Abstract
Objectives
The key component of neuroprotection after cerebral ischemia–reperfusion (I–R) injury is mitochondrial improvement. By focusing on the function of mitochondrial biogenesis and ATP-sensitive potassium (mK–ATP) channels and inflammatory responses, the current study assessed the neuroprotective potentials of lemon essential oil, D-limonene (LIM), in rats with cerebral I–R injury.
Methods
In order to simulate cerebral I–R injury, Sprague Dawley rats (n=72) were subjected to a two h local ischemia induced by middle cerebral artery blockage, followed by a 24 h reperfusion period. Five minutes before starting reperfusion, rats were intraperitoneally given LIM at doses of 10 or 100 mg/kg. Cerebral infarct volume was assessed by triphenyl-tetrazolium chloride staining, brain activity by behavioral tests and mitochondrial function/biogenesis, as well as proinflammatory cytokines by fluorometry, immunoblotting and other related techniques.
Results
When compared to the untreated control group, the administration of LIM substantially and dose-dependently decreased cerebral infarct volumes and neurological deficits (p<0.01). I–R injury-induced alterations in mitochondrial membrane depolarization, mitochondrial reactive oxygen species (mitoROS), and superoxide dismutase (mnSOD), as well as inflammatory cytokines TNF-α, IL-6 and IL-1β, were all significantly reversed after treatment with LIM 100 mg/kg (p<0.01). Additionally, this dose of LIM increased the expression of mitochondrial biogenesis proteins PGC-1α, TFAM, and NRF1. Interestingly, blockage of mK–ATP channels by 5-hydoxydecanoate diminished the effects of LIM on cerebral positive endpoints, cytokines production, and mitochondrial function/biogenesis.
Conclusions
Thus, the strong neuroprotective effects of LIM-postconditioning were mediated by an increase in mK–ATP channel activity, which improved mitochondrial biogenesis and suppressed inflammatory responses.
Introduction
Cerebral ischemic disease is one of the most prevalent conditions in the world that has significant rates of morbidity and death. Adults with brain injury often experience multiple pathophysiological alterations and pertinent deficits that are mostly brought on by acute ischemic stroke and the subsequent delayed reperfusion [1]. Various pathophysiological effects of cerebral ischemia–reperfusion (I–R) injuries include an increase in inflammatory responses, oxidative stress, mitochondrial malfunction, endothelial dysfunction, tissue necrosis, and cell death [1, 2]. Mitochondrial dysfunction, which can result from malfunctioning mechanisms disrupting mitochondrial homeostasis as well as its biogenesis, is connected to the increased prevalence of cerebral I–R disorders [3].
Earlier research has shown that after brain I–R injury, mitochondrial biogenesis is inhibited; therefore, enhancement of it would be neuroprotective [3, 4]. Peroxisome proliferator activated receptor gamma coactivator-1 (PGC-1α) is a transcriptional coactivator that is essential for sustaining mitochondrial biogenesis and improving mitochondrial normal activity [5]. Together with nuclear respiratory factor 1 (NRF1), PGC1α regulates the production of mitochondrial transcription factor A (TFAM), a protein that is expressed in the nucleus and governs the amount of mitochondrial DNA copies [6]. As a result, the whole mitochondrial biogenetic network is subjected to a strict regulation. However, the mitochondrial membrane’s ATP-dependent potassium (mK–ATP) channels also play a significant part in upholding this balance in mitochondrial biogenesis [7, 8]. Still, after I–R damage, these channels are inhibited, compromising mitochondrial integrity, and raising production of ROS and inflammatory cytokines such tumor necrosis factor α (TNF-α) and interleukin-1β (IL-1β) [9, 10]. Therefore, it is believed that mK–ATP channels and mitochondria can become the main pharmaceutical targets for protection of neuronal cells and the efficient suppression of cerebral I–R injury.
D-limonene (LIM) is a terpenoid compound naturally found in lemon essential oil and other citrus oils and has many biological properties that encourage its use for many diseases. According to various reports, this compound has robust antioxidant effects and strongly scavenger free radicals in different organs’ disorders like heart, kidney, liver, and skin [11], [12], [13]. Also, its anti-apoptotic and anti-inflammatory effects are more dominant among its other effects [12, 14]. Evidence shows that LIM has modulated synaptic activity in the brain, reduced neuronal death, and improved memory in stressed rats [15]. On the other hand, limonene has been able to greatly reduce apoptotic, inflammatory and oxidative cell damage by modulating the mitochondrial activity of heart and kidney cells [11, 12]. Despite LIM’s therapeutic potentials in conditions with inflammatory and mitochondrial dysfunction like cerebral I–R injury, its neuroprotective effects and the mitochondrial mechanisms involved in the actions of this multifunctional compound on the pathophysiology of stroke are not known. Therefore, we set out this study to assess the effects of LIM on the outcomes of cerebral infarction, mitochondrial biogenesis and activity, and pro-inflammatory cytokine production, as well as to investigate the possible contribution of mK–ATP channels in cerebral I–R injury.
