cat:G358) was utilized to assess cell proliferation. 8×103 cells were planted into 96-well cell culture plates with complete DMEM/F-12 cell culture medium at 200 µL final volume. All applications were performed as previously described, then the experiment was conducted.
Determination of specific gene expressions and measurement of CaMKII protein levels
The RT-PCR experiment analyzed surface antigen expressions and CaMKII isoforms (alpha-Qiagen:PPH02338A, beta-Qiagen:PPH01503E, gamma-Qiagen:PPH00525A, delta-Qiagen:PPH11180A) in WJ-MSCs from umbilical cords. Total RNA was isolated by using the MN Nucleospin II RNA isolation kit (Macherey-Nagel, Cat: 740-955.250) and RNA purity confirmed by Thermo NanoDrop 2000 device (A260/A280 ∼2). cDNA synthesis (2 μg) was done using the Qiagen RT2 First Strand Kit (Qiagen, Cat: 330404). qPCR was performed using specific primers and Qiagen RT2 SYBR Green Mastermix (Qiagen, Cat: 330600) on a LightCycler instrument. Expression of genes was standardized to GAPDH and quantified using the 2-(ΔΔCT) method. ΔCt was determined by subtracting GAPDH-CT from gene-CT, and relative expression was determined using control group data.
In this study, an ELISA experiment measured total CaMKII level in WJ-MSCs using Human CaMKII ELISA Kit (Fine Test, Cat: EH6990). Cells (200×103) were cultured in 6-well plates with DMEM/F-12 medium and incubated for 16–18 h at 37 °C in 5 % CO2. Inhibitor and hydrogen peroxide treatments were applied as described previously. Cell supernatants and lysates were collected and ELISA experiments were performed and protein concentrations were determined by BCA assay (ThermoFisher Scientific, Cat:23225) according to the manufacturer’s guidelines.
Statistical analysis
The data were analyzed using the SPSS Windows 25.0 software. Categorical variables were compared using the independent samples t-test, while continuous variables were assessed with the Mann-Whitney U test. Stem cells from the umbilical cords of seven pregnant women were utilized in the experiments, which were repeated between 3 and 6 times. Results are given as mean±standard error. p<0.05 values were considered statistically significant.
Results
Cell viability and proliferation levels of WJ-MSCs
MTT results showed no significant change in cell viability with 1 μM KN-93 or KN-92 inhibitors applied for 24 h compared to the control. In the group treated with 1 mM H2O2 for 30 min, cell viability decreased by 38.1 %, and with 1 μM KN-92 pre-application, it decreased by 39 %, both statistically significant. After 1 μM KN-93 pre-application, H2O2 treatment reduced viability by 57 % in comparison to the control and 28 % compared to the KN-92+H2O2 and H2O2-only groups, also statistically significant (p=0.0079) (Figure 1A).

The effect of CaMKII inhibitor and negative inhibitor pre-application and H2O2 applications (A) on viability of WJ-MSCs and (B) on proliferation of WJ-MSCs.
MTS test results showed no significant change in stem cell proliferation with 1 μM KN-93 or KN-92 applied for 24 h compared to the control. H2O2 (1 mM, 30 min) decreased proliferation by 73.7 %, and with 1 μM KN-92 pre-application 24 h, it decreased by 73.9 %, both statistically significant. With 1 μM KN-93 pre-application 24 h and H2O2 (1 mM, 30 min), proliferation decreased by 76 % compared to the control and 2.1 % compared to the H2O2 and KN-92+H2O2 groups, also statistically significant (p=0.0079) (Figure 1B).
Evaluation of CaMKII enzyme isoform levels by RT-qPCR
The results obtained by RT-qPCR method for CaMKII isoform changes for untreated-control WJ-MSCs show no statistically significant difference among the gene expressions of CaMKII alpha, beta, gamma and delta isoforms (p>0.05) (Figure 2).

Gene expression levels of CaMKII α, β, γ and δ isoforms in WJ-MSCs without any treatment.
