Abstract
Objectives
Vaccines are used on a large scale for prevention of disease. Preparing vaccines for administration can be a time consuming process. To increase efficacy of vaccine administration, the Vaxtractor was designed in January 2021. With the Vaxtractor, the desired volume of vaccine is drawn up automatically in syringes from two vials of vaccine simultaneously. We examined the quality of COVID-19 vaccines prepared with the Vaxtractor.
Methods
Sterility tests and uniformity of dosage units tests were performed. For the sterility test, 22 syringes were filled with 0.5 mL Tryptic Soy Broth and these were incubated at 25 °C for seven days followed by seven days at 30 °C. For the dosage unit test, the difference between the filled and empty syringe was used to compute the volume of the injectable volume. A time analysis was performed on manually and semi-automatically prepared vaccines.
Results
The sterility tests showed no signs of growth of micro-organisms. After optimizing the Vaxtractor, none of the Comirnaty® vaccines deviated more than 10 % and none of the Spikevax® vaccines deviated more than 5 % compared to the mean mass of the injectable volume. The acceptance value for uniformity of dosage units of both vaccines was below 4 (requirement <15). Preparing vaccines with the Vaxtractor was faster compared to manually prepared vaccines.
Conclusions
The Vaxtractor can be used to safely prepare Spikevax® and Comirnaty® vaccines. Further studies should explore the applicability of the Vaxtractor for the preparation of other vaccines. If applicable, this will contribute to effective upscaling of vaccination programs.
Introduction
Worldwide, a large number of vaccines are yearly administrated to increase public health [1]. For example, during the COVID-19 pandemic more than thirteen billion doses of COVID-19 vaccines were administrated to reduce mortality and morbidity [2, 3].
As preparing vaccines before administration can be a time consuming process, it is desirable that a device is developed which accelerates the process and increases efficacy for health care workers. Additionally, using a device to prepare vaccines can reduce the risk of needlestick injuries among health care workers. This is important because the risk of needlestick injuries is increased during mass vaccination programs [4, 5].
To overcome aforementioned drawbacks a device called ‘the Vaxtractor’ was developed in January 2021. With the Vaxtractor, the desired volume of vaccine is drawn up automatically in syringes from two vials of vaccine simultaneously. Until now, it is unknown if the Vaxtractor can be safely used to prepare vaccines. We aimed to examine the quality of COVID-19 vaccines prepared with the Vaxtractor.
Materials and methods
Setting
The study was performed at the Erasmus Medical Center from September to December 2021.
Specifications of the Vaxtractor
The Vaxtractor (©Atlas Metaal BV, Mierlo, the Netherlands) is a relatively small device (71 × 37.5 × 49 cm) (Figure 1). The device contains two holders (one at each site) for vials of vaccines which are available in different sizes depending on the type of vaccine. Besides that, it contains two removable syringe holders (one at each site) which can be loaded with a maximum of twelve syringes of 1 mL. With the Vaxtractor, the desired volume of vaccine is drawn up automatically in syringes from two vials of vaccine simultaneously.
