Abstract
Background and aims
People with shoulder pain often present with abnormal shoulder muscle function. It is not known whether shoulder pain causes or is the result of muscle dysfunction. If pain leads to muscle dysfunction, therapeutic interventions that produce shoulder pain may be contraindicated. Experimentally induced nociception can be used to investigate a causal relationship between shoulder pain and muscle dysfunction. However, the validity of current experimental shoulder pain protocols has not been established. The aim of this study was to determine whether current experimental shoulder pain protocols validly replicate the clinical experience of shoulder pain with respect to pain distribution, quality and behaviour.
Methods
Nine pain free participants received two injections of hypertonic saline, one into the subacromial space and one into supraspinatus, in random order, at least 1 week apart. Investigators blind to the injection site assessed pain distribution, pain response to clinical tests which provoke shoulder pain and pain quality assessed using the McGill Pain Questionnaire.
Results
Following hypertonic saline injection into both the subacromial space and supraspinatus: pain was most commonly reported in the deltoid region and did not extend beyond the elbow; the most common response to clinical tests which provoke shoulder pain was a decrease in pain; and the highest rating of pain quality was in the sensory domain with very few responses in the affective domain.
Conclusions
Experimental shoulder pain induced by injection of hypertonic saline into either the subacromial space or supraspinatus produced a pain distribution similar to that observed in clinical shoulder pain, but neither experimental pain protocol could reproduce the increases in pain intensity following shoulder provocation tests or the emotional distress commonly observed in people with clinical shoulder pain.
Implications
Pain induced by local shoulder nociception produced by hypertonic saline injection into shoulder structures has significant limitations as a model of clinical shoulder pain. While it is perhaps unsurprising that short duration, chemically-induced experimental pain does not replicate the quality of the clinical experience of shoulder pain, the validity of experimental shoulder pain models which produce the opposite response to provocation testing to clinical shoulder pain must be questioned.
1 Introduction
People with shoulder pain often demonstrate impaired shoulder movement patterns, muscle activity and muscle strength [1], [2]. It is not known whether shoulder pain causes changes in muscle function, or whether impaired shoulder muscle function causes shoulder pain. Without knowing the causal direction, clinicians cannot make effective decisions to manage and rehabilitate patients with shoulder pain. For example, clinicians do not know whether performing rehabilitation exercises with pain is potentially detrimental to patients.
Pain induced under experimental conditions in asymptomatic participants is a common method used to investigate the effect of pain on muscle function and local nociception induced by the injection of hypertonic saline is commonly used. Previous studies on the shoulder have induced pain by injecting hypertonic saline into the subacromial space [3], [4], [5] or into the supraspinatus muscle [3]. However, it is not known whether either of these models validly reproduces the clinical experience of shoulder pain.
Current literature suggests that people experience pain from nociception in the shoulder in the lateral proximal arm, which is aggravated during shoulder movements, passive shoulder impingement tests and isometric shoulder strength tests [6], [7]. People with clinical shoulder pain also describe the quality of pain across all domains of the McGill Pain Questionnaire [8]. An ideal model of experimental shoulder pain should reproduce the clinical experience in terms of the distribution, quality and behaviour of pain. The aim of this study was to determine whether injection of hypertonic saline into the subacromial space or supraspinatus constitutes such a model.
2 Materials and methods
2.1 Participants
Nine participants (five men) were recruited from staff and students of the University of Sydney and from professional colleagues. Subjects were excluded if they had current pain or pain in the previous 2 years which required treatment in the shoulder to be tested, if pain was produced during maximal isometric shoulder rotation strength testing, or if they were on immuno-suppressive medication. The study was approved by the Human Research Ethics Committee of The University of Sydney (Project No: 2014/857). Participants provided written informed consent.
2.2 Study design
The study design is illustrated in Fig. 1. Outcome data were collected on three separate testing sessions in all participants, with at least a 1-week rest period between sessions to “wash out” any residual effects of testing. Using a computer generated (Excel) randomised sequence, participants were randomly allocated to receive an injection of hypertonic saline either into the subacromial space or into the supraspinatus muscle. Subsequently, participants received two further injections in random order: an injection into the same site of isotonic saline that was matched in volume to the preceding injection of hypertonic saline; and an injection of hypertonic saline into the site that was not previously tested. The injection of isotonic saline served as the control condition to identify whether pain was due to chemical irritation from the hypertonic saline or to mechanical effects of the injection. Note that there were four test conditions but each participant only received three injections: all nine participants received hypertonic saline injection to each site, but isotonic saline injections were only given to a subset of participants for each site. This was done to minimise the total number of injections participants received. An investigator not involved in data collection performed all injections.
