Abstract
Objectives
Myeloperoxidase from polymorphonuclear leukocytes is an important enzyme in oxidative metabolism and has a key role in tissue injuries in oxidative stress and inflammatory conditions. Therefore, its inhibitors have become the focus of studies on new drug development in recent years. The aim of the study was to determine the inhibitory effect of organic acids on the peroxidation, chlorination, and nitration activities of myeloperoxidase.
Methods
Seven organic acids naturally abundant in plants were tested. Different activities of myeloperoxidase were measured in the presence of various amounts of organic acids, and inhibition rates and kinetic parameters were determined for each organic acid separately.
Results
All the organic acids examined had inhibitory effects on the different activities of myeloperoxidase. Comparison of the IC50 values obtained for peroxidation, chlorination, and nitration activities showed that oxalic acid was the strongest inhibitor of myeloperoxidase activity, while citric acid and succinic acid were the weakest.
Conclusions
The results suggested that all the organic acids examined are inhibitors of myeloperoxidase. In particular, oxalic acid and fumaric acid are popular candidates for drug development research. More studies are needed to determine the in vivo effects of organic acids and their effects in the treatment of disease.
Introduction
Myeloperoxidase (MPO; EC 1.11.1.7) is released in inflammation zones from activated polymorphonuclear leukocytes and monocytes and plays an obvious role in the generation of oxidants by the human immune system. These oxidants, with a number of bactericidal proteins and enzymes released from polymorphonuclear leukocytes and monocyte granules into the extracellular space or phagosomes, play a key role in the killing of infectious microorganisms [1, 2]. Although this process is highly coordinated and controlled, damage to the host tissue can occur, and this damage is the basis of the proposed links between damage by this enzyme and the pathology of numerous diseases associated with chronic inflammation [2, 3].
MPO has been indicated as a potential participant in atherosclerosis promotion and propagation. Abundant proof shows that MPO may play a role in human atherogenesis. Biochemical and immunohistochemical tests were used to detect the enzyme and its oxidation products in atherosclerotic lesions [4].
MPO has been found to be associated with various forms of cancer [5], neurodegenerative diseases [6], periodontal disease [7], hepatitis C virus-induced fibrosis [8], vasculitides [9], renal diseases [10], diabetic nephropathy [11], ischemia/reperfusion injury [12], chronic inflammatory diseases [13], inflammatory bowel disease [14], lung diseases like COPD [15], asbestos-induced injury [16], cystic fibrosis [17], ARDS [18], and bone marrow transplantation-induced injury [19].
MPO has become a popular target for drug development in recent years due to its correlation with an array of diseases. Many studies on MPO inhibition mechanisms and their inhibitors have been performed in the recent past, and the positive effect of MPO inhibition in the treatment of related diseases was shown. Potent irreversible MPO inhibitors are used to prevent and diagnose neural disorders like multiple system atrophy, Huntington’s disease, Parkinson’s disease, Alzheimer’s disease, and MS in mice [20]. It has been shown that inhibition of MPO decreases the inflammatory rate of atherosclerotic lesions [21]. As a group of natural products, flavonoids have been investigated in various studies as MPO inhibitors and antioxidants. Quercetin [22], resveratrol [23], myricitrin [24], cocoa flavanols (−)-epicatechin, and catechin [25] are the natural products in this field that are studied the most.
Organic acids are another group of natural products that are mostly known as antioxidants based on their radical scavenging properties. The antioxidant effect of some organic acids extracted from vegetables and mushrooms was described by Pereira et al. The IC50 values for their antioxidant effect varied from 7 to 1,458 μg/mL for organic acid extraction from different plants and different parts of plants based on ABTS and DPPH tests [26]. Multiple studies indicated the effect of antioxidant compounds on the treatment of inflammatory disease [27], [28], [29].
Several studies were carried out on organic acids as MPO inhibitors and they were mainly focused on ascorbic acid’s properties [30].
Both organic acids as a group of antioxidants and MPO inhibitors have a positive effect on the treatment of inflammatory and cardiovascular disease, so the determination of the relation between organic acids and MPO inhibition could be helpful for further studies in biochemical, pharmacological, and nutritional sciences. In the present study, inhibition of the peroxidation, chlorination, and nitration activities of MPO by citric acid, fumaric acid, malic acid, oxalic acid, succinic acid, tartaric acid, and trans-aconitic acid was demonstrated and the inhibition rate of these organic acids for each activity was compared.