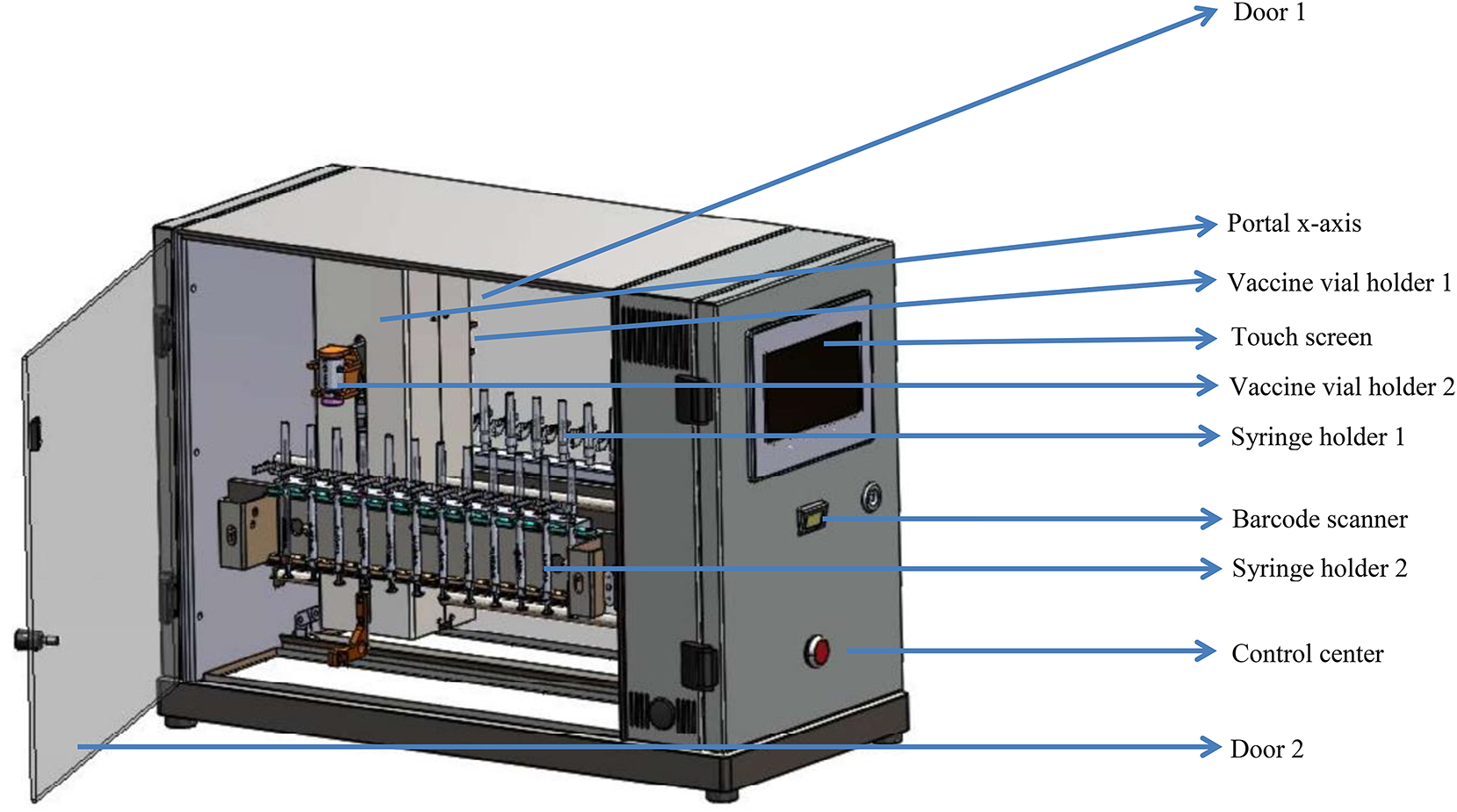
Lay-out of the Vaxtractor. The Vaxtractor is a symmetrical device with on both sides a vaccine vial holder and a syringe holder which can be loaded with a maximum of twelve syringes.
Operation of the Vaxtractor
Before the Vaxtractor can be used, two manual actions have to be performed. First, two vials of vaccine have to be placed in the vaccine vial holders (one at each side). Second, syringes have to be assembled and placed in the syringe holders. For the COVID-19 vaccines Spikevax® and Comirnaty® eleven and six syringes are positioned in one syringe holder, respectively.
Before the vaccines are drawn up, the type of vaccine, the batch number of the vaccine, and the type of syringe are set using the touchscreen or a 2D scanner. Consequently, the Vaxtractor activates the appropriate program. This program contains the number of syringes to prepare, the volume of each syringe and the speed of syringe filling.
After the two vials of vaccine and the two syringe holders are placed at each site of the Vaxtractor, the device starts to run after pressing the ‘play’-button on the touch screen. When the Vaxtractor starts, firstly it removes the protection cap from the needles. Then, the portal moves from left to right to position all syringes at the same height. Consequently, the portal moves from right to left to drawn up the desired volume of vaccine in the syringes. Each syringe punctures the rubber stopper of the vial of the vaccine at a slightly different position to prevent leakage of liquid from the vial. After the syringes are filled, the protection caps are replaced on the needles and the syringe holders are manually removed from the Vaxtractor.
Tests to examine the quality of COVID-19 vaccines prepared with the Vaxtractor
To determine the quality of Spikevax® and Comirnaty® vaccines prepared with the Vaxtractor, two tests were performed: a sterility test and a uniformity of dosage units test. Besides that, the vaccines were checked on the presence of visible particles and air bubbles. These tests were performed with a prototype of the Vaxtractor. This prototype was not able to automatically remove the protection cap from the needles. Because of this, these actions were manually performed. Besides that, the dilution step of the Spikevax® liquid was manually performed instead of using the Vaxtractor.