Materials and methods
Animals
In the present study, 72 Sprague-Dawley rats weighing 250–300 g were purchased from the animal center and housed in a 12 h cycle of light/dark at a typical animal housing temperature of around 24 °C and humidity of 50 %. All experiments and animal handling techniques were carried out with the agreement of the university Committee for Animal Ethics (no. 2022-AE305).
Cerebral I–R injury induction and study protocol
According to the previous studies, the middle cerebral artery occlusion (MCAO) model was used to develop cerebral I–R injury. The rats underwent an intraperitoneal chloral hydrate anesthesia (400 mg/kg). Then, the right carotid artery was exposed and a rounded filamentous thread with a diameter of 0.234 mm was conducted into the internal carotid from side of the external carotid trunk. The thread was slightly pushed forwardly for a length of 18–20 mm to block the MCA origin. After being left in place for 2 h (ischemic time), the thread was removed to begin reperfusion. During the course of the procedure, a heating pad was used to maintain a body temperature between 37 and 37.5 °C. The animals were anesthetized again 24 h after reperfusion and killed for sampling. In transient focal cerebral ischemia models, MCAO model is typically induced for durations of 60, 90, or 120 min. Various extent of resulting infarct volumes are measured in ipsilateral hemisphere after 24 h of reperfusion [16]. We chose 120 min ischemia and 24 h reperfusion. Rats in sham experienced the same procedures except for MCAO induction. Rats that had extensive bleeding or premature death during surgery were excluded from the study and sampling.
The grouping of animals for this experiment was done according to the following category: (1) Sham, (2) IR, (3) IR+LIM10, (4) IR+LIM100, (5) IR+5HD, and (6) IR+5HD+LIM100. Each group had six rats for each of the infarct volume determination or biochemical and molecular evaluations. In groups of rats receiving LIM, 10 mg/kg (LIM10) or 100 mg/kg of limonene (LIM100), dissolved in 1 % DMSO, was injected by intraperitoneal (i.p.) injection 5 min prior to reperfusion. Other groups received the same amount of 1 % DMSO, i.p. Also, the selective inhibitor of mK–ATP channels, 5-hydroxydecanoate (5HD), was prepared at a concentration of five μM and administered to the corresponding groups, 10 min before injecting 100 mg/kg LIM. Accurate determination of the effective dose of LIM in I–R studies has not yet been performed. In some studies of experimental brain damage in rats, different doses of the drug have been tested [17]. In one study, LIM 100 mg/kg was effective in protecting the brain against dementia [18]. While in another study, 10 mg/kg of it had a significant effect on improving the memory of rats [15]. However, there are reports of side effects such as organ toxicity and increased oxidative stress at higher doses [17]. Because of these findings, two doses of 10 and 100 mg/kg LIM, as the effective doses in previous reports, were chosen to evaluate their effects on cerebral I–R injury in the present study.
Grading of neurological deficits
Neurological deficits grading setting was performed 30 min after occlusion onset to confirm successful development of MCAO, and 24 h after occlusion immediately before euthanizing the rat. This setting’s higher grades designate higher degree of cerebral injury. To score neurological findings, a 5-point scale was used, with no symptoms of neurological deficit=0, failure to fully extend left paw, 1; circling to left, 2; falling to left, 3; no walking ability spontaneously; and depressed consciousness, 4.
Infarct volume determination
After anesthetizing six rats per group, their brain tissues were isolated and immediately frozen at −20 °C. The samples were sliced into two mm-thick coronal cuts and underwent staining with 2 % (w/v) 2,3,5-triphenyl tetrazolium chloride (Sigma-Aldrich, USA) for 30 min at 37 °C before being immersed overnight in 4 % (w/v) paraformaldehyde. Image J software (National Institutes of Health, Bethesda, USA) was used to photograph and analyze the slices. Each slice’s white zones represented infarcted areas. The brain infarct volumes were calculated in percentages and normalized to each brain volume.
Brain water content estimation
After isolating the brains of the rats under deep anesthesia, the contralateral cortex of their brains was separated and the weight of wet tissue was measured, and then the samples were put in an oven to dry for 48 h at a temperature of 105 °C, and the dry weight of the tissue was also obtained. To calculate the brain water content, the difference in the weights of dry and wet tissue was calculated and divided by the wet weight, and the resulting value was multiplied by 100 to obtain the percentage of water content changes.