Materials and methods
Materials
MPO from human neutrophils was obtained from Merck Millipore. Pure organic acids and other chemicals were obtained from the official distributor of Sigma-Aldrich in Türkiye. An Analytik Jena 210 Plus spectrophotometer with the software WinAspect Plus 4.2.0.0. was used for the spectrophotometric measurements and kinetic analyses.
Methods
Determination of the peroxidation activity of MPO
With some modifications, the method described by Suzuki et al. was used to measure the peroxidation activity of MPO [31]. The analyses were run in 50 mM phosphate buffer pH 5.4 containing 0.1 % hexadecyltrimethylammonium bromide (HETAB) and 0.05 mM ethylenediaminetetraacetic acid (EDTA) at 37 °C. The reaction mixtures were prepared with 100 µL of 10 mM H2O2, 100 µL of 16 mM 3,3′,5,5′-tetramethylbenzidine (TMB) as substrate (ε655=39,000 M−1 cm−1), and 10 µL of MPO from 100 U/mL stock solution, and were completed to 1 mL with phosphate buffer. Inhibition kinetic analyses were carried out in the same mixture by adding different amounts of organic acids to the reaction mixture in the final volume of 1 mL. MPO utilizes the oxidation of TMB to TMB diimine. The reaction was measured for 30 s at 655 nm. The enzyme activity was determined as Δ Abs per minute changes in absorbance at 655 nm.
Determination of the chlorination activity of MPO
Measurement of the chlorination of taurine by MPO is a widespread method for determining the chlorination activity of MPO. MPO chlorinates taurine to chlorotaurine and its concentration can be measured by following the reaction with 2-nitro-5-thiobenzoate (TNB). Several modified applications have been used in this manner. The method described by Saad Aissat et al. [32] was adapted to the macro method with some modifications and used in the present study. For the preparation of TNB solution, 1 mmol/L 5,5′-dithiobis(2-nitrobenzoic acid), 5 mmol/L EDTA, and 20 mmol/L NaBH4 were dissolved in phosphate buffer (50 mmol/L) at pH 6.6 and incubated at 37 °C for 30 min. The actual concentration of the prepared TNB solution was estimated directly by measuring the absorbance at 412 nm (ε412=13,600 M−1 cm−1). The reaction mixtures contained 30 µL of taurine (150 mM/L), 10 µL of MPO (0.1 mg/mL equal to 1 IU per assay), and 60 µL of NaCl (1.5 mol/L). Various amounts of organic acids were added and the total volume was adjusted to 540 µL with 100 mmol/L acetate buffer (pH 5.5). Then 60 µL of H2O2 (10 mM) was added and the final solution was incubated for 30 min at 37 °C. Next 100 µL of catalase was added followed by incubation for 15 min at 37 °C to stop the reaction. After that, 300 µL of TNB solution was added to the final solution and followed spectrophotometrically at 412 nm. Each molecule of chlorotaurine bleaches two molecules of TNB to form one molecule of DTNB. In addition, 100 % chlorination activity was determined by activity measurement without adding any organic acid.
Determination of the nitration activity of MPO
In the presence of H2O2 and NO2, MPO mediates the nitration of tyrosine to form 3-nitrotyrosine. MPO-catalyzed nitration of tyrosine was determined in 900-mL reaction mixtures containing 50 mM phosphate buffer, 0.1 mM EDTA, 0.1 % HETAB, 1 mM H2O2, 1 mM NO2, 1 mM tyrosine, and 5 µg of MPO (equal to 5 U per test). The reaction mixtures were incubated for 10 min at 37 °C. After that, 100 mL of 1 N NaOH was added to the mixture to stop the reactions and raise the pH of the mixture to 10 for 3-nitrotyrosine measurements. The absorbance of the mixtures was measured at 430 nm and 3-nitrotyrosine was determined from standards subjected to the same conditions.
Determination of the inhibitory effect of organic acids on the peroxidation, chlorination, and nitration activities of MPO
To determine the effective concentration ranges for inhibitor studies of organic acids, the peroxidation, chlorination, and nitration activities of MPO were tested by adding various organic acid concentrations. For peroxidation activity, the working concentrations were specified as 0–0.2 mM for oxalic acid (OA); 0–5 mM for fumaric (FA) acid and trans-aconitic acid (TAA); 0–10 mM for tartaric acid (TA), malic acid (MA), and citric acid (CA); and 0–35 mM for succinic acid (SA). The working concentrations for chlorination activity were specified as 0–4 mM for oxalic acid, 0–5 mM for fumaric acid, 0–8 for malic acid, 0–10 mM for tartaric acid and trans-aconitic acid, 0–15 mM for succinic acid, and 0–20 for citric acid. The working concentrations for nitration activity were specified as 0–1 mM for oxalic acid; 0–1.5 mM for fumaric acid, tartaric acid, trans-aconitic acid, and citric acid; 0–25 mM for succinic acid; and 0–5 for malic acid. For each organic acid 10 different reaction mixtures containing various previously specified concentrations of organic acids were prepared. All experiments were performed in triplicate. The maximum inhibition rate and maximum effective inhibitor concentration were determined from enzyme velocity (V) vs. inhibitor concentrations ([I]) plots. IC50 values were determined from V vs. Log[I] plots.