Sterility test
A sterility test was performed to examine microbiological contamination caused by the Vaxtractor or the process. The sterility test was performed in a non-classified area. The researcher (DN) who performed the sterility test wore sterile gloves and used sterile needles (BD safety needle ECLIPSE 23G 25 × 0.60 mm blue) and sterile syringes (BD Plastipak injection syringe 1 mL).
For the sterility test, 22 syringes were filled with 0.5 mL Tryptic Soy Broth (TSB-K111F009QQ) [6]. These syringes were incubated at 25 °C for seven days followed by a seven day incubation period at 30 °C. After these two weeks, five drops of each syringe were inoculated on a Tryptic Soy Agar (TSA) plate. Furthermore, each needle was rolled over a TSA contact plate. Both plates were incubated at 30 °C for two days. According to the European Pharmacopeia 2.6.1 Sterility no growth of micro-organisms should be detected after the incubation period [7]. Furthermore, the monograph advises a growth promotion test to determine if the used media was suitable for growth of micro-organisms [7]. For this test, three strains of test micro-organisms were used: Escherichia coli (ATCC 25922), Staphylococcus epidermidis (ATCC 12228), and Enterococcus faecalis (ATCC 29212). TSA plates loaded with these micro-organism strains were also incubated at 25 °C for seven days followed by a seven day incubation period at 30 °C.
Test on dosage units
To determine the uniformity of mass, the monographs of the European Pharmacopoeia for single-dose preparations and the guideline for uniformity of dosage units were applied [8, 9]. For this test, 60 syringes of each type of vaccine (30 syringes from each side of the device) were prepared with the Vaxtractor. For comparison, 30 Comirnaty® vaccines were prepared by pharmacy technicians. The Comirnaty® vaccines were selected over the day (from 8 am to 12 am) to monitor changes over time by the Vaxtractor and/or pharmacy technicians.
For both guidelines, the differences in mass between the filled and emptied syringes were calculated and the mean mass of the injectable volume was determined [8, 9]. The deviation between each individual measurement compared to the mean mass of the injectable volume was calculated. According to the European Pharmacopeia 2.9.5 uniformity of mass of single-dose preparations, a maximum of two units may deviate 5 % and none unit may deviate 10 % compared to the mean mass [9].
For the test on dosage units, the difference between the filled and empty syringe was used to compute the volume of the injectable volume; the mass of each was divided by the density of the liquid provided by the manufacturer (1.01 g/mL) [10]. As each unit in a batch should have active substance content within a narrow range around the labelled claim, we calculated the expected amount of active substance based on the calculated amount of injectable volume. Subsequently, the acceptance value was calculated based on the method described in European Pharmacopeia 2.9.40 uniformity of dosage units. The requirements for uniformity of dosage are met if the acceptance value of 30 dosage units is less than or equal to 15 and no individual content of the dosage units is less or more than 15 % compared to the labelled claim [8].
Absence of visible particles
As the same needle is used to drawn up the desired volume of vaccine with the Vaxtractor and to inject intramuscularly, a visible check on the presence of visible particles was applied. If particles derived from the rubber stopper of the vail were discovered in the syringes, the Vaxtractor should not be used to prepare COVID-19 vaccines.
Absence of air bubbles
As the presence of air bubbles can result in a change of the desirable volume that is injected and can result in health damage, we determined that the vaccines should be free of large air bubbles [11]. Because of this, each prepared vaccine was checked for the amount of air bubbles and the presence of large air bubbles. As there is no requirement for the maximum size of an air bubble in intramuscular syringes, we have chosen that air bubbles smaller than approximately 2 mm were acceptable (minimal effect on changing the desirable volume of the vaccines).
Time analysis
To determine the benefits in time of preparing COVID-19 vaccines for administration with the Vaxtractor, the mean time to manually prepare Spikevax® and Comirnaty® vaccines was determined. For this, the registration data of COVID-19 mass vaccinations days were used. The registration forms of different days, time of the day and pharmacy technicians were selected. For each pharmacy technician, the mean time to prepare one vial of Spikevax® and Comirnaty® was calculated. Afterwards, the overall mean time of preparing one vial of each vaccine was determined.
