Abstract
Objectives
Static mechanical allodynia (SMA), i. e., pain caused by normally non-painful static pressure, is a prevalent manifestation of neuropathic pain (NP). Although SMA may significantly affect the patient’s daily life, it is less well studied in the clinical context. We aimed to characterize SMA in women with chronic post-surgical NP (CPSNP) after breast cancer surgery. Our objective was to improve understanding of the clinical picture of this prevalent pain condition. This is a substudy of a previously published larger cohort of patients with intercostobrachial nerve injury after breast cancer surgery (Mustonen et al. Pain. 2019;160:246–56).
Methods
We studied SMA in 132 patients with CPSNP after breast cancer surgery. The presence, location, and intensity of SMA were assessed at clinical sensory examination. The patients gave self-reports of pain with the Brief Pain Inventory (BPI). We studied the association of SMA to type of surgery, oncological treatments, BMI, other pains, and psychological factors. General pain sensitivity was assessed by the cold pressor test.
Results
SMA was prevalent (84%) in this cohort whereas other forms of allodynia were scarce (6%). Moderate-to-severe SMA was frequently observed even in patients who reported mild pain in BPI. Breast and the side of chest were the most common locations of SMA. SMA was associated with breast surgery type, but not with psychological factors. Severe SMA, but not self-reported pain, was associated with lower cold pain tolerance.
Conclusions
SMA is prevalent in post-surgical NP after breast cancer surgery and it may represent a distinct NP phenotype. High intensities of SMA may signal the presence of central sensitization.
Implications
SMA should be considered when examining and treating patients with post-surgical NP after breast cancer surgery.
Introduction
Neuropathic pain (NP) is a predominant component in chronic post-surgical pain [1]. With a prevalence ranging from 14–31%, chronic post-surgical NP (CPSNP) is especially common after breast cancer surgery and may persist for several years [1], [2], [3]. Compared with other types of chronic pain, chronic NP is often more burdensome to patients and leads to decreased quality of life [4], [5].
The symptoms of NP are highly variable in terms of both sensory qualities and pain descriptors. Patients may experience spontaneous or evoked pain, or a combination of these [6], [7]. Different pain types and sensory alterations may involve distinct pathophysiological mechanisms [8], [9], [10]. Recent attempts in NP research have focused on identifying patient subsets who may respond differently to treatments [7], [8], [11], [12].
Allodynia is a type of evoked pain where pain is elicited by a normally non-painful stimulus. Different forms of allodynia are prevalent among NP patients [13]. Static mechanical allodynia (SMA), refers to pain evoked by a normally innocuous sustained blunt pressure stimulus. The prevalence of pressure-evoked pain was 52% in a study of NP patients with various etiologies [14]. SMA is likely mediated by sensitized C-fibers and possibly A-delta fibers, and its pathophysiology may differ from other forms of allodynia (e. g. punctate or dynamic mechanical allodynia) [13], [15]. Both peripheral and central sensitization mechanisms may contribute to the pathophysiology of SMA [16], [17].
The clinical impact of SMA has remained less well studied [13]. We have previously reported that pain evoked by static pressure was highly prevalent among patients with CPSNP after breast cancer surgery and that it could be clearly demonstrated during clinical examination [3]. Similarly, another study on chronic post-surgical pain after breast cancer operations reported lower thresholds for pressure pain at the painful surgical area [18]. These results suggest that SMA may play a significant role in the symptomology of CPSNP after breast cancer surgery.
In the current study, we aimed to characterize SMA in women with CPSNP after breast cancer surgery in order to assess how this pain type relates to the pain experience in CPSNP. Our objective was to thoroughly characterize the SMA phenotype by studying the prevalence, intensity and location of SMA assessed during clinical sensory examination in comparison with self-reported pain, i. e., pain reported in a pain questionnaire. We also tested how SMA associates with previously reported common risk factors of persistent post-surgical pain, including surgery type, oncological treatments, and psychological factors [19]. In addition, we tested whether SMA associates with general pain sensitivity as measured with the cold pressor test (CPT), which may reflect central sensitization [3], [20]. With these measures, we aimed to broaden the understanding of the pain experience in CPSNP, which may improve patient characterization and lead to more individualized treatments.
Methods
Patients
We studied 132 breast cancer operated patients who fulfilled the diagnostic criteria [21] of definite NP. The patients were clinically assessed on a research visit during 2014–2016, 4–9 years after surgery. Patient selection, diagnostic steps, and clinical sensory examination have previously been described in detail [3]. Sensory examination covered the whole upper body and included the following: static mechanical allodynia by finger compression, dynamic mechanical allodynia by a painter’s brush, tactile sensation by a cotton tuft, pinprick sensation by a sharp wooden cocktail stick, and cold and warm sensation by a metal roller.
After breast cancer surgery, the neuroanatomically plausible area for NP includes the operated breast and the area of intercostobrachial nerve (ICBN) innervation (axilla, upper side of the chest, lateral breast, or upper medial arm) [22].
To meet the NP grading system criteria [21], alterations in at least one sensory modality in the neuroanatomically plausible area of pain were required in the clinical examination. All patients had a surgeon-verified ICBN resection. To fulfill the criteria for definite NP, they all had pain and sensory changes in the area of ICBN innervation. The number of definite NP patients assessed in our previous study was 135, but we excluded three patients from the current analysis due to missing data in the intensity of SMA.
Pain measurements
We use the terms SMA (static mechanical allodynia) [23] for pain evoked by light static pressure by finger compression at clinical sensory examination and “self-reported pain” for pain reported by the patients in the pain questionnaire Brief Pain Inventory (BPI). We use the term “other pain” for pain outside the surgical and nearby area (e. g. back pain, joint pain etc.). From here on, we will use these terms to describe these different pain conditions.
We used a simple bedside test with the following standardization: first, the examiner presented the finger pressure to neutral regions such as the forehead and the sternum, so that the patient could experience a normal blunt pressure sensation. The finger pressure lasted for about 2 s, so no temporal summation was likely to develop. The pressure was light and it was performed in the same manner for all patients by the same examiner. The tip of the digit was perpendicular to the skin. SMA was tested all over the upper body in about 5 cm intervals and more densely (about 2 cm intervals) in the breast and the plausible innervation area of the ICBN.