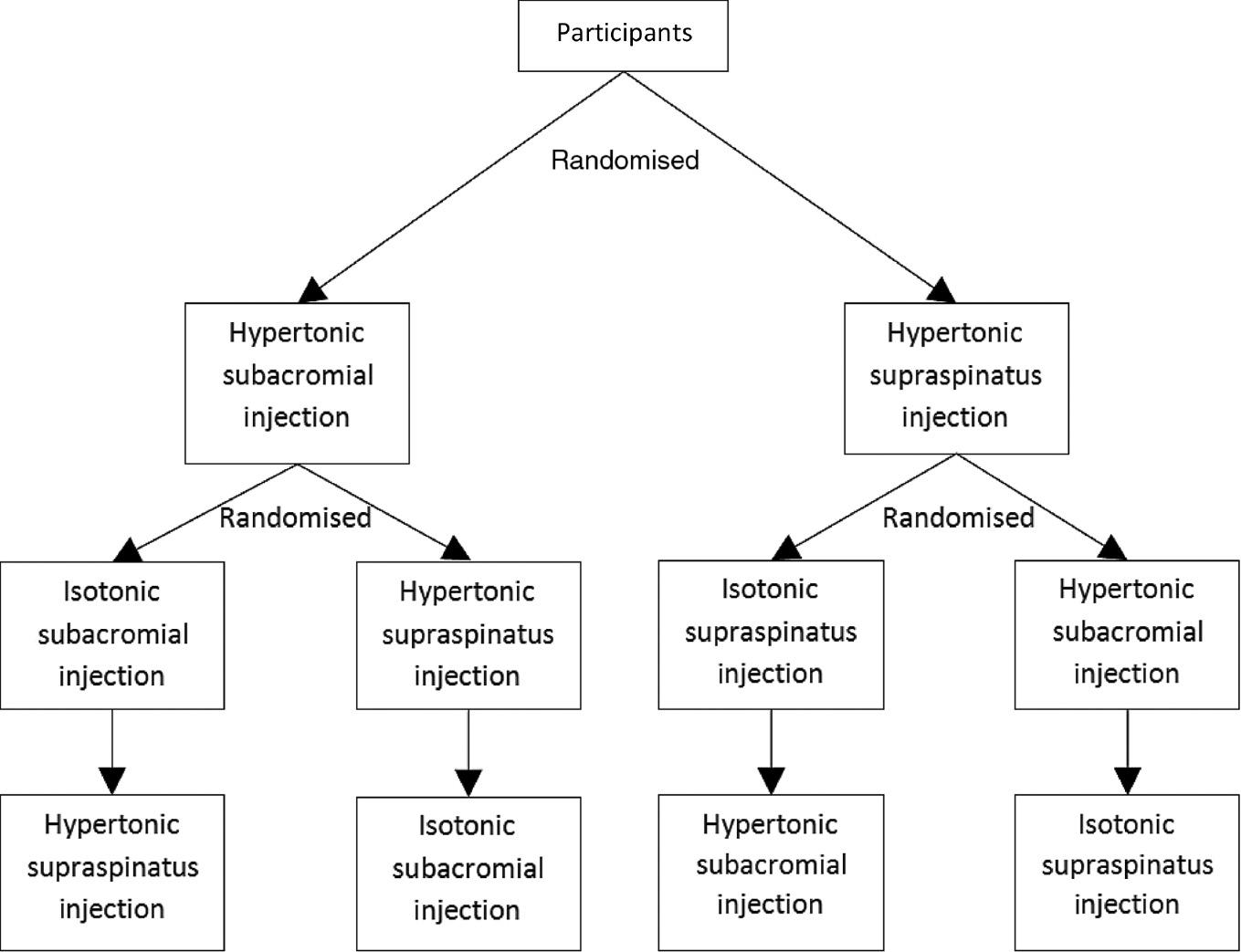
Study design.
2.3 Outcome measures
The distribution, quality, intensity and duration of pain, as well as the pain response to provocation tests, were assessed following hypertonic and isotonic saline injection.
2.3.1 Distribution of pain
Participants were asked to indicate the region of the body in which they experienced pain at five time points in the initial 7 min after injection (Table 1). This was recorded on a body chart by an investigator who was blind to the injection site, and participants visually confirmed that the region of pain recorded was accurate.
Timeline of outcome measures.
| Time | Outcome measure |
|---|---|
| 1 min | NRS+body chart |
| 2 min | NRS+body chart |
| 3 min | NRS+pain provocation testing |
| 4 min | NRS+body chart |
| 5 min | NRS+body chart |
| 6 min | NRS |
| 7 min | NRS+body chart |
| Each minute thereafter | NRS |
| Conclusion | McGill Pain Questionnaire |
-
NRS=11-point Numerical Rating Scale for pain.
2.3.2 Pain response to provocation tests
The response of experimental shoulder pain to provocation tests was examined using the empty can test [9], the Hawkins-Kennedy test [10] and active shoulder abduction. The tests were conducted in random order by an experienced physiotherapist with the participant seated. Pain intensity was measured using an 11-point Numerical Rating Scale (NRS) [11] with 0 representing “no pain” and 10 representing “worst possible pain”. The NRS is a valid [12], [13], responsive [14] and reliable [13] measure of pain. Participants were asked to verbally indicate their level of pain during and following each test. The empty can test was chosen as it is typically one of the most painful manual tests in patients with shoulder pain [15] and maximally activates nine muscles around the shoulder joint [16]. The empty can test was performed with the shoulder at 90 degrees abduction in full internal rotation with the arm in the scapular plane and the elbow extended. The assessor placed a hand on the participant’s distal forearm to resist a maximal effort abduction force [17]. The Hawkins-Kennedy test was chosen as it is the most commonly used manual test in the examination of subacromial pain [18] despite its low diagnostic sensitivity [19]. This test was performed with the shoulder at 90 degrees of flexion, elbow at 90 degrees flexion and the hand facing the ground. The assessor supported the arm to allow for maximal relaxation by the participant, stabilized the scapula with one hand and passively moved the shoulder into maximal internal rotation [10]. Active abduction was used at it is often painful [20] and is one of the cluster of tests commonly used in the examination of patients with shoulder pain [19]. Active abduction was performed by asking the participant to move the shoulder actively through full range abduction in the coronal plane with the elbow extended.