Results
Sterility test
All 22 syringes and the Tryptic Soy Agar plates were clear from visible growth of micro-organisms. Besides that, the growth promotion tests were positive.
Dosage units test
Spikevax® vaccines prepared with the Vaxtractor
All 60 vaccines deviated less than 5 % compared to the mean mass of the injectable volume (Figure 2). The mean volume of the prepared Spikevax® vaccines was 0.5 mL. The acceptance value for the left and right motor of the Vaxtractor was 2.5 and 3.4 respectively and no individual content of the dosage units was less or more than 15 percent compared to the labelled claim. Finally, there were no visible particles and the vaccines contained no (large) air bubbles or only a small amount of minuscule air bubbles.

Deviation between the individual and mean mass of injectable volume of Spikevax® vaccines. All Spikevax® vaccines prepared with the Vaxtractor deviated less than 5 % compared to the mean mass of the injectable volume.
Comirnaty® vaccines prepared by the Vaxtractor
Program setting one
In total, 22 out of the 60 vaccines deviated more than 5 % compared to the mean mass of the injectable volume (Figure 3). The mean volume of the prepared Comirnaty® vaccines was 0.29 mL. The acceptance value for the left and right motor of the Vaxtractor was 3.9 and 5.8 respectively and the individual content of one vaccine was less than 15 percent compared to the labelled claim. There were no visible particles, however large air bubbles (>2 mm) were observed in the vaccines.

Deviation between the individual and mean mass of injectable volume of Comirnaty® vaccines (setting one). Twenty two out of the 60 Comirnaty® vaccines prepared with the Vaxtractor (first setting) deviated more than 5 % compared to the mean mass of the injectable volume. One vaccine deviated 20.6 %, five vaccines had a deviation between the 10–15 %, and 16 vaccines had a deviation between the 5–10 %.
Program setting two
Because of above deviations, the manufacturer introduced a venting step in the program of the Vaxtractor to reduce the amount of air bubbles in the vaccines. With this setting, 2 of the 60 syringes deviated more than 5 % and 1 of the 60 syringes deviated 11.2 % compared to the mean mass of the injectable volume (Figure 4). The mean volume of the prepared Comirnaty® vaccines was 0.30 mL. The acceptance value for the left and right motor of the Vaxtractor was 1.5 and 1.8, respectively. No individual content of the dosage units was less or more than 15 percent compared to the labelled claim and there were no visible particles. When setting two was tested in clinical practice, 24 out of the 56 vaccines contained large air bubbles (>2 mm), and needed to be discarded.

Deviation between the individual and mean mass of injectable volume of Comirnaty® vaccines (setting two). Three out of 60 Comirnaty® vaccines prepared with the Vaxtractor (second setting) deviated more than 5 % compared to the mean mass of injectable volume.
Program setting three
As there were large air bubbles presented in the prepared vaccines of setting two, an additional venting step was introduced. Subsequently, 4 of the 60 Comirnaty® vaccines deviated more than 5 % (but less than 10 %) compared to the mean mass of the injected volume (Figure 5). The acceptance value for the left and right motor of the Vaxtractor was 2.9 and no individual content of the dosage units was less or more than 15 percent compared to the labelled claim. Furthermore, there were no signs of visible particles in the vaccines and the vaccines contain no (large) air bubbles or only a small amount of minuscule air bubbles. One vaccine of 60 was rejected due to the presence of a large air bubble (>2 mm).

Deviation between the individual and mean mass of injectable volume of manually and semi-automatically prepared Comirnaty® vaccines. Four of the 60 vaccines prepared with the Vaxtractor (third setting) and 1 out of the 30 manually prepared vaccines deviated more than 5 % (but less than 10 %) compared to the mean mass of injectable volume.
Comirnaty® vaccines prepared by pharmacy technicians
One of the 30 vaccines prepared by the pharmacy technicians deviated more than 5 % compared to the mean mass of injectable volume (Figure 5). The acceptance value was 3.4 and no individual content of the dosage units was less or more than 15 percent compared to the labelled claim. Furthermore, there were no signs of visible particles in the vaccines and the vaccines contain no (large) air bubbles or only a small amount of minuscule air bubbles.