If SMA was observed during the clinical sensory examination, patients rated the pain intensity by Numerical Rating Scale (NRS 0–10). For the self-reported pain intensity, the patients were asked to rate the worst pain in the neuroanatomically plausible area during the past week with a NRS 0–10 by using the BPI [24], [25]. The patients reported the intensity of other pains similarly, the worst pain intensity by BPI during past week with NRS 0–10. The 11-point NRS is a widely-used tool to assess pain intensity in chronic pain patients, including those with chronic NP [26], [27], [28]. The anchors used in both ratings were 0 for “no pain” and 10 for “the worst imaginable pain”. We considered NRS 1–3 as mild, NRS 4–6 as moderate and NRS 7–10 as severe pain [29], [30]. The clinical examiner was blinded to the BPI reports and the ICBN-status (total or partial resection) of the patients [3].
The clinical examiner marked the location of SMA on a upper torso body map. Pain drawings for patients were on similar type of body maps. For illustration, we divided the neuroanatomically plausible area into sections on the body map and gave number of patients reporting pain within each section.
Clinical and psychological variables
Multiple risk factors involve in chronic post-surgical pain and NP after breast cancer surgery. Although the results have varied across studies, certain patient – and treatment – related factors and psychological factors have often emerged as significant [19], [31], [32], [33], [34], [35], [36]. Based on these findings, we selected the following variables for our analysis: age; body mass index (BMI); other pains (0–10/10 NRS); type of breast surgery; type of axillary surgery; type of ICBN resection (total/partial); radiotherapy (yes/no); chemotherapy (yes/no); depressive symptoms (Hospital Anxiety and Depression Scale, HADS, total score for depression); anxiety (HADS, total score for anxiety); pain catastrophizing (Pain Catastrophizing Scale, PCS, total score). Since the follow-up time varied from 4 to 9 years, the time from surgery was also included in the analysis.
Since the studied patients are a subgroup from a previous larger prospective cohort of women treated for breast cancer [37], the information concerning surgery and breast cancer treatments was available. All patients had undergone either mastectomy or breast-conserving surgery (BCS) and axillary lymph node dissection (ALND), or sentinel lymph node biopsy (SLNB). All eligible patients (n=132) had a surgeon-verified ICBN resection during breast cancer surgery. The operating surgeon reported the type of ICBN resection (partial or total). The surgical process has been described in detail previously [3].
During the research visit, the research nurse recorded age, time from index surgery, and Body Mass Index (BMI) and the patients filled HADS and PCS questionnaires.
Cold pressor test
We performed a CPT to study sensitivity to cold pain. In the CPT, the patients immersed their contralateral (to the side of breast cancer operation) hand into circulating cold water (+2–4 °C) bath (JULABO USA Inc., Allentown, PA) up to the wrist for the maximum time tolerated but no longer than 90 s. During the CPT, patients reported pain intensity every 15 s and at the end of CPT on an NRS 0–10 by using the same anchors as for the pain in the surgical area.
Statistical analysis
We used SPSS 22.0 version for Windows (SPSS Inc., Chicago, IL., USA) to perform all statistical analyses. p-values below 0.05 were considered statistically significant. For bivariate analysis, we used Spearman’s rho (rs) for correlation of continuous variables with the SMA and self-reported pain intensity ratings. Additionally, the Mann–Whitney U-test or Kruskal–Wallis test was applied for the categorical variables. For multivariate analysis, linear regression was applied to test the association of the intensity of SMA and self-reported pain to clinical and psychological variables. We added all studied variables to one regression model to investigate the multivariate association of the variables on SMA and self-reported pain.
We used the Cox regression analysis to assess the CPT. We used time to withdrawal as the time to event. Data were right-censored if the participant endured the CPT the maximum 90 s. For the outcome variable of self-reported pain or SMA intensity, we categorized as no to low (0–3 NRS), moderate (4–6 NRS), and severe (7–10 NRS). The Cox regression models were adjusted for age, BMI, “other pain” and self-reported/SMA. Self-reported pain was used to adjust for the model of SMA, and vice versa.
Results
Patient characteristics
The demographic factors, cancer – and treatment-related factors, other pains, psychological factors and basic laboratory data of the studied patients have been described in detail previously [3]. All 132 patients had either self-reported pain, evoked pain at clinical examination, or both in the neuroanatomically plausible area. Static mechanical allodynia tested by finger compression was by far the most frequent type of evoked pain [3].
111 (84.1%) patients presented with SMA. 9/132 (6.8%) presented with some other form of allodynia or hyperalgesia at clinical sensory examination: pinprick hyperalgesia in six, dynamic mechanical allodynia in three, allodynia to light touch in two, and cold allodynia in one. All patients with other forms of allodynia also presented with SMA.
84/132 (63.6%) patients presented with both self-reported pain and SMA in the neuroanatomically plausible area. 21/132 (15.9%) patients had only self-reported pain, and 27/132 (20.5%) patients had only SMA pain (Figure 1A).

Prevalence and intensity of static mechanical allodynia and self-reported pain. (A) The prevalence and overlap of of static mechanical allodynia and self-reported pain. The Venn diagram is only illustrative and it has not been drawn to scale. (B) The overlap of no-mild (NRS 0–3) and moderate-severe (NRS 4–10) of static mechanical allodynia and self-reported pain. NRS, Numerical Rating Scale.
The overlap of self-reported pain and SMA intensities is illustrated in Figure 1B. 20/132 (15%) patients reported moderate to severe (NRS 4–10) pain in both intensity ratings and 38/132 (29%) had no more than mild pain (NRS 0–3) in both pain ratings. Only 9/132 (7%) patients had moderate to severe self-reported pain accompanied with no to mild intensity SMA. In contrast, 65/132 (49%) patients had moderate to severe SMA accompanied with no to mild self-reported pain. Only 7/132 (5%) of the patients reported severe (NRS 7–10) self-reported pain whereas SMA with severe intensity was reported by 35/132 (27%).
Location of pain in the neuroanatomically plausible area
Figure 2 illustrates the location of self-reported pain (A) and SMA (B) pain within the neuroanatomically plausible area and in the nearby areas. The neuroanatomically plausible area is marked with a dotted line.

Location of static mechanical allodynia and self-reported pain in the neuroanatomically plausible and nearby area. N=132. For clarity, right side is illustrated as the surgical side for all patients. The neuranatomically plausible area (breast and the area of ICBN innervation) is shown with a dotted line. (A) static mechanical allodynia and (B) self-reported pain.
62/132 (47%) and 70/132 (53%) of the surgeries were performed on the right and left sides, respectively. For clarity, all surgeries are shown on the right side in Figure 2.
