Abstract
Background and aims
Pain-related fear and its subsequent generalization is key to the development and maintenance of chronic pain disability. Research has shown that pain-related fear acquired through classical conditioning generalizes following a gradient, that is, novel movements that are proprioceptively similar to the original pain-associated movement elicit more fear. Studies suggest that classical conditioning can also modulate pain and conditioned fear seems to mediate this effect. However, it remains uninvestigated whether this is also the case for generalized fear.
Methods
In a voluntary joystick movement paradigm, one movement (conditioned stimulus; CS+) was followed by pain (pain-US), and another was not (CS−). Generalization to five novel movements (generalization stimuli; GSs) with varying levels of similarity to the CSs was tested when paired with an at-pain-threshold intensity stimulus (threshold-USs). We collected self-reported fear and pain, as well as eyeblink startle responses as an additional index of conditioned fear.
Results
Results showed a fear generalization gradient in the ratings, but not in the startle measures. The data did not support the idea that fear generalization mediates spreading of pain.
Conclusions
Despite the lack of effects in the current study, this is a promising novel approach to investigate pain modulation in the context of chronic pain.
Implications
This study replicates the finding that pain-related fear spreads selectively towards movements that are proprioceptively more similar to the original pain-eliciting movement. Although results did not support the idea that such generalized fear mediates spreading of pain, the study provides a promising approach to investigate pain modulation by pain-associated movements.
1 Introduction
Increasing empirical evidence [1], [2], [3] supports the idea that pain-related fear is key to the development and maintenance of chronic pain disability [4], [5], [6]. Research has shown that classical conditioning plays an important role in the acquisition of fear of movement-related pain. For example, when an initially pain-free movement (conditioned stimulus; CS+) is associated with a painful electrocutaneous stimulus (unconditioned stimulus; pain-US), it may start to elicit pain-related fear [7], [8], [9], [10], [11]. In general, such fear is adaptive as it triggers protective and recuperative responses (e.g. escape and avoidance), which help avert further bodily harm.
Pain-related fear can spread towards novel movements based on proprioceptive similarity to the original movement associated with pain [8], [10], [11] or movements belonging to the same conceptual category (e.g. function) [12], [13], despite that they were never paired with pain (i.e. stimulus generalization). Typically, generalization gradients are observed: the greater the proprioceptive similarity between novel movements (i.e. generalization stimuli; GSs) and the original CS+, the more fear they elicit [8], [11], [14], [15]. This is an adaptive mechanism as generalization of fear enables individuals to extrapolate knowledge to similar potentially harmful stimuli without having to experience them [16]. However, excessive spreading of pain-related fear towards movements and activities that are safe and not harmful may drastically interfere with daily life. Such overgeneralization of fear may play an important role in chronic pain disability [14], [17], [18].
Accumulating evidence suggests that classical conditioning not only plays a role in the development and spreading of pain-related fear, but also influences pain itself [19], [20], [21], [22]. Madden, Bellan, and colleagues demonstrated that at-pain-threshold stimuli (threshold-USs; i.e. ambiguous stimuli that were neither clearly painful or non-painful) are experienced as painful more often when paired with a CS+, compared to a CS− [23]. Pain modulation is mediated by expectations [19], [24], [25] and emotional states such as fear [9], [22], [26]. The question remains whether similar expectancies and fear elicited by GSs may play a mediating role in experiencing threshold-USs as painful or not.
Using the Voluntary Joystick Movement (VJM) paradigm [7], differential fear conditioning was established using joystick movements as CSs and a painful electrocutaneous stimulus as pain-US. During the generalization test, novel movements gradually varying in proprioceptive similarity to the CSs (GSs) were tested when being paired with a threshold-US. We hypothesized that (1) pain-related fear and expectancy would spread selectively to GSs that are more similar to the CS+ than to those similar to the CS− (fear generalization gradient), (2) the probability of a threshold-US being judged as painful, as well as pain intensity and unpleasantness would increase when paired with GSs increasing in similarity to the CS+ (pain generalization gradient), and (3) pain-related fear would (partially) mediate the pain modulating effect of GSs, with higher levels of pain-related fear resulting in increased pain.
2 Methods
2.1 Participants
In total, 50 healthy, pain-free individuals (16 males; Mage=27, SDage=9, range=19–60 years) voluntarily participated in this study and received €10 or course credits as compensation. They were recruited using the departmental Experiment Management System (EMS; http://psykuleuven.sona-systems.com), social media, flyers and through word-of-mouth. Exclusion criteria were: pregnancy, dyslexia, current or history of heart or cardiovascular disease, neurological disease (e.g. epilepsy), presence of any other severe medical condition, current or history of psychiatric disorder (e.g. clinical depression, anxiety disorder), presence of electronic medical devices (e.g. pacemaker), chronic pain, acute pain or problem at the dominant hand or wrist, uncorrected vision or hearing problems, or physician’s advice to avoid stressful situations. A standard health checklist was used to ensure candidate participants did not meet any of the exclusion criteria.
2.2 Experimental stimuli and apparatus
An adapted version of the VJM Paradigm [7] was employed in this experiment. Proprioceptive stimuli [i.e. moving a Paccus Hawk joystick (Paccus Interfaces BV, Almere, Netherlands) in different directions using the dominant hand][1] served as CSs and GSs. The CSs were joystick movements to the left and to the right in the horizontal plane at angles of 0° and 180°. Which direction served as the CS+ or CS− was counterbalanced across participants. The GSs were five intermediate movement directions (GS1-5) between the original CS+ and CS− movements: left and right at upward angels of 30° and 60° to the horizontal plane, and a vertical movement upward at 90°. The GSs gradually differed in terms of proprioceptive and visuospatial similarity to the original CS+ and CS− with GS1 being most similar to CS+ and GS5 being most similar to CS−.
Electrocutaneous stimuli served as the painful stimulus (pain-US) and at-pain-threshold stimulus (threshold-US). These stimuli consisted of a train of electrocutaneous pulses with duration of 1000 ms (1 ms pulse/1 ms no pulse; ×500). Electrical stimulation was delivered by a Digitimer DS5 commercial constant current stimulator (Digitimer Ltd, Welwyn Garden City, UK) through surface SensorMedics electrodes (Sensor Medics Corp, Homestead, FL, USA; 1 cm diameter, inter electrode distance of approximately 1–2 cm) filled with K-Y gel. These electrodes were attached to the wrist of the dominant hand. The intensities of the pain-US and threshold-US were calibrated for each participant individually before starting the experiment using two distinct calibration procedures.
During calibration of the pain-US, participants received electrocutaneous stimuli of increasing intensity and were asked to judge whether the stimulus was merely a sensation or painful. When the stimulus was judged to be painful, the participant was asked to rate the stimulus on a 10-point scale (1=A very light pain; 10=The worst pain imaginable). Participants were told that we targeted a stimulus that is significantly painful and demanding some effort to tolerate, corresponding roughly to a rating of 8/10 on the calibration scale. However, they were instructed to notify the experimenter at any time when they did not want to receive a stimulus of higher intensity, or when they wanted the intensity to be set back at a lower level. At the end of this procedure, participants were asked whether they agreed to repeatedly receive stimuli of maximally the selected intensity during the experiment (mean physical stimulus intensity was 7.45 mA, SD=2.65, range 2.75–14.50).
During calibration of the threshold-US, an alternate up-down staircase method was employed. The procedure started with an electrocutaneous stimulus that had the intensity of the very first stimulus rated as painful during the pain-US calibration procedure. The participant was again asked to judge whether this stimulus was merely a sensation or painful. If the participant rated this first stimulus intensity as a sensation, they received electrocutaneous stimuli of increasing intensity until a stimulus was rated as painful. Next, the participant received electrocutaneous stimuli of decreasing intensity until a stimulus was rated as a sensation. If the participant rated the first stimulus intensity (i.e. the first stimulus to be rated painful during pain-US calibration) as painful, the procedure started with electrocutaneous stimuli of decreasing intensity. This procedure was repeated (starting from the last stimulus administered) until an ambiguous stimulus was found (mean physical stimulus intensity was 3.84 mA, SD=2.13, range 1.25–10.00). The aim was to select a stimulus that was rated at least once as painful and once as sensation.
2.3 Experimental setting
Participants were invited to the Health Psychology lab at the Psychological Institute of the KU Leuven and were seated in a sound-attenuated experimental room. The computer screen on which the experimental stimuli were presented was positioned at eye level, approximately 60 cm in front of the participant. The joystick was mounted on a table in front of the computer screen. In an adjacent room, the experimenter could monitor the participants and their physiological responses (i.e. eyeblink startle responses). Two-way communication was possible through an intercom system.
2.4 Procedure
The experiment was conducted during a 90-min session and consisted of a preparation phase, a practice phase (PRAC), a startle probe habituation phase (see “Measures” section), an acquisition phase (ACQ), a transfer-of-acquisition phase (TRANS) and a generalization phase (GEN) (for the experimental design, see Table 1).