Statistical analyses
One-way and two-way ANOVA were used for analysis of the data. A p-value <0.05 indicated a significant difference. To compare the data with each other Bonferroni post hoc tests were used.
Results
The results of the experiments show that all the organic acids reduced MPO peroxidation, chlorination, and nitration activities in different ratios. Oxalic acid had the strongest antioxidant activity against all peroxidation, chlorination, and nitration activities of MPO. The other organic acids had approximately the same effects on the peroxidation, chlorination, and nitration activities of MPO.
Effect on peroxidation activity
There were significant differences between the activities of enzyme solutions with 0 and 4 mM organic acid contents in all types of organic acids tested (Figure 1). Comparison of the IC50 values showed that there were significant differences between the effects of oxalic acid and those of the other organic acids on MPO activity. No differences were found between effect of citric acid, fumaric acid, malic acid, and tartaric acid; there were no significant differences between succinic acid and trans-aconitic acid but both had significant differences with the other organic acids (Figure 3). However, no significant differences were found between 1, 2, and 4 mM concentrations of oxalic acid because of the potent inhibitory effect of this acid at lower concentrations (Figure 1). Further tests showed significant differences in µM concentrations (Figure 2).

Effect of different concentrations of organic acids on the peroxidation activity of MPO. The same concentrations were used to compare the effect of various organic acids. The p-values between 0 and 4 mM in all organic acids were <0.050, between the same concentrations of OA with other organic acids were <0.01, between TAA and the others were <0.05, and between all the others were >0.05.
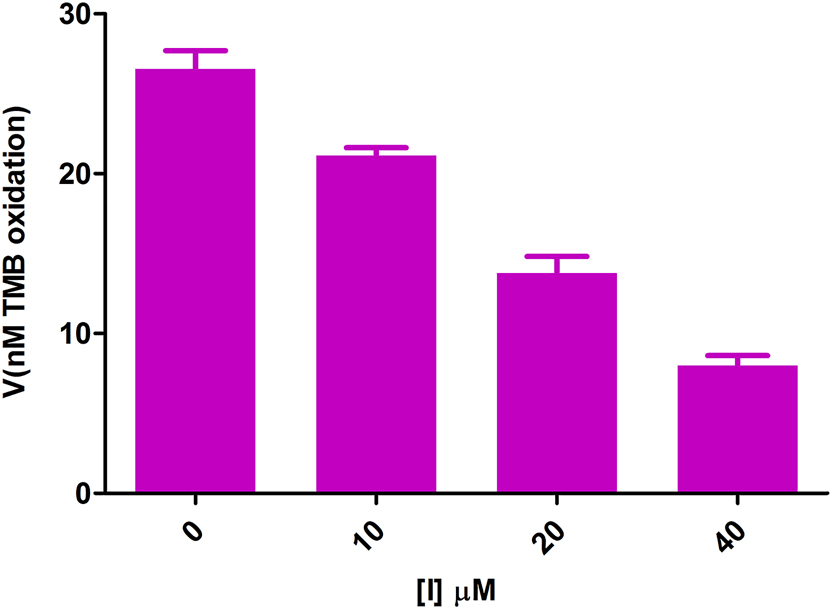
Effect of µM concentrations of oxalic acid on the peroxidation activity of MPO. p-values are <0.05 between all datasets.

Comparison of IC50 values of different organic acids for peroxidation activity of MPO. The p-values between OA and the others were <0.001, between SA and the others (except OA) were <0.05, and between CA, FA, MA, TA, and TAA were >0.05.
Effect on chlorination activity
All the organic acids studied inhibited MPO chlorination activity in different ratios. The differences between inhibition rates in different concentrations were significant for all organic acids except for 1 and 2 mM of citric acid (Figure 4). Oxalic acid was the strongest inhibitor of chlorination activity and citric acid the weakest. There were significant differences between the inhibitory effects of oxalic acid and citric acid with each other and the other organic acids. No differences were found between the other organic acids’ inhibitory effects (Figure 5).