Breast and upper side of the anterior chest wall were the most common locations for both self-reported pain and SMA (Figure 2). Self-reported pain was frequently located in the axilla: 43/132 (33%) gave self-reported pain in the anterior and 31/132 (23%) in the posterior side of axilla. SMA was less frequently found in the axilla: 16/132 (12%) patients had SMA in the anterior and 9/132 (7%) in the posterior side of axilla.
Self-reported pain was widely distributed within the neuroanatomically plausible area and the nearby areas (Figure 2). 93/132 (70%) patients self-reported pain in more than one of the locations depicted in Figure 2. In contrast, SMA was more confined within breast and the upper anterior chest wall (Figure 2). 59/132 (45%) patients had SMA pain in more than one location.
Factors associating with the intensity of static mechanical allodynia and self-reported pain
The results for the bivariate analysis and multivariate analysis (linear regression) are presented in Table 1.
Association of the intensity of static mechanical allodynia and self-reported pain to the clinical and psychological variables.
| Intensity of static mechianial allodynia (NRS 0–10) | Intensity of self-reported pain (NRS 0–10) | |||||||
|---|---|---|---|---|---|---|---|---|
| Bivariate analysis | Multivariate analysis | Bivariate analysis | Multivariate analysis | |||||
| rS or Median (IQR) | p-value | Beta | p-value | rS or Median (IQR) | p-value | t-value | p-value | |
| Static mechanical allodynia intensity (NRS 0–10) | – | – | – | – | 0.063 | 0.474 | 0.012 | 0.900 |
| Self-reported pain intensity (NRS 0–10) | 0.063 | 0.474 | 0.013 | 0.900 | – | – | – | – |
| Age (years) | 0.132 | 0.131 | −0.014 | 0.882 | 0.092 | 0.294 | 0.012 | 0.891 |
| BMI (kg/m2) | 0.190 | 0.029 | −0.009 | 0.918 | 0.046 | 0.601 | 0.022 | 0.794 |
| Time from surgery (months) | 0.005 | 0.956 | −0.048 | 0.563 | −0.084 | 0.339 | −0.077 | 0.340 |
| “Other pain” intensity (NRS 0–10)a | 0.190 | 0.040 | 0.071 | 0.509 | 0.418 | <0.001 | 0.483 | <0.001 |
| Breast surgery type | ||||||||
| BCS | 6 (4–8) | <0.001 | 0.470 | <0.001 | 2 (0–3) | 0.433 | −0.041 | 0.712 |
| Mastectomy | 3 (0–5) | [ref.] | 2 (1–4) | [ref.] | ||||
| Axillary surgery type | ||||||||
| ALND | 5 (3–7) | 0.528 | 0.122 | 0.238 | 2 (1–3) | 0.828 | 0.146 | 0.111 |
| SLNB | 4 (0–8) | [ref.] | 2 (0–3) | [ref.] | ||||
| ICBN resection type | ||||||||
| Total | 4 (2–7) | 0.748 | 0.008 | 0.926 | 2 (0–4) | 0.757 | −0.023 | 0.787 |
| Partial | 5 (3–7) | [ref.] | 2 (1–3) | [ref.] | ||||
| Radiotherapy | ||||||||
| Yes | 5 (3–7) | <0.001 | 0.084 | 0.400 | 2 (1–3) | 0.536 | −0.125 | 0.195 |
| No | 3 (0–5) | [ref.] | 2 (1–4) | [ref.] | ||||
| Chemotherapy | ||||||||
| Yes | 4 (3–7) | 0.388 | 0.011 | 0.898 | 2 (1–4) | 0.776 | 0.077 | 0.939 |
| No | 5 (4–8) | [ref.] | 2 (1–3) | [ref.] | ||||
| HADS-D, (total score)b | 0.200 | 0.022 | 0.158 | 0.266 | 0.267 | 0.002 | 0.113 | 0.412 |
| HADS-A (total score)c | 0.137 | 0.119 | −0.091 | 0.498 | 0.265 | 0.002 | 0.048 | 0.714 |
| PCS (total score) d | 0.129 | 0.146 | 0.103 | 0.307 | 0.373 | <0.001 | 0.063 | 0.516 |
| Adjusted R2 | 0.223 | 0.272 | ||||||
-
a 14 missing values.
-
b One missing value.
-
c One missing value.
-
d Four missing values.
-
p-values<0.05 are shown in bold.
-
Bivariate analysis reports the correlation for continuous variables to the intensity (0–10 NRS) of static mechanical allodynia and self-reported pain. For categorical variables, the median and IQR are reported. Due to the missing values, n=115 for linear regression.
-
ALND, axillary lymph node clearance; BMI, Body Mass Index; BCS, breast conserving surgery; HADS-A, Hospital Anxiety and Depression Scale, Anxiety; HADS-D, Hospital Anxiety and Depression Scale, Depression; IQR, interquartile range; PCS, Pain Catastrophizing Scale; rS, Spearman’s rho; SLNB, sentinel lymph node biopsy.
In the bivariate analyses, more intense other pains, higher BMI, BCS as breast surgery type, radiotherapy, and depressive symptoms were associated with higher intensities of SMA (Table 1). However, in the multivariate analysis, only the association to breast surgery type remained significant (Table 1).
In contrast to SMA, the intensity of self-reported pain did not associate with surgery – and treatment – related factors (Table 1). Higher intensities of self-reported pain were associated with “other pain” – intensity, higher psychological burden, and pain catastrophizing. However, in the linear regression model, the intensity of “other pains” overrode the effect of the psychological variables (Table 1).
Age and time from surgery showed no correlation with either SMA or self-reported pain intensities. There was no correlation between self-reported pain and SMA intensities. However, we observed a statistically-significant positive correlation (rs=0.24, p=0.026) for patients who presented with both self-reported pain and SMA (n=84).
Cold pain sensitivity
Patients with severe (NRS 7–10) SMA were significantly more sensitive to cold pain, i. e., they aborted the CPT after a shorter time than did patients with no to mild intensity SMA (Figure 3, Table 2). The association remained statistically significant even after multivariate adjustment (Table 2). Patients with moderate (NRS 4–6) SMA showed no significant increase in cold pain sensitivity (Table 2).