Quantification of indices of mitochondrial function
First, the mitochondrial content of the samples was isolated. For this purpose, peripheral zones of penumbra of ipsilateral cortex of the samples were placed in a solution containing isolating buffer plus a protease inhibitor cocktail (Sigma Aldrich, USA) to homogenize. Then the samples were centrifuged at 800 g and the resulting pellet was again centrifuged at 12,100 g. The BCA kit (Beyotime, Jiangsu, China) was also used to quantify the total protein of the mitochondrial fractions. In order to determine the levels mitochondrial ROS, the mitochondrial fraction was placed in a phosphate buffer solution containing 2 μmol DCFDA dye (Sigma-Aldrich, USA) at 37 °C for 30 min. Then, the absorbance of the solution was read at the excitation and emission wavelengths of 480 and 530 nm by a fluorometer. The absorbance values were normalized for the total protein of samples. Additionally, to estimate the mitochondrial membrane potential changes, the mitochondrial supernatant (100 μL) was added into a phosphate buffer solution containing 2 μL JC-1 dye- (Sigma-Aldrich, USA) and kept for 30 min at 37 °C. Using a fluorometer device, the red and green fluorescence intensities were obtained. The red to green intensity ratio was calculated to estimate the potential changes. Finally, the levels of manganese superoxide dismutase (MnSOD) were determined from the mitochondrial supernatant using the specific commercial ELISA kit (Cat. no: MBS1600375; MyBioSource, San Diego, California, USA), following the manufacturer’s instructions. The samples’ absorbance was measured at 450 nm, and the values were adjusted and presented as U/mg total protein content of mitochondrial samples determined using the BCA kit (Beyotime, Jiangsu, China).
Cytokines assays
Following 24 h of reperfusion, the ipsilateral cortex sample was homogenized in a lysis buffer (Beyotime, Jiangsu, China) and centrifuged at 8,000 g. Following the collection of sample supernatants, the levels of proinflammatory cytokines TNF-α (Cat. no: MBS175904), IL-1β (Cat. no: MBS825017), and IL-6 (Cat. no: MBS2021530) were measured by means of specialized ELISA kits according to manufacturer instructions (MyBioSource, San Diego, CA, USA). The cytokine levels of each sample were normalized by its protein contents (measured by the Bradford’s method), and the data were given as mg of protein per sample.
Immunoblotting
Proteins extracted from the samples underwent separation through polyacrylamide gel electrophoresis and subsequently transferred onto a polyvinylidene difluoride (PVDF) membrane. After washing steps and blocking the membranes in skim milk, they were treated with rabbit polyclonal antibodies against PGC-1α, NRF-1, and TFAM (1:1,000, Santa Cruz, USA) as well as beta-actin (1:2,000, Santa Cruz, USA). An enhanced chemiluminescence detection technique (Keygen Biotech, Nanjing, China) was employed for the visualization of the individual antibody–antigen responses. Quantity One 1-D Analysis Software (Bio-Rad, USA) was used to compute the target protein bands’ intensities, which were then adjusted to beta-actin intensity.
Statistical analysis
The data was presented as mean ± SD The one-way analysis of variance and Tukey post hoc test were used to analyze differences between groups. The p-value less than 0.05 was set to determine the minimal level of statistical significance.
Results
The neuroprotective action of LIM
After induction of cerebral I–R injury by MCAO in rats, their neurological deficits were scored using a 5-point scale. In comparison to the Sham group, MCAO-receiving rats showed a severe neurological deficit, demonstrating the success of induction of MCAO injury model. When compared to the IR group, LIM-treated rats had less neurological deficit 24 h after ischemia, but the higher dose of LIM had a superior impact (p<0.001) than the lower dose (p<0.05) (Figure 1A). Additionally, compared to IR rats receiving LIM 100 mg/kg, blockage of mK–ATP channels via 5HD dramatically eliminated the LIM’s protective impact on neurological results (p<0.01). Moreover, compared to the IR group, 100 mg/kg LIM significantly lowered the brain water content (p<0.05) (Figure 1B). Cerebral infarct volumes were also measured by the tetrazolium chloride staining method to validate the neuroprotective benefits of LIM. The results revealed that LIM treatment at both dosages significantly and dose-dependently decreased IR-induced cerebral infarct sizes (Figure 1C and D). Inhibiting mK–ATP channels considerably reduced the effects of 100 mg/kg LIM on cerebral water content (p<0.05) and infarct volumes (p<0.01).

Effect of LIM treatment on neuroprotective endpoints. (A) Grading of neurological deficits; (B) water contents of brain; (C) cerebral infarction volumes; (D) cerebral infarction images (n=6). **p<0.01, and ***p<0.001 vs. Sham group; #p<0.05, and ###p<0.001 vs. IR group; $p<0.05, and $$p<0.01 vs. IR+LIM100 group. IR, ischemia–reperfusion; LIM, limonene; 5HD, 5-hydroxydecanoate.
Effect of LIM on the mitochondrial activity
The measurement of mitochondrial ROS levels and mitochondrial membrane potential changes enabled the estimation of mitochondrial activity in this study. When compared to the Sham group, cerebral I–R damage significantly increased mitochondrial ROS production and depolarized mitochondrial membrane potential (p<0.001) (Figure 2A and B). But then again, LIM treatment in rats before reperfusion considerably and dose dependently reversed alterations in mitochondrial parameters induced by I–R injury (p<0.05 and p<0.001). Additionally, simultaneous administration of 5HD effectively eliminated the positive effects of LIM on the alterations in mitochondrial ROS and membrane potential (p<0.01) (Figure 2).