Effect of different concentrations of organic acids on the chlorination activity of MPO. The same concentrations were used to compare the effect of various organic acids. The p-values between 0 and 4 mM in all organic acids were <0.050, between the same concentrations of OA with other organic acids were <0.05, between citric acid and the others were <0.05, and between all others were >0.05.

Comparison of IC50 values of different organic acids for chlorination activity of MPO. The p-values between OA and the others were <0.05, between CA and the others were <0.05, and between FA, MA, SA, TA, and TAA were >0.05.
Effect on nitration activity
The inhibitory effect of all the organic acids studied on MPO chlorination activity was significant. The differences in enzyme activity between different exposed concentrations of inhibitors in all seven organic acids were also significant (Figure 6). By comparison of IC50 values, oxalic acid was found to be the strongest inhibitor of nitration activity and succinic acid the weakest. Oxalic acid and fumaric acid showed significant differences in activities with each other and the other organic acids. No differences were found between the other organic acids’ activities (Figure 7).

Effect of different concentrations of organic acids on the nitration activity of MPO. The same concentrations were used to compare the effect of various organic acids. The p-values between 0 and 0.8 mM in all organic acids were <0.050; between the same concentrations of SA with other organic acids except MA were >0.05; between MA and OA, FA, and TA were <0.05; and between all the others were >0.05.

Comparison of IC50 values of different organic acids for the nitration activity of MPO. The p-values between OA and CA, MA, SA, and TAA were <0.05; between FA and CA, MA, SA, and TAA were <0.05; between MA and SA was <0.05; and between the other couples were >0.05.
Discussion
The results of our investigation on the inhibitory effect of organic acids on the activities of human neutrophil myeloperoxidase indicate that the organic acids were suitable inhibitors for MPO. Comparison of the inhibition parameters (Table 1, Supplementary Material) shows that for all three activities oxalic acid is the most powerful inhibitor and low IC50 values in all experiments identify it as a strong candidate for further research. Although all organic acids are metal chelating agents, the high chelating power of oxalic acid, especially with calcium, probably made it the most powerful inhibitor among the tested organic acids [33].
Inhibition parameters of MPO inhibition by organic acids.
| Organic acid sample | Peroxidation | Chlorination | Nitration | ||||||
|---|---|---|---|---|---|---|---|---|---|
| IC50, mM | MIR, % | MEIC, mM | IC50, mM | MIR, % | MEIC, mM | IC50, mM | MIR, % | MEIC, mM | |
| Citric acid | 0.9256 ± 0.103 | 97.67 ± 2.0867 | 6.745 | 4.517 ± 0.134 | 85.49 ± 2.11 | 22.20 | 0.2516 ± 0.016 | 88.68 ± 0.174 | 1.192 |
| Fumaric acid | 0.4601 ± 0.0402 | 96.69 ± 1.89 | 3.0 | 1.803 ± 0.163 | 99.32 ± 0.674 | 4.8 | 0.1487 ± 0.015 | 82.86 ± 0.919 | 1.10 |
| Malic acid | 1.165 ± 0.252 | 79.44 ± 1.19 | 5.856 | 2.188 ± 0.093 | 99.95 ± 0.100 | 7.0 | 0.3279 ± 0.037 | 77.979 ± 1.120 | 1.864 |
| Oxalic acid | 0.0259 ± 0.003 | 93.30 ± 0.392 | 0.172 | 0.408 ± 0.123 | 90.76 ± 0.971 | 3.7 | 0.1660 ± 0.020 | 91.037 ± 0.380 | 0.923 |
| Succinic acid | 5.124 ± 0.466 | 90.62 ± 0.251 | 26.47 | 2.048 ± 0.128 | 99.9 ± 0.001 | 15.0 | 0.3825 ± 0.046 | 82.37 ± 0.768 | 1.96 |
| Tartaric acid | 1.084 ± 0.195 | 96.68 ± 0.271 | 5.77 | 1.693 ± 0.311 | 99.68 ± 2.32 | 8.0 | 0.2063 ± 0.025 | 87.12 ± 0.304 | 1.00 |
| Trans-aconitic acid | 0.3839 ± 0.0475 | 97.69 ± 0.111 | 3.40 | 1.348 ± 0.248 | 97.40 ± 0.319 | 8.82 | 0.2430 ± 0.037 | 89.15 ± 0.618 | 1.045 |
-
The maximum inhibition rate (MIR) demonstrates the inhibition rate that could be achieved practically and has been defined from the plateau at the bottom of the V vs. [I] plot and the maximum effective inhibitor concentration (MEIC) is the concentration of inhibitor at the beginning of this plateau.