Time analysis of semi-automatically vs. manually prepared COVID-19 vaccines
We used the registration data of 17 pharmacy technicians. The mean time to manually prepare 11 syringes (corresponding to one vial) of Spikevax® was 7.2 min. A run of the Vaxtractor (in which 22 vaccines were drawn up) takes about 5 min (Table 1). This includes filling and changing the syringe holders for the next run. Altogether, semi-automatically preparing of Spikevax® vaccines was two to three times faster than the manual preparation of the vaccines.
Comparison of the efficiency and accuracy of manually and semi-automatically prepared vaccines.
| Spikevax® vaccines | Comirnaty® vaccines | |||
|---|---|---|---|---|
| Manually prepared vaccines | Semi-automatically prepared vaccines (with the Vaxtractor) | Manually prepared vaccines | Semi-automatically prepared vaccines (with the Vaxtractor, setting 3) | |
|
|
||||
| Efficiency: | ||||
|
|
||||
| Time to prepare 2 vials of vaccine, min | 14.4 | 5 | 9.4 | 4 |
|
|
||||
| Accuracy: | ||||
|
|
||||
| Number of syringes with <5 % deviation compared to the mean mass of the injected volume | –a | 60 of the 60 vaccines | 29 of 30 vaccines | 56 of the 60 vaccines |
| Acceptance value | –a | 3.4 | 3.4 | 2.9 |
| Number of syringes with large air bubbles (>2 mm) | –a | 0 of the 60 vaccines | 0 of 30 vaccines | 1 of 60 vaccines |
-
aSpikevax vaccines were not available at the time of the manual vaccination campaign.
For Comirnaty® vaccines, the mean time of eight pharmacy technicians to prepare on average 6.5 vaccines (corresponding to one vial) was 4.7 min. Preparing 12 vaccines with the Vaxtractor and filling the syringe holders for the next run takes about 4 min (Table 1). Therefore, semi-automatically preparing of Comirnaty® vaccines was almost two times faster than manually preparing the vaccines.
Discussion
This study investigates the quality of COVID-19 vaccines prepared with a semi-automatical device. We observed no growth of micro-organism in the syringes filled with Tryptic Soy Broth. After the Vaxtractor settings were optimized, the Spikevax® and Comirnaty® vaccines prepared using the Vaxtractor complied with the requirements described in the European Pharmacopeia 2.9.40 uniformity of dosage units (acceptance value <15). Also, the prepared vaccines contained no visible particles and the vaccines contain no large air bubbles and none or only an acceptable amount of minuscule air bubbles.
In order to evaluate the accuracy of the Vaxtractor, we have chosen to also check whether the prepared vaccines complied to the requirements in the European Pharmacopeia 2.9.5 uniformity of mass of single-dose preparations.
This paragraph is a quality demand for drug manufacturing and is not generally used for ready-to-administer compounding activities. We observed that, even after optimizing the Vaxtractor, the Comirnaty® vaccines prepared with the Vaxtractor did not meet the requirement described in this paragraph. The Comirnaty® vaccines prepared by pharmacy technicians did not meet this requirement either. Therefore, we conclude that the European Pharmacopeia 2.9.5 uniformity of mass of single-dose preparations is too strict to test uniformity of dosage units of vaccines with a small volume. With a small declared volume in a relatively heavy container like the syringe with an attached needle, there is a big chance of observing a deviation of more than 5 %. For the Comirnaty® vaccines, a deviation of 5 % is already achieved when 0.28 mL instead of 0.30 mL vaccine was drawn up. The clinical relevance of this deviation can be questioned. Altogether, we suggest that the requirement described in the European Pharmacopeia 2.9.40 uniformity of dosage units seems more suitable to determine the uniformity of low-volume vaccines. However, the accepted deviation described in this monograph–an active substance may deviate a maximum of 15 % – might be too large [8]. We suggest that, like the maximum deviation of an active pharmaceutical ingredient compared to the declared content of an active pharmaceutical ingredient, an individual vaccine should have a maximum deviation of 10 % compared to the declared content of active substance [12]. Besides aforementioned monographs of the European Pharmacopeia, another monograph which can potentially be used to assess the quality of ready-to-use compounding is the European Pharmacopeia 2.9.17 Test for extractable volume of parenteral preparations [13]. Our results nearly met the requirement that the extractable volume is not less than the nominal volume. As multiple monographs can potentially be used to determine the quality of ready-to-administer compounding, it seems advisable to determine specific demands for this situation. If these demands are developed, the quality of the vaccines prepared with the Vaxtractor should be examined in a study with a larger sample size in order to draw a more robust conclusion.