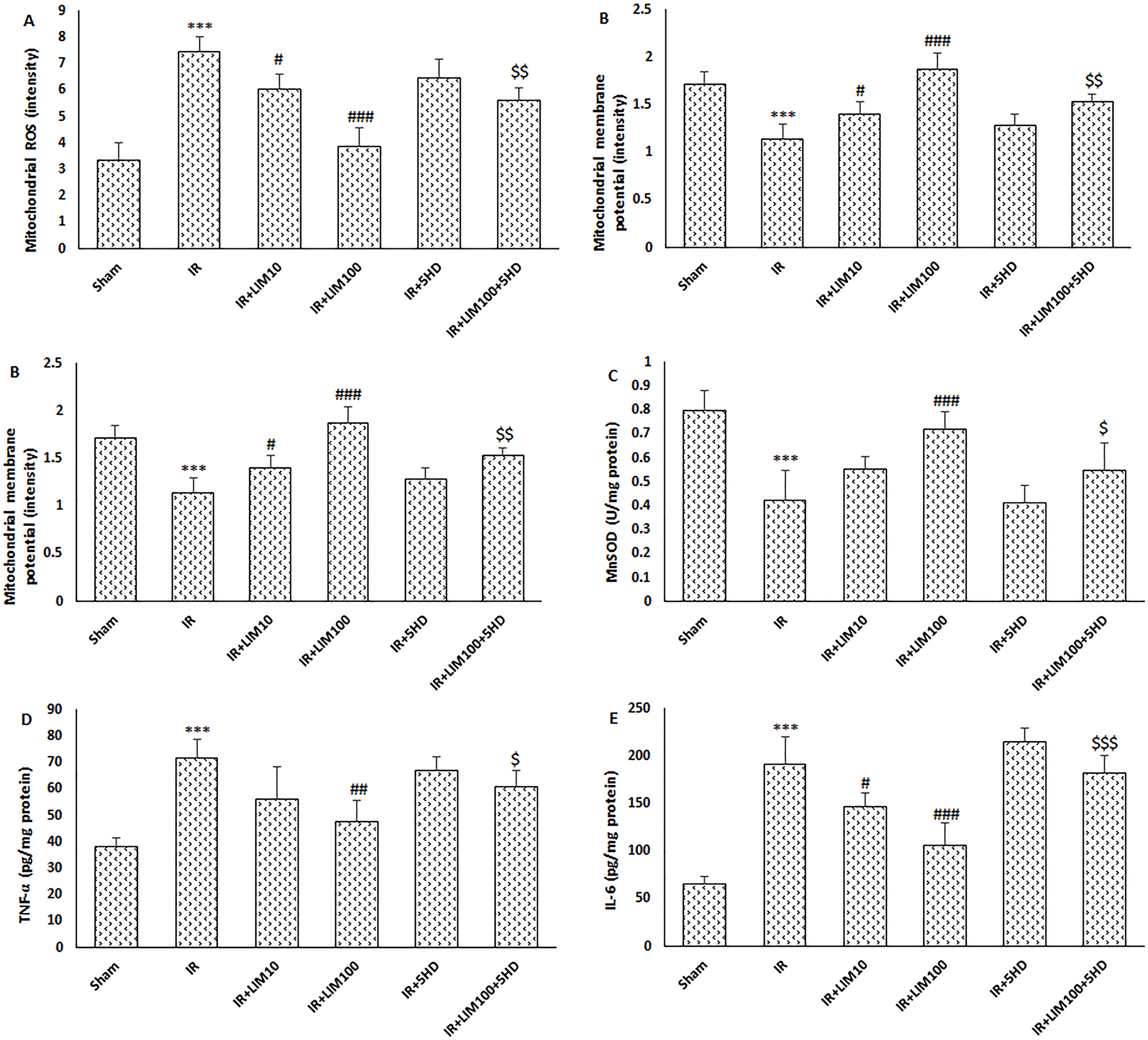
Effect of LIM treatment on the levels of brain mitochondrial endpoints and proinflammatory cytokines production. (A) Mitochondrial ROS; (B) mitochondrial membrane potential; (C) mitochondrial MnSOD; (D) TNF-α; (E) IL-6; (F) IL-1β (n=6). ***p<0.001 vs. Sham group; #p<0.05, and ###p<0.001 vs. IR group; $p<0.05, $$p<0.01, and $$$p<0.001 vs. IR+LIM100 group. IR, ischemia–reperfusion; LIM, limonene; 5HD, 5-hydroxydecanoate.
Effect of LIM on the MnSOD levels
MnSOD, acting endogenously as a powerful antioxidant enzyme, was found in considerably lower levels in the mitochondrial fraction of cerebral tissue of the IR group than the Sham group (p<0.001) (Figure 2C). LIM substantially increased the IR-induced decrease of MnSOD at 100 mg/kg but not at 10 mg/kg (p<0.001). In contrast to the IR+LIM100 group, the administration of 5HD to block the opening of mK–ATP channel considerably reduced the LIM’s ability to increase MnSOD levels (p<0.05).