The similarity of the organic acids’ action between peroxidation and nitration activities in comparison with chlorination activity shows that in the presence of chloride ions organic acids act as weak inhibitors of MPO chlorination activity. In contrast, in the absence of halogens they react as potent inhibitors for the peroxidation and nitration activities of MPO. Analysis of MPO’s reaction cycle leads to the conclusion that organic acids are good inhibitors of either MPO compound I or compound II but not native MPO.
Conclusions
A series of pathological conditions are associated with overexpressed and free MPO in the circulatory system. MPO mediates the oxidation of nucleic acids, proteins, and lipids, resulting in tissue injury, and is the cause of an array of diseases from cardiovascular to neurological disorders and cancer. MPO mediates the oxidation of biomolecules directly via its peroxidation activity or by generating hypochlorous acid, which is the product of MPO chlorination activity. MPO also nitrates tyrosine amino acids of proteins to form 3-nitrotyrosine, resulting in alterations in the function and stability of these proteins. Overexpression of this enzyme after inflammatory conditions usually caused biological damage and scientists are investigating potent but nontoxic inhibitors of it.
Organic acids have been described as antioxidant ingredients in fruits and vegetables in recent years [34], [35], [36]. Pereira et al. [26] identified the organic acids as weak antioxidants. Kayashima and Katayama [37] have shown the effect of oxalic and malic acids on reducing lipid peroxidation levels. In the present study, I demonstrated the effect of organic acids on myeloperoxidase peroxidation, chlorination, and nitration activities in different ratios in vitro conditions. The results of the present study showed that the existence of organic acids reduces the activity of MPO. With the antioxidant nature of organic acids, the reduction could be the result of radical scavenger activity of organic acids that alter the oxidative condition of media or direct inhibition of MPO. The scarcity of studies in this field is the most important limitation of the present study since there is not enough literature to allow comparisons with the results of this study. More in vitro and in vivo studies are needed to determine the mechanism of action and effect of different organic acids on MPO in vivo conditions. Since MPO is a key component in oxidative stress and its activities are the main factors in oxidative stress-induced tissue damage in inflammatory conditions, I suggest that organic acids could be potential inhibitors of MPO and described as anti-oxidative stress and anti-inflammatory agents in the prevention and treatment of related diseases. This preliminary study could be a useful source and starting point for further investigations in the field of the effect of organic acids on inflammatory diseases and as MPO inhibitors.
Funding source: Scientific Research Projects Department of Bolu Abant Izzet Baysal University
Award Identifier / Grant number: 2019.31.01.1412
-
Research funding: This work was supported by the Scientific Research Projects Department of Bolu Abant Izzet Baysal University (grant numbers 2019.31.01.1412). The funding organization played no role in the study design; in the collection, analysis, and interpretation of data; in writing of the report; or in the decision to submit report for publication.
-
Author contributions: Author has accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Author states no conflict of interest.
-
Informed consent: Not applicable.
-
Ethical approval: Not applicable.
References
1. Hampton, MB, Kettle, AJ, Winterbourn, CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood Am Soc Hematol 1998;92:3007–17. https://doi.org/10.1182/blood.V92.9.3007.Search in Google Scholar
2. Klebanoff, SJ. Myeloperoxidase: friend and foe. J Leukoc Biol 2005;77:598–625. https://doi.org/10.1189/jlb.1204697.Search in Google Scholar PubMed
3. Malle, E, Marsche, G, Arnhold, J, Davies, MJ. Modification of low-density lipoprotein by myeloperoxidase-derived oxidants and reagent hypochlorous acid. Biochim Biophys Acta Mol Cell Biol Lipids 2006;1761:392–415. https://doi.org/10.1016/j.bbalip.2006.03.024.Search in Google Scholar PubMed