CaMKII isoform changes were also examined in WJ-MSCs with H2O2 (1 mM, 30 min), showing decreased levels of CaMKII alpha, gamma and delta isoform gene expression levels relative to the control group, but increased CaMKII beta isoform gene expression level (p>0.05) (Figure 3) RT-qPCR results showed a decrease in gene expression in the 1 μM KN-93 and KN-92 application groups under 1 mM H2O2 stress, but it was not statistically significant (p>0.05). Also there was no statistically significant change in the KN-92 pre-treatment group in comparison to the control (p>0.05) (Figure 4). RT-qPCR results showed a 2.88-fold increase in CaMKII Beta gene expression under 1 mM H2O2 stress, and a 2.81-fold increase with 1 μM KN-92 pre-treatment. In the 1 μM KN-93 pre-treatment and H2O2 (1 mM, 30 min) group, expression decreased 0.5 times, and KN-93 alone reduced expression 8 times (p<0.05) (Figure 5).

Gene expression levels of CaMKII α, β, γ and δ isoforms in WJ-MSCs under 1 mM H2O2 treatment for 30 min.
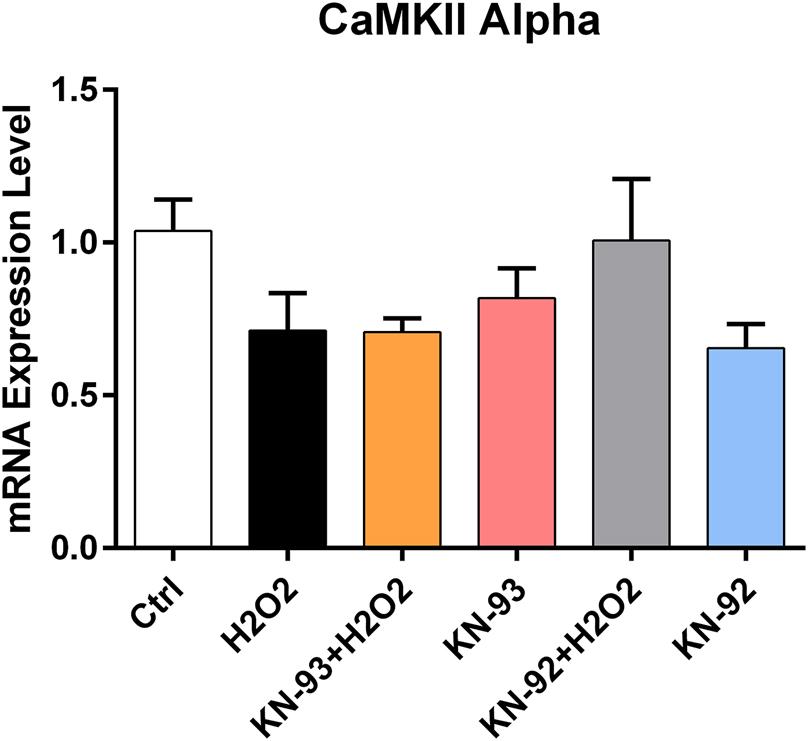
CaMKII alpha isoform gene expression change under CaMKII inhibitor and negative inhibitor pre-application and H2O2 applications in WJ-MSCs.

CaMKII beta isoform gene expression change under CaMKII inhibitor and negative inhibitor pre-application and H2O2 applications in WJ-MSCs.
The results showed a decrease in CaMKII Gamma gene expression in the 1 mM H2O2 stress group and the 1 μM KN-92 pre-treatment with H2O2 group, but the change was not statistically significant (p>0.05) (Figure 6). RT-qPCR data showed a significant 3-fold decrease in CaMKII Delta gene expression under 1 mM H2O2 stress (p=0.0079), and a 0.2-fold decrease with 1 μM KN-93 pre-applicaiton (p=0.0159). No notable changes were detected in CaMKII Gamma expression (Figure 7).

CaMKII gamma isoform gene expression change under CaMKII inhibitor and negative inhibitor pre-application and H2O2 applications in WJ-MSCs.

CaMKII delta isoform gene expression change under CaMKII inhibitor and negative inhibitor pre-application and H2O2 applications in WJ-MSCs.
Assessment of total CAMKII protein levels
A 64 % decline in total CAMKII protein level was observed in the 30 min 1 mM H2O2 stress condition in comparison to the control (p=0.0079). In the group in which 1 μM KN-92 pre-application for 24 h was applied and 1 mM H2O2 stress condition was applied for 30 min, a decrease of 62 % was found in comparison to the control group (p=0.0079). A decrease of 56 % was observed in the group in which 1 μM KN-93 CaMKII inhibitor pre-application for 24 h was applied and 1 mM H2O2 stress condition was applied for 30 min compared to the control group (p=0.0079) (Figure 8).