Experimental design summary.
| Practicea | Startle habituation | Acquisitionb | Transfer of acquisitionb | Generalizationc |
|---|---|---|---|---|
|
|
||||
| 8 Trials | 8 Trials | 2 Blocks of 8 trials | 8 Trials | 2 Blocks of 28 trials |
| 4 CS+ | 8 Startle probes | 2×4 CS+ | 4 CS+ | 2×4 CS+ |
| 4 CS− | 2×4 CS− | 4 CS− | 2×4 GS1-5 | |
| 2×4 CS− | ||||
-
CS=conditioned stimulus; GS=generalization stimulus. aNo pain- or threshold-US presented; b75% of CS+ trials were paired with the pain-US, CS− trials were never followed by the pain- or threshold-US; c50% of CS+ trials paired with the pain-US and the other 50% with the threshold-US, 50% of GS1-5 and CS− trials paired with the threshold-US.
A trial was structured as follows: 1000 ms after the start of a trial, the mouse cursor appeared on the screen and participants were requested to put the joystick in the upright position (i.e. moving the cursor to the middle of the screen). This was done to make sure the starting position of the joystick was the same before the movement was initiated on each trial. When in the correct position, the cursor disappeared and the starting signal “+” appeared (a white fixation cross presented in the middle of the screen after the cursor disappeared; see Fig. 1). Participants were instructed to move the joystick as fast and accurately as possible when this signal was presented. When a movement was successfully completed, a pain-US or threshold-US was delivered according to the experimental contingencies (see Table 1). After an intertrial interval (ITI) of 8 s, the next trial was initiated. In order to keep track of performed movements, counter bars (each divided into 4 equal segments) were displayed on the computer screen during the experiment (see Fig. 1). The positions of the counter bars corresponded with the different movement directions. Correctly performed movements resulted in a change of color of a segment in the corresponding counter bar. This way, participants received feedback on whether they performed a movement correctly and how many movements in each direction remained to be carried out during that block.
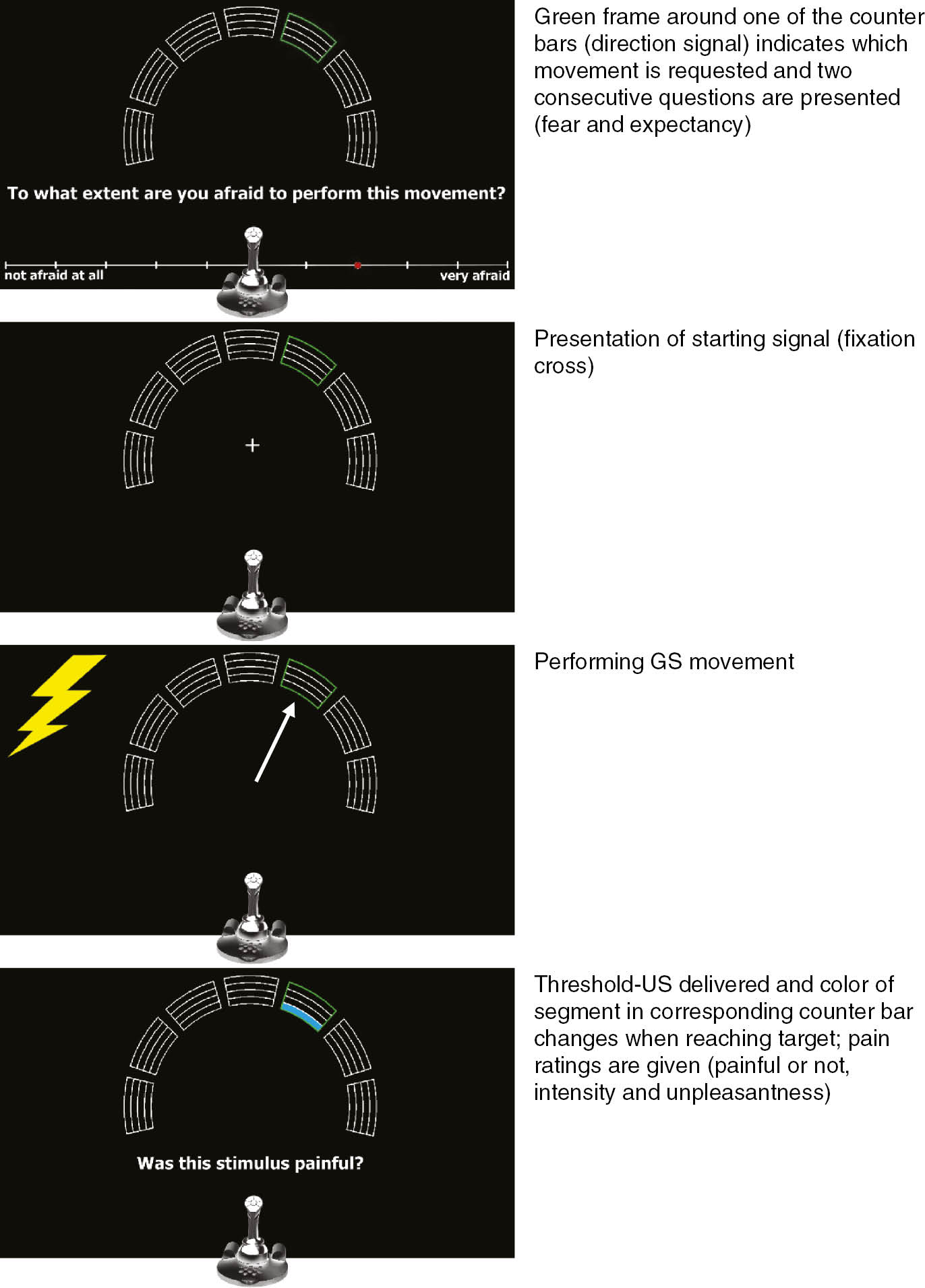
Schematic overview of the experimental task during generalization test. White arrow=movement direction; lightning bolt=presentation of pain-US or threshold-US (according to experimental contingencies). Joystick needs to be in central upright position to start trial.
2.4.1 Preparation phase
Before starting the experiment, all participants received an information sheet accompanying the informed consent form, which stated that participation was voluntary and that all gathered data would be processed and stored anonymously. Furthermore, it included a brief description of the experimental task and stated that painful but harmless electrocutaneous stimuli (pain-USs and threshold-USs) and harmless loud noises (startle probes) would be administered. It was also emphasized that they were allowed to decline participation at any time with no negative consequences and that they would receive their compensation regardless of whether they completed the experiment or not. After ensuring that participants had understood the provided information, they were asked to sign the informed consent form and complete the general health checklist. Next, the participants’ skin was peeled underneath the left eye and forehead to reduce inter-electrode resistance and electrodes were placed to measure eyeblink startle responses. Next, electrodes for delivering the pain- and threshold-USs were attached to the wrist of the dominant hand and the intensity of these stimuli was determined following the calibration procedures described in the “Experimental stimuli and apparatus” section.
2.4.2 Practice phase
First, detailed written instructions about the experimental task appeared on the computer screen. The goal of the practice phase was to familiarize participants with the procedure and use of the joystick. In total 8 movements were performed in the horizontal plane: 4 to the left and 4 to the right. Participants freely chose the order in which these movements were performed and received instant visual feedback during movements: the area in which they were allowed to move was delineated on the screen and turned green when they moved within it and red when they moved outside it. This way, participants learned what constituted a valid movement. The experimenter also provided online verbal feedback; no startle probes, pain-USs or threshold-USs were presented. At the end of the phase, participants answered questions regarding affective valence, arousal, and sense of being in control during CS movements.
2.4.3 Startle probe habituation phase
During the startle probe habituation phase, and the following phases as well, participants wore headphones and the lights were dimmed. This phase consisted of 8 trials, each lasting 13 s (with an ITI of 2 s). On each trial, a startle probe was presented between 8 and 12 s after trial-onset. The timing of the probes in this interval was randomized. The first responses to startle probes are usually relatively high; this phase was included to prevent distortions in the data (see Meulders et al. [8] for similar use of a habituation phase). No pain-USs or threshold-USs were presented during this phase.
2.4.4 Acquisition phase
The task during the acquisition phase was similar as in the practice phase. However, startle probes and pain-USs were presented, and the movement area was no longer delineated on the screen (i.e. no visual feedback through red/green color of movement area). Only the counter bars indicated whether a movement was successfully performed. This phase comprised 2 blocks of 8 trials (4 movements to the right and 4 movements to the left per block). The pain-US was presented on 75% of the CS+ trials, while CS− trials were never paired with the pain-US. Participants were not informed about this contingency. Note that no threshold-USs were delivered during this phase. During each trial, one startle probe was presented. Within each acquisition block, 4 probes occurred during movements: 2 during CS+ movements and 2 during CS− movements. These startle probes were presented 200 ms after participants started to move the joystick. The remaining 4 probes were presented during the ITI (i.e. context alone) following each trial at a random moment between 3000 and 6000 ms. After each block, participants were asked to rate the intensity and unpleasantness of the pain-US as well as their pain-related fear of both CS movements.
