1 Introduction
Although polyamines (PA) belong to relatively simple aliphatic substances, their role in life processes of animals and plants is of key importance [1–[5]. The group of the most important amines, called biogenic ones includes:
| Spermine (Spm): | H2N(CH2)3NH(CH2)4NH(CH2)3NH2 |
| Spermidine (Spd): | H2N(CH2)3NH(CH2)4NH2 |
| Putrescine (Put): | H2N(CH2)4NH2. |
Of secondary importance are homologues of biogenic amines, occurring in lower contents in living organisms [2, 6–8:
| 1,3-diaminopropan: | H2N(CH2)3NH2 |
| Cadaverine: | H2N(CH2)5NH2 |
| Homospermidine: | H2N(CH2)4NH(CH2)4NH2 |
| Norspermine (3,3,3-tet): | H2N(CH2)3NH(CH2)3NH(CH2)3NH2 |
| Thermospermine: | H2N(CH2)3NH(CH2)4NH(CH2)4NH2 |
| Caldopentamine: | H2N(CH2)3NH(CH2)3NH(CH2)3NH(CH2)3NH2. |
The first polyamine discovered in a living organism was tetramine, a spermine crystallised out of sperm in 1678 by Van Leewenkeuk [9]. Putrescine was discovered in the end of the 19th century in microbes and then triamine: spermidine was discovered in the beginning of the 20th century [2]. Later studies have shown that in animal cells spermidine and spermine occur at elevated levels, while in prokaryotes spermidine and putrescine contents are dominant. Putrescine, spermidine, 1,3-diaminopropan, homospermidine, norspermidine, and norspermine have been found in many gram-negative bacteria and algae [7, 10, 11].
Total concentration of PA in living organisms is on the order of millimols, however, the concentration of free polyamines is much lower. A low level of free amines follows from the fact that they are involved in noncovalent interactions with biomolecules occurring in living organisms such as nucleic acids, proteins, or phospholipids. High concentrations of non-bonded polyamines have been detected first of all in young molecules in the process of growth, in particular in rapidly proliferating cancer cells [6, 12]. Elevated levels of free polyamines have been observed, e.g. in breast, colon, lung, prostate, and skin tumours, accompanied by changed levels of enzymes responsible for biosynthesis and catabolism of polyamines. Because of the increased level of free polyamines and a tendency of their interaction with nucleic acids and other bioligands, these compounds have become objects of intense study [1, 13–19. There is no doubt that the regulation of biosynthesis of polyamines and catabolism is one of the most important pathways in the search strategy for chemoprevention and chemotherapeutic drugs [14, 15, 20–38]. The present state of knowledge of these processes, their significance in biological systems, and their application in medicine are presented in subsequent sections of this chapter.
2 Polyamines in living systems
Polyamines occur in practically all living organisms, although the level of their concentration depends on the type of species, type of tissue, and first of all on the age of the cells [10, 14, 39–45]. The organ in which the highest concentration of polyamines (2 mM spermidine and 4 mM spermine) has been detected, is the pancreas [10]. High levels of polyamines (spermine and spermidine) have also been detected in the kidneys, spleen, liver, lungs, and in semen [7, 20]. Spermidine and its analogues are also present in high levels in the central nervous system, and that is why spermidine is also called neuridine. It should be pointed out that spermidine is not uniformly distributed in the entire nervous system and occurs in high concentration in the white matter of the brain. Also, an untypical increase in the concentration of spermidine and spermine in the brain has been noted with increasing age of the organism [39, 40]. A similar situation as in the nervous system (different concentration of amine in the same tissue) has been noted in the skin. Measurements with liquid chromatography and fluorescence methods have proved much higher concentrations of spermidine and spermine in the epidermis than in the dermis of the skin. Regular measurements of changes in the PA level in the skin are necessary to control the condition of patients with dermatological diseases [46]. Small amounts of biogenic amines have been found in blood tissues, e.g. 0.01 mM in erythrocytes, and in urine (putrescine and spermidine) [41], in which an increase in their concentration is a symptom of disease.
Increasing concentration of polyamines is also observed in people with cystic fibrosis or mucoviscidosis, most probably spermidine and the products of its metabolic decomposition play an important role in the pathogenesis of dysfunction of cell membranes [47].
Another interesting fact is that polyamines, strongly basic substances, show a high affinity to protons. This affinity increases with an increasing methylene chain of the amine. In physiological conditions, at pH close to 7, biogenic amines are fully protonated. Amines with a methylene chain shorter than three methylene groups in physiological conditions can be partly protonated, which consequently show a lower affinity to polyanions than biogenic amines [48].
From a pharmacological point of view, polyamines are toxic [15], but at appropriate concentrations play various positive roles in living organisms, Figure. 1. These compounds interact with entire cells, cellular organelles, and nucleic acids, and participate in many metabolic reactions [5, 49]. Polyamines have been found to catalyse and control biosynthesis of nucleic acids and are directly responsible for macromolecular synthesis taking place upon cell growth (synthesis of DNA is stimulated by spermidine) [44]. Strongly basic polyamines show high affinity to anionic compounds and thus show the ability to bind to nucleic acids. When interacting with phosphate groups of polynucleotides, they prevent denaturation and stabilise the structure of the nucleic acid [6, 10, 50, 51.

Role of biogenic amines in living organisms.
Polyamines show a tendency towards initiation of t-RNA methylation in vitro, prevent enzymatic degradation and radiation-induced damage to ribosomes, and influence synthesis of nucleic acids and proteins [10, 41]. Moreover they affect the degree of DNA packing in a bacteriophage [52, 53].
Biogenic polyamines also influence the functioning of biological membranes (spermine stabilises membrane activity in bacteria), and their effect depends first of all on the positive charge of the amine chain (the greater the charge the better the stabilisation). A very important consequence of attachment of PA to double-layered membranes is protection against lipid peroxidation. Biogenic polyamines also modulate the synthesis of triacylglycerols necessary for membrane construction [24, 26, 27, 43, 49]. Spermidine is also known to exert an antimutagenic effect in bacteria [44].
Some polyamines are precursors of amino acids that are formed by oxidative deamination of amines. One of those amino acids (derivatives of amines) is putreanine, which is a natural component of the brains of vertebrates [42].
The pathway of polyamine biosynthesis has been for the first time defined on the basis of investigation of microorganisms. The later developed scheme of poly-amine biosynthesis for mammals proved almost identical, Figure. 2. In bacteria (e.g. in Escherichia Coli) and in plants, putrescine is synthesised along two parallel pathways:
from ornithine as a result of its decarboxylation in the presence of ornithine decarboxylase
from arginine in the process of decarboxylation to agmatine, which is then hydrolysed with involvement of agmatine ureohydrolase directly or through the intermediate product N-carbamyl-1, 4-diaminobutane to putrescine and urea.

The pathway of polyamine synthesis in living organisms. The enzymes involved are 1 – arginine decarboxylase, 2 – agmatine ureohydrolase, 3 – ornithine decarboxylase, 4 – S-adenosylmethionine synthase, 5 – S-adenosylmethionine decarboxylase, 6 – spermidine synthase, and 7 – spermine synthase.
Catabolism of polyamines in animals has been discovered as a two-stage process controlled by the enzymes spermidine/spermine N1-acetyltransferase (SSAT) [54–[59]. Thanks to the activity of these enzymes, acetylated polyamines become substrates for further conversion of spermidine to putrescine. SSAT catalyses the formation of N1-acetylspermine or N1-acetylspermidine by a transfer of acetyl group from acetyl-coenzyme A to the N1 position of spermidine or spermine. In the second stage of this pathway, peroxisomal flavin adenine dinucleotide (FAD) is formed, and its level is directly related to the activity of N1-acetylpolyamine oxidase (APAO) [60–[65]. APAO is the enzyme that catalyses the cleavage of acetylated polyamines and is responsible for formation of spermidine or spermine, 3-aceto-aminopropanal and H2O2 [62, 63, 66–68]. Alternatively, spermine can be oxidised back directly to spermidine with involvement of spermine oxidase (SMO) the enzyme dependent on Flavin adenine dinucleotide (FAD) [67–[69]. Induction of SMO has been reported to accompany the inflammatory conditions related to the colon and lung cancer, which suggests that SMO may be an attractive target for chemoprevention strategies [70–[74]. A side effect of the oxidation reaction is generation of toxic hydrogen peroxide (H2O2). The molecules of H2O2 are known to play an essential role in DNA damage, which leads, among other things, to catabolism of polyamines. Moreover, a significant reduction of the level of intracellular polyamines, which accompany the process of catabolism, is inhibited by an increase in the level of inhibitors of this process [42].
The best recognised transport of polyamines in animals suggests that in the first stage PAs enter the cell through as yet unidentified membrane transporter/ carrier. This process is powered by the membrane potential and then by rapid accumulation of polyamines into polyamine-sequestering vesicles driven by a vacuolar – ATPase pH gradient and proton exchange. This system of transportation permits explanation of the seemingly high total concentration of intracellular poly-amines, although the actual concentration of free PAs is believed to be relatively low [75–[78]. Another model of polyamines transportation assumes that spermine is bonded to heparin sulphate and glypican 1 (GPC1), which act as agents transporting amine inside the cell [72, 75, 76].
The enzymes and the amines synthesised (especially putrescine) are the direct target of the efforts undertaken to inhibit the synthesis of polyamines in the diseases in which the elevated level of polyamines is observed, particularly in cancer.
3 Polyamines as tumour markers
Neoplastic tumours, along with circulatory system diseases, are the leading causes of death in developed countries [79]. It has been recently established that increased incidence of inflammatory conditions and increased level of synthesis of polyamines are related to the development of intraepithelial neoplasia, which is a risk factor for cancer development. There is ample literature on the relation between the increased level of polyamines concentration and neoplastic diseases, which suggests a relation between induced biosynthesis of PAs and neoplasia [1, 72, 80]. Metabolism of polyamines is an integral component of the mechanisms of carcinogenesis in epithelium tissues. Development of neoplastic disease is a multistage process that involves specific transformations leading to progressive change from healthy normal cells to cancer cells. If the range of inflammatory conditions is irregular, the cell response leads to chronic inflammatory conditions in which the inflammatory focuses are dominated by macrophages and other inflammatory cells. This leads to generation of increased amount of growth agents and cytokines as well as reactive species of oxygen and nitrogen responsible for DNA damage [81, 82].
In persons with neoplastic diseases a considerable increase in the concentration of polyamines in blood and urine is observed; however, no specific values characteristic of the disease could be established because the level of polyamines depends on activity and degree of development of the disease [10, 15, 41, 83–85]. In many types of cancer, e.g. in persons with cancer of the stomach, pancreas, and breast, the concentration of polyamines is practically unchanged (only in 20% and 25% of patients is an increase in the concentration of spermidine and spermine observed, respectively), while an increased level of PAs is observed in about 50% of persons with malignant cancer of the lungs. In persons with cancer of the genital tract, prostate, or bladder, an increased level of PAs is noted in about 90% of patients [20, 45, 74]. Measurements of the concentrations of spermine and spermidine in urine and blood often facilitate diagnosis and evaluation of health conditions and can be used for monitoring the progress of therapy. If the therapy is effective, a significant decrease in the level of polyamines is observed starting from the first week of chemotherapy [15, 45, 86]. However, it has been established that analogues of biogenic amines (spermidine and spermine) inhibit the growth of tumours in model systems and show an antimalarial effect. These types of derivatives are mainly obtained by the Mitsunobu reaction (between alcohol and activated amine, leading to a chiral derivative of polyamine) [87]. In general, cells have well-developed mechanisms regulating the correct intracellular level of PAs, and deregulation of this metabolism (biosynthesis and catabolism) and transport is of key importance for cell growth. An increased level of polyamines (as a consequence of their increased synthesis) is noted as a result of inflammatory conditions or increased proliferation following from rapid growth of cancer cells (PA have pleiotropic effects on cell physiology) [88].
In the 1960s, Russell and Snyder for the first time noted a high level of concentration of ornithine decarboxylase (ODC) accompanying the occurrence of cancer [89]. High activity of ODC and a high level of PAs have been observed to accompany an increase in familial adenomatous polyposis in cases of genetic predispositions for colon cancer related to mutation of adenomatous polyposis coli [90]. Pioneer works aimed at checking correlations between a high level of polyamines and the occurrence of skin cancer have shown that an elevated level of ODC is necessary and often sufficient for triggering cancer in mice [73]. It has been also established that the level of ODC increases in persons with human non-melanoma skin cancer (NMSC) [91]. Induction of ODC and an increase in the level of PAs level have been correlated with breast cancer [16, 92] and prostate cancer [17]. Also other enzymes such as spermidine synthase and spermine synthase are closely related to carcinogenesis. The level of enzymes involved in catabolism is also related to carcinogenesis, e.g. increased activity of SMO is observed in inflammation conditions connected to neoplastic diseases such as infection and the ensuing cancer of alimentary tract (Heliobacter pylori) [93]. Infection of gastric epithelial cells provoked by H. pylori also leads to deregulation of SMO expression, leading to an increased level of DNA damage and apoptosis. Removal of the source of inflammation has been observed to be correlated with a reduction in SMO expression [94]. A decrease in the level of SMO can bring a reduction in the inflammatory condition caused by H. pylori and consequently inhibition of gastric cancer development. An elevated level of SMO expression, relative to its level in healthy prostate tissues, has been also found in tissues from patients with prostatic intraepithelial neoplasia and prostate cancer [95] and ulcerative colitis, the presence of which is related to a high risk of colon cancer [96]. Introduction to the organism of appropriate inhibitors of biosynthesis, e.g. those that inhibit the activity of ornithine decarboxylase, reduces the level of biogenic amines in the organism, Figure. 3. Of particular importance is α-difluormethylornithine (DFMO), obtained in 1978, Figure. 4. Significant inhibition of cell replication after the introduction of DFMO (or similar inhibitors) suggests a potential therapeutic strategy for the treatment of neoplastic diseases.
![Figure. 3 Targets in the polyamine metabolic pathway [72].](/document/doi/10.1515/psr-2016-0003/asset/graphic/j_psr-2016-0003_fig_003.jpg)
Targets in the polyamine metabolic pathway [72].
![Figure. 4 Structures of the classical inhibitors of polyamine biosynthesis [99].](/document/doi/10.1515/psr-2016-0003/asset/graphic/j_psr-2016-0003_fig_004.jpg)
Structures of the classical inhibitors of polyamine biosynthesis [99].
Interference into the metabolism of polyamines through the use of inhibitors of biosynthesis or catabolism seems promising in the treatment of inflammatory conditions bearing substantial risk of cancer development. Clinical trials using DFMO, a selective inhibitor of polyamines synthesis, have shown that after a year of DFMO application the size of enlarged prostates and the amount of serum prostate-specific antigen were reduced by half in persons genetically predisposed to prostate cancer. Similarly, the 3-year application of a combination of DFMO and sulindac, a non-steroid anti-inflammatory drug (NSAID), to persons with stage 1 colon cancer led to a 70% reduction in the amount of cancer cells [1, 97, 98].
4 Weak interactions of polyamines
The most common types of weak noncovalent interactions in biological systems are illustrated in Figure. 5.

Types of weak interactions.