4. Nicholls, SJ, Hazen, SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol 2005;25:1102–11. https://doi.org/10.1161/01.atv.0000163262.83456.6d.Search in Google Scholar
5. Mika, D, Guruvayoorappan, C. Myeloperoxidase: the yin and yang in tumour progression. J Exp Ther Oncol 2011;9:93–100.Search in Google Scholar
6. Ray, RS, Katyal, A. Myeloperoxidase: bridging the gap in neurodegeneration. Neuroscie Biobehav Rev 2016;68:611–20. https://doi.org/10.1016/j.neubiorev.2016.06.031.Search in Google Scholar PubMed
7. Polizzi, A, Torrisi, S, Santonocito, S, Di Stefano, M, Indelicato, F, Lo Giudice, A. Influence of myeloperoxidase levels on periodontal disease: an applied clinical study. Appl Sci 2020;10:1037. https://doi.org/10.3390/app10031037.Search in Google Scholar
8. Reynolds, WF, Patel, K, Pianko, S, Blatt, LM, Nicholas, JJ, Mchutchison, JG. A genotypic association implicates myeloperoxidase in the progression of hepatic fibrosis in chronic hepatitis C virus infection. Genes Immun 2002;3:345–9. https://doi.org/10.1038/sj.gene.6363880.Search in Google Scholar PubMed
9. Rutgers, A, Heeringa, P, Cohen Tervaert, JW. The role of myeloperoxidase in the pathogenesis of systemic vasculitis. Clin Exp Rheumatol 2003;21.Search in Google Scholar
10. Malle, E, Buch, T, Grone, HJ. Myeloperoxidase in kidney disease. Kidney Int 2003;64:1956–67. https://doi.org/10.1046/j.1523-1755.2003.00336.x.Search in Google Scholar PubMed
11. Saumya, M, Subin, EK, Suchithra, TV. Network analysis of MPO and other relevant proteins involved in diabetic foot ulcer and other diabetic complications. Interdiscip Sci Comput Life Sci 2019;11:180–90. https://doi.org/10.1007/s12539-017-0258-z.Search in Google Scholar PubMed
12. Matthijsen, RA, Huugen, D, Hoebers, NT, de Vries, B, Peutz-Kootstra, CJ, Aratani, Y, et al.. Myeloperoxidase is critically involved in the induction of organ damage after renal ischemia reperfusion. Am J Pathol 2007;171:1743. https://doi.org/10.2353/ajpath.2007.070184.Search in Google Scholar PubMed PubMed Central
13. Aratani, Y. Myeloperoxidase: its role for host defense, inflammation, and neutrophil function. Arch Biochem Biophys 2018;640:47–52. https://doi.org/10.1016/j.abb.2018.01.004.Search in Google Scholar PubMed
14. Hansberry, DR, Shah, K, Agarwal, P, Agarwal, N. Fecal myeloperoxidase as a biomarker for inflammatory bowel disease. Cureus 2017;9:e1004. https://doi.org/10.7759/cureus.1004.Search in Google Scholar PubMed PubMed Central
15. Zhu, A, Ge, D, Zhang, J, Teng, Y, Yuan, C, Huang, M, et al.. Sputum myeloperoxidase in chronic obstructive pulmonary disease. Eur J Med Res 2014;19:12. https://doi.org/10.1186/2047-783x-19-12.Search in Google Scholar PubMed PubMed Central
16. Haegens, A, van der Vliet, A, Butnor, KJ, Heintz, N, Taatjes, D, Hemenway, D, et al.. Asbestos-induced lung inflammation and epithelial cell proliferation are altered in myeloperoxidase-null mice. Cancer Res 2005;65:9670–7. https://doi.org/10.1158/0008-5472.can-05-1751.Search in Google Scholar
17. van der Vliet, A, Nguyen, MN, Shigenaga, MK, Eiserich, JP, Marelich, GP, Cross, CE. Myeloperoxidase and protein oxidation in cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 2000;279:L537–46. https://doi.org/10.1152/ajplung.2000.279.3.l537.Search in Google Scholar PubMed
18. Sugamata, R, Dobashi, H, Nagao, T, Yamamoto, K, Nakajima, N, Sato, Y, et al.. Contribution of neutrophil-derived myeloperoxidase in the early phase of fulminant acute respiratory distress syndrome induced by influenza virus infection. Microbiol Immunol 2012;56:171–82. https://doi.org/10.1111/j.1348-0421.2011.00424.x.Search in Google Scholar PubMed
19. Milla, C, Yang, S, Cornfield, DN, Brennan, M-L, Hazen, SL, Panoskaltsis-Mortari, A, et al.. Myeloperoxidase deficiency enhances inflammation after allogeneic marrow transplantation. 2004;287:706–14. https://journals.physiology.org/doi.org/101152/ajplung000152004.10.1152/ajplung.00015.2004Search in Google Scholar PubMed
20. Ahlberg, G, Eriksson, H. Combinations containing MPO inhibitors against neuroinflammatory disorders. WO/2009/025617, 2009.Search in Google Scholar
21. Roth Flach, RJ, Su, C, Bollinger, E, Cortes, C, Robertson, AW, Opsahl, AC, et al.. Myeloperoxidase inhibition in mice alters atherosclerotic lesion composition. PLoS One 2019;14:1–14. https://doi.org/10.1371/journal.pone.0214150.Search in Google Scholar PubMed PubMed Central