2.4.5 Transfer-of-acquisition phase
The procedure of this phase was very similar to the acquisition phase. The important difference was that participants no longer freely chose the order in which they performed movements. A green frame around one of the counter bars served as direction signal and indicated which movement was requested on a given trial (see Fig. 1). The phase was divided into 4 sub-blocks consisting of the 2 original CS movements (2 trials per sub-block); movements were performed in randomized order within a sub-block. Furthermore, two new questions were added: participants rated their pain-related fear and pain expectancy on each trial before performing the requested movement (after the direction signal was presented). Participants did not receive questions regarding affective valence, arousal, and sense of being in control after this phase.
2.4.6 Generalization phase
The basic procedure of the generalization phase was the same as the transfer-of-acquisition phase. The crucial differences were that participants performed 5 novel generalization movements in addition to the CS movements, and that threshold-USs were presented. Again, the direction signal indicated which of the 7 movements was requested on a given trial. The generalization phase consisted of 2 blocks each divided into 4 sub-blocks including the 2 original CSs and 5 GS movements (7 trials per sub-block); movements were again performed in randomized order within a sub-block. The threshold-US was presented in 50% of all trials (i.e. CS+, GS1-5 and CS− trials). To avoid extinction, the other 50% of CS+ trials were reinforced with the pain-US (i.e. CS+ trials were always paired with either a pain-US or threshold-US). Further differences with the previous phase were that all startle probes were presented during movements (never during the ITI) and that participants were no longer asked to rate pain intensity and unpleasantness after each block. However, when a pain-US or threshold-US occurred during a trial, participants were immediately asked to indicate whether the stimulus was painful or not. When the stimulus was perceived as painful, intensity and unpleasantness of the stimulus were rated. If the stimulus was not perceived as painful, only unpleasantness was rated. At the end of the phase, participants again answered questions regarding affective valence, arousal, and sense of being in control during CS movements.
2.5 Main outcome variables
2.5.1 Trial-by-trial pain-related fear ratings
During the transfer-of-acquisition and generalization phases, participants rated their pain-related fear on each trial using the joystick. This was done after presentation of the direction signal, but before performing the signaled movement. The following question was presented on the computer screen: “To what extent are you afraid to perform this movement?”. To answer, an 11-point Likert scale was presented underneath the question with labels “not afraid at all” and “very afraid” at the anchors.
2.5.2 Trial-by-trial pain-US expectancy ratings
Another question was presented after rating pain-related fear: “To what extent do you expect a painful electrical stimulus on your wrist after the movement you are about to perform?”. This question was answered using an 11-point Likert scale with labels “not at all” and “very much” at the anchors.
2.5.3 Eyeblink startle responses
In addition to self-reports, the eyeblink startle response was measured as a psychophysiological indicator of pain-related fear during all phases except the practice phase. This response can be triggered by startle-evoking stimuli and its amplitude will be larger when anticipating a threatening stimulus than when anticipating a neutral stimulus [27], [28]. In the present setup, the startle-evoking stimulus was a 100 dBA burst of white noise with instantaneous rise time (i.e. startle probe). This loud but harmless noise was delivered binaurally for 50 ms through Sennheiser HD 280 pro headphones (Sennheiser Belux BVBA, Asse, Belgium). During presentation of the startle probe, the electromyographic (EMG) activity of the orbicularis oculi muscles underneath the left eye was recorded as a measure of the eyeblink startle response. EMG activity was recorded using three Ag/AgCl Sensormedics electrodes (4 mm), filled with electrolyte gel: two electrodes were positioned under the left eye, another control electrode was placed on the forehead [29]. A Coulbourn isolated bioamplifier (Coulbourn instruments LLC, Holliston, MA, USA) with bandpass filter (LabLinc v75–04) was used to amplify the raw signal. The recording bandwidth of the EMG signal was between 13 Hz (low pass filter) and 500 Hz (high pass filter). The signal was rectified online and smoothed using a Coulbourn multifunction integrator (LabLinc v76–23 A) with a time constant of 20 ms. The EMG signal was digitized at 1000 Hz from 200 ms before the onset of the startle probe until 1000 ms after. Responses elicited by probes presented during the CS/GS movements served as an index of fear of movement-related, whereas responses elicited by probes during the ITI served as an index of contextual pain-related fear [10]. In the present setup, responses during ITI served as a control/baseline measure as it was assumed that contextual fear would be absent or low.
2.5.4 Pain ratings
2.5.4.1 Forced choice: painful or not?
During the generalization phase, the question “Was this stimulus painful?” was presented after each pain-US and threshold-US presentation. This forced choice question was answered by pressing one of two buttons on the joystick: the button labeled “Yes” or the one labeled “No”.
2.5.4.2 Trial-by-trial pain intensity and unpleasantness ratings
If the participant judged the stimulus to be painful on the forced choice test, the following questions were presented: “How painful did you find the electrical stimulus on your wrist?” and “How unpleasant did you find the electrical stimulus on your wrist?” These questions were answered using an 11-point Likert scale with “not painful/unpleasant at all” and “very painful/unpleasant” at the anchors. If the participant judged the stimulus not to be painful, they only rated the unpleasantness of the stimulus.
2.6 Manipulation checks
2.6.1 Retrospective pain-related fear ratings
After each block of the experiment except practice, participants rated their pain-related fear of both CS movements. The following question was presented: “To what extent were you afraid to perform the movement to the left/right?”. This question was answered using the same scale as the trial-by-trial pain-related fear ratings.
2.6.2 Retrospective pain-US intensity and unpleasantness ratings
After each block of the acquisition and transfer-of-acquisition phases, the following questions were presented: “How painful did you find the electrical stimulus on your wrist during the previous block?” and “How unpleasant did you find the electrical stimulus on your wrist during the previous block?”. These questions were answered using the same scales as the trial-by-trial pain intensity and unpleasantness ratings.
2.6.3 Retrospective affective valence, arousal, and sense of being in control of the CSs
After each phase except transfer-of-acquisition, participants indicated how they felt when performing movements to the left and to the right. The Self-Assessment Manikin scale (SAM) consisting of five pictographs [30] was used to assess affective valence, arousal, and sense of being in control during the CS movements performed. All responses were scored from 1 (very happy/not aroused at all/little sense of control) to 5 (very unhappy/very aroused/great sense of control).
2.7 Eyeblink startle response definition
PSychoPHysiological Analysis (PSPHA) [31] was used to process the startle data. Each startle waveform was visually inspected off-line. Responses showing technical abnormalities and artifacts were coded. They were marked as reject in case of an elevated baseline and as non-response when no response could be observed [29]. All data were included in the analyses (total of reject and non-response trials was lower than 20%). Startle peak amplitudes were defined as the maximum of the response curve between 21 and 175 ms after startle probe onset. Every peak amplitude was scored by subtracting its baseline score (the average EMG level between 1 and 20 ms after probe onset). These raw scores were transformed into Z-scores to account for inter-individual differences in physiological reactivity. A linear transformation of Z-scores into T-scores was done to optimize visualization of the data (i.e. to avoid negative values on the Y-axis).
2.8 Statistical analysis overview
To test our first hypothesis that pain-related fear would generalize following a gradient, we conducted repeated measures (RM) analyses of variance (ANOVAs) on the trial-by-trial pain-related fear ratings, trial-by-trial pain-US expectancy ratings and eyeblink startle responses during generalization. To test our second hypothesis that the probability of an at-pain-threshold intensity stimulus being rated as painful increases for GSs more similar to the CS+, a logistic regression was carried out on the forced choice data using a random intercept for each participant to account for correlations between repeated measurements. Further to test the pain generalization gradient, RM ANOVAs were employed on the trial-by-trial pain intensity and unpleasantness ratings during generalization. Because we were interested in pain modulation effects on the threshold-US, only pain ratings after threshold-US presentation were included in these analyses (i.e. judgments and ratings after pain-US presentation were excluded). All RM ANOVAs were further analyzed using trend analyses. Note that due to the unbalanced design of the experiment, the amount of data available for the intensity ratings was limited, thus reducing the power of the presented analyses. Because no differential pain modulation was observed, no mediation analysis was carried out. Therefore, the hypothesis that pain-related fear would (partly) mediate the pain modulating effect of GSs is not tested nor reported on in the “Results” section.
Further RM ANOVAs were employed to investigate the manipulation checks. As successful acquisition and transfer to the signaled setup were prerequisites to test for generalization effects on the trial-by-trial pain-related fear and pain-US expectancy ratings (see Appendix A in supplementary material), and startle amplitudes, responses during the acquisition and transfer-of-acquisition phases were analyzed. Retrospective pain-US intensity and unpleasantness ratings were analyzed for possible habituation or sensitization effects on both the affective and sensory dimension of pain. To test for differential fear learning and whether the effect remained stable throughout the experiment (i.e. transfer to the signaled setup and no extinction during generalization test), retrospective pain-related fear ratings were analyzed. Finally, three separate analyses were run on the retrospective SAM ratings for affective valence, arousal and sense of control (see Appendix B in supplementary material). All data were further analyzed using planned comparisons. Additionally, exploratory analyses were conducted on generalization data including trait questionnaire scores (Negative Affect, Trait Anxiety, Fear of Pain and Pain Catastrophizing standardized (total) scores), see Appendix C in supplementary material for a full report on the questionnaires and results.