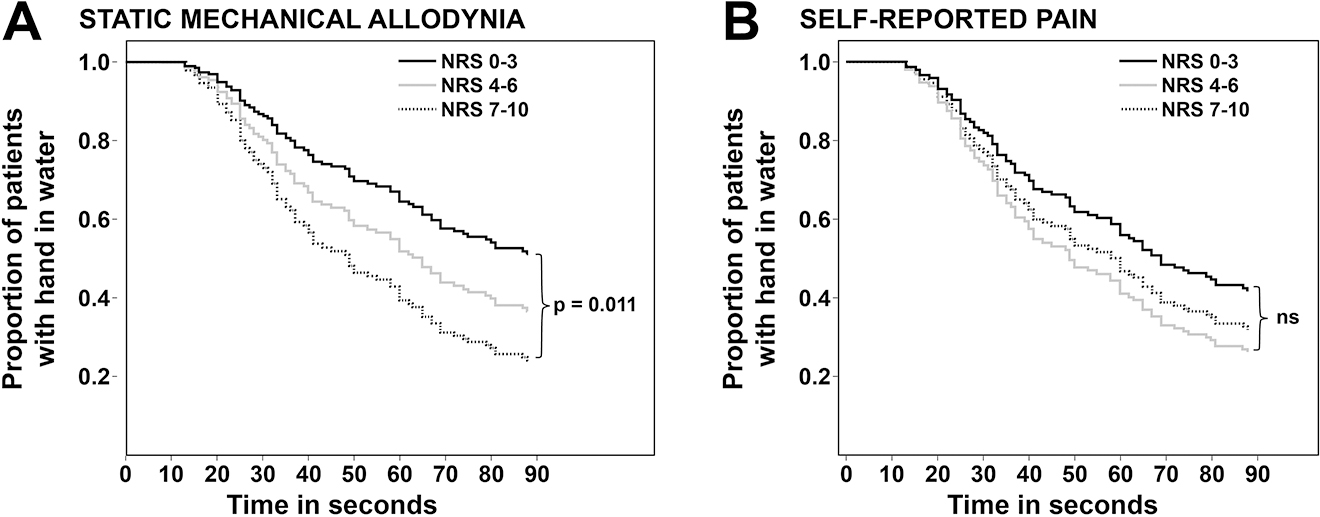
Cox regression analysis. Survival curves for cold pain tolerance for patients with no-mild (NRS 0–3), moderate (NRS 4–6), and severe (NRS 7–10) static mechanical allodynia (A) and self-reported pain (B). NRS, Numerical Rating Scale.
Cox’s regression models for cold pain tolerance: patients with no to mild (NRS 0–3), moderate (NRS 4–6), and severe (NRS 7–10) static mechanical allodynia and self-reported pain.
| n | Unadjusted model | Adjusted modela | |||||
|---|---|---|---|---|---|---|---|
| HR | CI (95%) | p-value | HR | CI (95%) | p-value | ||
| Static mechanical allodynia | |||||||
| NRS 0–3 | 47 | 1.00 | 1.00 | ||||
| NRS 4–6 | 50 | 1.49 | 0.87–2.56 | 0.15 | 1.51 | 0.85–2.65 | 0.17 |
| NRS 7–10 | 35 | 2.12 | 1.21–3.71 | 0.008 | 2.10 | 1.12–3.91 | 0.021 |
| Self-reported pain | |||||||
| NRS 0–3 | 103 | 1.00 | 1.00 | ||||
| NRS 4–6 | 22 | 1.53 | 0.87–2.70 | 0.14 | 1.59 | 0.80–3.16 | 0.19 |
| NRS 7–10 | 7 | 1.31 | 0.56–3.03 | 0.53 | 1.28 | 0.43–3.82 | 0.66 |
-
p-values < 0.05 are shown in bold.
-
aBoth models were adjusted for: age, BMI, other pain (NRS 0–10). n=118 for adjusted models. The static mechanical allodynia model was adjusted for self-reported pain and the self-reported pain model was adjusted for static mechanical allodynia.
-
BMI, Body Mass Index; CI, confidence interval; HR, hazard ratio; NRS, Numerical Rating Scale.
Patients with moderate (NRS 4–6) or severe (NRS 7–10) self-reported pain were not more sensitive to cold pain than patients with no or mild (NRS 0–3) self-reported pain (Table 2).
Discussion
In our cohort of post-surgical NP patients, the prevalence of SMA was high (84%) and overlap with self-reported pain common (64%). In contrast, the prevalence of other types of allodynia was low (6%).
Surprisingly, patients who self-reported none or mild pain anyhow presented with high intensities of SMA in the neuroanatomically plausible area. SMA was frequently confined to the areas of breast and side of chest whereas self-reported pain was more widely distributed within the operative and nearby areas.
Risk factors of post-surgical pain presented differently in SMA and self-reported pain. Higher intensities of SMA were observed in patients with BCS as the type of breast cancer surgery. Psychological factors did not associate with SMA intensity in multivariable analysis. Furthermore, patients with severe SMA showed increased general pain sensitivity in CPT. Overall, these results suggest that SMA represents a distinct pain type that frequently contributes to the symptomology of post-surgical NP in breast cancer survivors. It may reflect the presence of central sensitization and is not associated by psychological burden.
SMA is a common finding in NP conditions with a prevalence of 36–52% in NP of various etiologies and 51% in NP after peripheral nerve injury [14], [38], [39]. Nociceptor sensitization and altered function of spinal inhibition may play a role in SMA [13], [15], [40]. This is in line with our results linking higher intensities of SMA to central sensitization, reflected as increased cold pain sensitivity. These results may help to identify phenotypes within NP patients and in treatment strategies.
Nearly 50% of the patients in our cohort presented with moderate to severe evoked pain, but self-reported pain was only mild or none of it. This may indicate that patients may avoid even severe pain for blunt pressure in everyday life by behavioral adjustments, e. g., by avoiding pressure-provoking activities or clothing. Some of our patients shared these experiences with the examiner. Few studies have assessed the impact of SMA on daily life. In a Japanese population-based study, the report of pressure-pain in the pain detect questionnaire was associated with lower quality of life in patients with chronic NP [41]. Our results call for further studies on how different pain types affect the life of breast cancer survivors.
In the body maps, SMA was clearly more confined to the neuroanatomically plausible area compared with self-reported pain. This is in line with previous reports stating that SMA exhibits mainly in the areas of primary hyperalgesia (i. e., the area of damaged nerve endings) [13], [42]. This is in contrast to other forms of allodynia (thermal, punctate), which often spread to the areas of secondary hyperalgesia [13].
