Abstract
For most people, microorganisms are out of sight and therefore out of mind but they are large, extremely diverse group of organisms, they are everywhere and are the dominant form of life on planet Earth. Almost every surface is colonized by microorganisms, including our skin; however most of them are harmless to humans. Some microorganisms can live in boiling hot springs, whereas others form microbial communities in frozen sea ice. Among their many roles, microorganisms are necessary for biogeochemical cycling, soil fertility, decomposition of dead plants and animals and biodegradation of many complex organic compounds present in the environment. Environmental microbiology is concerned with the study of microorganisms in the soil, water and air and their application in bioremediation to reduce environmental pollution through the biological degradation of pollutants into non-toxic or less toxic substances. Field of environmental microbiology also covers the topics such as microbially induced biocorrosion, biodeterioration of constructing materials and microbiological quality of outdoor and indoor air.
Gentlemen, it is the microbes who will have the last word.
(Louis Pasteur)
1 Evolution of microorganisms
Earth is about 4.5 billion years old and scientists estimate that life first emerged at least 3.8 billion years ago after the surface of crust had cooled enough to allow liquid water to form. Early Earth was inhospitable from time to time because space rocks crushed into the Earth’s surface. Some impacts were powerful enough to vaporize oceans and create clouds of steam which sterilized the Earth’s surface. Nonetheless, some microorganisms were able to survive this period deep underground while some may have had the capacity of modern microorganism to produce survival forms called endospores. Early in the planet’s history conditions were harsh. The Earth’s surface was exposed to strong ultraviolet (UV) radiation because the ozone layer was not yet formed in the atmosphere. Nevertheless, the prokaryotic microorganisms began to develop. The first prokaryotic microorganisms lived in anaerobic environment because atmosphere was a mixture of CO2, N2, and H2O vapour and in traces H2. O2 gas began to appear in significant amount in the Earth’s atmosphere between 2.5 and 2 billion years ago as a result of microbial metabolic process called oxygenic photosynthesis. Oxygenic photosynthesis which started around 3 billion years ago differed from earlier forms of photosynthesis and the bacteria responsible for this type of photosynthesis are called cyanobacteria. Cyanobacteria brought the O2 level of the Earth’s atmosphere up to 10 % of today’s level and due to it the formation of ozone layer started. O2 level was high enough to enable evolution of oxygen-utilizing organisms [1, 2]. An approximate timeline of the development of life on Earth is presented in Figure 1. Since many eukaryotes are O2 dependent, researchers had theorized that protists first appeared around 2 billion years ago but according to recent evidence the first protists appeared about 3 billion years ago. Although bacteria and archaea are older than protists, an early appearance of the first eukaryotes is evidenced with a high degree of diversity in this group. Algae appeared after cyanobacteria within the last 2 billion years, because their chloroplasts were derived from cyanobacteria. Fungi appeared during the last several hundred million years.
![Figure 1: Approximate timeline in the history of life on Earth [1].](/document/doi/10.1515/psr-2016-0118/asset/graphic/j_psr-2016-0118_figure1.jpg)
Approximate timeline in the history of life on Earth [1].
2 The microbial world: classification, metabolism and growth
Microorganisms are the foundation for all life on Earth. They vary in their appearance, ability to carry out different biochemical transformations, ability to grow in wide variety of environments and in their interactions with other organisms. Due to a great variety of different organisms on Earth, a systematic approach to classifying these organisms is necessary. The science of classifying organisms is called taxonomy and the groups making up the classification hierarchy are called taxa. Nomenclature refers to the actual naming of organisms. The binominal system of nomenclature is used for microorganisms. The names are given in Latin or are Latinized. The first word in the name is the genus, with the first letter always capitalized; the second is the species name, which is not capitalized. Both words are always italicized. Classification of well-known bacterium Escherichia coli is presented in Example 1.
Example 1. Classification of bacterium Escherichia coli
E. coli is a member of genus Escherichia. It was named after the person who first isolated and described it, Theodor Escherich. The species name comes from the location where it was found, in this case in human intestinal tract. Its classification is as follows:
Domain: Bacteria, Phylum: Proteobacteria, Class: Gammaproteobacteria, Order: Enterobacteriales, Family: Enterobacteriaceae, Genus: Escherichia, Species: E. coli.
Traditional classification of living organisms was based on morphological differences like shape, colouring or appendages (flagella) extending from the cells. In the 1970s, a research by Woese and others suggested that life on Earth evolved along three evolutionary lineages. Analysis of 16S rRNA (ribosomal ribonucleic acid) has led to the modern phylogenetic classification of living organisms in three domains: Archaea, Bacteria and Eukarya. Phylogenetic information along with other taxonomic information has been used to construct the phylogenetic tree (Figure 2).

Schematics of universal phylogenetic tree evolved from a common ancestor.
Using the rRNA gene sequences of many different organisms resulted in formation of a tree with longer and shorter branches; the longer the branches lengths, the greater the diversity in the group. The branches lengths are shorter for newly evolved organisms such as fungi, plants and animals in comparison with the branches lengths for prokaryotic and lower eukaryotic microorganisms [1, 3].
Members of microbial world consist of two major cell types: the simple prokaryotic and the complex eukaryotic cells. Microscopically, the cells in domains Bacteria and Archaea look similar. They do not contain a membrane-bound nucleus or other intracellular lipid-bound organelles. Their genetic information is stored in a circular strand of deoxyribonucleic acid (DNA) in the nucleotide and cytoplasm is surrounded by a rigid cell wall. Due to structural similarities bacteria and archaea are prokaryotes but they differ in their chemical composition and therefore they are in different domains. The domain Eukarya is comprised of four kingdoms: Animalia, Fungi, Plantae and Protista. All members in this domain contain eukaryotic cells and this microbial world is composed of single-celled and multicellular organisms. The cells contain a membrane-bound nucleus and other internal organelles (mitochondria, chloroplast and cytoskeleton) that make eukaryotes more complex than the simple prokaryotes.
Term microorganism is used to describe an organism that is so small that it cannot be seen without the use of microscope. Microscopy plays an integral role in the study of microorganisms and can provide extremely useful information about them. Even today, one of the most important tools for studying microorganisms is the bright-field light microscope that can magnify images 1000×. Most living microorganisms are nearly transparent and move rapidly about the slide. Consequently, cells must be immobilised and stained with dyes. Simple staining employs one dye to stain the cells. Differential staining is used to distinguish one group of bacteria from another. One of most frequently used staining procedures is the Gram stain (Example 2).
Example 2. Gram staining is the procedure (Figure 3) by which bacteria can be separated in two major groups: Gram positive and Gram negative. Difference in the staining properties of these two groups is due to the difference in the chemical structure of their cell walls. This procedure was developed over a century ago by Dr. Hans Christian Gram.

Staining bacteria for microscopic observation.
The bacteria are the smallest prokaryotic cells and the least structurally complex unicellular microorganisms. They possess the greatest metabolic flexibility. Typical shapes of common bacteria are: (a) sphere (coccus), (b) rod (bacillus) and (c) spiral (spirillum). Diameter of a typical cell is 1 µm. A representative of rod-shaped bacteria is presented in Figure 4(a). The cell wall contains chemical compound peptidoglycan, which is not found in organisms in other domains. They multiply by binary fission in which one cell divides in two cells, and many can move using flagella. The archaea are a group of ancient organisms and subdivisions include methanogens (methane producing), halobacteria (live in high-salt environments) and thermoacidophiles (grow best under high temperature and high acidity). The cells of archaea are somewhat similar to bacteria in size and shape; however, they are genetically and biochemically quite different and may be the oldest form of life on Earth. They also multiply by binary fission and move by means of flagella, but archaea do not have peptidoglycan in their cell walls. Members in the domain Eukarya consist of eukaryotic cells and diameter of a typical cell is 10–100 µm. Many fungi are microscopic, while others, like mushrooms, are macroscopic. All fungi have chitin in their cell walls. Yeasts are single-celled fungi. Yeasts can be spherical, oval or cylindrical, and are usually 3–5 µm in diameter (Figure 4(b)). Yeasts can reproduce by asexual or sexual means. Asexual reproduction is by budding or fission. Moulds are filamentous fungi and have a mycelial structure. Long thin filaments on the mycelium are called hyphae. Some branches of mycelium may grow in the air and asexual spores (conidia) are formed on these aerial branches. Fungal spores are typically a single cell of about 3–30 µm in diameter, depending on the species (Figure 4(c)). Protozoa, slime moulds and unicellular algae as a single-celled organisms belong to the taxonomic kingdom of Protista. The algae are a diverse group of photosynthetic protists. Some of them are single-celled while others are multicellular, and they come in a great range of shapes and sizes, from spherical cells with 0.5 μm diameter (Prochlorococcus) to 60 m long multicellular thalli (Macrocystis). They all contain green pigment chlorophyll, but some of them also contain other pigments (Figure 4(d)). They reproduce asexually or sexually. The role of pigment is to absorb sunlight, which algae use as a source of energy. The cell walls of algae are rigid and are composed of cellulose. Diatoms are algae that have silicon dioxide incorporated into their cell walls. Some multicellular species contain other compounds such as carrageenan and agar in their cell walls. Protozoa are diverse groups of microscopic single-cell organisms and they are much larger (5–500 µm) than prokaryotes (Figure 4(e)). Protozoa are classified into three groups based on shape: ciliates, flagellates and amoebae. They do not have a rigid cell wall and many of them have a specific shape due to cytoskeleton just beneath the outer membrane of the cell. Metazoa – invertebrates are microscopic multicellular organisms (0.1–0.5 mm long) divided in several subgroups and among them are rotifers (Rotifera, Figure 4(f)). The body of a rotifer is divided into a head, trunk and foot, and is typically somewhat cylindrical. The organisms discussed above are all living members of the microbial world. Viruses are not living organisms and they consist of a piece of nucleic acid surrounded by protective protein coat. Viruses can only multiply inside living cells and therefore they are considered obligate intracellular parasites [1, 2, 3, 4].