Effect of LIM on the proinflammatory cytokines
Cerebral tissue TNF-α (Figure 2D), IL-1β (Figure 2E), and IL-6 (Figure 2F) production was considerably increased when cerebral I–R damage was induced in rats as compared to that in the Sham group (p<0.001) (Figure 2). TNF-α levels were unaffected by LIM at 10 mg/kg; however, this dose significantly decreased IL-6 and IL-1β levels as compared to the IR group (p<0.05) (Figure 2D–F). In brain samples of IR rats treated with 100 mg/kg LIM, all proinflammatory cytokines were shown to be significantly downregulated compared to the IR group (p<0.01). Finally, blockage of mK–ATP channels substantially abolished anti-inflammatory effects of LIM 100 mg/kg (p<0.05).
Effect of LIM on the mitochondrial biogenesis proteins
PGC-1α, TFAM, and NRF1, three important proteins that regulate mitochondrial biogenesis, were dramatically downregulated after in vivo cerebral I–R damage in rats (p<0.001) (Figure 3A–C). LIM administration at a dose of 10 mg/kg was unable to reverse the decreased protein expression following I–R damage. In contrast to the IR group, the expression levels of PGC-1α (p<0.001), NRF1, and TFAM (p<0.01) were considerably increased following LIM treatment at 100 mg/kg. When compared to the IR+LIM100 group, 5HD’s blockade of mK–ATP channels significantly reduced the impact of higher doses of LIM on the expression of the biogenesis proteins (p<0.05) (Figure 3).

Effect of LIM treatment on mitochondrial biogenesis proteins expression. (A) Representative immunoblots; (B) PGC-1α; (C) NRF1; (D) TFAM (n=4). ***p<0.001 vs. Sham group; ##p<0.01, and ###p<0.001 vs. IR group; $p<0.05 vs. IR+LIM100 group. IR, ischemia–reperfusion; LIM, limonene; 5HD, 5-hydroxydecanoate.
Discussion
Treatment of rats with LIM remarkably reduced the I–R injury-induced cerebral infarction and neurological impairments dose dependently. LIM at 100 mg/kg significantly enhanced mitochondrial activity and increased the protein expression of PGC-α, NRF1, and TFAM. Additionally, it impeded the elevation of cytokines and prevented mitochondrial oxidative damage after I–R injury. Importantly, the neuroprotective, anti-inflammatory, and mitochondrial-boosting actions of LIM were markedly diminished by blocking mK–ATP channels. The data showed that increasing mitochondrial activity in brain cells through LIM therapy could be a promising way to stop cerebral I–R injury. The earliest stages of reperfusion are associated with increased oxidative stress, inflammation, and apoptosis, which can exacerbate the ischemic injury [1]. Therefore, early intervention may help to mitigate these damaging effects and improve outcomes. Optimal timing of limonene treatment in rat MCAO model to induce neuroprotection is not clear from the available research. However, based on the general understanding of the pathophysiological mechanisms underlying I–R injury, administering it shortly after reperfusion may be more beneficial than waiting for late reperfusion.
Because of their wide activity, mitochondria have a specific role in preventing the fatal outcomes of I–R injury in brain tissues [4]. The majority of cellular oxygen is utilized by mitochondria during normal mitochondrial function, which lowers the chance of significant mitochondrial ROS generation and upregulates endogenously active antioxidant enzymes including MnSOD [19]. When reperfusion occurs suddenly, however, mitochondria otherwise become dysfunctional and are converted to the primary source of ROS and free radicals [4]. The production of proinflammatory cytokines is also enhanced once mitochondrial oxidative stress is amplified and mitochondrial function is disrupted [20]. This scenario causes the cerebral I–R damage to worsen. In the current investigation, treatment with LIM reduced the synthesis of interleukins and TNF-α, as well as it reduced the level of oxidative stress in the mitochondria (changes of ROS and MnSOD). These actions of LIM, along with its inhibitory impact on mitochondrial membrane depolarization, show that it has a significant ability to positively alter mitochondrial internal homeostasis under I–R circumstances. Similar reports claim that LIM has positive effects in organs with mitochondrial dysfunctions [11, 13, 17]. LIM pretreatment has protected osteoblasts against methylglyoxal-induced injury through stopping the dissipation of mitochondrial membrane potential and increasing the adenosine triphosphate production [21]. Additionally, this compound has been reported to reverse isoproterenol-induced myocardial infarction and heart damages by activating cardiac mitochondria and attenuating mitochondrial ROS [11]. Together with our findings, all of these studies point to the mitochondrial-targeted route may be a key target for this medication and a promising opportunity for lowering cerebral I–R injury.
Alterations in mitochondrial biogenesis are connected to mitochondrial dysfunction and the ensuing inflammatory and oxidative abnormalities [20]. There is growing evidence that encouraging mitochondrial biogenesis helps with neuroprotection [6, 20]. We revealed in the current study that LIM was able to trigger mitochondrial biogenesis, thereby providing supportive protection in cerebral I–R injury. At this point, LIM therapy elevated the expression of PGC-1α, TFAM, and NRF1 after I–R induction in rats’ brain. In consistent with our findings, the upregulation of mitochondrial biogenesis markers and mitochondrial improvement has been involved in the cytoprotective action of LIM treatment in prediabetic osteoblastic cells [21]. PGC-1α is the catalyst for mitochondrial biogenesis and, together with its transcriptional partner NRF1, stimulates the activity of TFAM [5, 6, 22]. The interaction between NRF1 and TFAM promotes mitochondrial oxidative-phosphorylation reactions and triggers mtDNA transcription [23]. The link between the neuroprotective impact of LIM and mitochondrial recovering action of PGC-1α needs further verification, even though our data suggest that neuroprotection by LIM may be achieved by boosting the mitochondrial biogenesis pathway.
