1 Introduction
As noted by the science journalist Robin Williams, “The 20th century was the century of physics, the 21st century will be the century of biology”. Knowledge of biological systems started firstly with observation or documentation, followed by understanding, then finally utilization or biomimicking. Whilst humans have been employing bioprocesses since as early as 7000 BC (as evidenced by Neolithic fermentation jars), our detailed understanding of biological processes in terms of genomics, metabolomics, and proteomics is very recent.
For a production process to be economically viable, the availability of the feedstock, the unit operations and the product must be understood. Lower value products (e.g. livestock feed), bulk commodities (e.g. sugar) or products with a number of competing sources (e.g. electricity) require scale, low cost feedstocks (e.g. sourced from broad-acre, agri-waste or animal tissues), and either low cost or efficient unit operations (e.g. high yields, low utility requirements). Higher value biomolecules (e.g. enzymes; biopharmaceuticals) can bear higher cost feedstocks (e.g. from fermentation) with higher cost intensity unit operations (e.g. centrifugation, spray drying and chromatography). Figure 1 shows some examples of biomass feedstocks or products and their associated value as a function of cellulose and hemicellulose content. Their abundance in many forms of biomass and their attractiveness for fuel drive down their price, but there is substantial opportunity for further value adding.
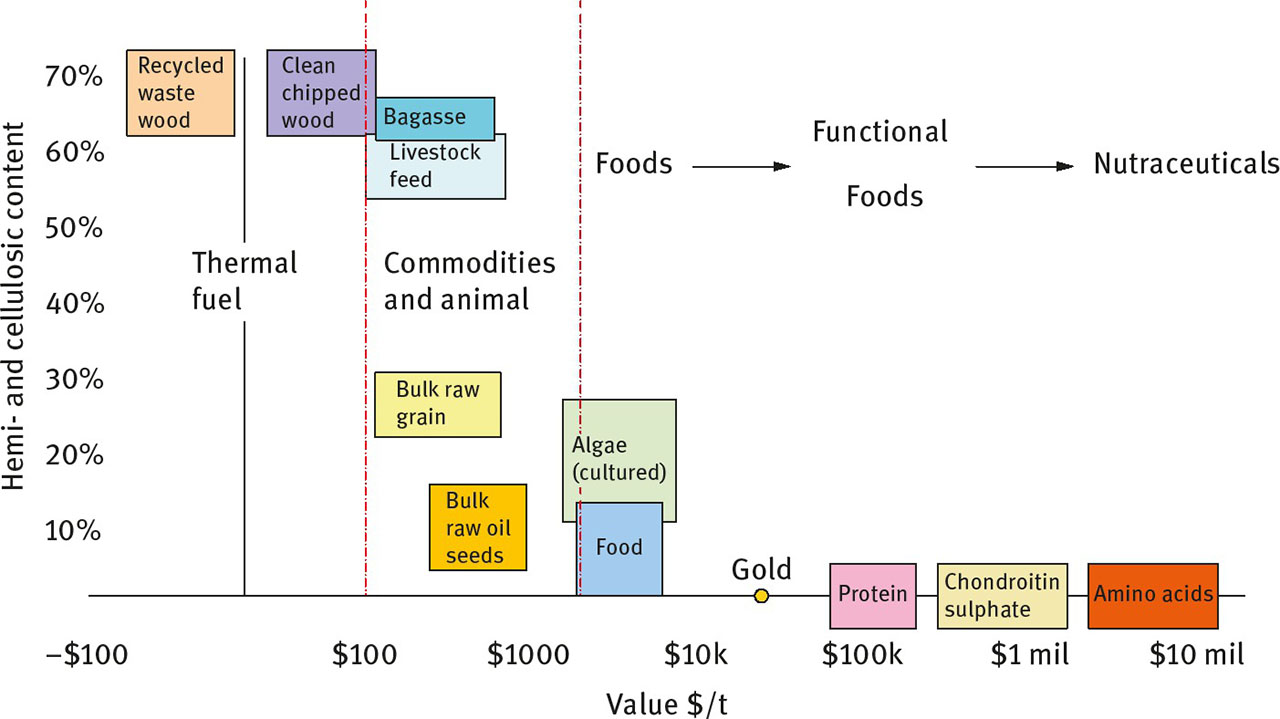
Correlation of dry weight cellulose and hemicellulose content to value in $ per ton. Note that the x-axis is logarithmic.
Biomass is inherent and, in the vast majority of cases, nontoxic as biological processes create products which serve as feedstocks for other processes (with notable exceptions, such as venoms and pathogens, representing extremely low percentages of the total global biomass). Hence, a well-engineered bioprocess should be “benign by design”. Bioprocess engineering is defined as the design and development of processes for the manufacture of products from biomass or via biological processes.
By using the inspiration offered by biological systems, bioprocess engineering should strive for creating circular processes where everything other than the end product can be a feedstock for another process. The inspiration to address many of society’s current challenges using biology has spawned the area of biomimetics, where an engineered biological system aims to mimic the inherent advantages of natural biological systems. An example is where humans look to biology for examples of biofixation of carbon dioxide (CO2). CO2 is taken from a gaseous phase and converted into biomass and biological compounds (e.g. carbohydrates, lipids, DNA, protein), then this biomass is reused as feedstock for products such as transport fuel, energy, polymers, food, food additives and fine chemicals. Whilst such systems may have inherent inefficiencies, if the net environmental impact is zero, then the economics may be improved through scale and smart engineering. Industry must find ways to achieve deep decarbonization of how energy, food and products (polymers, drugs, etc.) are created, that is, to stop the reliance on fossil fuels. Biomass processing coupled to innovative downstream processing provides one significant opportunity to decarbonize human society.
Use of life cycle analyses (LCAs) can provide insights for making long term decisions on sustainable processes. For example, the biopharmaceutical industry has one of the highest waste to product ratios of any industry (kg waste generated per kg product produced) compared to the oil and gas industry which has the lowest waste to product ratio. This high waste ratio is caused by the extensive use of disposables and cleaning requirements warranted in the manufacture of a biopharmaceutical. One option to improve the sustainability of bioindustries (refer Figure 2) is anaerobic digestion to produce bioenergy from organic byproducts, rather than chemical treatment or off-site disposal. Other examples include the recycling of nutrients or isolation of fractions from waste streams for other purposes such as carbon-fiber production from waste lignin.
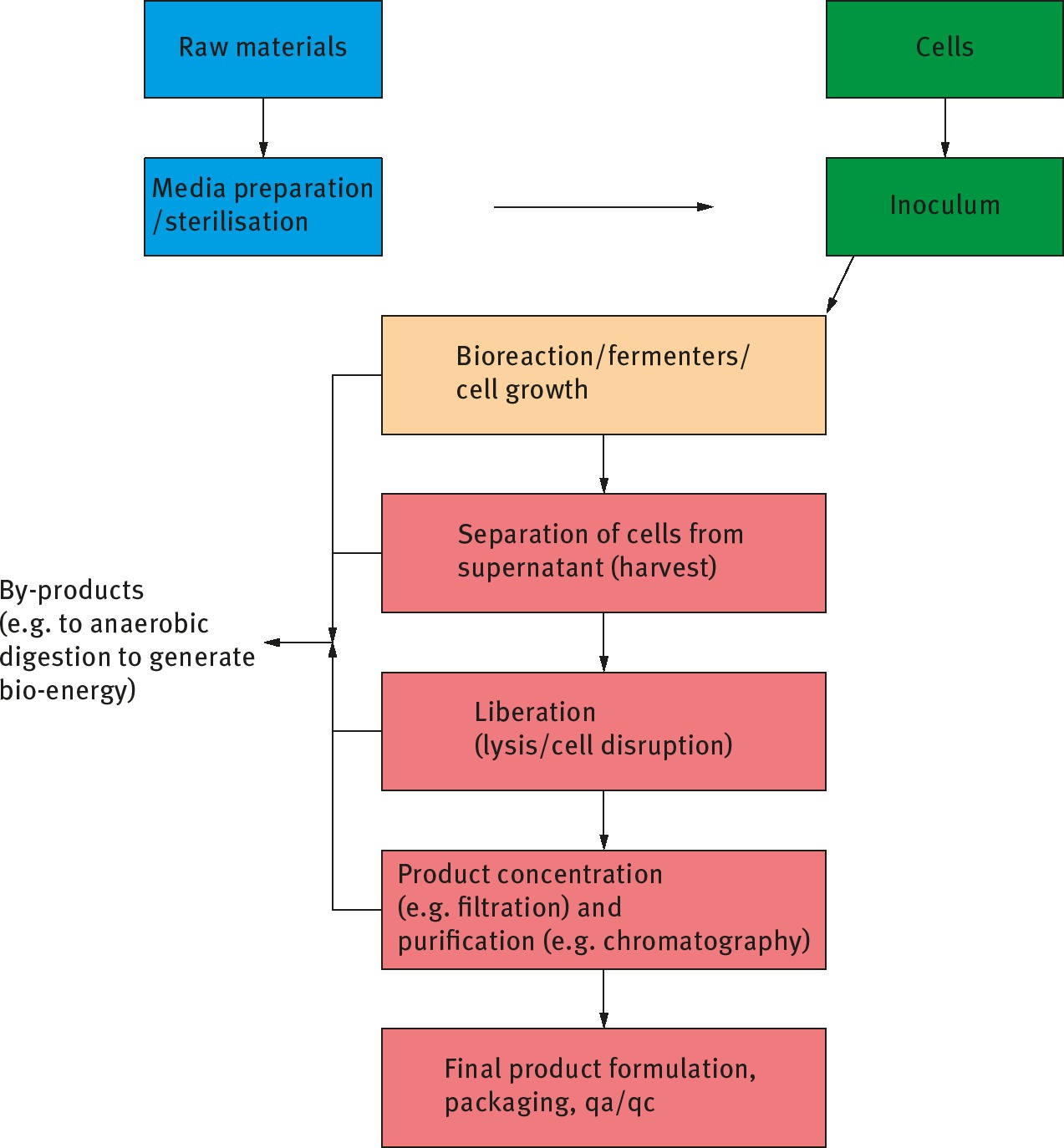
Block diagram of a bioprocess for the manufacture of a purified biomolecule via fermentation. The stage of product concentration and purification represents approximately 50 to 80% of total processing costs.
This chapter will consider which biomass feedstocks are most suited for the manufacture of bioproducts (e.g. bioenergy, chemicals, vaccines) via appropriate bioprocess engineering unit operations. For example, a low value biomass (chipped wood) requires simple, low cost and large scale unit operations whilst the manufacture of a high value bioproduct (e.g. a biopharmaceutical or functional food) utilizes a larger number of smaller scale and high cost unit operations to achieve appropriate purities. The chapter will consider upstream processes (the creation of the biomass), down-stream processes (the separation of the target biomolecules or value adding stages (refer Figure 2 for a sample flow diagram) and finally present some examples of the creation of products from biomass.
2 Upstream bioprocesses
Upstream biomass generation can be in “open” or “closed” systems, where open systems are traditionally utilized for the generation of food and energy crops using agricultural or cropping processes, whilst closed systems are routinely characterized by monocultures (e.g. bacterial, yeast) achieved via the use of sterile procedures with defined or semi-defined media.
2.1 Open systems
Food, forestry and energy crops are typically based around sugars, starch and lignocellulosic material. Harvesting operations for many food industries involve leaving some nonfood material in the field (e.g. stubble from grain or leaves from sugarcane) while the harvested material is separated into (i) food and (ii) nonfood material. The greater the chemical complexity of the nonfood material (e.g. lignocellulosics), the more challenging is the processing and value adding. Similarly timber is harvested, sawn to produce lumber and the residue is chemically complex which results in much of it being burned (Section 4.1). Notwithstanding, open systems can produce high value materials – an example is provided in Section 4.5.
Typical sources of biomass from open systems:
Food agriculture
Sugarcane industry
Grain industry
Fruit and vegetable
Other agriculture
Cotton
Dedicated energy crops (e.g.miscanthus)
Forestry
Timber
Bark
Woodchip
Leaves, branches, stumps
The products from biomass are wide (in order of increasing value):
Electricity
Buildingproducts
Transport fuels
Food
Pulp and paper products
Newsprint
Photocopier paper
Tissue
Niche materials (e.g.microfibrillated cellulose)
Typical upstream operations include:
– Planting
– Crop maintenance (e.g. fertilizing, spraying, pest control)
– Harvesting
– Transportation
– Coarse separation of the plant into different components (e.g. grain from husk and bark from the timber) by mechanical processing
– Combusting part of the feedstock may occur to assist upstream processing (e.g. sugarcane)
2.2 Closed systems
For closed systems biomass is composed of cells which may, from an engineering perspective, be considered as a highly organized molecular factory, see Figure 3. Our modern understanding of cells is not perfect clones of the same cell, but rather colonies of interacting cells with specialized functions.
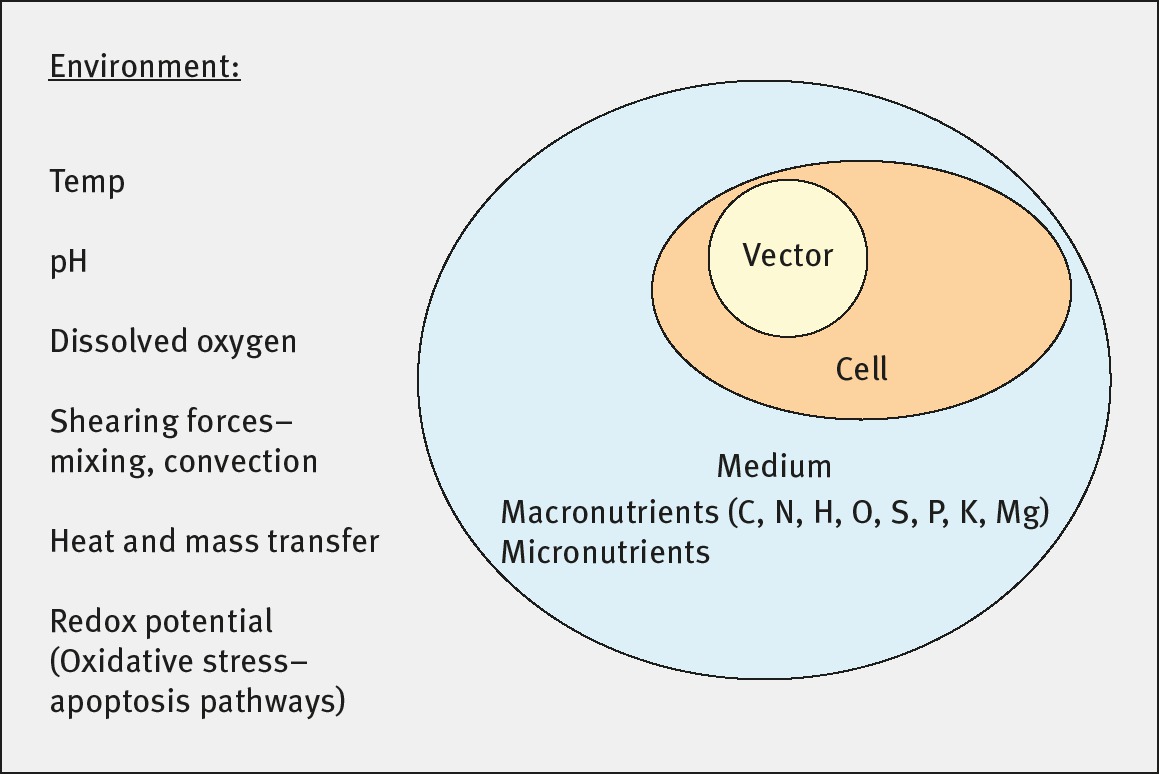
Upstream or BIOREACTION section: Selection and conversion.