Bright-field photomicrograph of cells: (a) rod-shaped bacterium Bacillus sp., (1000×), (b) yeast Saccharomyces sp., (400×), (c) mould Penicillium sp., (400×), (d) green algae Chlorella, (400×), (e) single-celled attached protist (stalked ciliates), (400×) and (f) multicellular Rotifera (100×).
Environmental factors that influence microbial growth are temperature, oxygen requirements and pH and water availability. The right temperature, pH and moisture levels vary from one organism to another. Some of them can grow at ‒20 °C in a brine to prevent freezing, while others can grow at 120 °C where water is under high pressure to prevent boiling. Microorganisms that grow optimally below 20 °C are called psychrophiles, those with temperatures optima in the range of 20 °C to 50 °C are mesophiles, while those that grow best at temperatures higher than 50 °C are thermophiles. Hyperthermophiles have an optimum growth temperature between 70 °C and 110 °C and these are usually members of the Archaea. Cells that require O2 for growth and metabolism are obligate aerobes; obligate anaerobes cannot multiply in the presence of O2, while facultative anaerobes grow best if O2 is present, but can also grow without it. Microaerophiles require small amounts of O2 and aerotolerant anaerobes (obligate fermenter) are indifferent to O2. Each bacterial species can survive within a range of pH values, but within this range it has a pH optimum. Neutrophiles multiply in the range of pH 5 to 8, acidophiles grow optimally at a pH below 5.5 and alkalophiles above 8.5. Finally, all microorganisms require water for growth. Growth of any microorganisms depends not only on a suitable physical environment, but also on available source of chemicals to use as nutrients. All cells contain the macromolecules as follows: carbohydrates, lipids, proteins, nucleotides. Nucleotides as monomers are needed for DNA and RNA synthesis and they play important role in cellular energetics. Elements that make up cell constituents are called macronutrients or major elements (C, O, H, N, S, P, K, and Mg). Trace elements are also essential to microbial nutrition. The most widely needed trace elements are Fe, Zn and Mn. The elements needed under specific growth condition are Cu, Co, Mo, Ca, Na, Cl, Ni and Se. Some bacteria, especially methanogens, need Na for ion balance. Element Na is important in the transport of charged species in eukaryotic cells. Some halobacteria and marine microorganisms need Cl‒. Microorganisms derive energy either from sunlight or by metabolising chemical compounds. Prokaryotes can live in many environmental habitats because they are able to use diverse sources of energy and carbon. Photoautotrophs use the energy of sunlight and CO2 in the atmosphere to synthesize organic compounds required by many other organisms. Cyanobacteria are important photoautotrophs that inhabit fresh and saltwater habitats. Chemoautotrophs or chemolithotrophs use inorganic compounds (H2, NH3, NO2‒, Fe2+, and H2S) for energy and derive their carbon from CO2. Photoheterotrophs use the energy of sunlight and derive their carbon from organic compounds. Chemoheterotrophs or chemoorganotrophs use organic compounds for energy and as a carbon source.
Cells synthesize only the enzymes they require for growth. Under the changed environmental conditions, additional or different enzymes may be important. Enzymes are proteins that act as biological catalysts, facilitating the conversion of substrate into product. They usually act on only one, or a very limited number of substrates. More than a 2000 different enzymes are known. They are named by adding the suffix –ase to the end of the substrate name, like amylase, or reaction catalysed, such as alcohol dehydrogenase. Some enzymes have a simple structure, while others have more than one subunit. Some protein enzymes require a non-protein group for their activity, such as cofactor (metal ions Mg, Zn, Cu), or coenzyme that are non-protein organic compounds (e.g. nicotinamide adenine dinucleotide – NAD+), or some vitamins.
Differences in microbial metabolisms can be attributed partly to genetic differences and partly to differences in their responses to changes in their environment. Even the same species may produce different products when grown under different nutritional and environmental conditions. Living cells require energy for biosynthesis, transport of nutrients, motility and maintenance. Two primary functions of cellular metabolism are catabolism and anabolism. Catabolism is the degradation of a substrate to more highly oxidized end products for the purpose of generating energy and reducing power. Anabolism is the biosynthesis of complex compounds from simpler compounds, usually with the consumption of energy and reducing power. Energy in biological systems is stored and transferred via adenosine triphosphate (ATP), which contains high energy phosphate bonds. Chemoheterotrophs use two processes to provide energy to form ATP: substrate-level phosphorylation and oxidative phosphorylation. Photosynthetic organisms can generate ATP using the process of photophosphorylation, utilizing radiant energy of the sun. There are other compounds analogue to ATP, which store and transfer high-energy phosphate bonds, but not to the extent of ATP. Glucose is a major carbon and energy source for many heterotrophic organisms. Several different metabolic pathways are used by different organisms for catabolism of glucose. Three of the most important metabolic pathways are:
Embden-Meyerhof-Parnas (EMP) pathway or glycolysis – converts glucose to pyruvate and produces initial amount of ATP.
Krebs, tricarboxylic acid cycle (TCA), or citric acid cycle – oxidizes pyruvate through acetyl-CoA into CO2 and H2O, and produces a large amount of ATP.
The pentose-phosphate or hexose-monophosphate (HMP) pathway – converts glucose-6-phosphate into variety of carbon skeletons (C3, C4, C5), with glyceraldehyde-3-phosphate as the end product.
Although all three pathways can have catabolic and anabolic roles, the EMP and TCA cycles are the primary means for energy generation, while HMP plays a key role in supplying carbon skeletons and reducing powers for use in biosynthesis. In prokaryotes a single glucose molecule will yield up to 24 mol of ATP, while in eukaryotes up to 36 mol of ATP. Reducing power is stored in coenzyme nicotinamide adenine dinucleotide NADH (oxidized form is NAD+) and nicotinamide adenine dinucleotide phosphate NADPH (oxidized form is NADP+). Reducing power can be used to generate ATP through electron transport chain. When O2 is the final electron acceptor for this reducing power, the process is called aerobic respiration. If another electron acceptor such as NO3‒ or SO42‒ is used in conjunction with electron transport chain, then the process is called anaerobic respiration. Anaerobic respiration is a less efficient form of energy transformation than aerobic respiration. Cells that obtain energy without using the electron transport chain use fermentation. Substrate-level phosphorylation supplies ATP. The end products of fermentative metabolism such as ethanol or lactic acid are formed in response to the cells’ need to balance consumption and the production of reducing power. Autotrophic organisms use CO2 as their carbon source to incorporate carbon from CO2 into cellular material. Energy is obtained either through sunlight (photoautotroph) or oxidation of reduced inorganic chemicals such as H2S, NH3 or Fe2+ (chemoautotroph).
By knowing environmental and nutritional factors that influence growth of specific prokaryotes, it is possible to provide appropriate conditions for their cultivation in laboratory. These include a medium on which to grow the organism and a suitable atmosphere. For routine purposes many types of complex media are used. Commonly used complex medium, nutrient broth, consists of only peptone (protein hydrolysed to amino acids and short peptides), beef extract and water. Nutrient agar results if agar (polysaccharide from algae) is added to nutrient broth. Chemically defined media are composed of mixtures of pure chemicals. An example is glucose-salts medium, which supports the growth of bacterium E. coli, and contains ingredients as follows: glucose, K2HPO4, KH2PO4, MgSO4, (NH4)2SO4, CaCl2, FeSO4 and water. Selective and differential media are used for the purpose of detecting or isolating an organism that is a part of a mixed bacterial population. Selective medium types are formulated to support the growth of one group of organisms such as Gram-negative bacteria, but inhibit the growth of other like Gram-positive bacteria. Differential media are widely used for differentiating closely related organisms or groups of organisms. Some media may possess both, selective and differential properties, allowing them to grow certain groups of organisms of interest and give the investigator a way to discern differences in the group based on a visible reaction with the media. These media can be powerful diagnostic tools in environmental settings. Furthermore, an appropriate atmospheric condition for microbial growth has to be obtained. Obligate aerobes grow best when tubes or flasks containing media are shaken, providing maximum aeration. Some other microbial species requires atmospheric condition with increased CO2, microaerophilic or anaerobic conditions.
The most widely used methods [1, 2, 3, 5] in the study of microorganisms present in different samples is their growth in a liquid medium, followed by dilution of the sample and plating on a solid agar medium. The theory is that one colony arises on a solid agar medium from one microbial cell. Each colony is then referred to as a colony forming unit (CFU) and the result of the analysis expressed in terms of CFU/mL or CFU/g of sample. Except for providing an estimate of bacterial numbers, this procedure allows obtaining pure culture isolates. Bacterial growth can be measured by direct cell counts and viable cell counts. Direct microscopic count is one of the most rapid methods of determining the number of cells in a suspension, but generally does not distinguish living and dead cells. Viable cell counts are used to quantify the number of cells capable of multiplying. Plate counts and membrane filtration both measure the concentration of cells by determining the number of colonies that arise from a sample added to an agar plate. The two different plating methods pour plate and spread plate, differ in how the suspension of microorganisms is applied to the agar plate. A simple count of the colonies determines how many cells were in the initial sample. The ideal number of colonies to count is between 30 and 300. However, samples frequently contain many more microbial cells than this number and therefore it is necessary to dilute the samples before plating out the cells. The sample is diluted in 10-fold increments and a sterile physiological saline (0.9 % NaCl in water) is used to make the dilutions (1 mL sample in 9 mL saline solution). In the pour plate method, 1.0 mL of the final dilution is transferred into sterile Petri dish and then overlaid with melted nutrient agar that has been cooled to 45 °C. At this temperature, agar is still liquid. Petri dish is then gently swirled to mix microbial cells with liquid agar. When the agar hardens the individual cells are fixed in place and, after incubation, distinguishable colonies appear on the agar surface (Figure 5).