22. Momić, T, Vujčić, Z, Vasić, V. Kinetics of inhibition of peroxidase activity of myeloperoxidase by quercetin. Int J Chem Kinet 2008;40:384–94. https://doi.org/10.1002/kin.20319.Search in Google Scholar
23. Kohnen, S, Franck, T, Van Antwerpen, P, Boudjeltia, KZ, Mouithys-Mickalad, A, Deby, C, et al.. Resveratrol inhibits the activity of equine neutrophil myeloperoxidase by a direct interaction with the enzyme. J Agric Food Chem 2007;55:8080–7. https://doi.org/10.1021/jf071741n.Search in Google Scholar PubMed
24. Meotti, FC, Senthilmohan, R, Harwood, DT, Missau, FC, Pizzolatti, MG, Kettle, AJ. Myricitrin as a substrate and inhibitor of myeloperoxidase: implications for the pharmacological effects of flavonoids. Free Radic Biol Med 2008;44:109–20. https://doi.org/10.1016/j.freeradbiomed.2007.09.017.Search in Google Scholar PubMed
25. Schewe, T, Sies, H. Myeloperoxidase-induced lipid peroxidation of LDL in the presence of nitrite. Protection by cocoa flavanols. Biofactors 2005;24:49–58. https://doi.org/10.1002/biof.5520240106.Search in Google Scholar PubMed
26. Pereira, DM, Valentão, P, Andrade, PB. Organic acids of plants and mushrooms: are they antioxidants? Funct Plant Sci Biotechnol 2009;3:103–13.Search in Google Scholar
27. Tang, X, Liu, J, Dong, W, Li, P, Li, L, Lin, C, et al.. The cardioprotective effects of citric acid and L-malic acid on myocardial ischemia/reperfusion injury. Evid base Compl Alternative Med 2013;2013. https://doi.org/10.1155/2013/820695.Search in Google Scholar PubMed PubMed Central
28. Saita, E, Kondo, K, Momiyama, Y. Anti-inflammatory diet for atherosclerosis and coronary artery disease: antioxidant foods. Clin Med Insights Cardiol 2014;8:61–5. http://journals.sagepub.com/doi/10.4137/CMC.S17071.10.4137/CMC.S17071Search in Google Scholar PubMed PubMed Central
29. de Vargas, FS, Almeida, PDO, de Boleti, APA, Pereira, MM, de Souza, TP, de Vasconcellos, MC, et al.. Antioxidant activity and peroxidase inhibition of Amazonian plants extracts traditionally used as anti-inflammatory. BMC Compl Alternative Med 2016;16:1–8. https://doi.org/10.1186/s12906-016-1061-9.Search in Google Scholar PubMed PubMed Central
30. Al-Asmari, AK, Khan, AQ, Al-Qasim, AQ, Al-Yousef, Y. Ascorbic acid attenuates antineoplastic drug 5-fluorouracil induced gastrointestinal toxicity in rats by modulating the expression of inflammatory mediators. Toxicol Rep 2015;2:908–16. https://doi.org/10.1016/j.toxrep.2015.06.006.Search in Google Scholar PubMed PubMed Central
31. Suzuki, K, Ota, H, Sasagawa, S, Sakatani, T, Fujikura, T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal Biochem 1983;132:345–52. https://doi.org/10.1016/0003-2697(83)90019-2.Search in Google Scholar PubMed
32. Aissat, S, Benbarek, H, Franck, T, Kohnen, S, Serteyn, D, Ahmed, M, et al.. Effect of honey on oxidation, chlorination and nitration by purified equine myeloperoxidase. Medicine 2017;5:398–402. https://doi.org/10.12980/jclm.5.2017J7-84.Search in Google Scholar
33. Çalişkan, M. The metabolism of oxalic acid. Turk J Zool 2000;24:103–6.Search in Google Scholar
34. Zitouni, H, Hssaini, L, Ouaabou, R, Viuda-Martos, M, Hernández, F, Ercisli, S, et al.. Exploring antioxidant activity, organic acid, and phenolic composition in strawberry tree fruits (arbutus unedo L.) growing in Morocco. Plants 2020;9:1–24. https://doi.org/10.3390/plants9121677.Search in Google Scholar PubMed PubMed Central
35. Liu, Q, Tang, G-Y, Zhao, C-N, Gan, R-Y, Li, H-B. Antioxidant activities, phenolic profiles, and organic acid contents of fruit vinegars. Antioxidants 2019;8:78. https://doi.org/10.3390/antiox8040078.Search in Google Scholar PubMed PubMed Central
36. Zafra-Rojas, Q, Cruz-Cansino, N, Delgadillo-Ramírez, A, Alanís-García, E, Añorve-Morga, J, Quintero-Lira, A, et al.. Organic acids, antioxidants, and dietary fiber of Mexican blackberry (rubus fruticosus) residues cv. Tupy. J Food Qual 2018;2018:5950761. https://doi.org/10.1155/2018/5950761.Search in Google Scholar
37. Kayashima, T, Katayama, T. Oxalic acid is available as a natural antioxidant in some systems. Biochim Biophys Acta Gen Subj 2002;1573:1–3. https://doi.org/10.1016/s0304-4165(02)00338-0.Search in Google Scholar PubMed
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/tjb-2022-0260).