The effect of CaMKII inhibitor, negative inhibitor pre-application and H2O2 applications on total CaMKII protein levels in WJ-MSCs.
All results are presented as mean ± SEM.
Discussion
This study aimed to address the limited understanding of CaMKII isoforms in regulating the survival and proliferation of mesenchymal stem cells (MSCs) under oxidative stress. While CaMKII is well-studied in neuronal systems, its role in MSCs, especially under stress, remains unclear. The study examined the effects of H2O2-induced oxidative stress and CaMKII inhibition on Wharton’s Jelly-derived MSCs (WJ-MSCs). The results showed that oxidative stress significantly impaired WJ-MSC viability and proliferation, with KN-93-mediated CaMKII inhibition exacerbating these effects. Additionally, H2O2 exposure induced isoform-specific changes in CaMKII gene expression, including upregulation of the beta isoform and downregulation of the delta isoform. These findings highlight CaMKII’s crucial role in MSC responses to oxidative stress and its potential as a target for improving MSC resilience in regenerative therapies.
The source and protocols for obtaining stem cells are essential for their potential in treating degenerative and proliferative diseases. While bone marrow stem cells are commonly used, MSCs from Wharton’s Jelly possess stem cell characteristics and a reduced risk of tissue rejection due to the lack of associated proteins [27]. There has been growing interest in mesenchymal stem cells properties, particularly their ability to self-renewal and differentiation into various cell lines, and MSCs obtained from perinatal tissues are believed to possess greater differentiation potential than most adult MSCs [28]. Musiał-Wysocka et al. (2019) found that WJ-MSCs express pluripotency markers such as NANOG, OCT-4, and SSEA-4, but at lower levels than induced pluripotent stem cells (iPS), with expression potentially enhanced under hypoxic conditions. Notably, WJ-MSCs don’t create tumors in vivo, highlighting their promise in regenerative medicine [29].
Limited information exists on protocols for extracting stem cells from Wharton’s jelly and their stress responses. This study isolated MSCs from Wharton’s Jelly under laboratory conditions, revealing minimal stem cell properties [26]. These cells were examined, and their characteristics were also identified in the current study. Experiments showed that oxidative stress from H2O2 reduced cell viability and proliferation by approximately 40–50 % compared to the control group. The optimal dose and duration for WJ-MSCs were determined based on the dose-response curves for the KN-93 CaMKII inhibitor and the KN-92 negative inhibitor. Doses of 5 and 10 μM of KN-93 decreased cell viability and were excluded. Given that the IC50 for KN-93 is 0.37 μM, a 1 μM dose, which had no negative effects on cell viability, was used in all experiments. This dose has also been employed in similar studies for 24 h with both inhibitors [30]. This study assessed the effects of the KN-93 CaMKII inhibitor on WJ-MSC viability, proliferation, and changes in gene expression of CaMKII isoforms and total CaMKII protein levels under H2O2-induced stress. The results showed that combining KN-93 with H2O2 significantly reduced cell viability and proliferation compared to both the H2O2-stressed and control groups, highlighting the crucial role of CaMKII in these processes. This supports the hypothesis that “the living cell learns, and the learning cell lives,” emphasizing CaMKII’s role as an enzyme in learning.
One study previously showed that CaMKII has no impact on BMSCs proliferation, but can hinder their chondrogenic potential by impacting their differentiation [20]. Another study showed that the CaMKII mRNA levels were significantly upregulated when treated with H2O2 in BMSCs [31]. In WJ-MSCs, the application of H2O2 stress significantly increased the gene expression of the CaMKII Beta isoform but returned to control levels in both the KN-93 and H2O2 applied groups. H2O2 was shown to activate CaMKII in astrocytes, and this activation can be blocked by KN-93 [32]. H2O2 stress increased CaMKII Beta isoform gene expression, suggesting the enzyme’s role in maintaining cell viability and proliferation. Additionally, H2O2 application increased CaMKII activity due to methionine oxidation effect of H2O2 itself [33]. An increase in CaMKII Beta isoform was observed in groups treated with both KN-92 and H2O2, as well as in the H2O2-only group, indicating that KN-93 significantly affected its expression. A statistically significant decline in CaMKII Delta isoform was found only in the H2O2 stressed group compared to controls, with levels approaching control in the KN-93 and H2O2 combination group. No significant changes were observed for CaMKII Alpha and Gamma isoforms.