2.3.3 Quality of pain
The quality of pain was assessed using the McGill Pain Questionnaire (MPQ). The MPQ is sensitive to changes in pain [21] and is a valid [22] and reliable [13] measure of pain quality.
2.4 Procedure
2.4.1 Pre-pain induction
Betadine was applied to the skin over the injection site to minimise infection risk. A small amount of lignocaine (<0.5 mL, Xylocaine 2%) was then injected to minimise skin sensation during subsequent injections.
2.4.2 Pain induction session 1
Participants received an injection of sterile 6% hypertonic saline (MucoClear, 6% Sodium Chloride) either into the subacromial space or into the supraspinatus muscle belly, using a 25-gauge needle (Fig. 1). The subacromial injection was performed by an experienced rheumatologist using an anterolateral approach; the injection site was located approximately 2 cm distal to the lateral edge of the acromion and 1 cm posterior to the anterior edge of the acromion. Needle insertion was directly in line with the axial plane. The intramuscular supraspinatus injection was performed by injecting into the medial third of the muscle belly immediately superior to the scapular spine. Needle insertion was perpendicular to surface of the skin.
The volume of hypertonic saline was progressively increased until the participant indicated that they experienced a moderately high level of pain (at least 7/10 on the NRS). The needle was withdrawn and the injection site was covered with an adhesive bandage. An adhesive bandage was also applied to the second potential injection site to keep assessors blind to the location of the injection.
2.4.3 Pain induction session 2
Participants received either an injection of sterile 6% hypertonic saline into the site not injected during session 1, or an injection of isotonic saline (0.9% Sodium Chloride) into the same site as session 1 (Fig. 1). The procedure for injection of the 6% hypertonic saline into the alternative site was identical to session 1. The volume of isotonic saline injected was matched to the hypertonic saline used for that site. Again, adhesive bandages were applied to both sites to maintain assessor blinding.
2.4.4 Pain induction session 3
Participants either received an injection of 6% hypertonic saline into the site that was not injected in session 1, or an injection of isotonic saline into the site that was injected in session 1 (Fig. 1). The testing procedure was identical to sessions 1 and 2.
2.4.5 Post pain-induction
Outcome measures were recorded according to the schedule summarised in Table 1 to document the time course of pain. Pain intensity was measured with the participant sitting at rest every minute after injection and during pain provocation tests. This was done until two consecutive scores of 0/10 were obtained. Participants were asked to indicate the location of their pain at 1 min intervals for 7 min, except at minute 3 when pain provocation testing took place and at minute 6. Participants completed the MPQ when the induced pain had resolved.
2.5 Statistical analysis
The pain data was tested for normality using the Kolmogorov-Smirnov test and found not to be normally distributed. Therefore, the Wilcoxon’s paired non-parametric test was used to compare the pain levels from the hypertonic saline between subacromial and supraspinatus sites at each time point (IBM SPSS Statistics Version 24). A significance level of 0.05 was used. Descriptive statistics were used to compare response to pain provocation tests and distribution of pain. Pain rating indices based on rank values of the descriptors were calculated using the MPQ [21] and the most common words for each condition were recorded.
3 Results
The mean (SD, range) volume of 6% hypertonic saline required to obtain an NRS score of 7 was 2.4 mL (0.7 mL, 1.7–4.0 mL) for subacromial injections and 2.2 mL (0.5 mL, 1.5–3.0 mL) for supraspinatus injections.
The distribution of pain is summarised in Fig. 2 and Table 2. All hypertonic subacromial injections produced pain in the anterolateral shoulder region. Hypertonic supraspinatus injections produced pain in the anterolateral region of the shoulder in eight out of nine participants. Hypertonic subacromial injections produced pain across a wider area compared with hypertonic supraspinatus injections. No participant experienced pain outside the areas shown in Fig. 2. Pain experienced during isotonic injections was localised to the site of the injection.