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review
- Exploring nanotechnology-based approaches using miRNAs to treat neurodegenerative disorders
- Research Articles
- Rhesus factor is a stronger predictor for the risk of Sars-CoV-2 and mortality than ABO blood types
- Clinical laboratory testing in the emergency department: a six-year analysis
- New data for endemic Phlomis brevibracteata Turrill from North Cyprus: biological activities and chemical composition
- Inhibitory effect of organic acids on human neutrophil myeloperoxidase’s peroxidation, chlorination, and nitration activities
- Prevalence and association of sIgA in saliva and Pseudomonas aeruginosa infection in TB patients: a cross-sectional study
- Within- and between-subject biological variation of hemostasis parameters in a study of 26 healthy individuals
- Nasal fluid sample as a reliable matrix for determination of cytokine levels in childhood asthma
- Evaluation of the monocyte-to-lymphocyte ratio (MLR) and C-reactive protein (CRP) as diagnostic biomarkers in different lung diseases, especially for SCLC
- The association between plasma concentration of pigment epithelium-derived factor and diabetic retinopathy
- Can preoperative neopterin levels predict acute kidney injury in patients undergoing on-pump cardiac surgery?
- Exosomal prognostic biomarkers predict metastatic progression and survival in breast cancer patients
- miR-145-5p suppresses cell proliferation by targeting IGF1R and NRAS genes in multiple myeloma cells
- miR-564 and miR-718 expressions are downregulated in colorectal cancer tissues
- Ischemic cerebrovascular disease caused by genetic mutation and patent foramen ovale
- Comprehensive geriatric assessment and drug burden in elderly chronic kidney disease patients
- Exploring the enzyme inhibitory properties of Antarctic algal extracts
Articles in the same Issue
- Frontmatter
- Review
- Exploring nanotechnology-based approaches using miRNAs to treat neurodegenerative disorders
- Research Articles
- Rhesus factor is a stronger predictor for the risk of Sars-CoV-2 and mortality than ABO blood types
- Clinical laboratory testing in the emergency department: a six-year analysis
- New data for endemic Phlomis brevibracteata Turrill from North Cyprus: biological activities and chemical composition
- Inhibitory effect of organic acids on human neutrophil myeloperoxidase’s peroxidation, chlorination, and nitration activities
- Prevalence and association of sIgA in saliva and Pseudomonas aeruginosa infection in TB patients: a cross-sectional study
- Within- and between-subject biological variation of hemostasis parameters in a study of 26 healthy individuals
- Nasal fluid sample as a reliable matrix for determination of cytokine levels in childhood asthma
- Evaluation of the monocyte-to-lymphocyte ratio (MLR) and C-reactive protein (CRP) as diagnostic biomarkers in different lung diseases, especially for SCLC
- The association between plasma concentration of pigment epithelium-derived factor and diabetic retinopathy
- Can preoperative neopterin levels predict acute kidney injury in patients undergoing on-pump cardiac surgery?
- Exosomal prognostic biomarkers predict metastatic progression and survival in breast cancer patients
- miR-145-5p suppresses cell proliferation by targeting IGF1R and NRAS genes in multiple myeloma cells
- miR-564 and miR-718 expressions are downregulated in colorectal cancer tissues
- Ischemic cerebrovascular disease caused by genetic mutation and patent foramen ovale
- Comprehensive geriatric assessment and drug burden in elderly chronic kidney disease patients
- Exploring the enzyme inhibitory properties of Antarctic algal extracts