CaMKII was initially discovered in the brain, and it is regarded as a key target for cerebral ischemic nerve injury; phosphorylated CaMKII translocates from the cytoplasm to the cell membrane [34]. The total flavonoid extract of Dracocephalum moldavica L. (TFDM) has been shown to protect astrocytes against H2O2-induced apoptosis by attenuating a CaMKII-dependent mitochondria pathway [35]. Another study in mesenchymal stem cells has demonstrated that Ganoderic Acid D (GA-D) prevented oxidative stress induced senescence by the activation of CaM/CaMKII/NRF2 signaling pathway [36]. In our study, total CaMKII protein levels significantly decreased under both H2O2 stress and H2O2 stress with KN-92 pre-application conditions. Furthermore, H2O2 stress with KN-93 CaMKII inhibitor caused a dramatic decrease compared to controls, likely due to rapid degeneration of phosphorylated proteins. While phosphorylated forms were not assessed in our ELISA tests, they showed an increase compared to the H2O2-only group and a slight increase relative to the KN-92+H2O2 group. Although H2O2 typically increases CaMKII activity, our findings suggest that hydrogen peroxide-induced cytotoxicity led to a decrease in the enzyme’s protein level. This reduction may have triggered an increase in gene expression, particularly of the beta isoform, as a cellular response.
The latest findings indicate that each of the four isoforms serves distinct functions and in some cases, these roles is entirely independent of their enzymatic function. Recent research highlights the Delta isoform’s crucial role in memory maintenance and persistence through the sustained expression of its gene [37]. Also, it has been shown that in rats, CaMKII-delta protein expression increase for as long as 5 days after brain injury [38] and as long as 7 days in homogenates form ventricles following transverse aortic constriction [39]. In our study, H2O2 application significantly decreased the total CaMKII expression level, especially for the delta isoform. The detection of CaMKII Delta in both presynapses and the nucleus is a key finding that deserves further investigation.
Conclusions
This study represents the first to explore and demonstrate the critical role of CaMKII in regulating the viability of mesenchymal stem cells (MSCs) under oxidative stress conditions induced by H2O2. CaMKII is integral to multiple signaling pathways that control cell survival, proliferation, and differentiation. A deeper understanding of its function offers valuable insights into the mechanisms through which MSCs sustain their effectiveness under stressful environments, thereby supporting their regenerative potential. Notably, CaMKII acts as a molecular memory mechanism, allowing cells to retain and adapt to previous signaling events. This capability enhances the adaptability and long-term functionality of MSCs under adverse conditions. Additionally, CaMKII plays a significant role in modulating cellular responses to oxidative stress, a hallmark of many pathophysiological processes. Understanding the specific impact of CaMKII on MSC behavior during oxidative stress can inform the development of targeted strategies to improve MSC resilience and effectiveness in regenerative medicine applications.
Acknowledgments
The author Tugba SAN ERKOC is supported by the Council of Higher Education (CoHE, Yuksekogretim Kurulu, YÖK) with 100/2000 Ph.D. Scholarship in Human Brain and Neuroscience and also supporting by The Scientific and Technological Research Council of Turkiye (TUBITAK) with BIDEB 2211/A National PhD Scholarship Program. The authors would like to thank the support provided by YÖK (Turkiye), TUBITAK, Dokuz Eylul University Department of Medical Biochemistry for the opportunity to use the laboratories and Dr. Simon Mumford for English grammar & spelling corrections.
-
Research ethics: This study was approved by the Dokuz Eylul University Non-Interventional Research Ethics Committee with the 4061-GOA protocol and decision number 2019/08–35 on 03.04.2019. Informed written consent was obtained from all women included in this study.
-
Informed consent: Informed consent was obtained from all individuals included in this study, or their legal guardians or wards.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission. MSc. Tugba San Erkoc: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – Original Draft, Review & Editing, Visualization and Funding acquisition. MSc. Irem Nur Gokbayrak Atay: Resources, Data Curation, Writing – Original Draft, Review & Editing, Visualization. Assoc. Prof. Dr. Deniz Oztekin: Resources, Writing – Review & Editing. Dr. Mehmet Emin Gunes: Resources, Writing – Review & Editing. Prof. Dr. Bekir Ugur Ergur: Resources, Visualization, Writing – Review & Editing. Prof. Dr. Pinar Akan: Conceptualization, Methodology, Writing – Review & Editing, Supervision, Project administration, Funding acquisition.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest and no known competing financial interest which could influence the work reported in this study.