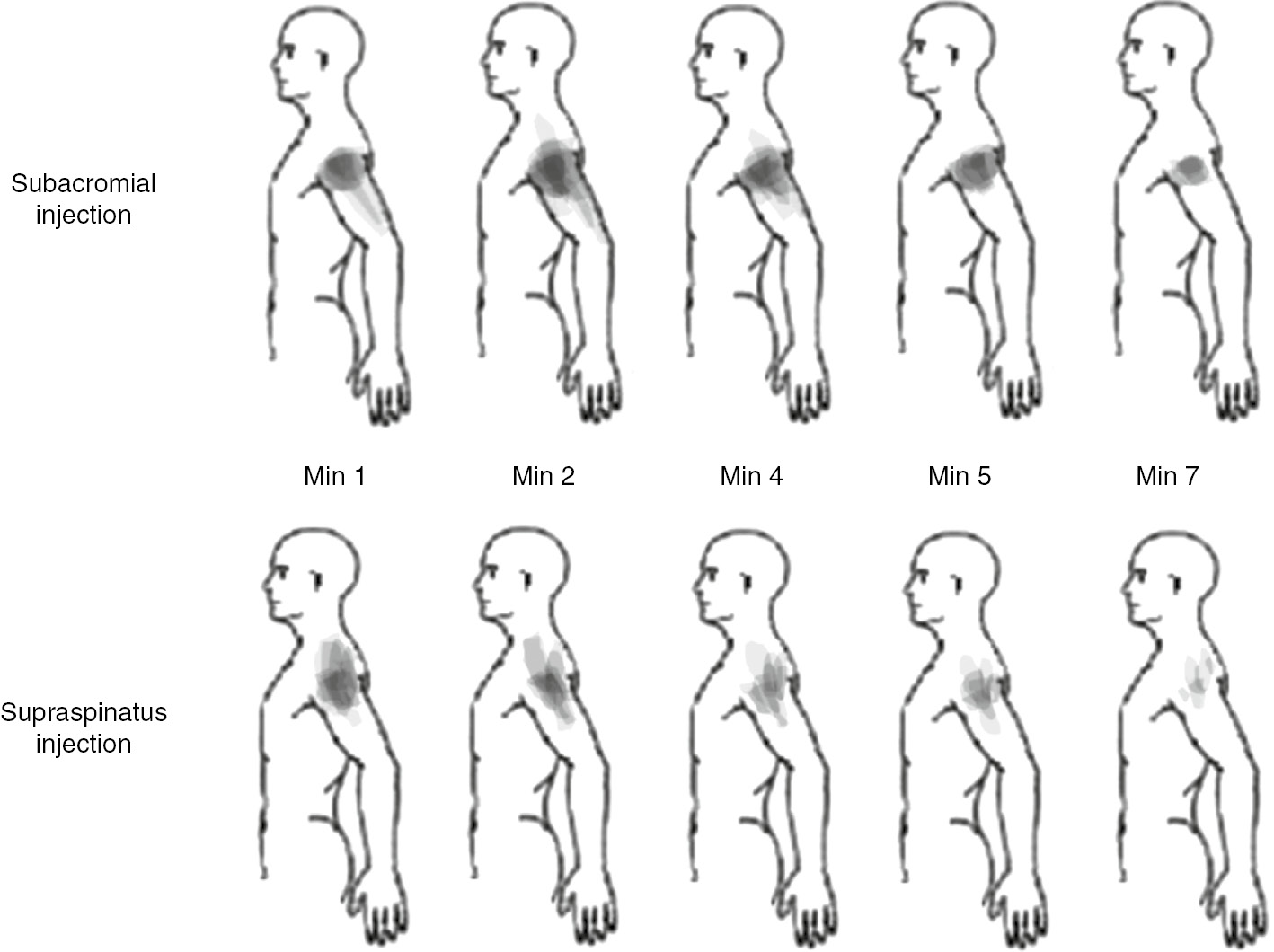
Composite body charts representing changes in pain distribution following injection of hypertonic saline. Coloured regions were formed by overlaying regions of pain for each participant. Darker colours indicate more participants reported pain in that area.
Distribution and time course of pain in participants.
| Minute 1 | Minute 2 | Minute 4 | Minute 5 | Minute 7 | |
|---|---|---|---|---|---|
| Subacromial injection | |||||
| Proximal upper arm (n) | 9 | 9 | 9 | 8 | 7 |
| Distal upper arm (n) | 2 | 3 | 3 | 0 | 0 |
| Neck (n) | 2 | 2 | 1 | 0 | 0 |
| Supraspinatus injection | |||||
| Proximal upper arm (n) | 8 | 8 | 7 | 6 | 4 |
| Distal upper arm (n) | 0 | 1 | 1 | 1 | 0 |
| Neck (n) | 5 | 4 | 3 | 3 | 3 |
-
This is a numerical representation of information provided in Fig. 2.
Following hypertonic saline injection into both the subacromial space and supraspinatus the most common response to pain provocation testing was a decrease in pain levels (Table 3). After each provocation test, the reported level of shoulder pain was substantially less than 7/10 in most participants.
Response to pain provocation testing showing pain change scores for those who reported an increase in pain, no change in pain, or a decrease in pain on the NRS.
| Hypertonic subacromial injection |
Hypertonic supraspinatus injection |
|||||
|---|---|---|---|---|---|---|
| Increase | No change | Decrease | Increase | No change | Decrease | |
| Hawkins-Kennedy test | ||||||
| n | 1 | 2 | 6 | 0 | 3 | 6 |
| Mean (SD) change in pain | 1 (NA) | – | 2.7 (1.3) | NA | – | 3.8 (2.1) |
| Empty can test | ||||||
| n | 1 | 3 | 5 | 2 | 3 | 4 |
| Mean (SD) change in pain | 1 (NA) | – | 3.6 (2.1) | 1 (0) | – | 3.5 (2.5) |
| Active abduction | ||||||
| n | 0 | 2 | 7 | 1 | 2 | 6 |
| Mean (SD) change in pain | NA | – | 2.9 (1.8) | 1 (NA) | – | 2.7 (1.4) |
The results of the MPQ are summarised in Table 4. There was no difference in the quality of pain reported from subacromial or supraspinatus hypertonic saline injections. The most common descriptor used for both conditions was “aching” (six for each condition) while five participants described the subacromial injection as “throbbing” compared with two for supraspinatus injection.