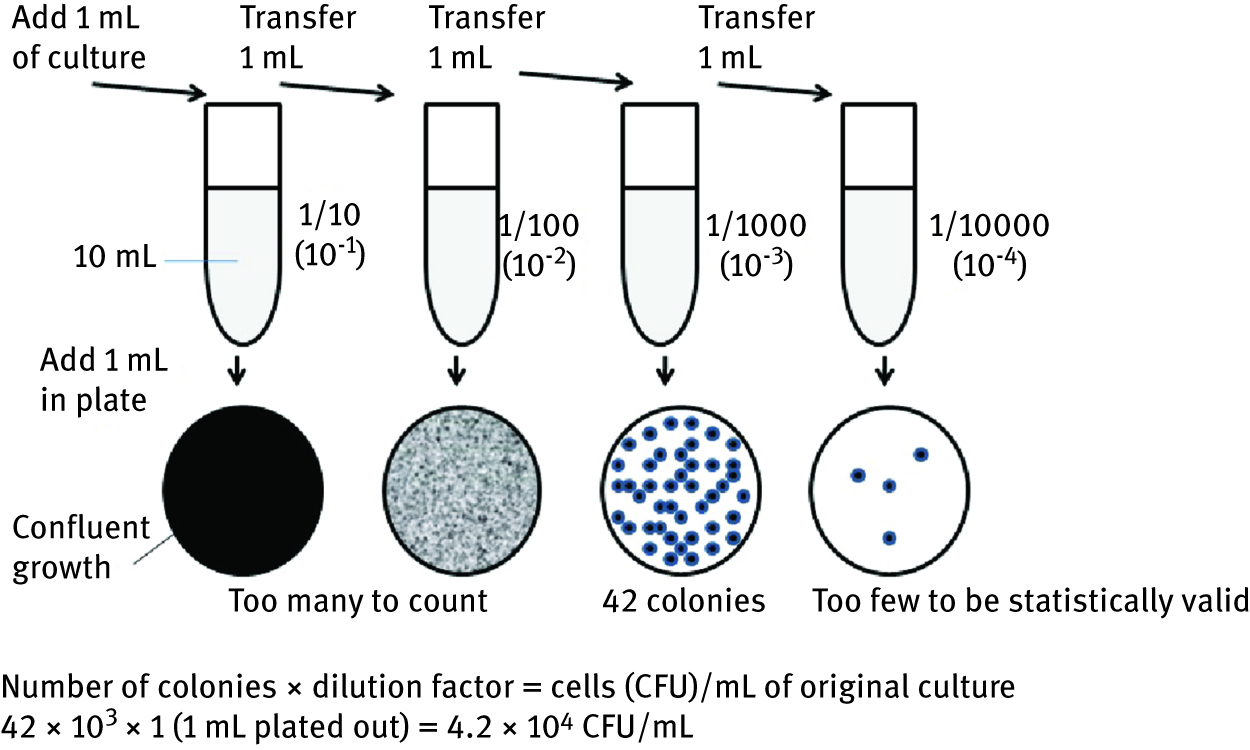
Pour plate method – serial dilutions for the estimation of viable cell count.
In the spread plate method 0.1 mL of the final dilution is transferred directly onto the plate already containing a solidified nutrient agar medium. This solution is then spread over the surface of agar with previously sterilized bent glass rod. Membrane filtration is used when the numbers of organisms in a sample are relatively low, as might occur in dilute environment such as natural waters. This method concentrates the bacteria by filtration before they are plated. A known amount of liquid is passed through a sterile membrane filter (pore sizes 0.2–0.45 µm), the filter is placed on an appropriate agar medium and then incubated. The number of colonies that grow on the filter indicates the number of microbial cells that were in the volume filtered and the result is reported per 100 mL of original sample. The most probable number (MPN) method is a statistical assay of cell numbers based on the theory of probability. To determine the MPN, three sets of three or five tubes containing the same growth medium are prepared. Each set receives a measured amount of sample such as water or soil. What is important is that the second set receives 10-fold less than the first and the third set 100-fold less. After incubation, the presence (e.g. gas production or precipitate) or absence of growth in each set is noted. The results are then compared against an MPN table, which gives a statistical estimate of the cell concentration, and expressed as MPN/100 mL of original sample. Instead of measuring the number of cells the cell mass can be determined. This can be done by measuring the turbidity or dry weight. A spectrophotometer is used to measure cloudiness or turbidity of a bacterial suspension as a broth culture. To measure the wet weight, cells growing liquid mediums are centrifuged down, liquid removed and cell mass weighted. The dry weight of the cell mass is determined by drying the centrifuged cells at 105 °C for several hours before weighing them. In the laboratory, bacteria are typically grown in broth contained in a tube or flask. When grown under batch conditions a population of bacteria goes through four distinct stages: in lag phase cells synthesize macromolecules prior to active multiplication; in exponential or log phase, cells divide at a constant rate and their numbers increase by the same percentage during each time interval (generation time is measured during this active multiplication); cells enter the stationary phase when they have exhausted their supply of energy and nutrients and the total number of viable cells in the population remains relatively constant; death phase is the period when total number of viable cells in the population decreases as cells die off at a constant rate. Theoretically, the time taken for cell division to occur is the mean generation time or doubling time. This varies depending on the species of microorganisms and conditions in which it is grown. For example, one of the most intensively studied prokaryotic and eukaryotic model single-celled organisms bacterium E. coli can double in 20 minutes under the laboratory conditions and yeast Saccharomyces cerevisiae in 90 to 120 minutes. Most bacteria reproduce by binary fission, which results in doubling of the number of viable bacterial cells. The relationship of cell numbers and generations can be expressed in a series of equations. Starting with an initial population N0, the total population N at the end of a given time period would be expressed as eq. (1):
where 2n is the bacterial population after n generations.
Solving the equation for n, eq. (2):
The number of generations can be calculated if the initial population N0 and the population N after time t are known. The generation time tg is equal to t (the time which elapsed between N0 and N) divided by the number of generations n, eq. (3):
Calculation of mean generation time of bacterial population is presented in Example 3.
Example 3. What is a generation time of bacterial population that increases from 1 × 104 cells per 1 mL to 1 × 107 cells per 1 mL in four hours of growth?
Answer:tg = 24 minutes
The selection of methods used for microbial identification depends on the type and nature of the microorganism. The methods commonly used in identification and substantiation of taxonomic classification of microorganisms are phenotypic analysis and molecular methods. Phenotypic analysis often involves growth of microorganisms as pure culture on an artificial media, followed by visual observation of colony morphology such as shape, size, surface characteristics and pigmentation and cell morphology (cell wall characteristics - Gram-staining, sporulation characteristics, mechanisms of motility and other cellular inclusions) using microscopy. For biochemical test specific growth media, nutrients, chemicals or growth conditions are used. These tests include: utilization of carbon and nitrogen sources, growth requirements (anaerobic or aerobic; temperature-optimum and range, pH optimum and range), generation of fermentation products, production of extracellular enzymes (e.g. amylase, protease and cellulase, which hydrolyses starch, protein and cellulose, respectively; Figure 6) or other compounds.

Amylolytic activity (a) and proteolytic activity (b) indicated by haloes around a colonies of mould Aspergillus niger and filamentous bacterium Streptomyces sp. and (c) cellulolytic activity indicated by growth of mould Trichoderma viride on wood chip as a sole source of carbon.
Identification, using molecular methods, relies on the comparison of the nucleic acid sequences (DNA, RNA) or protein profiles of a microorganism with documented data on known organisms. The molecular methods are considered sensitive enough to allow detection of low concentrations of viable or non-viable microorganisms in both pure cultures and complex samples (e.g. soil, water and other samples). One of the methods is polymerase chain reaction (PCR), which consists of sequence comparisons of conserved genomic regions such as 16S or 18S rRNA. Reliable genotypic identification requires databases with accurate and complete sequence information from a large number of taxa. Commonly used gene sequence database is GenBank provided by the National Centre for Biotechnology Information (NCBI, http:://www.ncbi.nih.gov). Microbial identification of bacterium Pseudomonas aeruginosa is described in Example 4.
Example 4. A nicotine degrading bacterium was isolated from the composting mass [6]. To identify isolated culture phenotypic and molecular method were conducted. According to phenotypic analysis colonies of isolated pure culture were circular, raised and produced water soluble yellow-green pigment. Bacterial cells observed with a bright field microscope were rod-shaped and motile, and after Gram staining cell were Gram negative. A series of biochemical tests were carried out applying API 20 NE test. The biochemical reactions were read according to the Reading Table and by referring to the Analytical Profile Index, the bacterial culture was identified as Pseudomonas aeruginosa. Although it is known that the maximum temperature for growth of P. aeruginosa is 42 °C, this isolated species was quite actively degrading nicotine at 50 °C during composting tobacco waste. The molecular characterisation that uses 16S rRNA gene sequence to identify bacterium, confirmed that isolated species is P. aeruginosa FN (FN-fast nicotine degrading).
3 Microbial diversity in the environment
Microorganisms inhabit terrestrial and aquatic ecosystems, including geographical locations considered to be extreme to life [2, 5, 7]. Among their many roles, they are necessary for cycling of carbon, nitrogen, phosphorus and sulphur (biogeochemical cycles), all essential components of living organisms. Since the Earth is a closed system, in order for life to continue, dead organisms must be degraded to their component parts to provide the ingredients needed to grow new living organisms. Microorganisms are recyclers of biomass on Earth and without their recycling activities life on Earth would quickly come to a halt. Microorganisms are also food for some higher organisms and they are at the base of all the Earth’s food chains. Apart from recycling dead biological material, some microorganisms also utilize metals and thus recycle non-biological materials. The biodeterioration of man-made compounds by some microorganisms can be a problem (e.g. in the case of paints and plastics). Some fungi attack and degrade man-made materials such as paper, leather, jet fuel, etc.