In the systems of polycations of biogenic amines with other bioligands, the dominant types of interactions are ion–ion or ion–dipole, with involvement of protonated PAs and biomolecules that contain atoms or a group of atoms of negative charge or high electron density [8]. The main factor determining the possibility of occurrence of weak interactions is high basicity of polyamines. In physiological environments, polyamines occur in protonated form [2], and -NHx+ groups are potential positive centres of interactions of electrostatic type with other biomolecules such as nucleosides, nucleotides, amino acids, proteins, nucleic acids, or phospholipids [86]. Protonation of primary amine groups in the PA molecule is more exothermic than protonation of secondary groups. Differences in the subsequent ∆H values decrease with increasing length of the chain, which is a consequence of changes in the transmission of inducing effect depending on the number of methylene groups. If the chain is shorter than three methylene groups, at least one pK value is less than seven, and the molecule is partly protonated in the physiological medium [99–[101]. This explains a low activity of short polyamines in noncovalent interactions in biological systems [48].
Reliable information on the formation of molecular complexes as a result of non-covalent interactions with involvement of protonated polyamines have been published about 40 years ago [102, 103]. As a result of interactions of this type, electron density at the reaction centre changes, which leads to a shift in NMR signals and permits identification of the sites of interactions. On the basis of NMR studies, the protonated -NHx+ groups of spermidine and spermine have been found to react with deprotonated phosphate groups and high electron density fragments of AMP, ADP or ATP (5’-adeninomono-, di- and triphosphate, respectively) [102, 104].
According to the polyelectrolytic Manning theory, the main factor determining the mode of interactions is the charge of reagents [105, 106]. However, this approach does not explain the specificity of certain reactions. Analysis of the character of weak interactions of polyamines has revealed that their structure influences the character of the reaction and formation of adducts with other biomolecules. PAs cannot be approximated as a point charge, as has been assumed in analysis of the reaction of biomolecules with metal ions, e.g. magnesium(II) [107]. Spatial matching of polyamine to other bioligands determines the stability of adducts formed. According to the calorimetric studies of polyamine interaction with DNA, much better agreement between theory and experiment is obtained when taking into account the structural actors [108]. Particular improvement in this agreement is obtained for the biogenic amines spermidine and spermine. For biogenic amines the effectiveness of interactions related to structural factors increases in the order Put < Spd < Spm, which corresponds not only to the number of amine groups but also to the length of these PA molecules. As the formation of molecular complexes is accompanied by a shift of the acid-base equilibrium in the reversible process:
the liberation of hydrogen cations permits determination of thermodynamic stability of adducts by a potentiometric method with computer analysis of experimental data. Such determinations were performed for a series of binary and ternary systems of polyamines with nucleosides, nucleotides, and amino acids [8, 109–115. A scheme of the adduct forming in the system of AMP/(3,3-tri), 3,3-tri=NH2(CH2)3NH(CH2)3NH2 is presented in Figure. 6[110]. Interestingly, according to NMR results, the longer polyamine, spermine, in the system with the same nucleotide, interacts only with endocyclic nitrogen atoms from AMP (at different spatial arrangement), see Figure. 6[111].

Tentative mode of interactions (noncovalent interaction).
Molecular complexes form in the pH range in which amine is protonated and act as a positive centre of interaction, while the nucleoside (nucleotide, amino acid) is deprotonated and acts as a negative centre of interaction. Dissociation of polyamine leads to adduct decomposition (Figure. 7). In comparison to the system with cytidine (Cyd) the pH range of adduct formation in the solution with uridine (Urd) is significantly shifted towards higher pH values, which is a consequence of greater basicity of the endocyclic nitrogen atom from the second nucleoside (log KHCyd = 4.5, log KHUrd = 9.2) and a different pH range of its dissociation (Figure. 7a, b) [8, 114, 115].

Distribution diagram for (a): Urd/Spm, (b): Cyd/Spm, (c): CMP/Spm systems; (a): 1-(Urd)H3(Spm), 2 – (Urd)H2(Spm), 3 – (Urd)H(Spm); (b): 1 – (Cyd)H4(Spm), 2 – (Cyd)H3(Spm), 3 – (Cyd)H2(Spm); (c): 1 – (CMP)H5(Spm), 2 – (CMP)H4(Spm), 3 – (CMP)H3(Spm).
The presence of an additional reaction centre, such as a phosphate group from CMP, causes a shift in the pH range of adduct formation towards higher acidity (Figure. 7c). Dissociation of the first proton from the nucleotide takes place at a lower pH than that of the dissociation of nucleoside. Of importance is also the ionic composition of the solution. The presence of Na+ or K+ cations weakens the effectiveness of the interactions. The observed formation of complexes of polyamines with inorganic ions and the salt effect changing the acid-base character of the ligands [116–[118] supports the thesis that formation of adducts of polyamines with other bioligands is of ion-ion and ion-dipole nature. In general, the thermodynamically stable molecular complexes have been found to form only when at least two centres of interaction between the ligands are present [107]. The NMR results have also revealed differences in the types of interactions between different polyamines and ATP molecule. It has been suggested that spermine in contrast to spermidine, prefers pyrimidine base to triphosphate group [119, 120].
Other information provided by NMR results is that in the systems of PA with amino acids the inversion effect takes place. The amine groups from PA can be positive or negative reaction centres depending on the degree of amine protonation [112, 113, 121]. The above discussed type of interactions and noncovalent interactions in the system with metal ions is essential for molecular recognition and self-assembly of biomolecules in living organisms [122, 123].
In the systems with nucleic acids, putrescine polycation at a low concentration locates in and interacts with minor and major DNA grooves, while spermidine and spermine locate only in the major groove. In contrast to putrescine the interaction of spermidine when at high concentrations is much stronger, and this amine is located in major grooves, as indicated by Infrared spectroscopy (IR) results. The authors of [124] suggest the presence of hydrophobic interactions and, apart from them, the presence of electrostatic interactions between polyamines and DNA phosphate groups. The results obtained for the system of DNA and spermine also point to the occurrence of electrostatic interactions [125, 126]. The interactions of spermine polycation in the system with DNA and metal ions have been studied by X-ray diffraction method. The polyamine joins in the crystal lattice the duplexes of nucleic acid through a system of hydrogen bonds between phosphate groups and nitrogen atoms of the bases [127]. Significant differences have been noted between the interaction of biogenic amines and their analogues. The former bind to DNA through major and minor grooves and phosphate groups, while e.g. 1,11-diamine-4,8-diazaundecane (analogue of spermine shorter by one -CH2 group) binds mainly through the N7 atom from guanine and phosphate groups. Moreover, DNA complexes with analogues of biogenic amines are thermo-dynamically weaker than those with spermine, spermidine, or putrescine. Bonding of amines with DNA leads to partial transformation of B-DNA to A-DNA, which has not been observed for biogenic amines [128]. The study of polyamine interactions with dendrimers has revealed that biogenic amines make more stable complexes than their synthetic analogues and the amine affinity to dendrimers depends on the charge of the polycation [129]. The reactions of a series of polyamines with phytic acid (myo-inositolhexakisphosphate acid, IP) were investigated by potentiometric measurements and 31P NMR. As a result of multifunctional noncovalent interactions, stable complexes of the type (IP)Hn(PA) of the molar ratio IP:PA 1:1 and of a different number of protons, are formed in the system. Nonselective interactions in the adducts take place between the positive protonated -NHx+ groups from polyamines and negative centres of deprotonated phosphate groups from IP [130, 131]. Biogenic amines have been also found to show antioxidative activity. Spermine is a stronger antioxidant than spermidine, which is suggested to be related to the higher ability of Spm for metal chelation and damage of radicals [132].
The role of polyamines in processes taking place in living organisms has stimulated further studies of this group of ligands. The application of complexes in medicine is described in another part of this chapter. Recently, a number of review papers have been published on the significance of PA in living systems, describing their role as necessary components of biological systems and their negative influence on the health [133–[136].
5 Complex formation of bioamines
The complexing properties of aliphatic polyamines depend on the number and nature of -NH2 groups and the length of methylene chains in the molecules. Literature data on the coordination compounds, mainly in binary systems, have been reviewed by D.A. House [137]. One of the most important and earliest studied ligands is ethylene diamine (en), which in metal-free systems assumes trans or cis (gauche) conformation, the transformation energy is low, close to 4 kJ/mol. Bonding with metal ions usually leads to formation of a five-membered ring. Such species can assume enantiomeric conformations λ (left-handed helicity) or δ (right-handed helicity). The energy barrier of λ D δ inversion is low and equal to about 20 kJ/mol [138]. Although en is a typical chelating ligand, its complexes with monofunctional coordination or the complexes in which it is a bridging ligand are also known [139–[143]. The number of stereochemical conformations in bis(en) complexes is greater. If the two ligands are trans isomers there are λλ, δδ, and λδ possible conformations. The first two are energetically equivalent, while λδ is less stable by about 4 kJ/mol [137].
The simplest triamine, diethylenetriamine (dien), forms six-membered rings with metal ions, and the compounds prefer chair-type coordination, although, particularly in systems with alkyl derivatives, the compounds making twist conformation are also known [139]. Linear triamines react with formation of meridional or facial conformation, while tripodal-type amines, RC(CH2NH2)3, where R = H, Me, Et, occur only in the facial form, both in solution [144, 145] and in solid state [146, 147].
The length of a polyamine chain has a significant effect on the character of interaction with metal ions. In solid state complexes, of ML2 type made by Cd(II) with dien, 2,3-tri and 3,3-tri, all six donor nitrogen atoms are involved in the coordination. In the complex with dien, the cadmium ion is sandwiched between two ligands of crystal-lographic symmetry C2, in contrast to the complexes with 2,3-tri and 3,3-tri, in which deformed octahedron was detected to be formed, Figure. 8[148].

Structures of complexes in Cd(II)/polyamine systems.
Results of the equilibrium studies of metal/amine systems have been presented in a number of papers [8, 137, 142, 149–151], and also of anhydrous systems [152]. Kinetic studies of formation of complexes with polyamines and their analogues have been reported in [153–[156]. In the formulae of the complexes the charge values have been omitted, unless necessary for understanding of the text.
The literature on metal complexes with biogenic amines: putrescine, spermi-dine, and spermine is rather poor relative to that on metal complexes with other amines, especially in binary systems. Only in the end of the last century was the formation of copper(II) complexes with putrescine finally confirmed, after many years of controversies in this matter [157]. The influence of the size of the ring on processes of chelation has been observed [158]. A larger ring of Put than shorter diamines lead to downfield shift in 59Co NMR signal because of a smaller overlap of metal-ligand σ-bond [159, 160]. In a series of platinum complexes of biogenic amines and their analogues, a clear correlation between the rate of ring-closing and the size of the ring has been found [161]. The differences in reactivity of particular ligands are related rather to enthalpy than entropy of activation. In some platinum complexes amines act as a bridging ligand [162]. Results of the studies of bis(platinum) reactions have proved that dimers are kinetically more active than their monomer analogues [163]. The differences in the spectra of ternary complexes Co(NH3)5[NH2(CH2)nNH2] (n = 2, 8, 10) and Co(NH3)5[NH2(CH2)nNH3] are assigned to the formation of the charge-transfer bands, which is related to the formation of intramolecular hydrogen bonds between free amino groups and protons from coordinated amino groups [140]. After many attempts, 1,ω-diamine copper(II) complex with putrescine was successfully isolated in solid phase [164]. In the solid complex [Co(PA)3]Br3 the chair conformation of a seven-membered ring with the {N6} coordination geometry, trigonally elongated [165–[167]. The seven-membered ring is stabilised by the metal ion, although in solution the conformation can change as a result of solvation effect and ion pairs formation [158]. In the systems with amines of long chains, the tendency to formation of monomeric forms and bridging structures dominates over the formation of chelate complexes [168–[170]. The series of bis and tris complexes: [Ni(Put)3]Cl2·H2O, [Ni(Put)2(H2O)2]Br2, [Ni(Put)2](NCS)2, and many products of their pyrolysis are characterised by the symmetry Oh[171]. Also halogen and pseudo-halogen complexes of Zn(II), Cd(II), and Hg(II) with 1,3-dia-minopropane, putrescine, and cadaverine have been isolated. Diamines behave as bridging or chelating ligands [137]. Investigation of copper(II) complexes with linear triamines has shown that the most stable complexes form five- and six-ring sequency [99, 100, 172, 173. In the series of tetramines, the value of log K increases in the order: Spm < 3,3,3-tet < 2,2,2-tet < 3,2,3-tet < 2,3,2-tet and depends on the tension in the ring and on the ability to assume the chair or twist-chair conformation. It does not explain the low stability of spermine complex, although it correlates with the observation that Spm often behaves as two independent fragments of NH2(CH2)3NH separated by a long methylene chain, which is supported by formation of {N2} type complex with Cu(II) and Spm [111]. A similar behaviour has been reported to occur in the system with Spm analogue 4,9-dioxa-1,12-dodecanediamine. This mode of coordination permits noncovalent interactions with the protonated amine groups from Spm. The heat of formation of protonated complexes of spermidine with copper is similar to that for Cu(tn)2 complex, but much lower than the heat of formation of complexes with five-membered rings, which indicates the formation of six-membered ring in Cu(HSpd) species [174, 175]. As follows from analysis of thermo-dynamic data, in Cu(Spd) the six-membered ring is fused into the seven-membered ring with involvement of three donor nitrogen atoms in the coordination. The enthalpy of Cu(Spd) formation is by 12.5 kJ/mol higher than that of Cu(HSpd) formation. Stability of particular copper(II) complexes forming in the systems with triamines and tetramines is determined by the enthalpy and entropy, respectively [176]. Computer analysis of the potentiometric data in combination with spectroscopic analysis permits the choice of a coordination model, including the stoichiometric composition of the complexes and their thermodynamic stability. Determination of the solution structure in binary systems of metal/polyamine is relatively easy and boils down to finding the number of donor nitrogen atoms from polyamine in the internal coordination zone.
Low stability of the seven-membered ring in the systems with putrescine explains the presence of monofunctional coordination in the complex and formation of the species Cu(HPut) at pH close to 7 [157]. In the systems of copper(II) ions with longer biogenic amines the metalation usually begins at pH close to 5 with formation of the six-membered species Cu(HSpd) and Cu(H2Spm). The thermody-namically less preferred seven-membered ring is stabilised by the presence of one or two six-membered rings in the Spd and Spm complexes, respectively. The formation of the three-ring chelate corresponds to a high increase in the stability constant of Cu(Spm) complex (log K = 14.70) relative to that of Cu(Spd) (log K = 11.7). Although the absorption bands in electron spectra can differ for different systems, the value λmax = 564 nm suggests the coordination with {N4} chromophore. Decrease in the ligand field strength results in a shift of the absorption energy towards weaker fields, λmax = 626 nm in Cu(Spd), {N3} coordination but λmax = 655 nm in Cu(HSpd), {N2} coordination [13, 177, 178]. For monofunctional coordination, as e.g. in Cu(HPut), λmax = 705 nm [157]. Analysis of these results points to the equatorial geometry of species in {N4} coordination. In the electron spectrum of the complex with {N5} coordination, a red shift appears as a result of localisation of the fifth nitrogen atom in the axial position [142, 177, 179]. The geometry of the complexes is supported by the studies of Cu(II), Zn(II) and Co(II) reactions with long-chain polyamines [180, 181]. The above conclusions on the mode of coordination in solution correlate with the results of a study of the complexes in solid state. The isolated [Cu(Spd)]X2 (X = Cl,Br,I) assume the structure of a square pyramid with one halogen atom at the axial position [182]. The stability of triamine complexes with Hg(II) increases in the series en < tn < Put, which can explain the tendency of this metal ion to linear coordination. The tension in seven-membered ring is relatively the lowest [183]. In the series of solid state complexes of rhodium with tetra-mines, the shortest ligand 2,2,2-tet makes only cis-α isomer, while 2,3,2-tet makes cis-α and trans isomers, and the longer ones 3,2,3-tet, 3,3,3-tet, and Spm assume a trans configuration [184]. These differences are related to different tensions in particular rings. The size of the ring also determines the kinetic properties of the complexes. In an acidic medium, [Cu(Spd)]Cl3 immediately undergoes hydrolysis and shows increased lability of the coordinated chlorine ion with respect to the shorter amines 2,3-tri and 3,3-tri. The change in the rate of Co(PA)(H2O)n complex reduction to Co(II) increases in the order: Spd < 3,3-tri < dien, which corresponds to the changes in the activation energy [185].