Deloitte Touche Tohmatsu [1] reports that global fermentation industries are valued at over US $ 127 billion per annum made up of ethanol (87% of value) with the next two largest groups being amino acids (8.7%) and organic acids (2.8%). The projected annual growth to 2020 (excluding alcohols) for fermentation products is 6.5%, in which the highest growth area is polymers.
The primary characteristics of a cell are the cell membrane (or plasma membrane), cytoplasm and organelles and the nuclear region. The similarities between all cells are:
Cell membrane
Contains DNA
Composed of the same basis chemicals: carbohydrates, proteins, nucleic acids, minerals, fats and vitamins
All cells regulate the flow of nutrients and wastes that enter and leave the cell
All cells reproduce and are the result of reproduction
All cells require a supply of energy
All cells responding to stimuli are highly regulated by elaborate sensing systems
Biomolecules come from a vast number of sources:
From all types of cells imaginable
Synthesized (peptides and oligos)
Biomolecules have an even larger number of applications, in increasing value:
Livestock feed
Human food and beverage
Function foods e.g. enhanced foods
Nutraceuticals e.g. amino acids, proteins
Finechemicals
Enzymes
Biopharmaceuticals e.g. vaccines, monoclonal antibodies, gene therapy agents
Typical upstream unit operations include:
Medium formulation
Sterilization
Cellculturing
Propagation
Biomass production
Metabolite biosynthesis
Aggregation/ harvesting
Hydrolysis
Biotransformations
One of the main actions for bulk biomass and cell cultures is the reduction of water content. The composition of a typical cell is 70% or more water with the other mass (dry weight) consisting of the molecules outlined below (Table 1).
Moisture contents of biomolecules.
| Class | % dry weight | Constituents |
|---|---|---|
| Protein | 55 | amino acids |
| Carbohydrates | 10 | sugars |
| Lipid | 9 | fatty acids, glycerol, phosphate |
| RNA | 20 | purines, pyrimidines, phosphate, and ribose |
| DNA | 3 | purines, pyrimidines, phosphate, and deoxyribose |
| Small molecules | 3 | metabolic intermediates and inorganic ions (e.g. metals ~1%) |
Big, glycosylated, complex molecules are more likely to be sourced from biomass, whilst smaller, simpler molecules are likely to be generated by synthetic means (Table 2).
KPIs of cellular systems for rDNA protein production.
| Key Performance lndicator | E. coli | Yeast | lnsect | Plant | Mammalian |
|---|---|---|---|---|---|
| High μ | 4 | 3 | 0.5 | 0.5 | 0.5 |
| Expression Ieveis | 4 | 3 | 1.5 | 1.5 | 1.5 |
| Low-cost media | 4 | 4 | 0 | 1 | 0 |
| Protein folding | 1 | 1.5 | 3.5 | 4 | 4 |
| Simple posttranslational processing | 0 | 2 | 3 | 4 | 4 |
| Complex posttranslational processing | 0 | 0 | 0 | 2 | 4 |
| Low proteolyic degradation | 1.5 | 2 | 3 | 3 | 3 |
| Secretion | 0 | 3 | 3 | 4 | 4 |
| Safety | 3 | 4 | 4 | 3 | 2 |
| Sealability | 4 | 3.5 | 3 | 2 | 2.5 |
| Posttranslational processing required (e.g. glycosylation) | 26 | 21.5 | 25 | 25.5 | |
| Posttranslational processing and secretion NOT required (e.g. glycosylation) | 21.5 | 21 | 15.5 | 15 | 13.5 |
3 Downstream bioprocesses
3.1 Overview of downstream bioprocesses
Downstream processing refers predominantly to the physical and chemical processing to extract, purify then concentrate the target molecule from the water and biomass created in the upstream stage. The processing of the bulk feed material (e.g. whole cells suspended in cell culture) must ensure that an appropriate chemical structure and biochemical activity is maintained whilst ensuring the entire process is economically viable. The process must have sufficient yield and appropriate capital and operating costs.
The level of processing depends on the difficult degree of purification, the intended use of the final product and the final required specification. Industrial fuels, chemicals and materials will generally require fewer processing steps than a product that will be consumed by humans or animals. Even within human use products, the level of bioprocessing increases as the therapeutic use of the molecule increases (i.e. administered intravenously rather than orally and must have a specific efficacy rather than general effects). For example, some food supplements may be simply dried and milled, whilst a biopharmaceutical may undergo a dozen or more downstream unit operations to achieve the required purity and sterility.
The principal aims of downstream processing are to:
maximize target product/biomolecule yield
minimize the number of unit operations (because product loss occurs at each purification step)
minimize processing time and cost
maintain biomolecule activity/integrity by preventing degradation (due to temperature, pH, chemical reactions, enzyme hydrolysis, shear, UV degradation, etc.)
ensure product quality in terms of specified product composition and reproducibility (i.e. no variation between doses, batches or during storage)
provide a product with an acceptable concentration, volume, and guaranteed activity
provide a safe product that is, for example, free of toxins (endotoxins/lipopolysaccharides), viruses, chemical contaminants and pathogens
where required, uses a validated process such as a current Good Manufacturing Process (cGMP)
The aims of downstream processing can be counterproductive, with a compromise or economic optimization being required. For example, whilst chromatography may provide a fast and single unit operation for the purification of a recombinant protein, it may result in a cost impost which is unacceptable (due to equipment, resin, buffer media, and operating costs). As a further example, if the aim is to create a high purity and high biological activity enzyme, more unit operations contribute to achieving a higher purity but often reduce biological activity through increased opportunity for degradation and denaturation.
Downstream processing is routinely utilized for the manufacture of food supplements, nutraceuticals and biopharmaceuticals. Biopharmaceuticals represent a new generation of therapeutics of rapidly increasing importance: in 2002 the total pharma market was US $ 390 billion, of which biopharmaceuticals accounted for 7% (US $ 27.3 billion); in 2005 it was 12% (US $ 70.8 billion); in 2010 about 50% of drugs in development were biopharmaceuticals [2]; in 2013 it was estimated to represent around 20% (approximately US $ 199.7 billion) with the predicted biopharma market in 2020 as high as US $ 490 billion [3]. The global nutraceuticals product market was US $ 142.1 billion in 2011 and is expected to reach US $ 204.8 billion by 2017, with ingredients for the nutraceutical industry valued at US $ 33.6 billion [4]. The functional food and beverage market reached US $ 93 billion in 2011. Figure 4 shows how the value of a biomolecule increases via additional processing and as the therapeutic requirements increase in complexity. That is, small batch size therapeutic proteins for low frequency disease states and gene therapy molecules are much more expensive to make than large production runs of vaccines and monoclonal antibodies.
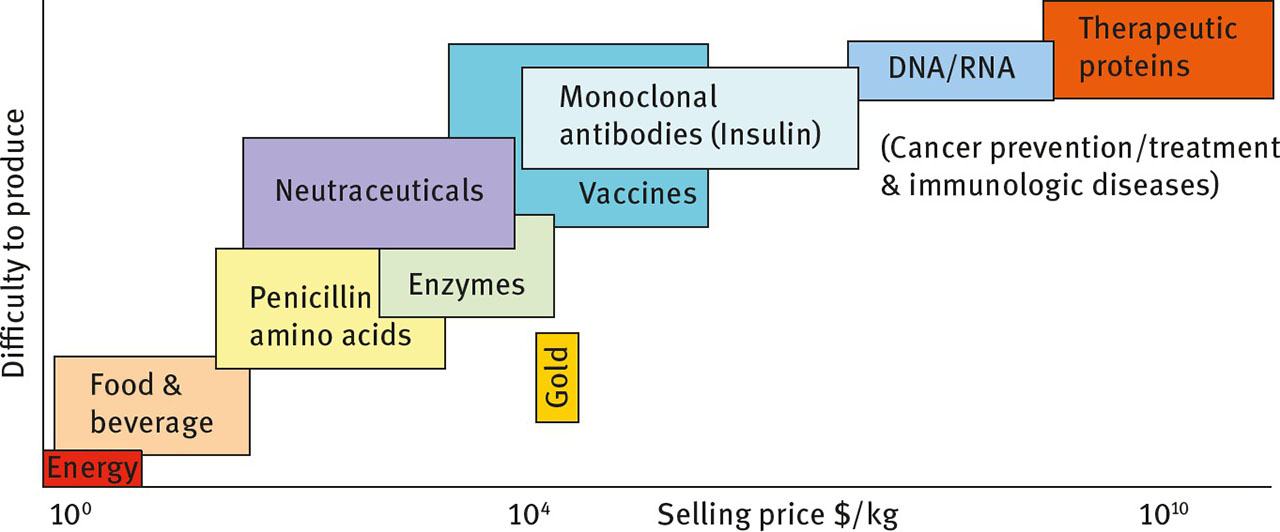
Value in US $ per kg (x-axis) versus indicative difficulty to produce different products sourced from biomass and biomass-derived sugars. As the requirement for high purity increases, so too does the number of unit operations required to produce the final product. Note that the x-axis is a logarithmic scale.
3.2 Downstream bioprocessing unit operations
Listed below are the most common unit operation employed in downstream processing. This is not an exhaustive list, nor does it detail the specifics of each unit operation as this is left for other text books dedicated to these topics. The list provides an indicative ranking of unit operations from cheapest to most expensive on a $/ton processed basis. The first few unit operations (coarse size separation, comminution and biomass dewatering) are the only unit operations routinely used for biomass energy applications as further processing is usually too expensive. To make a food supplement or high purity biomolecule injectable a large number of these unit operations may be required.
Typical downstream[1]unit operations include:
1. Coarse size separation: sieving, sorting, sizing decks
2. Comminution: crushing, grinding, milling
3. Biomass dewatering: centrifugation, gravity separation, flocculation, rotating drum
4. Cell lysis/disruption or liberation: homogenization/pressure, chemical cell wall rupture
5. Extra-cellular product concentration:
(a) Mechanical: tangential flow filtration, centrifugation
(b) Evaporative: spray drying, freeze drying
6. Purification:
(a) Ultrafiltration
(b) Chromatography
(c) Precipitation (e.g. via the use of salts)
(d) Extraction (e.g. liquid-liquid)
(e) Crystallization
(f) Dia-filtration/dialysis
(g) Sterile filtration
7. Stabilization/formulation/vialing/dosing/packaging
3.3 Specific processing considerations for biomolecules
Improving the economics of the production of a biomolecule is not only about the upstream volumetric yield (i.e. grams of biomolecule per liter of cell culture) and upstream specific yield (i.e. grams of biomolecule per gram of biomass) but also:
time yield, for example, grams per hour of production time (bioprocessing plants are expensive to construct and operate, hence efficient utilization is paramount for a profitable biomolecule production process),
bioactivity (i.e. percentage of biomolecules that are biologically active),
cell and product substrate yield e.g. grams of cells and/ or secondary metabolites produced per gram of glucose or other fermentable sugar consumed (or more generally, the yield of carbon in the product compared to the carbon source used),
cell and product macro-/micronutrient yields e.g. where the creation of cells and/ or secondary metabolites depends upon the presence of a particular additive being in the growth media, the efficient use of this additive is vital for a process to be economically viable, and
maintaining acceptable yields and bioactivities for all downstream unit operations.
As mentioned in Section 3.1, aims of downstream processing are sometimes counterproductive, especially from an economic perspective. For example, when making a dietary supplement processing the biomolecule to a purity that is fit for purpose may be more economically viable than achieving the highest purity possible. This point is emphasized by the example nutraceutical product chondroitin presented in Section 4.2, where the profit after tax for the “value adder” (stage in the supply chain where the target biomass is isolated) equates to 36%, compared to 9% for the manufacturer and 16% for the retailer (refer Figure 5).
![Figure 5: Coumaryl, coniferyl, and sinapyl lignin [12].](/document/doi/10.1515/psr-2016-0046/asset/graphic/psr-2016-0046_fig_005.jpg)
Coumaryl, coniferyl, and sinapyl lignin [12].
Generally, as purity requirements increase for a biomolecule, so too does the number of unit operations (or processing stages) employed. Additionally, certain unit operations or processes may destroy the target biomolecule, including:
Shearing e.g. pumps and mixing
Hydrolysis or the cleavage of molecular bonds e.g. via enzymes
Inhibition via oxidation or chelation with metal ions
Irreversible aggregation/ precipitation
Extremes of pH
Hence, bioactivity yield refers to both retaining the target molecule as well as the biological activity of the molecule (i.e. the amount of the molecule that it is present and that can perform its function). Table 3 shows the relationship between the number of stages or unit operations and the percentage bioactivity yield.
Overall bioactivity (%) for multiple stage processes.
| Percentage (%) bioactivity yield at each stage | After 3 stages | After 5 stages | After 10 stages |
|---|---|---|---|
| 99 | 97 | 95 | 90 |
| 95 | 86 | 77 | 60 |
| 90 | 73 | 59 | 35 |
| 80 | 51 | 33 | 11 |
3.4 Chromatography
Chromatography has been singled out as a unit operation of specific interest for the manufacture of higher value biomolecules. It is particularly important in the biopharmaceutical industry, however is not widely utilized in other process industries due to its batch-wise nature. Chromatography separates mixtures into components by passing a fluid (i.e. mobile or pumpable phase containing molecules in suspension) through a bed of adsorbent (i.e. stationary phase), followed by elution of the target molecule.
Of particular importance within a bioprocessing context is High Pressure Liquid Chromatography (HPLC). The choice of the stationary phase and consequently the type of chromatography depends on the nature of the solutes and process goals. The column is the mechanical device which holds the adsorbent in place and is normally rated to a specific operating pressure. Separation is achieved on the basis of:
Charge (ion exchange or IE; most common in the area of protein purification)
Size
Hydrophobicity
Affinity (highly specific molecule to ligand adsorption/ desorption).
Chromatography uses the following mode of operation:
Equilibration (washing of the column with equilibrating/ running buffer)
Sample is loaded (with associating binding to the adsorbent)
Washing to remove contaminants until absorbance returns to base line
Elution (recovering the target molecule)
Cleaning/ regeneration
The process is then started again until the adsorbent no longer provides an acceptable performance, which could be overpressure due to clogging or compression of the adsorbent, a reduction in binding capacity (g target/mL adsorbent) or lack of specificity for the target biomolecule.
The Key Performance Indicators (KPIs) of chromatography are:
Retention factor: used to describe the migration rate of an analyte through a column. It should not be too slow so as to impact cycle time nor too fast so as to result in low binding capacity or low purity. It is also known as the capacity factor.
Selectivity factor: describes the degree of separation between molecules.
Resolution: a combination of the degree of separation between the peaks eluted from the column (selectivity), the ability of the column to produce narrow, symmetrical peaks (efficiency) and the amount (mass) of sample applied. It is defined as the distance between peak maxima compared with the average base width of the peaks.
Partition coefficient: concentration of analyte in the stationary phase divided by the molar concentration of the analyte in the mobile phase. It is also known as the equilibrium constant.
Scale up of chromatography is achieved by firstly optimizing the purification scheme on the laboratory scale, then increasing one of the following parameters (in order of approximate preference): column diameter (cm), column volume (L), sample load (g), and finally volumetric flow rate (l/hr). The following should be endeavored to be kept constant: bed height (cm), linear flow rate (cm/h), and sample concentration (g/l). A common scale-up issue is increasing pressure drop with increasing linear flow rate leading to chromatography medium (i.e. the solid adsorbent) deformation. Hence, industrial chromatography systems are routinely less than 1.0 min height. Liquid distribution across large bed diameters is also a key challenge for scale-up (i.e. prevention of channeling, ensuring an even binding density throughout the bed, complete washing and elution of the column).