-
Research funding: This study was supported by Dokuz Eylul University Scientific Research Projects Coordination Unit with 2019.KB.SAG.026 code.
-
Data availability: The raw data can be obtained on request from the corresponding author.
References
1. Burnett, G, Kennedy, EP. The enzymatic phosphorylation of proteins. J Biol Chem [Internet] 1954;211:969–80. https://doi.org/10.1016/s0021-9258(18)71184-8.Search in Google Scholar
2. Swulius, MT, Waxham, MN. Ca2+/calmodulin-dependent protein kinases. Cell Mol Life Sci 2008;65:2637–57. https://doi.org/10.1007/s00018-008-8086-2.Search in Google Scholar PubMed PubMed Central
3. Miller, CA, Gavin, CF, White, JA, Parrish, RR, Honasoge, A, Yancey, CR, et al.. Cortical DNA methylation maintains remote memory. Nat Neurosci 2010;13:664–6. https://doi.org/10.1038/nn.2560.Search in Google Scholar PubMed PubMed Central
4. Yamauchi, T. Neuronal Ca2+/calmodulin-dependent protein kinase II - discovery, progress in a quarter of a century, and perspective: implication for learning and memory. Biol Pharm Bull 2005;28:1342–54. https://doi.org/10.1248/bpb.28.1342.Search in Google Scholar PubMed
5. Xu, J, Wang, H, Hu, Y, Zhang, YS, Wen, L, Yin, F, et al.. Inhibition of CaMKIIα activity enhances antitumor effect of fullerene C60 nanocrystals by suppression of autophagic degradation. Adv Sci 2019;6:1801233. https://doi.org/10.1002/advs.201801233.Search in Google Scholar PubMed PubMed Central
6. Zhan, Q, Jeon, J, Li, Y, Huang, Y, Xiong, J, Wang, Q, et al.. CAMK2/CaMKII activates MLKL in short-term starvation to facilitate autophagic flux. Autophagy 2021;18:726–44. https://doi.org/10.1080/15548627.2021.1954348.Search in Google Scholar PubMed PubMed Central
7. Cui, C, Wang, C, Cao, M, Kang, X. Ca2+/calmodulin-dependent protein kinases in leukemia development. J Cell Immunol 2021;3:144–50. https://doi.org/10.33696/immunology.3.091.Search in Google Scholar PubMed PubMed Central
8. Nicole, O, Pacary, E. Camkiiβ in neuronal development and plasticity: an emerging candidate in brain diseases. Int J Mol Sci 2020;21:7272. https://doi.org/10.3390/ijms21197272.Search in Google Scholar PubMed PubMed Central
9. Rostas, JAP, Spratt, NJ, Dickson, PW, Skelding, KA. The role of Ca2+-calmodulin stimulated protein kinase II in ischaemic stroke – a potential target for neuroprotective therapies. Neurochem Int 2017;107:33–42. https://doi.org/10.1016/j.neuint.2017.01.012.Search in Google Scholar PubMed
10. Hoffman, L, Stein, RA, Colbran, RJ, McHaourab, HS. Conformational changes underlying calcium/calmodulin-dependent protein kinase II activation. EMBO J 2011;30:1251–62. https://doi.org/10.1038/emboj.2011.40.Search in Google Scholar PubMed PubMed Central
11. Ashpole, NM, Song, W, Brustovetsky, T, Engleman, EA, Brustovetsky, N, Cummins, TR, et al.. Calcium/calmodulin-dependent protein kinase II (CaMKII) inhibition induces neurotoxicity via dysregulation of glutamate/calcium signaling and hyperexcitability. J Biol Chem 2012;287:8495–506. https://doi.org/10.1074/jbc.m111.323915.Search in Google Scholar PubMed PubMed Central
12. Yu, J, Xie, X, Ma, Y, Yang, Y, Wang, C, Xia, G, et al.. Effects and potential mechanism of Ca2+/calmodulin-dependent protein kinase II pathway inhibitor KN93 on the development of ovarian follicle. Int J Mol Med 2022;50:121. https://doi.org/10.3892/ijmm.2022.5177.Search in Google Scholar PubMed PubMed Central
13. Liang, H, Qin, L, Feng, R, Shim, J, Huang, X, Xu, X, et al.. Increased NaV1.2 expression and its interaction with CaM contribute to the hyperexcitability induced by prolonged inhibition of CaMKII. Epilepsia 2025;1–17. https://doi.org/10.1111/epi.18377.Search in Google Scholar PubMed