Although all patients had a surgeon-verified ICBN-resection, the breast region was also important for SMA pain especially in patients with BCS. We have previously reported that BCS associates significantly with the development of NP after ICBN resection [3]. Here, we observed that BCS associated with higher intensities of SMA, but not with self-reported pain. The reason for this is unclear. Radiotherapy could mediate the effect, since it is commonly administered after BCS. However, radiotherapy did not show significant association in multivariable analysis. All in all, our findings are in line with a recent study on persistent post-surgical pain after breast cancer surgery reporting lower pressure pain thresholds in quantitative sensory testing in patients with BCS [18]. The authors referred to the intact nociceptor hypothesis as a possible mechanistic explanation to the findings.
Perception of chronic pain is influenced by an affective component, encompassing mood and beliefs [43], [44], [45]. In line with this, anxiety, depression, and pain catastrophizing have emerged as risk factors for persistent post-surgical pain after breast cancer surgery [19]. In our study, higher intensities of self-reported pain positively associated with depressive symptoms, anxiety, and pain catastrophizing, although the association was mediated by “other pains” in our multivariate model (Table 1). In contrast, more severe SMA did not associate with anxiety or pain catastrophizing and the positive correlation to depressive symptoms was rendered non-significant in multivariable analysis. Our results are in line with a previous study [44] showing that in NP patients, psychological factors are stronger predictors for spontaneous pain than for evoked pain supporting the hypothesis of distinct pain types and psychological correlates.
Different forms of allodynia are common in NP patients [13]. For example, the prevalence of dynamic mechanical allodynia (i. e., pain caused by light brushing of skin) ranged from 12–49% in different NP etiologies in a large study based on quantitative sensory testing [38]. However, in our cohort of breast cancer treated patients with CPSNP, other forms of allodynia were scarce (6%) at clinical sensory examination. This is in line with previous studies on sensory dysfunction of post-surgical pain after breast cancer surgery [18].
Sensory profiling has gained recognition in NP research as a means to pursue more individualized and mechanism-based treatment [8], [14]. Previous clinical trials have used different forms of allodynia either to predict treatment responses in post hoc analyses [46], [47], [48], [49] or as an outcome measure [50]. However, studies that have assessed mechanical allodynia, have mainly included punctate or dynamic mechanical allodynia. These may differ mechanistically from SMA [13], [15] and are rare in our cohort of CPSNP after breast cancer surgery. Further studies are needed to assess whether SMA could be used to predict treatment responses in this patient group. For now, the pharmacological treatment of neuropathic pain with SMA is based on the general guidelines for treating neuropathic pain [51]. Patients can be advised to avoid triggers of SMA, e. g., pressure by tight clothing, and apply psychological or physiotherapeutic interventions, when needed.
Strengths and limitations of the study
Strengths of the study include a fairly large cohort of well phenotyped NP patients with extensive clinical and psychological data. The patients underwent a thorough clinical examination and were classified according to the latest NP grading system.
As limitations, our cohort consisted of women treated for breast cancer. Therefore, our results may not be generalized to men or to other traumatic nerve injuries. Secondly, we compared SMA to self-reported pain assessed by BPI, which does not distinguish spontaneous and evoked components of pain and may therefore include both components.
Conclusions
Our results show that SMA is an important component of post-surgical NP after breast cancer treatment whereas other forms of allodynia are scarce. A clinician can readily test SMA in a standard clinical setting and it may provide an easy method to characterize patients. We demonstrated that high intensities of SMA associate with general sensitivity to experimental pain and may reflect central sensitization. Our results confirm previous findings that NP consists of different pain components that may differ between individuals. This may be important to consider in individualized pain management.
Funding source: European Union FP7
Funding source: Neuro Pain
Funding source: Finnish Governmental subsidiary grant (VTR)
Acknowledgments
We thank our research nurse Eija Ruoppa for her excellent work. We are grateful to all patients who participated in the study.
-
Research funding: This study was funded by the European Union FP7 (# Health_F2-2013-602891) project Neuro Pain and Finnish Governmental subsidiary grant (VTR). The funders did not influence the design, conduct or publication of this study.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: Dr. Kalso reports personal fees from Orion Pharma, personal fees from Pfizer, personal fees from Gruenenthal, outside the submitted work. Other authors state no conflict of interest.
-
Informed consent: Informed consent has been obtained from all individuals included in this study.
-
Ethical approval: The research related to human use complies with all the relevant national regulations, institutional policies and was performed in accordance with the tenets of the Helsinki Declaration, and has been approved by the Coordinating Ethics Board of the Helsinki and Uusimaa Hospital District (149/13/03/00/14).
References
1. Haroutiunian, S, Nikolajsen, L, Finnerup, NB, Jensen, TS. The neuropathic component in persistent postsurgical pain: a systematic literature review. Pain 2013 Jan;154:95–102. https://doi.org/10.1016/j.pain.2012.09.010.Search in Google Scholar PubMed
2. Ilhan, E, Chee, E, Hush, J, Moloney, N. The prevalence of neuropathic pain is high after treatment for breast cancer: a systematic review. Pain 2017 Nov;158:2082–91. https://doi.org/10.1097/j.pain.0000000000001004.Search in Google Scholar PubMed
3. Mustonen, L, Aho, T, Harno, H, Sipila, R, Meretoja, T, Kalso, E. What makes surgical nerve injury painful? A 4-year to 9-year follow-up of patients with intercostobrachial nerve resection in women treated for breast cancer. Pain 2019 Jan;160:246–56. https://dx.doi.org/10.1097%2Fj.pain.0000000000001398.10.1097/j.pain.0000000000001398Search in Google Scholar PubMed PubMed Central