3.5 Stabilization and formulation
Selecting the correct stabilization and formulation for biomolecules is vital as suitable vialing, labeling, dosing and packaging is critical for storage, distribution and at the point of use. Maintenance of the structural integrity of biomolecules, and in particular therapeutics, is essential for its physiological and pharmacological efficacy. Biomolecule stabilizing strategies include:
Native structure stabilization (excipients/molecular engineering)
Prevention of aggregation (excipients)
Avoid or block unwanted hydrophobic surfaces (excipients and avoidance of headspace) and
Reduce shear forces (avoid having a headspace)
The most common long term storage options for biomolecules are in solid form (e.g. freeze drying/lyophilization) or liquid form. For freeze drying, excipients are added to protect biomolecules during freezing/ drying and thawing/rehydrating. For freeze drying, molecular mobility is drastically reduced thereby preventing or reducing the majority of the deactivation mechanisms: oxidation, aggregation, hydrolysis (breaking down of the chemical structure), and deamidation (nonenzymatic covalent modification which occurs to asparagine and glutamine). One negative of freeze drying is that liquid buffer needs to be added to re-suspend the dry powder before administration.
Liquid solutions of biomolecules are commonly a formulation with excipients in a pH buffered. A key limitation of liquid solutions is the requirement for continuous refrigeration until the point of administration (e.g. a cold chain requiring a storage temperature of 2–8 °C; freezing generally damages biomolecules due to creation and melting of solid ice crystals). Excipients are auxiliary substances used in suspensions with examples including buffering agents, isotonicity modifiers, preservatives, stabilizers and complexing agents. Table 4 provides additional information on excipients. The use of excipients can be a delicate balance between achieving maximum shelf life, maximum bioactivity and minimum pain for the recipient (e.g. pain can result if the salt concentration is either too low or too high). Crystallization is another stabilization method but used for niche applications in the areas of formulation of proteins for X-ray crystallography, pharmaceuticals, and antibiotics. Crystallization operates at low temperatures to minimize thermal degradation and prevent resolubalization with associated high concentrations of the biomolecule being required. Optimization of crystallization conditions performed via empirical experimentation as phase diagrams and kinetic info are usually not available for new and novel biomolecules. Crystals are ultimately recovered via centrifugation or filtration.
When choosing the vial and packaging options, consideration must be given to the light sensitivity of the molecule, diffusion of small molecules/gases and heat/ cold through the packaging, usability at point of use, recyclability of the packaging, packaging weight, and packaging cost.
Examples of excipients and associated purposes.
| Excipient | Purpose | Example |
|---|---|---|
| Buffering agents | Target pH maintenance | Sodium phosphate (PBS, pH 7.4), sodium bicarbonate, sodium citrate, sodium acetate. |
| Isotonicity modifiers | Minimize cell damage and pain | Glycerin (16mg/ml), sodium chloride (7mg/ml), Target osmolality of 285 mOsmol/kg. |
| Preservatives | Antimicrobials | Phenol, m-cresol, methylparaben, chlorobutanol, benzyl alcohol, sorbic acid, potassium sorbate, benzoic acid, chlorocresol. Crystal growth is a main issue. |
| Stabilizers | Impart stability to particles or the entire suspension | Metal ions (zinc, calcium), salts, organic molecules. May be used to induce crystallization. |
| Complexing or for- mulation agents | Create stable complexes or particle formulations | Protamine sulfate (also inhibits protease activity). Wetting agents, surfactants, colloids, electrolytes, viscosity modifiers. |
3.6 Mechanical considerations: Pumps, valves, piping, mixing
Biomolecule manufacturing plants have unique requirements in terms of selecting the correct mechanical equipment and materials of construction. For example, pumps and valves are a major source of contamination in bioprocesses. Ball valves and centrifugal pumps that are static and not continuously in operation will generate “dead” areas where water, process liquid or biofilms can accumulate. The US Food and Drug Administration (FDA) notes that firms should install a drain from the low point in equipment housing.
Pumps can burn out and parts can wear resulting in direct contamination (e.g. metal shavings) and failure has the potential to disrupt a process, resulting in contamination via dead zones in the process equipment. Consideration needs to be given to shear forces and pressure changes imposed during pumping of cells and bioproducts suspensions. This pressure change issue is of concern to viable cells pumped through heat exchangers external to a bioreactor. Rotary or positive displacement pumps (as opposed to centrifugal pumps) are almost exclusively used in pharmaceutical/biotech industries, utilizing electrical drives with mechanical seals or magnetic drives. Specific examples of rotary pumps include:
Gear pumps. These pumps are comparably continuous, produce nonpulsating flow and they are good for viscous material.
Lobe pumps. Uses rotating lobes to direct the flow. These pumps reduce shear effects, can drive large solids and slurry-laden media, are suitable for high viscosity liquids, have high efficiency, are corrosion resistant, and have high reliability.
Diaphragm pumps. These pumps are well suited for sterile/ aseptic applications as the diaphragm separates the pump chambers and the pumped material. They can be air or hydraulically driven.
Screw pumps. Twin screw pumps are generally more reliable and result in fewer blockages (as opposed to single screw), however, metal-on-metal wearing is a source of contamination.
Piston pumps. Used for very accurate pumping and dispensing of a wide range of fluids. May only have one moving part hence reduces change of blockages or valve failures. Well suited to corrosive and aggressive media; suitable for high discharge pressure applications.
The valve closing mechanism must be isolated from the contents of the pipe, that is the mechanical mechanisms must be isolated from the process fluid. Diaphragm valves are most commonly used in bioprocesses, such as diaphragm valves (or pinch valves) and weir-type diaphragm valves. Diaphragm valves can wear and ultimately fail during service and so routine inspection is required as well as replacing the diaphragm. Ball, butterfly and plug valves are commonly used on the utility side to reduce costs (e.g. chilled water, cooling water, hot water, steam), but are not ideal for liquids that contact the process fluid.
Bioprocess facilities should have no “dead-legs”, which refers to areas that do not experience constant process flows (e.g. t-sections off the main pipe run that end in a blind flange). A design rule commonly used is to ensure that the unused portion of a pipe is not greater in length than six diameters of the unused pipe measured from the axis of the pipe in use for hot circulating streams (75–80 °C). For colder systems (65–75 °C) any dead-legs have potential for the formation of a biofilm and should be eliminated or have special sanitizing procedures. Bioprocess facilities should have no threaded fittings. All pipe joints should utilize sanitary fittings or be butt welded. A firm’s procedures for sanitization, as well as the actual piping, should be reviewed and evaluated during the design stages.
For mixing, shear and power considerations are of primary concern. The main mechanical device selection relates to the impeller. Two common types are:
Flat bladed/Rushton impeller: exhibits higher shear, higher mass/energy transfer. Normally suited to bacteria, yeast and the preparation of suspensions and buffers.
Marine impeller: lower shear. Better suited for mammalian cell cultures.
Surfaces in contact with process fluids must be constructed from easily cleaned materials of construction (e.g. stainless steel 316; appropriate polymers) and be resistant to the temperature variations and cleaning chemicals used in the process. Copper and copper alloys should not be used; nor should polymers that generate pyrogens (fever-producing agents).
4 Sample bioprocesses
4.1 Bioenergy
Replacing fossil fuel based energy with renewable energy is essential to minimize global warming. Around 10% of global energy needs are derived from bioenergy [5]. Bioenergy takes many forms however. Traditional burning of fiber (e.g. from wood) for household heating and cooking remains the largest form of bioenergy generation worldwide. Bioenergy is a major contributor to renewable electricity and transportation in many countries, such as Australia. The main bioenergy bioproducts considered here are electricity, biogas, ethanol, biodiesel and biocrude oils. This subsection elucidates the relationship between feedstocks, the bioproducts and the processes to make them.
Although wind, hydroelectricity and solar power are significant sources of renewable energy, bioenergy is likely to remain a key technology for achieving greenhouse gas reduction targets. Biomass is well suited for producing liquid fuels for combustion in reciprocating engines used for transportation. Unlike many other forms of renewable energy, liquid biofuels can be blended with the existing transportation fuel supply and delivery infrastructure, allowing a gradual transition to a renewable fuel supply. It is the only renewable energy technology which has the potential to be carbon negative by absorbing carbon dioxide out of the atmosphere and returning some of it to the soil in the form of solid residue (char).
First generation biofuels involve edible feedstocks and are produced by conventional processes. These feedstocks are generally sugars (e.g. sugarcane molasses), starches (corn/maize) and vegetable oils that can be readily converted into liquid fuels. These raw materials have been cultivated by mankind for thousands of years as a food source and consequently have also been explored in depth as a fuel source. To this end, first generation feedstocks are grown on arable land. Using arable land to make a fuel feedstock renders it incapable of producing food, and thereby reducing food supply and potentially increasing world food prices. This situation is known as the “food versus fuel” debate.
Further to this, first generation feedstocks are limited in their potential supply and their environmental credentials. Regrettably, a small proportion of the expansion of first generation biofuels comes as a result of deforestation to grow biofuel crops. This deforestation can also lead to soil erosion, increased levels of fertilizer application and emissions of nitrous oxides and chemical run-off.
Second generation biofuels have the potential to overcome many of the negative issues associated with first generation biofuels. They are made from inedible feedstocks, which are often wastes from other processes. Typically these are lignocellulosic materials from wood and agricultural and other waste from agriculture (grown on arable land), urban centers or industry. They greatly increase the availability of the feedstock supply and have improved environmental credentials. However, second generation biofuels require large research and development costs to commercialize.
Third generation biofuels have a distinct advantage over first and second generation biofuels in that the feedstock does not require arable land. The ability to use nonarable land, in principle, allows for potentially large enough volumes to replace the fossil fuel supply if other constraints could be overcome. Further, the use of wastes or byproducts from other industries is a common characteristic for third generation biofuels. It was originally envisaged that algae would use industrial wastewater to provide the nutrients for microorganisms with a high lipid content. Third generation biofuels have an inherent problem – if grown in open ponds, a large water supply is required with associated mass transfer challenges (e.g. high energy, high capital cost equipment). If grown in photo-bioreactors, the high water requirements can be overcome, but at present, capital costs are high.
4.1.1 Ligno-cellulosic wastes
Ligno-cellulosic waste is produced by numerous industries:
Forestry sector waste. This includes material which comes from saw mill operations (e.g. bark and saw dust), logging (stumps) or plantation forestry maintenance such as thinnings (i.e. branches). This material is most commonly burned in a boiler for steam or electricity generation.
Pulp and paper industry black liquor. In order to make white papers, the industry takes woodchips, adds chemicals and dissolves the natural brown resin, lignin. In this process, the lignin is dissolved in the chemicals to form black liquor which must be burned as an economic and environmental measure. In doing so, the combustion of the lignin and incidental carbohydrate in the black liquor makes it a green energy source.
Sugarcane industry waste. Sugarcane is harvested and crushed to extract the juice which contains the sugar. The fiber that is left behind is known as bagasse and the leaves left in the field are known as cane trash. Bagasse is most commonly used for steam and electricity generation in the factory but the electricity can also be exported for use in the community [6–10].
Other agricultural waste. The waste of many other agricultural industries is left in the field, such as straw. Some fruit and vegetable wastes are potential sources for bioenergy through anaerobic digestion.
The chemical composition of a ligno-cellulosic feedstock affects the potential bioproduct and the amount of energy produced. Compared to fossil fuels, biomass contains large amounts of moisture and oxygen which reduces the energy conversion efficiency.
Apart from water, the most abundant chemical in ligno-cellulosic material is cellulose, which is a polymer of glucose, and has the formula (C6H10O5)n. The proportion of cellulose in biomass can vary widely up to 90% in cotton, although 20–60% is more typical for wood and most agricultural crops. Cellulose can be broken down into its monomers by biological or chemical hydrolysis routes. However, accessibility of the cellulose depends on chemical composition and structure of the biomass.
Hemicellulose is a random polymer of five and six carbon sugar monomers which is more prevalent in agricultural crops than in wood. The sugar monomers include glucose, mannose (C6), xylose, and arabinose (C5). Hemicellulose is readily broken down under acidic or basic conditions, as occurs in pulp production during paper manufacture.
Lignin is a complex hydrophobic polymer composed of linked aromatic rings. Lignin provides the plant with a rigid structure and protects it from microbial attack. It inhibits fermentation and since it contains aromatic groups, when partially combusted it can produce toxic emissions. Its removal is necessary to allow fermentation to second generation biofuel and for the remaining cellulose and hemicellulose to be almost white and malleable, which is a requirement for white paper production. It is present in wood 25–40%, and sugarcane 15–25%[11] although other grasses contain almost no lignin (this is apparent by their color and lack of rigidity).
There are three primary lignin monomers: p-Coumaryl, Coniferyl and Sinapyl alcohols (see Figure 5). These monolignins differ in their reactivity due to the number of methoxy sites and each monolignin is present to varying extents in softwood, hardwood and grasses.
4.1.2 Urban center waste
The main potential feedstocks from urban centers are:
– Municipal solid waste. Although this material contains significant amounts of plastics from fossil fuels, it has a high proportion of ligno-cellulosic content. This occurs due to the high amount of cardboard packaging society uses. Developed countries typically use over 100 kg of paper per capita each year.
– Recycled fiber. The lignin content of this feedstock depends on whether it is generated from office waste (white papers) or household waste which includes boxes, which still contains significant amounts of lignin.
– Sewage contains a very high organic content on a dry basis, although it has very high water content which is eliminated in many processing routes as conversion efficiency would be too low.
4.1.3 Regional considerations
The availability of a feedstock depends on a variety of factors which must be assessed on a case by case basis. An approach to measuring the potential availability of feed-stock is provided by Kosinkova et al. [13]. Kosinkova et al. used Australia, which was investigated as being a microcosm to demonstrate some of these issues as shown in Figure 6. These considerations include [14, 15]:
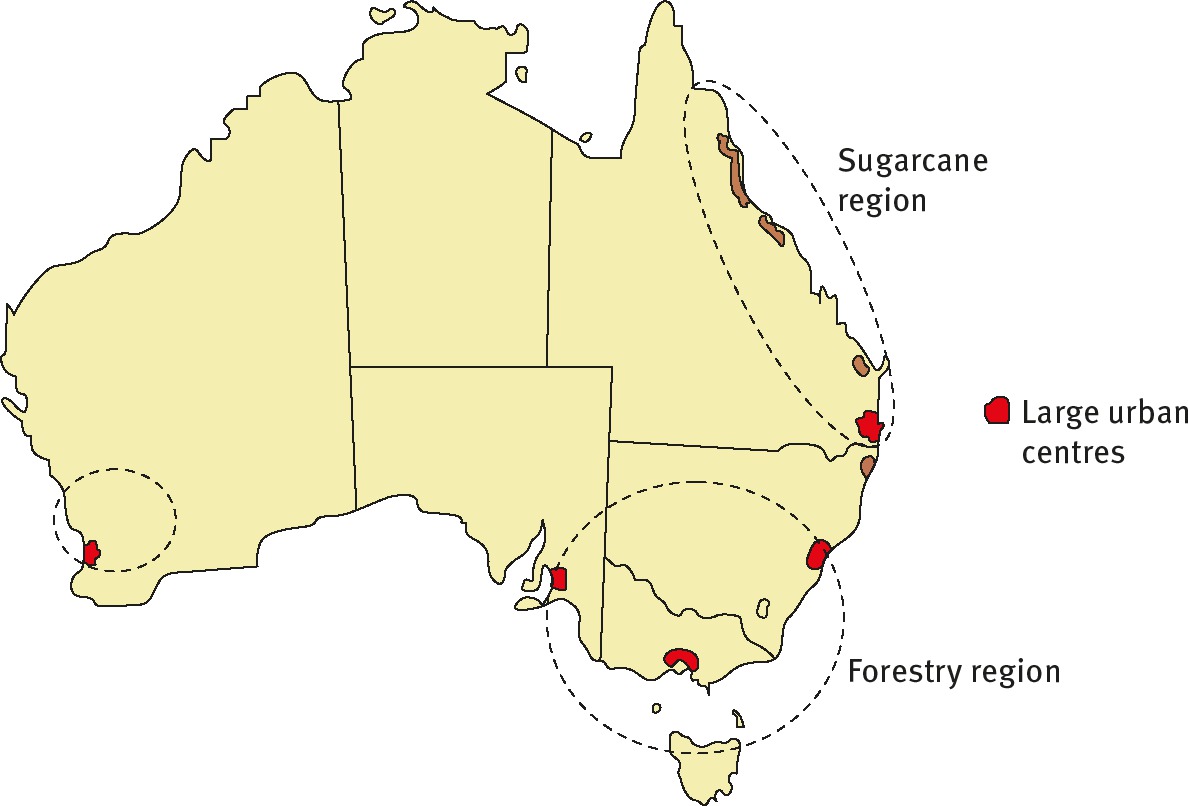
Distribution of bioenergy feedstocks in Australia.