Analyses were performed on the mean trial-by-trial ratings and startle amplitudes, and calculated per stimulus type in each block. Note that data of the second generalization block (including retrospective ratings) was missing for one participant due to technical difficulties. This participant was completely omitted in RM ANOVAs conducted on generalization data. When appropriate, Greenhouse-Geisser corrections [32] are reported: uncorrected degrees of freedom and corrected p-values are reported together with ε. The indication of effect size
3 Results
3.1 Main hypotheses
Hypothesis 1.1: Is there a generalization gradient in trial-by-trial pain-related fear ratings?
To test our first hypothesis that pain-related fear would generalize following a gradient, a 2×7 [Block (GEN1/GEN2)×Stimulus Type (CS+/GS1-5/CS−)] RM ANOVA was run on the trial-by-trial pain-related fear ratings to test for a generalization effect. The main effect of Block was not significant, F(1, 48)=2.42, p=0.13 (see Fig. 2). The main effect of Stimulus Type did reach significance, F(6, 288)=35.42, p<0.001, ε=0.31,
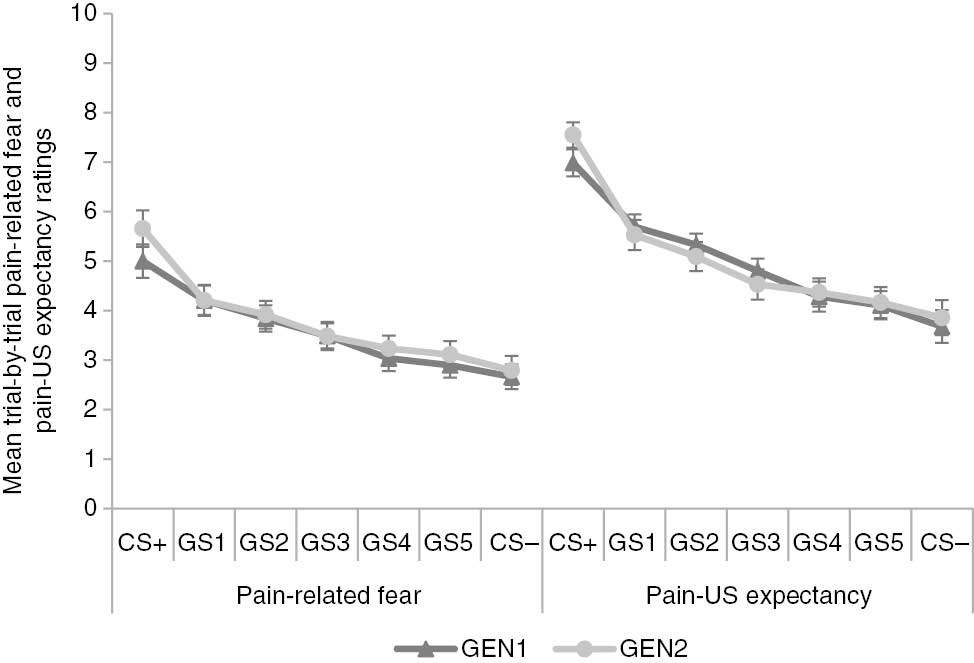
Mean trial-by-trial pain-related fear and pain-US expectancy ratings for the original conditioned (CS+/−) and generalization (GS1-5) movements during both generalization blocks (GEN1-2). Error bars represent standard errors.
Pairwise comparisons between trial-by-trial pain-related fear and pain-US expectancy ratings for each movement direction during generalization.
| p-Values | GS1 | GS2 | GS3 | GS4 | GS5 | CS− |
|---|---|---|---|---|---|---|
| Trial-by-trial pain-related fear ratings | ||||||
| GEN1 | ||||||
| CS+ | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| CS− | <0.001 | <0.001 | <0.001 | 0.90 | 1 | |
| GEN2 | ||||||
| CS+ | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| CS− | <0.001 | <0.001 | <0.05 | <0.05 | 0.54 | |
| Trial-by-trial pain-US expectancy ratings | ||||||
| GEN1 | ||||||
| CS+ | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| CS− | <0.001 | <0.001 | <0.001 | 0.10 | 0.21 | |
| GEN2 | ||||||
| CS+ | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| CS− | <0.001 | <0.001 | 0.09 | 0.37 | 1.00 | |
-
p-Values of pairwise comparisons between trial-by-trial pain-related fear and pain-US expectancy ratings for the original conditioned (CS+/−) and generalization (GS1-5) movements during both generalization blocks (GEN1-2). Bonferroni corrections were applied. Total degrees of freedom are 49 for all comparisons.
Hypothesis 1.2: Is there a generalization gradient in trial-by-trial pain-US expectancy ratings?
A 2×7 [Block (GEN1/GEN2)×Stimulus Type (CS+/GS1-5/CS−)] RM ANOVA run on the expectancy ratings during generalization revealed a pattern similar as observed in the fear ratings. The analysis showed no significant main effect of Block, F<1, while the main effect of Stimulus Type did reach significance, F(6, 288)=46.86, p<0.001, ε=0.35,
Hypothesis 1.3: Is there a generalization gradient in eyeblink startle responses?
A 2×7 [Block (GEN1/GEN2)×Stimulus Type (CS+/GS1-5/CS−)] RM ANOVA was run on the startle data to test for a generalization effect. The analysis revealed significant main effects of Block, F(1, 48)=13.57, p<0.001,
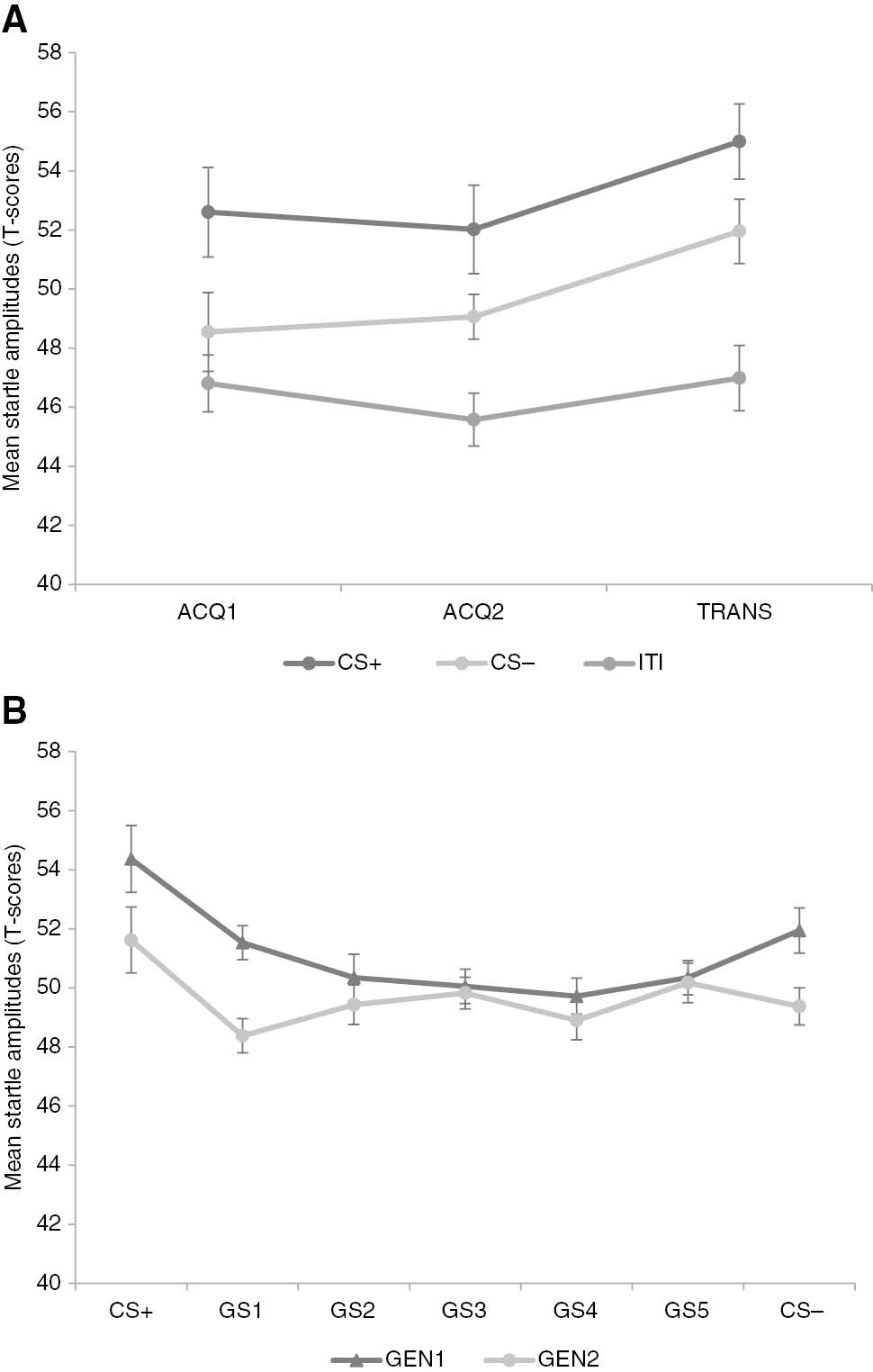
Mean startle amplitudes during the original conditioned movements (CS+/−), intertrial intervals (ITI) and generalization movements (GS1-5) in acquisition (ACQ1-2), transfer-of-acquisition (TRANS) (A) and both generalization blocks (GEN1-2) (B). Error bars represent standard errors.