4. Attal, N, Lanteri-Minet, M, Laurent, B, Fermanian, J, Bouhassira, D. The specific disease burden of neuropathic pain: results of a French nationwide survey. Pain 2011 Dec;152:2836–43. https://doi.org/10.1016/j.pain.2011.09.014.Search in Google Scholar PubMed
5. Torrance, N, Smith, BH, Bennett, MI, Lee, AJ. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain 2006 Apr;7:281–9. https://doi.org/10.1016/j.jpain.2005.11.008.Search in Google Scholar PubMed
6. He, D, Grant, B, Holden, RR, Gilron, I. Methodology for self-report of rest pain (or spontaneous pain) vs evoked pain in chronic neuropathic conditions: a prospective observational pilot study. Pain Rep 2017 Mar 10;2:e587. https://dx.doi.org/10.1097%2FPR9.0000000000000587.10.1097/PR9.0000000000000587Search in Google Scholar PubMed PubMed Central
7. Truini, A, Garcia-Larrea, L, Cruccu, G. Reappraising neuropathic pain in humans--how symptoms help disclose mechanisms. Nat Rev Neurol 2013 Oct;9:572–82. https://doi.org/10.1038/nrneurol.2013.180.Search in Google Scholar PubMed
8. Baron, R, Maier, C, Attal, N, Binder, A, Bouhassira, D, Cruccu, G, et al. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. Pain 2017 Feb;158:261–72. https://dx.doi.org/10.1097%2Fj.pain.0000000000000753.10.1097/j.pain.0000000000000753Search in Google Scholar PubMed PubMed Central
9. von Hehn, CA, Baron, R, Woolf, CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 2012 Feb 23;73:638–52. https://doi.org/10.1016/j.neuron.2012.02.008.Search in Google Scholar PubMed PubMed Central
10. Woolf, CJ, Mannion, RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet 1999 Jun 5;353:1959–64. https://doi.org/10.1016/S0140-6736(99)01307-0.Search in Google Scholar PubMed
11. Baron, R, Binder, A, Wasner, G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 2010 Aug;9:807–19. https://doi.org/10.1016/S1474-4422(10)70143-5.Search in Google Scholar PubMed
12. Demant, DT, Lund, K, Vollert, J, Maier, C, Segerdahl, M, Finnerup, NB, et al. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double-blind, placebo-controlled phenotype-stratified study. Pain 2014 Nov;155:2263–73. https://doi.org/10.1016/j.pain.2014.08.014.Search in Google Scholar PubMed
13. Jensen, TS, Finnerup, NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol 2014 Sep;13:924–35. https://doi.org/10.1016/S1474-4422(14)70102-4.Search in Google Scholar PubMed
14. Attal, N, Fermanian, C, Fermanian, J, Lanteri-Minet, M, Alchaar, H, Bouhassira, D. Neuropathic pain: are there distinct subtypes depending on the aetiology or anatomical lesion?. Pain 2008 Aug 31;138:343–53. https://doi.org/10.1016/j.pain.2008.01.006.Search in Google Scholar PubMed
15. La, JH, Chung, JM. Peripheral afferents and spinal inhibitory system in dynamic and static mechanical allodynia. Pain 2017 Dec;158:2285–9. https://doi.org/10.1097/2Fj.pain.0000000000001055.10.1097/j.pain.0000000000001055Search in Google Scholar PubMed PubMed Central
16. Colloca, L, Ludman, T, Bouhassira, D, Baron, R, Dickenson, AH, Yarnitsky, D, et al. Neuropathic pain. Nat Rev Dis Primers 2017 Feb 16;3:17002. http://dx.doi.org/10.1038/nrdp.2017.2.10.1038/nrdp.2017.2Search in Google Scholar PubMed PubMed Central
17. Meacham, K, Shepherd, A, Mohapatra, DP, Haroutounian, S. Neuropathic pain: central vs. peripheral mechanisms. Curr Pain Headache Rep 2017 Jun;21:28-017-0629-5. https://doi.org/10.1007/s11916-017-0629-5.Search in Google Scholar PubMed
18. Andersen, KG, Duriaud, HM, Kehlet, H, Aasvang, EK. The relationship between sensory loss and persistent pain 1 year after breast cancer surgery. J Pain 2017 Sep;18:1129–38. https://doi.org/10.1016/j.jpain.2017.05.002.Search in Google Scholar PubMed
19. Tait, RC, Zoberi, K, Ferguson, M, Levenhagen, K, Luebbert, RA, Rowland, K, et al. Persistent post-mastectomy pain: risk factors and current approaches to treatment. J Pain 2018 Dec;19:1367–83. https://doi.org/10.1016/j.jpain.2018.06.002.Search in Google Scholar PubMed PubMed Central
20. Johansen, A, Schirmer, H, Stubhaug, A, Nielsen, CS. Persistent post-surgical pain and experimental pain sensitivity in the Tromso study: comorbid pain matters. Pain 2014 Feb;155:341–48. https://doi.org/10.1016/j.pain.2013.10.013.Search in Google Scholar PubMed
21. Finnerup, NB, Haroutounian, S, Kamerman, P, Baron, R, Bennett, DL, Bouhassira, D, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain 2016 Aug;157:1599–606. https://doi.org/10.1097/2Fj.pain.0000000000000492.10.1097/j.pain.0000000000000492Search in Google Scholar PubMed PubMed Central
22. Andersen, KG, Aasvang, EK, Kroman, N, Kehlet, H. Intercostobrachial nerve handling and pain after axillary lymph node dissection for breast cancer. Acta Anaesthesiol Scand 2014 Nov;58:1240–8. https://doi.org/10.1111/aas.12393.Search in Google Scholar PubMed
23. Baron, R. Peripheral neuropathic pain: from mechanisms to symptoms. Clin J Pain 2000 Jun;16(2 Suppl):S12–20. https://doi.org/10.1097/00002508-200006001-00004.Search in Google Scholar PubMed
24. Cleeland, CS, Ryan, KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singapore 1994 Mar;23:129–38.Search in Google Scholar
25. Tan, G, Jensen, MP, Thornby, JI, Shanti, BF. Validation of the brief pain inventory for chronic nonmalignant pain. J Pain 2004 Mar;5:133–7. https://doi.org/10.1016/j.jpain.2003.12.005.Search in Google Scholar PubMed
26. Farrar, JT, Young, JPJr, LaMoreaux, L, Werth, JL, Poole, RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001 Nov;94:149–58. https://doi.org/10.1016/S0304-3959(01)00349-9.Search in Google Scholar PubMed
27. Ferreira-Valente, MA, Pais-Ribeiro, JL, Jensen, MP. Validity of four pain intensity rating scales. Pain 2011 Oct;152:2399–404. https://doi.org/10.1016/j.pain.2011.07.005.Search in Google Scholar PubMed
28. Hjermstad, MJ, Fayers, PM, Haugen, DF, Caraceni, A, Hanks, GW, Loge, JH, et al. European palliative care research collaborative (EPCRC). studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage 2011 Jun;41:1073–93. https://doi.org/10.1016/j.jpainsymman.2010.08.016.Search in Google Scholar PubMed
29. Gerbershagen, HJ, Rothaug, J, Kalkman, CJ, Meissner, W. Determination of moderate-to-severe postoperative pain on the numeric rating scale: a cut-off point analysis applying four different methods. Br J Anaesth 2011 Oct;107:619–26. https://doi.org/10.1093/bja/aer195.Search in Google Scholar PubMed