– Local population size. Is the region a significant urban center or an agricultural area?
– The climate. Is the temperature better suited to forestry, agricultural fiber or a tropical crop such as sugarcane?
– Availability of water. Agriculture and algal cultures benefit from an abundance of water supply.
– Logistical and political considerations. How does the waste management supply chain operate?
– Availability of local infrastructure to export material. E.g. electricity transmission lines, ports, roads, railways.
4.1.4 Processes and energy bioproducts
The feedstocks are matched to the bioproducts by the conversion processes.
Combustion to produce electricity
The oldest biomass to energy conversion process is simple combustion. Fiber is a form of hydrocarbon, and so burns in the presence of oxygen provided there is an ignition source. This is the basis for traditional household cooking and heating. Industrially, biomass is fed into the furnace of a large industrial boiler to generate steam and electricity via a turbine. The generalized formula for combustion is:
Fuel + oxygen + nitrogen → water + carbon dioxide + nitrogen (+ carbon monoxide) ,
where carbon monoxide is produced as a result of incomplete combustion.
Anaerobic digestion to produce biogas
Microorganisms break biomass down in the absence of oxygen in a series of steps which produce methane and carbon dioxide. Lignin is a problem for the initial degradation step, and so it is not suitable for wood. Apart from this, the process can be used for grasses, sewage, delignified waste (e.g. waste paper), abattoir waste, some household waste, fats and oils. The process can be tailored to the moisture content of the feedstock and multiple feedstocks can sometimes be used.
Fermentation to produce fuel ethanol
Ethanol can be used as a liquid fuel and is particularly well suited for blending with petroleum gasoline. The conversion of sugars (sucrose, glucose) to ethanol involves converting glucose into ethanol and carbon dioxide through the action of microorganisms, namely
C6H12O6 → 2C2H5OH + 2CO2 .
Transesterification to produce biodiesel
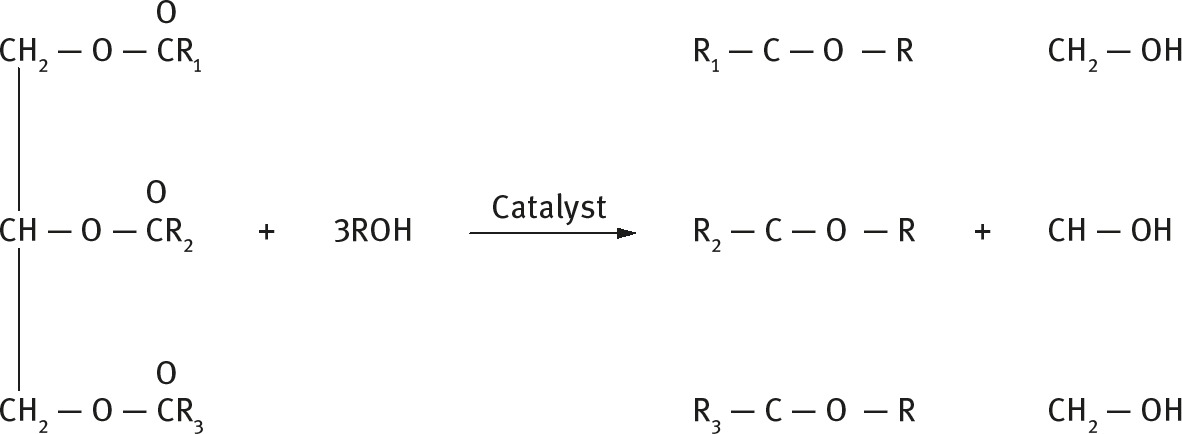
Vegetable and animal oils consist of triglycerides which are reacted with methanol or ethanol in the presence of an acid or base catalyst in order to produce biodiesels. Glycerol is a byproduct of this reaction. The conversion to fatty acid methyl esters (i.e. FAME) is necessary to reduce the viscosity and improve atomization and combustion efficiency.
Thermochemical conversion to produce electricity or liquid fuel
This is a family of routes which involve subjecting biomass to elevated temperatures in a tightly controlled oxygen environment (Figure 7). Gasification occurs with a limited amount of oxygen present. Carbon monoxide and possibly carbon dioxide and carbon are produced, but these molecules react with water to produce hydrogen. The high temperatures result in high conversion efficiencies. Apart from hydrogen, the syngas can be used to produce methanol or liquid fuels (diesel) by the Fischer–Tropsch process [16].
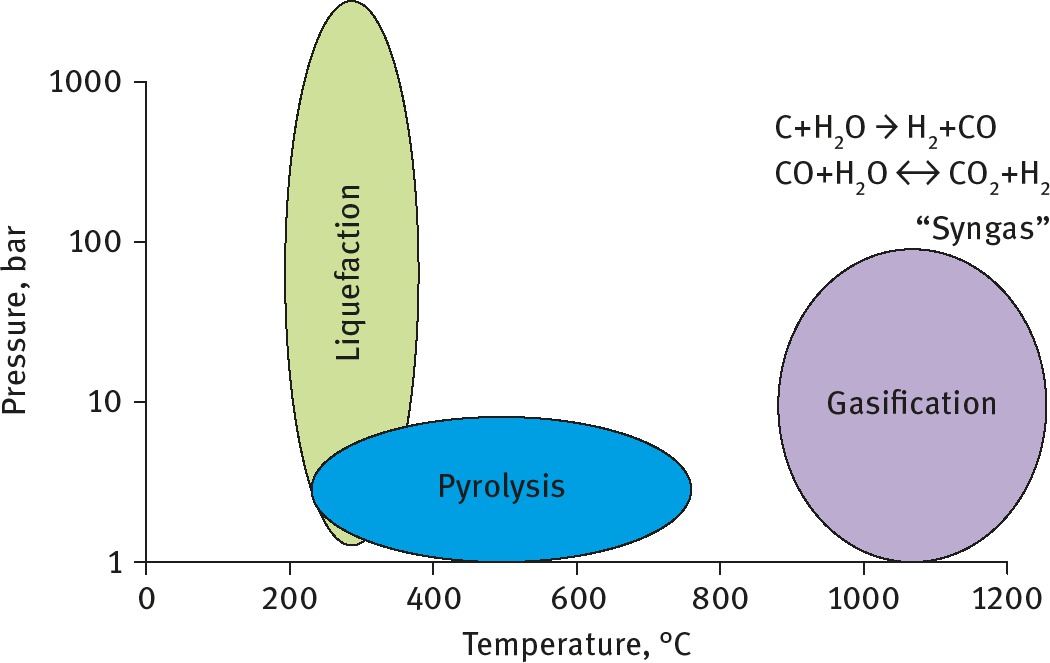
Thermochemical conversion pathways.
Pyrolysis occurs at more modest temperatures in the absence of oxygen and water. The conditions can be varied to target different products but generally bio-oil, solid char and volatile gases (e.g. methane) are produced. Flash pyrolysis, whereby the biomass is heated within just a few seconds, achieves better yields than slow pyrolysis.
Liquefaction occurs at lower temperatures but high pressures are required. Liquefaction results in increased oil yields compared to pyrolysis, but the main advantage is that the process is highly tolerant of water and no pre-drying is needed, unlike in pyrolysis. The main disadvantage is that the equipment tolerant of these high temperatures and pressures is expensive.
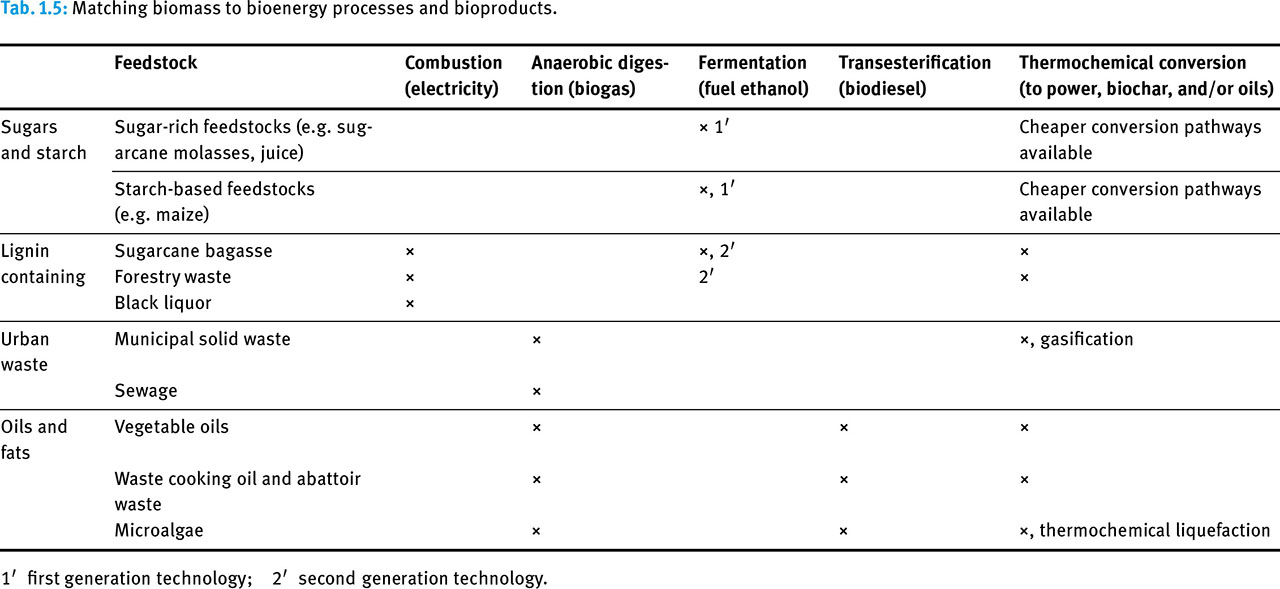
Table 5 shows typical matching of the feedstock to the process and bioenergy product. Sugars and starches are well suited for producing fuel ethanol, while combustion is best suited for drier lignified biomass. However, lignified biomass can be used to produce ethanol via second generation technology or converted via thermochemical processing. Urban wastes and oils are well suited to anaerobic digestion and thermochemical processing but oils are also used for biodiesel.
4.2 Nutraceutical example: Chondroitin
Chondroitin sulfate is an important structural component of cartilage and provides resistance to compression. Chondroitin sulfate is used as a supplement for treatment of osteoarthritis and is believed to help draw water and nutrients into the cartilage, keeping it spongy and healthy.
Chondroitin is a naturally occurring biomolecule found in the body and is a sulfated glycosaminoglycan (GAG) attached to proteins as part of a proteoglycan (hence the need to remove proteins during downstream processing via proteolysis). Made traditionally from shark cartilage, shark is considered a less sustainable source of chondroitin due to declining shark populations, hence bovine (cow) sources are more widely used. As a result, demand exists for “clean” sources of bovine chondroitin from a known and traceable source. An example process for the manufacture of chondroitin sulfate is presented in Figure 8.
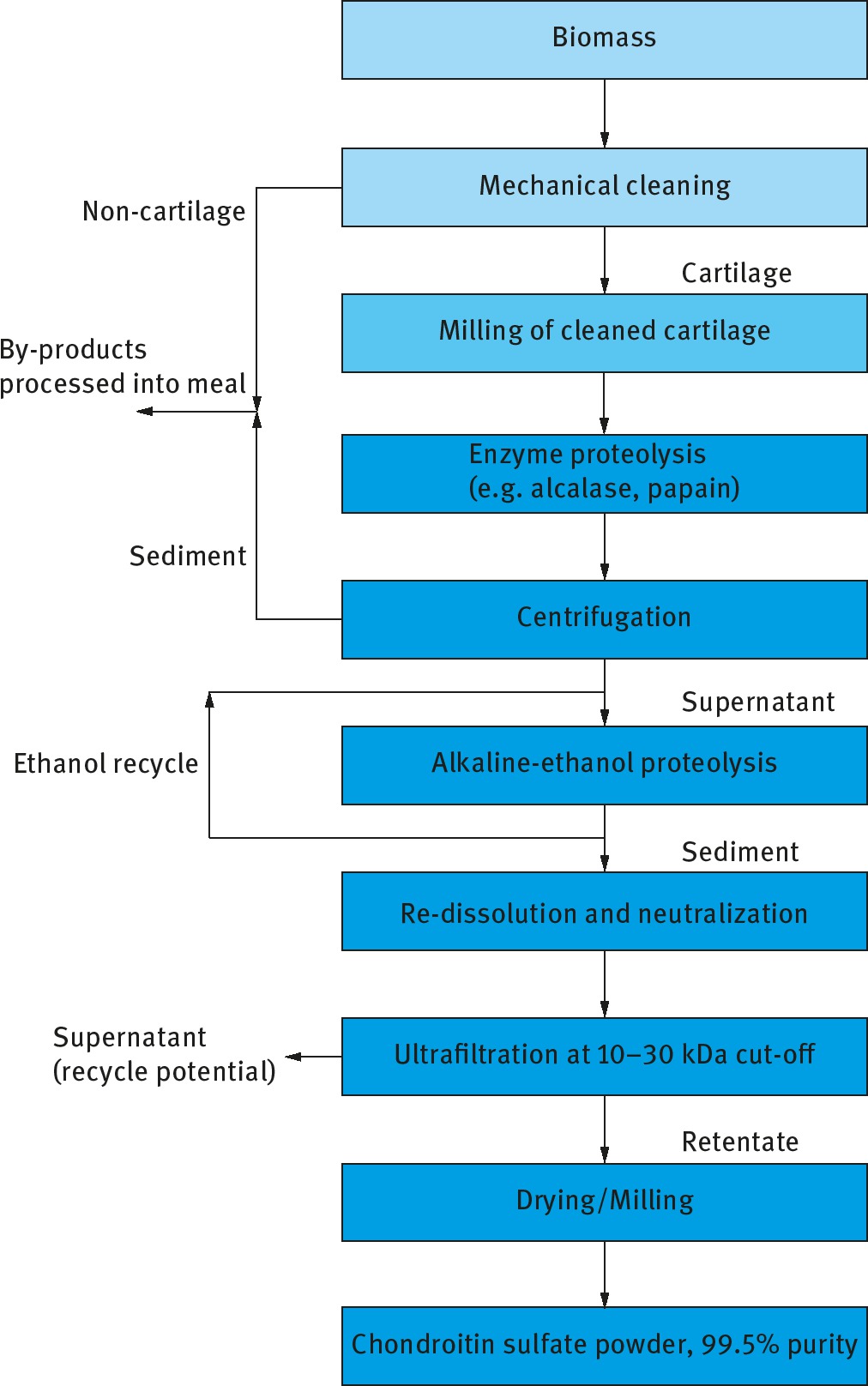
Block diagram of a bioprocess for the manufacture of 99.5% purity chondroitin sulfate.