Hypothesis 2.1: Is there a generalization gradient in the forced choice data?
To test our hypothesis on the spreading of pain, a logistic regression was carried out on the forced choice data. Contrary to our expectations, this analysis showed no significant association between Stimulus Type and forced choice ratings, F(6, 1329)<1, p=0.54 (see Fig. 4). The effect of Block did reach significance, F(1, 1329)=8.04, p<0.005. The proportion of threshold-USs being rated as painful was generally higher during the second block compared to the first block. Pooled data from both generalization blocks showed that threshold-USs on CS+ trials were rated as painful 23.74% (SD=42.66) of the time, and threshold-USs on CS− trials 24.24% (SD=42.96) of the time.
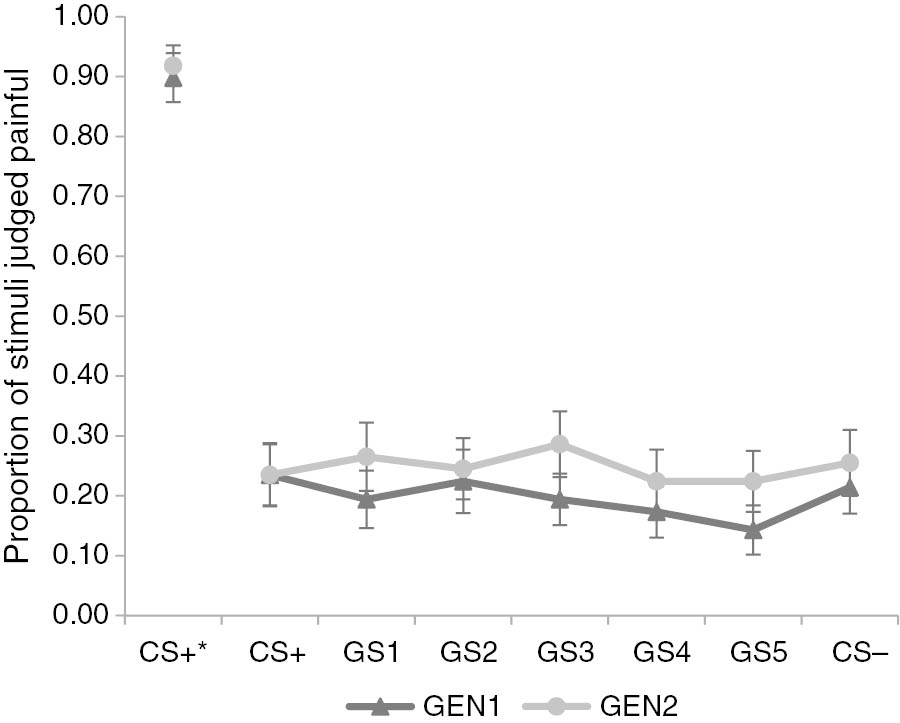
*=After presentation of pain-US. Proportion of pain- and threshold-USs judged painful on forced choice question during generalization blocks (GEN1-2) after threshold-US presentation on conditioned (CS+/−) and generalization (GS1-5) movements, and after pain-US presentation on CS+ movements. Error bars represent standard errors.
Hypothesis 2.2: Is there a generalization gradient in the trial-by-trial pain intensity ratings?
A 2×7 [Block (GEN1/GEN2)×Stimulus Type (CS+/GS1-5/CS−)] RM ANOVA was run on the intensity ratings to test for a generalization effect. There was no main effect of Block, F(1, 2)=1.74, p=0.32, and against our expectations, also no main effect of Stimulus Type, F(6, 12)=1.79, p=0.30, ε=0.22 (see Fig. 5). The interaction effect between Block and Stimulus type did not reach significance either, F(6, 12)=1.12, p=0.41, ε=0.31. These results indicated there was no generalization gradient in the trial-by-trial pain intensity ratings. Pooled data from both generalization blocks and all trial types showed that the threshold-US was rated as painful 22.51% of the time, indicating that 77.49% of all threshold-US trials were not rated on pain intensity.
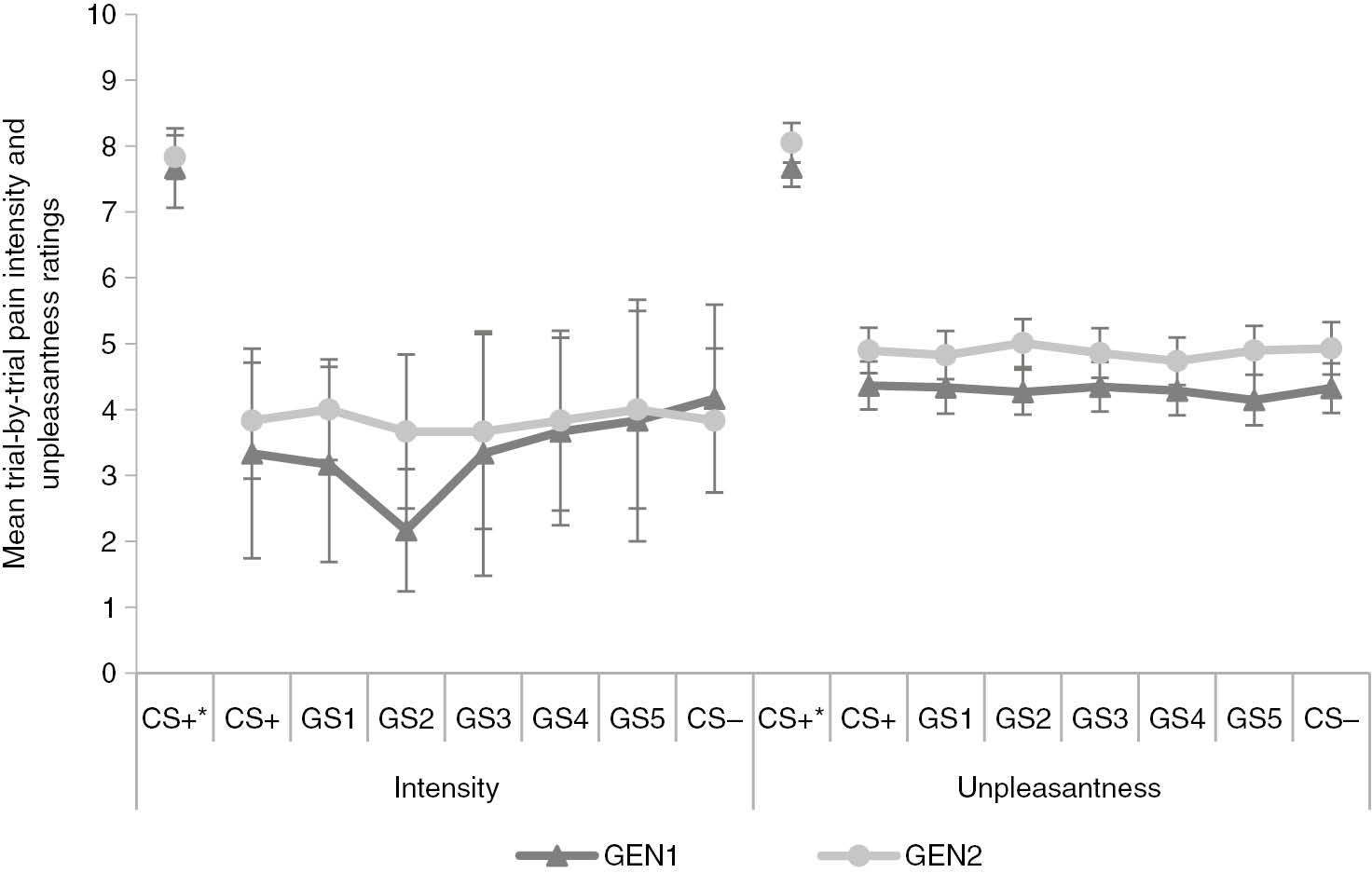
*=After presentation of pain-US. Mean trial-by-trial pain intensity and unpleasantness ratings during generalization blocks (GEN1-2) after threshold-US presentation on conditioned (CS+/−) and generalization (GS1-5) movements, and after pain-US presentation on CS+ movements. Error bars represent standard errors.
Hypothesis 2.3: Is there a generalization gradient in the trial-by-trial pain unpleasantness ratings?