30. Woo, A, Lechner, B, Fu, T, Wong, CS, Chiu, N, Lam, H, et al. Cut points for mild, moderate, and severe pain among cancer and non-cancer patients: a literature review. Ann Palliat Med 2015 Oct;4:176–83.Search in Google Scholar
31. Andersen, KG, Duriaud, HM, Jensen, HE, Kroman, N, Kehlet, H. Predictive factors for the development of persistent pain after breast cancer surgery. Pain 2015 Dec;156:2413–22. https://doi.org/10.1097/j.pain.0000000000000298.Search in Google Scholar PubMed
32. Gartner, R, Jensen, MB, Nielsen, J, Ewertz, M, Kroman, N, Kehlet, H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA 2009 Nov 11;302:1985–92. https://doi.org/10.1001/jama.2009.1568.Search in Google Scholar PubMed
33. Meretoja, TJ, Leidenius, MH, Tasmuth, T, Sipila, R, Kalso, E. Pain at 12 months after surgery for breast cancer. JAMA 2014 Jan 1;311:90–2. https://doi.org/10.1001/jama.2013.278795.Search in Google Scholar PubMed
34. Meretoja, TJ, Andersen, KG, Bruce, J, Haasio, L, Sipila, R, Scott, NW, et al. Clinical prediction model and tool for assessing risk of persistent pain after breast cancer surgery. J Clin Oncol 2017 May 20;35:1660–7. http://dx.doi.org/10.1200/JCO.2016.70.3413.10.1200/JCO.2016.70.3413Search in Google Scholar PubMed
35. Pereira, S, Fontes, F, Sonin, T, Dias, T, Fragoso, M, Castro-Lopes, J, et al. Neuropathic pain after breast cancer treatment: characterization and risk factors. J Pain Symptom Manage 2017 Dec;54:877–88. https://doi.org/10.1016/j.jpainsymman.2017.04.011.Search in Google Scholar PubMed
36. Chapman, CR, Vierck, CJ. The transition of acute postoperative pain to chronic pain: an integrative overview of research on mechanisms. J Pain 2017 Apr;18:359.e1–38. https://doi.org/10.1016/j.jpain.2016.11.004.Search in Google Scholar PubMed
37. Kaunisto, MA, Jokela, R, Tallgren, M, Kambur, O, Tikkanen, E, Tasmuth, T, et al. Pain in 1,000 women treated for breast cancer: a prospective study of pain sensitivity and postoperative pain. Anesthesiology 2013 Dec;119:1410–21. https://doi.org/10.1097/ALN.0000000000000012.Search in Google Scholar PubMed
38. Maier, C, Baron, R, Tölle, TR, Binder, A, Birbaumer, N, Birklein, F, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. PAIN 2010 September 2010;150:439–50. https://doi.org/10.1016/j.pain.2010.05.002.Search in Google Scholar PubMed
39. Schuning, J, Scherens, A, Haussleiter, IS, Schwenkreis, P, Krumova, EK, Richter, H, et al. Sensory changes and loss of intraepidermal nerve fibers in painful unilateral nerve injury. Clin J Pain 2009 Oct;25:683–90. https://doi.org/10.1097/AJP.0b013e3181a1260e.Search in Google Scholar PubMed
40. Benarroch, EE. Dorsal horn circuitry: complexity and implications for mechanisms of neuropathic pain. Neurology 2016 Lippincott Williams & Wilkins;86:1060–9. http://dx.doi.org/10.1212/WNL.0000000000002478.10.1212/WNL.0000000000002478Search in Google Scholar PubMed
41. Inoue, S, Taguchi, T, Yamashita, T, Nakamura, M, Ushida, T. The prevalence and impact of chronic neuropathic pain on daily and social life: A nationwide study in a Japanese population. Eur J Pain 2017 Apr;21:727–37. https://doi.org/10.1002/ejp.977.Search in Google Scholar PubMed PubMed Central
42. Arning, K, Baron, R. Evaluation of symptom heterogeneity in neuropathic pain using assessments of sensory functions. Neurotherapeutics 2009 Oct;6:738–48. https://doi.org/10.1016/j.nurt.2009.07.002.Search in Google Scholar PubMed PubMed Central
43. Gatchel, RJ. Comorbidity of chronic pain and mental health disorders: the biopsychosocial perspective. Am Psychol 2004 Nov;59:795–05. https://doi.org/10.1037/0003-066X.59.8.795.Search in Google Scholar PubMed
44. Sullivan, MJ, Lynch, ME, Clark, AJ. Dimensions of catastrophic thinking associated with pain experience and disability in patients with neuropathic pain conditions. Pain 2005 Feb;113:310–15. https://doi.org/10.1016/j.pain.2004.11.003.Search in Google Scholar PubMed
45. Turk, DC, Okifuji, A. Psychological factors in chronic pain: evolution and revolution. J Consult Clin Psychol 2002 Jun;70:678–90. https://doi.org/10.1037/0022-006X.70.3.678.Search in Google Scholar
46. Wasner, G, Kleinert, A, Binder, A, Schattschneider, J, Baron, R. Postherpetic neuralgia: topical lidocaine is effective in nociceptor-deprived skin. J Neurol 2005 Jun;252:677–86. https://doi.org/10.1007/s00415-005-0717-z.Search in Google Scholar PubMed
47. Finnerup, NB, Sindrup, SH, Bach, FW, Johannesen, IL, Jensen, TS. Lamotrigine in spinal cord injury pain: a randomized controlled trial. Pain 2002 Apr;96:375–83. https://doi.org/10.1016/S0304-3959(01)00484-5.Search in Google Scholar PubMed
48. Attal, N, Rouaud, J, Brasseur, L, Chauvin, M, Bouhassira, D. Systemic lidocaine in pain due to peripheral nerve injury and predictors of response. Neurology 2004 Jan 27;62:218–25. https://doi.org/10.1212/01.WNL.0000103237.62009.77.Search in Google Scholar
49. Simpson, DM, Rice, AS, Emir, B, Landen, J, Semel, D, Chew, ML, et al. A randomized, double-blind, placebo-controlled trial and open-label extension study to evaluate the efficacy and safety of pregabalin in the treatment of neuropathic pain associated with human immunodeficiency virus neuropathy. Pain 2014 Oct;155:1943–54. https://doi.org/10.1016/j.pain.2014.05.027.Search in Google Scholar PubMed
50. Cruccu, G, Nurmikko, TJ, Ernault, E, Riaz, FK, McBride, WT, Haanpaa, M. Superiority of capsaicin 8% patch versus oral pregabalin on dynamic mechanical allodynia in patients with peripheral neuropathic pain. Eur J Pain 2018 Apr;22:700–6. https://doi.org/10.1002/ejp.1155.Search in Google Scholar PubMed PubMed Central
51. Finnerup, NB, Attal, N, Haroutounian, S, McNicol, E, Baron, R, Dworkin, RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015 Feb;14:162–73. https://doi.org/10.1016/S1474-4422(14)70251-0.Search in Google Scholar PubMed PubMed Central
© 2020 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial Comment
- The history of the idea of widespread pain and its relation to fibromyalgia
- Clinical Pain Research
- Pain perception in chronic knee osteoarthritis with varying levels of pain inhibitory control: an exploratory study
- Fibromyalgia 2016 criteria and assessments: comprehensive validation in a Norwegian population
- Development and preliminary validation of the Chronic Pain Acceptance Questionnaire for Clinicians
- Static mechanical allodynia in post-surgical neuropathic pain after breast cancer treatments
- Preoperative quantitative sensory testing and robot-assisted laparoscopic hysterectomy for endometrial cancer: can chronic postoperative pain be predicted?