It must be noted that the effectiveness of chondroitin and the combined use of glucosamine/chondroitin for osteoarthritis remains unclear; some studies have found chondroitin reduces pain more than a placebo, however several newer studies have found no improvement in pain with chondroitin; some evidence exists that chondroitin supplements slow cartilage breakdown or repair damaged cartilage from knee osteoarthritis [17]. Studies have found that glucosamine and chondroitin supplements may interact with the anticoagulant (blood-thinning) drug warfarin (Coumadin). Overall, studies have not shown any other serious side effects [18].
Typical doses of chondroitin sulfate for osteoarthritis are 200–400 mg two to three times daily or 1000–1200mg as a single daily dose; when applied to the skin in a creamfor osteoarthritis typical doses are 50 mg chondroitin sulfate/g for up to 8 weeks [19]. Due to feedstock limitations, chondroitin sulfate sourced from shark cartilage is around five times the cost of chondroitin sulfate from bovine trachea.
As an example of an alternative chondroitin containing product in the area of functional foods, dried whole beef tracheas containing high levels of naturally occurring glucosamine and chondroitin retail for approximately AU$ 51/kg retail finished product.
In Figure 9, the upstream process may be considered the “Producer” and “Processor” whilst the downstream process is represented by the “Value adder” (concentration into an impure bulk), and “Manufacturer” (purification). The retailer may then package the final purified product. As can be seen, the profit after tax (PAT) for the upstream sections is negligible whilst for the value adder PAT equates to 36%, for the manufacturer 9% and for the retailer 16%.
![Figure 9: Graph showing the costs for producing chondroitin sulfate at various stages in the value chain. SG&A: Selling, General and Administrative Expenses. COGS: Cost of Goods Sold: the costs of making the products to be sold later. PAT: Profit after tax [20].](/document/doi/10.1515/psr-2016-0046/asset/graphic/psr-2016-0046_fig_009.jpg)
Graph showing the costs for producing chondroitin sulfate at various stages in the value chain. SG&A: Selling, General and Administrative Expenses. COGS: Cost of Goods Sold: the costs of making the products to be sold later. PAT: Profit after tax [20].
4.3 Fermentation example: Fermentation products from ligno-cellulose
Given the abundance of ligno-cellulose and its availability as a co-product from industrial processes such as sugar milling, it is an attractive potential carbon source for industrial fermentations to produce a range of fuels and industrial chemicals. The majority of existing industrial microbial fermentation processes use glucose derived from corn starch or sucrose from sugarcane (in the form of molasses or cane juice) as the carbon source. To use ligno-cellulose as the feedstock, it is necessary to break down the constituent polymers to fermentable sugars, such as the conversion of cellulose to glucose [21, 22]. The challenge in the approach to use ligno-cellulosicmaterials arises from the recalcitrance of the feedstock. A major role of ligno-cellulose in nature is to provide mechanical strength to plant materials and resist degradation, meaning that significant time and energy is usually required to break it down. Further, once degraded, the economics of the fermentation processes would be maximized if all product streams (C5 and C6 sugars as well as lignin) could be utilized to generate products. To use all available sources of carbon means that multiple or highly adaptable processes are required (for example using microbes that can utilize both C5 and C6 sugars simultaneously) [23].
The overall process for the production of fermentation products from lignocellulose usually first involves a pretreatment step to mechanically and thermochemically degrade the material and separate the fibers, as well as degrade some carbohydrate polymers. This step converts the fibrous dry material to a liquid form where the remaining constituent polymers are accessible to enzymes for the second step of saccharification to generate fermentable sugars such as glucose or xylose. These sugars are then available as a carbon source for microbial fermentation to generate the desired end product chemical that must then be isolated and purified. Various purification and fractionation steps may be required during the entire process to isolate various constituents depending on the specific carbon source required for the fermentation and the need to remove microbial inhibitors such as furfural and phenolic compounds that may be produced during pretreatment [24].
The improvement of the overall process is an ongoing area of research and numerous options are being examined to increase process efficiency and reduce costs. The aims and goals of this research include reducing the temperature and overall severity of the pretreatment process to reduce the required energy, cost and levels of production of inhibitory molecules; the combination of enzymatic saccharification and fermentation into one step; the utilization of microorganisms that can both produce carbohydrase enzymes and ferment the resulting sugars to products in a so called consolidated bioprocess; and the use of microorganisms that can utilize both C5 and C6 sugars to produce the desired fermentation product.
4.3.1 Novel pretreatment strategies
Typical pretreatment strategies usually employ a mechanical milling step to reduce particle size followed by a chemical modification step using dilute acid or alkaline hydrolysis along with further mechanical processing such as steam explosion [24]. Processes such as dilute acid hydrolysis typically employ temperatures from 120 to 210 °C and lead to significant hydrolysis of xylan to xylose as well as making cellulose available for enzymatic saccharification. Often such pretreatments lead to the formation of unwanted compounds that can inhibit the subsequent fermentation [24]. One approach to reduce the severity of the pretreatment and therefore reduce both energy costs and byproduct formation is to use co-solvents. Ionic liquids have been investigated for this purpose [25, 26] as well as acidified glycerol [27, 28], where increased enzymatic digestibility has been observed with increasing concentrations of glycerol. An ammonia-based process to expand the fiber in a processed termed AFEX™ has also been developed (for example see [29–31]). A direct comparison of dilute acid, ionic liquid and AFEX™pretreatment methods was performed using corn stover [32]. The study found that similar ethanol production metabolic yields were observed for all three methods although the required enzyme combinations varied considerably to achieve the yields and the need for additional nutrient supplementation, which was not required after AFEX™ pretreatment. AFEX™ is being developed and commercialized by the not-for-profit company MBI in collaboration with Michigan State University. The technology has been tested in collaboration with Deinove using specific Deinococcus bacteria in a simultaneous saccharification and fermentation process that achieved high levels of utilization of the available sugars [33].
4.3.2 Use of specific ligno-cellulose fractions
Once glucose can be generated from cellulose, it should be compatible with existing fermentation processes that utilize glucose derived from conventional sources, depending on the presence of inhibitory compounds. Industrial fermentations based on C5 sugars such as xylose and arabinose are not currently common in industry given that glucose or sucrose are generally more available feedstocks. Unlike cellulose that only generates glucose upon degradation, hemicellulose is a heterogeneous polymer that produces a range of both C5 and C6 (pentose and hexose) sugars and as such represents additional challenges for full utilization of the available sugars. Overall, to fully utilize the available sugars in ligno-cellulose, strains that can co-metabolize both C5 and C6 sugars are required. Ethanol production using Saccharomyces cerevisiae is one of the world’s oldest and largest bioprocesses with cellulosic ethanol production now entering commercial scale manufacturing [34]. Furthermore, this yeast is the basis for extensive metabolic engineering toward new chemical products [35], with some in commercial production (for example see the development of the farnesene process by the company Amyris). As such, S. cerevisiae has been a popular organism for the development of strains that can use C5 sugars such as xylose aswell as glucose [36, 37]. These engineered strains must match the robustness of existing industrial ethanol strains and further, must ideally be tolerant of inhibitors and other constituents found in ligno-cellulosic hydrolysates, such as high levels of salt from pretreatment chemicals. For example, it has been shown that the presence of chloride and sulfate salts with sodium, potassium and ammonium cations reduced biomass growth, ethanol production and glucose consumption as well as reduced xylose utilization in the presence of sodium chloride in a S. cerevisiae strain capable of co-utilization of glucose and xylose [38]. An acetic acid reduction pathway to generate ethanol has also been integrated with C5/ C6 co-utilization in an S. cerevisiae strain [39].
Overall, S. cerevisiae has been extensively engineered to use a variety of carbon sources and generate a wide range of products [35] and remains a key platform organism in industrial biotechnology. Nevertheless, a range of other organisms have also been explored for similar aims, including Escherichia coli [40], Rhodococcus opacus [41] and Zymomonas mobilis [42].
Lignin is a significant constituent of ligno-cellulose and presents numerous challenges for use associated with its natural role in providing protection and mechanical strength to biomass. It is also a heterogeneous and noncarbohydrate based polymer, meaning the breakdown products aren’t as readily utilized by microorganisms as glucose from cellulose. Lignin is generated at large scale in the pulp and paper industry and would be a major co-product from a future ligno-cellulosic ethanol industry. Lignin can be utilized by microorganisms for growth and metabolism offering a possible route to convert this challenging but highly available and cheap material to both biomass and chemical products. It has been known for some time that organisms such as white rot fungi are able to degrade ligno-cellulose using a suite of enzymes that break down lignin and enhance the availability of the carbohydrate polymer fractions to enzymatic saccharification [43]. The main classes of ligninolytic enzymes include lignin peroxidases, manganese-dependent peroxidases and laccases and these enzymes have been widely studied for the biocatalytic degradation of lignin [44, 45]. These enzymes can be isolated and used as biocatalysts to degrade and modify lignin although a collection of different enzymes are usually required in concert to achieve degradation. The use of enzymes has so far mostly been targeted at enhancing the pretreatment of biomass to isolate cellulose for breakdown to glucose [46].
As well as using isolated enzymes to transform the lignin polymer and associated monomers, the use of whole cell microorganisms and the manipulation of in vivo metabolic pathways for the degradation and assimilation of lignin derived chemicals is emerging as an option to generate specific products [44]. Lignin associated monomers such as ferulic acid and vanillic acid can be metabolized in vivo to protocatachuic acid and on to beta-ketoadipic acid and acetyl-CoA for incorporation into the TCA cycle and other pathways such as for fatty acid and isoprenoid synthesis. This channeling or funneling of a variety of lignin derived chemicals towards acetyl-CoA has been proposed as a solution to the problem of heterogeneity of the lignin substrate [47]. In this example, a specific strain of the bacterium Pseudomonas putida that is naturally able to catabolize aromatic molecules was used. Both lignin model compounds and heterogeneous lignin derived material could be converted to medium chain length polyhydroxyalkanoates. The same microorganism has also been metabolically engineered to produce cis,cis-muconate, an adipic acid precursor, from both the lignin model compound p-coumaric acid and a biomass-derived lignin stream [48]. The yield obtained on the model compound in fed-batch fermentations was 13.5 g/l and over 15 times what was achieved in shake flask cultures. Using the lignin stream in shake flask cultures produced 0.7 g/ l of product at a molar yield of 67% from the p-coumarate and ferulate substrates that were detected in the substrate. This work represents an encouraging first step towards further development of the process in bioreactors and additional improvements as both the lignin stream and microbial degradation metabolic pathways are further understood and optimized.
An assessment of fourteen bacteria, including the P. putida strain described above, that secrete ligninolytic enzymes was performed to assess the formation of molecules that could be used for industrial applications such as fuels, chemicals and materials [49]. This work provided information on particular species that could not only break down lignin but also metabolize the breakdown products to biomass and compounds such as polyhydroxyalkanoates, particularly in nitrogen limited conditions.
4.3.3 Consolidated bioprocessing
As seen above for the consolidated bioprocessing of lignin directly to products using a single organism and process, the same concept can be applied to the processing of the cellulose and hemicellulose biomass fractions. There are two general strategies for the generation of microbial strains that can both break down ligno-cellulose to sugars and then convert them to chemical products. Such organisms generally do not exist in nature and so one must either endow the ability to produce cellulolytic enzymes on an existing production strain [50] or engineer a strain that already produces cellulolytic enzymes to be able to generate the product of interest [51].
There are many examples in the academic literature of where consolidated bioprocessing has been demonstrated, but commercial reality remains to be achieved due to cost, yield, efficiency and scalability issues in addition to the challenge of heterologous expression of cellulase enzymes [52]. An important and immediate application is the conversion of cellulose to glucose for the subsequent production of ethanol [53–55]. The production of ethanol in this way builds on the existing and extensive ethanol biofuel industry. Making an established product such as ethanol is less risky than developing both cellulose degradation and engineering new molecule production at the same time. The relatively low selling price of ethanol as a fuel compared to producing higher value commodity or specialty chemicals means that commercial success for this concept remains challenging however.
For reasons related to the co-utilization of hexose and pentose sugars, the utilization of hemicellulose in a consolidated bioprocess represents an additional challenge over and above the conversion of cellulose [56]. As well as requiring an organism that can produce relevant hydrolase enzymes (such as xylanases) and generate an industrial chemical product, the organism must also readily metabolize C5 and C6 sugars. The susceptibility of hemicellulose to degradation during acidic pretreatment steps, however, may alleviate the need for extensive enzymatic degradation. Nevertheless, the conversion of hemicellulose has been reported. One example involves the generation of succinic acid in an engineered strain of E. coli [57]. In this case, endoxylanases and xylosidases were selected for their suitability in a consolidated bioprocess, for example to have good activity at the ideal growth temperature of the bacterium. Enzyme production levels were optimized but secretion was found to be a limiting factor. Integration of the xylan utilization capability into an E. coli succinate production strain enabled the production of this industrially important chemical from xylan.
4.3.4 Commercial applications
With an established and large-scale ethanol biofuel industry, particularly in the USA and Brazil using corn and sugarcane feedstocks respectively, there has been significant industrial focus on generating commercial value from the ligno-cellulosic co-streams such as corn stover and bagasse. The large volumes of ligno-cellulosic biomass that could be available from these industries and others (including forestry) coupled with the lack of competition with human or animal food make this feedstock attractive despite the challenges of accessing the fermentable sugars. To this end the world’s major enzyme producing companies and research scientists backed by substantial government support have made significant progress in the development of improved cellulose enzymes. Along with pretreatment and process innovations, the first large-scale cellulosic ethanol production facilities have emerged. These facilities include Beta Renewables plant at Crescentino in Italy that has the potential to process 270 000 tons per year of biomass and generate up to 60 000 tons of ethanol. The first cellulosic ethanol facility in the USA was established in Iowa in 2014 by the POET-DSM joint venture [58]. It has the capacity to process 770 tons of biomass per day (281 050 tons per year operating 365 days per year) and generate 20–25 million gallons (60 000–75000 tons) of ethanol per year. There exists significant scope for expansion of both the number and size of cellulosic ethanol production facilities once the technology and costs are fully established and proven. In 2015 there were 195 fuel ethanol plants with a mean production capability of 75 million gallons per year per plant according to U.S. Energy Information Administration statistics [59].
In Brazil GranBio is using sugarcane straw and bagasse at a facility in Alagoas and has the capacity to generate 82 million liters (65 000 tons) of ethanol per year. GranBio has also established a partnership with the chemical company Rhodia around using similar approaches for the production of n-butanol [60]. Research and development towards the production of 1,4-butanediol from ligno-cellulose has also been disclosed by the company Genomatica [61]. This work involved engineering E. coli for co-fermentation of C5 and C6 sugars, strain engineering towards 1,4-butanediol production from this feedstock and development of a fermentation process. A range of different hydrolysates from different processes and biomass types were also tested. Using a concentrated (700 g/lmonomeric sugar) and clean hydrolysate, a 1,4-butanediol titer of 119 g/l and productivity approaching 2.5 g/ l/h could be achieved.
Overall, ligno-cellulosic biomass represents a significant resource for the future manufacture of liquid fuels and biochemical. The recalcitrant and heterogeneous nature of the material along with variations in composition between and within plant species raises many challenges for its economic and scalable use. These challenges also raise many opportunities for advancements in plant biotechnology for new biomass types, improved pretreatment technologies and the development of new microbial strains and fermentation processes to utilize as much of the available biomass and generate chemical products in the most efficient and cost effective manner.