A 2×7 [Block (GEN1/GEN2) x Stimulus Type (CS+/GS1-5/CS−)] RM ANOVA run on the online unpleasantness ratings showed a main effect of Block, F(1, 48)=10.48, p<0.005,
3.2 Manipulation checks
3.2.1 Retrospective pain-related fear ratings
On the retrospective pain-related fear ratings, a 2×5 [Stimulus Type (CS+/CS−)×Block (ACQ1/ACQ2/TRANS/GEN1/GEN2)] RM ANOVA was run to test for the acquisition effect and whether it remained present throughout the experiment. The analysis showed a significant main effect of Stimulus Type, F(1, 48)=93.55, p<0.001,
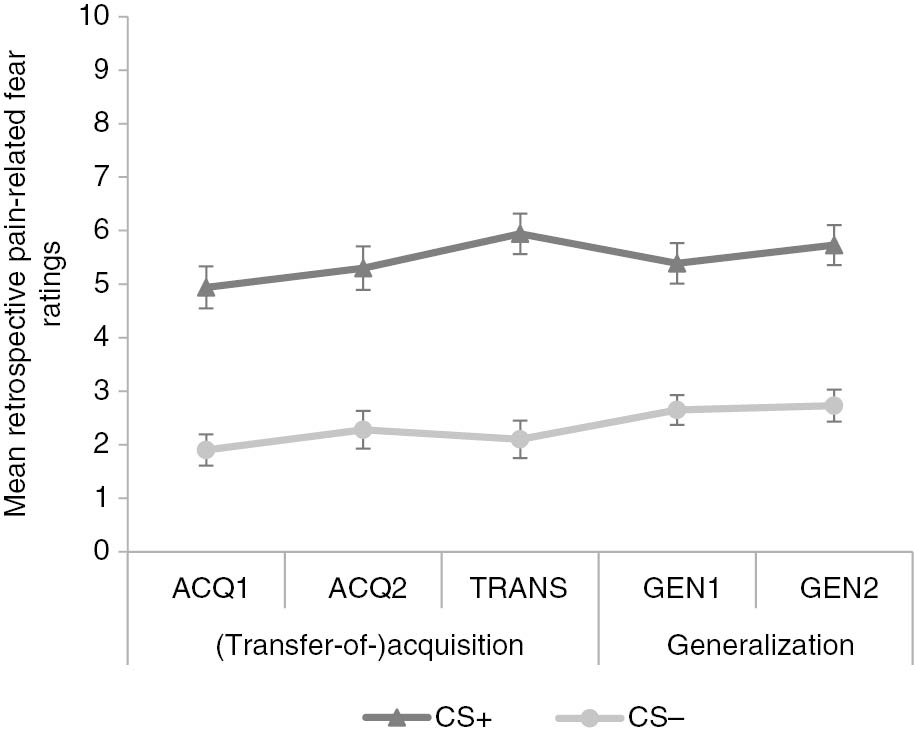
Mean retrospective pain-related fear ratings for conditioned movements (CS+/−) after acquisition (ACQ1-2), transfer-of-acquisition (TRANS) and generalization (GEN1-2) blocks. Error bars represent standard errors.
3.2.2 Eyeblink startle modulation during (transfer-of-)acquisition
To analyze the mean startle amplitudes during acquisition and transfer-of-acquisition, a 3×3 [Stimulus Type (CS+/CS−/ITI)×Block (ACQ1/ACQ2/TRANS)] RM ANOVA was performed. The analysis showed significant main effects of Stimulus Type, F(2, 98)=22.19, p<0.001, ε=0.98,
3.2.3 Retrospective pain-US intensity and unpleasantness ratings
A 2×3 [Rating (Intensity/Unpleasantness)×Block (ACQ1/ACQ2/TRANS)] RM ANOVA was run on the retrospective pain-US intensity and unpleasantness ratings and revealed a significant main effect of Rating, F(1, 49)=12.76, p<0.001,
Mean (retrospective) pain(-US) intensity and unpleasantness ratings and standard deviations.
| n=50 | Intensity |
Unpleasantness |
||
|---|---|---|---|---|
| M | SD | M | SD | |
| ACQ1a | 5.82 | 2.34 | 6.74 | 2.26 |
| ACQ2a | 6.10 | 2.27 | 6.66 | 2.30 |
| TRANSa | 6.30 | 2.22 | 6.82 | 2.47 |
| GEN1 | 7.67 | 1.04 | 7.67 | 2.03 |
| GEN2 | 7.83 | 0.58 | 8.05 | 2.10 |
-
a=Retrospective ratings. Means (M) and standard deviations (SD) of the retrospective pain-US intensity and unpleasantness ratings after acquisition (ACQ1-2) and transfer-of-acquisition (TRANS) blocks and trial-by-trial pain intensity ratings (after pain-US presentation) during generalization blocks (GEN1-2).
4 Discussion
The present study employed the VJM Paradigm [7] to examine the generalization of pain-related fear towards novel movements following differential fear conditioning and the mediating role of generalized fear in pain modulation. We hypothesized that (1) pain-related fear and expectancy would spread selectively to GSs that are more similar to the CS+ than to those similar to the CS−, (2) the probability of a threshold-US being judged as painful, as well as pain intensity and unpleasantness would increase when paired with GSs increasing in similarity to the CS+, and (3) pain-related fear would (partially) mediate the pain modulating effect of GSs, with higher levels of pain-related fear resulting in increased pain.
First, we successfully demonstrated a generalization gradient in pain-related fear and pain-US expectancy: ratings were highest in response to the CS+ and gradually decreased as GSs decreased in similarity to the CS+. These results were consistent with previous work [8], [11], [15], thus replicate the findings that the more proprioceptive similarity between a novel movement and the original painful movement, the more pain-related fear and expectancy it elicits. However, eyeblink startle responses were not in line with previous work as we were unable to test for a gradient due to the lack of differential responding to CSs during the generalization phase. An important difference between the current study and previous work is that during the generalization test of the current study, half of all movements were paired with the threshold-US, including CS− movements. The expectancy-violation on CS− trials (paired with no electrocutaneous stimuli during acquisition and transfer-of-acquisition phases), may have led to increased attention toward the CS−. As the eyeblink startle response is not only modulated by fear, but also by attention [28], [34], this may partly explain the absence of differential fear responding. Additionally, the number of threshold-USs presented during generalization and their relative unpredictability (i.e. on 50% of the trials and not stimulus-specific) may have made this phase more aversive in general compared to the previous phases [35]. This non-specific aversiveness may further explain the absence of differential fear responding [28], [34]. Note that this post-hoc explanation is not directly testable using the current design. However, both the positive forced choice judgments and trial-by-trial unpleasantness ratings for threshold-USs significantly increased over the course of the generalization phase. These sensitization effects may be a consequence of the aversiveness of the phase as affective valence plays a mediating role in pain modulation [9], [26], [36].
Second, we did not find a gradient in pain ratings for the threshold-US: forced choice judgments, and trial-by-trial pain intensity and unpleasantness ratings did not differ between GSs. In other words, we did not find evidence for our hypothesis that the probability of a threshold-US being judged as painful, as well as pain intensity and unpleasantness would increase when paired with GSs increasing in similarity to the CS+. Consequently, we were unable to investigate the mediating role of pain-related fear. Results did not corroborate the findings of Madden, Bellan, and colleagues [23]. A possible explanation is that the difference in intensity between the pain-US and threshold-US was too large in the current experiment, thus creating two very distinct USs. During acquisition, the pain-US may have become the anchor point for a “painful” stimulus (possibly reinforced by retrospective pain-US intensity questions as they referred to the pain-US as “painful”). This anchor may subsequently have been used to answer pain ratings during generalization, thus leading to the threshold-US to be judged as non-painful. Assimilation-contrast theory [37] suggests that the distance between prior experience and the actual stimulus is crucial. When the distance is small (i.e. when the stimulus is not too discrepant from our existing anchor), pain perception will be biased towards the expected level of pain (i.e. assimilation) [25]. However, when the reality is very different to our expectations, “we will experience the real, discrepant stimulus as even more discrepant compared to our expectations” ([19], p. 182). In other words, because the difference between the pain-US and threshold-US was obvious to participants, this may have impeded assimilation (i.e. threshold-US experienced as more painful) from happening and the contrary may have happened (i.e. threshold-US was experienced as even less painful). It should be noted that the proportion of threshold-USs being rated as painful was surprisingly low compared to the study by Madden, Bellan, and colleagues [23]. This may not be explained by assimilation-contrast theory alone and suggests that a habituation effect may have been present on the threshold-US, thus increasing the difference in subjective intensity between the pain-US and threshold-US even more. Madden and colleagues used laser stimuli as USs in their study, which are less prone to habituation effects as they have a higher intrinsic threat value (i.e. risk of skin damage) [38].