14. Di Marzo, N, Chisci, E, Giovannoni, R. The role of hydrogen peroxide in redox-dependent signaling: homeostatic and pathological responses in mammalian cells. Cells 2018;7:156. https://doi.org/10.3390/cells7100156.Search in Google Scholar PubMed PubMed Central
15. Cai, H, Davis, ME, Drummond, GR, Harrison, DG. Induction of endothelial NO synthase by hydrogen peroxide via a Ca2+/calmodulin-dependent protein kinase II/janus kinase 2-dependent pathway. Arterioscler Thromb Vasc Biol 2001;21:1571–6. https://doi.org/10.1161/hq1001.097028.Search in Google Scholar PubMed
16. Kimura, W, Xiao, F, Canseco, DC, Muralidhar, S, Thet, S, Zhang, HM, et al.. Hypoxia fate mapping identifies cycling cardiomyocytes in the adult heart. Nature 2015;523:226–30. https://doi.org/10.1038/nature14582.Search in Google Scholar PubMed
17. Xu, X, Li, D, Li, X, Shi, Q, Ju, X. Mesenchymal stem cell conditioned medium alleviates oxidative stress injury induced by hydrogen peroxide via regulating miR143 and its target protein in hepatocytes. BMC Immunol 2017;18:51. https://doi.org/10.1186/s12865-017-0232-x.Search in Google Scholar PubMed PubMed Central
18. Aji, K, Maimaijiang, M, Aimaiti, A, Rexiati, M, Azhati, B, Tusong, H, et al.. Differentiation of human adipose derived stem cells into smooth muscle cells is modulated by CaMKII γ. Stem Cells Int 2016:1267480. https://doi.org/10.1155/2016/1267480.Search in Google Scholar PubMed PubMed Central
19. Hwang, NS, Varghese, S, Elisseeff, J. Controlled differentiation of stem cells. Adv Drug Deliv Rev 2008;60:199–214. https://doi.org/10.1016/j.addr.2007.08.036.Search in Google Scholar PubMed PubMed Central
20. Qu, F, Zhao, Z, Yuan, B, Qi, W, Li, C, Shen, X, et al.. CaMKII plays a part in the chondrogenesis of bone marrow-derived mesenchymal stem cells. Int J Clin Exp Pathol 2015;8:5981–7.Search in Google Scholar
21. Saitta, B, Elphingstone, J, Limfat, S, Shkhyan, R, Evseenko, D. CaMKII inhibition in human primary and pluripotent stem cell-derived chondrocytes modulates effects of TGFβ and BMP through SMAD signaling. Osteoarthr Cartil 2019;27:158–71. https://doi.org/10.1016/j.joca.2018.08.017.Search in Google Scholar PubMed PubMed Central
22. Ma, L, He, X, Wu, Q. The molecular regulatory mechanism in multipotency and differentiation of Wharton’s jelly stem cells. Int J Mol Sci 2023;24:12909. https://doi.org/10.3390/ijms241612909.Search in Google Scholar PubMed PubMed Central
23. Junho, CVC, Caio-Silva, W, Trentin-Sonoda, M, Carneiro-Ramos, MS. An overview of the role of calcium/calmodulin-dependent protein kinase in cardiorenal syndrome. Front Physiol 2020;11:735. https://doi.org/10.3389/fphys.2020.00735.Search in Google Scholar PubMed PubMed Central
24. Saleh, M, Fotook Kiaei, SZ, Kavianpour, M. Application of Wharton jelly-derived mesenchymal stem cells in patients with pulmonary fibrosis. Stem Cell Res Ther 2022;13:71. https://doi.org/10.1186/s13287-022-02746-x.Search in Google Scholar PubMed PubMed Central
25. Li, Y, Ahrens, MJ, Wu, A, Liu, J, Dudley, AT. Calcium/calmodulin-dependent protein kinase II activity regulates the proliferative potential of growth plate chondrocytes. Development 2011;138:359–70. https://doi.org/10.1242/dev.052324.Search in Google Scholar PubMed PubMed Central
26. San, T, Bora, U, Sayın, O, Gunes, ME, Oztekin, D, Ergur, BU, et al.. The effect of antioxidant culture conditions and isolation methods in obtaining of mesenchymal stem cells from the Wharton’s jelly of human umbilical cord. J Deu Med 2021;35:43–60. https://doi.org/10.5505/deutfd.2021.27132.Search in Google Scholar
27. Kim, DW, Staples, M, Shinozuka, K, Pantcheva, P, Kang, SD, Borlongan, CV. Wharton’s jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci 2013;14:11692–712. https://doi.org/10.3390/ijms140611692.Search in Google Scholar PubMed PubMed Central