By blocking mK–ATP channels with 5HD, it was possible to learn more about how LIM regulates mitochondrial activity, mitochondrial biogenesis, and inflammatory responses in cerebral I–R conditions. This investigation also aimed to determine whether these channels were involved in the LIM’s neuroprotective effects. In addition to eliminating LIM’s neuroprotective properties, impeding mK–ATP channels opening also rendered it’s anti-inflammatory and mitochondrial activities ineffective. These results demonstrate that the therapeutic effects of LIM in cerebral I–R injury are mediated by mK–ATP channels opening following reperfusion. The mK–ATP channel plays a crucial role in regulating PGC-1α activity to affect homeostatic balance of the mitochondria [7, 8]. This channel in the brain has a higher density than that of other organs [7]. This strongly highlights the significance of this channel in mediating LIM actions on mitochondrial biogenesis and homeostasis by avoiding mitochondrial swelling and membrane potential collapse, as well as delaying inflammation. The protective effects of LIM on lowering cerebral I–R endpoints are mediated by these important channels, which is thought to be one of the primary elements of mitochondria in their function as the ultimate effectors of neuroprotection [24]. Consequently, the mitochondrial K–ATP channels/biogenesis/anti-inflammatory pathway should be modulated to achieve the neuroprotective effects of LIM in cerebral I–R injury. Despite this, it is still not clear how LIM leads to the opening of mK–ATP channels. In this context, it is clear that increasing nitric oxide significantly enhances the activity of these channels at reperfusion [25, 26]. In this regard, a study has shown that LIM is able to increase the activity of heme-oxygenase-1 and intracellular concentration of nitric oxide in diabetic osteopathy [21]. Therefore, this effect of LIM can be one of the mechanisms involved in regulating the activity of the channels, which was not investigated in our study. Further research is also necessary to determine the role of other critical mediators, such as the PKG/cGMP and PI3K/AKT pathways, in this protection.
Conclusions
LIM demonstrated potent neuroprotective features in cerebral I–R injury by eliciting anti-inflammatory, antioxidant, and mitochondrial improvements. The positive effects of LIM were significantly mediated by improved mK–ATP channel activity during reperfusion. This study showed that this medication has the potential to be among the most effective pharmacological strategies for correcting mitochondrial dysfunction in I-R situations.
Funding source: Hebei Medical Science Research Project 0000-0002-1748-0421
Award Identifier / Grant number: 20220371
-
Research ethics: All experiments and animal handling techniques were carried out with the agreement of the local Institutional Review Board (no. 20220371).
-
Informed consent: Not applicable.
-
Author contributions: LeZ, ZZ, and CZ conceptualized and designed the project; ZZ, JJ, LiZ, and RX performed the experimentations; LeZ, RX, and CZ analyzed, and interpreted the data. LZ and CZ were the major contributors in writing the manuscript. All authors have read, critically revised, and accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Authors state no conflict of interest.
-
Research funding: This study was supported by a grant from Hebei Medical Science Research Project (no: 20220371).
-
Data availability: The raw data can be obtained on request from the corresponding author.
References
1. Kuriakose, D, Xiao, Z. Pathophysiology and treatment of stroke: present status and future perspectives: Int J Mol Sci 2020;21:7609, https://doi.org/10.3390/ijms21207609.Search in Google Scholar PubMed PubMed Central
2. Ansari, J, Gavins, FNE. Ischemia-reperfusion injury in sickle cell disease: from basics to therapeutics. Am J Pathol 2019;189:706–18, https://doi.org/10.1016/j.ajpath.2018.12.012.Search in Google Scholar PubMed PubMed Central
3. Liu, F, Lu, J, Manaenko, A, Tang, J, Hu, Q. Mitochondria in ischemic stroke: new insight and implications. Aging Dis 2018;9:924–37, https://doi.org/10.14336/ad.2017.1126.Search in Google Scholar PubMed PubMed Central
4. Carinci, M, Vezzani, B, Patergnani, S, Ludewig, P, Lessmann, K, Magnus, T, et al.. Different roles of mitochondria in cell death and inflammation: focusing on mitochondrial quality control in ischemic stroke and reperfusion. Biomedicines 2021;9:169, https://doi.org/10.3390/biomedicines9020169.Search in Google Scholar PubMed PubMed Central