Binary systems were studied mainly in the 1970s and 1980s. Although many problems in bioorganic and bioinorganic chemistry have not been solved yet, the simple structure of amines soon diminished the interest in this group of compounds [186]. At present the research work has been concentrated on more complex, multicomponent model systems that are better approximations of the in vivo systems. Much attention has been paid to the interactions and processes related to the transfer of genetic information, mainly to the reactions of polyamines with nucleic acids in the model systems with DNA or RNA fragments (nucleosides, nucleotides) and metal ions [8, 179, 187, 188. As mentioned above, the interactions of protonated amines stabilise DNA (or RNA) mainly as a result of charge neutralisation. Because of their coordinating abilities, transition metal ions should be treated as factors interfering with the system of noncovalent bonds between the negative fragments of nucleic acid and the polyamine that is a positive centre. The potential centres of the reaction of polyamine with a nucleotide simultaneously make the metalation sites (Figure. 9). In ternary complexes of metal/PA/PNuc (PNuc = nucleotides), besides the phosphate residues, the donor atoms N(3) from pyrimidine nucleosides and N(1) and N(7) atoms from purine nucleosides are the preferred sites of metal binding with fragments of nucleic acids.

A scheme of interactions in metal/nucleotide/polyamine systems, R = phosphate groups (red arrows indicate potential sites of interactions).
Analysis of the mode of coordination in the systems containing purine nucleosides or nucleotides shows with no doubt that simultaneous bonding of the metal with N(1) and N(7) is impossible for steric reasons. However, in a series of metal/nucleotide and metal/nucleotide/polyamine systems, the coordination dichotomy with monofunctional metalation and formation of a mixture of isomers in which the ions Cu(II), Ni(II), Co(II), Zn(II), Cd(II), Hg(II) were bonded either to N(1) or to N(7) atoms [178, 189–193.
The presence of a phosphate group in nucleotides significantly changes the mode of interaction of this ligand with respect to that of nucleoside. For instance, in the system Cu(II)/Nuc/Spm the complexes with {N5} type coordination are formed, in which the metal ion binds four nitrogen atoms from the polyamine in equatorial position and the nitrogen atom from Nuc in the axial position (Nuc = Ado or Cyd). In the system Cu(II)/PNuc/Spm, (PNuc = AMP or CMP), meta-lation is realised only via the phosphate group, while spermine is engaged in noncovalent interactions with nitrogen atoms of high density from a purine base, Figure. 10 [111, 194].
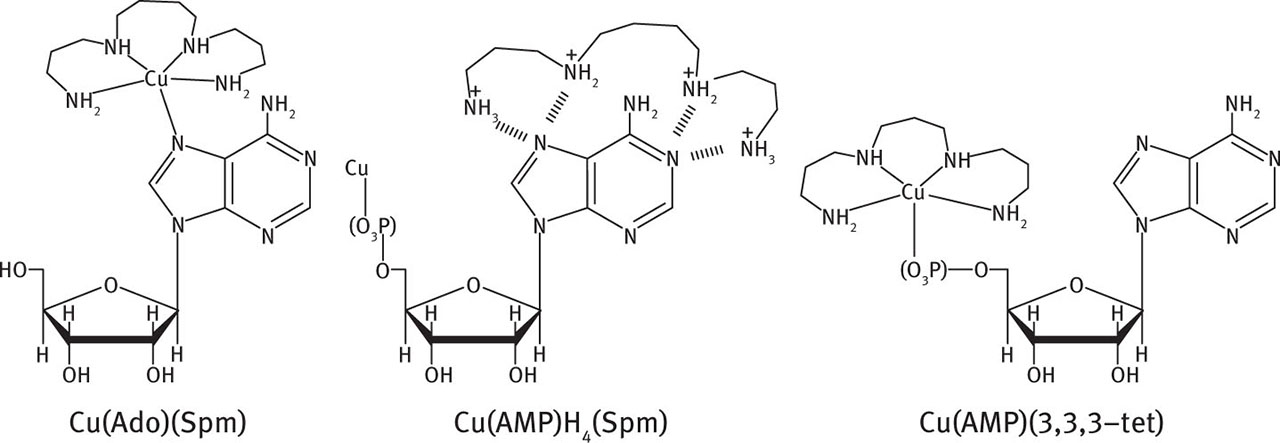
Tentative mode of coordination.
Solution structures presented in Figure. 10 are just a few examples from many illustrating that even small differences in the length of polyamines exert significant effects on the character of interactions determining the specificity of the reaction. Of course, also the nature of the metal influences the character of interactions in ternary systems. The effect of metal is best illustrated by the differences between Co(II) and Ni(II). The nickel (II) ions show low effectiveness of binding to phosphate groups from the nucleotide, while cobalt (II) binds both the endocyclic nitrogen atoms and coordinates with involvement of the phosphate groups [189]. In many ternary systems, not all donor atoms from the polyamine are engaged in the metal ions binding in MLL Hx species (L = polyamine, L = nucleotide) that permits the appearance of noncovalent interactions with the protonated –NHx+ groups from the polyamine, as illustrated in Figure. 11. Such an intramolecular interaction has a stabilising effect on the complex [8, 110, 111, 195.

Tentative mode of coordination in Cu(ATP)H3(Spm).
Also intramolecular interactions between ligands in the inner coordination sphere of the species metal/nucleotide and the polyamine located in the external sphere lead to the formation of molecular complex of ML····L type [196, 197]. The mode of interaction is significantly dependent on the concentration of polyamine [198]. The formation of ML····L type complexes has been also observed in the systems of metal/polyamine/amino acid [112, 113].
The influence of copper ions on the copper srtructure is also illustrated on Figure. 12. The introduction of Cu(II) ions to the binary system eliminates the phosphate groups from noncovalent interactions [110].

Tentative mode of interaction.
However, polyamine can be treated as an agent interfering into the metal-nucleotide interactions. In the system Cu(II)/CMP, Cu(II) ions bind the phosphate group from the nucleotide and the endocylic N(3) atom. The polyamine introduced into the system blocks N(3) atom as a result of the noncovalent interaction, while the copper ion coordinates to the donor nitrogen atom from putrescine and to the phosphate group from CMP [110]. Moreover, uridine introduced to the ternary system of Cu(II)/ ATP/3,3,3-tet is involved in noncovalent interactions with the purine base from the nucleotide, while the presence of metal ions and the polyamine changes the specific mode of interactions of complementary bases (according to Watson-Crick), observed in the metal-free system, Figure. 13[199].

Tentative mode of interaction in Cu(ATP)(3,3,3-tet)H(Urd).
6 Application of polyamine complexes in medicine
The majority of currently used therapeutic drugs are organic compounds, while much less attention has been paid to the effect of inorganic compounds. Metals in coordination or organometallic compounds offer great chemical diversity and thus larger therapeutic use [4]. Inorganic compounds have been shown to be particularly effective in therapy for malignant forms of neoplasms, as their activity is based on the specific interaction with DNA, which leads to cell damage and eventually cell death [200–[206].
The coordination compounds of platinum have been used for chemotherapy since 1978, when cisplatin (cis-diamminedichloroplatinum (II)) was first introduced in the USA as a component of chemotherapeutic treatment [207]. Although thousands of mononuclear analogues of cisplatin have been synthesised and tested as potential anticancer drugs, only two compounds, carboplatin and oxaliplatin, have been used in clinical treatment of neoplastic diseases, while other analogues were therapeutically inactive [200, 207–223.
It is known now that the antineoplastic properties of platinum complexes are related to their selective reaction with DNA and possible involvement in the formation of bridging systems with donor nitrogen atoms N(7) and N(1) [210, 224], which affects the processes of replication and transcription. The very promising results of the studies on mononuclear complexes of platinum have aroused interest in the polynuclear platinum complexes containing two or three metallic centres. The polynuclear compounds may be more cytotoxic because they cause considerable irreparable damage to DNA. From among them, the polynuclear platinum complexes containing bridging polyamines make a new class of compounds potentially more effective in antineoplastic diseases therapy than the hitherto used ones, Figure. 14 [211, 215, 217, 224. The introduction of biogenic amines to the first generation platinum complexes has an essential influence on their cytotoxic properties [217].
![Figure. 14 Polyamine–transition metal complexes with antitumour activity [99].](/document/doi/10.1515/psr-2016-0003/asset/graphic/j_psr-2016-0003_fig_014.jpg)
Polyamine–transition metal complexes with antitumour activity [99].
The cytotoxic effect of these compounds is higher than that of cisplatin [209, 215, 225, 226. Dimers of this new class of complexes show a wider range of activity than their monomer analogues because of the possibility of intra- and inter-strand cross-links formation [211, 215]. Small differences in the structure of polyamines were found to lead to considerable changes in the cytotoxic properties of their complexes with platinum. Besides the correlation between the structure and activity, the cytotoxic effectiveness of biomolecules also depends on the charge, flexibility, and noncovalent interactions [206, 215]. One such trinuclear Pt(II) compounds, BBR3464, successfully passed clinical trials, Figure. 15[227]. Also, cytotoxicity of the new complexes of polyamines with platinum (IV) has been tested [224, 228, 229]. Therapeutic possibilities of platinum (IV) complexes are similar to those of platinum (II) because, as has been suggested, in vivo the Pt(IV) ions are reduced. In the search for anticancer drugs, the better solubility of platinum (IV) compounds should be taken into account [216]. Many polyfunctional chelates containing di, tri, or tetramines as linkers have been the subject of intense studies [225, 230–236. Intense studies of anticancer therapeutic drugs of a wide pharmacological spectrum of activity, high therapeutic profile, and low toxicity have shown that polynu-clear platinum complexes are very promising candidates for replacement of cisplatin and carboplatin in therapeutic applications in which the latter are ineffective. Biogenic amines, putrescine, spermidine, and spermine and their N-alkylated correspondents are used in particular as bridging ligands in the complexes of Pt(II) and Pd(II) [212, 215, 216, 233, 237–251]. The presence of connectivities of this type permits the occurrence of untypical mechanisms of interaction with DNA such as “long-distance” inter- and intrastrand crosslinks inside the DNA helix [252], which contribute to improvement in the anticancer activity shown by some of the complexes, e.g. they give a positive response in the treatment of cancers usually resistant to cisplatin [253, 254].
Besides the complexes with platinum, also those with palladium (II) have been tested for cytotoxic activity. The value of ID50 of palladium (II) complexes with putrescine and spermidine is better than for cis-DDP, but much worse for the complexes with spermine, which corresponds to the fact that the latter is not able to produce conformational changes in DNA [212, 213]. Higher anticancer activity of the compounds in which Pd(II) has replaced Pt(II) as the central atom, has been already described in literature [255–[257]. A number of trinuclear complexes of polyamines with Pt(II) and Pd(II) have been synthesised and tested for the structure-activity relationship with regard to their potential cytotoxic properties [258]. Complexes of palladium have been found to reduce the cell activity of the ornithine decarboxylase enzyme more then complexes of platinum [259]. The already recognised functions of polyamines in the processes taking place in living organisms include not only the effect on cell growth and differentiation or cell death, but also the protection of nucleic acids against damage caused by reactive oxidative species (ROS) generated by different substances and also by transition metal ions Cu(II) and Fe(II) [260–[262]. Moreover, the influence of BBR3464 complex on the shortening of cell lifetimes was much lower than that produced by cisplatin [253].
The application of polynuclear compounds has brought promising therapeutic effects in the treatment of melanoma, pancreatic, lung, and ovarian cancers [263–[265]. The cytotoxic effect on the cell lines of human cancer (HSC-3) has been also checked for the dinuclear complex of Pd(II) spermine (Spm) chelate, (PdCl2)2(Spm) and compared with the effect of the earlier described Pt(II) analogues [215]. The results confirmed higher cytotoxic effect of the compounds with Pd(II) than those with Pt(II) [266].
It is known that Pd(II) complexes are usually very labile and their deactivation by cis-trans isomerisation is unlikely. Spermine makes extremely strong chelate bonds with Pd(II) because of a high chelating effect [240]. Spm, while strongly bonded to Pd(II), imposes the cis coordination on the labile ligands such as e.g. Cl–, and prevents such deactivation. Deactivation as a result of interaction with the cell components other than DNA, thanks to the high lability of Pd(II) complexes, is possible.
For the complexes with Pd(II), the range of ligands undergoing exchange is much wider than for the analogous species with Pt(II). It is important that inside the cell, Figure. 16, (PdCl2)2(Spm) undergoes a fast hydrolysis that permits the appearance of hydrated species (e.g. thiols) that interact with cell components other than DNA more strongly and earlier than with DNA. Although the rate of water exchange is 106 times higher for Pd(H2O)42+ than Pt(H2O)42+ [240, 250], for other systems the Pd(II) and Pt(II) complexes may show the same rate of ligand exchange [268–[270].

Tentetive mode of coordination in (PdCl2)2(Spm).
The effect of polyamine analogues, N1,N11-bis(ethyl)norspermine (BENSpm) and N1-cyclo-propylmethyl-N11-ethylnorspermine (CPENSpm), as well as the synthesised dinuclear complexes Pd2(BENSpm), Pt2(CPENSpm), and Pd2(Spm) on the heathy cells of breast epithelial MCF-10A and the breast cancer cell lines JIMT-1 and L56BR-C1, has been studied to evaluate their activities. It has been established that palladination of BENSpm increases cytotoxicity, while platination of CPENSpm leads to its reduction. Moreover, Pd2(BENSpm) was the most effective compound damaging DNA and reducing the population of bacteria colony. However, Pt2(CPENSpm) and Pd2(Spm) show lower toxicity in all tests. Pd2(Spm) efficiently reduces the level of cell glutathione, which probably causes their metabolic deactivation by linking thiols to their endoge-nic parts. The formation of many cross-link bonds connectivities with DNA leads to distortion of the DNA particle, thus the key biological processes such as DNA replication and transcription are inhibited, which causes inappropriate synthesis of proteins and inhibition of cell proliferation [271, 272]. The problems related to side effects such as neurotoxicity, nephrotoxicity, and development of an acquired resistance to cisplatin are the main factors limiting the effectiveness of therapy with this drug [271, 272]. Another group of profound interest in experimental cancer research is that of polyamine analogues [273].
Platinum-based therapeutic drugs, such as cisplatin, are very effective but show undesirable side effects, and their effectiveness is limited to a few types of cancer cells. Therefore, intense research work on development of new drugs of a wider range of activity and lower toxicity is continued. The effectiveness of cisplatin and its analogues (II generation drugs) as anticancer drugs make them the basis for designing alternative drugs showing other modes of activity and less acute side effects [274–[277]. The medical use of such metals as Ag, Au, and Cu is well known. Au(III) complexes with porphyrin, dithiocarbamate, and polyamine show in vitro high stability constants and much effort has been made to evaluate the anticancer effects of such complexes [278–[280].