28. Pittenger, MF, Discher, DE, Péault, BM, Phinney, DG, Hare, JM, Caplan, AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med 2019;4:22. https://doi.org/10.1038/s41536-019-0083-6.Search in Google Scholar PubMed PubMed Central
29. Musiał-Wysocka, A, Kot, M, Sułkowski, M, Badyra, B, Majka, M. Molecular and functional verification of Wharton’s jelly mesenchymal stem cells (WJ-MSCs) pluripotency. Int J Mol Sci 2019;20:1807. https://doi.org/10.3390/ijms20081807.Search in Google Scholar PubMed PubMed Central
30. An, P, Zhu, JY, Yang, Y, Lv, P, Tian, YH, Chen, MK, et al.. KN-93, a specific inhibitor of CaMKII inhibits human hepatic stellate cell proliferation in vitro. World J Gastroenterol 2007;13:1445–8. https://doi.org/10.3748/wjg.v13.i9.1445.Search in Google Scholar PubMed PubMed Central
31. Wang, Y, Zhao, R, Liu, D, Deng, W, Xu, G, Liu, W, et al.. Exosomes derived from miR-214-enriched bone marrow-derived mesenchymal stem cells regulate oxidative damage in cardiac stem cells by targeting CaMKII. Oxid Med Cell Longev 2018;2018:4971261. https://doi.org/10.1155/2018/4971261.Search in Google Scholar PubMed PubMed Central
32. Haskew-Layton, RE, Mongin, AA, Kimelberg, HK. Hydrogen peroxide potentiates volume-sensitive excitatory amino acid release via a mechanism involving Ca2+/calmodulin-dependent protein kinase II. J Biol Chem 2005;280:3548–54. https://doi.org/10.1074/jbc.m409803200.Search in Google Scholar
33. Burgoyne, JR, Oka, SI, Ale-Agha, N, Eaton, P. Hydrogen peroxide sensing and signaling by protein kinases in the cardiovascular system. Antioxidants Redox Signal 2013;18:10421052. https://doi.org/10.1089/ars.2012.4817.Search in Google Scholar PubMed PubMed Central
34. Xu, M, Zhang, HL. Death and survival of neuronal and astrocytic cells in ischemic brain injury: a role of autophagy. Acta Pharmacol Sin 2011;32:1089–99. https://doi.org/10.1038/aps.2011.50.Search in Google Scholar PubMed PubMed Central
35. Zheng, RF, Du, YW, Zeng, C, Wang, HF, Xing, JG, Xu, M. Total flavones of Dracocephalum moldavica L. protect astrocytes against H2O2-induced apoptosis through a mitochondria-dependent pathway. BMC Complement Med Ther 2020;20:78. https://doi.org/10.1186/s12906-020-2846-4.Search in Google Scholar PubMed PubMed Central
36. Yuan, H, Xu, Y, Luo, Y, Zhang, JR, Zhu, XX, Xiao, JH. Ganoderic acid D prevents oxidative stress-induced senescence by targeting 14-3-3ε to activate CaM/CaMKII/NRF2 signaling pathway in mesenchymal stem cells. Aging Cell 2022;21:1–19. https://doi.org/10.1111/acel.13686.Search in Google Scholar PubMed PubMed Central
37. Zalcman, G, Federman, N, Romano, A. CaMKII isoforms in learning and memory: localization and function. Front Mol Neurosci 2018;11:1–14. https://doi.org/10.3389/fnmol.2018.00445.Search in Google Scholar PubMed PubMed Central
38. Zhang, M, Shan, H, Gu, Z, Wang, D, Wang, T, Wang, Z, et al.. Increased expression of calcium/calmodulin-dependent protein kinase type II subunit delta after rat traumatic brain injury. J Mol Neurosci 2012;46:631–43. https://doi.org/10.1007/s12031-011-9651-y.Search in Google Scholar PubMed
39. Zhang, T, Maier, LS, Dalton, ND, Miyamoto, S, Ross, J, Bers, DM, et al.. The δc isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res 2003;92:919–21. https://doi.org/10.1161/01.res.0000069686.31472.c5.Search in Google Scholar
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/tjb-2024-0265).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review
- Progression in the study of protective effect of microRNAs against sevoflurane-induced postoperative cognitive dysfunction and potential mechanisms
- Research Articles
- Evaluating novel therapeutics in type 2 diabetes: a systematic review and meta-analysis of bexagliflozin and tirzepatide
- Comparison of lipid, liver, and renal parameters between fasting and non-fasting states in apparently healthy adults