Terrestrial environments are the richest and the most complex and vary from a rain forest to a desert, but all soils contain a rich diversity of microorganisms. Soil is three-phase system consisting of: (a) a solid or mineral inorganic phase that is often associated with organic matter; (b) a liquid or solution phase that contains almost all essential minerals (e.g. NO3‒, Cl‒, CO32‒, SO42‒, Ca2+, Mg2+, Na+, K+), which are primary source of inorganic nutrients for plant roots and (c) a gas phase or atmosphere. The density and composition of microbial species of the soil is affected by environmental conditions. For example, wet soils are unfavourable for aerobic microorganisms because the free air spaces are filled up with water. When the water content of soil drops to a very low level (drought or desert) the metabolic activity and number of soil microorganisms decrease. Acidity, temperature and nutrient supply also have influence on microbial activity and diversity. Humification – humus as regulator of soil structure, source of plant nutrients, carbon pool and carbon – CO2 sequestration. Representative sampling of soils is crucial for determination of microbial diversity, interactions between them and shifts in microbial population in soil. Soil samples are obtained with a shovel or a soil auger. Sampling strategy requires taking into consideration the objective of the study, a level of precision of the data needed and sampling requirements (sampling sites, numbers and types of samples, size of sample units, etc.). Methods of soil sampling can be random, transect, two-stage or systematic grid [5, 7, 8]. The traditional methods of microbiological analysis of soil samples include cultural assays utilizing dilution and plating methodology (Figure 7 and Figure 8) or direct count assays. The number of bacteria in sample is expressed in terms of CFU/g soil (dry weight). As moist soil is originally weighed out, therefore actual dry weight of soil depends on soil moisture at the time of diluting. Molecular methods such as DNA or RNA sequencing are applied for identification of nonculturable microorganisms.

Bacterial colonies that result from incubation following dilution and plating of soil sample. The plate on the left (a) has the most bacterial colonies (lowest soil dilution). The other two plates (b and c) result from subsequent dilutions. From these three plates, plate B should be counted since it has between 30 and 300 colonies.

Fungal colonies that result from incubation following dilution and plating of soil sample. The plate on the left (a) has the most fungal colonies (lowest soil dilution). The other two plates (b and c) result from subsequent dilutions. From these three plates, plate C should be counted since it has between 30 and 300 colonies.
Surface soils are occupied by indigenous populations of archaea, bacteria, fungi, algae, and protozoa, and the microbial distribution depends on soil texture and structure. The microbial communities within the subsurface are lower in number and less diverse than in surface soils. Since nutrients are much more limited in the subsurface, a greater proportion of the population may be in a nonculturable state. In deep saturated zones, the numbers are even lower with limited microbial diversity and include a range of aerobic and facultative anaerobic chemoheterotrophs such as denitrifiers, methanogens, sulphate-reducers, sulphur-oxidizers, nitrifiers, and nitrogen-fixing bacteria.
Prokaryotes are the most numerous soil inhabitants. Gram-positive (G+) bacteria are more abundant in soils than Gram-negative (G-) bacteria. Among the most common are G (+) spore-forming, rod-shaped bacteria of genera Bacillus and Clostridium. These species form endospores inside vegetative cells, enabling them to survive adverse periods such as drought or extreme heat. Filamentous G (+) bacteria of the genus Streptomyces produce desiccation-resistant spores unrelated to endospores. They also produce metabolites called geosmin, which give soil its characteristic musty odour. Some G (-) chemoheterotrophic bacteria adapted to live in terrestrial environments are species of Azotobacter, Agrobacterium, Rhizobium and Pseudomonas, and among chemoautotrophs nitrifying bacteria such as species of Nitrosomonas (transform ammonium to nitrite) and Nitrobacter (oxidize nitrite to nitrate). Importance and practical use of nitrogen-fixing bacteria is for bacterisation of legume seeds (symbiotic – Rhisobium sp. and non-symbiotic Azotobacter chroococum). Prokaryotes are most numerous in soil, but biomass of fungi (eukaryotes) is much greater. Most fungi are aerobes and they are usually found in the top 10 cm of soil. Fungi derive energy from organic material and they are found wherever organic materials are present. The soil fungi are crucial in decomposing plant matter, degrading and using complex macromolecules such as lignin and cellulose. Many soil bacteria and fungi are important in nutrient cycling and removal of organic contaminants by the processes of biodegradation and bioremediation. Various protists are also found in most soils. Algae generally depend on sunlight for energy and most protozoans require O2, so they are found near the soil surface [9].
Aquatic environments occupy more than 70 % of the Earth’s surface and main types are: surface waters (springs, streams, rivers and lakes), marine environments (seas, oceans) and ground waters. There are many types of springs (cold, thermal, hot and specialized – iron, sulphur, radioactive). Cold springs are habitat for bacteria and algae, thermal or hot springs for many different species of archaea and thermophilic bacteria, iron springs for bacterial species Gallionella and Sphaerotilus and sulphur springs for photosynthetic and non-photosynthetic sulphur bacteria. Water from a source like a spring, rain or snow melt begins to flow down, and small streams are formed. These streams may slowly join together to form a larger stream or river. Streams and rivers accumulate organic matter and heterotrophic population from surrounding terrestrial environment thus a profile of microorganisms in streams and rivers resembles terrestrial. The types and relative numbers of microorganisms inhabiting lakes and marine environments depend on concentration of dissolved O2 and nutrients, temperature and sunlight. The upper layer is oxygen-rich due to the activities of many photosynthetic organisms such as cyanobacteria. In contrast, the bottom layer of lakes may be anaerobic due to the consumption of O2 by heterotrophs. In deep sea, water is O2-saturated due to mixing associated with tides, currents, and wind action. Ground waters are found inland in the subsurface zone and include shallow or deep aquifers. Microorganisms are the sole inhabitants and bacteria are predominant. In general, here microbial activity is low due to the poor concentration of nutrients. Ground water, rivers and lakes are important sources of fresh water. Man-made aquatic environment comprises of stagnant ponds, swimming pools, drainage ditches and wastewaters treatment plants in industrial and urbanized regions [9].
Water and wastewater sampling is generally performed by one of two methods; grab sampling or composite sampling. Grab samples are single samples collected from a specific spot at a site over a short period of time (typically seconds or minutes) for: protected groundwater supplies, water supplies receiving conventional treatment, some well-mixed surface waters, wastewater streams, rivers, large lakes, shorelines or estuaries. Composite samples can be obtained by combining portions of multiple grab samples or by using specially designed automatic sampling devices. Membrane filtration (Figure 9(a)) and multiple-tube MPN method (Figure 10(a)) are applied for microbiological analysis of natural waters and wastewater streams; however more polluted water will likely require a greater dilution range [10]. Total and faecal coliform bacteria (TC and FC) have been used to evaluate the general quality of water. Faecal coliform bacteria and their representative E. coli originate specifically from the intestinal tract of warm-blooded species (e.g. humans, beavers, racoons, etc.) and are cultured by increasing the incubation temperature to 44.5 C and using selective growth medium such as mFC broth or mFC agar (Figure 9(b)).

(a) Membrane filtration apparatus: 1. water sample, filtration funnel, 3. vacuum pump, 4. selective agar media; and (b) appearance of blue colonies of faecal coliform bacteria on membrane filter after incubation on mFC agar for 24 hours at 44.5 °C

MPN dilution series: (a) test tubes after incubation - three series with five replicate tubes, (b) positive test tube in comparison with negative one and (c) dark colour colonies of iron bacteria with metallic shine on Winogradsky agar.
Two other groups of bacteria that are present in faeces are: faecal streptococci (FS) and Clostridium. Clostridia spores can survive a long time during adverse conditions. These genera occur naturally in soils and polluted waters, but also indicate a faecal contamination by warm-blooded animals. The ratio of faecal coliforms to faecal streptococci (FC:FS) can provide information on the source of contamination. Human faeces have much more FC than FS, while FS are more common in animal faeces. Comparing the numbers of FC to FS is handy for getting an idea as to whether the water has been polluted with human faecal wastes (\gt4:1 ratio) or animal wastes (\lt1:1). For example FC:FS ratio from human faecal wastes is 4.4:1, while from duck and cow 0.6:1 and 0.2:1, respectively.
While faecal coliform bacteria and their representative E. coli are a health problem, iron bacteria are not hazardous to health, but in some cases they cause problems in wells, which are a part of water distribution system. Iron bacteria are a natural part of the soil and aqueous environments (ocean, river, or groundwater) in most parts of the world. Iron bacteria use iron as an energy source for their life functions and form chains of cells encased within a tube, or sheath. They are also characterized by the deposition of ferric hydroxide in their sheaths. This clustering within a sheath enables iron bacteria to attach to a solid surface such as rocks or wood in flowing water. The genera Gallionella, Leptothrix and Crenothrix, which are Gram-negative rods, belong to the iron bacteria group. If surface water or soil enters a well, the iron bacteria may be introduced and begin to thrive if conditions are favourable. A major problem associated with iron in wells is production of reddish-brown slime as a metabolic by-product from the oxidation of iron. The slime generated by iron bacteria can corrode pipes and plumbing equipment, and clog pipes, screens, and other components of the well system. To estimate the number of iron bacteria in water well the MPN method may be applied (Example 5).
Example 5. The number of iron bacteria in water sample from well was estimated by applying MPN dilution series. Replicates of five test tubes (Winogradsky liquid medium [11],) of each dilution (10‒1, 10‒2 and 10‒3 mL) of water sample were prepared (Figure 10(a)) and incubated at 17 °C for three days. Tubes were scored positive on the basis of bacterial growth and formation of brown precipitate (Figure 10(a) and Figure 10(b)). According to statistical MPN table, the estimated number of iron bacteria was 1.4 × 103/100 mL of original water sample. From positive test tube culture was transferred onto Winogradsky agar plate to observe the growth of colonies of iron bacteria (Figure 10(c)). After incubation, a small amount of culture from one of the colonies was transferred onto slide and cells stained by Meyers method [11]. By examination under the light microscope, cells of iron bacteria were red and deposits of iron were blue. According to formation of chains of cells, isolated bacterium present in the water sample belong to the genus Leptothrix.