Some limitations and methodological considerations for future research should be outlined as well. Future research may consider not presenting the pain-US during the generalization test to prevent it from being used as anchor point during pain ratings, or using a pain-US of lower intensity for the whole experiment, thus decreasing the distance between the pain-US and threshold-US. Additionally, considering assimilation-contrast theory, future studies may want to pair CS− movements during acquisition with a non-painful stimulus that is not too discrepant from the threshold-US. In the current study, CS− trials were paired with no electrocutaneous stimulus during acquisition and paired with the threshold-US during generalization. This significant change may have impeded assimilation effects on CS− trials (i.e. threshold-US being experienced as less painful) and increased fear responding due to expectancy-violation (as discussed earlier). Another possible limitation is the use of forced choice questions. This led to a small percentage of threshold stimuli to be rated on pain intensity, limited statistical power may have impeded observing a gradient. Future research may consider having participants rate pain intensity immediately instead of using a forced choice question. This allows for a more sensitive measurement while still being able to distinguish between painful and not painful. A final consideration concerns the use of electrocutaneous stimuli as USs and their calibration. Future research may control for the potential confound of expectancy effects (e.g. by randomizing presentation order) during calibration: systematic expectancies may have developed due to the ascending/descending presentation order of stimuli. Furthermore, habituation or sensitization may have occurred during calibration (due to the variable repetitions of stimuli) and/or during the experiment. Future research may circumvent such effects during calibration, or consider using USs less prone to such effects (e.g. laser stimuli) [38] or recalibrating the threshold-US throughout the experiment [39]. The latter approach was not used in the current experiment for reasons of ecological validity, as there is no “real life” equivalent to recalibrating pain stimuli.
The current study aimed to extend research on fear generalization and integrate it with the field of pain modulation. As there are often misunderstandings about the relationship between classical conditioning and pain modulation in clinical practice [40], this field deserves further attention. Even though the data of the current study do not support the idea that generalized pain-related fear plays a mediating role in pain modulation, it remains a plausible mechanism contributing to the spreading of pain in chronic pain disability. The current study did replicate generalization gradients in pain-related fear. Multiple studies observed that across patients with different pain disorders, pain-related fear generalizes more to stimuli similar to the CS− (i.e. higher fear responses) in comparison to healthy controls [14], [17], [18], while fear responses to stimuli similar to the CS+ do not seem to differ between patients and healthy controls. If pain-related fear indeed plays a mediating role, a similar pattern could be anticipated in pain responses. In other words, such overgeneralization of pain-related fear may be of particular relevance in the transition to and maintenance of chronic pain disability. Even though the existence of a causal relationship needs to be investigated, these findings may be of clinical importance. First, the conclusion from generalization research that fear can be triggered by stimuli that never featured in a pain episode should be considered in treatments of chronic pain that target pain-related fear (e.g. exposure-based therapy). Second, as generalization mechanisms may play an important role in the transition to disability, this may be an interesting target in the prevention of chronic pain (e.g. discrimination training [41]).
To conclude, the current study was unable to provide evidence for the idea that generalization of pain-related fear might mediate the subsequent spreading of pain. However, it provided a promising approach to investigate pain modulation by pain-associated movements. Despite its methodological limitations, the current study successfully replicated important findings on generalization of pain-related fear. The results revealed more spreading of fear toward novel movements that were proprioceptively similar to the original painful movement than to the ones similar to the non-painful movement. Future research might focus on the conditions under which generalization gradients may be reduced in order to develop new methods to limit the spreading of fear and avoidance in chronic pain disability.
Supplementary material: (A) Statistical analyses of trial-by-trial pain-related fear and pain-US expectancy ratings during transfer-of-acquisition; (B) Statistical analysis of retrospective affective valence, arousal, and sense of being in control of the CSs; (C) Exploratory analyses: influence of psychological traits on generalization gradient.
-
Authors’ statements
-
Research funding: This research is supported by a Vidi grant from the Netherlands Organization for Scientific Research (NWO), The Netherlands (grant ID 452-17-002) granted to Ann Meulders. Ann Meulders is also a postdoctoral researcher of the Research Foundation Flanders (FWO-Vlaanderen), Belgium, (grant ID: 12E3717N).
-
Conflict of interest: Authors state no conflict of interest.
-
Informed consent: Informed consent has been obtained from all individuals included in this study.
-
Ethical approval: The Social and Societal Ethics Committee of the KU Leuven approved the experimental protocol (SMEC registration number: G-2015 03 189).
References
[1] Crombez G, Eccleston C, Van Damme S, Vlaeyen JWS, Karoly P. Fear-avoidance model of chronic pain: the next generation. Clin J Pain 2012;28:475–83.10.1097/AJP.0b013e3182385392Suche in Google Scholar PubMed
[2] Leeuw M, Goossens MEJB, Linton SJ, Crombez G, Boersma K, Vlaeyen JWS. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med 2007;30:77–94.10.1007/s10865-006-9085-0Suche in Google Scholar PubMed
[3] Zale EL, Lange KL, Fields SA, Ditre JW. The relation between pain-related fear and disability: a meta-analysis. J Pain 2013;14:1019–30.10.1016/j.jpain.2013.05.005Suche in Google Scholar PubMed PubMed Central
[4] Vlaeyen JWS, Crombez G, Linton SJ. The fear-avoidance model of pain. Pain 2016;157:1588–9.10.1097/j.pain.0000000000000574Suche in Google Scholar PubMed
[5] Vlaeyen JWS, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain 2000;85:317–32.10.1016/S0304-3959(99)00242-0Suche in Google Scholar PubMed
[6] Vlaeyen JWS, Linton SJ. Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain 2012;153:1144–7.10.1016/j.pain.2011.12.009Suche in Google Scholar PubMed
[7] Meulders A, Vansteenwegen D, Vlaeyen JWS. The acquisition of fear of movement-related pain and associative learning: a novel pain-relevant human fear conditioning paradigm. Pain 2011;152:2460–9.10.1016/j.pain.2011.05.015Suche in Google Scholar PubMed
[8] Meulders A, Vandebroek N, Vervliet B, Vlaeyen JWS. Generalization gradients in cued and contextual pain-related fear: an experimental study in healthy participants. Front Hum Neurosci 2013;7:345.10.3389/fnhum.2013.00345Suche in Google Scholar PubMed PubMed Central
[9] Meulders A, Vansteenwegen D, Vlaeyen JWS. Women, but not men, report increasingly more pain during repeated (un)predictable painful electrocutaneous stimulation: evidence for mediation by fear of pain. Pain 2012;153:1030–41.10.1016/j.pain.2012.02.005Suche in Google Scholar PubMed
[10] Meulders A, Vlaeyen JWS. The acquisition and generalization of cued and contextual pain-related fear: an experimental study using a voluntary movement paradigm. Pain 2013;154:272–82.10.1016/j.pain.2012.10.025Suche in Google Scholar PubMed
[11] Geschwind N, Meulders M, Peters ML, Vlaeyen JWS, Meulders A. Can experimentally induced positive affect attenuate generalization of fear of movement-related pain? J Pain 2015;16:258–69.10.1016/j.jpain.2014.12.003Suche in Google Scholar PubMed
[12] Glogan E, van Vliet C, Roelandt R, Meulders A. Generalization and extinction of concept-based pain-related fear. J Pain 2018;20:325–38.10.1016/j.jpain.2018.09.010Suche in Google Scholar PubMed
[13] Meulders A, Vandael K, Vlaeyen JWS. Generalization of pain-related fear based on conceptual knowledge. Behav Ther 2017;48:295–310.10.1016/j.beth.2016.11.014Suche in Google Scholar PubMed
[14] Meulders A, Meulders M, Stouten I, De Bie J, Vlaeyen JWS. Extinction of fear generalization: a comparison between fibromyalgia patients and healthy control participants. J Pain 2017;18:79–95.10.1016/j.jpain.2016.10.004Suche in Google Scholar PubMed
[15] Niederstrasser NG, Meulders A, Meulders M, Struyf D, Vlaeyen JW. Executive functions deficits impair extinction of generalization of fear of movement-related pain. Eur J Pain (United Kingdom) 2017;21:886–99.10.1002/ejp.991Suche in Google Scholar PubMed
[16] Ghirlanda S, Enquist M. A century of generalization. Anim Behav 2003;66:15–36.10.1006/anbe.2003.2174Suche in Google Scholar
[17] Meulders A, Jans A, Vlaeyen JWS. Differences in pain-related fear acquisition and generalization: an experimental study comparing patients with fibromyalgia and healthy controls. Pain 2015;156:108–22.10.1016/j.pain.0000000000000016Suche in Google Scholar PubMed
[18] Meulders A, Harvie DS, Bowering JK, Caragianis S, Vlaeyen JWS, Moseley GL. Contingency learning deficits and generalization in chronic unilateral hand pain patients. J Pain 2014;15:1046–56.10.1016/j.jpain.2014.07.005Suche in Google Scholar PubMed
[19] Jepma M, Wager TD. Conceptual conditioning: mechanisms mediating conditioning effects on pain. Psychol Sci 2015;26:1728–39.10.1177/0956797615597658Suche in Google Scholar PubMed PubMed Central