As yet, the main group of inorganic anticancer drugs includes the compounds containing platinum (II) and platinum (IV), palladium (II), gold (I) and gold (III), ruthenium (II) and ruthenium (III), bismuth (III), rhenium (I), and copper (II) as well as gallium (III) and tin (IV). Many of them show in vitro much higher cytotoxicity than cisplatin [281].
This article is also available in: Jastrząb, Tylkowski, New-Generation Bioinorganic Complexes. De Gruyter (2016), isbn 978-3-11-034890-3.
References
[1] Gerner EW, Meyskens FL Jr. Polyamines and cancer: Old molecules, new understanding. Nat Rev Cancer 2004;4:781–92.10.1038/nrc1454Search in Google Scholar
[2] Cohen SSA. Guide to the Polyamines. Oxford University Press, New York, USA, 1998.Search in Google Scholar
[3] Marton LJ, Pegg AE. Polyamines as targets for therapeutic intervention. Annu Rev Pharmacol Toxicol 1995;35:55–91.10.1146/annurev.pa.35.040195.000415Search in Google Scholar
[4] Pegg AE. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res 1988;48:759–74.Search in Google Scholar
[5] Wallace HM, Fraser AV, Hughes A. A perspective of polyamine metabolism. Biochem J 2003;376(Pt 1):1–14.10.1042/bj20031327Search in Google Scholar
[6] Ganem B. New chemistry of naturally occurring polyamines. Acc Chem Res 1982;15:290–8.10.1021/ar00081a004Search in Google Scholar
[7] Yamamoto S, Koumoto Y, Shikami S, Shinoda S. Effect of norspermidine and its related triamines on the cell-free polyphenylalanine synthesizing system from Vibrio parahaemolyticus. Microbiol Immunol 1990;34:575–85.10.1111/j.1348-0421.1990.tb01034.xSearch in Google Scholar
[8] Lomozik L, Gasowska A, Bregier-Jarzebowska R, Jastrzab R. Coordination chemistry of polyamines and their interactions in ternary systems including metal ions, nucleosides and nucleotides. Coord Chem Rev 2005;249:2335–50.10.1016/j.ccr.2005.05.002Search in Google Scholar
[9] Bachrach H. The early history of polyamine research. Plant Physiol Biochem 2010;48:490–5.10.1016/j.plaphy.2010.02.003Search in Google Scholar
[10] Raina A, Jänne J. Physiology of the natural polyamines putrescine, spermidine and spermine. Med Biol 1975;53:121–47.Search in Google Scholar
[11] Scherer P, Kneifel H, Bacteriol J. Distribution of polyamines in methanogenic bacteria. J Bacteriol 1983;154:1315–22.10.1128/jb.154.3.1315-1322.1983Search in Google Scholar
[12] Bratek-Wiewiorowska MD, Alejska M, Figlerowicz M, Barciszewski J, Wiewiorowski M, Jaskolski M. Interaction of polyamines, their protonated salts and metal complexes with nucleic acid fragments. Pure Appl Chem 1987;59:407–14.10.1351/pac198759030407Search in Google Scholar
[13] Antonelli ML, Balzamo S, Carunchio V, Cernia E, Purrello R. Thermodynamics of simple and mixed complexes of copper(II) with adenosine 5′-monophosphate and spermine. J Inorg Biochem 1988;32:153–61.10.1016/0162-0134(88)80023-0Search in Google Scholar
[14] Tabor H, Tabor CW. Spermidine, spermine, and related amines. Pharmacol Rev 1964;16:245–300.Search in Google Scholar
[15] Jänne J, Alhonen L, Leinonen P. Polyamines: From molecular biology to clinical applications. Ann Med 1991;23:241–59.10.3109/07853899109148056Search in Google Scholar
[16] Manni A. Involvement of the polyamine pathway in breast cancer progression. Cancer Lett 1995;92:49–57.10.1016/0304-3835(95)03763-MSearch in Google Scholar
[17] Gupta S, Ahmad N, Marengo SR, MacLennan GT, Greenberg NM, Mukhtar H. Chemoprevention of prostate carcinogenesis by alpha-difluoromethylrnithine in TRAMP mice. Cancer Res 2000;60:5125–33.Search in Google Scholar
[18] Gilmour SK. Polyamines and nonmelanoma skin cancer. Toxic Appl Pharm 2007;224:249–56.10.1016/j.taap.2006.11.023Search in Google Scholar PubMed PubMed Central
[19] Upp JR Jr, Saydjari R, Townsend CM Jr, Singh P, Barranco SC, Thompson JC. Polyamine levels and gastrin receptors in colon cancers. Ann Surg 1989;207:662–9.10.1097/00000658-198806000-00004Search in Google Scholar PubMed PubMed Central
[20] Williams-Ashman HG, Canellakis ZN. Polyamines in mammalian biology and medicine. Perspect Biol Med 1979;22:421–53.10.1353/pbm.1979.0013Search in Google Scholar PubMed
[21] Jänne J, Raina A, Siimes M. Spermidine and spermine in rat tissues at different ages. Acta Physiol Scand 1964;62:352–8.10.1111/j.1748-1716.1964.tb10433.xSearch in Google Scholar PubMed
[22] Shukla OP. Polyamine metabolism as a target for chemotherapy of parasite infection. J Sci Ind Res 1990;49:263–82.Search in Google Scholar
[23] Frydman B, Westler WM, Samejima K. Spermine binds in solution to the TpsiC loop of tRNA(Phe): Evidence from a 750 MHz (1)H-NMR analysis. J Org Chem 1996;61:2588–9.10.1021/jo9601775Search in Google Scholar PubMed
[24] Feuerstein BG, Williams LD, Basu HS, Marton LJ. Implications and concepts of polyaminenucleic acid interactions. J Cell Biochem 1991;46:37–47.10.1002/jcb.240460107Search in Google Scholar PubMed
[25] Hobbs CA, Gilmour SK. In polyamine cell signaling: Physiology, pharmacology, and cancer research. In: Wang J-Y. & Casero RA, eds. Totowa: Humana Press; 2006;75–89.10.1007/978-1-59745-145-1_5Search in Google Scholar
[26] Ha HC, Sirisoma NS, Kuppusamy P, Zweier JL, Woster PM, Casero RA Jr. The natural polyamine spermine functions directly as a free radical scavenger. Proc Natl Acad Sci USA 1998;95:11140–5.10.1073/pnas.95.19.11140Search in Google Scholar PubMed PubMed Central
[27] Kurata HT, Marton LJ, Nichols CG. The polyamine binding site in inward rectifier K+ channels. J Gen Physiol 2006;127:467–80.10.1085/jgp.200509467Search in Google Scholar
[28] Park MH. The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A). J Biochem-Tokyo 2006;139:161–9.10.1093/jb/mvj034Search in Google Scholar
[29] Park JH, Aravind L, Wolf EC, Kaevel J, Kim YS, Park MH. Molecular cloning, expression, and structural prediction of deoxyhypusine hydroxylase: A HEAT-repeat-containing metalloenzyme. Proc Natl Acad Sci USA 2006;103:51–6.10.1073/pnas.0509348102Search in Google Scholar
[30] Tobias KE, Shor J, Kahana C. c-Myc and Max trans regulate the mouse ornithine decarboxylase promoter through interaction with two downstream CACGTG motifs. Oncogene 1995;11:1721–7.Search in Google Scholar
[31] Shantz LM, Levin VA. Regulation of ornithine decarboxylase during oncogenic transformation: Mechanisms and therapeutic potential. Amino Acids 2007;33:213–23.10.1007/s00726-007-0531-2Search in Google Scholar
[32] Holtta E, Sistonen L, Alitalo K. The mechanisms of ornithine decarboxylase deregulation in c-Ha-ras oncogene-transformed NIH 3T3 cells. J Biol Chem 1988;263:4500–7.10.1016/S0021-9258(18)68954-9Search in Google Scholar
[33] Ignatenko NA, Babbar N, Mehta D. Suppression of polyamine catabolism by activated Ki-ras in human colon cancer cells. Mol Carcinog 2004;39:91–102.10.1002/mc.10166Search in Google Scholar
[34] Celano P, Berchtold CM, Giardiello FM, Casero RA Jr. Modulation of growth gene expression by selective alteration of polyamines in human colon carcinoma cells. Biochem Biophys Res Commun 1989;165:384–90.10.1016/0006-291X(89)91082-6Search in Google Scholar
[35] Packham G, Bello-Fernandez C, Cleveland JL. Position and orientation independent transactivation by c-Myc. Cell Mol Biol Res 1994;40:699–706.Search in Google Scholar
[36] Tabib A, Bachrach U. Activation of the protooncogene c-myc and c-fos by c-ras: Involvement of polyamines. Biochem Biophys Res Commun 1994;202:720–7.10.1006/bbrc.1994.1990Search in Google Scholar
[37] Nilsson JA, Maclean KH, Keller UB, Pendeville H, Baudino TA, Cleveland JL. Mnt loss triggers Myc transcription targets, proliferation, apoptosis, and transformation. Mol Cell Biol 2004;24:1560–9.10.1128/MCB.24.4.1560-1569.2004Search in Google Scholar
[38] Sauders LR, Verdin E. Polyamines regulate sensitivity to HDAC inhibitor-induced apoptosis. Proc Am Assoc Cancer Res 2006;47:1093.Search in Google Scholar
[39] Harik SI, Snyder SH. Putrescine: Regional distribution in the nervous system of the rat and the cat. Brain Res 1974;66:328–31.10.1016/0006-8993(74)90152-8Search in Google Scholar
[40] Shaw GG, Pateman AJ. The regional distribution of the polyamines spermidine and spermine in brain. J Neurochem 1973;20:1225–30.10.1111/j.1471-4159.1973.tb00091.xSearch in Google Scholar
[41] Tabor CW, Tabor H. Polyamines. Annu Rev Biochem 1984;53:749–90.10.1146/annurev.bi.53.070184.003533Search in Google Scholar
[42] Seiler N. Polyamines. J Chromatogr 1986;379:157–76.10.1007/978-1-4757-0614-7_9Search in Google Scholar
[43] Schuber F. Influence of polyamines on membrane functions. Biochem J 1989;260:1–10.10.1042/bj2600001Search in Google Scholar
[44] Tabor CW, Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem 1976;45:285–306.10.1146/annurev.bi.45.070176.001441Search in Google Scholar
[45] Jänne J, Pösö H, Raina A. Polyamines in rapid growth and cancer. Biochim Biophys Acta 1978;473:241–93.10.1016/0304-419X(78)90015-XSearch in Google Scholar
[46] Baze PE, Milano G, Verrando P, Renée N, Ortonne JP. Polyamine levels in normal human skin. A comparative study of pure epidermis, pure dermis, and suction blister fluid. Arch Dermatol Res 1983;275:218–21.10.1007/BF00416663Search in Google Scholar PubMed
[47] Pegg AE. Regulation of ornithine decarboxylase. J Biol Chem 2006;281:14529–32.10.1074/jbc.R500031200Search in Google Scholar PubMed
[48] Takeda Y, Sameima K, Nagano k, Watanabe M, Sugeta H, Kyogoku Y. Determination of protonation sites in thermospermine and in some other polyamines by 15N and 13C nuclear magnetic resonance spectroscopy. Eur J Biochem 1983;130:383–9.10.1111/j.1432-1033.1983.tb07164.xSearch in Google Scholar PubMed
[49] Matthews HR. Polyamines, chromatin structure and transcription. Bioessays 1993;15: 561–6.10.1002/bies.950150811Search in Google Scholar PubMed
[50] Cohen SS. What do polyamines do? Nature 1978;274:209–10.10.1038/274209a0Search in Google Scholar
[51] Saenger W. Principles of nucleic acid structure. New York, Springer Verlag, 1984.10.1007/978-1-4612-5190-3Search in Google Scholar
[52] Braunlin WH, Strick TJ, Record MT. Equilibrium dialysis studies of polyamine binding to DNA Record. Biopolymers 1982;21:1301–14.10.1002/bip.360210704Search in Google Scholar
[53] Fang Y, Hoh JH. Early intermediates in spermidine-induced DNA condensation on the surface of mica. J Am Chem Soc 1998;120:8904–9.10.1021/ja981332vSearch in Google Scholar
[54] Matsui I, Wiegand L, Pegg AE. Properties of spermidine N-acetyltransferase from livers of rats treated with carbon tetrachloride and its role in the conversion of spermidine into putrescine. J Biol Chem 1981;256:2454–9.10.1016/S0021-9258(19)69802-9Search in Google Scholar
[55] Pegg AE, Matsui I, Seely JE, Pritchard ML, Poso H. Formation of putrescine in rat liver. Med Biol 1981;59:327–33.Search in Google Scholar
[56] Persson L, Pegg AE. Studies of the induction of spermidine/spermine N1-acetyltransferase using a specific antiserum. J Biol Chem 1984;259:12364–7.10.1016/S0021-9258(18)90754-4Search in Google Scholar
[57] Casero RA Jr, Pegg AE. Spermidine/spermine N1-acetyltransferase the turning point in polyamine metabolism. FASEB J 1993;7:653–61.10.1096/fasebj.7.8.8500690Search in Google Scholar
[58] Casero RA Jr, Celano SJ, Ervin NB, Applegren L, Wiest, Pegg AE. Isolation and characterization of a cDNA clone that codes for human spermidine/spermine N1-acetyltransferase. J Biol Chem 1991;266:810–4.10.1016/S0021-9258(17)35245-6Search in Google Scholar
[59] Casero RA, Pegg AE. Polyamine catabolism and disease. Biochem J 2005;421:323–38.10.1042/BJ20090598Search in Google Scholar PubMed PubMed Central
[60] Holtta E. Oxidation of spermidine and spermine in rat liver: Purification and properties of polyamine oxidase. Biochem 1977;16:91–100.10.1021/bi00620a015Search in Google Scholar PubMed
[61] Holtta E. Polyamine oxidase (rat liver). Methods Enzymol 1983;94:306–11.10.1016/S0076-6879(83)94054-5Search in Google Scholar
[62] Vujcic S, Liang P, Diegelman P, Kramer DL, Porter CW. Genomic identification and biochemical characterization of the mammalian polyamine oxidase involved in polyamine back-conversion. Biochem J 2003;370:19–28.10.1042/bj20021779Search in Google Scholar
[63] Wu T, Yankovskaya V, McIntire WS. Cloning, sequencing, and heterologous expression of the murine peroxisomal flavoprotein, N1-acetylated polyamine oxidase. J Biol Chem 2003;278:20514–25.10.1074/jbc.M302149200Search in Google Scholar
[64] Wang Y, Hacker A, Murray-Steward T. Properties of recombinant human N1-acetylpolyamine oxidase (hPAO): Potential role in determining drug sensitivity. Cancer Chemother Pharmacol 2005;56:83–90.10.1007/s00280-004-0936-5Search in Google Scholar
[65] Wallace HM, Duthie J, Evans DM, Lamond S, Nicoll KM, Heys SD. Alterations in polyamine catabolic enzymes in human breast cancer tissue. Clin Cancer Res 2000;6:3657–61.Search in Google Scholar
[66] Xie X, Gillies RJ, Gerner EW. Characterization of a diamine exporter in Chinese hamster ovary cells and identification of specific polyamine substrates. J Biol Chem 1997;272: 20484–9.10.1074/jbc.272.33.20484Search in Google Scholar