Various layers in the atmosphere can be recognized up to the height of about 1000 km [9]. Although air content and atmospheric pressure vary at different layers, air suitable for the survival of terrestrial plants and terrestrial animals is currently only known to be found in the Earth’s troposphere. This layer is the nearest to the Earth. In temperate regions, troposphere extends up to about 11 km while in tropics up to about 16 km. In addition to water droplets, dust particles and other matter, air also contains microorganisms. Atmosphere is not a hospitable environment for microorganisms due to temperature variations, low amount of organic matter and a scarcity of available water. Airborne biological particles are called bioaerosols. They are generated as polydispersed droplets or particles of different sizes (0.5 to 30 µm). Pathway of bioaerosols is as follows: launching into the air from soil or water surfaces, the subsequent transport via dispersion in air and deposition on surface. Transport of bioaerosols can be defined in terms of time and distance and it ranges from minutes to hours or days and from 100 m up to 100 km. The transport and settling of bioaerosols are affected by their physical properties and environmental factors. The size, density and shape of the droplets or particles are the most important physical characteristics, while magnitude of air currents, temperature and relative humidity are environmental factors. Temperature and relative humidity also contribute to the influx of airborne microorganisms. Increased concentrations of some fungal spores (e.g., Cladosporium sp. and Nigrospora) in outdoor air and increased numbers of bacteria released from plant surfaces have been associated with high temperatures and low relative humidity. In wet weather the rain washes the microorganisms from the air. In order to survive in the air, it is important that these microorganisms adapt to environmental stresses (desiccation, humidity and radiation). While harsh environmental conditions tend to decrease the number of viable airborne microorganisms, there is variability in survival between groups of microorganisms and within genera. In general, fungal spores and enteric viruses are somewhat resistant to environmental stress during transport through air. Bacteria and algae are more susceptible while bacterial endospores (e.g. Bacillus sp.) are quite resistant.
For collection of air samples, many devices are used on the basis of their sampling methods, but most samplers are based on impaction and impingement [12]. Impingement is the trapping of airborne practices in a liquid matrix by drawing air through the inlet and into a liquid. Impaction is the forced deposition of airborne particles on a solid surface. The impaction method separates particles from the air by utilizing the inertia of the particles to force their deposition onto a solid or semi-solid surface. The collection surface is usually an agar medium such as nutrient agar for bacteria or malt agar for fungi (Figure 11).

Sampling of bioaerosols by the impaction method: (a) microbial air sampler and after incubation of Petri dishes, (b) bacterial colonies on nutrient agar and (c) fungal colonies on malt agar.
Agricultural practices, wastewater and solid waste treatments (sanitary landfill operations and composting) serve as the origin of bioaerosols in outdoor environments. In summer and autumn moulds such as genera of Cladosporium, Alternaria, Penicillium and Aspergillus may be present in outdoor air in city parks, near busy streets genus Cladosporium and near buildings genus Stachybotrys. Deterioration of building materials, offensive odours, and adverse human health effects are associated with microbial contamination of indoor environments. Bacteria and algae grow in areas with standing water such as humidification systems and sites where water leaking (flooding and condensation) has occurred. Fungi, which have lower water activity requirements than other microorganisms tend to colonize a wide variety of building materials. Penicillium sp. and Aspergillus versicolor are often isolated on wallpaper and drier margins of wetted walls, while Cladosporium sp. proliferates as secondary colonizer. The fungus Stachybotrys chartarum is most commonly found in homes or buildings which have sustained flooding or water damage from broken pipes, roof, wall or floor leaks, condensation, etc. Wet conditions are required to initiate and maintain growth. It is most common on the paper covering of gypsum wall board, but can be found on wallpaper, cellulose based ceiling tiles, paper products, carpets with natural fibres, paper covering on insulated pipes, in insulation material, on wood and wood panelling, and on general organic debris. The fungus will usually produce large amounts of conidiophores and conidia giving the substrate a black appearance. This fungus is a serious problem in homes and buildings and one of the causes of the “sick building syndrome”. S. chartarum produces a mycotoxin that can cause animal and human mycotoxicosis. Some other fungi can attack materials such as leather, cloth and hydrocarbons, but can also cause degradative change in glass and metal because of their ability to produce acid as they grow.
The conditions of extreme environment greatly restrict the range of microbial species that can grow in such habitat. The extremes of environmental conditions that microbes must be able to tolerate include high temperatures near to boiling, low and high pH values, high salt concentrations, low water availability, low concentration of nutrients, high concentration of toxic compounds and high irradiation. In hot springs and thermal vents there are obligate thermophilic archaea belonging to the genera Methanothermus, Sulfolobus, Pirodictium, and Pyrococcus. Acidic environment such as mining waste streams and various mineral oxidizing environments are inhabited by bacteria that belong to genus Thiobacillus. Some species oxidize only sulphur compounds, while others, such as Thiobacillus ferrooxidans, also oxidize ferrous to ferric iron for ATP generation. Halotolerant organisms require salt concentrations for growth that are higher than those found in sea water. The examples of extreme halophilic bacteria belong to the genera Halobacterium and Halanaerobium. Very few microorganisms are known to survive in nutrient-free extreme environments (ultra pure water used in semiconductor). It can cause flaws in crystal design of computer chips. Caulobacter and Pseudomonas fluorescens are known to be present there. Radiation resistance of bacterium Deinococcus radiodurans is presented in Example 6.
Example 6. Bacterial biofilms have been found growing on the surfaces of spent nuclear fuel rods stored in nuclear reactor facilities. An example of bacterium that is unusually resistant to ultraviolet (λ = 100 to 295 nm) and ionizing (up to 5 kGy) radiation is Deinococcus radiodurans. This bacterium can survive doses of radiation that would kill other living organisms. Its ability to do these results from an unusually active DNA (deoxynucleic acid) repair system, and over a period of 3–4 hours, overlapping fragments of DNA are spliced together into complete chromosomes, and the cells soon resume normal growth [13]. Attempts to understand the molecular basis of this phoenix-like capability has given rise to numerous hypotheses. Notable among them are proposals that the condensed nature of the Deinococcus genome or an unusual capacity to avoid protein oxidation is keys to radiation resistance. Bacterium D. radiodurans is of interest to scientists developing new bioremediation strategies because it can survive the radiation found in sites contaminated with high levels of radioactive substances. Cleaning up pollution at such sites manually is expensive, while seeding the site with bacteria, which then carry out the work of decontamination has economic and safety advantages. After cloning to introduce genes into D. radiodurans it would allow this bacterium to degrade toxic pollutants such as trichloroethylene or chlorobenzene [14].
4 Eutrophication process
Why would the topic of eutrophication be included in environmental microbiology? First, the organisms involved in the eutrophication process and the subsequent degradation of water quality are microorganisms: picocyanobacteria, nitrogen-fixing cyanobacteria, diatoms, dinoflagellates, sulfide-oxidizing bacteria, and the suite of aerobic and anaerobic bacteria involved in biogeochemical processes. Second, the process, the symptoms, and the geographic locations of eutrophication are all increasing worldwide at an accelerated rate. Eutrophication is the increase in the rate of production of carbon or the accumulation of carbon in an aquatic ecosystem. The source of the increased organic carbon may come from within the system (autochthonous) or from outside the system (allochthonous).
Historical course of eutrophication: Changes in nutrients as well as the symptoms of cultural eutrophication follow similar time courses on a global scale among developed countries. This same sequence of events is becoming more evident in developing countries (Figure 12). As scientists began documenting the sequence of symptoms progressing toward eutrophication and correlating them with changes in water quality, particularly nutrient loads, the patterns and similar trajectories became evident globally in developed countries.

Period of the explosive increase in coastal eutrophication in relation to global additions of anthropogenically fixed nitrogen.
Given the trajectories of future nutrient loads in both developed and developing countries, principally from terrestrial drainage, atmospheric deposition, and urban discharges, it is likely that coastal eutrophication will continue to expand globally. Populations will continue to expand with higher consumptive requirements for food and fuel, and the current trend is for fertilizer use to escalate as the industrialization of agriculture intensifies in the developed world and spreads even more rapidly in developing countries. Coastal ecosystems in many parts of the world, where conditions are conducive to stratification and retention of water, are at high risk for developing eutrophication [15, 16]. “Given the growing magnitude of the problem and the significance of the resources at risk, nutrient over-enrichment represents the greatest pollution threat faced by the coastal marine environment” [17].
Symptoms? The initial response of an estuarine or coastal system to an increase in limiting nutrients is an increase in phytoplankton growth rate and biomass accumulation, growth of filamentous macroalgae, blooms of noxious or toxic algae, reduction in water clarity, shifts in phytoplankton community structure, or combinations of these. A key element of eutrophication is change. Upwelling systems cycle through phases of increased nutrient availability, high primary and secondary productivity, and often, oxygen depletion in the lower water column. The causes may include changes in physical characteristics of the system such as changes in hydrology, changes in biological interactions such as reduced grazing, or an increase in the input of organic and inorganic nutrients. Although the causes may include direct natural or anthropogenic carbon enrichment, eutrophication in the twentieth and twenty-first century is more often caused by excess nutrients that would otherwise limit the growth of phytoplankton [15].
5 Microorganisms in biodegradation and bioremediation
The quality of life on Earth is linked inextricably to the overall quality of the environment. In early times, we believed that we had an unlimited abundance of land and resources; today, however, the resources in the world show, in greater or lesser degree, our carelessness and negligence in using them. Due to rapid industrialization and large-scale anthropogenic activities, the pollution level is increasing at a rapid rate, which is a major concern. Though many remediation techniques are available, the use of microorganisms has many advantages like cost effectiveness, few or no by-products, reusability, and more. Microorganisms are readily available, rapidly characterized, highly diverse, omnipresent, and can use many noxious elements as their nutrient source. They can be applied in both in situ and ex situ conditions; in addition, many extreme environmental conditions can be cleaned by such entities. Most countries do not restrict industrialization in spite of increased pollution levels; however, these can be minimized using suitable remedial measures, particularly where microorganisms provide a useful tool for a better alternative.