5. Xie, Y, Li, J, Fan, G, Qi, S, Li, B. Reperfusion promotes mitochondrial biogenesis following focal cerebral ischemia in rats. PLoS One 2014;9:e92443, https://doi.org/10.1371/journal.pone.0092443.Search in Google Scholar PubMed PubMed Central
6. Gureev, AP, Shaforostova, EA, Popov, VN. Regulation of mitochondrial biogenesis as a way for active longevity: interaction between the Nrf2 and PGC-1α signaling pathways. Front Genet 2019;10:435, https://doi.org/10.3389/fgene.2019.00435.Search in Google Scholar PubMed PubMed Central
7. Peng, K, Hu, J, Xiao, J, Dan, G, Yang, L, Ye, F, et al.. Mitochondrial ATP-sensitive potassium channel regulates mitochondrial dynamics to participate in neurodegeneration of Parkinson’s disease. Biochim Biophys Acta, Mol Basis Dis 2018;1864:1086–103, https://doi.org/10.1016/j.bbadis.2018.01.013.Search in Google Scholar PubMed
8. Nikbakht, F, Khanizadeh, AM, Golab, F, Baluchnejadmojarad, T, Vazifehkhah, S, Moeinsadat, A. Mitochondrial ATP-sensitive potassium channel, MitoKATP, ameliorates mitochondrial dynamic disturbance induced by temporal lobe epilepsy. J Chem Neuroanat 2021;113:101808, https://doi.org/10.1016/j.jchemneu.2020.101808.Search in Google Scholar PubMed
9. Vishwakarma, VK, Upadhyay, PK, Chaurasiya, HS, Srivasatav, RK, Ansari, TM, Srivastava, V. Mechanistic pathways of ATP sensitive potassium channels referring to cardio-protective effects and cellular functions. Drug Res 2019;69:365–73, https://doi.org/10.1055/a-0806-7207.Search in Google Scholar PubMed
10. Szeto, V, Chen, N, Sun, H, Feng, Z. The role of KATP channels in cerebral ischemic stroke and diabetes. Acta Pharmacol Sin 2018;39:683–94, https://doi.org/10.1038/aps.2018.10.Search in Google Scholar PubMed PubMed Central
11. Rhana, P, Barros, GM, Santos, VCO, Costa, AD, Santos, DMD, Fernandes-Braga, W, et al.. S-limonene protects the heart in an experimental model of myocardial infarction induced by isoproterenol: possible involvement of mitochondrial reactive oxygen species. Eur J Pharmacol 2022;930:175134, https://doi.org/10.1016/j.ejphar.2022.175134.Search in Google Scholar PubMed
12. Babaeenezhad, E, Hadipour Moradi, F, Rahimi Monfared, S, Fattahi, MD, Nasri, M, Amini, A, et al.. D-limonene alleviates acute kidney injury following gentamicin administration in rats: role of NF-κB pathway, mitochondrial apoptosis, oxidative stress, and PCNA. Oxid Med Cell Longev 2021;2021:6670007, https://doi.org/10.1155/2021/6670007.Search in Google Scholar PubMed PubMed Central
13. Anandakumar, P, Kamaraj, S, Vanitha, MK. D-limonene: a multifunctional compound with potent therapeutic effects. J Food Biochem 2020;45:e13566, https://doi.org/10.1111/jfbc.13566.Search in Google Scholar PubMed
14. Zhao, C, Zhang, Z, Nie, D, Li, Y. Protective effect of lemon essential oil and its major active component, D-limonene, on intestinal injury and inflammation of E. coli-challenged mice. Front Nutr 2022;9:843096, https://doi.org/10.3389/fnut.2022.843096.Search in Google Scholar PubMed PubMed Central
15. Bigdeli, Y, Asle-Rousta, M, Rahnema, M. Effects of limonene on chronic restraint stress-induced memory impairment and anxiety in male rats. Neurophysiol 2019;51:107–13, https://doi.org/10.1007/s11062-019-09800-0.Search in Google Scholar
16. Xu, WW, Zhang, YY, Su, J, Liu, AF, Wang, K, Li, C, et al.. Ischemia reperfusion injury after gradual versus rapid flow restoration for middle cerebral artery occlusion rats. Sci Rep 2018;8:1638, https://doi.org/10.1038/s41598-018-20095-9.Search in Google Scholar PubMed PubMed Central
17. Eddin, LB, Jha, NK, Meeran, MFN, Kesari, KK, Beiram, R, Ojha, S. Neuroprotective potential of limonene and limonene containing natural products. Molecules 2021;26:4535, https://doi.org/10.3390/molecules26154535.Search in Google Scholar PubMed PubMed Central
18. Zhou, W, Fukumoto, S, Yokogoshi, H. Components of lemon essential oil attenuate dementia induced by scopolamine. Nutr Neurosci 2009;12:57–64, https://doi.org/10.1179/147683009x388832.Search in Google Scholar
19. Shields, HJ, Traa, A, Van Raamsdonk, JM. Beneficial and detrimental effects of reactive oxygen species on lifespan: a comprehensive review of comparative and experimental studies. Front Cell Dev Biol 2021;9:628157, https://doi.org/10.3389/fcell.2021.628157.Search in Google Scholar PubMed PubMed Central
20. Patergnani, S, Bouhamida, E, Leo, S, Pinton, P, Rimessi, A. Mitochondrial oxidative stress and “Mito-Inflammation”: actors in the diseases. Biomedicines 2021;9:216, https://doi.org/10.3390/biomedicines9020216.Search in Google Scholar PubMed PubMed Central
21. Suh, KS, Chon, S, Choi, EM. Limonene protects osteoblasts against methylglyoxal-derived adduct formation by regulating glyoxalase, oxidative stress, and mitochondrial function. Chem Biol Interact 2017;278:15–21, https://doi.org/10.1016/j.cbi.2017.10.001.Search in Google Scholar PubMed