[67] Wang Y, Devereux W, Woster PM, Stewart TM, Hacker A, Casero RA Jr. Cloning and characterization of a human polyamine oxidase that is inducible by polyamine analogue exposure. Cancer Res 2001;61:5370–3.Search in Google Scholar
[68] Vujcic S, Diegelman P, Bacchi CJ, Kramer DL, Porter CW. Identification and characterization of a novel flavin-containing spermine oxidase of mammalian cell origin. Biochem J 2002;367:665–75.10.1042/bj20020720Search in Google Scholar
[69] Wang Y, Murray-Stewart T, Devereux W, Hacker A, Frydman B, Woster PM, Casero RA Jr. Properties of purified recombinant human polyamine oxidase, PAOh1/SMO. Biochem Bioph Res Com 2003;304:605–11.10.1016/S0006-291X(03)00636-3Search in Google Scholar
[70] Babbar N, Casero RA. Tumor necrosis factor-alpha increases reactive oxygen species by inducing spermine oxidase in human lung epithelial cells: A potential mechanism for inflammation-induced carcinogenesis. Cancer Res 2006;66:11125–30.10.1158/0008-5472.CAN-06-3174Search in Google Scholar PubMed
[71] Goodwin AC, Shields CED, Wu S, Huso DL, Wu X, Murray-Stewart TR, Hacker-Prietz A, Rabizadeh S, Woster PM, Sears CL, Casero RA. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilisinduced colon tumorigenesis. P Natl Acad Sci USA 2008;108:15354–9.10.1073/pnas.1010203108Search in Google Scholar PubMed PubMed Central
[72] Casero RA Jr, Marton LJ. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev Drug Discov 2007;6:373–90.10.1038/nrd2243Search in Google Scholar PubMed
[73] Pegg AE. Mammalian polyamine metabolism and function. IUBMB Life 2009;61:880–94.10.1002/iub.230Search in Google Scholar
[74] Wallace HM. The physiological role of the polyamines. Eur J Clin Invest 2000;30:1–3.10.1046/j.1365-2362.2000.00585.xSearch in Google Scholar
[75] Soulet D, Gagnon B, Rivest S, Audette M, Poulin RA. Fluorescent probe of polyamine transport accumulates into intracellular acidic vesicles via a twostep mechanism. J Biol Chem 2004;279:49355–66.10.1074/jbc.M401287200Search in Google Scholar
[76] Belting M, Persson S, Fransson LA. Proteoglycan involvement in polyamine uptake. Biochem J 1999;338:317–23.10.1042/bj3380317Search in Google Scholar
[77] Belting M, Mani K, Jönsson M, Cheng F, Sandgren S, Jonsson S, Ding K, Delcros JG, Fransson LA. Glypican-1 is a vehicle for polyamine uptake in mammalian cells: A pivital role for nitrosothiol-derived nitric oxide. J Biol Chem 2003;278:47181–9.10.1074/jbc.M308325200Search in Google Scholar
[78] Poulin R, Casero RA, Soulet D. Recent advances in the molecular biology of metazoan polyamine transport. Amino Acids 2012;42:711–23.10.1007/s00726-011-0987-ySearch in Google Scholar
[79] Cragg GM, Grothaus PG, Newman DJ. Impact of natural products on developing new anti-cancer agents. Chem Rev 2009;109:3012–43.10.1021/cr900019jSearch in Google Scholar
[80] Casero RA Jr, Wang Y, Stewart TM, Devereux W, Hacker A, Wang Y, Smith R, Woster PM. The role of polyamine catabolism in anti-tumour drug response. Biochem Soc Trans 2003;31:361–5.10.1042/bst0310361Search in Google Scholar
[81] Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? Lancet 2001;357:539–45.10.1016/S0140-6736(00)04046-0Search in Google Scholar
[82] Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7.10.1038/nature01322Search in Google Scholar PubMed PubMed Central
[83] Holm I, Persson L, Heby O, Seiler N. Feedback regulation of polyamine synthesis in Ehrlich ascites tumor cells. Analysis using nonmetabolizable derivatives of putrescine and spermine. Biochim Biophys Acta 1988;972:239–48.10.1016/S0005-2728(88)80054-9Search in Google Scholar
[84] Anchini A, Fabbrizzi L, Barbucci R, Mastroianni A. Thermodynamic and electron spin resonance spectroscopy investigation of the co-ordinating properties of 4-azaoctane-1,8 diamine, (spermidine) in aqueous solution. J Chem Soc Dalton Trans 1977;2224–8.10.1039/dt9770002224Search in Google Scholar
[85] Horn Y, Beal SL, Walach N, Lubich WP, Spigel L, Marton LJ. Further evidence for the use of poliamines as biochemical markers for malignant tumors. Cancer Res 1982;42:3248–51.Search in Google Scholar
[86] Lomozik L. Metal complexes with polyamines. In: Berthon G, ed. Handbook of metal-ligand interaction in biological fluids, v. I, New York, Basel, Hong Kong: Marcel Dekker Inc.; 1995; 686–97.Search in Google Scholar
[87] Edwards ML, Stemerick DM, McCarthy JR. Use of the Mitsunobu reaction in the synthesis of polyamines. Tetrahedron 1994;50:5579–90.10.1016/S0040-4020(01)85630-1Search in Google Scholar
[88] Thomas T, Thomas TJ. Polyamines in cell growth and cell death: Molecular mechanisms and therapeutic applications. Cell Mol Life Sci 2001;58:244–58.10.1007/PL00000852Search in Google Scholar PubMed
[89] Russell D, Snyder SH. Amine synthesis in rapidly growing tissues: Ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. P Natl Acad Sci USA 1968;60:1420–7.10.1073/pnas.60.4.1420Search in Google Scholar PubMed PubMed Central
[90] Giardiello FM, Hamilton SR, Hylind LM. Ornithinedecarboxylase and polyamines in familial adenomatous polyposis. Cancer Res 1997;57:199–201.Search in Google Scholar
[91] Elmets CA, Athar M. Targeting ornithine decarboxylase for the prevention of nonmelanoma skin cancer in humans. Cancer Prev Res 2010;3:8–11.10.1158/1940-6207.CAPR-09-0248Search in Google Scholar PubMed PubMed Central
[92] Manni A, Mauger D, Gimotty P, Badger B. Prognostic influence on survival of increased ornithine decarboxylase activity in human breast cancer. Clin Cancer Res 1996;2:1901–6.Search in Google Scholar
[93] Chaturvedi R, Asim M, Romero-Gallo J, Barry DP, Hoge S, de Sablet T, Delgado AG, Wroblewski LE, Piazuelo MB, Yan F, Israel DA, Casero RA Jr, Correa P, Gobert AP, Polk DB, Peek RM Jr, Wilson KT. Spermine oxidase mediates the gastric cancer risk associated with Helicobacter pylori CagA. Gastroenterology 2011;141:1696–708.10.1053/j.gastro.2011.07.045Search in Google Scholar PubMed PubMed Central
[94] Xu H, Chaturvedi R, Cheng Y, Bussiere FI, Asim M, Yao MD, Potosky D, Meltzer SJ, Rhee JG, Kim SS, Moss SF, Hacker A, Wang Y, Casero RA, Wilson KT. Spermine oxidation induced by Helicobacter pylori results in apoptosis and DNA damage: Implications for gastric carcinogenesis. Cancer Res 2004;64:8521–5.10.1158/0008-5472.CAN-04-3511Search in Google Scholar PubMed
[95] Goodwin AC, Jadallah S, Toubaji A, Lecksell K, Hicks JL. Increased spermine oxidase expression in human prostate cancer and prostatic intraepithelial neoplasia tissues. Prostate 2008;68:766–72.10.1002/pros.20735Search in Google Scholar PubMed PubMed Central
[96] Alhonen L, Halmekyto M, Kosma VM. Life-long over-expression of ornithine decarboxylase (ODC) gene in transgenic mice does not lead to generally enhanced tumorigenesis or neuronal degeneration. Int J Cancer 1995;63:402–4.10.1002/ijc.2910630317Search in Google Scholar PubMed
[97] Huang L, Zhu C, Sun Y, Xie G, Mackenzie GG, Qiao G, Komninou D, Rigas B. Phospho-sulindac (OXT-922) inhibits the growth of human colon cancer cell lines: A redox/polyamine-dependent effect. Carcinogenesis 2010;31:1982–90.10.1093/carcin/bgq149Search in Google Scholar
[98] Babbar N, Ignatenko NA, Casero RA Jr, Gerner EW. Cyclooxygenase-independent induction of apoptosis by sulindac sulfone is mediated by polyamines in colon cancer. J Biol Chem 2003;278:47762–75.10.1074/jbc.M307265200Search in Google Scholar
[99] Senanayake MDT, Amunugama H, Boncher TD, Casero RA, Woster PM. Design of polyamine-based therapeutic agents: new targets and new directions. Essays Biochem 2009;46:77–94.10.1042/bse0460006Search in Google Scholar
[100] Palmer BN, Pawell HKJ. Complex Formation between 4,9-diazadodecane-1,12-diamine (spermine) and copper(II) ions and protons in aqueous solution. J Chem Soc Dalton Trans 1974:2086–9.10.1039/dt9740002086Search in Google Scholar
[101] Palmer BN, Pawell HKJ. Polyamine complexes with seven-membered chelate rings: Complex formation of 3-azaheptane-1,7-diamine, 4-azaoctane-1,8-diamine (spermidine), and 4,9-diazadodecane-1,12-diamine (spermine) with copper(II) and hydrogen ions in aqueous solution. J Chem Soc Dalton Trans 1974;2089–92.10.1039/dt9740002089Search in Google Scholar
[102] Bunce S, Kong ESW. The interactions between nucleic acids and polyamines. I. High resolution carbon-13 and hydrogen-1 nuclear magnetic resonance studies of spermidine and 5′-AMP. Biophys Chem 1978;8:357–68.10.1016/0301-4622(78)80017-9Search in Google Scholar
[103] Nakai C, Glinsmann W. Interactions between polyamines and nucleotides. Biochem 1977;16:5636–41.10.1021/bi00644a039Search in Google Scholar PubMed
[104] Corazza A, Di Paolo ML, Skarpa M, Zennaro L, Rigo A. Interactions between polyamines and nucleotides studied by 31P and 1H NMR. Apel Magn Reson 1994;7:89–94.10.1007/BF03162549Search in Google Scholar
[105] Manning GS. Limiting laws and counterion condensation in polyelectrolyte solutions. 8. Mixtures of counterions, species selectivity, and valence selectivity. J Phys Chem 1984;88:6654–61.10.1021/j150670a030Search in Google Scholar
[106] Manning GS. Is the counterion condensation point on polyelectrolytes a trigger of structural transition? J Phys Chem 1988;85:3772–7.10.1063/1.454899Search in Google Scholar
[107] Kimura E, Kodama m, Yatsunami T. Macromonocyclic polyamines as biological polyanion complexons. 2. Ion-pair association with phosphate and nucleotides. J Am Chem Soc 1982;104:3182–7.10.1021/ja00375a042Search in Google Scholar
[108] Esposito D, Del Veccio P, Barone G. Interactions with natural polyamines and thermal stability of DNA. A DSC study and a theoretical reconsideration. J Am Chem Soc 1997;119:2606–13.10.1021/ja962449rSearch in Google Scholar
[109] Lomozik L, Gasowska A, Bolewski L. Noncovalent interactions In polyamine/nucleoside (or diaminocarboxylate) systems studied by potentiometric and NMR techniques. J Chem Soc Perkin Trans 1997;2:1161–5.10.1039/a607656dSearch in Google Scholar
[110] Gasowska A, Lomozik L, Jastrzab R. Mixed ligand complexes of copper(II) ions with AMP and CMP In the systems with polyamines and non-covalent interaction between bioligands. J Inorg Biochem 2000;78:139–47.10.1016/S0162-0134(99)00223-8Search in Google Scholar
[111] Lomozik L, Gasowska A. Complexes of copper(II) with spermine and non-covalent interactions in the systems including nucleosides and nucleotides. J Inorg Biochem 1998;72:37–47.10.1016/S0162-0134(98)10060-0Search in Google Scholar
[112] Bregier-Jarzebowska R, Lomozik L. Noncovalent interactions and copper (II) coordination in systems containing L-aspartic acid and triamines. Polyhedron 2010;29:3294–303.10.1016/j.poly.2010.09.005Search in Google Scholar
[113] Bregier-Jarzebowska R, Gasowska A, Jastrzab R, Lomozik L. Noncovalent interactions and coordination reaction In the systems containing of copper(II) ions, aspartic acid and diamines. J Inorg Biochem 2009;103:1228–35.10.1016/j.jinorgbio.2009.07.001Search in Google Scholar
[114] Lomozik L, Jastrzab R, Gasowska A. Interactions in binary and ternary systems including Cu(II), uridine, uidine 5’-monophosphate or diamine. Polyhedron 2000;19:1145–54.10.1016/S0277-5387(00)00375-2Search in Google Scholar
[115] Lomozik L. Jastrzab R. Non-covalent and coordination interactions in Cu(II) systems with uridine, uridine 5’-monophosphate and triamine as biogenic amine analogues in aqueous solution. J Inorg Biochem 2003;97:179–90.10.1016/S0162-0134(03)00276-9Search in Google Scholar
[116] De Robertis A, De Stefano C, Patane G. Salt effects on the protonation of diethylenetriamine: A complex formation model. Termochem Acta 1992;209:7–24.10.1016/0040-6031(92)80180-5Search in Google Scholar
[117] De Robertis, De Stefano C, Gianguzza A, Sammartano S. Binding of polyanions by biogenic amines. I. Formation and stability of protonated putrescine and cadaverine complexes with inorganic anions. Talanta 1998;46:1085–93.10.1016/S0039-9140(97)00388-3Search in Google Scholar
[118] De Robertis, De Stefano C, Patane G, Sammartano S. Effects of salt on the protonation in aqueous solution of triethylenetetramine and tetraethylenepentamine. J Sol Chem 1993;22:927–40.10.1007/BF00646604Search in Google Scholar
[119] Maruyoshi K, Yamaguchi T, Demura T, Matsumori N, Oishi T, Murata M. Conformations of spermine in adenosine triphosphate complex: The structural basis for weak bimolecular interactions of major cellular electrolytes. Chem Eur J 2011;17:4788–95.10.1002/chem.201002759Search in Google Scholar PubMed
[120] Maruyoshi K, Nonaka K, Sagane T, Demura T. Yamaguchi T, Matsumori N, Oishi T, Murata M. Conformational change of spermidine upon interacting with ATP in aqueous solution. Chem Eur J 2009;15:1618–26.10.1002/chem.200801961Search in Google Scholar PubMed
[121] Bregier-Jarzebowska R. Complexes of copper(II) with L-aspartic acid in systems with tetramines and non-covalent interactions between bioligands. J Coord Chem 2013;66: 1287–302.10.1080/00958972.2013.780050Search in Google Scholar