McGill Pain Questionnaire rating values for hypertonic supraspinatus and subacromial injection conditions.
| Component (maximum score) | Hypertonic supraspinatus injection | Hypertonic subacromial injection |
|---|---|---|
| Sensory (out of 42) | ||
| Mean (SD) | 7.9 (4.1) | 7.9 (2.2) |
| Percentage of maximum score | 18.8 | 18.8 |
| Affective (out of 14) | ||
| Mean (SD) | 0.3 (0.7) | 0.1 (0.3) |
| Percentage of maximum score | 2.1 | 0.7 |
| Evaluative (out of 5) | ||
| Mean (SD) | 1.1 (1.6) | 1.2 (1.9) |
| Percentage of maximum score | 22.0 | 24.0 |
| Miscellaneous (out of 17) | ||
| Mean (SD) | 1.4 (2.0) | 1.2 (1.8) |
| Percentage of maximum score | 8.2 | 7.1 |
| Total score (out of 78) | 10.8 (5.0) | 10.4 (4.1) |
| Percentage of maximum score | 13.8 | 13.3 |
All participants experienced pain following hypertonic saline injection into the supraspinatus muscle and into the subacromial space. Pain scores over time are shown in Fig. 3. There were no significant differences between the amount of pain experienced from subacromial or supraspinatus hypertonic saline injections at any time point (p=0.157–0.916). Following hypertonic saline subacromial injection, all participants were pain-free by 10 min post injection. Following supraspinatus hypertonic saline injection, two out of the nine participants had an NRS score of 1 at 10 min post-injection.
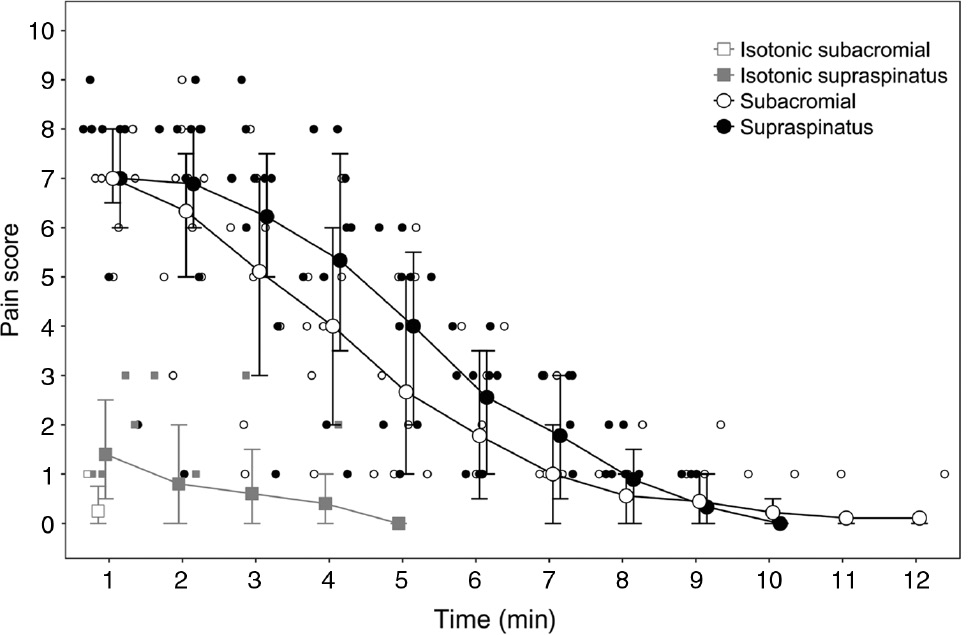
Time-course of pain following injection of either hypertonic or isotonic saline in both supraspinatus and the subacromial space. Group median (error bars: interquartile range) are shown with larger markers. Individual data for the nine participants are shown with smaller markers.
Isotonic saline injections produced a mean (SD) peak NRS of 1.4 (1.1) at the supraspinatus site and 0.3 (0.5) at the subacromial space site. Isotonic saline injections at either site produced a shorter duration of pain than hypertonic saline injections.
4 Discussion
The results of this study indicate no difference between the experimental shoulder pain protocols investigated. Injection of hypertonic saline into the subacromial space and into supraspinatus muscle consistently reproduced the location of clinical shoulder pain i.e. in the lateral shoulder and proximal upper arm. However, both protocols failed to replicate the quality of the clinical experience and the behaviour of clinical shoulder pain in response to provocation tests. Indeed, pain provocation tests generally alleviated rather than provoked experimental shoulder pain. These results indicate that pain induced by local shoulder nociception produced by hypertonic saline injection has significant limitations as a model of clinical shoulder pain.
The pattern of experimental shoulder pain induced in the current study is consistent with the distribution of clinical shoulder pain. An investigation of 285 patients who had posterosuperior rotator cuff tear found that the majority (86%) reported pain in the anterolateral region of the shoulder and upper arm which rarely extended past the elbow [23]. In patients with various structural deficits at the shoulder, the most common location of pain was the anterolateral shoulder and upper arm [24], [25]. The pattern of pain induced in the current study is broadly consistent with these clinical findings.