Biodegradation is nature’s way of recycling wastes, or breaking down organic matter into smaller compounds by living microbial organisms. In the microbiological sense, “biodegradation” means that the decaying of all organic materials is carried out by a huge assortment of life forms comprising mainly bacteria, and fungi, and possibly other organisms. Complete biodegradation or mineralisation involves oxidation of parent compound to form carbon dioxide and water, a process providing both carbon and energy for growth and reproduction of microbial cells. Mineralisation of an organic compound (taking glucose, as an example) under aerobic and anaerobic conditions is shown in Figure 13. Irrespective of the structure of the carbon source, each degradation step in the pathway is catalysed by a specific enzyme made by the degrading microbial cell. Lack of appropriate biodegrading enzyme is one common reason for the persistence of organic pollutants in environment. Thus, pollutants that have structures similar to those of natural substrates are normally easily degraded. However, in most cases the term biodegradation is generally used to describe almost any biologically mediated change in a substrate. Biotransformation is the metabolic modification of the molecular structure of a compound, resulting in the loss or alteration of some characteristic properties of the original compound, with no (or only minor) loss of molecular complexity. Biotransformation may affect the solubility, mobility in the environment, or toxicity of the organic compound. Bioremediation is the use of naturally occurring microorganisms to degrade the environmental contaminants into less toxic forms. Bioremediation and biotransformation methods endeavour to harness the astonishing, naturally occurring, microbial catabolic diversity to degrade, transform or accumulate a huge range of compounds including hydrocarbons (e.g. oil), polychlorinated biphenyls (PCBs), polyaromatic hydrocarbons (PAHs), radionuclides and metals [7, 18, 19, 20, 21].

Aerobic or anaerobic biodegradation of an organic compound.
In the last few decades, highly toxic organic compounds have been synthesized and released into the environment for direct or indirect application over a long period of time. The problem is worldwide, and the estimated number of contaminated sites is significant. It is now widely recognized that contaminated land is a potential threat to human health, and its continual discovery over recent years has led to international efforts to remedy many of these sites, either as a response to the risk of adverse health or environmental effects caused by contamination or to enable the site to be redeveloped for use.
What are xenobiotics?Xenobiotic compounds are chemicals which are foreign to the biosphere. Depending on their fate in air, water, soil, or sediment, xenobiotic pollutants may become available to microorganisms in different environmental compartments. Actually, the dominant means of transformation and degradation of xenobiotic compounds on Earth resides in microorganisms. In natural habitats, the physicochemical properties of the environment may affect and even control biodegradation performance. Xenobiotics may resist biodegradation, or they undergo incomplete biodegradation or just biotransformation. The definition of xenobiotics as compounds ‘foreign to life’ exhibiting ‘unnatural’ structural features does not necessarily imply that xenobiotics are toxic compounds, but many xenobiotics indeed are harmful to living organisms [18].
Why are some compounds degraded by microorganisms? The degradation is done not as a favour to us but because the organisms gain energy needed for growth, reproduction, and other biological functions needed for survival. Microbial activities are very important for the renewal of our environment and maintenance of the global carbon cycle. These activities are included in the term biodegradation. Microorganisms can degrade numerous of organic pollutants owing to their metabolic machinery and to their capacity to adapt to inhospitable environments. Their efficiency depends on many factors, including the chemical nature and the concentration of pollutants, their availability to microorganisms, and the physicochemical characteristics of the environment. So, factors that influence the rate of pollutants degradation by microorganisms are either related to the microorganisms and their nutritional requirements or associated to the environment. A biotic factor is the metabolic ability of microorganisms. The extent to which contaminants are metabolized is largely a function of the specific enzymes involved and their “affinity” for the contaminant and the availability of the contaminant. In addition, sufficient amounts of nutrients and oxygen must be available in a usable form and in proper proportions for unrestricted microbial growth to occur. Other factors that influence the rate of biodegradation are temperature, pH and moisture [7, 18, 19, 20, 21].
6 Biodegradation of organic pollutants
Many environmental contaminants are subject to chemical or photochemical reactions. However, biological organisms – particularly microorganisms – play a more important role in the removal of many hazardous organics from the environment. Thermodynamically feasible contaminant transformations often do not occur in the absence of a biological catalyst, due to kinetic limitations, but are facilitated by microorganisms via enzymes, which lower the activation energy that must be overcome for a reaction to proceed, and the investment of biochemical energy to convert oxygen and other key co-reactants to more reactive forms [15].
The term biodegradation is often used in relation to ecology, waste management and mostly associated with environmental remediation (bioremediation). A huge number of bacterial and fungal genera possess the capability to degrade organic pollutants. Biodegradation is defined as the biologically catalysed reduction in complexity of chemical compounds. The process of biodegradation requires an understanding of the microorganisms that make the process work. The microbial organisms transform the substance through metabolic or enzymatic processes. It is based on two processes: growth and co-metabolism. In growth, an organic pollutant is used as sole source of carbon and energy. This process results in a complete degradation of organic pollutants. Co-metabolism is defined as the metabolism of an organic compound in the presence of a growth substrate that is used as the primary carbon and energy source. Compounds that support microbial growth are known as primary substrates. Co-metabolic substrates are called secondary substrates because they do not support growth. Several microorganisms, including fungi and bacteria are involved in biodegradation process. Biodegradation processes vary greatly, but frequently the final product of the degradation is carbon dioxide. Organic material can be degraded aerobically, with oxygen, or anaerobically, without oxygen [19, 21].
Whereas xenobiotics may persist in the environment for months and years, most biogenic compounds are biodegraded rapidly. However, it is not always easy to determine which structural moieties indeed are xenobiotic in the sense of ‘foreign to life’. It should be noted that organic chemicals of anthropogenic origin are not necessarily recalcitrant. There are a number of industrial products that are degraded by microorganisms. These compounds obviously are readily recognized by microbial catabolic enzymes. Figure 14 summarizes the possible fate of xenobiotic compounds [18].

Possible environmental fate of xenobiotic compound.
Example 7. The products of partial biodegradation, or biotransformation, or co-metabolic conversion of a xenobiotic may be less harmful as the original compound, or they may be as hazardous or even more hazardous than the original compound. For example, tetrachloroethane and trichloroethane can be microbially reduced to vinyl chloride, a known carcinogen, in anoxic habitats. In natural environments, the products of bioconversion processes may be further transformed or degraded by other microorganisms, maybe eventually leading to complete degradation by the microbial consortium. Co-metabolic processes and biodegradation by microbial consortia are thought to be of enormous ecological importance. However, persistent xenobiotics and metabolic dead-end products will accumulate in the environment, become part of the soil humus, or enter the food chain leading to biomagnification. Biomagnification is the increase in concentration of substance that occurs in a food chain. It is a result of, for instance, heavy metal contamination or in other words persistence of contamination which cannot be decomposed by environmental processes [18].
How to describe biodegradation of organic matter? Microbial degradation is generally defined as biological oxidation of organic matter. In natural environments, biodegradation conditions are very complex, and the rate and degree of biodegradation depends on chemical, physical, and biological factors that can differ from one ecosystem to another. Though microbial processes are very complex, some events or groups of events can be presented using a model. Several kinetic models have been developed to describe biological degradation of organic matter. The models are used to gain insight into the applicability and restrictions of treatment processes. The substrate concentration surrounding the microorganisms within the microbial ecosystem is important for the determination of kinetic parameter [15, 21].
Any factor that affects concentration of contaminant, the number of microorganisms present or the expression of specific enzymes by the cells can increase or decrease the rate of contaminant degradation.
Performing the substrate and biomass balance on the batch reactor at constant volume yields the equation for biomass growth rate which is described by the following first-order kinetic eq. (4):
where rx is biomass growth rate, g/L·d; X is the biomass concentration, g/L, t is the time, d, and μ is the specific growth rate of biomass, 1/d. At the same time as the production of the biomass, the substrate is degraded and the equation for the substrate degradation rate is:
where rS is substrate consumption rate, g/L·d, S is the limiting substrate concentration, g/L, and Y is the growth yield coefficient, g/g. The substrate degradation rate can also be expressed by the following eq. (6):
where q is the specific substrate degradation rate, g/g·d, the single parameter which characterises the degradation process. Re-arranging eqs (4)–(6), the equation for biomass growth rate can be presented by the expressions:
There are several expressions which relate the specific rates (μ and q) to the substrate concentration. How to calculate μ and q is presented in Example 8.
Example 8. How to calculate specific growth rate and specific substrate consumption rate directly from the experimental data?
Specific growth rate, µ, can be calculated directly from experimental data based on biomass concentration change (X) in time, using eq. (9):
Specific substrate consumption q, calculated using eq. (10), represents the rate at which the substrate is consumed in relation to biomass growth.
In bioprocess modelling, limiting substrate concentration which may occur during the process, may be modelled by various kinetic models. Due to their reliability and simplicity, the Monod model, the modified Monod model with endogenous metabolism, the Haldane model, and the Endo-Haldane model are commonly used [6, 9, 22]. Most frequently applied is the Monod model, which describes the dynamic behaviour of the process, i.e. it shows the relationship between the specific growth rate of the biomass and the limiting substrate:
In this traditional Monod model, µmax represents the maximum specific growth rate, 1/d, and KS is the substrate saturation constant, g/L, defined as the concentration of substrate at half the maximum specific growth rate. The biodegradation process results in microbial growth with the removal of substrate. At the end of the process, when most of the organic matter is removed, due to the lack of substrate, a weaker cell population becomes food for the healthier one. Due to biomass decay, the endogenous or decay coefficient, kd, must be incorporated in the original Monod model. This coefficient corresponds to the endogenous metabolism which involves reactions in cells that consume cell substances.
where kd is the decay coefficient, 1/d. Cell concentration reduction is known as endogenous respiration stage. How to calculate important parameters is presented in Example 9.
Example 9. How to calculate growth yield coefficient and the biomass decay rate?
Growth yield coefficient Y is one of the most important parameters used in biological kinetics models. It represents biomass concentration produced by unit of removed substrate. Endogenous respiration rate kd is the biomass decay rate. The dependency of consumed organic substrate to the production of microorganism cells is shown by eq. (13).
Linear regression of µ and q dependency, based on eqs (9) and (10), produces Y and kd parameters from the slope and intercept of plot, respectively.
When a substrate inhibits its own biodegradation, the Monod model is inadequate and must be modified by incorporating the inhibition constant Ki:
This is the Haldane model which takes into account the inhibition constant Ki, g/L·d, which is a measure of sensitivity to inhibition by inhibitory substances. However, some authors have proposed that the decline in cell population, i.e. biomass decay, after complete consumption of the substrate, should be taken into account. Therefore, after attaching the coefficient of microbial decay, kd, in the expression of the Haldane model, the equation assumes the following form:
This is the modified Haldane model, subsequently referred to as the Endo-Haldane model. This model is frequently used because of its ability to assess the effect of inhibition at high concentrations, and cell death and/or maintenance metabolism at low concentrations. The inhibition constant corresponds to the highest substrate concentration at which the specific growth rate equals one-half of the maximum specific growth rate without inhibition.