Finally, it is important to mention that the Vaxtractor is not qualified nor intended as a medical device [14]. Because of this, the Vaxtractor does not need to meet Medical Device Regulation requirements to ensure that all prepared vaccines contain the declared volume. To guarantee patient safety, the volume of the prepared vaccines and the absence of visible particles and air bubbles have to be visually checked by a health care worker before they are administered to a person. This additional manual check was incorporated in our time analysis. So, despite the visual check after preparing the vaccines, the Vaxtractor was still faster compared to manually preparation of the vaccines.
A strength of this study is that the dosage unit test was performed with both manually and semi-automatically prepared vaccines. We observed that both manually and semi-automatically prepared Comirnaty® vaccines did not meet the requirements described in the European Pharmacopeia 2.9.5 uniformity of mass of single-dose preparation, but they both do meet the requirements described in the European Pharmacopeia 2.9.40 uniformity of dosage units. Because of this, we have demonstrated that the quality of the semi-automatically prepared vaccines was non-inferior compared to manually prepared vaccines.
For the sterility test, a prototype of the Vaxtractor was used. This version of the device was not able to remove and replace protection caps of the needles. As these operations were manually performed, there was a higher risk of contamination of micro-organism compared to the final version of the Vaxtractor. As there was no growth of micro-organism in the sterility test performed with the prototype, the chance of micro-organism contamination in the final version of the Vaxtractor is small. Furthermore, a ready-to-use formulation of Comirnaty® is recently developed [15]. As a consequence, the manual dilution is no longer required.
We conclude that the Vaxtractor can be safely used to prepare Spikevax® and Comirnaty® vaccines. The prepared vaccines complied with the sterility and uniformity of dosage units test. Furthermore, this device reduced the time to prepare vaccines and may reduce the risk of needlestick injuries. Further studies should explore the applicability of the Vaxtractor for the preparation of other vaccines, for current and upcoming vaccination programmes the Vaxtractor can be used at large scale at the in-and out-of-hospital setting to accelerate vaccination programs.
-
Informed consent: Not applicable.
-
Ethical approval: Not applicable.
-
Competing interests: Authors state no competing of interest.
-
Research funding: None declared.
-
Author contributions: AD, DN, LR and LvR designed the study. DN and LvR collected the data. DN analysed and interpreted the data, and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
References
1. World Health Organization. Immunization coverage. 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/immunization-coverage.Search in Google Scholar
2. Global Change Data Lab. Coronavirus (COVID-19) deaths. 2023. Available from: https://ourworldindata.org/covid-deaths.Search in Google Scholar
3. Liu, Y, Sandmann, FG, Barnard, RC, Pearson, CA, Pastore, R, Pebody, R, et al.. Optimising health and economic impacts of COVID-19 vaccine prioritisation strategies in the WHO European Region: a mathematical modelling study. Lancet Reg Heal – Eur 2022;12:100267. https://doi.org/10.1016/j.lanepe.2021.100267.Search in Google Scholar PubMed PubMed Central
4. Persaud, E, Mitchell, A. Needlestick injuries among healthcare workers administering COVID-19 vaccinations in the United States. New Solut 2021;31:16–9. https://doi.org/10.1177/10482911211001483.Search in Google Scholar PubMed
5. Williams, NJ, Ghosh, TS, Vogt, RL. Needlestick injury surveillance during mass vaccination clinics: lessons learned and why more is needed—Tri-County (Denver Metropolitan) region, Colorado, 2009. Am J Infect Control 2012;40:768–70. https://doi.org/10.1016/j.ajic.2011.09.014.Search in Google Scholar PubMed
6. Biotrading. TSB. 2021. Available from: https://biotrading.com/product/tsb-k111f009qq/.Search in Google Scholar
7. European Directorate for the Quality of Medicines & HealthCare. European Pharmacopeoia. In: Section 6.2.1 sterility, 10th ed. Strasbourg: Council of Europe; 2008:191–4 pp.Search in Google Scholar