[122] Yamauchi O, Odani A, Hirota S. Metal ion-assisted weak interactions involving biological molecules. From small complexes to metalloproteins. Bull Chem Soc Jpn 2001;1525:45.10.1246/bcsj.74.1525Search in Google Scholar
[123] Yamauchi O, Odani A, Masuda H, Sigel H. Stacking interactions involving nucleotides and metal ion complexes. In: Sigel A, Sigel H, eds. Metal ions in biological systems. 32 New York: Marcel Dekker; 1996;207–70.Search in Google Scholar
[124] Ouameur AA, Tajmir-Riahi H-A. Structural analysis of DNA interactions with biogenic polyamines and cobalt(III)-hexamine studied by Fourier transform infrared and capillary electrophoresis. J Biol Chem 2004;279:42041–51.10.1074/jbc.M406053200Search in Google Scholar PubMed
[125] Terrier P, Tortajada J, Zin G, Buchmann W. Noncovalent complexes between DNA and Basic polypeptides or polyamines by MALDI-TOF. J Am Soc Mass Spectrm 2007;18:1977–89.10.1016/j.jasms.2007.07.028Search in Google Scholar PubMed
[126] Terrier P, Tortajada J, Zin G, Buchmann W. A study of noncovalent complexes ilvolving single-stranded DNA and polybasic compounds using nanospray mass spectrometry. J Am Soc Mass Spectrm 2007;18:346–58.10.1016/j.jasms.2006.09.027Search in Google Scholar PubMed
[127] Drozdzal P, Gilski M, Kierzek R, Lomozik L, Jaskolski M. Ultrahigh resolution crystal structures of Z-DNA In complex with Mn2+ and Zn2+ ions. Acta Cryst 2013;D69:1180–90.10.1107/S0907444913007798Search in Google Scholar PubMed
[128] N’Soukpoé-Kossi CN, Ouameur AA, Thomas T, Shirahata A, Thomas TJ, Tajmir-Riahi HA. DNA interaction with antitumor polyamine analogues: A comparison with biogenic polyamines. Biomacromolecules 2008;9:2712–8.10.1021/bm800412rSearch in Google Scholar PubMed
[129] Mandeville J-S, Bourassa P, Thomas TJ, Tajmir-Riahi HA. Biogenic and sytthetic polyamines bind cationic dendrimers. PLoS ONE 2012;7:e36087.10.1371/journal.pone.0036087Search in Google Scholar PubMed PubMed Central
[130] Odani A, Jastrzab R, Lomozik L. Equilibrium study on the interaction of phytic acid with polyamines and metal ions. Metallomics 2011;3:735–43.10.1039/c1mt00031dSearch in Google Scholar PubMed
[131] De Stefano C, Giuffrè O, Milea D, Rigano C, Sammartano S. Speciation of phytate ion in aqueous solution. Noncovalent interactions with biogenic polyamines. Chem Spec Bioavailab 2002;15:29–36.10.3184/095422903782775235Search in Google Scholar
[132] Toro-Funes N, Bosch-Fuste J, Veciana-Nogues MT, Izquierdo-Pulido M, Vidal-Carou MC. In vitro antioxidant activity of dietary polyamines. Food Res Int 2013;51:141–7.10.1016/j.foodres.2012.11.036Search in Google Scholar
[133] Moinard C, Cynober L, de Bandt J-P. Polyamines: Metabolism and implications in human diseases. Clin Nutr 2005;24:184–97.10.1016/j.clnu.2004.11.001Search in Google Scholar PubMed
[134] Minois N, Carmona-Gutierrez D, Madeo F. Polyamines in aging and disease. Aging 2011;3:716–32.10.18632/aging.100361Search in Google Scholar PubMed PubMed Central
[135] Pegg AE. Toxicity of polyamines and their metabolic products. Chem Res Toxicol 2011;26:1782–899.10.1021/tx400316sSearch in Google Scholar PubMed
[136] Pegg AE. The function of spermine. IUBMB Life 2014;66:8–18.10.1002/iub.1237Search in Google Scholar PubMed
[137] House DA. Ammonia and amines. In: Wilkinson G, Gillard RD, McCleverty J, eds. Comprehensive Coordination Chemistry. Oxford: Pergamon Press; 1987;23–72.Search in Google Scholar
[138] Gollogly JR, Hawkins CJ, Beattie JK. Conformational analysis of coordination compounds. IV. Conformational energies and activation energies for ring inversion of ethylenediamine complexes. Inorg Chem 1971;317–23.10.1021/ic50096a020Search in Google Scholar
[139] Alexander MD, Kilcrease HG. Binuclear chloropentaaminecobalt(III) complexes. J Inorg Nucl Chem 1973;35:1583–90.10.1016/0022-1902(73)80249-0Search in Google Scholar
[140] Lomozik L, Bolewski L, Dworczak-Jastrzab R. Complex formation in copper(II) ternary systems involving polyamines and diaminocarboxylates studied by potentiometric and spectroscopic techniques. J Coord Chem 1997;41:261–74.10.1080/00958979708045504Search in Google Scholar
[141] Ogino H, Orihara Y, Tanaka N. Syntheses and properties of pentaamminecobalt(III) complexes containing N-methyl-, N,N-dimethyl-, and N,N′-dimethylethylenediamine and (2-aminoethyl)trimethylammonium ion. Inorg Chem 1980;3178–80.10.1021/ic50212a071Search in Google Scholar
[142] Ogino H. Acid-base properties of (alpha, omega-alkanediamine) pentaaminecobalt(III) complexes in aqueous solution. Inorg Chem 1980;19:1619–22.10.1021/ic50208a037Search in Google Scholar
[143] Lomozik L, Bolewski L, Bregier-Jarzebowska R. Stability and structure studiem of copper(II) complexes with polyamines and related ligands In aqueous solution. Polish J Chem 1995;69:197–205.Search in Google Scholar
[144] Micheloni M, Sabatini A, Vacca A. Nickel(II), copper(II) and zinc(II) complexes of 1,1,1-tris(aminomethyl)propane. A calculation procedure of stepwise formation constants and their standard errors from the Values Obtained for the Cumulative Equilibria. Inorg Chim Acta 1977;25:41–8.10.1002/chin.197803132Search in Google Scholar
[145] Boloni L, MIcheloni M, Sabatini A, Vacca A. Complex formation equilibria between 1,1,1-tris (N-methylaminomethyl)ethane and nickel(II), copper(II), zinc(II) and hydrogen ions. Inorg Chim Acta 1983;70:117–9.10.1016/S0020-1693(00)82788-7Search in Google Scholar
[146] Fluckiger JR, Schlapfer CW, Couldwell C. Preparation of [Co(η2-Htame)(η3-tame)X]n, a new type of cobalt(III)-acidopentaamine complex with a dangling amine, and the crystal structure of [Co(η2-Htame)(η3-tame)Cl]Cl 3·2H2O. Inorg Chem 1980;19:2493–500.10.1002/chin.198048066Search in Google Scholar
[147] Ahlgren M, Turpeinen U. The structure of μ-Aqua-bis(μ-trifluoroacetato-O,O′)-bis[(N,N,N′,N′-tetramethylethylenediamine)(trifluoroacetato)nickel(II)]. Acta Crystallogr B 1982;38:276–9.10.1107/S0567740882002611Search in Google Scholar
[148] Bartoszak-Adamska E, Bregier-Jarzebowska R, Lomozik L. Crystal and molecular structures of cadmium(II) nitrate complexes with triamines: 1,5-diamino-3-azapentane, 1,6-diamino-3-azahexane and 1,7-diamino-4-azaheptane. Polyhedron 2002;21:739–44.10.1016/S0277-5387(02)00848-3Search in Google Scholar
[149] Barbucci R, Fabbrizzi L, Paoletti P. Thermodynamics of complex formation with linear aliphatic tetra-amines. Part III. Enthalpy and entropy contributions to the stability of metal complexes of 4,7-diazadecane-1,10-diamine. J Chem Soc Dalton Trans 1973;1763–7.10.1039/dt9730001763Search in Google Scholar
[150] Anderegg G, Blauenstein P. Polyamines XV. Thermodynamics of metal complex formation of linear tetraamines containing two ethylenediamine residues. Helv Chim Acta 1982;65:913–23.10.1002/hlca.19820650324Search in Google Scholar
[151] Hague DN, Moreton AD. Zinc(II) complexes of 4,7-diazadecane-1,10-diamine, 4,8-diazaundecane-1,11-diamine, and tetraethylenepentamine: A carbon-13 nuclear magnetic resonance study. J Chem Soc Dalton Trans 1989:1171–7.10.1039/dt9890001171Search in Google Scholar
[152] Cassol A, Di Bernardo P, Zanonato P, Portanova R, Tolazzi M, Tomat G. Equilibrium measurements for silver(I) complexes with polyamines in dimethyl sulphoxide. J Chem Soc Dalton Trans 1988;1781–3.10.1039/dt9880001781Search in Google Scholar
[153] Weatherburn DC. Dissociation kinetics of metal complexes in acid. II. Copper complexes of linear polyamines. Aust J Chem 1983;36:433–9.10.1071/CH9830433Search in Google Scholar
[154] Diaz G, Bustos C, Shepherd RE. Low frequency infrared modes of copper(II) polyamine complexes and related kinetic factors of ligand dissociation. Inorg Chim Acta 1987;133:23–6.10.1016/S0020-1693(00)84364-9Search in Google Scholar
[155] House DA, Tobe ML. Kinetics of aquation and base hydrolysis of some trans-[Co(tn)2(B)Cl]n+(tn = 1,3-diaminopropane; B = OH, NCS, N3, NO2, NH3, or CN) complexes. The effect of ring size on reactivity. J Chem Soc Dalton Trans 1989:853–8.10.1039/dt9890000853Search in Google Scholar
[156] Massoud SS, Milburn RM. Kinetics of solvolysis of dihalo-(3,3′,3″-triaminotripropylamine) cobalt(III) perchlorate. Polyhedron 1989;8:415–18.10.1016/S0277-5387(00)80735-4Search in Google Scholar
[157] Lomozik L, Wojciechowska-Gasowska A. Complexation of putrescine with copper(II), zinc(II), lead(II) and magnesium(II) in aqueous solution. Polyhedron 1989;8:2645–8.10.1016/S0277-5387(00)80434-9Search in Google Scholar
[158] Tesic ZL, Janjic TJ, Malinar MJ, Celap MB. Effect of the chelate ring size of diamine cobalt(III) complexes on their RF values obtained by paper chromatography. J Chromatogr 1989;481:471–6.10.1016/S0021-9673(01)96799-8Search in Google Scholar
[159] Craighead LK, Jones P, Bryant RG. Nuclear magnetic resonance investigation of cobalt(III) outer-sphere complexes in aqueous solutions. J Phys Chem 1975;79:1868–74.10.1021/j100584a023Search in Google Scholar
[160] Juranic N, Malinar MJ, Radivoja PN, Juranic J, Celap MP. Influence of diamine ligands on the 59Co NMR chemical Shift In kobalt(III) complexes. J Serb Chem Soc 1986;51:417–20.Search in Google Scholar
[161] Romeo R, Lanza S, Minniti L, Tobe L. Kinetics of the chelate effect. Ring closing and ring opening in cis-dichloro(dimethyl sulfoxide)(3-aminopropyl)ammonium)- and -((4-aminobutyl)ammonium)platinum(II) chloride. Effect of ring size. Inorg Chem 1978;17:2436–41.10.1021/ic50187a021Search in Google Scholar
[162] Romeo R, Minniti D, Lanza S, Tobe ML. Platinum(II) complexes containing dimethylsulphoxide and linear aliphatic diamines formation of a seven-membered chelate ring. Inorg Chim Acta 1977;22:87–91.10.1016/S0020-1693(00)90904-6Search in Google Scholar
[163] Farrell N, Qu Y. Chemistry of bis(platinum) complexes. Formation of trans derivatives from tetraamine complexes. Inorg Chem 1989;28:3416–20.10.1021/ic00317a005Search in Google Scholar
[164] Pfeiffer P, Böhm A, Schmitz E. Einfaehe Metallkomplexsalze mit höheren Diaminen. Naturwissenschften 1948;35:190–1.10.1007/BF00627390Search in Google Scholar
[165] Fujita J, Ogino H. Preparation and optical resolution of tris(tetramethylenediamine) cobalt(III) complex. Chem Lett 1974:57–8.10.1246/cl.1974.57Search in Google Scholar
[166] Sato S, Saito Y. The crystal structure and absolute configuration of (+)589-tris-(1,4-diaminobutane)cobalt(III) bromide. Acta Crystallogr B 1975;31:1378–81.10.1107/S0567740875005237Search in Google Scholar
[167] Sato S, Saito Y, Fujita J, Ogino H. The absolute configuration of (+)589-tris(1,a-diaminobutane)-cobalt(III) ion, (+)589-[Co(tmd)3]3+. Inorg Nucl Chem Lett 1974;10:669–73.10.1016/0020-1650(74)80011-5Search in Google Scholar
[168] Kojima M, Morita K, Fujita J. Preparation and spectroscopic studies of cobalt(III) complexes containing optically active seven-membered chelate ligands. Bull Chem Soc Jpn 1981;54: 2947–55.10.1246/bcsj.54.2947Search in Google Scholar
[169] Ogino H. Cobalt(III) complexes containing large chelate rings. II. Syntheses and properties of cobalt(III)–ammine complexes containing α,ω-alkanediamines. Bull Chem Soc Jpn 1977;50:2459–63.10.1246/bcsj.50.2459Search in Google Scholar
[170] Kapanadze TS, Kokunov YV, Tsintsadze GV. Diastereoisomerism of cobalt(III) complexes with ethylenediamine, 1,3-propylenediamine and 1,4-butylenediamine. Koord Khim 1990;16:666–72.Search in Google Scholar
[171] De G, Chaudhuri NR. Thermal investigations of 1,4-butanediamine complexes of nickel(II) in the solid phase. Bull Chem Soc Jpn 1985;58:715–20.10.1246/bcsj.58.715Search in Google Scholar
[172] Weatherburn DC, Billo FJ, Jones JP, Margerum DW. Effect of ring size on the stability of polyamine complexes containing linked consecutive rings. Inorg Chem 1970;9:1557–9.10.1021/ic50088a050Search in Google Scholar
[173] Paleotti P, Fabbrizzi L, Barbucci R. Linear relation between the enthalpy of formation and the frequency of the maximum in the electronic absorption spectra of the copper(II)-tetramine complexes. Inorg Chem 1973;12:1961–2.10.1021/ic50126a064Search in Google Scholar
[174] Anichini A, Fabbrizzi L, Barbucci R, Mastroianni A. Thermodynamic and electronic and electron spin resonance spectroscopic investigation of the co-ordinating properties of 4-azaoctane-1,8-diamine (spermidine) in aqueous solution. J Chem Soc Dalton Trans 1977;2224–8.10.1039/dt9770002224Search in Google Scholar
[175] Holmes F, Williams DR. Thermodynamic considerations in co-ordination. Part III. The copper(II) and nickel(II) complexes of 1,2-diaminoethane, 1,3-diaminopropane, 4(5)-aminomethylimidazole, 4(5)-2′-aminoethylimidazole, 2-aminomethylpyridine, and 2-2′-aminoethylpyridine. J Chem Soc A 1967;1702–4.10.1039/J19670001702Search in Google Scholar
[176] Gold M, Powell HKJ. Thermochemical study of the stepwise protonation of 3-azaheptane-1,7-diamine and 4-azaoctane-1,8-diamine, and of complex formation between copper(II) and 3-azaheptane-1,7-diamine and 4,9-diazadodecane-1,12-diamine. J Chem Soc Dalton Trans 1976;230–3.10.1039/dt9760000230Search in Google Scholar