The results of this study support the findings of previous research reporting pain distribution following hypertonic saline injection into the subacromial space [4], [26], where all participants experienced pain over the lateral proximal arm. However, in these previous studies a small proportion of participants also reported pain distal to the elbow. No previous studies have described pain distribution following injection of hypertonic saline into the supraspinatus muscle.
The provocation tests performed in the current study represent a typical range of tests used to elicit clinical shoulder pain. To validly reflect the clinical experience, an experimental model should be associated with increased induced shoulder pain during provocation testing. However, following injection of hypertonic saline, the most common experience reported by participants in the current study was a decrease in shoulder pain intensity regardless of whether the provocation test was performed passively, dynamically, or isometrically. A total of 54 provocation tests were performed and an increase in experimental shoulder pain intensity occurred in only five of these tests (two following hypertonic saline injection into the subacromial space and three following injection into supraspinatus). These results indicate that neither of the experimental models in the current study validly replicated the clinical experience of shoulder pain with respect to pain behaviour.
This is the only study to investigate the experimental pain response to typically provocative manoeuvres following hypertonic saline injection into the subacromial space and supraspinatus. However, our finding that there was no change or, more commonly, a decrease in experimental pain intensity following typical tests known to increase shoulder pain in patients is consistent with a previous study at the shoulder investigating the experimental pain response to provocation following hypertonic saline injection into the acromioclavicular joint [26]. In this previous study, provocation testing did not change experimental pain intensity in the majority of subjects.
There were no differences in the quality of pain experienced following hypertonic saline injection into either the subacromial space or supraspinatus. The score on the evaluative component of the MPQ, considered a measure of the intensity of the pain experience [21], was approximately 23% of the highest possible score for both experimental shoulder pain conditions. Most words chosen were in the sensory component of the MPQ with a rating of approximately 19% of the highest possible value for both conditions. Low pain descriptor scores were recorded in the miscellaneous (approximately 8% of the highest possible score) and affective components (less than 3% of the highest possible score) of the MPQ for both the conditions.
Quality of pain reported during clinical shoulder pain has been investigated in one study [8], which found higher ratings in the affective component of the MPQ (35.5% of total MPQ score) compared with the current study (supraspinatus injection site 2.1% of total MPQ score; subacromial injection site 0.7% of total MPQ score). As affective descriptors of pain are a measure of emotional distress [21] it is not surprising that short duration, chemically-induced experimental pain is unable to replicate the emotional state associated with clinical shoulder pain.
Isotonic saline injections produced either no pain or very mild pain. When participants did report mild pain, it was located in the immediate vicinity of the injection site with no pain referral. These results indicate that the pain felt during the experimental pain protocols is more likely due to the chemical irritation from the hypertonic saline rather than the mechanical effects of the volume of saline injected.
In the current study highly consistent results were demonstrated in our sample of nine participants. Both experimental shoulder pain protocols investigated consistently reproduced the most common location of clinical shoulder pain, scored similarly on the MPQ and, in over 90% of tests designed to provoke clinical shoulder pain, did not result in an increase in experimental pain intensity. As stated above, it is probably unsurprising that these commonly used experimental shoulder pain models are unable to replicate the quality of the clinical pain experience. However, it is concerning that they do not replicate clinical pain behaviour. Not only did experimental shoulder pain not increase following provocation testing, the majority of participants in this study reported a decrease in shoulder pain. The validity of experimental pain models which produce the opposite response to provocation testing to clinical pain must be questioned. It has to be concluded, therefore, that pain induced by local shoulder nociception produced by hypertonic saline injection has significant limitations as a model of clinical shoulder pain.
-
Authors’ statements
-
Research funding: Authors state no funding involved.
-
Conflict of interest: Authors state no conflict of interest.
-
Informed consent: Informed consent has been obtained from all individuals included in this study.
-
Ethical approval: The research related to human use complies with all the relevant national regulations, institutional policies and was performed in accordance with the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional human ethics review board.
-
Author contributions: KG conceived the study and KG, BF, MC, MH & JD participated in the design of the study, data collection, data analysis and preparation of the manuscript.