7 Principles of bioremediation
7.1 What is bioremediation?
Bioremediation uses microorganisms to reduce pollution through the biological degradation of pollutants into non-toxic or less toxic substances. Bioremediation is an alternative process that involves accelerated natural biodegradation. This can involve either aerobic or anaerobic microorganisms that often use this breakdown as an energy source. Microorganisms might be considered excellent pollutant removal tools in soil, water, and sediments, mostly due to their advantage over other bioremediation procedures. Moreover, bioremediation using biodegradation represents a high impact strategy, but still a low-cost tool for removing pollutants, hence a very viable process to be applied. The principles of bioremediation are based on natural attenuation, bioaugmentation and biostimulation. The simplest method of bioremediation is natural attenuation, in which soils are only monitored for variations in pollution concentrations to ensure that the pollutant transformation is active. Bioaugmentation is usually applied in cases where natural active microbial communities are present in low quantities or even absent, wherein the addition of contaminant degrading organisms can accelerate the transformation rates. In such cases, the adaptation of exogenous strains that exert highly efficient activities for pollutant transformation to new environments is a key challenge in implementation. The capacity of a microbial population to degrade pollutants can be enhanced also by biostimulation of the indigenous microorganisms by addition of nutrients and oxygen [19, 20].
Bioremediation is considered as one of the safest, cleanest, cost effective and environmentally friendliest technology for site decontamination which is contaminated with wide range of pollutants. If the natural attenuation is not quick enough or complete enough, bioremediation will be enhanced either by biostimulation or bioaugmentation.
The popularity of bioremediation is increasing because it often consumes less energy and fewer resources and thus is less expensive and more sustainable than physicochemical treatment approaches, such as landfilling or incineration. Further, many alternative remediation techniques simply transfer organic contaminants to another medium without detoxifying the compounds [15].
History of bioremediation: Bioremediation is not a new concept: microbiologists have studied the process since the 1940s. The first commercial use of naturally occurring microbes to safely and effectively clean up a toxic environmental disaster occurred in the late 1960s following an accidental oil spill in Cat Canyon (located in Santa Barbara, California, USA) after an oil pump shaft broke loose. The spill over went into the drainage system, into a stream and eventually into the nearest drinking water supply. George M. Robinson, assistant county petroleum engineer, treated the oil spill sumps with bacterial cultures that he had isolated in home experiments begun in the 1960s. The new treatment technology of bioremediation grew out of these early studies on petroleum hydrocarbon degradation. The first patent for a biological remediation agent was registered in 1974, a strain of Pseudomonas putida that was able to degrade petroleum [19, 20].
The invisible workforce, microorganisms can be isolated from almost any environmental conditions. Microbes can adapt and grow at sub-zero temperatures, as well as extreme heat, desert conditions, in water, with an excess of oxygen and in anaerobic conditions, with the presence of hazardous compounds or on any waste stream. Because of the adaptability of microbes and other biological systems, these can be used to degrade or remediate environmental hazards. The main requirements are an energy source and a carbon source. Although the microorganisms are present in contaminated soil, they cannot necessarily be there in the numbers required for bioremediation of the site. Their growth and activity must be stimulated. Biostimulation usually involves the addition of nutrients and oxygen to help indigenous microorganisms. These nutrients are the basic building blocks of life and allow microbes to create the necessary enzymes to break down the contaminants. All of them will need nitrogen, phosphorous, and carbon. Carbon is the most basic element of living forms and is needed in greater quantities than other elements. In addition to hydrogen, oxygen, and nitrogen it constitutes about 95 % of the weight of cells. The rate of biodegradation is decreased by roughly one-half for each 10 °C decrease in temperature. Biodegradation can occur under a wide-range of pH; however, a pH of 6.5 to 8.5 is generally optimal for biodegradation in most aquatic and terrestrial systems. Moisture influences the rate of contaminant metabolism because it influences the kind and amount of soluble materials that are available as well as the osmotic pressure and pH of terrestrial and aquatic systems [18, 19, 20].
Natural organisms, either indigenous or extraneous (introduced), are the prime agents used for bioremediation. The organisms that are utilized vary, depending on the chemical nature of the polluting agents, and are to be selected carefully as they only survive within a limited range of chemical contaminants. Since numerous types of pollutants are to be encountered in a contaminated site, diverse types of microorganisms are likely to be required for effective mediation (Table 1) [19, 23].
Microorganisms having biodegradation potential for xenobiotics.
| Organism | Toxic chemicals |
|---|---|
| Pseudomonas spp | Benzene, anthracene, hydrocarbons, PCBs |
| Alcaligenes spp | Halogenated hydrocarbons, linear alkylbenzene sulfonates, polycyclic aromatics, PCBs |
| Arthrobacter spp | Benzene, hydrocarbons, pentachlorophenol, phenoxyacetate, polycyclic aromatic, aromatics, long chain alkanes, phenol, cresol |
| Bacillus spp | Halogenated hydrocarbons, phenoxyacetates |
| Flavobacterium | Naphthalene, biphenyl, aromatics, branched hydrocarbons |
| Rhodococcus spp | Aromatics |
| Mycobacterium spp | Hydrocarbons, polycyclic hydrocarbons |
| Streptomyces spp | Phenoxyacetate, halogenated hydrocarbon, diazinon |
| Candida tropicalis | PCBs, formaldehyde |
7.2 Some groups of microbes
1. Aerobic: Examples of aerobic bacteria recognized for their degradative abilities are Pseudomonas, Alcaligenes, Sphingomonas, Rhodococcus, and Mycobacterium. These microbes have often been reported to degrade pesticides and hydrocarbons, both alkanes and polyaromatic compounds. Many of these bacteria use the contaminant as the sole source of carbon and energy.
2. Anaerobic. Anaerobic bacteria are not as frequently used as aerobic bacteria. There is an increasing interest in anaerobic bacteria used for bioremediation of polychlorinated biphenyls (PCBs) in river sediments, dechlorination of the solvent trichloroethylene (TCE) and chloroform.
3. Ligninolytic fungi. Fungi such as the white rot fungus Phanerochaete chrysosporium have the ability to degrade an extremely diverse range of persistent or toxic environmental pollutants. Common substrates used include straw, saw dust, or corn cobs.
4. Methylotrophs. Aerobic bacteria grow utilizing methane for carbon and energy. The initial enzyme in the pathway for aerobic degradation, methane monooxygenase, has a broad substrate range and is active against a wide range of compounds, including the chlorinated aliphatic trichloroethylene and 1,2-dichloroethane.
Microorganisms play a more important role in the removal of many hazardous organics from the environment. Many microorganisms possess the ability to transform hazardous compounds. However, the long-term persistence of many of these contaminants in the environment is a testament to the fact that these naturally occurring processes often do not occur at rates that are fast enough to protect ecosystem and human health. Frequently, the microorganisms are limited by the availability of the pollutant or another key substrate or are not present in sufficient numbers. In many cases, bioremediation can overcome these limitations through careful engineering of the contaminated environment, thereby enhancing the rates of key microbial processes. Thus, successful bioremediation involves the integration of environmental microbiology and engineering techniques with other disciplines, such as geochemistry and hydrology.
Biodegradation is nature’s way of recycling wastes, or breaking down organic matter into nutrients that can be used by other organisms. In nature, there is no waste because everything gets recycled. The waste products from one organism become the food for others, providing nutrients and energy while breaking down the waste organic matter. Some organic materials will break down much faster than others, but all will eventually decay. By harnessing these natural forces of biodegradation, people can reduce wastes and clean up some types of environmental contaminants. Through composting, we accelerate natural biodegradation and convert organic wastes to a valuable resource. Wastewater treatment also accelerates natural forces of biodegradation. In this case the purpose is to break down organic matter so that it will not cause pollution problems when the water is released into the environment. Through bioremediation, microorganisms are used to clean up oil spills and other types of organic pollution. Treatments can be either ex situ or in situ. The technology can involve aerobic and/or anaerobic bioreactors, biofiltration, air sparging, bioventing, composting, landfarming, and biopiles. Intrinsic remediation refers to the combined effects of all natural processes in contaminated environments that reduce the mobility, mass, and risks of pollutants [15, 20].
8 Composting
8.1 What is composting? Composting is nature’s way of recycling
Composting is the controlled aerobic decomposition of organic matter by microorganisms into a stable, humus-like soil amendment. The processes used in composting occur in nature, but systems can be designed and managed to enhance and accelerate the process. The by-product of composting consists of the biomass of dead and living microorganisms, non-degradable raw material, and stable by-products of decomposition. Compost is an organic soil conditioner that has been sufficiently stabilized to minimize or eliminate unpleasant odours, substantially reduce or eliminate viable pathogens and weed seeds, facilitate storage and handling without attracting insects and vectors, and provide plants with nutrients that become available throughout the growing season. Composting stabilizes organic matter by converting the readily degradable portion of the organic matter into carbon dioxide and water. Complete stabilization is neither practical nor desirable because complete stabilization would destroy all the slowly degradable organic matter that gives compost its soil-building properties. The degree of stabilization and pathogen destruction desired in compost is dependent on the purpose for composting and intended use of the compost by-product [24, 25].
Microorganisms in composting process decompose the more readily available carbonaceous compounds. Composting involves various types of microorganisms which are all vital to the process. In the process of composting, microorganisms such as bacteria, fungi, and actinomycetes, break down organic matter and produce carbon dioxide, water, heat, and humus, the relatively stable organic end product. Bacteria are most numerous and are the primary biodegraders. The most common microorganisms involved in the composting process are genera Bacillus, Pseudomonas, Cellulomonas, Trichoderma, Trichosporon, Cladosporium, Thermoactinomyces, Streptomyces. Under optimal conditions, composting proceeds through three phases: (1) the mesophilic, or moderate-temperature phase, which lasts for a couple of days, (2) the thermophilic, or high-temperature phase, which can last from a few days to several weeks, and finally, (3) a several-month cooling and maturation phase.