8. European Directorate for the Quality of Medicines & HealthCare. European Pharmacopoeia. In: Section 2.9.40 uniformity of dosage units, 10th ed. Strasbourg: Council of Europe; 2017:398–400 pp.Search in Google Scholar
9. European Directorate for the Quality of Medicnes & HealthCare. European Pharmacopoeia. In: Section 2.9.5 uniformity of mass of single-dose preparations, 10th ed. Strasbourg: Council of Europe; 2017:335–6 pp.Search in Google Scholar
10. BioNTech/Pfizer. Comirnaty (COVID-19 mRNA vaccine): density. Mainz: BioNTech Manufacturing GmbH; 2021:1 p.Search in Google Scholar
11. Rodger, MA, King, L. Drawing up and administering intramuscular injections: a review of the literature. J Adv Nurs 2000;31:574–82. https://doi.org/10.1046/j.1365-2648.2000.01312.x.Search in Google Scholar PubMed
12. Bouwman-Boer, Y, Le Brun, P, Oussoren, C, Tel, R, Woerdenbag, H. Recepteerkunde: productzorg en bereiding van geneesmiddelen, 5th ed. Houten: Bohn Stafleu van Loghum; 2009.10.1007/978-90-313-8032-9Search in Google Scholar
13. European Directorate for the Quality of Medicines & HealthCare. European pharmacopeoia. In: Section 2.9.17 test for extractable volume of parenteral preparations, 10th ed. Strasbourg: Council of Europe; 2008:347 p.Search in Google Scholar
14. The European Parliament and the Council of the European Union. Medical devices regulation. Strasbourg Cedex, France: Council of Europe Publishing; 2017:1–175 pp.Search in Google Scholar
15. European Medicnes Agency. New manufacturing sites and new formulation approved for COVID-19 vaccine from BioNTech/Pfizer. 2021. Available from: https://www.ema.europa.eu/en/news/new-manufacturing-sites-new-formulation-approved-covid-19-vaccine-biontech-pfizer.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Air contamination, syringe contamination, and cross-contamination when using an automatic compounding device for sensitizing drugs
- Long term physicochemical stability study of novel ophthalmic formulations combining ceftazidime and vancomycin with and without cyclodextrins
- Semi-automatic COVID-19 vaccine preparation for upscaling of vaccination: a descriptive study
- Physicochemical stability of Cabazitaxel Zentiva® solution in vials after opening and diluted solutions in three infusion bags
- Physicochemical stability of durvalumab (Imfinzi®) concentrate for solution in original vials after first opening
- Physicochemical stability of urea-containing Mitomycin C preparations in glass vials (1.0 mg/mL) and plastic syringes (2.0, 0.4, 0.2 mg/mL)
- Physicochemical stability study of a biosimilar of Bevacizumab in vials and after dilution in 0.9% NaCl in polyolefin intravenous bags
- Assessment of the relevance of osmolality measurement as a criterion for the stability of solutions
- Short Communications
- An exploratory study of a simplified approach to evaluate drug solubility in milk related vehicles
- Use of a liquid chromatography-tandem mass spectrometry method to assess the concentration of epinephrine, norepinephrine, and phenylephrine stored in plastic syringes
Articles in the same Issue
- Research Articles
- Air contamination, syringe contamination, and cross-contamination when using an automatic compounding device for sensitizing drugs
- Long term physicochemical stability study of novel ophthalmic formulations combining ceftazidime and vancomycin with and without cyclodextrins
- Semi-automatic COVID-19 vaccine preparation for upscaling of vaccination: a descriptive study
- Physicochemical stability of Cabazitaxel Zentiva® solution in vials after opening and diluted solutions in three infusion bags
- Physicochemical stability of durvalumab (Imfinzi®) concentrate for solution in original vials after first opening
- Physicochemical stability of urea-containing Mitomycin C preparations in glass vials (1.0 mg/mL) and plastic syringes (2.0, 0.4, 0.2 mg/mL)
- Physicochemical stability study of a biosimilar of Bevacizumab in vials and after dilution in 0.9% NaCl in polyolefin intravenous bags
- Assessment of the relevance of osmolality measurement as a criterion for the stability of solutions
- Short Communications
- An exploratory study of a simplified approach to evaluate drug solubility in milk related vehicles
- Use of a liquid chromatography-tandem mass spectrometry method to assess the concentration of epinephrine, norepinephrine, and phenylephrine stored in plastic syringes