References
[1] Chester R, Smith TO, Hooper L, Dixon J. The impact of subacromial impingement syndrome on muscle activity patterns of the shoulder complex: a systematic review of electromyographic studies. BMC Musculoskelet Disord 2010;11:45.10.1186/1471-2474-11-45Suche in Google Scholar PubMed PubMed Central
[2] Kibler WB, Sciascia A. Current concepts: scapular dyskinesis. BJSM Online 2010;44:300–5.10.1136/bjsm.2009.058834Suche in Google Scholar PubMed
[3] Diederichsen LP, Winther A, Dyhre-Poulsen P, Krogsgaard MR, Norregaard J. The influence of experimentally induced pain on shoulder muscle activity. Exp Brain Res 2009;194: 329–37.10.1007/s00221-008-1701-5Suche in Google Scholar PubMed
[4] Stackhouse SK, Eisennagel A, Eisennagel J, Lenker H, Sweitzer BA, McClure PW. Experimental pain inhibits infraspinatus activation during isometric external rotation. J Shoulder Elbow Surg 2013;22:478–84.10.1016/j.jse.2012.05.037Suche in Google Scholar PubMed
[5] Wassinger CA, Sole G, Osborne H. The role of experimentally-induced subacromial pain on shoulder strength and throwing accuracy. Man Ther 2012;17:411–5.10.1016/j.math.2012.03.008Suche in Google Scholar PubMed
[6] Diercks R, Bron C, Dorrestijn O, Meskers C, Naber R, de Ruiter T, Willems J, Winters J, van der Woude HJ; Dutch Orthopaedic Association. Guideline for diagnosis and treatment of subacromial pain syndrome A multidisciplinary review by the Dutch Orthopaedic Association. Acta Orthop 2014;85:314–22.10.3109/17453674.2014.920991Suche in Google Scholar PubMed PubMed Central
[7] Ludewig PM, Cook TM. Alterations in shoulder kinematics and associated muscle activity in people with symptoms of shoulder impingement. Phys Ther 2000;80:276–91.10.1093/ptj/80.3.276Suche in Google Scholar
[8] Camargo P, Haik M, Mattiello-Rosa S, Salvini T. Pain in workers with shoulder impingement syndrome: an assessment using the DASH and McGill pain questionnaires. Braz J Phys Ther 2007;11:161–7.10.1590/S1413-35552007000200012Suche in Google Scholar
[9] Jobe FW, Moynes DR. Delineation of diagnostic-criteria and a rehabilitation program for rotator cuff injuries. Am J Sports Med 1982;10:336–9.10.1177/036354658201000602Suche in Google Scholar PubMed
[10] Hawkins RJ, Kennedy JC. Impingement syndrome in athletes. Am J Sports Med 1980;8:151–8.10.1177/036354658000800302Suche in Google Scholar PubMed
[11] Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94:149–58.10.1016/S0304-3959(01)00349-9Suche in Google Scholar PubMed
[12] Downie WW, Leatham PA, Rhind VM, Wright V, Branco JA, Anderson JA. Studies with pain rating-scales. Ann Rheum Dis 1978;37:378–81.10.1136/ard.37.4.378Suche in Google Scholar PubMed PubMed Central
[13] Ferraz MB, Quaresma MR, Aquino LRL, Atra E, Tugwell P, Goldsmith CH. Reliability of pain scales in the assessment of literate and illiterate patients with rheumatoid-arthritis. J Rheumatol 1990;17:1022–4.Suche in Google Scholar
[14] Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain 2011;152:2399–404.10.1016/j.pain.2011.07.005Suche in Google Scholar PubMed
[15] Timmons MK, Ericksen JJ, Yesilyaprak SS, Michener LA. Empty can exercise provokes more pain and has undesirable biomechanics compared with the full can exercise. J Shoulder Elbow Surg 2016;25:548–56.10.1016/j.jse.2015.08.046Suche in Google Scholar PubMed
[16] Boettcher CE, Ginn KA, Cathers I. Standard maximum isometric voluntary contraction tests for normalizing shoulder muscle EMG. J Orthop Res 2008;26:1591–7.10.1002/jor.20675Suche in Google Scholar PubMed
[17] Kelly BT, Kadrmas WR, Speer KP. The manual muscle examination for rotator cuff strength an electromyographic investigation. Am J Sports Med 1996;24:581–8.10.1177/036354659602400504Suche in Google Scholar PubMed
[18] Ganestam A, Attrup ML, Hølmich P, Barfod KW. Evaluation of the clinical practice of shoulder examination among ten experienced shoulder surgeons. J Orthop Res Physiother 2015;1:008.10.24966/ORP-2052/100008Suche in Google Scholar
[19] Hegedus EJ. Which physical examination tests provide clinicians with the most value when examining the shoulder? Update of a systematic review with meta-analysis of individual tests. BJSM Online 2012;46:964–78.10.1136/bjsports-2012-091066Suche in Google Scholar PubMed
[20] Kessel L, Watson M. The painful arc syndrome. Clinical classification as a guide to management. Bone Joint J 1977;59:166–72.10.1302/0301-620X.59B2.873977Suche in Google Scholar PubMed
[21] Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain 1975;1:277–99.10.1016/0304-3959(75)90044-5Suche in Google Scholar PubMed
[22] Byrne M, Troy A, Bradley LA, Marchisello PJ, Geisinger KF, Van der Heide LH, Prieto EJ. Cross-validation of the factor structure of the Mcgill Pain Questionnaire. Pain 1982;13:193–201.10.1016/0304-3959(82)90029-XSuche in Google Scholar PubMed
[23] Gumina S, Candela V, Passaretti D, Venditto T, Carbone S, Arceri V, Giannicola G. Intensity and distribution of shoulder pain in patients with different sized postero-superior rotator cuff tears. J Shoulder Elbow Surg 2014;23:807–13.10.1016/j.jse.2013.09.011Suche in Google Scholar PubMed
[24] Bayam L, Ahmad MA, Naqui SZ, Chouhan A, Funk L. Pain mapping for common shoulder disorders. Am J Orthop 2011;40:353–8.Suche in Google Scholar
[25] Singh S, Mohammad F, Gill S, Kumar D, Kumar S. Role of pain mapping in shoulder disorders. Int J Orthop 2015;2:323–7.10.17554/j.issn.2311-5106.2015.02.80Suche in Google Scholar
[26] Gerber C, Galantay RV, Hersche O. The pattern of pain produced by irritation of the acromioclavicular joint and the subacromial space. J Shoulder Elbow Surg 1998;7:352–5.10.1016/S1058-2746(98)90022-2Suche in Google Scholar PubMed
Article note
Some of the data in this manuscript have been published as an abstract of a presentation at the World Congress of Physiotherapy in Cape Town in 2017: Ford B et al “Determining a valid experimental shoulder pain model” RR-PO-08-08.