[20] Madden VJ, Harvie DS, Parker R, Jensen KB, Vlaeyen JWS, Moseley GL, Stanton TR. Can pain or hyperalgesia be a classically conditioned response in humans? A systematic review and meta-analysis. Pain Med 2016;17:1094–111.10.1093/pm/pnv044Suche in Google Scholar PubMed
[21] Miguez G, Laborda MA, Miller RR. Classical conditioning and pain: conditioned analgesia and hyperalgesia. Acta Psychol (Amst) 2014;145:10–20.10.1016/j.actpsy.2013.10.009Suche in Google Scholar PubMed PubMed Central
[22] Price DD, Finniss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annu Rev Psychol 2008;59:565–90.10.1146/annurev.psych.59.113006.095941Suche in Google Scholar PubMed
[23] Madden VJ, Bellan V, Russek LN, Camfferman D, Vlaeyen JWS, Moseley GL. Pain by association? experimental modulation of human pain thresholds using classical conditioning. J Pain 2016;17:1105–15.10.1016/j.jpain.2016.06.012Suche in Google Scholar PubMed
[24] Atlas LY, Wager TD. How expectations shape pain. Neurosci Lett 2012;520:140–8.10.1016/j.neulet.2012.03.039Suche in Google Scholar PubMed
[25] Hoskin R, Berzuini C, Acosta-Kane D, El-Deredy W, Guo H, Talmi D. Sensitivity to pain expectations: a Bayesian model of individual differences. Cognition 2019;182:127–39.10.1016/j.cognition.2018.08.022Suche in Google Scholar PubMed
[26] Rhudy JL, Meagher MW. The role of emotion in pain modulation. Curr Opin Psychiatry 2001;14:241–5.10.1097/00001504-200105000-00012Suche in Google Scholar
[27] Lissek S, Biggs AL, Rabin SJ, Cornwell BR, Alvarez RP, Pine DS, Grillon C. Generalization of conditioned fear-potentiated startle in humans: experimental validation and clinical relevance. Behav Res Ther 2008;46:678–87.10.1016/j.brat.2008.02.005Suche in Google Scholar PubMed PubMed Central
[28] Filion DL, Dawson ME, Schell AM. The psychological significance of human startle eyeblink modification: a review. Biol Psychol 1998;47:1–43.10.1016/S0301-0511(97)00020-3Suche in Google Scholar
[29] Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, Van Boxtel A. Committee report: guidelines for human startle eyeblink electromyographic studies. Psychophysiology 2005;42:1–15.10.1111/j.1469-8986.2005.00271.xSuche in Google Scholar PubMed
[30] Bradley MM, Lang PJ. Measuring emotion: the self-assessment manikin and the semantic differential. J Behav Ther Exp Psychiatry 1994;25:49–59.10.1016/0005-7916(94)90063-9Suche in Google Scholar PubMed
[31] De Clercq A, Verschuere B, De Vlieger P, Crombez G. Psychophysiological Analysis (PSPHA): a modular script-based program for analyzing psychophysiological data. Behav Res Methods 2006;38:504–10.10.3758/BF03192805Suche in Google Scholar
[32] Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika 1959;24:95–112.10.1007/BF02289823Suche in Google Scholar
[33] Cohen J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Hillsdale, NJ: Erlbaum, 1988.Suche in Google Scholar
[34] Lipp OV, Cox D, Siddle DAT. Blink startle modulation during anticipation of pleasant and unpleasant stimuli. J Psychophysiol 2001;15:155–62.10.1027//0269-8803.15.3.155Suche in Google Scholar
[35] Grillon C. Startle reactivity and anxiety disorders : aversive. Biol Psychiatry 2002;52:958–75.10.1016/S0006-3223(02)01665-7Suche in Google Scholar PubMed
[36] Rhudy JL, Meagher MW. Fear and anxiety: divergent effects on human pain thresholds. Pain 2000;84:65–75.10.1016/S0304-3959(99)00183-9Suche in Google Scholar PubMed
[37] Hovland CI, Harvey OJ. Assimilation and contrast effects in reactions to communication and attitude change. J Abnorm Soc Psychol 1957;55:244–52.10.1037/h0048480Suche in Google Scholar PubMed
[38] Madden VJ, Catley MJ, Grabherr L, Mazzola F, Shohag M, Moseley GL. The effect of repeated laser stimuli to ink-marked skin on skin temperature–recommendations for a safe experimental protocol in humans. PeerJ 2016;4:e1577.10.7717/peerj.1577Suche in Google Scholar PubMed PubMed Central
[39] Traxler J, Madden VJ, Moseley GL, Vlaeyen JWS. Modulating pain thresholds through classical conditioning. PeerJ 2019;7:e6486.10.7717/peerj.6486Suche in Google Scholar PubMed PubMed Central
[40] Madden VJ, Moseley GL. Do clinicians think that pain can be a classically conditioned response to a non-noxious stimulus? Man Ther 2016;22:165–73.10.1016/j.math.2015.12.003Suche in Google Scholar PubMed
[41] Vervliet B, Kindt M, Vansteenwegen D, Hermans D. Fear generalization in humans: impact of verbal instructions. Behav Res Ther 2010;48:38–43.10.1016/j.brat.2009.09.005Suche in Google Scholar PubMed
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/sjpain-2019-0065).
©2020 Scandinavian Association for the Study of Pain. Published by Walter de Gruyter GmbH, Berlin/Boston. All rights reserved.
Artikel in diesem Heft
- Frontmatter
- Editorial
- Change in Editorship: A Tribute to the Outgoing Editor-in-Chief
- Editorial comments
- Laboratory biomarkers of systemic inflammation – what can they tell us about chronic pain?
- Considering the interpersonal context of pain catastrophizing
- Systematic review
- Altered pain processing and sensitisation is evident in adults with patellofemoral pain: a systematic review including meta-analysis and meta-regression
- Topical reviews
- Pain revised – learning from anomalies
- Role of the immune system in neuropathic pain
- Clinical pain research
- Cryoneurolysis for cervicogenic headache – a double blinded randomized controlled study
- Interpersonal problems as a predictor of pain catastrophizing in patients with chronic pain
- Pain and small-fiber affection in hereditary neuropathy with liability to pressure palsies (HNPP)
- Predicting the outcome of persistent sciatica using conditioned pain modulation: 1-year results from a prospective cohort study
- Observational studies
- Revised chronic widespread pain criteria: development from and integration with fibromyalgia criteria
- The relationship between patient factors and the refusal of analgesics in adult Emergency Department patients with extremity injuries, a case-control study
- Chronic neuropathic pain after traumatic peripheral nerve injuries in the upper extremity: prevalence, demographic and surgical determinants, impact on health and on pain medication
- Tramadol prescribed use in general and chronic noncancer pain: a nationwide register-based cohort study of all patients above 16 years
- Changes in inflammatory plasma proteins from patients with chronic pain associated with treatment in an interdisciplinary multimodal rehabilitation program – an explorative multivariate pilot study
- Original experimental
- The pro-algesic effect of γ-aminobutyric acid (GABA) injection into the masseter muscle of healthy men and women
- The relationship between fear generalization and pain modulation: an investigation in healthy participants
- Experimental shoulder pain models do not validly replicate the clinical experience of shoulder pain
- Computerized quantification of pain drawings
- Head repositioning accuracy is influenced by experimental neck pain in those most accurate but not when adding a cognitive task
- Short communications
- Dispositional empathy is associated with experimental pain reduction during provision of social support by romantic partners
- Superior cervical sympathetic ganglion block under ultrasound guidance promotes recovery of abducens nerve palsy caused by microvascular ischemia
Artikel in diesem Heft
- Frontmatter
- Editorial
- Change in Editorship: A Tribute to the Outgoing Editor-in-Chief
- Editorial comments
- Laboratory biomarkers of systemic inflammation – what can they tell us about chronic pain?
- Considering the interpersonal context of pain catastrophizing
- Systematic review
- Altered pain processing and sensitisation is evident in adults with patellofemoral pain: a systematic review including meta-analysis and meta-regression
- Topical reviews
- Pain revised – learning from anomalies
- Role of the immune system in neuropathic pain
- Clinical pain research
- Cryoneurolysis for cervicogenic headache – a double blinded randomized controlled study
- Interpersonal problems as a predictor of pain catastrophizing in patients with chronic pain
- Pain and small-fiber affection in hereditary neuropathy with liability to pressure palsies (HNPP)
- Predicting the outcome of persistent sciatica using conditioned pain modulation: 1-year results from a prospective cohort study
- Observational studies
- Revised chronic widespread pain criteria: development from and integration with fibromyalgia criteria
- The relationship between patient factors and the refusal of analgesics in adult Emergency Department patients with extremity injuries, a case-control study
- Chronic neuropathic pain after traumatic peripheral nerve injuries in the upper extremity: prevalence, demographic and surgical determinants, impact on health and on pain medication
- Tramadol prescribed use in general and chronic noncancer pain: a nationwide register-based cohort study of all patients above 16 years
- Changes in inflammatory plasma proteins from patients with chronic pain associated with treatment in an interdisciplinary multimodal rehabilitation program – an explorative multivariate pilot study
- Original experimental
- The pro-algesic effect of γ-aminobutyric acid (GABA) injection into the masseter muscle of healthy men and women
- The relationship between fear generalization and pain modulation: an investigation in healthy participants
- Experimental shoulder pain models do not validly replicate the clinical experience of shoulder pain
- Computerized quantification of pain drawings
- Head repositioning accuracy is influenced by experimental neck pain in those most accurate but not when adding a cognitive task
- Short communications
- Dispositional empathy is associated with experimental pain reduction during provision of social support by romantic partners
- Superior cervical sympathetic ganglion block under ultrasound guidance promotes recovery of abducens nerve palsy caused by microvascular ischemia