- Exploring the impact of pain management programme attendance on complex regional pain syndrome (CRPS) patients’ decision making regarding immunosuppressant treatment to manage their chronic pain condition
- Preliminary validity and test–retest reliability of two depression questionnaires compared with a diagnostic interview in 99 patients with chronic pain seeking specialist pain treatment
- Pain acceptance and its impact on function and symptoms in fibromyalgia
- Observational studies
- Cooled radiofrequency for the treatment of sacroiliac joint pain – impact on pain and psychometrics: a retrospective cohort study
- Pain perception during colonoscopy in relation to gender and equipment: a clinical study
- The association between initial opioid type and long-term opioid use after hip fracture surgery in elderly opioid-naïve patients
- Pain in adolescent chronic fatigue following Epstein-Barr virus infection
- Patients with shoulder pain referred to specialist care; treatment, predictors of pain and disability, emotional distress, main symptoms and sick-leave: a cohort study with a six-months follow-up
- Original Experimental
- The effect of periaqueductal gray’s metabotropic glutamate receptor subtype 8 activation on locomotor function following spinal cord injury
- Baseline pain characteristics predict pain reduction after physical therapy in women with chronic pelvic pain. Secondary analysis of data from a randomized controlled trial
- A novel clinical applicable bed-side tool for assessing conditioning pain modulation: proof-of-concept
- The acquisition and generalization of fear of touch
- Associations of neck and shoulder pain with objectively measured physical activity and sedentary time among school-aged children
- Health-related quality of life in burning mouth syndrome – a case-control study
- Stretch-induced hypoalgesia: a pilot study
- Educational Case Report
- Erector spinae plane and intra thecal opioid (ESPITO) analgesia in radical nephrectomy utilising a rooftop incision: novel alternative to thoracic epidural analgesia and systemic morphine: a case series
- Short Communication
- Above and beyond emotional suffering: the unique contribution of compassionate and uncompassionate self-responding in chronic pain
- Letter to the Editor
- Labor pain, birth experience and postpartum depression
- Reply: Response to Letter to the Editor “Labor pain, birth experience and postpartum depression”
- Corrigendum
- Corrigendum to: Are labor pain and birth experience associated with persistent pain and postpartum depression? A prospective cohort study
Articles in the same Issue
- Frontmatter
- Editorial Comment
- The history of the idea of widespread pain and its relation to fibromyalgia
- Clinical Pain Research
- Pain perception in chronic knee osteoarthritis with varying levels of pain inhibitory control: an exploratory study
- Fibromyalgia 2016 criteria and assessments: comprehensive validation in a Norwegian population
- Development and preliminary validation of the Chronic Pain Acceptance Questionnaire for Clinicians
- Static mechanical allodynia in post-surgical neuropathic pain after breast cancer treatments
- Preoperative quantitative sensory testing and robot-assisted laparoscopic hysterectomy for endometrial cancer: can chronic postoperative pain be predicted?
- Exploring the impact of pain management programme attendance on complex regional pain syndrome (CRPS) patients’ decision making regarding immunosuppressant treatment to manage their chronic pain condition
- Preliminary validity and test–retest reliability of two depression questionnaires compared with a diagnostic interview in 99 patients with chronic pain seeking specialist pain treatment
- Pain acceptance and its impact on function and symptoms in fibromyalgia
- Observational studies
- Cooled radiofrequency for the treatment of sacroiliac joint pain – impact on pain and psychometrics: a retrospective cohort study
- Pain perception during colonoscopy in relation to gender and equipment: a clinical study
- The association between initial opioid type and long-term opioid use after hip fracture surgery in elderly opioid-naïve patients
- Pain in adolescent chronic fatigue following Epstein-Barr virus infection
- Patients with shoulder pain referred to specialist care; treatment, predictors of pain and disability, emotional distress, main symptoms and sick-leave: a cohort study with a six-months follow-up
- Original Experimental
- The effect of periaqueductal gray’s metabotropic glutamate receptor subtype 8 activation on locomotor function following spinal cord injury
- Baseline pain characteristics predict pain reduction after physical therapy in women with chronic pelvic pain. Secondary analysis of data from a randomized controlled trial
- A novel clinical applicable bed-side tool for assessing conditioning pain modulation: proof-of-concept
- The acquisition and generalization of fear of touch
- Associations of neck and shoulder pain with objectively measured physical activity and sedentary time among school-aged children
- Health-related quality of life in burning mouth syndrome – a case-control study
- Stretch-induced hypoalgesia: a pilot study
- Educational Case Report
- Erector spinae plane and intra thecal opioid (ESPITO) analgesia in radical nephrectomy utilising a rooftop incision: novel alternative to thoracic epidural analgesia and systemic morphine: a case series
- Short Communication
- Above and beyond emotional suffering: the unique contribution of compassionate and uncompassionate self-responding in chronic pain
- Letter to the Editor
- Labor pain, birth experience and postpartum depression
- Reply: Response to Letter to the Editor “Labor pain, birth experience and postpartum depression”
- Corrigendum
- Corrigendum to: Are labor pain and birth experience associated with persistent pain and postpartum depression? A prospective cohort study