4.4 Biopharmaceutical example: Monoclonal antibodies
The rise of biopharmaceuticals has been unprecedented since the turn of the 21st century. Today biopharmaceuticals constitute around 20% of the global pharmaceutical market, having a growth rate above 8% per annum which is double that of chemical pharmaceuticals. Current biopharmaceutical revenues are estimated to be $ 163 billion [62]. Overall, biopharmaceuticals are the highest value biomolecules being produced from industrial processes but they also have a higher cost of manufacture than any other class of industrial biomolecules. Manufacturing costs are comparatively high for several reasons: facility outlay costs, regulatory compliance, lengthy processing times, low yields, expensive raw materials, costs of skilled staff and short market life cycles (e.g. competition from generics). In the case of the facility outlay, the costs to build a biopharmaceutical manufacturing facility start at $ 200–500 million and can take up to five years to complete depending on the nature of the facility and the biomolecules to be manufactured within the facility, whilst comparable chemical pharmaceutical facilities cost in the order of $ 30–100 million.
The potential for disruptive process innovation in the biopharmaceutical sector is very high. However, the limitations are the cost-of-entry to develop and deliver a disruptive process which fits into existing facility designs, meets project budgets, project timelines and complies with regulatory requirements for validation, characterization and manufacture. Because of these limitations there is a constant cycle of reassessment of processes and investment in exploring new technology within the existing manufacturing landscape. Overall, process advancements have proven to be largely conservative and predominantly driven by project requirements to meet production needs and product quality. This has especially been the case for mammalian cell derived biopharmaceuticals which represent the highest cost biomolecules currently manufactured within the biopharmaceutical sector.
For mammalian cell derived biopharmaceuticals themain innovations have come from:
Improvements to cell lines through metabolic engineering
Metabolic engineering can improve consistency of fermentation, reduce media complexity and improve product yields and quality. For example, engineering the inclusion of glutamine synthetase allows a condensation reaction of glutamate and ammonia to form glutamine. For some cell lines – such as murine myeloma cells, there is no intrinsic glutamine synthetase and so engineering the cell line with glutamine biosynthetic capacity removes the challenges of glutamine supplementation to the media such as glutamine depletion/limitation or deamidation post-formulation of the media or during the course of fermentation. Such cell line developments produce clones with higher specific productivities for antibodies (exceeding 50 pg/ cell/day) compared to the undeveloped base cell lines (less than 10 pg/ cell/day).
Molecular biology improvements to the DNA vector
Improvements to the vector allow greater consistency of insertion into the host genome, thereby producing more stable clones and better transcription initiation. Stability is particularly important for mammalian cell lines such as murine and hamster cell lines as a large percentage of the genome (around 30%) is composed of transposable elements that potentially can rearrange and insert into different regions of the genome. Industrial cell lines are qualified and validated to be stable for the duration of the manufacturing run (from vial thaw, through seed train expansion, to beyond the cell age seen at the end of the production cycle). Depending on the nature of the process, this can be up to 120 days where a cell line must remain consistent in terms of stability and productivity.
Process analytical technologies (PATs)
PATs allow novel real-time monitoring of fermentation metrics and metabolism that provide greater elements of control and understanding of upstream processes. Greater uptake is now occurring for PATs that are proving to be fit-for-purpose for biopharmaceutical manufacture with the most pre-eminent being near infrared spectroscopy. Regulators are now setting in place guidelines for the use of PATs with a strong desire to see the uptake and implementation of PATs to improve consistency and quality of manufacture.
Biomass media and feed-media development
Media and feedstocks are typically highly complex formulations and always provide the greatest increase in yield and quality metrics in simple batch and fed-batch fermentations. Due to regulatory constraints against the use of animal-derived materials (which may contain biological hazards such as bacteria, viruses, prions and endotoxins), industry has a preference for protein-free media with optimized concentrations of amino acids, salts, co-factors, vitamins and lipids, despite the high costs associated with Good Manufacturing Practice (GMP)-certified formulations.
Typical yield increases from first generating a single stable clone to selection of the media and optimizing a feed strategy for batch fermentation are between two to tenfold for biopharmaceuticals. For fed-batch antibody processes of 10–21 days duration, titers in the range of 1–5mg/l are common, but yields as high as 10–15 g/l have been reported. The advantages of optimized media include consistency of upstream biomass generation, the biopharmaceutical is safer (less contamination from difficult-to-purify animal products), the quality is more easily planned and controlled in the final product (referred to as quality by design or QBD) and the productivity of the biomass is higher. The focus of media development is to maximize the total viable cell density for as long as possible, which leads to significant biomass increases in fed-batch cultures. In conjunction with high specific productivity from engineered clones, economically viable processes can be established from relatively small bioreactor volumes. Optimized media will improve specific and volumetric yields as well as “time yields” (e.g. g product/hour of fermentation), a performance metric of interest for facilities that have capacity or fermentation volume limitations.
Single-use bioreactors (SUBs)
The advent of SUBs represents a novel, disruptive technology for the generation of upstream biomass to produce biopharmaceuticals. The single-use bioreactor technology utilizes a sterilized disposable plastic bag as the bioreactor vessel which is then held in a temperature controlled jacketed support frame. SUB systems are complete, including all mixing, aeration and exhaust requirements (i.e. impellers, spargers and exhaust filters are integral to the sterilized disposable bags). The SUB systems are expensive – nowadays approximating the costs of cheaply sourced steel, but are considered to be economical when factoring all aspects of the pharmaceutical upstream process. This includes:
Production time considerations – sterilization using steam-in-place systems are not required and so turnaround times are improved
Batch loss and facility down-time due to contamination is greatly reduced
Flexibly designed facilities, which can be open-plan as SUB systems can be portable within a facility and therefore accommodate upstream and downstream changes or whole process differences for different biopharmaceuticals manufactured within the same facility. This decreases the costs of building a rigid facility which can be expensive to modify for evolving process requirements, and
Because the scales of the SUB technology are relatively small (usually 50 to 2000 l); the technology is more congenial for expansion by scaling-out rather than scaling-up, which simplifies operational expansion as the systems are the same and the complexities of different equipment at different scales are eliminated
The SUB systems have the potential to be disruptive technology for the expensive and intensive biopharmaceutical workflow by allowing small operations and biotech start-ups to operate in niche areas and compete with large pharma at a greatly reduced cost. An example of niche manufacturing is monoclonal antibodies developed to Phase I levels by start-up pharmaceutical companies using wave-induced motion bioreactors.
Wave-induced motion bioreactors were first described by Singh [63] and utilize the disposable, flexible and sterile plastic bag (cellbag) with controlled headspace gassing supported on a rocking thermo-regulated platform. The rocking platform is responsible for a distinctive undulating wave movement created in the bioreactor cellbag. This different, low-shear, mass and energy transfer mechanism ensures good off-bottomsuspension, efficient nutrient distribution and a high oxygen transfer from a large surface mixing area.
Cell growth is controlled by adjusting the rocking rate, the rocking angle, the fill level, headspace aeration rate and headspace gas composition. Customized and more complex systems have been described that control cell growth with nutrient feed controls, integrated perfusion and online monitoring-feedback controls of dissolved oxygen (DO) and pH (controlled via O2, CO2 and base addition) [64, 65].
Wave-induced motion bioreactors can be used as a process bioreactor on a range of different cells for both stable and transient systems, or as a unit operation for biomass expansion for seeding process bioreactors and high-density cell banking. Though simple in design and operation, the performance characteristics have been demonstrated to be distinct – but similar – to more complex stirred tank bioreactors [66]. Simple changes such as the percentage of CO2 in the headspace can control the pH of the media or potentially alter the pH outside of a normal operating range. The hydrodynamic flow (mixing and shear behavior) is not necessarily the same in different sized cellbags or with different media volumes. However, there is generally a high mass transfer coefficient (kLa), that is unusually steady over a broad range of operational parameters (the kLa is reported to be as high as 20–30/h at operation ranges from 20–30 rpm, 7.5° [67]). The kLa can be simply manipulated by changing the rocking angle, rocking rate and, to a very superficial extent due to the large surface area of exchange, the headspace gassing. As such, most operational conditions for the wave-induced motion bioreactors can be achieved in a short amount of time and manipulated simply; for example, it is known that subtle changes in the percentage of CO2 in the headspace can elicit the effect of either stripping or enriching the pCO2 as desired to influence the media pH in a reasonable timeframe of just over 10 min for a 50 l bioreactor without any other control system for the pH [68]. Furthermore, scaling can be readily achieved when considering the media: cellbag ratio and adjusting for differences in the hydrodynamic flow changes for different sized cellbags and different media volumes. The “kLa fixing” to match different scales typically requires manipulation of the integral power density via aeration and/ or agitation rates, with smaller media volumes having higher kLa values and requiring less power for kLa fixing. A smaller media to cellbag ratio is predicted to have higher kLa values and more readily become influenced by the power terms (rocking angles and rocking rates) and gas velocity (headspace gassing). Successful and simple scaling therefore allows a smaller fermentation model to be established to reduce the costs associated with development and characterization.
For monoclonal antibody production, a small-scale model system using a wave-induced motion bioreactor of 10–50 l media can be used as an affordable development platform to establish a process to then scale to kLa fixing anywhere up to 500 l. Using a perfusion system, a continuous cell culture process can be established at a very modest scale whereby biomass is retained in the wave-induced motion bioreactor, while new culture media is continuously added at approximately one (1) bioreactor volumeper day and culture supernatant is removed at the same rate to keep the bioreactor volume constant. In this respect a 10 l scale-down perfusion model can be used to economically develop a Phase I process. When scaled to a modest 100 l wave-induced motion bioreactor, the operation in perfusion mode from day 7 to day 21 (14 day perfusion) is equivalent to operating in batch or fed-batch at a scale of 1400 l. If a conservative 1 g/ l volumetric yield is achieved from such a process and assuming a low 30% yield from downstream unit operations, such a process could produce approximately 400 g of monoclonal antibodies and therefore be suitable to initiate Phase I investigations at a greatly reduced cost.
4.5 Novel material example: Cellulose nanofibers
4.5.1 Manufacture and properties of cellulose nanofibers
Cellulose is the most abundant natural polymer on earth. The fundamental building block of cellulose in all ligno-cellulosicmaterials is of crystallites of 4–5 nm in diameter. Thus, given the degree of polymerization of cellulose, the natural organization of cellulose in all ligno-cellulosicmaterial is as cellulose nanofibers [69]. Cellulose nanomaterials are produced by breaking down ligno-cellulosicmaterial, either partially or fully, into the constituent nanofibers.
The terminology around cellulose nanofibers is still in flux. Broadly the materials may be divided into two classes, based on crystallinity and aspect ratio. Cellulose nanocrystals or nanocrystalline cellulose are treated with acid to hydrolyze the cellulose. Mechanical treatment, to break down the structure produces 5–10 nm diameter, low aspect ratio cylinders of crystalline cellulose. Microfibrillated cellulose (MFC) or cellulose nanofibers are high aspect ratio material, often only partially separated, produced by combining mechanical treatment, typically with a chemical pretreatment to reduce the energy required for separation. Cellulose nanofibers are typically more heterogeneous than nanocrystalline cellulose, both in the average fiber diameter for a given batch and the distribution of fiber diameters [70].
In the rest of this brief discussion, the focus will be on the applications of cellulose nanofibers. A characteristic of this class of material is that it can be made from almost any ligno-cellulosic material including hardwoods and softwoods [71] and many different non-wood fibers [72] and grasses [73], including a variety of agricultural residues. It is generally considered that for optimal separation of fibers into cellulose nanofibers, all or most of the lignin should be removed prior to processing [74].
Many different methods can be used to separate the starting material into cellulose nanofibers, including homogenization [75], grinding [76], ball milling [77] and refining. The common elements are applying shear force to a low consistency (often around 1%) suspension. Without appropriate pretreatment, the energy consumption required to fully separate the starting material into nanofibers in a homogenizer is very large, perhaps up to 20 000 kWh/t, thus leading researchers to investigate more energy efficient ways to treat the fibers or developing pretreatments to reduce energy consumption by enhancing separation efficiency. Currently available pretreatments include enzymatic treatment [75], carboxymethylation or TEMPO assisted oxidation [78]. Pretreatment typically also produces more uniform fibers.
4.5.2 Applications of cellulose nanofibers
Applications of cellulose nanofibers can be broadly divided into additive applications and sheet and/ or film applications.
Cellulose nanofibers have been widely investigated as a reinforcing fiber in composites. Matrices investigated include petrochemical derived matrix materials such as polypropylene [79]. However, the use of bioderived materials such thermoplastic starch [80] or PLA [81] to make all biocomposites, seems to be the most promising approach. The fibers do not require additional treatment to compatibilize themwith the biopolymer matrix and the final product is renewable and biodegradable. Adding only 5 wt.% of cellulose nanofibers to PLA, increased tensile strength and elastic modulus by almost 25% [81]. Cellulose nanofibers can also be added directly to the matrix in twin-screw extrusion [81], where wood pulp fibers cannot, as they are three orders of magnitude larger.
Cellulose nanofibers also show considerable promise as an additive in conventional papermaking. Cellulose nanofiber addition improves both wet and dry strength and increases the ratio of wet to dry strength [82, 83], while reducing permeability and increasing sheet resistance to drainage [84]. Sheet strength was doubled [83] by using a combination 50–100mg/g of nanofibers and 5mg/g of PAE, a polymeric strength additive.
The improvement in mechanical strength is because the aspect ratio of the cellulose nanofibers is much higher [85] than all hardwood and many softwood fibers. The strength of short fiber nonwovens such as paper typically increases with aspect ratio and is only controlled by fiber length if the diameter is constant. Higher aspect ratio increases the efficiency of stress transfer within the network, by reducing the importance of load build up from the ends of the fibers [86].
Sheet or film applications include as membranes, as barrier layers and for substrates for flexible electronics [87], which will not otherwise be discussed here. Standalone sheets are formed from suspensions either by filtration [70, 88, 89] or casting [90]. Casting is extremely time consuming and is not likely to be a commercially viable solution for large-scale production. Filtration can be time consuming due to the dimensions of the nanofibers [89]. The small size of the fibers means that the filtration conditions must be carefully selected in order to retain the nanofibers on the filter media. In addition, as the filter mat forms, the small size of the pores in the structure reduces the flow through the filter mat. Sheet preparation time can be significantly reduced by using high solids content to minimize water to be removed, combined with filters with large mesh openings to minimize filter resistance [70, 88]. The addition of small amounts of polyelectrolytes such as CPAM and PAE will also further improve the drainage speed [91, 92]. Methods of application of nanofiber layers onto substrates include filtration [93], spray coating [94] and contact coating [95].
One of the most interesting commercial possibilities for the use of cellulose nanofibers is as a barrier layer. Paper as a packaging material has significant weaknesses. The fibers are hydrophilic and make a network with high porosity composed of large micron sized pores, which pose little barrier to water or gas transport. Up until now, paper based packaging requiring excellent barrier properties has been made as polymer composites or laminates, with the paper providing the stiffness and the polymer materials or layers controlling the permeability. However, such composite materials are difficult to recycle. Cellulose nanofiber barrier layers are effective, firstly, because the nanofibers typically form a denser network than sheets made from conventional wood fibers. Sheet densities ranging from 780 kg/m3 to 1400 kg/m3 have been reported, in comparison to the density of cellulose of approximately 1500 kg/m3. Secondly, the flow rate through a structure of a given porosity, for a given pressure drop, will scale with the size of the pores, which in turn scale with the fiber diameter. Reducing the fiber diameter by three orders of magnitude and increasing the network density, reduces the network permeability to close to that of typical polymer laminate layers, while remaining fully compatible and recyclable with the packaging.