22. Liu, L, Zhang, W, Wang, L, Li, Y, Tan, B, Lu, X, et al.. Curcumin prevents cerebral ischemia reperfusion injury via increase of mitochondrial biogenesis. Neurochem Res 2014;39:1322–31, https://doi.org/10.1007/s11064-014-1315-1.Search in Google Scholar PubMed
23. Kang, I, Chu, CT, Kaufman, BA. The mitochondrial transcription factor TFAM in neurodegeneration: emerging evidence and mechanisms. FEBS Lett 2018;592:793–811, https://doi.org/10.1002/1873-3468.12989.Search in Google Scholar PubMed PubMed Central
24. Wrzosek, A, Augustynek, B, Żochowska, M, Szewczyk, A. Mitochondrial potassium channels as druggable targets. Biomolecules 2020;10:1200, https://doi.org/10.3390/biom10081200.Search in Google Scholar PubMed PubMed Central
25. Saeid, F, Aniseh, J, Reza, B, Manouchehr, VS. Signaling mediators modulated by cardioprotective interventions in healthy and diabetic myocardium with ischaemia–reperfusion injury. Eur J Prev Cardiol 2018;25:1463–81, https://doi.org/10.1177/2047487318756420.Search in Google Scholar PubMed
26. Wrzosek, A, Gałecka, S, Żochowska, M, Olszewska, A, Kulawiak, B. Alternative targets for modulators of mitochondrial potassium channels. Molecules 2022;27:299, https://doi.org/10.3390/molecules27010299.Search in Google Scholar PubMed PubMed Central
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorial
- Time to treat the climate and nature crisis as one indivisible global health emergency
- Review
- Critical evaluation of publications and patents in nanobiotechnology-based research in the last decade
- Mini Review
- Current evaluation and recommendations for the use of artificial intelligence tools in education
- Research Articles
- Improvement of the post-analytical phase by means of an algorithm based autoverification
- Decision support system for the classification of Downey cells as a pre-diagnostic tool
- Prediction of LDL in hypertriglyceridemic subjects using an innovative ensemble machine learning technique
- Researching of resistance to etravirine in some HIV-1 low-level viremia strains by in-silico methods
- Enhancement of chondrogenic differentiation in ATDC5 cells using GFOGER-modified peptide nanofiber scaffold
- Zeolite nanomaterial-modified dielectrode oxide surface for diagnosing Alzheimer’s disease by dual molecular probed impedance sensor
- Cloning and in silico investigation of a putative voltage-gated calcium channel gene and protein in Astacus leptodactylus
- Postconditioning with D-limonene exerts neuroprotection in rats via enhancing mitochondrial activity
- Investigation of the effect of CA IX enzyme inhibition on the EZH2 gene and histone 3 modifications
- Midkine can not be accepted as a new biomarker for unexplained female infertility
- Silibinin reduces cell proliferation and migration via EMT pathway in TFK-1 cell line
- Fetuin A and fetuin B as an indicator of liver fibrosis in hepatitis B
- Acknowledgment
- Acknowledgment
Articles in the same Issue
- Frontmatter
- Editorial
- Time to treat the climate and nature crisis as one indivisible global health emergency
- Review
- Critical evaluation of publications and patents in nanobiotechnology-based research in the last decade
- Mini Review
- Current evaluation and recommendations for the use of artificial intelligence tools in education
- Research Articles
- Improvement of the post-analytical phase by means of an algorithm based autoverification
- Decision support system for the classification of Downey cells as a pre-diagnostic tool
- Prediction of LDL in hypertriglyceridemic subjects using an innovative ensemble machine learning technique
- Researching of resistance to etravirine in some HIV-1 low-level viremia strains by in-silico methods
- Enhancement of chondrogenic differentiation in ATDC5 cells using GFOGER-modified peptide nanofiber scaffold
- Zeolite nanomaterial-modified dielectrode oxide surface for diagnosing Alzheimer’s disease by dual molecular probed impedance sensor
- Cloning and in silico investigation of a putative voltage-gated calcium channel gene and protein in Astacus leptodactylus
- Postconditioning with D-limonene exerts neuroprotection in rats via enhancing mitochondrial activity
- Investigation of the effect of CA IX enzyme inhibition on the EZH2 gene and histone 3 modifications
- Midkine can not be accepted as a new biomarker for unexplained female infertility
- Silibinin reduces cell proliferation and migration via EMT pathway in TFK-1 cell line
- Fetuin A and fetuin B as an indicator of liver fibrosis in hepatitis B
- Acknowledgment
- Acknowledgment