©2020 Scandinavian Association for the Study of Pain. Published by Walter de Gruyter GmbH, Berlin/Boston. All rights reserved.
Artikel in diesem Heft
- Frontmatter
- Editorial
- Change in Editorship: A Tribute to the Outgoing Editor-in-Chief
- Editorial comments
- Laboratory biomarkers of systemic inflammation – what can they tell us about chronic pain?
- Considering the interpersonal context of pain catastrophizing
- Systematic review
- Altered pain processing and sensitisation is evident in adults with patellofemoral pain: a systematic review including meta-analysis and meta-regression
- Topical reviews
- Pain revised – learning from anomalies
- Role of the immune system in neuropathic pain
- Clinical pain research
- Cryoneurolysis for cervicogenic headache – a double blinded randomized controlled study
- Interpersonal problems as a predictor of pain catastrophizing in patients with chronic pain
- Pain and small-fiber affection in hereditary neuropathy with liability to pressure palsies (HNPP)
- Predicting the outcome of persistent sciatica using conditioned pain modulation: 1-year results from a prospective cohort study
- Observational studies
- Revised chronic widespread pain criteria: development from and integration with fibromyalgia criteria
- The relationship between patient factors and the refusal of analgesics in adult Emergency Department patients with extremity injuries, a case-control study
- Chronic neuropathic pain after traumatic peripheral nerve injuries in the upper extremity: prevalence, demographic and surgical determinants, impact on health and on pain medication
- Tramadol prescribed use in general and chronic noncancer pain: a nationwide register-based cohort study of all patients above 16 years
- Changes in inflammatory plasma proteins from patients with chronic pain associated with treatment in an interdisciplinary multimodal rehabilitation program – an explorative multivariate pilot study
- Original experimental
- The pro-algesic effect of γ-aminobutyric acid (GABA) injection into the masseter muscle of healthy men and women
- The relationship between fear generalization and pain modulation: an investigation in healthy participants
- Experimental shoulder pain models do not validly replicate the clinical experience of shoulder pain
- Computerized quantification of pain drawings
- Head repositioning accuracy is influenced by experimental neck pain in those most accurate but not when adding a cognitive task
- Short communications
- Dispositional empathy is associated with experimental pain reduction during provision of social support by romantic partners
- Superior cervical sympathetic ganglion block under ultrasound guidance promotes recovery of abducens nerve palsy caused by microvascular ischemia
Artikel in diesem Heft
- Frontmatter
- Editorial
- Change in Editorship: A Tribute to the Outgoing Editor-in-Chief
- Editorial comments
- Laboratory biomarkers of systemic inflammation – what can they tell us about chronic pain?
- Considering the interpersonal context of pain catastrophizing
- Systematic review
- Altered pain processing and sensitisation is evident in adults with patellofemoral pain: a systematic review including meta-analysis and meta-regression
- Topical reviews
- Pain revised – learning from anomalies
- Role of the immune system in neuropathic pain
- Clinical pain research
- Cryoneurolysis for cervicogenic headache – a double blinded randomized controlled study
- Interpersonal problems as a predictor of pain catastrophizing in patients with chronic pain
- Pain and small-fiber affection in hereditary neuropathy with liability to pressure palsies (HNPP)
- Predicting the outcome of persistent sciatica using conditioned pain modulation: 1-year results from a prospective cohort study
- Observational studies
- Revised chronic widespread pain criteria: development from and integration with fibromyalgia criteria
- The relationship between patient factors and the refusal of analgesics in adult Emergency Department patients with extremity injuries, a case-control study
- Chronic neuropathic pain after traumatic peripheral nerve injuries in the upper extremity: prevalence, demographic and surgical determinants, impact on health and on pain medication
- Tramadol prescribed use in general and chronic noncancer pain: a nationwide register-based cohort study of all patients above 16 years
- Changes in inflammatory plasma proteins from patients with chronic pain associated with treatment in an interdisciplinary multimodal rehabilitation program – an explorative multivariate pilot study
- Original experimental
- The pro-algesic effect of γ-aminobutyric acid (GABA) injection into the masseter muscle of healthy men and women
- The relationship between fear generalization and pain modulation: an investigation in healthy participants
- Experimental shoulder pain models do not validly replicate the clinical experience of shoulder pain
- Computerized quantification of pain drawings
- Head repositioning accuracy is influenced by experimental neck pain in those most accurate but not when adding a cognitive task
- Short communications
- Dispositional empathy is associated with experimental pain reduction during provision of social support by romantic partners
- Superior cervical sympathetic ganglion block under ultrasound guidance promotes recovery of abducens nerve palsy caused by microvascular ischemia