[177] Templeton DM, Sarkar B. Fletcher-Powell minimization of analytical potentiometric data by microcomputer: application to the Cu(II) complexes of biological polyamines. Can J Chem 1985;63:3122–38.10.1139/v85-515Search in Google Scholar
[178] Wojciechowska A, Bolewski L, Lomozik L. A study of polyamine complex formation with H+ Cu(II), Zn(II), Pb(II) and Mg(II) in aqueous solution. Monatsh Chem 1991;122:131–8.10.1007/BF00809357Search in Google Scholar
[179] Alves da Silva J, Felcman J, Merce ALR, Mangrich AS, Lopes RSC, Lopes CC. Study of binary and ternary complexes of copper(II) with some polyamines and adenosine 5′ triphosphate. Inorg Chim Acta 2003;356:155–66.10.1016/S0020-1693(03)00462-6Search in Google Scholar
[180] Aquilar J, Diaz P, Escarti F, Garcia-Espana E, Gil L, Soriano C, Verdejo B. Cation and anion recognition characteristics of open-chain polyamines containing ethylenic and propylenic chains. Inorg Chim Acta 2002;339:307–16.10.1016/S0020-1693(02)00947-7Search in Google Scholar
[181] Allan JR, Beaumont PC, Milburn GHW, Wood I. Structural and thermal studies of the chloro complexes of cobalt, nickel and copper with 1,6-hexanediamine. Thermochim Acta 1993;219:159–65.10.1016/0040-6031(93)80493-TSearch in Google Scholar
[182] Barbucci R, Campbell MJM, Cannas MG, Marongiu G. Seven membered chelate rings in copper(II) complexes with spermidine. Inorg Chim Acta 1980;46:135–8.10.1016/S0020-1693(00)84181-XSearch in Google Scholar
[183] Lomozik L, Bregier-Jarzebowska R. Complexes of cadmium(II) and mercury(II) with polyamines, nucleosides and nucleotides. Polish J Chem 1999;73:927–40.Search in Google Scholar
[184] Halliday RW, Court RH. Some rhodium(III) complexes of linear tetraamines. Can J Chem 1974;52:3469–73.10.1139/v74-516Search in Google Scholar
[185] House DA, Moon RP. 1,2,6-trianiono(spermidine)cobalt(III) complexes. J Inorg Nucl Chem 1981;43:2572–4.10.1016/0022-1902(81)80308-9Search in Google Scholar
[186] Bratek-Wiewiórowska MD, Alejska M, Figlerowicz M, Barciszewski J, Wiewiorowski M, Jaskolski M, Zielenkiewicz W, Zielenkiewicz A, Kamiński M. Interaction of polyamines, their protonated salts and metal complexes with nucleic acid fragments. Pure Appl Chem 1987;59:407–14.10.1351/pac198759030407Search in Google Scholar
[187] Szyfman NW, Loureiro NP, Tenorio T, Merce ALR, Mangrich AS, Rey NA, Felcman J. Study of copper(II) ternary complexes with phosphocreatine and some polyamines in aqueous solution. J Inorg Biochem 2011;105:1712–9.10.1016/j.jinorgbio.2011.09.027Search in Google Scholar
[188] De Almeida BL, Versiani O, Sousa M, Merce ALR, Mangrich AS, Felcman J. Synthesis and characterization of new ternary Cu(II)-polyamine-ATP cpmplexes. Inorg Chim Acta 2009;362:2447–51.10.1016/j.ica.2008.11.001Search in Google Scholar
[189] Gasowska A, Lomozik L. Spectroscopic and potentiometric investigation of the solution structure and stability of Ni(II) and Co(II) complexes with adenosine 5′-monophosphate and 1,12-diamino-4,9-diazadodecane (spermine) or 1,11-diamino-4,8-diazadodecane. Polyhedron 2002;21:745–51.10.1016/S0277-5387(02)00849-5Search in Google Scholar
[190] Lomozik L, Gasowska A, Bolewski L. Copper(II) ions as a factor interfering in the interaction between bioligands systems with adenosine and polyamines. J Inorg Biochem 1996;63:191–206.10.1016/0162-0134(95)00215-4Search in Google Scholar
[191] Martin RB. Nucleoside sites for transition metal ion binding. Acc Chem Res 1985;18:32–8.10.1021/ar00110a001Search in Google Scholar
[192] Lomozik L, Gasowska A, Bregier-Jarzebowska R. Coordination mode of adenosine 5’-diphosphate in ternary systems containing Cu(II), Cd(II) or Hg(II) ions and polyamines. J Inorg Biochem 2004;98:1319–30.10.1016/j.jinorgbio.2004.04.007Search in Google Scholar PubMed
[193] Martin RB. Dichotomy in metal ions binding to N1 and N7 purines. In: Sigel H, ed. Metal ions in biological systems. NY 1996 Marcel Deccer Inc. 1996;61–87.Search in Google Scholar
[194] Gasowska A. Interaction centres of purine nucleotides: Adenosine-5′-diphosphate and adenosine-5’-triphosphate in their reactions with tetramines and Cu(II) ions. J Inorg Biochem 2003;96:346–56.10.1016/S0162-0134(03)00150-8Search in Google Scholar
[195] Gasowska A, Lomozik L, Bregier-Jarzebowska R. Solution structure of Cu(II) complexes with spermine and nucleosides or nucleotides In ternary systems. Polish J Chem 1999;73:909–13.Search in Google Scholar
[196] Gasowska A. Coordination centres of purine nucleotides: Adenosine-5′-diphosphate and adenosine-5’-triphosphate in their reactions with nickel(II), cobalt(II) and tetramines. Z Anorg Allg Chem 2006;632:2281–7.10.1002/zaac.200600114Search in Google Scholar
[197] Lomozik L, Odani A, Yamauchi O. Spectroscopic studies on complex formation and non covalent interactions in ternary palladium(II) systems involving spermidine and 2,3 diaminopropionate or 2,4 diamonobutyrate. Inorg Chim Acta 1994;219:107–14.10.1016/0020-1693(94)03837-6Search in Google Scholar
[198] Jastrzab R, Runka T, Skowronek P, Lomozik L. The effect of spermine concentration on the solution structure of complexes formed in copper(II)/adenosine-5’-triphosphate/phosphoserine system. J Inorg Biochem 2010;104:868–76.10.1016/j.jinorgbio.2010.04.006Search in Google Scholar PubMed
[199] Gasowska A, Jastrzab R, Lomozik L. Specific type of interactions In the quaternary system of Cu(II), adenosine 5’-triphosphate, 1,11-diamino-4,8-diazaundecane and uridine. J Inorg Biochem 2007; 101:1362–9.10.1016/j.jinorgbio.2007.05.009Search in Google Scholar PubMed
[200] Dyson PJ, Sava G. Metal-based antitumour drugs in the post genomic era. Dalton Trans 2006;16:1929–33.10.1039/b601840hSearch in Google Scholar PubMed
[201] Gielen M. Metal Based Antitumor Drugs. Freud, London UK 1988.Search in Google Scholar
[202] Farrell N. Uses of Inorganic Chemistry in Medicine. Royal society of chemistry, Cambridge, UK, 1999.10.1039/9781847552242Search in Google Scholar
[203] Fricker SP. Metal based drugs: from serendipity to design. Dalton Tran 2007;43:4903–17.10.1039/b705551jSearch in Google Scholar PubMed
[204] Hannon MJ. Metal-based anticancer drugs: From a past anchored in platinum chemistry to a post-genomic future of diverse chemistry and biology. Pure Appl Chem 2007;79:2243–61.10.1351/pac200779122243Search in Google Scholar
[205] Rijt SH, Sadler PJ. Current applications and future potential for bioinorganic chemistry in the development of anticancer drugs. Drug Discov Today 2009;14:1089–97.10.1016/j.drudis.2009.09.003Search in Google Scholar PubMed PubMed Central
[206] Farrell N. Polynuclear platinum drugs. In: Sigel H. Metal Ions in Biological Systems: Volume 42: Metal Complexes in Tumor Diagnosis and as Anticancer Agents. 2004;251–96.10.1201/b12414-8Search in Google Scholar
[207] Lebwohl D, Canetta R. Clinical development of platinum complexes in cancer therapy: An historical perspective and an update. Eur J Cancer 1998;34:1522–34.10.1016/S0959-8049(98)00224-XSearch in Google Scholar
[208] Brunner H, Hankofer P, Holzinger U, Treittinger B, Schonenberger H. Synthesis and antitumor activity of platinum(II) complexes containing substituted ethylenediamine ligands. Eur J Med Chem 1990;25:35–44.10.1016/0223-5234(90)90162-VSearch in Google Scholar
[209] Farrell N, Qu Y, Hacker MP. Cytotoxicity and antitumor activity of bis(platinum) complexes. A novel class of platinum complexes active in cell lines resistant to both cisplatin and 1,2-diaminocyclohexane complexes. J Med Chem 1990;33:2179–84.10.1021/jm00170a021Search in Google Scholar
[210] Farrell N, Qu Y, Feng L, Van Houten B. A comparison of chemical reactivity, cytotoxicity, interstrand crosslinking and DNA sequence specificity of bis(platinum) complexes containing monodentate or bidentate coordination spheres with their monomeric analogs. Biochem 1990;29:9522–31.10.1021/bi00493a005Search in Google Scholar
[211] Roberts JD, Van Houten B, Qu Y, Farrell NP. Interaction of novel bis(platinum) complexes with DNA. Nucleic Acids Res 1989;17:9719–33.10.1093/nar/17.23.9719Search in Google Scholar
[212] Navarro-Ranninger C, Zamora F, Perez JM, Lopez-Solera I, Martinez-Carrera S, Masaguer JR, Alonso C. Palladium(II) salt and complexes of spermidine with a six-member chelate ring. Synthesis, characterization, and initial DNA-binding and antitumor studies. J Inorg Biochem 1992;46:267–79.10.1016/0162-0134(92)80037-VSearch in Google Scholar
[213] Navarro-Ranninger C, Perez JM, Zamora F, Gonzales VM, Masaguer JR, Alonso C. Palladium(II) compounds of putrescine and spermine. Synthesis, characterization, and DNA-binding and antitumor properties. J Inorg Biochem 1993;52:37–49.10.1016/0162-0134(93)85621-ESearch in Google Scholar
[214] Alvarez-Valdes A, Perez JM, Lopez-Solera I, Lannegrand R, Continente JM, Amo-Ochoa P, Camazon MJ, Solans X, Font-Bardia M, Navarro-Ranninger C. Preparation and characterization of platinum(II) and (IV) complexes of 1,3-diaminepropane and 1,4-diaminebutane: Circumvention of cisplatin resistance and DNA interstrand cross-link formation in CH1cisR ovarian tumor cells. J Med Chem 2002;45:1835–44.10.1021/jm010968lSearch in Google Scholar
[215] Teixeira LJ, Seabra M, Reis E, Girao da Cruz MT, Pedroso de Lima MC, Pereira E, Miranda MA, Marques MPM. Cytotoxic activity of metal complexes of biogenic polyamines: Polynuclear platinum(II) chelates. J Med Chem 2004;47:2917–25.10.1021/jm0311238Search in Google Scholar
[216] Navarro-Ronninger C, Amo-Ochoa P, Perez JM, Gonzalez VM, Masaguer JM, Alonso C. Platinum (II) and (IV) spermidine complexes. Synthesis, characterization, and biological studies. J Inorg Biochem 1994;53:177–90.10.1016/0162-0134(94)80003-0Search in Google Scholar
[217] Rauter H, Di Domenico R, Menta E, Oliva A, Qu Y, Farrell N. Selective platination of biologically relevant polyamines. Linear coordinating spermidine and spermine as amplifying linkers in dinuclear platinum complexes. Inorg Chem 1997;36:3919–27.10.1021/ic9701827Search in Google Scholar
[218] Jung Y, Lippard SJ. Direct cellular responses to platinum-induced DNA damage. Chem Rev 2007;107:1387–407.10.1021/cr068207jSearch in Google Scholar
[219] Rau T, van Eldik R. Metal Ions in Biological Systems. NewYork, NY, USA, 1996.Search in Google Scholar
[220] Orvig C, Abrams MJ. Medicinal inorganic chemistry: Introduction. Chem Rev 1999;99:2202–3.10.1021/cr980419wSearch in Google Scholar
[221] Allardyce CS, Dorcier A, Scolaro C, Dyson PJ. Development of organometallic (organo-transi-tion metal) pharmaceuticals. Appl Organomet Chem 2005;19:1–10.10.1002/aoc.725Search in Google Scholar
[222] Garbutcheon-Singh KB, Grant MP, Harper BW. Transition metal based anticancer drugs. Curr Top Med Chem 2011;11:521–42.10.2174/156802611794785226Search in Google Scholar
[223] Gomez-Ruiz S, Maksimovic-Ivanic D, Mijatovic S, Kaluderovic GN. On the discovery, biological effects, and use of cisplatin and metallocenes in anticancer chemotherapy. Bioinorg Chem Appl 2012;2012, Article ID 140284, 14 pages.10.1155/2012/140284Search in Google Scholar
[224] Amo-Ochoa P, Gonzalez VM, Perez JM, Masaguer JR, Alonso C, Navarro-Ranninger C. Cytotoxicity, DNA binding, and reactivity against nucleosides of platinum (II) and (IV) spermine compounds. J Inorg Biochem 1996;64:287–99.10.1016/S0162-0134(96)00082-7Search in Google Scholar
[225] Farrell N. Nonclassical platinum antitumor agents: perspectives for design and development of new drugs complementary to cisplatin. Cancer Invest 1993;11:578–89.10.3109/07357909309011676Search in Google Scholar
[226] Jansen BAJ, Van der Zwan J, Reedijk J, Den Dulk H, Brouwer JA. A tetranuclear platinum compound designed to overcome cisplatin resistance. Eur J Inorg Chem 1999;9:1429–33.10.1002/(SICI)1099-0682(199909)1999:9<1429::AID-EJIC1429>3.0.CO;2-8Search in Google Scholar
[227] Calvert AH, Thomas H, Colombo N, Gore M, Earl H, Sena L, Camboni G, Liati P, Sessa C. Phase II clinical study of BBR 3464, a novel, bifunctional platinum analogue, in patients with advanced ovarian cancer. Eur J Cancer 2001;37:260–7.10.1016/S0959-8049(01)81457-XSearch in Google Scholar
[228] Souzu H. Fluorescence polarization studies on Escherichia coli membrane stability and its relation to the resistance of the cell to freeze-thawing. I. Membrane stability in cells of differing growth phase. Biochem Biphys Acta 1986;861:353–60.10.1016/0005-2736(86)90438-4Search in Google Scholar
[229] Alvarez-Valdes A, Perez JM, Lopez-Solera I, Lannegrand R, Continente JM, Amo-Ochoa P, Camazon MJ, Solans X, Font-Bardia M, Navarro-Ranninger C. Preparation and characterization of platinum(II) and (IV) complexes of 1,3-diaminepropane and 1,4-diaminebutane: Circumvention of cisplatin resistance and DNA interstrand cross-link formation in CH1cisR ovarian tumor cells. J Med Chem 2002;45:1835–44.10.1021/jm010968lSearch in Google Scholar PubMed
[230] Nishioka K. Polyamines in Cancer: Basic Mechanisms and Clinical Approaches, Springer, Berlin, Germany, 1966.Search in Google Scholar