Because the cellulose is hydrophilic, cellulose nanofiber layers typically have poorer resistance to water vapor transport. Cellulose nanofiber layers of 35 grams per square meter (gsm) had water vapor transmission rate (WVTR) of 215 g/ m2/day/ m compared to low density polyethylene (LDPE) which had WVTR of 100 g/ m2/day/ m [90]. The WVTR could be reduced to below that of the LDPE film by either adding mineral filler or an additional coating of cooked starch [90], neither of which would be expected to affect the recyclability of the film. In contrast, the oxygen permeability is more than two orders of magnitude lower than either low or high density polyethylene [96] and is only slightly worse than specialist barrier polymers such as EVOH. Treatment with TEMPO mediated oxidation to produce uniform, very low diameter nanofibers has been shown to produce excellent oxygen barrier properties even with a film thickness of only 0.4 μm[95]. The wide range of application modes and the ability to tailor the barrier performance suggests products are likely to be commercialized in the near term.
Applications of cellulose nanofiber sheets as ultrafiltration membranes have been demonstrated [93]. The experiments prepared membranes by filtering nanofiber suspensions through a support layer, followed by pressing and conventional drying. Membrane layer grammage ranged from 5 to 30 gsm, using commercial MFC, with some of the larger fibers removed and the layer density estimated from other studies is expected to be 780 kg/m3 [92]. Porosity and pore size were also tailored by adding silica nanoparticles. The results showed that MFC alone produced a porous structure with the pores too large for conventional ultrafiltration, but that addition of 10% by weight of silica nanoparticles reduced both the average size and dispersion of the pore sizes, producing a membrane that performed adequately for higher end ultrafiltration applications, although the performance still needs further improvement compared to commercial membranes. The major possible application of the material is as an ultrafiltration membrane, separating based on size.
Another application has recently been demonstrated in oil-water separation. Oil-watermixtures are extremely heterogeneous, ranging from fully or partially separated phases to dispersed emulsions. However, even well-emulsifiedmixtures will typically have a particle size in the micron range. So the pore size required for size separation is much higher than for ultrafiltration applications, and separation is often only gravity driven. As well as size exclusion, separation is also engineered by chemical compatibility, i.e. by using the hydrophilicity of the cellulose or by modifying the cellulose fibers to make them hydrophobic [97], oleophobic or oleophilic. Pore size has been increased compared to conventional sheets by freeze drying partially separated MFC, where the energy consumption in fibrillation was reduced so as to only partially separate the fibers [97]. This process has been shown to produce sheet materials with density as low as 0.002 kg/m3 [97]. This field of research is still in development.
It has been the intent of this brief survey to highlight the potential of cellulose nanofibers as a renewable, recyclable nanomaterial, which can greatly extend the property space achievable with conventional renewable fiber materials.
5 Conclusion
Dramatic increases in the demand for energy and products over the past decades with an associated requirement for lower greenhouse gas (GHG) emissions has led to the need to revisit the feedstocks and processes that society uses to generate power, fuels and goods. The consumption of fossil fuels has resulted in a number of negative environmental outcomes, including acid rain, water contamination and global warming. The challenge is to utilize low cost feedstocks to create products and energy in ways that are technically, environmentally and economically viable. Hence, there exists an urgent need for the development of innovative, flexible, efficient and low emissions technologies that utilize low cost feedstocks.
Future works can utilize computer based process modelling to rapidly consider and optimize a huge range of feedstock-unit operation-product combinations for meeting the future needs of society. Such work will highlight knowledge gaps that need to be filled with regards to physical data, reaction kinetics, rates of reaction, plant design and quality/ composition requirements for creating the highest value products for the lowest cost. For example, algae offer the opportunity to biosequester carbon via the creation of biochar from a pyrolysis process, however, may firstly require the creation of several higher value biomolecules to be economically viable. Conversely, the co-processing of low cost feedstocks such as bagasse, green wastes, and/ or coal can provide feedstock reliability and economies of scale.
Bioprocess engineers can address the major challenges facing the development and application of new industries by:
Addressing the limited data on the kinetics of complex reactions involved in processing biomass and co-processing of mixed feedstocks
Higher capital and operating costs that represent obstacles for adopting these technologies
Restrictive and continually more constraining emissions to air requirements to ensure that industry is sustainable
Rapidly changing operating environments which will require industrial facilities to be flexible with regards to feedstocks due to dramatic changes to commodities prices, changes to regional and global climates, and extreme weather events
Life cycle analysis/quadruple bottom line analysis (economic, environmental, societal, GHG)
Acknowledgment
This article is also available in: Luque/Xu, Biomaterials. De Gruyter (2016), isbn 978–3–11–034230–7.
References
[1] Deloitte Touche Tohmatsu, Opportunities for the fermentation-based chemical industry. 2014.Search in Google Scholar
[2] Data Monitor,Pharmaceuticals: Global Industry Guide. Research and Markets Publishing, Dublin, Ireland, 2010.Search in Google Scholar
[3] Industry Experts. Biopharmaceuticals- A Global Market Overview. 2013.Search in Google Scholar
[4] Transparency Market Research, Neutraceutical ingredientsmarket. Albany, 2014.Search in Google Scholar
[5] Chum H, Faaij A, Moreira J, Berndes G, Dhamija P, Dong H, Gabrielle B, Gosseng A, Lucht W, Ma-pako M, Masera Cerutti O, McIntyre T, Minowa T, Pingoud K. Bioenergy, in EdenhoferO et al., editors. IPCC Special Report on Renewable Energy Sources and Climate Change Mitigation, Cambridge University Press: Cambridge, 2011.10.1017/CBO9781139151153.006Search in Google Scholar
[6] Rainey TJ, Doherty B, Martinez DM, Brown R, Kelson NA. The effect of flocculants on the filtra-tion properties of bagasse pulp, Tappi Journal, 2010 (May), 7–14.10.32964/TJ9.5.7Search in Google Scholar
[7] Rainey TJ, Doherty WOS, Martinez DM, Brown RJ, Kelson NA. Pressure filtration of bagasse pulp, Transport in Porous Media, 2010, 86 (3), 737–751.10.1007/s11242-010-9649-xSearch in Google Scholar
[8] Rainey TJ, O’Hara I, Mann AP, Bakir CH. Reducing bagasse dust emissions from stockpile op-erations by depithing, in Proceedings of the Australian Society for Sugarcane Technologists, 2012.Search in Google Scholar
[9] Rainey TJ, O’Hara I, Mann AP, Bakir CH, Plaza F. Effect of depithing on the safety and environ-mental aspects of bagasse stockpiling, Process Safety and Environmental Protection, 2013, 91 , 378–385.10.1016/j.psep.2012.09.002Search in Google Scholar
[10] Rainey TJ. A comparison between highly depithed and conventionally depithed bagasse pulp, Appita Journal, 2012, 65 (2), 178–183.Search in Google Scholar
[11] Rainey TJ. A study into the permeability and compressibility properties of Australian bagasse pulp, in Faculty of Built Environment and Engineering, Queensland University of Technology: Brisbane, 2009.Search in Google Scholar
[12] Doherty B, Rainey T. Bagasse fractionation by the soda process, in Proc. Aust. Soc. Sugar Cane Technol.Mackay, 2006.Search in Google Scholar
[13] Kosinkova J, Doshi A, Maire J, Ristovski Z, Brown R, Rainey TJ. Measuring the regional availabil-ity of biomass for biofuels and the potential for microalgae, Renewable & Sustainable Energy Reviews, 2015, 49 , 1271–1285.10.1016/j.rser.2015.04.084Search in Google Scholar
[14] Covey G, Rainey TJ, Shore D. A new opportunity to pulp bagasse in Australia, in Proc. Aust. Soc. Sugar Cane Technol.Mackay, 2006.Search in Google Scholar
[15] Rainey TJ, Covey G, Shore D. An analysis of Australian sugarcane regions for bagasse paper manufacture, International Sugar Journal, 2006, 108 (1295), 640–644.Search in Google Scholar
[16] Ramirez J, Brown RJ, Rainey TJ A Review of Hydrothermal Liquefaction Bio-Crude Properties and Prospects for Upgrading to Transportation Fuels, Energies, 2015, 8 , 6765–6794.10.3390/en8076765Search in Google Scholar
[17] Arthritis Australia. Glucosamine and Chondroitin.2014 [Accessed on 26 February 2016] http:// www.arthritisvic.org.au/Complementary-Therapies/Complementary-Therapies/Glucosamine-and-Chondroitin.Search in Google Scholar
[18] National Center for Complementary and Integrative Health. Glucosamine and Chon-droitin for Osteoarthritis: What You Need To Know 2014 [Accessed on 26 February 2016] https://nccih.nih.gov/health/glucosaminechondroitin.Search in Google Scholar
[19] U.S. National Library of Medicine. Chondroitin sulfate. 2015 [Accessed on 26 February 2016] http://www.nlm.nih.gov/medlineplus/druginfo/natural/744.html.Search in Google Scholar
[20] Greenwoods W, editor. Bioactive market study of beef, steep and goat meat: value chain analy-sis. M.a.L.A. Ltd, 2011.Search in Google Scholar
[21] Chundawat SPS, Beckham GT, Himmel ME, Dale BE. Deconstruction of lignocellulosic biomass to fuels and chemicals, Annual Review of Chemical and Biomolecular Engineering, 2011, 2 (2), 121–145.10.1146/annurev-chembioeng-061010-114205Search in Google Scholar PubMed
[22] Blanch HW, Simmons BA, Klein-Marcuschamer D. Biomass deconstruction to sugars, Biotech-nology Journal, 2011, 6 (9), 1086–1102.10.1002/biot.201000180Search in Google Scholar PubMed
[23] Kim SR, Ha SJ, Wei N, Oh EJ, Jin YS. Simultaneous co-fermentation of mixed sugars: a promising strategy for producing cellulosic ethanol, Trends in Biotechnology, 2012, 30 (5), 274–282.10.1016/j.tibtech.2012.01.005Search in Google Scholar PubMed
[24] van der Pol EC, Bakker RR, Baets P, Eggink G. By-products resulting from lignocellulose pre-treatment and their inhibitory effect on fermentations for (bio)chemicals and fuels, Appl Microbiol Biotechnol, 2014, 98 (23), 9579–93.10.1007/s00253-014-6158-9Search in Google Scholar PubMed
[25] Vancov T, Alston AS, Brown T, McIntosh S. Use of ionic liquids in converting lignocellulosic material to biofuels, Renewable Energy, 2012, 45 , 1–6.10.1016/j.renene.2012.02.033Search in Google Scholar
[26] Klein-Marcuschamer D, Simmons BA, Blanch HW. Techno-economic analysis of a ligno-cellulosic ethanol biorefinery with ionic liquid pre-treatment, Biofuels Bioproducts & Biorefining-Biofpr, 2011, 5 (5), 562–569.10.1002/bbb.303Search in Google Scholar
[27] Zhang Z, Wong HH, Albertson PL, Doherty WO, O’Hara IM. Laboratory and pilot scale pretreat-ment of sugarcane bagasse by acidified aqueous glycerol solutions, Bioresour Technol, 2013, 138 , 14–21.10.1016/j.biortech.2013.03.065Search in Google Scholar PubMed
[28] Zhang Z, Wong HH, Albertson PL, Harrison MD, Doherty WO, O’Hara IM. Effects of glycerol on enzymatic hydrolysis and ethanol production using sugarcane bagasse pretreated by acidified glycerol solution, Bioresour Technol, 2015, 192 , 367–73.10.1016/j.biortech.2015.05.093Search in Google Scholar PubMed
[29] Bals BD, Gunawan C, Moore J, Teymouri F, Dale BE. Enzymatic Hydrolysis of Pelletized AFEX (TM)-Treated Corn Stover at High Solid Loadings, Biotechnology and Bioengineering, 2014, 111 (2), 264–271.10.1002/bit.25022Search in Google Scholar PubMed
[30] Jin MJ, Gunawan C, Balan V, Lau MW, Dale BE. Simultaneous saccharification and co-fermentation (SSCF) of AFEX (TM) pretreated corn stover for ethanol production using commercial enzymes and Saccharomyces cerevisiae 424A(LNH-ST), Bioresource Technology, 2012, 110 , 587–594.10.1016/j.biortech.2012.01.150Search in Google Scholar PubMed
[31] Chundawat SPS, Bals B, Campbell T, Sousa L, Gao D, Jin M, Eranki P, Garlock R, Teymouri F, Balan V, Dale BE. Primer on Ammonia Fiber Expansion Pretreatment, in Aqueous Pretreatment of Plant Biomass for Biological and Chemical Conversion to Fuels and Chemicals, 2013, pp. 169–200.10.1002/9780470975831.ch9Search in Google Scholar
[32] Uppugundla N, Sousa LD, Chundawat SPS, Yu XR, Simmons B, Singh S, Gao X.D, Kumar R, Wyman CE, Dale BE, Balan V. A comparative study of ethanol production using dilute acid, ionic liquid and AFEX (TM) pretreated corn stover, Biotechnology for Biofuels, 2014, 7 .10.1186/1754-6834-7-72Search in Google Scholar PubMed PubMed Central
[33] Deinove. DEINOVE teams up with MBI, pioneer of AFEX technology, to evaluate its process on industrial biomass. 2014 [Accessed on 26 February 2016] http://www.deinove.com/en/news/all-press-releases/deinove-teams-mbi-pioneer-afex-technology-evaluate-its-process-industrial-biomass.Search in Google Scholar
[34] Dionisi D, Anderson JA, Aulenta F, McCue A, Paton G. The potential of microbial processes for lignocellulosic biomass conversion to ethanol: a review, J. Chem. Technol. Biotechnol, 2015, 90 , 366–383.10.1002/jctb.4544Search in Google Scholar
[35] Borodina I, Nielsen J. Advances in metabolic engineering of yeast Saccharomyces cerevisiae for production of chemicals, Biotechnology Journal, 2014, 9 , 609–620.10.1002/biot.201300445Search in Google Scholar PubMed
[36] Weber C, et al. Trends and challenges in the microbial production of lignocellulosic bioalcohol fuels, Applied Microbiology and Biotechnology 2010, 87 (4), 1303–1315.10.1007/s00253-010-2707-zSearch in Google Scholar PubMed
[37] Matsushika A, et al. Ethanol production from xylose in engineered Saccharomyces cerevisiae strains: current state and perspectives, Applied Microbiology and Biotechnology, 2009, 84 (1), 37–53.10.1007/s00253-009-2101-xSearch in Google Scholar PubMed
[38] Casey E, Mosier NS, Adamec J, Stockdale Z, Ho N, Sedlak M. Effect of salts on the Co-fermentation of glucose and xylose by a genetically engineered strain of Saccharomyces cerevisiae, Biotechnology for Biofuels, 2013, 6 (83).10.1186/1754-6834-6-83Search in Google Scholar PubMed PubMed Central