Different communities of microorganisms predominate during the various composting phases. Initial decomposition is carried out by mesophilic microorganisms, which rapidly break down the soluble, readily degradable compounds. The heat they produce causes the compost temperature to rapidly rise. As the temperature rises above about 40 °C, the mesophilic microorganisms become less competitive and are replaced by others that are thermophilic, or heat-loving. At temperatures of 55 °C and above, many microorganisms that are human or plant pathogens are destroyed. Because temperatures over about 65 °C kill many forms of microbes and limit the rate of decomposition, compost managers use aeration and mixing to keep the temperature below this point. During the thermophilic phase, high temperatures accelerate the breakdown of proteins, fats, and complex carbohydrates like cellulose and hemicellulose, the major structural molecules in plants. As the supply of these high-energy compounds becomes exhausted, the compost temperature gradually decreases and mesophilic microorganisms once again take over for the final phase of “curing” or maturation of the remaining organic matter.
How does composting happen? For successful composting microbes need nutritious “food”, suitable moisture, pH, temperature, and oxygen. The supply of carbon (C) relative to nitrogen (N) is an important quality of compost feedstocks. It is designated as the C:N ratio. The ideal starting range is C:N 25–35:1. Microorganisms such as bacteria, fungi and actinomycetes account for most of the decomposition, as well as the rise in temperature that occurs in the composting process. The ideal moisture content of the compost pile is between 45 and 60 % by weight, and pH 6.5–8.0.
The rate of decomposition or process limitation is a function of microbial activity. As shown in Figure 15, temperature rises from ambient to mesophilic and then to thermophilic. As temperature in the compost pile increases, thermophiles (microorganisms that function at temperatures above 40 oC) take over. The temperature in the compost pile typically increases rapidly to 55–65 oC within 24–72 hours of pile formation, which is maintained for several weeks. This is called the active phase of composting.

Temperature changes in an average compost pile.
In the active “thermophilic” phase, temperatures are high enough to kill pathogens and weed seeds and to break down phytotoxic compounds (organic compounds toxic to plants). Common pathogens killed in this phase are E. coli, Staphylococcus aureus, Bacillus subtillus, and Clostridium botulinum. During this phase, oxygen must be replenished through passive or forced aeration, or turning the compost pile. As the active composting phase subsides, temperature gradually declines to under 40 oC. The mesophilic microorganisms recolonize the pile, and the compost enters the curing phase. The rate of oxygen consumption declines to the point where compost can be stockpiled without turning. During curing, organic matter continues to decompose and are converted to biologically stable and mature compost [25].
References
[1] Salyers AA, Whit DD. Microbiology: diversity, disease, and the environment. Bethesda, MA: Fitzgerald Science Press; 2001: 19–32, 439–452.Search in Google Scholar
[2] Environmental IV. Microbiology for engineers, 2nd ed. Boca Raton, FL: CRC Press, 2010: 1–15, 265–293.Search in Google Scholar
[3] Nester EW, Anderson DG, Roberts CE, Pearsall NN, Nester MT, Hurley D. Microbiology: a human perspective. New York, NY: McGraw Hill, 2004: 83–103, 785–797.Search in Google Scholar
[4] Shuler ML, Kargi F. Bioprocess engineering: basic concepts. Upper Saddle River, NJ: Prentice Hall PTR, 2002: 133–155.Search in Google Scholar
[5] Pepper IL, Gerba CP. Environmental microbiology: a laboratory manual, 2nd ed. Burlington, MA: Elsevier Academic Press, 2004: 17–49, 113–139, 169–174.Search in Google Scholar
[6] Briški F, Kopčić N, Ćosić I, Kučić D, Vuković M. Biodegradation of tobacco waste by composting: Genetic identification of nicotine-degrading bacteria and kinetic analysis of transformations in leachate. Chem Paper. 2012;66:1103–1110.10.2478/s11696-012-0234-3Search in Google Scholar
[7] Sharma PD. Environmental microbiology. Harrow, UK: Alpha Science International Ltd., 2005: 66–94.Search in Google Scholar PubMed
[8] Van Elsas JD, Smalla K. Methods for sampling soil microbes. In: (Hurst CJ, editor. in Chief), Manual of environmental microbiology. Washington, DC: ASM Press, 1997: 383–391.Search in Google Scholar
[9] Briški F Zaštita okoliša (in Croatian), Fakultet kemijskog inženjerstva i tehnologije Sveučilišta u Zagrebu i Element, Zagreb, 2016: 46–50, 87–91, 113–134, 179–185.Search in Google Scholar
[10] APHA. Standard methods for the examination of water and wastewater, 2nd ed. Washington, DC: American Public Health Association, 1998.Search in Google Scholar
[11] Rodina AG. Methods in aquatic microbiology. Baltimore, Maryland: University Park Press, 1972: 358–368.Search in Google Scholar
[12] Stetzenbach LD. Introduction to aerobiology. In: Hurst CJ, Knudsen GR, McInerney MJ, Stetzenbach LD, Walter MV, editors, Manual of environmental microbiology. Washington, DC: ASM Press, 1997: 620–621.Search in Google Scholar
[13] Slade D, Radman M. Oxidative stress resistance in Deionococcus radiodurans. Microbiol Mol Biol Rev. 2011;75:133–191.10.1128/MMBR.00015-10Search in Google Scholar
[14] Lange CC, Wackett LP, Minton KW, Daly MJ. Engineering a recombinant Deionococcus radiodurans for organopollutant degradation in radioactive mixed waste environments. Nature Biotechnol. 1998;16:9229–9233.10.1038/nbt1098-929Search in Google Scholar
[15] Mitchell R, Gu JD. Environmental Microbiology. New Jersey, USA: John Wiley & Sons, Inc., 2010: 115–135, 177–212.Search in Google Scholar
[16] Rosenberg R. Eutrophication: the future marine coastal nuisance?. Mar Pollut Bull. 1985;16:227–231.10.1016/0025-326X(85)90505-3Search in Google Scholar
[17] NRC (National Research Council). Clean coastal waters: understanding and reducing the effects of nutrient pollution. Washington, DC: National Academies Press; 2000.Search in Google Scholar
[18] Doelle HW, Rokem S, Berovic M. Biotechnology- volume X: fundamentals in biotechnology. Oxford, UK: Eolss Publishers Co. Ltd, 2009: 215–246.Search in Google Scholar
[19] Kumar A, Bisht BS, Joshi V, Dhewa T. Review on bioremediation of polluted environment: a management tool. Int J Environ Sci Technol. 2011;6:1079–1093.Search in Google Scholar
[20] Adams GO, Fufeyin PT, Okoro SE, Ehinomen I. Bioremediation, biostimulation and bioaugmention: a review. Int J Environ Biorem Biodeg. 2015;3:28–39.Search in Google Scholar
[21] Chamy R, Rosenkranz F. Biodegradation – life of science. Rijeka: InTech, 2013: 289–320.10.5772/52777Search in Google Scholar
[22] Vuković Domanovac M, Ćosić I, Sojčić M, Briški F. Treatment of tobacco dust leachate by activated sludge- Evaluation of biokinetic parameters. Cabeq. 2013;27:51–56.Search in Google Scholar
[23] Vidali M. Bioremediation an overview. Pure Appl Chem. 2001;73:1163–1172.10.1351/pac200173071163Search in Google Scholar
[24] USDA. National engineering handbook, Part 637, Chapter 2, Composting. Washington, DC: NRCS, 2000.Search in Google Scholar
[25] Epstein E. Industrial composting: environmental engineering and facilities management. Boca Raton, FL: CRC Press, 2011: 15–24.10.1201/b10726-3Search in Google Scholar
A Appendix
Questions and Problems
In what ways did the cyanobacteria make possible the later evolutions of living organisms?
The prokaryotic members of the microbial world include: 1. algae, 2. fungi, 3, bacteria, 4. archaea, 5. viruses. Which of the following is correct answer?
A. 1, 2 B. 2, 3 C. 3, 4 D. 1, 5
The eukaryotic members of the microbial world include: 1. protozoa, 2. algae, 3. bacteria, 4. viruses. Which of the following is correct answer?
A. 1, 2 B. 2, 3 C. 3, 4
From the following data calculate the mean generation time if at the beginning of exponential growth t = 0 initial cell concentration is 2.5 · 103/mL, and t = 10 hours cell concentration is 1 · 105/mL.
Describe the role of bacterial species Nitrosomonas and Nitrobacter in soil.
What is the definition of coliform bacteria and name one bacterium which is the member of this group?
After bacteriological analysis of water sample from nearby river the number of faecal coliform on mFC plate was 1.7 · 102/100 mL and faecal streptococci on FS plate was 3.0 · 101/100 mL. Calculate the FC:FS ratio and deduce what is the source of contamination.
What effect causes the microbial contamination of indoor environment and describe the adverse effect of fungus Stachybotrys chartarum.
What is eutrophication?
What is the difference between biodegradation and biotransformation?
What are xenobiotics?
How to describe biodegradation of organic matter?
How to calculate specific growth rate and specific substrate consumption rate directly from the experimental data?
What is bioremediation?
What is the difference between bioaugmentation and biostimulation?
Describe the process of composting.
Notes
The authors of this chapter are thankful to Mrs. Marijana Vidakovic on technical support in preparation of photomicrographs for Figure 4 and photographs for Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11.
This article is also available in: Environmental Engineering, Tomašic/Zelic. De Gruyter (2017), isbn 978‒3‒11‒046801‒4
© 2017 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- The halogen bond: Nature and applications
- Soil treatment engineering
- Effect of protonation, composition and isomerism on the redox properties and electron (de)localization of classical polyoxometalates
- Environmental microbiology
- Gas-phase high-resolution molecular spectroscopy for LAV molecules
- Modeling of Azobenzene-Based Compounds
Articles in the same Issue
- The halogen bond: Nature and applications
- Soil treatment engineering
- Effect of protonation, composition and isomerism on the redox properties and electron (de)localization of classical polyoxometalates
- Environmental microbiology
- Gas-phase high-resolution molecular spectroscopy for LAV molecules
- Modeling of Azobenzene-Based Compounds