[231] Qu Y, Scarsdale NJ, Tran MC, Farrell NP. Cooperative effects in long-range 1,4 DNA-DNA interstrand cross-links formed by polynuclear platinum complexes: An unexpected syn orientation of adenine bases outside the binding sites. J Biol Inorg Chem 2003;8:19–28.10.1007/s00775-002-0383-xSearch in Google Scholar PubMed
[232] Chvalova K, Kasparkova J, Farrell N, Brabec V. Deoxyribonuclease I footprinting reveals different DNA binding modes of bifunctional platinum complexes. FEBS J 2006;273:3467–78.10.1111/j.1742-4658.2006.05356.xSearch in Google Scholar PubMed
[233] Monti E, Gariboldi M, Maiocchi A. Cytotoxicity of cisplatinum(II) conjugate models. The effect of chelating arms and leaving groups on cytotoxicity: A quantitative structure-activity relationship approach. J Med Chem 2005;48:857–66.10.1021/jm049508tSearch in Google Scholar PubMed
[234] Costa Couri MR, Vieira de Almeida M, Soares Fontes AP. Synthesis of polyamines from ethylenediamine and their platinum(II) complexes, potential antitumor agents. Eur J Inorg Chem 2006;9:1868–74.10.1002/ejic.200501024Search in Google Scholar
[235] Liu Q, Qu Y, van Antwerpen R, Farrell N. Mechanism of the membrane interaction of polynuclear platinum anticancer agents. Implications for cellular uptake. Biochem 2006;45:4248–56.10.1021/bi052517zSearch in Google Scholar PubMed
[236] Williams JW, Qu Y, Bulluss GH, Alvorado E, Farrell NP. Dinuclear platinum complexes with biological relevance based on the 1,2-diaminocyclohexane carrier ligand. Inorg Chem 2007;46:5820–2.10.1021/ic700410ySearch in Google Scholar PubMed
[237] Komeda S, Moulaei T, Chikuma M. The phosphate clamp: A small and independent motif for nucleic acid backbone recognition. Nucleic Acids Res 2011;39:325–36.10.1093/nar/gkq723Search in Google Scholar PubMed PubMed Central
[238] Fiuza SM, Amado AM, Oliveira PJ, Sardão VA, Batista de Carvalho LAE, Marques MPM. Pt(II) vs Pd(II) polyamine complexes as new anticancer drugs: A structureactivity study. Lett Drug Des Discov 2006;3:149–51.10.2174/157018006776286989Search in Google Scholar
[239] Marques MPM, Girão T, Pedroso de Lima MC, Gameiro A, Pereira E, Garcia P. Cytotoxic effects of metal complexes of biogenic polyamines. I. Platinum(II) spermidine compounds: Prediction of their antitumour activity. BBA-Mol Cell Res 2002;1589:63–70.10.1016/S0167-4889(01)00186-0Search in Google Scholar
[240] Soares AS, Fiuza SM, Gonçalves MJ, Batista de Carvalho LAE, Marques MPM, Urbano AM. Effect of the metal center on the antitumor activity of the analogous dinuclear spermine chelates (PdCl2)2(Spermine) and (PtCl2)2(Spermine). Lett Drug Des Discov 2007;4:460–3.10.2174/157018007781788516Search in Google Scholar
[241] Navarro-Ranninger C, Perez JM, Zamora F, Gonzalez VM, Masaguer JR, Alonso C. Palladium(II) compounds of putrescine and spermine. Synthesis, characterization, and DNA-binding and antitumor properties. J Inorg Bioch 1993;52:37–49.10.1016/0162-0134(93)85621-ESearch in Google Scholar
[242] Navarro M, Peña NP, Colmenares I, González T, Arsenak M, Taylor P. Synthesis and characterization of new palladium-clotrimazole and palladium-chloroquine complexes showing cytotoxicity for tumor cell lines in vitro. J Inorg Biochem 2006;100:152–7.10.1016/j.jinorgbio.2005.10.013Search in Google Scholar PubMed
[243] Hegmans A, Kasparkova J, Vrana O, Kelland LR, Brabec V, Farrell NP. Amide-based prodrugs of spermidinebridged dinuclear platinum. Synthesis, DNA binding, and biological activity. J Med Chem 2008;51:2254–60.10.1021/jm070813zSearch in Google Scholar PubMed PubMed Central
[244] Fiuza SM, Amado AM, dos Santos HF, Marques MPM, Batista de Carvalho LAE. Conformational and vibrational study of cis-diamminedichloropalladium(II). Phys Chem Chem Phys 2010;12:14309–21.10.1039/c0cp00957aSearch in Google Scholar PubMed
[245] Tummala R, Diegelman P, Fiuza SM. Characterization of Pt-, Pd-spermine complexes for their effect on polyamine pathway and cisplatin resistance in A2780 ovarian carcinoma cells. Oncol Rep 2010;24:15–24.Search in Google Scholar
[246] Tummala R, Diegelman P, Hector S. Combination effects of platinum drugs and N1, N11 diethylnorspermine on spermidine/spermine N1-acetyltransferase, polyamines and growth inhibition in A2780 human ovarian carcinoma cells and their oxaliplatin and cisplatin-resistant variants. Cancer Chemoth Pharm 2011;67:401–14.10.1007/s00280-010-1334-9Search in Google Scholar PubMed PubMed Central
[247] Marques MPM. Platinum and Palladium Polyamine Complexes as Anticancer Agents: The Structural Factor, ISRN Spectroscopy 2013; article ID 287353, 29 pages.10.1155/2013/287353Search in Google Scholar
[248] Corduneanu O, Chiorcea-Paquim AM, Fiuza SM, Marques MPM, Oliveira-Brett AM. Polynuclear palladium complexes with biogenic polyamines: AFM and voltammetric characterization. Bioelectrochemistry 2010;78:97–105.10.1016/j.bioelechem.2009.08.003Search in Google Scholar PubMed
[249] Corduneanu O, Chiorcea-Paquim AM, Diculescu V, Fiuza SFM, Marques MPM, Oliveira-Brett AM. DNA interaction with palladium chelates of biogenic polyamines using atomic force microscopy and voltammetric characterization. Anal Chem 2010;82:1245–52.10.1021/ac902127dSearch in Google Scholar PubMed
[250] Fiuza SM, Holy J, Batista de Carvalho LAE, Marques MPM. Biologic activity of a dinuclear Pd(II)-spermine complex toward human breast cancer. Chem Biol Drug Des 2011;77:477–88.10.1111/j.1747-0285.2011.01081.xSearch in Google Scholar
[251] Batista de Carvalho ALM, Fiuza SM, Tomkinson J, Batista de Carvalho LAE, Marques MPM. Pt(II) complexes with linear diamines-part i: Vibrational study of Ptdiaminopropane. Inter J Spect 2012;27:403–13.10.1155/2012/206297Search in Google Scholar
[252] Kasparaková J, Zehnulova J, Farrell N, Brabec V. DNA: Replication repair and recombination. J Biol Chem 2002;277:48076–86.Search in Google Scholar
[253] Manzotti C, Pratesi G, Menta E, Di Domenico R, Cavalletti E, Fiebig HH, Kelland LR, Farrell N, Polizzi D, Supino R, Pezzoni G, Zunino F. BBR 3464: A novel triplatinum complex, exhibiting a preclinical profile of antitumor efficacy different from cisplatin. Clin Cancer Res 2000;6:2626–34.Search in Google Scholar
[254] Perego P, Caserini C, Gatti L, Carenini N, Romanelli S, Supino R, Colangelo D, Viano I, Leone R, Spinelli S, Pezzoni G, Manzotti G, Farrell N, Zunino F. A novel trinuclear platinum complex overcomes cisplatin resistance an osteosarcoma cell system. Mol Pharmacol 1999;55:528–34.Search in Google Scholar
[255] Friebolin W, Schilling G, Zöller M, Amtmann E. Antitumoral activity of non-platinum xanthate complexes. J Med Chem 2005;48:7925–31.10.1021/jm040899lSearch in Google Scholar
[256] Messere A, Fabri E, Borgatti M, Gambari R, Di Blasio B, Pedone C, Romanelli A. Antiproliferative activity of Pt(II) and Pd(II) phosphine complexes with thymine and thymidine. J Inorg Biochem 2007;101:254–60.10.1016/j.jinorgbio.2006.09.022Search in Google Scholar
[257] Alverdi V, Giovagnini L, Marzano C, Seraglia R, Bettio F, Sitran S, Graziani R, Fregona D. Characterization studies and cytotoxicity assays of Pt(II) and Pd(II) dithiocarbamate complexes by means of FT-IR, NMR spectroscopy and mass spectrometry. J Inorg Biochem 2004;98:1117–28.10.1016/j.jinorgbio.2004.03.011Search in Google Scholar
[258] Marques MPM, Batista de Carvalho LAE. Vibrational spectroscopy studies on linear polyamines. Biochem Soc Trans 2007;35:374–80.10.1042/BST0350374Search in Google Scholar
[259] Silva TM, Andersson S, Sukumaran SK, Maria Paula Marques MP, Persson L, Oredsson S. Norspermidine and novel Pd(II) and Pt(II) polynuclear complexes of norspermidine as potential antineoplastic agents against breast cancer. PLoS ONE 2013;8:e55651.10.1371/journal.pone.0055651Search in Google Scholar
[260] Tadolini B. The influence of polyamine-nucleic acid complexes on Fe2+ autoxidation. Mol Cell Biochem 1988;83:179–85.10.1007/BF00226145Search in Google Scholar
[261] Matkovics B, Kecskemeti V, Varga SI, Novak Z, Kertesz Z. Antioxidant properties of DI- and polyamines. Comp Biochem Physiol B 1993;104:475–9.10.1016/0305-0491(93)90269-BSearch in Google Scholar
[262] Pedreno E, Lopez-Contreras AJ, Cremades A, Penafiel R. Protecting or promoting effects of spermine on DNA strand breakage induced by iron or copper ions as a function of metal concentration. J Inorg Biochem 2005;99:2074–80.10.1016/j.jinorgbio.2005.07.005Search in Google Scholar PubMed
[263] Kelland L. The resurgence of platinum-based cancer chemotherapy. Nature Rev Cancer 2007;7:573–84.10.1038/nrc2167Search in Google Scholar
[264] Gatti L, Perego P, Leone R. Novel bis-platinum complexes endowed with an improved pharmacological profile. Mol Pharmaceut 2010;7:207–16.10.1021/mp900211jSearch in Google Scholar
[265] Jodrell DI, Evans TRJ, Steward W. Phase II studies of BBR3464, a novel tri-nuclear platinum complex, in patients with gastric or gastro-oesophageal adenocarcinoma. Eur J Cancer 2004;40:1872–7.10.1016/j.ejca.2004.04.032Search in Google Scholar
[266] Bonomi R, Saielli G, Scrimin P, Mancin F. An experimental and theoretical study of the mechanism of cleavage of an RNA-model phosphate diester by mononuclear Zn(II) complexes. Supramol Chem 2013;25:665–71.10.1080/10610278.2013.830724Search in Google Scholar
[267] Harris AL, Yang X, Hegmans A. Synthesis, characterization, and cytotoxicity of a novel highly charged trinuclear platinum compound. Enhancement of cellular uptake with charge. Inorg Chem 2005;44:9598–600.10.1021/ic051390zSearch in Google Scholar
[268] Hallinan N, Besançon V, Forster M, Elbaze G, Ducommun Y, Merbach AE. High-pressure NMR kinetics. 47. Solvent-exchange mechanisms of nonaqueous square-planar tetrasolvates: A high-pressure proton NMR investigation. Inorg Chem 1991;30:1112–4.10.1021/ic00005a043Search in Google Scholar
[269] Frey U, Elmroth S, Moullet B, Elding LI, Merbach AE. Proton NMR kinetic study of ligand exchange on bis(1,4-dithiane)platinum(II), bis(1,4-dithiane)palladium(II), and tetrakis(dimethyl sulfide)platinum(II). Inorg Chem 1991;30:5033–7.10.1021/ic00026a034Search in Google Scholar
[270] Hohmann H, Hellquist B, Van Eldik R. Effect of steric hindrance on kinetic and equilibrium data for substitution reactions of diaqua(N-substituted ethylenediamine)palladium(II) with chloride in aqueous solution. Inorg Chim Acta 1991;188:25–32.10.1016/S0020-1693(00)80912-3Search in Google Scholar
[271] Brabec V, Kasparkova J. Modifications of DNA by platinum complexes. Relation to resistance of tumors to platinum antitumor drugs. Drug Resist Updat 2005;8:131–46.10.1016/j.drup.2005.04.006Search in Google Scholar PubMed
[272] Esteban-Fernández D, Moreno-Gordaliza E, Cañas B, Palacios MA, Gómez-Gómez MM. Analytical methodologies for metallomics studies of antitumor Pt-containing drugs. Metallomics 2010;2:19–38.10.1039/B911438FSearch in Google Scholar PubMed
[273] Zhu Q, Huang Y, Marton LJ, Woster PM, Davidson NE, Casero RA. Polyamine analogues modulate gene expression by inhibiting lysine-specific demethylase 1(LSD1) and altering chromatin structure in human breast cancer cells. Amino Acids 2011;42:887–98.10.1007/s00726-011-1004-1Search in Google Scholar
[274] Rosenberg B, Camp LV, Mansour JE, Mansour VH. Platinum compounds: A new class of potent antitumour agents. Nature 1969;222:385–6.10.1038/222385a0Search in Google Scholar
[275] Alderden RA, Hall MD, Hambley TW. The discovery and development of cisplatin. J Chem Educ 2006;83:728–34.10.1021/ed083p728Search in Google Scholar
[276] Wong E, Giandomenico CM. Current status of platinum-based antitumor drugs. Chem Rev 1999;99:2451–66.10.1021/cr980420vSearch in Google Scholar
[277] Sedletska Y, Giraud-Panis MJ, Malinge JM. Cisplatin is a DNA-damaging antitumour compound triggering multifactorial biochemical responses in cancer cells: Importance of apoptotic pathways. Curr Med Chem Anticancer Agents 2005;5:251–65.10.2174/1568011053765967Search in Google Scholar
[278] Farrell N, McCleverty JA, Meyer TJ. Metal complexes as drugs and chemotherapeutic agents. In: Comprehensive Coordination Chemistry II. Pergamon, Oxford, UK, 2003:809–40.10.1016/B0-08-043748-6/09021-6Search in Google Scholar
[279] Guo ZJ, Sadler PJ. Metals in medicine. Angew Chem Int Edit 1999;38:1513–31.10.1002/(SICI)1521-3773(19990601)38:11<1512::AID-ANIE1512>3.0.CO;2-YSearch in Google Scholar
[280] Messori L, Marcon G. Gold complexes as antitumor agents. In: Metal Ions in Biological Systems, Vol 42: Metal complexes in tumor diagnosis and as anticancer agents. Marcel Dekker, New York, USA, 2004;385–424.10.1201/b12414-12Search in Google Scholar
[281] Galanski M, Arion VB, Jakupec MA, Keppler BK. Recent developments in the eld of tumor-inhibiting metal complexes. Curr Pharm Des 2003;9:2078–89.10.2174/1381612033454180Search in Google Scholar
© 2016 by Walter de Gruyter Berlin/Boston

![Figure. 15 Polyamine-bridged polynuclear Pt(II) complexes: BBR3464 (Triplatin) [254] (a), Pt2Spm (Spm = spermine) [240] (b), and TriplatinNC [267] (c) [247].](/document/doi/10.1515/psr-2016-0003/asset/graphic/j_psr-2016-0003_fig_015.jpg)