[39] Wei N, Oh EJ, Million G, Cate JHD, Jin YS. Simultaneous utilization of cellobiose, xylose, and acetic acid from lignocellulosic biomass for biofuel production by an engineered yeast plat-form, Synthetic Biology, 2015, 4 (6), 717–713.10.1021/sb500364qSearch in Google Scholar PubMed
[40] Kim SM. Simultaneous utilization of glucose and xylose via novel mechanisms in engineered Escherichia coli, Metabolic Engineering, 2015, 30 , 151–148.10.1016/j.ymben.2015.05.002Search in Google Scholar PubMed
[41] Fei Q. High-cell-density cultivation of an engineered Rhodococcus opacus strain for lipid production via co-fermentation of glucose and xylose, Process Biochemistry, 2015, 50 (4), 500–506.10.1016/j.procbio.2015.01.008Search in Google Scholar
[42] Dunn KL, Rao CV. Expression of a xylose-specific transporter improves ethanol production by metabolically engineered Zymomonas mobilis, Appl Microbiol Biotechnol, 2014, 98 (15), 6897–905.10.1007/s00253-014-5812-6Search in Google Scholar PubMed
[43] Leonowicz A, Matuszewska A, Luterek J, Ziegenhagen D, Wojtas-Wasilewska M, Cho NS, Hofrichter M, Rogalski J. Biodegradation of lignin by white rot fungi, Fungal Genet Biol, 1999, 27 (2–3), 175–85.10.1006/fgbi.1999.1150Search in Google Scholar PubMed
[44] Bugg TDH, Rahmanpour R. Enzymatic conversion of lignin into renewable chemicals, Current Opinion in Chemical Biology, 2015, 29 , 10–17.10.1016/j.cbpa.2015.06.009Search in Google Scholar PubMed
[45] Wong DWS. Structure and Action Mechanism of Ligninolytic Enzymes, Applied Biochemistry and Biotechnology, 2009, 157 (2), 174–209.10.1007/s12010-008-8279-zSearch in Google Scholar PubMed
[46] Rico A, Rencoret J, del Rio JC, Martinez AT, Gutierrez A. Pretreatment with laccase and a phe-nolic mediator degrades lignin and enhances saccharification of Eucalyptus feedstock, Biotechnology for Biofuels, 2014, 7 .10.1186/1754-6834-7-6Search in Google Scholar PubMed PubMed Central
[47] Linger JG, Vardon DR, Guarnieri MT, Karp EM, Hunsinger GB, FrandenMA, Johnson CW, Chupka G, Strathmann TJ, Pienkos PT, Beckham GT. Lignin valorization through integrated biologi-cal funneling and chemical catalysis, Proceedings of the National Academy of Sciences of the United States of America, 2014, 111 (33), 12013–12018.10.1073/pnas.1410657111Search in Google Scholar PubMed PubMed Central
[48] Vardon DR, Franden MA, Johnson CW, Karp EM, Guarnieri MT, Linger JG, Salm MJ, Strathmann TJ, Beckham GT. Adipic acid production from lignin, Energy & Environmental Science, 2015, 8 (2), 617–628.10.1039/C4EE03230FSearch in Google Scholar
[49] Salvachua D, Karp EM, Nimlos CT, Vardon DR, Beckham GT. Towards lignin consolidated bio-processing: simultaneous lignin depolymerization and product generation by bacteria, Green Chemistry, 2015.10.1039/C5GC01165ESearch in Google Scholar
[50] den Haan R, et al. Progress and challenges in the engineering of non-cellulolytic microorgan-isms for consolidated bioprocessing, Current Opinion in Biotechnology, 2015, 33 , 32–38.10.1016/j.copbio.2014.10.003Search in Google Scholar PubMed
[51] Hasunuma T, Okazaki F, Okai N, Hara K.Y, Ishii J, Kondo A. A review of enzymes and microbes for lignocellulosic biorefinery and the possibility of their application to consolidated biopro-cessing technology, Bioresour Technol, 2013, 135 , 513–22.10.1016/j.biortech.2012.10.047Search in Google Scholar PubMed
[52] Lambertz C, et al. Challenges and advances in the heterologous expression of cellulolytic en-zymes: a review, Biotechnology for Biofuels, 2014, 7 (1), 135.10.1186/s13068-014-0135-5Search in Google Scholar PubMed PubMed Central
[53] Gholamreza SJ, Taherzadeh MJ. Advances in consolidated bioprocessing systems for bioethanol and butanol production from biomass: a comprehensive review, Biofuel Research Journal, 2015, 2 (1), 152–195.10.18331/BRJ2015.2.1.4Search in Google Scholar
[54] Hasunuma T, Kondo A. Development of yeast cell factories for consolidated bioprocessing of lignocellulose to bioethanol through cell surface engineering, Biotechnology Advances, 2012, 30 (6), 1207–1218.10.1016/j.biotechadv.2011.10.011Search in Google Scholar PubMed
[55] Hasunuma T, Kondo A. Consolidated bioprocessing and simultaneous saccharification and fermentation of lignocellulose to ethanol with thermotolerant yeast strains, Process Biochem-istry, 2012, 47 (9), 1287–1294.10.1016/j.procbio.2012.05.004Search in Google Scholar
[56] Girio FM. Hemicelluloses for fuel ethanol: A review, Bioresource Technology, 2010, 101 (13), 4775–4880.10.1016/j.biortech.2010.01.088Search in Google Scholar PubMed
[57] Zheng Z, Chen T, Zhao M, Wang Z, Zhao X. Engineering Escherichia coli for succinate produc-tion from hemicellulose via consolidated bioprocessing, Microb Cell Fact, 2012, 11 , 37.10.1186/1475-2859-11-37Search in Google Scholar
[58] POET-DSM. First commercial-scale cellulosic ethanol plant in the U.S. opens for business. 2014 [Accessed on 26 February 2016] http://www.dsm.com/corporate/media/informationcenter-news/2014/09/29-14-first-commercial-scale-cellulosic-ethanol-plant-in-the-united-states-open-for-business.html.10.1016/S1351-4180(14)70420-9Search in Google Scholar
[59] U.S. Energy Information Administration. U.S. Fuel Ethanol Plant Production Capacity. 2015 [Accessed on 26 February 2016] http://www.eia.gov/petroleum/ethanolcapacity/.Search in Google Scholar
[60] GranBio. GranBio-Rhodia. 2015 [Accessed on 26 February 2016] http://www.granbio.com.br/en/conteudos/biochemicals/.Search in Google Scholar
[61] Burk M, Barton N, Trawick J. Development of an Integrated Biofuel and Chemical Refinery. Final report for Genomatica. [Accessed on 26 February 2016] http://www.genomatica.com/_uploads/pdfs/Genomatica,BiomassAdvances,TechnicalPaper,June2015.pdf.Search in Google Scholar
[62] Otto R, Santagostino A, Schrader U. The beauty and the beast: A perspective on biopharmaceu-ticals, in Otto R, Santagostino A, Schrader U, editors. From Science to Operations Questions, Choices and Strategies for Success in Biopharma, McKinsey & Company, 2014, p. 10.Search in Google Scholar
[63] Singh V. Disposable bioreactor for cell culture using Wave-induced agitation, Cytotechnology, 1999, 30 , 149–158.10.1007/0-306-46860-3_74Search in Google Scholar
[64] Clincke M, Mölleryd C, Zhang Y, Lindskog E, Walsh K, Chotteau V. Study of a recombinant CHO cell line producing a monoclonal antibody by ATF or TFF external filter perfusion in a WAVE Bioreactor™, in 22nd European Society for Animal Cell Technology (ESACT) Meeting on Cell Based Technologies. Vienna, Austria, 2011, p. P105.10.1186/1753-6561-5-S8-P105Search in Google Scholar PubMed PubMed Central
[65] Clincke M, Mölleryd C, Zhang Y, Lindskog E, Walsh K, Chotteau V. Very High Density of CHO Cells in Perfusion by ATF or TFF in WAVE Bioreactor™. Part I. Effect of the Cell Density on the Process, Biotechnol. Prog, 2013, 29 (3), 754–767.10.1002/btpr.1704Search in Google Scholar PubMed PubMed Central
[66] Rodrigues ME, Costa A.R, Henriques M, Azeredo J, Oliveira R. Wave characterization for mam-malian cell culture: residence time distribution, New Biotechnology, 2012, 29 (3), 402–408.10.1016/j.nbt.2011.10.006Search in Google Scholar PubMed
[67] Tao Y, Shih J, Sinacore M, Ryll T, Yusuf-Makagiansar H. Development and Implementation of a Perfusion-Based High Cell Density Cell Banking Process, Biotechnol. Prog, 2011, 27 (3), 924–929.10.1002/btpr.599Search in Google Scholar PubMed
[68] Yuk IH, Baskar D, Duffy PH, Hsiung J, Leung S, Lin AA. Overcoming challenges in WAVE Biore-actors without feedback controls for pH and dissolved oxygen, Biotechnol. Prog, 2011 27 (5), 1397–406.10.1002/btpr.659Search in Google Scholar PubMed
[69] Isogai A. Wood nanocelluloses: fundamentals and applications as new bio-based nanomateri-als, Journal of Wood Science, 2013, 1–11.10.1007/s10086-013-1365-zSearch in Google Scholar
[70] Zhang L, Batchelor W, Varanasi S, Tsuzuki T, Wang X. Effect of cellulose nanofiber dimensions on sheet forming through filtration, Cellulose, 2012, 19 (2), 561–574.10.1007/s10570-011-9641-9Search in Google Scholar
[71] Syverud K, Chinga-Carrasco G, Toledo J, Toledo PG. A comparative study of Eucalyptus and Pinus radiata pulp fibers as raw materials for production of cellulose nanofibrils, Carbohydrate Polymers, 2011, 84 (3), 1033–1038.10.1016/j.carbpol.2010.12.066Search in Google Scholar
[72] Alemdar A, Sain M. Isolation and characterization of nanofibers from agricultural residues - Wheat straw and soy hulls, Bioresource Technology, 2008, 99 (6), 1664–1671.10.1016/j.biortech.2007.04.029Search in Google Scholar PubMed
[73] Amiralian N, Annamalai PK, Memmott P, Schmidt S, Taran E, Martin D. Easily deconstructed, high aspect ratio cellulose nanofibers from Triodia pungens; an abundant grass of Australia’s arid zone, RSC Advances, 2015.10.1039/C5RA02936HSearch in Google Scholar
[74] Spence KL, Venditti RA, Habibi Y, Rojas OJ, Pawlak JJ. The effect of chemical composition on microfibrillar cellulose films from wood pulps: Mechanical processing and physical properties, Bioresource Technology, 2010, 101 (15), 5961–5968.10.1016/j.biortech.2010.02.104Search in Google Scholar PubMed
[75] Henriksson M, Henriksson G, Berglund LA, Lindström T. An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers, European Polymer Journal, 2007, 43 (8), 3434–3441.10.1016/j.eurpolymj.2007.05.038Search in Google Scholar
[76] Abe K, Iwamoto S, Yano H. Obtaining cellulose nanofibers with a uniform width of 15 nm from wood, Biomacromolecules, 2007, 8 (10), 3276–3278.10.1021/bm700624pSearch in Google Scholar PubMed
[77] Zhang L, Tsuzuki T, Wang X. Preparation of cellulose nanofiber from softwood pulp by ball milling, Cellulose, 2015.10.1007/s10570-015-0582-6Search in Google Scholar
[78] Saito T, Nishiyama Y, Putaux J.-L, Vignon M, Isogai A. Homogeneous suspensions of individu-alized microfibrils from TEMPO-catalyzed oxidation of native cellulose, Biomacromolecules, 2006 7 (6), 1687–1691.10.1021/bm060154sSearch in Google Scholar PubMed
[79] Suzuki K, Okumura H, Kitagawa K, Sato S, Nakagaito A, Yano H. Development of continu-ous process enabling nanofibrillation of pulp and melt compounding, Cellulose, 2013, 20 (1), 201–210.10.1007/s10570-012-9843-9Search in Google Scholar
[80] Hietala M, Mathew AP, Oksman K. Bionanocomposites of thermoplastic starch and cellulose nanofibers manufactured using twin-screw extrusion, European Polymer Journal, 2012.10.1016/j.eurpolymj.2012.10.016Search in Google Scholar
[81] Jonoobi M, Harun J, Mathew AP, Oksman K. Mechanical properties of cellulose nanofiber (CNF) reinforced polylactic acid (PLA) prepared by twin screw extrusion, Composites Science and Technology, 2010 70 (12), 1742–1747.10.1016/j.compscitech.2010.07.005Search in Google Scholar
[82] Su J, Mosse WKJ, Sharman S, Batchelor WJ, Garnier G. Effect of tethered and free microfibril-lated cellulose (MFC) on the properties of paper composites, Cellulose, 2013, 20 , 1925-1935.10.1007/s10570-013-9955-xSearch in Google Scholar
[83] Ahola S, Österberg M, Laine J. Cellulose nanofibrils – Adsorption with poly(amideamine) epichlorohydrin studied by QCM-D and application as a paper strength additive, Cellulose, 2008, 15 (2), 303–314.10.1007/s10570-007-9167-3Search in Google Scholar
[84] Taipale T, Österberg M, Nykänen A, Ruokolainen J, Laine J. Effect of microfibrillated cellulose and fines on the drainage of kraft pulp suspension and paper strength, Cellulose, 2010, 17 (5), 1005–1020.10.1007/s10570-010-9431-9Search in Google Scholar
[85] Varanasi S, He R, Batchelor WJ. Estimation of cellulose nanofiber aspect ratio from measure-ments of fiber suspension gel point, Cellulose, 2013, 20 (4), 1885–1896.10.1007/s10570-013-9972-9Search in Google Scholar
[86] Batchelor WJ. An analytical solution for the load distribution along a fiber in a nonwoven net-work, Mechanics of Materials, 2008, 40 (12), 975–981.10.1016/j.mechmat.2008.07.003Search in Google Scholar
[87] Zhu H, Fang Z, Preston C, Li Y, Hu L. Transparent paper: fabrications, properties, and device applications, Energy & Environmental Science, 2014, 7 (1), 269–287.10.1039/C3EE43024CSearch in Google Scholar
[88] Varanasi S, Batchelor WJ. Rapid preparation of cellulose nanofiber sheet, Cellulose, 2013, 20 (1), 211–215.10.1007/s10570-012-9794-1Search in Google Scholar
[89] Sehaqui H, Liu A, Zhou Q, Berglund LA. Fast preparation procedure for large, flat cellulose and cellulose/inorganic nanopaper structures, Biomacromolecules, 2010, 11 (9), 2195–2198.10.1021/bm100490sSearch in Google Scholar PubMed
[90] Spence KL, Venditti RA, Rojas OJ, Pawlak JJ, Hubbe MA. Water vapor barrier properties of coated and filled microfibrillated cellulose composite films, BioResources, 2011, 6 (4), 4370–4388.10.15376/biores.6.4.4370-4388Search in Google Scholar
[91] Raj PR, Varanasi S, Batchelor WJ, Garnier G. Effect of cationic polyacrylamide on the process-ing and properties of nanocellulose films, Journal of Colloid & Interface Science, 2015, 447 , 113–119.10.1016/j.jcis.2015.01.019Search in Google Scholar PubMed
[92] Varanasi S, Batchelor WJ, Superior non-woven sheet forming characteristics of low-density cationic polymer-cellulose nanofiber colloids, Cellulose, 2014, 21 (5), 3541–3550.10.1007/s10570-014-0370-8Search in Google Scholar
[93] Varanasi S, Low Z-X, Batchelor WJ. Cellulose nanofiber composite membranes – biodegradable and recyclable UF membranes, Chemical Engineering Journal, 2015 265 , 138–146.10.1016/j.cej.2014.11.085Search in Google Scholar
[94] Beneventi D, Chaussy D, Curtil D, Zolin L, Gerbaldi C, Penazzi N. Highly porous paper loading with microfibrillated cellulose by spray coating on wet substrates, Industrial & Engineering Chemistry Research, 2014, 53 (27), 10982–10989.10.1021/ie500955xSearch in Google Scholar
[95] Fukuzumi H, Saito T, Wata T, Kumamoto Y, Isogai A. Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation, Biomacromolecules, 2009, 10 (1), 162–165.10.1021/bm801065uSearch in Google Scholar PubMed
[96] Syverud K, Stenius P. Strength and barrier properties of MFC films, Cellulose, 2009, 16 , 75–85.10.1007/s10570-008-9244-2Search in Google Scholar
[97] Wang S, Peng X, Zhong L, Tan J, Jing S, Cao X, Chen W, Liu C, Sun R. An ultralight, elastic, cost-effective, and highly recyclable superabsorbent frommicrofibrillated cellulose fibers for oil spillage cleanup, Journal of Materials Chemistry A, 2015, 3 (16), 8772–8781.10.1039/C4TA07057GSearch in Google Scholar
© 2016 by Walter de Gruyter Berlin/Boston