- Serum adipokines and gene polymorphisms in peripheral vascular disease
- Eosin-5′-maleimide (EMA)-binding assay as a diagnostic method of hereditary spherocytosis
- Evaluating serum FOXs transcription factor proteins in Hashimoto’s thyroiditis: a comparative analysis
- Assessment of circadian rhythm protein levels in the pathogenesis of infantile colic
- lncRNA SNHG6 regulates neuroinflammation in traumatic brain injury by inhibiting the expression of miR-101-3p
- Serum miR-142-5p serves as a biomarker to predict onset and short-term prognosis in acute coronary syndrome patients
- Predictive value and clinical significance of miR-186-5p in the development of esophageal varices in cirrhosis patients
- miR-199a-5p inhibits proliferation and migration of burn-denatured fibroblasts by targeting VEGFA in burn patients
- Development of rapid diagnostic tests for multiplexed detection of COVID-19 and Influenza diseases
- Can new laboratory parameters be added for clinical scoring systems to determine the severity of RSV bronchiolitis?
- Kushen Herpes Tincture confront senile herpes zoster by inhibiting STAT3 and EGFR
- Modulation of CaMKII levels in Wharton’s jelly mesenchymal stem cells under hydrogen peroxide induced stress conditions
- Tumor-endothelium reciprocal interactions in co-culture: changes of genetics and phenotypic characteristics of endothelial and laryngeal cells
- Evaluation of hesperidin and taurine for mitigating letrozole-induced uterine toxicity in rats: histopathological, molecular, and biochemical insights
- Assessment of the anticancer function of Coronilla orientalis MILLER through comprehensive in vitro and computational studies
Articles in the same Issue
- Frontmatter
- Review
- Progression in the study of protective effect of microRNAs against sevoflurane-induced postoperative cognitive dysfunction and potential mechanisms
- Research Articles
- Evaluating novel therapeutics in type 2 diabetes: a systematic review and meta-analysis of bexagliflozin and tirzepatide
- Comparison of lipid, liver, and renal parameters between fasting and non-fasting states in apparently healthy adults
- Serum adipokines and gene polymorphisms in peripheral vascular disease
- Eosin-5′-maleimide (EMA)-binding assay as a diagnostic method of hereditary spherocytosis
- Evaluating serum FOXs transcription factor proteins in Hashimoto’s thyroiditis: a comparative analysis
- Assessment of circadian rhythm protein levels in the pathogenesis of infantile colic
- lncRNA SNHG6 regulates neuroinflammation in traumatic brain injury by inhibiting the expression of miR-101-3p
- Serum miR-142-5p serves as a biomarker to predict onset and short-term prognosis in acute coronary syndrome patients
- Predictive value and clinical significance of miR-186-5p in the development of esophageal varices in cirrhosis patients
- miR-199a-5p inhibits proliferation and migration of burn-denatured fibroblasts by targeting VEGFA in burn patients
- Development of rapid diagnostic tests for multiplexed detection of COVID-19 and Influenza diseases
- Can new laboratory parameters be added for clinical scoring systems to determine the severity of RSV bronchiolitis?
- Kushen Herpes Tincture confront senile herpes zoster by inhibiting STAT3 and EGFR
- Modulation of CaMKII levels in Wharton’s jelly mesenchymal stem cells under hydrogen peroxide induced stress conditions
- Tumor-endothelium reciprocal interactions in co-culture: changes of genetics and phenotypic characteristics of endothelial and laryngeal cells
- Evaluation of hesperidin and taurine for mitigating letrozole-induced uterine toxicity in rats: histopathological, molecular, and biochemical insights
- Assessment of the anticancer function of Coronilla orientalis MILLER through comprehensive in vitro and computational studies

